Abstract
Background
Lung mucociliary clearance provides the first line of defense from lung infections and is impaired in individuals who consume heavy amounts of alcohol. Previous studies have demonstrated that this alcohol-induced ciliary dysfunction (AICD) occurs through impairment of the nitric oxide (NO) and cyclic nucleotide-dependent kinase-signaling pathways in lung airway ciliated epithelial cells. Recent studies have established that all key elements of this alcohol-driven signaling pathway co-localize to the apical surface of the ciliated cells with the basal bodies. These findings led us to hypothesize that alcohol activates the cilia stimulation pathway at the organelle level. To test this hypothesis we performed experiments exposing isolated demembranated cilia (isolated axonemes) to alcohol and studied the effect of alcohol-stimulated ciliary motility on the pathways involved with isolated axoneme activation.
Methods
Isolated demembranated cilia were prepared from bovine trachea and activated with adenosine triphosphate (ATP). Ciliary beat frequency (CBF), NO production, adenylyl and guanylyl cyclase activities, cAMP- and cGMP-dependent kinase activities were measured following exposure to biologically relevant concentrations of alcohol.
Results
Alcohol rapidly stimulated axoneme beating 40% above baseline at very low concentrations of alcohol (1-10 mM). This activation was specific to ethanol, required the synthesis of NO, the activation of soluble adenylyl cyclase (sAC) and the activation of both cAMP- and cGMP-dependent kinases (PKA and PKG), all of which were present in the isolated organelle preparation.
Conclusions
Alcohol rapidly and sequentially activates the eNOS→NO→GC→cGMP→PKG and sAC→cAMP→ PKA dual signaling pathways in isolated airway axonemes. These findings indicate a direct effect of alcohol on airway cilia organelle function and fully recapitulate the alcohol-driven activation of cilia known to exist in vivo and in intact lung ciliated cells in vitro following brief moderate alcohol exposure. Furthermore, these findings indicate that airway cilia are exquisitely sensitive to the effects of alcohol and substantiate a key role for alcohol in the alterations of mucociliary clearance associated with even low levels of alcohol intake. We speculate that this same axoneme-based alcohol activation pathway is down regulated following long term high alcohol exposure and that the isolated axoneme preparation provides an excellent model for studying the mechanism of alcohol-mediated cilia dysfunction.
Keywords: cilia, axonemes, mucociliary clearance, alcohol, ethanol, cAMP, cGMP, PKA, PKG, lung epithelium
Introduction
Cilia are the fingerlike motor-driven organelles that provide the force required to expel inhaled particles and mucus from the lung airways. While motile lung cilia are normally part of the airway epithelial cell and covered with the plasma membrane, they can function as isolated demembranated motile organelles or axonemes that beat when activated by exogenous adenosine triphosphate (ATP; (Hastie et al., 1986). Studies of ATP-activated axonemes have provided valuable insights into the signaling molecules that regulate ciliary motility (Wyatt et al., 2005).
Key cilia regulatory molecules that are tightly associated with axonemes include nitric oxide synthase, adenylyl and guanylyl cyclases and the cyclic nucleotide-dependent kinases, PKA and PKG (Stout et al., 2007). A number of physiologic and pharmacologic compounds, such as beta agonists, can stimulate cilia to beat faster through this pathway. Surprisingly, alcohol is also a potent short-term activator of ciliary motility (Sisson, 1995).
Moderate brief alcohol exposure is known to stimulate airway ciliary motility through a nitric oxide- and protein kinase-dependent pathway, which in theory should enhance mucociliary clearance (Sisson, 1995; Sisson et al., 1999; Wyatt et al., 2003). In contrast, heavy and prolonged alcohol intake is known to impair mucociliary clearance through a subsequent downregulation of this same cyclic nucleotide-dependent kinase-signaling pathway (Wyatt et al., 2004). Because alcohol can modulate this critical cilia signaling mechanism, we hypothesized that alcohol directly activates isolated demembranated axonemes. To test this hypothesis we studied the effects of alcohol on airway axoneme motility and the axoneme-associated ciliary regulatory proteins using isolated demembranated bovine tracheal axonemes. We found that alcohol, at very low and biologically relevant concentrations, triggers the production of nitric oxide, stimulates a soluble adenylyl cyclase and sequentially activates PKG followed by PKA. These findings indicate that the cilia signaling molecules that alcohol acts through to stimulate motility are tightly associated with the axoneme and provide a unique model for studying alcohol effects on motile organelles.
Materials & Methods
Materials
Reagents used include alcohol (Decon Labs, King of Prussia, PA), Heptapeptide substrate (RKRSRAE) for the PKG activity assay (Gift of Dr. Sam Sanderson), Kemptide (LRRASLG) for the PKA activity assay (Bachem Biosciences, Inc., Torrance, CA). All other reagents were obtained from Sigma-Aldrich, St. Louis, MO.
Airway axoneme extraction and preparation
Airway axonemes were isolated from bovine ciliated epithelium by a modification of a previously described method (Hastie, 1995; Wyatt et al., 2005). Briefly, isolated axonemes were extracted from fresh bovine trachea obtained from a local abattoir. Excess adipose and connective tissue were removed from the trachea followed by two washes with phosphate buffered saline (PBS). The proximal and distal tracheal ends were closed with large hemostats after the addition of 15 ml of extraction buffer containing 20 mM Tris-HCl, 50 mM NaCl, 10 mM calcium chloride, 1 mM EDTA, 7 mM 2-mercaptoethanol, 100 mM Triton X-100, and 1 mM dithiothreitol. The trachea was then shaken for 90 seconds after which the extraction buffer containing released axonemes was filtered through a 100-μm polyproplylene mesh and centrifuged at 17,250g for 7 min. The pelleted axonemes were resuspended after discarding the supernatant, to a concentration of 1 mg/ml in resuspension buffer consisting of 20 mM Tris HCl, 50 mM KCl, 4 mM MgCl2, 0.5 mM EDTA, 1 mM dithiothreitol, 10 mM soybean trypsin inhibitor, and 25% sucrose (w/v). Axoneme preparations from four different trachea were used in this study with consistent results. Isolated axonemes were stored in aliquots at -80°C for use up to six months after isolation.
Experimental treatment of axonemes
Frozen aliquots of isolated axonemes were thawed and maintained at 4°C on ice for up to 4 hours. For each experimental condition, isolated axoneme samples were diluted to a final concentration of 0.25 mg/ml in microcentrifuge tubes by adding various reagents in resuspension buffer and incubating at room temperature. At each condition measured, isolated axonemes were removed from the sample microcentrifuge tube, pipetted onto one well of a 48-well polystyrene tissue culture plate along with 10 μl resuspension buffer and spun in a Sorvall T6000D for 2 minutes at 400g. Following centrifugation, the plate of isolated axonemes was returned to the Sisson-Ammons Video Analysis (SAVA, Sisson et al., 2003; Wyatt et al., 2005) system where 10 μl aliquots of ATP and other reagents for specific experiments and a volume of resuspension buffer were added to equilibrate volumes between conditions.
Measurement of isolated ciliary beat frequency
The ciliary beat frequency (CBF) of isolated demembranated axonemes was derived using the SAVA system. Axonemes were anchored by centrifugation to prevent motion, with the majority of axonemes bending in each field of view. The experiments were captured as described previously (Wyatt et al., 2005) with temperature of all experiments controlled by a thermostatic stage to remain constant at 26°C. The sampling rate was set at 85 frames per second for all experimental conditions. Axonemes with a frequency of ≤ 2 Hz were not analyzed and considered nonmotile, as were data points with less than 10% of the original numbers of beating points. For each experimental condition, a minimum of six separate fields were captured, analyzed, and expressed as mean ± SEM for each data point. Significance for paired samples was determined using the Student's t test with P value < 0.05. Significance for more than two conditions was determined using a one-way ANOVA with P value < 0.05.
Cyclic nucleotide-dependent kinase activity assays
PKA activity was determined in isolated axonemes extracted from bovine trachea prepared as described above. The isolated axoneme protein (1 mg/ml) was assayed in the presence or absence of 10 μM cAMP by a modification of the methods previously described (Wyatt et al., 2005) using a reaction mix consisting of 130 mM PKA heptapeptide substrate in a buffer containing 20 mM Tris HCl (pH 7.5), 100 μM IBMX, 20 mM magnesium-acetate, and 200 μM [γ-32P] ATP. PKG activity was assayed with PKG heptapeptide substrate, the addition of 10 μM cGMP, and in the presence of 2 μg/ml protein kinase inhibitor peptide (PKI) (Wyatt et al., 2005). Isolated axonemes (20 μl) were added to 50 μl of the reaction mix described above and incubated for 15 min at 30°C. Reactions were halted in 75 mM phosphoric acid after spotting the assay mix (60 μl) onto P-81 phosphocellulose papers (Whatman, Hillsboro, OR). Papers were then washed five times for 5 min in 75 mM phosphoric acid and washed once in ethanol. Dried papers were counted in non-aqueous scintillation fluid, and enzyme activity was expressed as pmol of phosphate incorporated into substrate peptide per min per mg of total assay protein. Each experiment was conducted three separate times (n=3) and the results expressed as the mean ± SEM for each data point. Significance was determined using a one-way ANOVA and accepted at the 95% Confidence Interval if the P value < 0.05.
Nitric oxide analysis
Isolated axonemes were treated with 10 mM ethanol in a final volume of 50 μl at room temperature for 30 minutes prior to the addition of ATP. Following a 10 minute incubation, the supernatants were harvested and stored at -80°C for NO analysis. For the experiments using L-arginine, the ethanol was simultaneously pre-incubated for 30 minutes prior to addition of ATP. NO was measured indirectly in the liquid phase, using a Sievers NOA 280 chemiluminescence analyzer (Boulder, CO) using vanadium chloride reduction to convert oxidation products back to nitric oxide (Wyatt et al., 2005). The level of NO detected in axoneme supernatants was compared to the amount of NO present in resuspension buffer incubated under the same conditions in the absence of axonemes. Calculations are extrapolated from a standard curve made by injections of 1 nM – 100 μM sodium nitrate. For each experimental condition, a minimum of six separate measurements was expressed as mean ± SEM for each data point. Significance was determined using a one-way ANOVA with P value < 0.05.
Results
Alcohol stimulates isolated axonemal beating in time- and concentration-dependent fashion
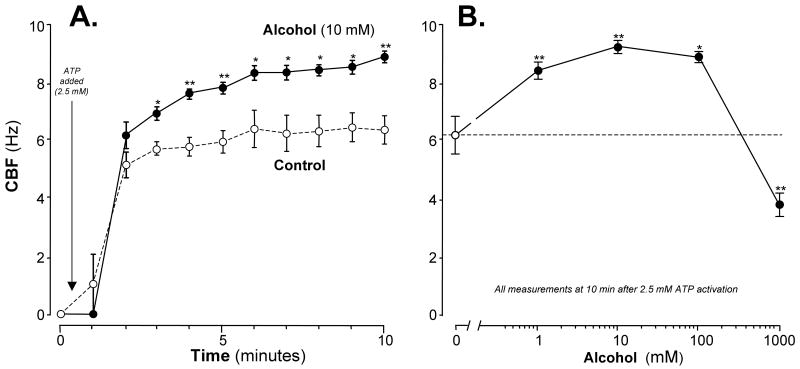
Isolated cilia, activated by 2.5 mM ATP, beat at an average CBF of ∼6 Hz during the 10 minute exposure. In contrast, isolated axonemes pre-incubated for up to 30 min at 0°C with 10 mM alcohol beat much faster with an average CBF of 8.5 Hz, representing a net stimulation over control of ∼40% (Figure 1A). Very low concentrations of alcohol stimulated isolated axonemes beating with significantly detectable stimulation at 1 mM (Figure 1B). CBF stimulation was maximal at 10 mM alcohol and was actually slower than control CBF at 1 M alcohol suggesting ethanol toxicity. Based on these findings all subsequent experiments were conducted after a 10-minute exposure and at a concentration of 10 mM alcohol.
Figure 1. Alcohol stimulates isolated cilia in a time- and concentration-dependent manner.
Panel A: Time course. Ciliary beat frequency (CBF) is represented on the vertical axis in cycles/sec (Hz). Time is represented on the horizontal axis in minutes. A pre-activation baseline CBF measurement was made at time = 0. Adenosine triphosphate (ATP), at a concentration of 2.5 mM, was added at T=0.5 minutes to initiate cilia activation. The open circles with dashed lines indicate CBF of isolated cilia resuspended in buffer alone. The closed circles with solid lines indicate CBF of isolated cilia resuspended in buffer containing 10 mM alcohol. Within 3 minutes of activation, alcohol-exposed cilia beat faster than control-exposed cilia by approximately 2.5 Hz. Each data point represents the mean ± SEM of three experiments. * P < 0.05 or ** P < 0.01 for each alcohol value compared to control by the Student's t test.
Panel B: Alcohol concentration response. CBF is represented on the vertical axis. All CBF measurements were made after 10 minutes activation with 2.5 mM ATP. The open circle and the dashed line indicates CBF in control buffer with no alcohol (0 mM). The closed circles and solid line indicate alcohol concentrations represented on the horizontal axis in millimolar (mM) in a log10 scale. Alcohol stimulated CBF above control at a concentration of 1 mM with maximal stimulation occurring at 10 mM alcohol. At the highest concentration (1000 mM), alcohol reduced CBF compared to control-cilia. Each data point represents the mean ± SEM of three experiments. Each data point represents the mean ± SEM of three experiments. * P < 0.05 or ** P < 0.01 compared to control by one-way ANOVA.
Stimulation of isolated axoneme motility is specific to ethanol
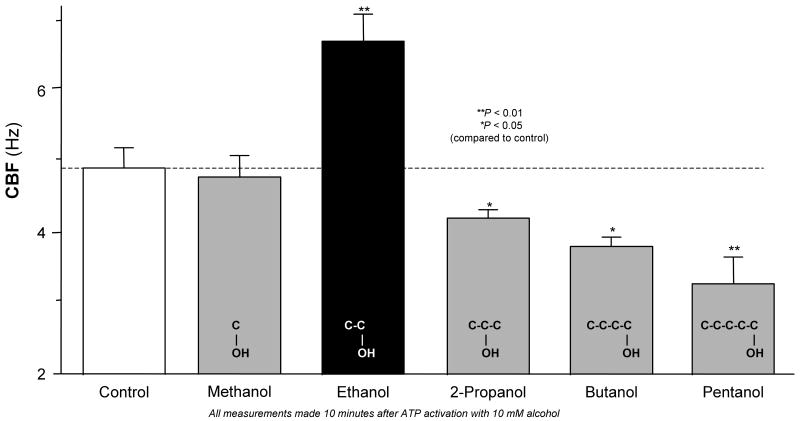
The rapid stimulation of isolated axonemes at extremely low alcohol concentrations was striking and suggested the interaction of alcohol with cilia regulatory proteins. We hypothesized that this alcohol effect on isolated axonemes was specific to ethanol. To test this hypothesis, we pretreated isolated cilia with short chain alcohols ranging from 1-5 carbons in length. While 10 mM ethanol stimulated CBF ∼40% over control following ATP activation, equimolar (10 mM) concentrations of methanol, 2-propanol, butanol and pentanol failed to stimulate CBF. In contrast to ethanol, cilia motility was progressively slowed when isolated cilia were pretreated with > 2-carbon alcohols (Figure 2). Indeed, pentanol reduced CBF ∼1.8 Hz below control values. Taken together, these data suggest that ethanol specifically stimulates isolated axonemes through a specific signaling pathway.
Figure 2. Effects of ethanol and other short-chain alcohols on insolated axoneme beating.
CBF is represented on the vertical axis. All CBF measurements were made after 10 minutes activation with 2.5 mM ATP. The open bar and the dashed line indicate CBF in control buffer with no alcohol. The solid bars represent CBF following exposure to various short chain alcohols at a 10 mM concentration. Gray bars represent non-ethanol short chain alcohols. The black bar represents 10 mM ethanol, which specifically stimulated CBF above baseline compared to the other short chain alcohols and control. Longer chain alcohols, including 2-propanol, butanol and pentanol, reduced CBF compared to control. Each data point represents the mean ± SEM of three experiments. * P < 0.05 or ** P < 0.01 compared to control by one-way ANOVA.
Isolated axoneme stimulation by alcohol requires nitric oxide production
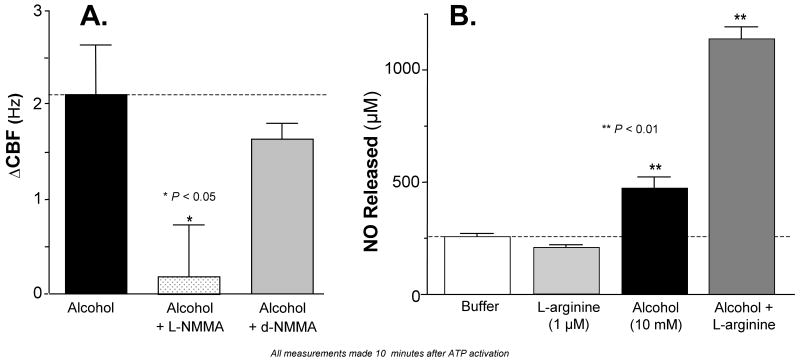
We have previously demonstrated that alcohol stimulation of CBF in intact airway cells requires the production of NO (Wyatt et al., 2003) and that the endothelial isoform of nitric oxide synthase (eNOS) co-localizes with the basal bodies in intact ciliated airway cells (Stout et al., 2007). We therefore hypothesized that alcohol activates cilia-associated eNOS in this isolated organelle preparation. To test this hypothesis, we pre-incubated isolated axonemes with a NOS inhibitor, NG-monomethyl-L-arginine (L-NMMA; 10 μM). Inhibition of NOS by L-NMMA completely blocked alcohol stimulation of isolated axoneme beating while the control inactive D-enantiomer, D-NMMA, had no effect on alcohol stimulation of isolated axoneme beating (Figure 3, panel A). Additional control experiments demonstrated that the addition of L-arginine (1 μM) had no impact on alcohol-stimulated CBF (data not shown). These findings support previous work suggesting that the isolated axoneme preparation contains NOS (Stout et al., 2007), leading us to hypothesize that alcohol stimulates the production of NO by isolated cilia. To test this, we measured NO accumulation in the cilia suspension buffer after preincubating isolated cilia with alcohol (10 mM) with and without exogenous eNOS substrate L-arginine (1 μM). Exposure of isolated cilia to 10 mM alcohol alone stimulated NO approximately two-fold over buffer alone. The addition of L-arginine alone did not alter NO release but when combined with 10 mM alcohol, NO release increased 3.5-fold over baseline (Figure 3, panel B). Taken together, these findings indicate that alcohol requires the production of NO from the isolated cilia preparation to stimulate cilia beating as evidenced by the release of NO into the axoneme suspension buffer.
Figure 3. Alcohol stimulates the release of nitric oxide (NO) and NO-dependent stimulation of CBF in isolated axonemes.
Panel A: Effect of NOS inhibition on alcohol stimulation of CBF. The change in CBF over buffer control is represented on the vertical axis in Hz. All CBF measurements were made after 10 minutes activation with 2.5 mM ATP. Alcohol (10 mM, black bar, dotted line) simulated CBF 2.2 Hz over buffer control. Pre-incubation of axonemes with 1 μM NG-monomethyl-L-arginine (L-NMMA; speckled bar) completely blocked alcohol stimulation of CBF (** P < 0.05 compared to alcohol alone by ANOVA). Pre-incubation of axonemes with 1 μM D-NMMA did not significantly alter alcohol stimulation of CBF.
Panel B: Alcohol-stimulated nitric oxide (NO) release. NO release is represented on the vertical axis as the NO concentration in μM. Experimental conditions are represented on the horizontal axis. Measurements of NO released into the buffer were made after 10 minutes activation with 2.5 mM ATP. Baseline NO release is represented in the white bar. L-arginine did not alter NO release compared to buffer alone. Alcohol alone (10mM; black bar) increased NO release 2-fold over baseline. When axonemes pretreated with 1 μM arginine were exposed to 10 mM alcohol (dark gray bar) NO release increased 4-fold compared to buffer. Each data point represents the mean ± SEM of three experiments. ** P < 0.01 for both Alcohol and Alcohol + L-arginine compared to buffer by one-way ANOVA.
Soluble adenylyl cyclase (sAC) inhibition blocks alcohol stimulation of isolated axonemes
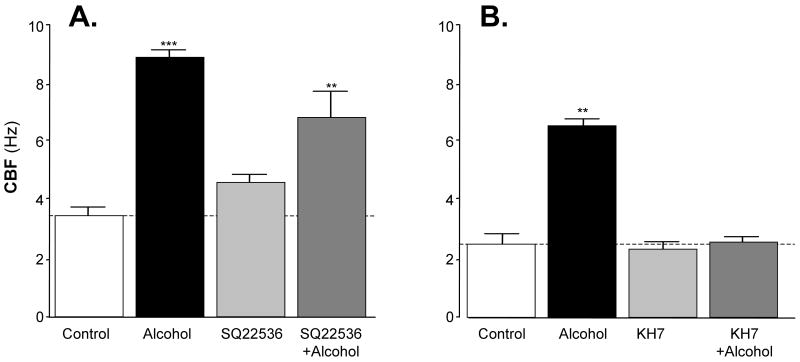
We have previously shown that alcohol stimulates adenylyl cyclase (AC) in intact bovine ciliated airway epithelial cells (Wyatt et al., 2003). While G-protein responsive trans-membrane adenylyl cyclases (tmACs) are certainly important in intact cells, a non-membrane bound source of cAMP, sAC, was recently demonstrated to play an important role in the cAMP-dependent regulation of CBF in intact airway cells (Schmid et al., 2007). To test the hypothesis that sAC could be activated by alcohol in isolated axonemes, we pretreated isolated axonemes with specific inhibitors of both tmAC and sAC followed by exposure to 10 mM alcohol. Alcohol stimulation of CBF was not significantly inhibited by the tmAC-selective inhibitor, SQ22536 (Figure 4, panel A). In contrast, alcohol stimulation of CBF was completely blocked by the sAC inhibitor, KH7 (Figure 4, panel B). These data highlight the role AC activation plays in alcohol stimulation of isolated axonemes and further substantiate the likely intrinsic importance of sAC in cilia regulation at the axoneme/basal body level. They also imply key roles for cyclic nucleotides and their target kinases in the regulation of CBF.
Figure 4. Alcohol stimulation of CBF in isolated cilia requires activation of the soluble adenylyl cyclase (sAC).
CBF is represented on the vertical axes and the experimental condition is represented on the horizontal axes. The open bars indicate buffer-exposed (control) axonemes. The black bars indicate axonemes exposed to 10mM alcohol. The light gray bars indicate axonemes pretreated with each specific cyclase inhibitor. The dark gray bars indicate axonemes pretreated with each specific cyclase inhibitor followed by exposure to 10 mM alcohol. All CBF measurements were made 10 minutes after cilia activation by 2.5 mM ATP. Each data point represents the mean ± SEM of three experiments. ** P < 0.01 and *** P < 0.001 compared to control by one-way ANOVA.
Panel A: Effect of a transmembrane adenylyl cyclase (tmAC) inhibitor, SQ22536, on alcohol-stimulated CBF. Pre-incubation of isolated axonemes for 30 minutes with 1 μM SQ22536 did not significantly change alcohol stimulation of CBF.
Panel B: Effect of a soluble adenylyl cyclase (sAC) inhibitor, KH7, on alcohol-stimulated CBF. Pre-incubation of isolated axonemes for 10 minutes with 10 μM KH7 completely blocked alcohol stimulation of CBF suggesting that alcohol activates a sAC in isolated axonemes.
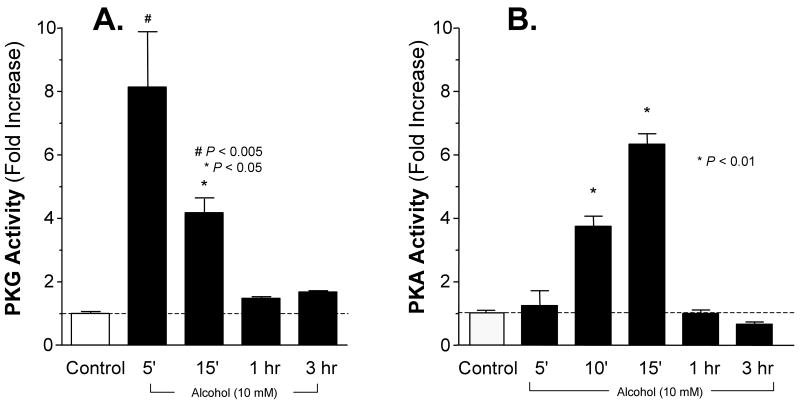
Alcohol drives sequential activation of PKG and PKA in isolated axonemes
We have demonstrated that both PKA and PKG are localized to the axoneme fraction in intact ciliated epithelial cells (Stout et al., 2007). Furthermore, studies of intact airway ciliated cells demonstrate that alcohol activation of PKA requires the precedent activation of PKG (Wyatt et al., 2003). We hypothesized the same kinase sequential activation dependency in alcohol-stimulated isolated axonemes. To test this hypothesis, we measured direct PKG and PKA activity in isolated axonemes exposed to alcohol. Alcohol (10 mM) produced an eight-fold activation of PKG after 5 minutes of alcohol exposure, which returned to baseline levels by 1 hour (Figure 5, panel A). Alcohol similarly activated PKA six-fold but was slower in onset and peaked at 15 minutes after alcohol exposure (Figure 5, panel B). These findings recapitulate that alcohol activates PKG precedent to PKA similar to the sequential PKG-PKA activation observed in intact airway ciliated cells (Wyatt et al., 2003).
Figure 5. Alcohol rapidly and transiently activates cyclic nucleotide-dependent kinases in isolated axonemes.
Kinase activities are represented on the vertical axes expressed as fold increase over Control (white bars, dotted lines). Experimental conditions are represented on the horizontal axes.
Panel A: cGMP-dependent kinase (PKG) activation. Alcohol (10 mM; black bars) activated PKA eight-fold over baseline, which was maximal by 5 minutes and returned to baseline by 1 hour. cGMP (10 μM) caused a 23-fold activation and served as a positive control (data not shown). Each data point represents the mean ± SEM of three experiments. * P < 0.01 and # P < 0.005 compared to Control by one-way ANOVA.
Panel B: cAMP-dependent kinase (PKA) activation. Alcohol (10 mM; black bars) activated PKA six-fold over baseline, which was detectable by 10 minutes, maximal by 15 minutes and returned to baseline by 1 hour. cAMP (10 μM) caused a 9-fold activation and served as a positive control (data not shown) Each data point represents the mean ± SEM of three experiments. * P < 0.01 compared to Control by one-way ANOVA.
Discussion
Alcohol, at very low and biologically relevant concentrations (1, 10 and 100 mM), rapidly stimulated ATP-activated axonemes to beat ∼40% faster than control axonemes. Alcohol's ability to stimulate axoneme beating was unique to ethanol, among low-carbon alcohols tested, indicating high specificity for this ethanol response in cilia. This biologically significant degree of isolated axoneme stimulation by alcohol is comparable to that observed with alcohol exposure of intact cultured bovine ciliated airway cells in vitro (50% CBF stimulation over baseline; (Sisson, 1995). Importantly, it is also equivalent to the CBF stimulation (40% over baseline) present in rat tracheal epithelial cilia after approximately 1 week of 36% alcohol consumption (Wyatt et al., 2004). The findings of the current study parallel this alcohol cilia stimulation in isolated axonemes and strongly supports the hypothesis that the alcohol-sensitive signaling molecules that govern cilia stimulation are tightly associated with the axoneme and are likely modified with long-term high alcohol exposure, leading to alcohol-induced cilia dysfunction.
These and previous studies collectively define a central role for alcohol modifying lung functions related to the production of nitric oxide (Sisson, 2007). These alcohol-modified functions include the regulation of lung vasculature (Polikandriotis et al., 2007; Polikandriotis et al., 2005), alterations of lung immune effectors cells (Greenberg et al., 1998; Greenberg et al., 1999; Zhao et al., 1997; Zhao et al., 1998), and modulation of mucociliary clearance (Sisson, 1995; Sisson et al., 1999; Wyatt et al., 2003). With regards to the latter lung function, the current studies provide important insight into a specific site of action of ethanol, namely the cilia axoneme and associated basal body. In retrospect, this is not surprising since most NO exhaled from the normal lung is derived from the airway epithelium and localized to the base of cilia (Ricciardolo et al., 2004; Stout et al., 2007). This is further supported by our anecdotal observation that isolated cilia produce the highest amount of NO on a per milligram of protein basis (data not shown). The isolation/concentration of axonemes from the ciliated airway epithelium results in high concentrations of eNOS that drive the downstream signaling kinases stimulating increased motility. We acknowledge that the bovine trachea axoneme model is limited to acute exposure experiments in vitro. Chronically feeding cattle controlled amounts of ethanol are prohibitively expensive and not practical. For this reason we limited our experiments to the acute effects of alcohol on isolated axoneme function. Experiments to explore the effects of chronic ethanol on airway axoneme function will require either new approaches to airway cilia functions using small numbers of axonemes (e.g. from mice or rats) or harvesting axonemes from larger animals (e.g. pigs or sheep) that can be chronically fed alcohol.
The two key downstream cyclic nucleotide-dependent kinases mediating the action of alcohol in these studies are PKG and PKA. Similar to our findings in intact primary ciliated airway epithelial cells (Wyatt et al., 2003), alcohol activated both of these kinases and that PKG must be activated before PKA in for cilia to beat faster. The requirement of sequential activation both of these kinases in this dual signaling pathway is a hallmark of alcohol's effect on cilia and highlights the linkage between these two kinase-dependent pathways in the regulation of cilia by various physiologic and pharmacologic agents (Wyatt et al., 2005; Wyatt et al., 2003; Yang et al., 1996; Yang et al., 1997). Indeed, we have previously demonstrated the sequential PKG-PKA activation in isolated bovine axonemes using kinase inhibitor studies (Wyatt et al., 2005). Importantly, as demonstrated by the current study in isolated demembranated axonemes, membrane bound receptors are not required for ethanol-stimulated CBF and highlight the ability of alcohol to freely diffuse into intracellular domains and directly act upon intracellular signaling pathways.
A key upstream enzyme activated by alcohol is adenylyl cyclase. We originally postulated that alcohol stimulation of intact ciliated cells worked by activating a classic particulate trans-membrane bound AC (tmAC) because that cyclase is known to be active in a variety of motile cilia systems (Allen-Gipson et al., 2004; Lieb et al., 2002; Schultz et al., 1992; Weber et al., 2004). More recently, studies of human airway cilia indicate that the AC activated by low pH is a soluble AC (sAC), which led us to challenge our original assumption. Indeed, the detection of AC activity in our demembranated cilia preparation made the presence of membrane-associated AC highly unlikely. Our experiments with inhibitors of both tmAC and sAC strongly support the hypothesis that alcohol directly activates the sAC further highlighting the importance of this axoneme-associated AC in the regulation of ciliary motility as part of a larger cilia activation-signaling pathway.
The alcohol-driven cilia activation pathway demonstrates how closely associated the NOS, cyclases and kinases are with the axoneme. Based on our recent studies that co-localize these regulatory enzymes to the axoneme-associated basal body (Stout et al., 2007), our current findings further substantiate this localization since, from a functional standpoint, all of the alcohol-stimulatory molecules are present in the isolated axoneme preparation. This ciliary metabolon appears to be extremely sensitive to alcohol and suggests an important and novel alcohol mechanism of action.
Acknowledgments
Drs. Sisson and Wyatt are supported by the National Institute on Alcohol Abuse and Alcoholism (5R37AA008769 and R01AA017663) and by a VA Merit Review Grant. This material is based upon work supported in part by the Office of Research and Development, Research Service, Department of Veterans Affairs. The authors thank Dr. Jochen Buck and Dr. Lonny R. Levin of the Department of Pharmacology at Weill Medical College of Cornell University for contributing the KH7 used for experiments reported in this paper. The authors also wish to acknowledge Ms. Lisa Chudomelka for her valuable assistance in the preparation of this manuscript.
Sources of Support: NIH grants R37 AA008769, R01 AA017663 and VA Merit Grant.
Literature Cited
- Allen-Gipson DS, Romberger DJ, Forget MA, May KL, Sisson JH, Wyatt TA. IL-8 inhibits isoproterenol-stimulated ciliary beat frequency in bovine bronchial epithelial cells. J Aerosol Med. 2004;17(2):107–15. doi: 10.1089/0894268041457138. [DOI] [PubMed] [Google Scholar]
- Greenberg SS, Jie O, Zhao X, Wang JF. Role of PKC and tyrosine kinase in ethanol-mediated inhibition of LPS-inducible nitric oxide synthase. Alcohol. 1998;16(2):167–75. doi: 10.1016/s0741-8329(97)00187-0. [DOI] [PubMed] [Google Scholar]
- Greenberg SS, Ouyang J, Zhao X, Parrish C, Nelson S, Giles TD. Effects of ethanol on neutrophil recruitment and lung host defense in nitric oxide synthase I and nitric oxide synthase II knockout mice. Alcohol Clin Exp Res. 1999;23(9):1435–45. [PubMed] [Google Scholar]
- Hastie AT. Isolation of respiratory cilia. Methods Cell Biol. 1995;47:93–8. doi: 10.1016/s0091-679x(08)60795-5. [DOI] [PubMed] [Google Scholar]
- Hastie AT, Dicker DT, Hingley ST, Kueppers F, Higgins ML, Weinbaum G. Isolation of cilia from porcine tracheal epithelium and extraction of dynein arms. Cell Motil Cytoskeleton. 1986;6(1):25–34. doi: 10.1002/cm.970060105. [DOI] [PubMed] [Google Scholar]
- Lieb T, Frei CW, Frohock JI, Bookman RJ, Salathe M. Prolonged increase in ciliary beat frequency after short-term purinergic stimulation in human airway epithelial cells. J Physiol. 2002;538(Pt 2):633–46. doi: 10.1113/jphysiol.2001.013222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polikandriotis JA, Rupnow HL, Brown LA, Hart CM. Chronic ethanol ingestion increases nitric oxide production in the lung. Alcohol. 2007;41(5):309–16. doi: 10.1016/j.alcohol.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polikandriotis JA, Rupnow HL, Hart CM. Chronic ethanol exposure stimulates endothelial cell nitric oxide production through PI-3 kinase-and hsp90-dependent mechanisms. Alcohol Clin Exp Res. 2005;29(11):1932–8. doi: 10.1097/01.alc.0000187597.62590.a4. [DOI] [PubMed] [Google Scholar]
- Ricciardolo FL, Sterk PJ, Gaston B, Folkerts G. Nitric oxide in health and disease of the respiratory system. Physiol Rev. 2004;84(3):731–65. doi: 10.1152/physrev.00034.2003. [DOI] [PubMed] [Google Scholar]
- Schmid A, Sutto Z, Nlend MC, Horvath G, Schmid N, Buck J, Levin LR, Conner GE, Fregien N, Salathe M. Soluble adenylyl cyclase is localized to cilia and contributes to ciliary beat frequency regulation via production of cAMP. J Gen Physiol. 2007;130(1):99–109. doi: 10.1085/jgp.200709784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz JE, Klumpp S, Benz R, Schurhoff-Goeters WJ, Schmid A. Regulation of adenylyl cyclase from Paramecium by an intrinsic potassium conductance. Science. 1992;255(5044):600–3. doi: 10.1126/science.1371017. [DOI] [PubMed] [Google Scholar]
- Sisson JH. Ethanol stimulates apparent nitric oxide-dependent ciliary beat frequency in bovine airway epithelial cells. Am J Physiol. 1995;268(4 Pt 1):L596–600. doi: 10.1152/ajplung.1995.268.4.L596. [DOI] [PubMed] [Google Scholar]
- Sisson JH. Alcohol and airways function in health and disease. Alcohol. 2007;41(5):293–307. doi: 10.1016/j.alcohol.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisson JH, May K, Wyatt TA. Nitric oxide-dependent ethanol stimulation of ciliary motility is linked to cAMP-dependent protein kinase (PKA) activation in bovine bronchial epithelium. Alcohol Clin Exp Res. 1999;23(9):1528–33. [PubMed] [Google Scholar]
- Sisson JH, Stoner JA, Ammons BA, Wyatt TA. All-digital image capture and whole-field analysis of ciliary beat frequency. J Microsc. 2003;211(Pt 2):103–11. doi: 10.1046/j.1365-2818.2003.01209.x. [DOI] [PubMed] [Google Scholar]
- Stout SL, Wyatt TA, Adams JJ, Sisson JH. Nitric oxide-dependent cilia regulatory enzyme localization in bovine bronchial epithelial cells. J Histochem Cytochem. 2007;55(5):433–42. doi: 10.1369/jhc.6A7089.2007. [DOI] [PubMed] [Google Scholar]
- Weber JH, Vishnyakov A, Hambach K, Schultz A, Schultz JE, Linder JU. Adenylyl cyclases from Plasmodium, Paramecium and Tetrahymena are novel ion channel/enzyme fusion proteins. Cell Signal. 2004;16(1):115–25. doi: 10.1016/s0898-6568(03)00129-3. [DOI] [PubMed] [Google Scholar]
- Wyatt TA, Forget MA, Adams JM, Sisson JH. Both cAMP and cGMP are required for maximal ciliary beat stimulation in a cell-free model of bovine ciliary axonemes. Am J Physiol Lung Cell Mol Physiol. 2005;288(3):L546–51. doi: 10.1152/ajplung.00107.2004. [DOI] [PubMed] [Google Scholar]
- Wyatt TA, Forget MA, Sisson JH. Ethanol stimulates ciliary beating by dual cyclic nucleotide kinase activation in bovine bronchial epithelial cells. Am J Pathol. 2003;163(3):1157–66. doi: 10.1016/S0002-9440(10)63475-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt TA, Gentry-Nielsen MJ, Pavlik JA, Sisson JH. Desensitization of PKA-stimulated ciliary beat frequency in an ethanol-fed rat model of cigarette smoke exposure. Alcohol Clin Exp Res. 2004;28(7):998–1004. doi: 10.1097/01.ALC.0000130805.75641.F4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, Schlosser RJ, McCaffrey TV. Dual signal transduction mechanisms modulate ciliary beat frequency in upper airway epithelium. Am J Physiol. 1996;270(5 Pt 1):L745–51. doi: 10.1152/ajplung.1996.270.5.L745. [DOI] [PubMed] [Google Scholar]
- Yang B, Schlosser RJ, McCaffrey TV. Signal transduction pathways in modulation of ciliary beat frequency by methacholine. Ann Otol Rhinol Laryngol. 1997;106(3):230–6. doi: 10.1177/000348949710600309. [DOI] [PubMed] [Google Scholar]
- Zhao X, Jie O, Li H, Xie J, Giles TD, Greenberg SS. Ethanol inhibits inducible nitric oxide synthase transcription and post-transcriptional processes in vivo. Alcohol Clin Exp Res. 1997;21(7):1246–56. [PubMed] [Google Scholar]
- Zhao X, Jie O, Li H, Xie J, Greenberg SS. Ethanol inhibits the potentiation of endotoxin by dibutyryl cAMP and 2-methylthio ATP in vivo. Alcohol Clin Exp Res. 1998;22(8):1662–72. [PubMed] [Google Scholar]