Summary
A conserved herpesviral kinase has been shown to play multiple vital roles in the life cycle of herpesviruses. ORF36, the kinase of murine gamma-herpesvirus 68 (MHV-68), was identified to counteract antiviral type I interferon (IFN) response through the screening of mutant viruses. ORF36 binds to activated interferon regulatory factor 3 (IRF-3) in the nucleus and inhibits the interaction between the IRF-3 and the co-transcriptional activator CBP, thereby suppressing the recruitment of RNA polymerase II to interferon beta promoter. Although the conserved kinase activity is not absolutely required for this interaction, the anti-IFN function of ORF36 is conserved among all herpesvirus subfamilies. Mutant viruses without ORF36 induce more interferon response and are attenuated both in vitro and in vivo. Our data suggest that herpesviruses have evolved an inhibitor of antiviral IFN defense within their conserved kinase, which is critical for herpesvirus to evade host immune control and persist in a host.
Introduction
Viral infection induces a variety of immune responses in the host that control viral replication. In the absence of pre-existing adaptive immunity, the non-specific innate immune responses are critical for restricting viral invasion and replication. Type I interferons (henceforth IFNs), a family of cytokines that includes IFN-β and multiple IFN-α species, are likely to be the most critical components of the innate immune defense against viruses. The secretion of IFNs by virus-infected cells is the foremost step of activating an antiviral state through autocrine and paracrine signaling (Taniguchi and Takaoka, 2002). Direct antiviral effects of IFN include inhibition of viral RNA expression (Li et al., 1998), degradation of viral mRNA (Player and Torrence, 1998), inhibition of viral protein synthesis, and induction of apoptosis (Garcia et al., 2006). Indirectly, IFN activates lymphocytes, NK cells, and macrophages, and it enhances antigen presentation on cell surface (Stark et al., 1998). Therefore, IFNs are the main component of host antiviral defense, which links the innate and adaptive wings of the host immune system (Mossman and Ashkar, 2005).
IFNs have been shown to be induced by different pathways, including Toll like receptor (TLR)-dependent (TLR-3, -4, -7 and -9) and independent (RIG-I and Mda5) pathways (Hiscott, 2007). Those inducing signals are thought to activate latent transcription factors in the cytoplasm, such as interferon regulatory factors (IRFs) and nuclear factor kappa B (NF-kB). The activated transcription factors translocate into the nucleus and activate IFN promoters in both temporally and spatially controlled manner. Among those transcription factors, IRF-3 is the central player in the IFN system. IRF-3 is expressed constitutively but activated immediately upon viral infection (Hiscott, 2007). Virus infection activates cellular kinases, such as TBK-1, and leads to the phosphorylation of IRF-3. The phosphorylation induces a conformation change in IRF-3, which relieves autoinhibitory intra-molecular binding within IRF-3. The uninhibited form of IRF-3 forms a dimer, which translocates into the nucleus, then recruits the CBP/p300 transcriptional coactivator and activates promoters, including IFN-β promoter. Eventually, virus-induced phosphorylation and activation of IRF-3 leads to the proteasome-mediated degradation of IRF-3 (Hiscott, 2007).
The produced IFN-β is secreted, and it binds to IFN receptors on cell surfaces. This binding causes cascades of phosphorylation events of Janus kinase (JAK) as well as signal transducer and activator of transcription (STAT) proteins, leading to the nuclear translocation of phosphorylated STAT complex (Katze et al., 2002). Binding of the complex to the IFN stimulated response element (ISRE) within several promoters initiates transcription of interferon-stimulated genes (ISGs) (Mossman and Ashkar, 2005; Taniguchi and Takaoka, 2002). The protein products of these ISGs subsequently function to control virus replication and propagation. One of the induced ISG products, IRF-7, is further activated by virus infection and induces the full array of IFNs cooperatively with IRF-3, resulting in the induction of a larger subset of ISGs (Hiscott, 2007).
Viruses have evolved under the hostile pressure of the host’s antiviral immune responses. In proportion to the importance of the IFN system in controlling viral infection and replication, viruses have developed a variety of strategies to antagonize it. Herpesviruses are large DNA viruses that can establish a lifelong persistence in a host by evading the host immune surveillance and cause various diseases during their persistent infection. Thus, understanding the mechanism of herpesviral immune evasion is essential in controlling the herpesviral diseases. To investigate the immune evasion mechanisms of tumorigenic gamma-herpesviruses, we used an animal model, murine gamma-herpesvirus 68 (MHV-68), which is biologically and genetically related to the human gamma-herpesviruses. Through screening of the mutant library of MHV-68 in IFN receptor knock-out mice, we found that the conserved herpesviral kinase ORF36 suppresses IFN-mediated response by inhibiting IRF-3. Although the conserved kinase activity is not absolutely required for this inhibitory function, it is shared by the ORF36 homologs among all herpesvirus families. Therefore, we have identified a novel function of the conserved herpesviral kinase that is essential for herpesvirus to evade host immune surveillance and persist in a host.
Results
Identification of ORF36 as a necessary gene of MHV-68 to counteract IFN response
To systematically analyze the role of viral genes in virus-host interaction, we have generated a random insertion mutant library of MHV-68 by in vitro Mu transposase-mediated signature tagged mutagenesis (Song et al., 2005). Signature tag allows simultaneous screening of multiple mutants in vivo by tagging each mutant with a unique short DNA sequence for PCR-mediated identification and quantification. We conducted in vivo screening to identify viral genes that counteract antiviral IFN response, which is the first line of host immune defense. A pool of MHV-68 mutants were infected into BALB/c mice or type I interferon receptor knock-out mice (IFNAR−/−). Each mouse was infected with a total of 500 plaque forming unit (pfu) of mixed mutant viruses with distinct tags (50 pfu/mutant). The in vivo growth of mutants was analyzed for acute replication in the lung at 7 days post infection (dpi) and for establishment of latency in the spleen at 14 dpi by real-time quantitative PCR (q-PCR) as previously described (Song et al., 2005). After comparing the growth of each mutant in two groups of mice, one mutant virus was identified to show significant attenuation (<1/50 of average growth of mutants) in normal mice and enhanced growth (~10 fold higher than average growth of all mutants) in IFNAR−/− mice in both acute replication in the lung and latency in the spleen (Fig. 1A–B). The mutant virus had a transposon-insertion in orf36 region (nucleotide 52925 in U97553, corresponding to the 27th amino acid (aa) in the total 437 aa protein).
Figure 1. Identification of MHV-68 mutants impaired in counteracting IFN response (A).
Growth of mutants by acute infection in the lung and latent infection in the spleen of normal BALB/c mice. The ratios of in vivo to in vitro replication of each mutant are shown. The ratio of mutant that shows same level of replication in vivo and in vitro, relative to the other mutants in a pool, is defined as 1. Individual mutants in a pool are labeled with the numbers 1 to 10. Mutant #6 is the ORF36-null mutant (36T). (B) The same pool of mutant viruses was used to infect IFNAR−/− mice, and the same analysis as (A) was performed. (C) Acute replication of MHV-68 in the lung after infection with WT and 36T individually. (D) Latency establishment of MHV-68 in the spleen after infection with WT and 36T individually. I.C.: infectious center; sp.: splenocytes. 4 mice per group and per time point. Data are represented as mean + standard deviation (SD).
To confirm the screening result using the pooled mutant infection, BALB/c and IFNAR−/− mice were infected with 50 pfu of either wildtype (WT) or ORF36-null transposon mutant (36T) MHV-68. With 50 pfu/mouse infection, the peak time of acute replication in the lung was determined as 9 dpi in separate experiment. Thus, at the peak time of acute replication in the lung and latency in the spleen (14 dpi), the organs were harvested and analyzed for infectious/reactivatable virus and viral genome copy number. 36T was attenuated in normal BALB/c mice (~1/400 of WT in both acute and latent replication) (Fig. 1C–D). However, its attenuation was significantly rescued in IFNAR−/− mice (~1/10 of WT in acute and ~1/50 of WT in latent replication). Therefore, the attenuation of 36T in normal mice and the partial recovery of its attenuated growth in IFNAR−/− mice are due to the intrinsic growth defect of 36T in vivo, rather than by an unexpected effect of infection with pooled mutants. Furthermore, these data validate the effectiveness of our screening method of using the pool of mutants to identify critical viral genes in virus-host interaction.
Lack of ORF36 delays the establishment of splenic latency
To further analyze the role of ORF36 in the establishment of MHV-68 latency, we generated a mutant virus with nonsense mutation on the 30th aa of ORF36 (36S) and analyzed the establishment of splenic latency at 14 dpi and 21 dpi after intranasal infection with WT or 36S. In the spleen of 36S infected mice at the peak of latency (14 dpi), latently infected cells were almost undetectable (Fig. 2A). This represents at least a 104-fold decrease in latency compared with that of the WT virus. There was also no evidence of the latency-associated splenomegaly normally driven by MHV-68, which resembles lymphoproliferative responses that occur in humans during infectious mononucleosis (Sunil-Chandra et al., 1994) (Fig. 2B). However, the analysis of the number of latently-infected cells at 21 dpi showed that 36S established latency similar to WT. These data indicate that the lack of ORF36 expression delays, but does not completely impair, the establishment of gamma-herpesvirus latency.
Figure 2. Analysis of the in vivo response to 36S.
C57BL/6 mice were intranasally infected with 1000 pfu of WT or 36S. (A) The number of latently infected spleen cells was determined using an infectious center assay at 14 and 21 dpi. (B) Analysis of spleen cell numbers at 14 and 21 dpi. (C) Absolute numbers of CD8-positive ORF6487–495/Db (left panel) and ORF61524–531/Kb (right panel) in the lung of infected mice. (D) Absolute numbers of CD8-positive ORF6487–495/Db (left panel) and ORF61524–531/Kb (right panel) in the spleen of infected mice. 5 mice per group and per time point. Data are represented as mean + SD.
Next, we analyzed the 36S for its capacity to elicit an adaptive T-cell immune response, which has been known to control the in vivo replication of MHV-68 (Braaten et al., 2005). To monitor the virus-specific CD8 T cell response, we used tetrameric reagents against two well-defined MHV-68 epitopes, ORF6487–495/Db and ORF61524–531/Kb. The absolute numbers of ORF6487–495/Db- and ORF61524–531/Kb-specific CD8 T cells at the mucosal site of infection (lung) (Fig. 2C) were reduced 10 to 100-fold in 36S-infected mice at 14 dpi, but no differences of the size of the response were found at 21 dpi. The analysis of spleen cells (Fig. 2D) showed that the numbers of MHV-68-specific CD8 T cells were slightly reduced in 36S-infected mice at 14 dpi but they were comparable in 36S- or WT-infected mice at 21 dpi, a time point when 36S- and WT-infected mice show similar levels of latency (Fig. 2A). These data suggest that the magnitude of the early MHV-68-specific CD8 T cell response is reduced in 36S-infected mice as a consequence of its reduced replication in lung and delayed latency in spleen.
ORF36 inhibits IRF-3 mediated activation of interferon beta promoter
Virus-infected cells produce a mixture of IFNs depending on the cell type. In general, fibroblast and epithelial cells predominantly produce IFN-β, whereas dendritic cells, leukocytes and macrophages express multiple IFN-α (Katze et al., 2002). For efficient IFN response, IFN-β producing cells mainly rely on autocrine feedback, but IFN-α producing cells constitutively express IRF-7 to rapidly produce high levels of IFN-α (Mossman and Ashkar, 2005). Since intranasal infection of MHV-68 can reach both types of cells, we first investigated the effect of ORF36 expression on the activity of IFN-β promoter, which is a common denominator in both systems (Hiscott, 2007).
While the IFN-β promoter was induced by the known transactivators, such as IRF-3, IRF-7, or NF-kB, ORF36 specifically inhibited the IRF-3 mediated activation of IFN-β promoter in a dose dependent manner (Fig. 3A). These data suggest that ORF36 may inhibit the production of IFN-β through IRF-3. We further pursued this possibility by testing upstream activators of IRF-3 signal transduction pathway, such as TANK Binding Kinase 1 (TBK-1), Toll-like receptor 4 (TLR-4), or Sendai virus infection. TBK-1 phosphorylates and activates IRF-3 upon viral infection (Sharma et al., 2003); TLR-4 detects microbial invasion and activates IRF-3 pathway (In this study, we used constitutively activated form of TLR-4 by the conjugation with extracellular domain of CD4 (Doyle et al., 2002)); Sendai virus infection is a potent activator of IFN pathway (Lin et al., 1998). As shown in Figure 3B, all three different inducers successfully activated IFN-β promoter, but ORF36 inhibited these activations in a dose dependent manner. These data suggest that ORF36 can inhibit the antiviral signal transduction pathway from viral-invasion detection to IFN-β promoter activation by inhibiting the function of IRF-3.
Figure 3. Impacts of ORF36 on IFN activation pathway (A).
The effect of ORF36 expression on the activation of IFN-β reporter which is induced by the co-transfected plasmids expressing IRF-3, IRF-7 or NF-kB (p65), respectively. (B) The effect of ORF36 expression on the activation of IFN-β reporter which is induced by upstream activators of IRF-3 signal transduction pathway, TBK-1, TLR-4 and Sendai virus infection. (C) The effect of ORF36 expression on the activation of interferon stimulatory response element containing reporter. (D) The effect of ORF36 and dominant negative form of IRF-3 (IRF-3dDBD) expression on the activation of IFN-β reporter induced by TBK-1 expression, and the effect of TBK-1 or IRF-3 over-expression in addition to ORF36 or IRF-3dDBD. All the reporter assays were performed in human 293T cells, and the data are represented as mean + SD.
Consistently, ORF36 inhibited the downstream reporter (ISRE-luc; IFN stimulatory response element) of IFN receptor signaling when it was activated by the TBK-1 over-expression, suggesting that the inhibitory effect of ORF36 can significantly reduce the production of the downstream antiviral effecters (Fig. 3C). Furthermore, the inhibitory effect of ORF36 on IFN-β promoter can be reversed by the over-expression of either TBK-1 or IRF-3 (Fig. 3D). In contrast, the inhibition by dominant negative form of IRF-3 (IRF-3dDBD, which lacks DNA binding domain but maintains interaction and activation domain) can be reversed by IRF-3 but not by TBK-1. This prompted us to test whether ORF36 inhibits the function of IRF-3 directly.
ORF36 inhibits the interaction between IRF-3 and CBP
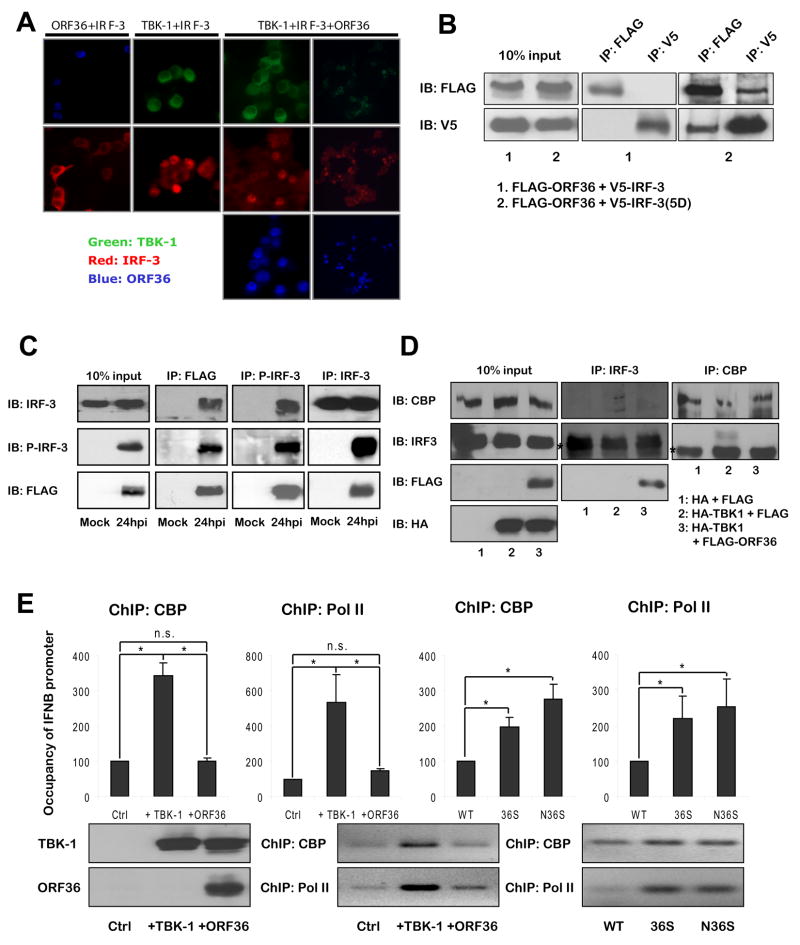
To further elucidate the inhibitory mechanism of ORF36, we investigated the effect of ORF36 on each step of IRF-3 mediated activation of IFN-β promoter. We first found that increasing the expression of ORF36 has no effect on the expression level of IRF-3 and the phosphorylation of IRF-3 mediated by TBK-1 (Fig. S1A). In fact, activated IRF-3 by TBK-1 over-expression translocates into the nucleus, but ORF36, which localizes in the nucleus, did not affect this translocation (Fig. 4A). Consistently, when we activated IFN-β promoter with IRF-3(5D), which mimics the phosphorylated and activated form of IRF-3 by mutation of the five serine/threonine at the C-term to aspartic acid (Lin et al., 1999), ORF36 can inhibit IRF-3(5D) mediated activation of IFN-β promoter (Fig. S1B). Altogether, these data clearly demonstrate that ORF36 inhibits a step downstream of IRF-3 nuclear translocation.
Figure 4. Molecular interaction between IRF-3 and ORF36 (A).
Subcellular localization of IRF-3 upon TBK-1/ORF36 expression in 293T cells. Anti-FLAG and anti-mouse-Alexa405 (Blue) for FLAG-tagged ORF36; anti-HA-FITC (Green) for HA-tagged TBK-1; anti-IRF-3 and anti-rabbit-Cy3 (Red) for endogenous IRF-3. (B) Interaction between ORF36 and the native or activated form of IRF-3 by co-immunoprecipitation assay. 293T cells were transfected with the plasmids expressing the indicated proteins (bottom) and lysed and analyzed at 48 hours post-transfection as described in method. IP: immunoprecipitation using antibody against indicated epitope, IB: immunoblot using antibody against indicated epitope. (C) Interaction between the endogenous IRF-3 activated upon viral infection and the FLAG-tagged ORF36 expressed from MHV-68 during natural infection. 293T cells were uninfected (mock) or infected with a recombinant MHV-68 expressing FLAG-tagged ORF36 at MOI=10 and harvested and analyzed at 24 hours post infection (hpi). (D) Interaction between the endogenous IRF-3 and CBP in the absence/presence of TBK-1/ORF36 in 293T cells. (E) The effect of ORF36 on the recruitment of CBP and RNA Pol II transcription complex to the IFN-β promoter in the transfected 293T cells or infected NIH3T12 cells. (top) Relative amount of the IFN-β promoter precipitated by anti-CBP or anti-Pol II, quantitated by q-PCR. (bottom) The PCR products were run on agarose gels. A set of representative data is shown here. Data are represented as mean + SD. n.s.: not significant; *, P < 0.05.
To test whether ORF36 can inhibit the transactivation function of IRF-3 bound to DNA by a heterologous DNA binding domain, we analyzed the activation of a reporter controlled by GAL4 binding site and GAL4-DBD fused form of IRF-3. ORF36 still inhibited the activation of the reporter (Fig. S1C), implying that ORF36 interferes the transactivation function of IRF-3, independently of the DNA binding activity of IRF-3. Next, we examined whether ORF36 can physically interact with IRF-3. Although we could not detect any interaction between ORF36 and the inactive native form of IRF-3, a physical interaction was observed between ORF36 and IRF-3(5D) representing an activated form of IRF-3 (Fig. 4B). This data suggests that ORF36 may bind to the activated IRF-3 during the viral infection. Therefore, to further confirm the interaction between active IRF-3 and ORF36 during the natural infection of MHV-68, we constructed a recombinant MHV-68, in which triple FLAG-epitope coding sequence is inserted into the 5′ of ORF36 gene in MHV-68 genome, thus expressing FLAG-tagged ORF36 during viral replication. Infection of the recombinant MHV-68 at high MOI caused the phosphorylation/activation of endogenous IRF-3, and, in consistent with Fig. 4B, the FLAG-tagged ORF36 expressed from the virus bound to this slower-migrating phosphorylated IRF-3 (Fig. 4C and S1E). This data suggests that, during the natural infection of MHV-68, the host may detect/inhibit the infection of MHV-68 through the activation of IRF-3 but the ORF36 of MHV-68 can counteract this antiviral defense of host by directly binding to the phosphorylated/activated IRF-3. Furthermore, purified ORF36 from bacteria can bind to in vitro translated IRF-3(5D), supporting the direct interaction between ORF36 and the activated IRF-3 (Fig. S2A).
Through the binding assay of IRF-3 deletion mutants to ORF36, we found that IRF association domain (IAD, aa 193 ~ 350) of IRF-3 is necessary and sufficient for binding to ORF36 (Fig. S2B). IAD domain has been shown to be required for binding to CBP as well as dimerization (Qin et al., 2005), suggesting that the binding of ORF36 to IRF-3 may inhibit the interaction between the activated IRF-3 and CBP inside the nucleus. Therefore, we investigated whether the physical binding between ORF36 and IRF-3 affects the interaction between IRF-3 and CBP. When the activated form of IRF-3 was co-expressed with CBP, both IRF-3 and CBP was co-immunoprecipitated reciprocally. However, in the presence of ORF36, the amounts of co-immunoprecipitated IRF-3 and CBP were significantly reduced (Fig. S1D). Indeed, when we over-expressed TBK-1, endogenous IRF-3 bound to CBP. ORF36 inhibited this interaction, suggesting that ORF36 may inhibit the recruitment of CBP by IRF-3 to IFN-β promoter (Fig. 4D).
We further analyzed the recruitment of CBP and RNA Polymerase II (Pol II) to IFN-β promoter by performing chromatin immunoprecipitation (ChIP) analysis of IFN-β promoter using anti-CBP and anti-Pol II antibody. As shown in Figure 4E, over-expression of TBK-1 induced occupancy of IFN-β promoter with both CBP and Pol II. However, when ORF36 was co-expressed, the recruitment of both CBP and Pol II was significantly reduced, indicating that ORF36 inhibited the recruitment of general transcription complex to IFN-β promoter by activated IRF-3. Moreover, there was more recruitment of CBP and Pol II to IFN-β promoter in the mutant virus infected cells than wildtype infected cells. Taken together, all the data suggest that ORF36 binds to active IRF-3 in the nucleus and inhibits the IRF-3 mediated recruitment of general transcription complex to IFN-β promoter, thus inhibiting IFN-β production.
Anti-IFN function of ORF36 is conserved in herpesviruses
ORF36 is a kinase conserved among all the subfamilies of herpesvirus [e.g. alpha - Herpes Simplex Virus (HSV) UL13; beta - Human Cytomegalovirus (HCMV) UL97; gamma - Epstein Barr Virus (EBV) BGLF4 and Kaposi’s Sarcoma-associated Herpesvirus (KSHV) ORF36) (Gershburg and Pagano, 2008)]. We tested whether this anti-IFN function of ORF36 is also conserved. When we expressed the homologs of ORF36, all the homologs inhibited the TBK-1 mediated activation of IFN-β promoter in a dose dependent manner (Fig. 5A). Further, when the production of IFN-β was induced by treating cells with double-strand RNA (ds polyIC), mimicking the infection of RNA viruses, all the homologs suppressed the activation of IFN-β promoter (Fig. 5D). The ORF36 homologs also suppressed the production of endogenous IFN-β and downstream effecters (e.g. Mx1) after selecting the transfected cells by drug-resistance, due to the low efficiency of transfection (Fig. 5B). Collectively, these data suggest that the anti-IFN function of ORF36 may be also conserved among the conserved herpesviral kinases.
Figure 5. Impact of ORF36 homologs and kinase-null mutant on IFN activation (A).
The effect of homologs of ORF36 in all subfamilies of herpesvirus - alpha (HSV-1 UL13), beta (HCMV UL97) and gamma (EBV BGLF4 and KSHV ORF36) - on the activation of IFN-β reporter induced by TBK-1 expression in human 293T cells. (B) The effect of homologs of ORF36 on the production of IFN-β and the transcription of IFN-β and Mx1 induced by ds polyIC treatment. The murine NIH3T3 cells were transfected as indicated and selected for 3 days with 6 ug/ml of puromycin by the co-transfected pBabe-puro. 3 days after selection, the transfected/selected cells were treated with 10 ug/ml of ds polyIC in liposome-complex. 1 day post treatment, cell supernatant and RNA extracted from the cells were analyzed for endogenous IFN-β production by enzyme-linked immunosorbent assay (ELISA) (top) and transcription level of IFN-β (middle) and Mx1 (bottom) by q-PCR. (C) The effect of ORF36(K107Q), a kinase-null mutant of ORF36, on the activation of IFN-β reporter induced by TBK-1 in human 293T cells. (D) The effect of homologs and a kinase-null mutant of ORF36 on the activation of IFN-β reporter with or without induction by ds polyIC treatment in NIH3T3 cells. Data are represented as mean + SD. *, P < 0.05.
Subsequently, the next question was whether this inhibitory activity requires the conserved kinase function of ORF36. We constructed a kinase-null mutant of ORF36 by mutating the invariantly conserved lysine residue in catalytic core to glutamine (K107Q), and the kinase activity is indeed knocked-out in the mutant (Tarakanova et al., 2007). This kinase-null mutant of ORF36 still inhibited the IFN-β reporter although less potent than the wildtype (Fig. 5B-D). Consistently, the part of ORF36 containing conserved kinase domain was sufficient but not necessary for binding to IRF-3 (Fig. S2C). The same inhibition was also observed with the kinase-null mutant of EBV BGLF4 (K102I, data not shown). Although there is a possibility that the kinase activity may indirectly contribute to the anti-IFN function of ORF36, these data suggest that the kinase activity of ORF36 is not absolutely required for inhibition of IFN-β promoter by ORF36.
Kinase-dependent/independent function of ORF36 is required for efficient replication of MHV-68
The ChIP data suggest that MHV-68 without functional ORF36 induces more transcription complex recruitment to IFN-β promoter, which will lead to more IFN-β production. Previously, we have found that 36T was attenuated even in in vitro cell culture system (Song et al., 2005). Thus, we hypothesized that MHV-68 without functional ORF36 may induce more antiviral IFN response than wildtype, and this may lead to the attenuation of ORF36-null viruses. Indeed, ORF36-null mutant viruses induced more IFN-β and ISRE response than wildtype at the same multiplicity of infection (MOI = 0.05) (Fig. S3A). This induction was proportional to the extent of viral replication, because both WT and the mutants induced more IFN reporter activity at higher MOI (data not shown). To further confirm the specific phenotype of ORF36-null mutants, we generated additional mutant viruses: another nonsense mutant of ORF36 (N36S, nonsense mutation on the 107th aa), the kinase-null mutant of ORF36 (36KN, K107Q), and wildtype revertant of ORF36-null mutant (36R). Like the other ORF36-null mutants, N36S induced more IFN response than wildtype (Fig. 6A). However, both 36KN and 36R showed similar phenotype to wildtype, supporting the specificity of ORF36-null phenotypes.
Figure 6. The role of ORF36 and its kinase activity in the IFN induction and replication of MHV-68 in vitro and in vivo (A).
The induction of IFN response by WT, N36S, 36KN and 36R. NIH3T3 cells were infected with WT, N36S, 36KN or 36R virus at MOI=0.05. At 24 hours post infection, cell supernatant and RNA extracted from the infected cells were analyzed for endogenous IFN-β production by ELISA (left) and transcription level of IFN-β (middle) and Mx1 (right) by q-PCR. For ELISA, samples from three independent experiments were combined and tittered. (B) The multi-step growth curve of WT, N36S, 36KN, and 36R. Samples from two independent experiments were combined, tittered, and shown here. (C) Transcript level of ISGs in the lung after WT or 36S MHV-68 infection examined by q-PCR. Viral genome and cellular actin transcript level were also measured as control. Data are represented as mean + SD. (D) Acute replication in the lung after intranasal infection of the indicated viruses in normal C57BL/6 (left) and IFNAR−/− (right) mice. (E) Latency establishment in the spleen after intranasal infection of the indicated viruses in normal C57BL/6 (left) and IFNAR−/− (right) mice. I.C.: infectious center; sp.: splenocytes. 500 pfu/mouse and 4~5 mice per group and per time point. Data are represented as mean + SD. n.s.: not significant; *, P < 0.05.
Next, we investigated the importance of antiviral IFN response in controlling the in vitro replication of MHV-68 by generating stable cell lines that express either IRF-3dDBD or IRF-3(5D). All ORF36-null mutants, but not 36R, showed attenuated growth in the parental cell line. Their attenuated growth was significantly rescued in the cells expressing IRF-3dDBD and further reduced in the cells expressing IRF-3(5D) (Fig. 6B and S3B). Furthermore, the growth of 36T and 36S was also attenuated in primary mouse embryonic fibroblast (MEF) cells, but this attenuation was also rescued in MEF cells derived from IFNAR−/− mice (Fig. S3B). Interestingly, 36KN also showed similarly attenuated growth like ORF36-null mutant viruses in vitro (Fig. 6B), although it did not significantly induce IFN response (Fig. 6A). Collectively, these data suggest that ORF36 is required for MHV-68 to counteract IFN response and to grow efficiently in vitro.
Next, we investigated whether the same phenomenon happens in vivo. When we measured IFN level in the lung of WT and 36S infected mice, however, we could not detect any significant level of IFN-β production by ELISA to measure secreted IFN-β nor by q-PCR to measure the transcription of IFN-β (data not shown). This may be due to the extremely low level of IFN-β production after MHV-68 infection, because it is known that MHV-68 is a poor inducer of IFN production in vivo (Weslow-Schmidt et al., 2007). Knocking out ORF36 may be not enough to increase IFN in the entire lung to measurable levels by our assay systems, but may cause significant difference of IFN level only at the microenvironment level. Thus, we attempted to measure the transcript level of ISGs, such as Mx1 and IRF-7, as representatives of the amplified downstream effectors of IFN signaling pathway (Doyle et al., 2002). In the same RNA harvested from either WT or 36S infected lung, we detected a similar level of Mx1 and IRF-7 (Fig. 6C), showing that 36S can induce the same level of ISG response to WT even when its replication was severely attenuated in vivo. Taken together, these data suggest that MHV-68 without ORF36 may induce more antiviral IFN response in vivo as well as in vitro than wildtype at the same level of viral replication.
We next examined the phenotype of ORF36-null mutants by infecting wildtype and mutant MHV-68 into normal and IFNAR−/− mice. During the acute replication of MHV-68 in the lung, 36R replicates just like WT, but both N36S and 36KN were significantly attenuated (~1/1000 and ~1/100 of WT by infectious viral titer, respectively) (Fig. 6D). However, N36S could be partially rescued in the IFNAR−/− mice (~1/100 by infectious viral titer), but not 36KN. Therefore, during acute infection in the lung, it appears that the anti-IFN function of ORF36 is largely mediated by a kinase-independent mechanism. These data further suggest that the kinase activity of ORF36 may play another role in the replication of MHV-68 in the lung, independently of anti-IFN function.
We also examined the level of viral latency established in the spleen at 14dpi. 36R behaved indistinguishably from WT, while both N36S and 36KN were attenuated in normal mice (Fig. 6E). However, in contrast to the acute replication in the lung, the attenuation of both mutants could be mostly rescued in the IFNAR−/− mice. Thus, the kinase activity of ORF36 seems to be mainly responsible for the anti-IFN function during the latency in the spleen. Altogether, these data indicate that the anti-IFN function of ORF36 is essential for viral infection in the host, and can be mediated through both kinase-dependent and -independent mechanisms. Furthermore, the fact that the attenuation of N36S could not be fully restored in the IFNAR−/− mice, suggest additional in vivo roles of ORF36 other than the anti-IFN function (Lee et al., 2007; Tarakanova et al., 2007).
The critical role of ORF36 in the normal replication kinetics of MHV-68
For the continuous monitoring of the interaction between MHV-68 and the host, we recently developed a bioluminescent imaging system using a recombinant MHV-68, in which viral M3 promoter drives firefly luciferase expression (M3FL). Our results suggests that the replication kinetics of M3FL is similar to that of parental wildtype MHV-68 and that M3FL is an effective model for studying the in vivo interaction of gamma-herpesvirus with its host (Hwang et al., 2008). To further examine the systemic infection of MHV-68 without ORF36, we generated the ORF36-null stop-codon mutant in the background of M3FL (M3FL-36S). Normal BALB/c mice were intranasally infected with 5×105 pfu of either wildtype M3FL or M3FL-36S, and bioluminescent images were obtained every other day post infection. In contrast to the normal progression of wildtype from lung to spleen, which is from the primary site infection to the major reservoir of viral latency, the mutant virus showed relatively normal acute replication in the lung but could not progress to the spleen (Fig. 7). This imaging result is consistent to the virological assays presented in Figure 1 and 6. Furthermore, increasing the inoculum dose by 10000 fold (compared to 50 pfu shown in Fig. 1) cannot overcome the attenuation caused by the loss of ORF36.
Figure 7. Spatial and temporal progression of M3FL and M3FL-36S replication in vivo (A).
Bioluminescence imaging of in vivo replication of wildtype M3FL and mutant M3FL-36S in normal mice. Images at different time point are shown to represent the peak of acute infection in the lung (D6 with 5×105 pfu/mouse), the transition of replication from the lung to the spleen (D10), and the establishment of viral replication in the spleen (D14). (B) Bioluminescence imaging of in vivo replication of wildtype M3FL and mutant M3FL-36S in IFNAR−/− mice.
However, when IFNAR−/− mice were infected with 5×105 pfu of the same viruses, there was no significant difference between two viruses in replication or distribution. Eventually, IFNAR−/− mice infected with either M3FL or M3FL-36S succumbed to infection during 6–8 dpi (Fig. 7). This systemic analysis of infection in vivo clearly demonstrated that the defective replication/progression of MHV-68 without ORF36 can be rescued by inhibiting IFN response. Moreover, these data suggest that ORF36 is the gene important for MHV-68 to counteract antiviral IFN response in order to replicate efficiently inside immune-competent host/cells.
Discussion
The critical role of IFN system in defending host from viral invasion can be attested by the diverse antagonistic strategies of various viruses. Herpesviruses have developed multiple ways to counteract this critical antiviral host response (Mossman and Ashkar, 2005; Stevenson and Efstathiou, 2005). Here we identified a novel function of a conserved herpesviral protein which antagonizes IFN response during viral replication through the systematic screening of MHV-68 mutant library in mice with different genetic backgrounds. As in this study, screening pools of mutant viruses in hosts with different genetic backgrounds will provide efficient screening environment with competition among viruses, which will expedite the identification process. Furthermore, by combining this genetic screening with bioluminescence whole body imaging, screening and validation processes can be accelerated.
Many viral and cellular proteins have been shown to be phosphorylated by ORF36 homologs, including ORF36 itself and elongation factor 1δ. Multiple functions of ORF36 homologs have been proposed in the various stages of viral life cycle, such as cellular and viral gene regulation, nuclear egress, virus maturation and replication, chromosome condensation and tissue tropism (Asai et al., 2007; Gershburg and Pagano, 2008; Hamza et al., 2004; Izumiya et al., 2007; Kawaguchi and Kato, 2003; Lee et al., 2007; Michel and Mertens, 2004). The ORF36 also plays a major role in sensitizing gamma-herpesvirus to nucleoside analog drugs, such as ganciclovir (Davis et al., 2007). However, the biological significance of many of the proposed targets and functions has not been clearly demonstrated in the life cycle of the virus during natural infection in vivo. In this study, we identified a new function of ORF36 via an unbiased screen. The biological significance of this function in the virus life cycle can be demonstrated by the attenuation of the ORF36-null mutant in normal cells/mice and the significant rescue of that attenuation in IFNAR−/− cells and mice.
Intriguingly, ORF36 interacts only with active IRF-3 which localizes in the nucleus. The finding is further supported by the nuclear localization of ORF36 (Fig 4A) and the binding of ORF36 to IAD of IRF-3 (Fig S2B), which is buried by the autoinhibitory elements in inactive IRF-3 (Qin et al., 2005). It may represent the beauty of viral evolution: the virus targets only the activated form of IRF-3, rather than all IRF-3 molecules. Majority of IRF-3 in the cell is inactive form. By targeting activated IRF-3 specifically, virus can achieve the inhibitory goal more efficiently with small amount of ORF36 proteins.
Through the point- and deletion-mutant study of ORF36 protein in vitro, we found that the kinase domain of MHV-68 ORF36 is sufficient but not necessary for this inhibition (Fig. 5 and S2C). Consistently, the mutant MHV-68 without functional kinase did not significantly induce the production of IFN response (Fig. 6A). However, MHV-68 with the kinase-null mutation did not replicate competently both in vitro and in vivo, and this attenuated growth was significantly rescued when the antiviral IFN response was compromised (Fig. 6B and 6D–E). These data suggest that ORF36 counteract antiviral IFN responses by both kinase-dependent and -independent manners. Although the kinase activity was not absolutely required for ORF36 to interact with IRF-3 and to suppress the induction of IFN-β promoter by the activated IRF-3, the kinase activity may affect other cellular or viral proteins indirectly to reduce the antiviral effect of IFN, augmenting direct inhibitory effect of ORF36 on IRF-3. This may be more crucial in the real life cycle of MHV-68, in which multiple viral proteins act together to subvert the antiviral host defense for efficient replication.
Moreover, the fact that the ORF36-null mutant could not be fully rescued in the IFNAR−/− mice (Fig. 6D–E) suggest that, in addition to antagonizing the host IFN responses, ORF36 has other functions that are important for viral replication in vivo. This is consistent with notion that ORF36 may have multiple functions that are important for the efficient replication of MHV-68, such as the initiation of DNA damage response and chromosome condensation (Lee et al., 2007; Tarakanova et al., 2007).
IFN response is an intruder alerting system of host cells to defend themselves, so invading viruses need to subvert this alarm system immediately to infect and replicate successfully. It has been shown that herpesvirus virions, especially glycoproteins on the envelope mediating attachment and fusion of virus to the host cells, induce interferon response (Barchet et al., 2002; Compton et al., 2003; Dalod et al., 2002; Lund et al., 2003; Morrison, 2004; Simmen et al., 2001). Although ORF36 is an early protein expressed several hours after virus entry (Ebrahimi et al., 2003; Martinez-Guzman et al., 2003), it was also detected in the infectious virion (Asai et al., 2006; Bechtel et al., 2005; Bortz et al., 2003; Overton et al., 1992; Varnum et al., 2004; Zhu et al., 2005). Therefore, it can be released into the cytoplasm of host cells immediately upon viral entry and may down-regulate the host IFN response to modify the host cell physiology in favor of viral gene expression and replication. This function will be further enhanced after ORF36 is expressed in the infected cells. Alternatively but not exclusively, ORF36 may also be needed for the latent virus to efficiently reactivate. In fact, ORF36 is one of the genes directly activated from latent KSHV in a hypoxia-induced reactivation (Haque et al., 2006). IFN has been shown to regulate the reactivation of latent MHV-68 (Barton et al., 2005). Without IFN, MHV-68 reactivated more readily, suggesting that IFN reduces the efficiency of latent virus reactivation. To maintain a lifelong persistent infection, MHV-68 would need to reactivate to transmit and replenish the latent pool. Thus, MHV-68 needs to overcome the restraining effect of IFN when it reactivates, where ORF36 may also be required in this process.
Innate immunity affecting adaptive immune response
Herpesvirus replication is significantly affected by IFN response both in vitro and in vivo (Barton et al., 2005; Mossman and Ashkar, 2005). Depletion of IFN in wildtype mice during the establishment of latency does not enhance MHV-68 reactivation compared to the reactivation of MHV-68 in IFNAR−/− mice, suggesting that IFN-mediated innate and adaptive immune responses during the acute infection of MHV-68 may be required to control the reactivation of MHV-68 (Barton et al., 2005). These data imply that the effect of IFN responses is beyond the immediate non-specific immune defense against viral invasion, further affecting the later stage of viral replication and persistent infection in vivo. IFNs induced during viral infection have been considered to be an important link between the innate and adaptive immune responses to viruses. For example, IFNs stimulate antigen-presenting cells to initiate cross-priming for the activation of CD8+ T cell response (Durand et al., 2004; Le Bon et al., 2003), and promotes proliferation and maintenance of CD8+ cytotoxic T lymphocytes (CTL) (Agnello et al., 2003; Marrack et al., 1999). Given the critical role of T lymphocytes in controlling MHV-68 replication in vivo (Braaten et al., 2005; Sparks-Thissen et al., 2004), it is possible that the anti-IFN function of ORF36 is required not only to breach the immediate innate immune defense upon viral infection/reactivation but also to hinder the development of adaptive T-cell response.
The early reduction of the size of the virus specific T-cell response, induced by a replication attenuated 36S, correlates with a critical role of the magnitude of antigenic stimulation for T cell activation and differentiation (Wherry et al., 1999). Intriguingly, the ratio of the virus-specific CD8 T cell response per virus titer is higher for 36S than WT (Fig. 2C–D). Further, MHV-68 without functional ORF36 induced more IFN response in vitro (Fig. 6A) and similar level of IFN response in vivo (Fig. 6C) in comparison with WT even when its replication was attenuated. Therefore, it is tempting to speculate that the lack of an anti-IFN function of ORF36 in 36S contributes to induce an enhanced innate anti-viral state with higher IFN signaling in vivo (Fig. 6C) that also will add to the expansion of T cells (Kolumam et al., 2005), even in the presence of very low levels of replicating virus. One prediction based on this idea is that a herpesvirus vaccine without ORF36 will be safer and more effective since it will replicate less and still generate a strong T-cell response.
The outcome of a herpesvirus infection depends on the delicate balance between the strength of the host immune system and the ability of the virus to counteract. The identification of viral genes responsible for different immune evasion strategies of herpesvirus will provide not only a new ground for basic mechanistic research but also more opportunities to develop novel preventive and therapeutic approaches against persistent herpesviral infection and associated diseases.
EXPERIMENTAL PROCEDURES
Preparation and Quantitation of virus
The transposon-inserted mutant MHV-68 viruses were generated using our BAC MHV-68 and in vitro MuA transposition system (Finzyme, Finland) as described (Song et al., 2005). To make specific mutants of MHV-68 (i.e. 36S, N36S, 36KN, 36R, M3FL, M3FL-36S), a two-step allelic exchange method was performed (Smith and Enquist, 1999).
Mouse experiments
All animal handling was performed in accordance with University of California, Los Angeles, and Animal Research Committee guidelines and the Institutional Animal Care and Use Committee at the Research Institute at Nationwide Children’s Hospital. All mice were infected after anesthesia, and the infected mice were sacrificed at 7 days post-infection (dpi) (500~5000 pfu/mouse) or 9 dpi (50 pfu/mouse) to measure the acute viral infection in the lung or at 14 dpi to measure the viral latent load in the spleen.
Plasmids and transfection
All ORF36 homologs were amplified from the viral DNA by PCR and cloned into Entry vector of Gateway system (Invitrogen). Murine IRF-3 and IRF-3(5D) were derived from the original pEBB-IRF-3 and pBabe-IRF-3(5D) (Doyle et al., 2002), respectively, and further transferred into Gateway system.
Supplementary Material
Acknowledgments
We thank the members of Sun and Cheng laboratories for helpful discussions; Janet Weslow-Schmidt for her technical assistance; and Dr. Herbert W. Virgin and Dr. Vera L. Tarakanova for sharing reagents and information. This work was supported by NIH grants R01-DE15752, R21-CA120761, P01-DE019085, DE015612 and P50-CA86306. S.H. was supported by UCLA AIDS Institute and UCLA Center for AIDS Research (AI28697) and Universitywide AIDS Research Program Dissertation Award (D06-LA-4). E.F. was supported by NIH grant R01-AI59603. R.M.O. was funded by NIH T32AI060567 and the Irvington Institute for Immunological Research.
Footnotes
Detailed experimental procedures and information are provided in “Expanded Experimental Procedures” in the Supplemental Data.
The authors have no conflicting financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agnello D, Lankford CS, Bream J, Morinobu A, Gadina M, O’Shea JJ, Frucht DM. Cytokines and transcription factors that regulate T helper cell differentiation: new players and new insights. Journal of clinical immunology. 2003;23:147–161. doi: 10.1023/a:1023381027062. [DOI] [PubMed] [Google Scholar]
- Asai R, Kato A, Kato K, Kanamori-Koyama M, Sugimoto K, Sairenji T, Nishiyama Y, Kawaguchi Y. Epstein-Barr virus protein kinase BGLF4 is a virion tegument protein that dissociates from virions in a phosphorylation-dependent process and phosphorylates the viral immediate-early protein BZLF1. Journal of virology. 2006;80:5125–5134. doi: 10.1128/JVI.02674-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai R, Ohno T, Kato A, Kawaguchi Y. Identification of proteins directly phosphorylated by UL13 protein kinase from herpes simplex virus 1. Microbes and Infection. 2007;9:1434–1438. doi: 10.1016/j.micinf.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Barchet W, Cella M, Odermatt B, Asselin-Paturel C, Colonna M, Kalinke U. Virus-induced Interferon {alpha} Production by a Dendritic Cell Subset in the Absence of Feedback Signaling In Vivo. 2002:507–516. doi: 10.1084/jem.20011666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton ES, Lutzke ML, Rochford R, Virgin HWIV. Alpha/Beta Interferons Regulate Murine Gammaherpesvirus Latent Gene Expression and Reactivation from Latency. Journal of virology. 2005;79:14149–14160. doi: 10.1128/JVI.79.22.14149-14160.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechtel JT, Winant RC, Ganem D. Host and viral proteins in the virion of Kaposi’s sarcoma-associated herpesvirus. Journal of virology. 2005;79:4952–4964. doi: 10.1128/JVI.79.8.4952-4964.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortz E, Whitelegge JP, Jia Q, Zhou ZH, Stewart JP, Wu TT, Sun R. Identification of Proteins Associated with Murine Gammaherpesvirus 68 Virions. J Virol. 2003;77:13425–13432. doi: 10.1128/JVI.77.24.13425-13432.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braaten DC, Sparks-Thissen RL, Kreher S, Speck SH, Virgin HWIV. An Optimized CD8+ T-Cell Response Controls Productive and Latent Gammaherpesvirus Infection. J Virol. 2005;79:2573–2583. doi: 10.1128/JVI.79.4.2573-2583.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton T, Kurt-Jones EA, Boehme KW, Belko J, Latz E, Golenbock DT, Finberg RW. Human Cytomegalovirus Activates Inflammatory Cytokine Responses via CD14 and Toll-Like Receptor. 2003;2:4588–4596. doi: 10.1128/JVI.77.8.4588-4596.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalod M, Salazar-Mather TP, Malmgaard L, Lewis C, Asselin-Paturel C, Briere F, Trinchieri G, Biron CA. Interferon {alpha}/{beta} and Interleukin 12 Responses to Viral Infections: Pathways Regulating Dendritic Cell Cytokine Expression In Vivo. 2002:517–528. doi: 10.1084/jem.20011672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis DA, Singer KE, Reynolds IP, Haque M, Yarchoan R. Hypoxia Enhances the Phosphorylation and Cytotoxicity of Ganciclovir and Zidovudine in Kaposi’s Sarcoma-Associated Herpesvirus Infected Cells. Cancer Res. 2007;67:7003–7010. doi: 10.1158/0008-5472.CAN-07-0939. [DOI] [PubMed] [Google Scholar]
- Doyle S, Vaidya S, O’Connell R, Dadgostar H, Dempsey P, Wu T, Rao G, Sun R, Haberland M, Modlin R, et al. IRF3 mediates a TLR3/TLR4-specific antiviral gene program. Immunity. 2002;17:251–263. doi: 10.1016/s1074-7613(02)00390-4. [DOI] [PubMed] [Google Scholar]
- Durand V, Wong SY, Tough DF, Le Bon A. Shaping of adaptive immune responses to soluble proteins by TLR agonists: a role for IFN-alpha/beta. Immunology and cell biology. 2004;82:596–602. doi: 10.1111/j.0818-9641.2004.01285.x. [DOI] [PubMed] [Google Scholar]
- Ebrahimi B, Dutia BM, Roberts KL, Garcia-Ramirez JJ, Dickinson P, Stewart JP, Ghazal P, Roy DJ, Nash AA. Transcriptome profile of murine gammaherpesvirus-68 lytic infection. J Gen Virol. 2003;84:99–109. doi: 10.1099/vir.0.18639-0. [DOI] [PubMed] [Google Scholar]
- Garcia MA, Gil J, Ventoso I, Guerra S, Domingo E, Rivas C, Esteban M. Impact of Protein Kinase PKR in Cell Biology: from Antiviral to Antiproliferative Action. 2006:1032–1060. doi: 10.1128/MMBR.00027-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershburg E, Pagano JS. Conserved herpesvirus protein kinases. Biochimica et Biophysica Acta (BBA) - Proteins & Proteomics. 2008;1784:203–212. doi: 10.1016/j.bbapap.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamza MS, Reyes RA, Izumiya Y, Wisdom R, Kung HJ, Luciw PA. ORF36 Protein Kinase of Kaposi’s Sarcoma Herpesvirus Activates the c-Jun N-terminal Kinase Signaling Pathway. J Biol Chem. 2004;279:38325–38330. doi: 10.1074/jbc.M400964200. [DOI] [PubMed] [Google Scholar]
- Haque M, Wang V, Davis DA, Zheng ZM, Yarchoan R. Genetic Organization and Hypoxic Activation of the Kaposi’s Sarcoma-Associated Herpesvirus ORF34-37 Gene Cluster. J Virol. 2006;80:7037–7051. doi: 10.1128/JVI.00553-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiscott J. Triggering the innate antiviral response through IRF-3 activation. J Biol Chem. 2007;282:15325–15329. doi: 10.1074/jbc.R700002200. [DOI] [PubMed] [Google Scholar]
- Hwang S, Wu TT, Tong LM, Kim KS, Martinez-Guzman D, Colantonio AD, Uittenbogaart CH, Sun R. Persistent Gamma-Herpesvirus Replication and Its Dynamic Interaction with Host In Vivo. Journal of virology. 2008 doi: 10.1128/JVI.01152-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumiya Y, Izumiya C, Van Geelen A, Wang DH, Lam KS, Luciw PA, Kung HJ. Kaposi’s Sarcoma-Associated Herpesvirus-Encoded Protein Kinase and Its Interaction with K-bZIP. J Virol. 2007;81:1072–1082. doi: 10.1128/JVI.01473-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katze MG, He Y, Gale M. VIRUSES AND INTERFERON: A FIGHT FOR SUPREMACY. Nature Reviews Immunology. 2002;2:675–687. doi: 10.1038/nri888. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Kato K. Protein kinases conserved in herpesviruses potentially share a function mimicking the cellular protein kinase cdc2. Reviews in medical virology. 2003;13:331–340. doi: 10.1002/rmv.402. [DOI] [PubMed] [Google Scholar]
- Kolumam GA, Thomas S, Thompson LJ, Sprent J, Murali-Krishna K. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J Exp Med. 2005;202:637–650. doi: 10.1084/jem.20050821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bon A, Etchart N, Rossmann C, Ashton M, Hou S, Gewert D, Borrow P, Tough DF. Cross-priming of CD8+ T cells stimulated by virus-induced type I interferon. Nat Immunol. 2003;4:1009–1015. doi: 10.1038/ni978. [DOI] [PubMed] [Google Scholar]
- Lee CP, Chen JY, Wang JT, Kimura K, Takemoto A, Lu CC, Chen MR. Epstein-Barr Virus BGLF4 Kinase Induces Premature Chromosome Condensation through Activation of Condensin and Topoisomerase II. J Virol. 2007;81:5166–5180. doi: 10.1128/JVI.00120-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XL, Blackford JA, Hassel BA. RNase L Mediates the Antiviral Effect of Interferon through a Selective Reduction in Viral RNA during Encephalomyocarditis Virus Infection. 1998:2752–2759. doi: 10.1128/jvi.72.4.2752-2759.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin R, Heylbroeck C, Pitha PM, Hiscott J. Virus-dependent phosphorylation of the IRF-3 transcription factor regulates nuclear translocation, transactivation potential, and proteasome-mediated degradation. Mol Cell Biol. 1998;18:2986–2996. doi: 10.1128/mcb.18.5.2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin R, Mamane Y, Hiscott J. Structural and functional analysis of interferon regulatory factor 3: localization of the transactivation and autoinhibitory domains. Mol Cell Biol. 1999;19:2465–2474. doi: 10.1128/mcb.19.4.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund J, Sato A, Akira S, Medzhitov R, Iwasaki A. Toll-like Receptor 9-mediated Recognition of Herpes Simplex Virus-2 by Plasmacytoid Dendritic Cells. 2003:513–520. doi: 10.1084/jem.20030162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrack P, Kappler J, Mitchell T. Type I interferons keep activated T cells alive. J Exp Med. 1999;189:521–530. doi: 10.1084/jem.189.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Guzman D, Rickabaugh T, Wu TT, Brown H, Cole S, Song MJ, Tong L, Sun R. Transcription program of murine gammaherpesvirus 68. Journal of virology. 2003;77:10488–10503. doi: 10.1128/JVI.77.19.10488-10503.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel D, Mertens T. The UL97 protein kinase of human cytomegalovirus and homologues in other herpesviruses: impact on virus and host. Biochimica et Biophysica Acta (BBA) - Proteins & Proteomics. 2004;1697:169–180. doi: 10.1016/j.bbapap.2003.11.022. [DOI] [PubMed] [Google Scholar]
- Morrison LA. The Toll of herpes simplex virus infection. Trends in Microbiology. 2004;12:353–356. doi: 10.1016/j.tim.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Mossman KL, Ashkar AA. Herpesviruses and the innate immune response. Viral immunology. 2005;18:267–281. doi: 10.1089/vim.2005.18.267. [DOI] [PubMed] [Google Scholar]
- Overton HA, McMillan DJ, Klavinskis LS, Hope L, Ritchie AJ, Wong-kai-in P. Herpes simplex virus type 1 gene UL13 encodes a phosphoprotein that is a component of the virion. Virology. 1992;190:184–192. doi: 10.1016/0042-6822(92)91204-8. [DOI] [PubMed] [Google Scholar]
- Player MR, Torrence PF. The 2–5 A system: Modulation of viral and cellular processes through acceleration of RNA degradation. Pharmacology & Therapeutics. 1998;78:55–113. doi: 10.1016/S0163-7258(97)00167-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin BY, Liu C, Srinath H, Lam SS, Correia JJ, Derynck R, Lin K. Crystal structure of IRF-3 in complex with CBP. Structure. 2005;13:1269–1277. doi: 10.1016/j.str.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Sharma S, tenOever BR, Grandvaux N, Zhou GP, Lin R, Hiscott J. Triggering the interferon antiviral response through an IKK-related pathway. Science. 2003;300:1148–1151. doi: 10.1126/science.1081315. [DOI] [PubMed] [Google Scholar]
- Simmen KA, Singh J, Luukkonen BGM, Lopper M, Bittner A, Miller NE, Jackson MR, Compton T, Fruh K. Global modulation of cellular transcription by human cytomegalovirus is initiated by viral glycoprotein B. 2001:7140–7145. doi: 10.1073/pnas.121177598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GA, Enquist LW. Construction and transposon mutagenesis in Escherichia coli of a full-length infectious clone of pseudorabies virus, an alphaherpesvirus. Journal of virology. 1999;73:6405–6414. doi: 10.1128/jvi.73.8.6405-6414.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song MJ, Hwang S, Wong WH, Wu TT, Lee S, Liao HI, Sun R. Identification of viral genes essential for replication of murine gamma-herpesvirus 68 using signature-tagged mutagenesis. Proc Natl Acad Sci U S A. 2005;102:3805–3810. doi: 10.1073/pnas.0404521102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparks-Thissen RL, Braaten DC, Kreher S, Speck SH, Virgin HWt. An optimized CD4 T-cell response can control productive and latent gammaherpesvirus infection. Journal of virology. 2004;78:6827–6835. doi: 10.1128/JVI.78.13.6827-6835.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark GR, Kerr IM, Williams BRG, Silverman RH, Schreiber RD. HOW CELLS RESPOND TO INTERFERONS. 1998:227–264. doi: 10.1146/annurev.biochem.67.1.227. [DOI] [PubMed] [Google Scholar]
- Stevenson PG, Efstathiou S. Immune mechanisms in murine gammaherpesvirus-68 infection. Viral immunology. 2005;18:445–456. doi: 10.1089/vim.2005.18.445. [DOI] [PubMed] [Google Scholar]
- Sunil-Chandra NP, Arno J, Fazakerley J, Nash AA. Lymphoproliferative disease in mice infected with murine gammaherpesvirus 68. Am J Pathol. 1994;145:818–826. [PMC free article] [PubMed] [Google Scholar]
- Taniguchi T, Takaoka A. The interferon-alpha/beta system in antiviral responses: a multimodal machinery of gene regulation by the IRF family of transcription factors. Curr Opin Immunol. 2002;14:111–116. doi: 10.1016/s0952-7915(01)00305-3. [DOI] [PubMed] [Google Scholar]
- Tarakanova VL, Leung-Pineda V, Hwang S, Yang CW, Matatall K, Basson M, Sun R, Piwnica-Worms H, Sleckman BP, Virgin Iv HW. [gamma]-Herpesvirus Kinase Actively Initiates a DNA Damage Response by Inducing Phosphorylation of H2AX to Foster Viral Replication. Cell Host & Microbe. 2007;1:275–286. doi: 10.1016/j.chom.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varnum SM, Streblow DN, Monroe ME, Smith P, Auberry KJ, Pasa-Tolic L, Wang D, Camp DG, 2nd, Rodland K, Wiley S, et al. Identification of proteins in human cytomegalovirus (HCMV) particles: the HCMV proteome. Journal of virology . 2004;78:10960–10966. doi: 10.1128/JVI.78.20.10960-10966.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weslow-Schmidt JL, Jewell NA, Mertz SE, Simas JP, Durbin JE, Flano E. Type I Interferon Inhibition and Dendritic Cell Activation during Gammaherpesvirus Respiratory Infection. J Virol. 2007;81:9778–9789. doi: 10.1128/JVI.00360-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wherry EJ, Puorro KA, Porgador A, Eisenlohr LC. The Induction of Virus-Specific CTL as a Function of Increasing Epitope Expression: Responses Rise Steadily Until Excessively High Levels of Epitope Are Attained. J Immunol. 1999;163:3735–3745. [PubMed] [Google Scholar]
- Zhu FX, Chong JM, Wu L, Yuan Y. Virion proteins of Kaposi’s sarcoma-associated herpesvirus. Journal of virology. 2005;79:800–811. doi: 10.1128/JVI.79.2.800-811.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.