Abstract
Background and Aims
Microsporogenesis in monocots is often characterized by successive cytokinesis with centrifugal cell plate formation. Pollen grains in monocots are predominantly monosulcate, but variation occurs, including the lack of apertures. The aperture pattern can be determined by microsporogenesis features such as the tetrad shape and the last sites of callose deposition among the microspores. Potamogeton belongs to the early divergent Potamogetonaceae and possesses inaperturate pollen, a type of pollen for which it has been suggested that there is a release of the constraint on tetrad shape. This study aimed to investigate the microsporogenesis and the ultrastructure of pollen wall in species of Potamogeton in order to better understand the relationship between microsporogenesis features and the inaperturate condition.
Methods
The microsporogenesis was investigated using both light and epifluorescence microscopy. The ultrastructure of the pollen grain was studied using transmission electron microscopy.
Key Results
The cytokinesis is successive and formation of the intersporal callose wall is achieved by centrifugal cell plates, as a one-step process. The microspore tetrads were tetragonal, decussate, T-shaped and linear, except in P. pusillus, which showed less variation. This species also showed a callose ring in the microsporocyte, and some rhomboidal tetrads. In the mature pollen, the thickening observed in a broad area of the intine was here interpreted as an artefact.
Conclusions
The data support the view that there is a correlation between the inaperturate pollen production and the release of constraint on tetrad shape. However, in P. pusillus the tetrad shape may be constrained by a callose ring. It is also suggested that the lack of apertures in the pollen of Potamogeton may be due to the lack of specific sites on which callose deposition is completed. Moreover, inaperturate pollen of Potamogeton would be better classified as omniaperturate.
Key words: Alismatales, callose, microsporogenesis, pollen aperture, Potamogeton illinoensis, P. polygonus, P. pusillus, tetrad shape
INTRODUCTION
Microsporogenesis is the process that yields four haploid microspores from a single diploid microsporocyte through meiosis and cytokinesis (Furness et al., 2002). Two types of cytokinesis, successive and simultaneous, are traditionally recognized. In the first type a cell plate is formed after meiosis I, giving rise to a dyad stage. In the simultaneous type, the cell partitioning takes place only after meiosis II (Maheshwari, 1950). In monocots, cytokinesis is predominantly successive (Furness and Rudall, 1999a; Ressayre, 2001; Furness et al., 2002; Penet et al., 2005; Nadot et al., 2006) and occurring through centrifugal cell plates (Penet et al., 2005; Nadot et al., 2006), whereas in eudicots, cytokinesis is commonly simultaneous (Furness and Rudall, 1999a; Ressayre, 2001) with centripetal cell plates (Ressayre, 2001; Ressayre et al., 2005), but other combinations exist in both groups (Nadot et al., 2008).
Features such as cytokinesis type and cell plate formation have systematic value in some monocots groups, viz. Dioscoreales, Asparagales and Poales (Furness and Rudall, 1999a). Although the relationship between the type of cytokinesis and the type of pollen aperture is not straightforward (Furness and Rudall, 1999a; Nadot et al., 2008), the type of cytokinesis has an influence on the type of tetrad formed at the end of meiosis, which in turn can have an impact on the aperture type. These factors greatly determine the pattern observed in pollen aperture (Furness and Rudall, 1999a; Furness et al., 2002; Penet et al., 2005; Nadot et al., 2006, 2008; Sannier et al., 2006).
Angiosperms display a diversity of pollen aperture patterns (Walker and Doyle, 1975), which are defined in terms of shape, structure, number and distribution of apertures on the pollen wall (Ressayre et al., 2002). In monocots, the basic pattern is that of a single distal aperture, but the aperture may be missing, characterizing the inaperturate pollen type. The lack of apertures is usually associated with a reduction in exine thickness and complexity (Zavada, 1983). The exine may be either absent or present as a non-ornamented thin layer (Pettitt and Jermy, 1975; Zavada, 1983; Furness and Rudall, 1999b). Two types of inaperturate pollen grains are recognized: omniaperturate (Thanikaimoni, 1984), i.e. the entire wall functions as a potential site for pollen tube germination; and functionally monoaperturate (Furness and Rudall, 1999b), i.e. a clearly defined site for pollen tube growth is indicated by a localized thickening of the intine.
In Alismatales, the largest clade of aquatic Angiosperms with 14 families (Angiosperm Phylogeny Group II, 2003; Chase, 2004), the inaperturate pollen is the predominant type, being associated with hydrophily (Furness and Rudall, 1999b). This is the pollen type found in Potamogetonaceae (Sorsa, 1988). In earlier accounts on pollen development of Potamogeton it was pointed out that cytokinesis during microsporogenesis is of the successive type (Stenar, 1925; Schnarf, 1931). In some representatives of this genus, the inaperturate pollen has been described as functionally monoaperturate (Furness and Rudall, 1999b).
The present study aims to investigate the microsporogenesis and the ultrastructure of the pollen wall in three species of Potamogeton, to address the following two questions. Is there a release of developmental constraints during microsporogenesis due to the inaperturate condition of pollen grains in Potamogeton? To which inaperturate subtype do the pollen grains of the three Potamogeton species studied belong?
MATERIALS AND METHODS
Material from three selected Potamogeton L. species was collected from rivers and lakes of Brazil (vouchers deposited in the Herbário do Departamento de Botânica, Universidade Federal do Paraná, Curitiba, Paraná, Brazil – UPCB): Potamogeton illinoensis Morong (UPCB 60501), Potamogeton polygonus Cham. and Schltdl. (UPCB 61311) and Potamogeton pusillus L. (UPCB 60500).
Inflorescences of each species were collected at various developmental stages and fixed in 1 % glutaraldehyde and 4 % formaldehyde in 0·1 m phosphate buffer, pH 7·2 (McDowell and Trump, 1976), unless otherwise stated. For light microscope examination, samples were rinsed in phosphate buffer, dehydrated through a graded ethanol series and embedded in historesin (Leica Historesin Embedding Kit, Nussloch, Germany). Sections (2–5 µm) were cut on a rotary microtome RM2145 (Leica Microsystems, Wetzlar, Germany) using glass knives (Leica Microsystems, Vienna, Austria), stained with toluidine blue (O'Brien et al., 1965) and mounted in Permount (Fisher Scientific, Fair Lawn, USA). Photomicrographs were taken using a Zeiss Axiolab microscope with a digital camera.
To observe microsporogenesis in progress, fixed anthers at appropriate stages were selected. They were washed in phosphate buffer several times, carefully opened on slides, and covered with a drop of 0·05 % aniline blue. After 5 min, the excess stain was removed and the material was mounted in glycerine gelatine. Aniline blue preparations were observed using an epifluorescence Zeiss Axiophot microscope with a DAPI filter.
For scanning electron microscope observations, the inflorescences were dehydrated through a graded ethanol series until 70 % ethanol, then dissected, transferred to a graded propanone series until 100 % propanone, critical point dried, coated with gold and examined using a JEOL JSM 6360LV (JEOL Ltd, Tokyo, Japan) scanning electron microscope.
For transmission electron microscope observations, pre-anthesis anthers were placed in modified Karnovskýs fixative (2·5 % glutaraldehyde and 2 % paraformaldehyde in 0·1 m phosphate buffer, pH 7·2; Karnovsky, 1965), deaerated under vacuum and fixed for 12–24 h at 10 °C. Samples were rinsed in cold phosphate buffer, post-fixed in the dark with 2 % osmium tetroxide for 2 h at 10 °C, rinsed again, and dehydrated through a cold graded propanone series. Material was embedded in epoxy resin (Spurr, 1969). Ultrathin (gold) sections were cut using a diamond knife on a Leica Ultracut R (Leica Microsystems,Vienna, Austria), stained with uranyl acetate and lead citrate (Reynolds, 1963). Observations were made with a JEOL JEM-1200EXII (JEOL Ltd) transmission electron microscope.
RESULTS
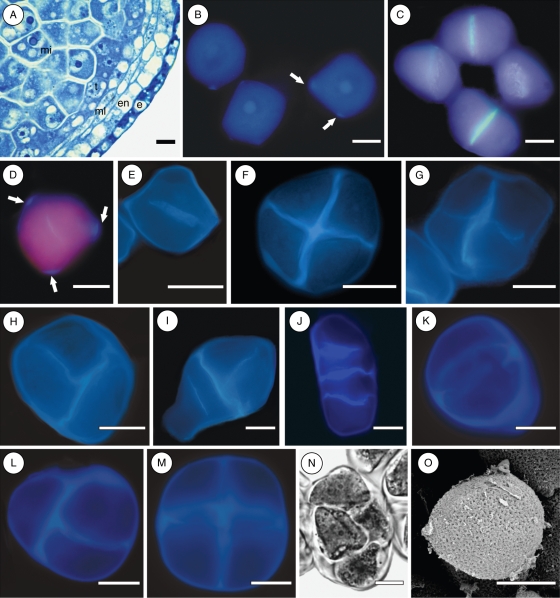
In all species studied, the microsporocytes completely fill the anther locule, and present a prominent nucleus and a dense cytoplasm (Fig. 1A). The anther wall of the three species is composed of an epidermis, an endothecium, two middle layers and a tapetum layer (Fig. 1A).
Fig. 1.
Microsporogenesis and pollen sculpturing in Potamogeton: P. polygonus in (A–D, F, I); P. illinoensis in (E, G, H, J, O); P. pusillus in (K–N). (A) Cross-section of a pre-meiotic anther; (B) microsporocytes with initial callose deposition on the corners (arrows); (C) centrifugal cell plates in the first meiotic division and dyad stage; (D) cytokinesis through centrifugal cell plate in a microsporocyte with callose deposits on its corners (arrows); (E) cytokinesis through centrifugal cell plate in a microsporocyte without conspicuous callose deposits on its corners; (F) regular tetragonal tetrad; (G) irregular tetragonal tetrad; (H) decussate tetrad; (I) T-shaped tetrad; (J) linear tetrad; (K) microsporocyte with a conspicuous callose ring; (L) decussate tetrad; (M) tetragonal tetrad; (N) rhomboidal tetrad; (O) pollen grain with reticulate exine sculpturing. Abbreviations: e, epidermis; en, endothecium; mi, microsporocyte; ml, middle layers; t, tapetum. Scale bars = 10 µm.
Microsporogenesis in progress was observed for only two of the three species, namely P. illinoensis and P. polygonus. In both species the meiotic cytokinesis is successive (Fig. 1C–J). After meiosis I, a thin callosic wall is laid down by centrifugal progression of the cell plate, giving rise to a dyad stage (Fig. 1C–E). No conspicuous additional callose deposition was observed. At the end of meiosis II the resulting tetrads were mainly of tetragonal (Fig. 1F, G) and decussate shapes (Fig. 1H), but some T-shaped (Fig. 1I) and linear tetrads (Fig. 1J) were also observed in the same anther locule. Although several attempts were made, it was not possible to observe microsporogenesis in progress in Potamogeton pusillus. Only pre-meiotic and post-meiotic stages were observed. Microsporocytes in this species showed a conspicuous ring of callose (Fig. 1K), and tetrads were predominantly decussate (Fig. 1L) and tetragonal (Fig. 1M), mixed with a few rhomboidal tetrads (Fig. 1N). In Potamogeton polygonus, microsporocytes have a polygonal shape and are surrounded by a callose wall which is thicker at the corners (Fig. 1B, D, arrows), which is different from the others species.
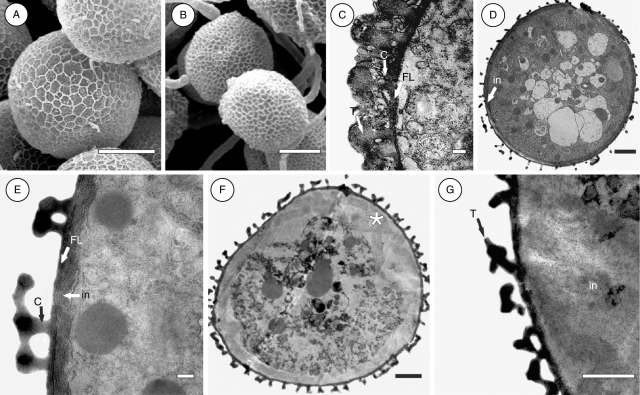
Scanning electron microscope observations showed that the mature pollen is inaperturate with a reticulate exine (Figs 1O and 2A, B). The pattern of reticulation differs among species, being narrow in P. illinoensis (Fig. 1O) wide in P. pusillus (Fig. 2A), and intermediary in P. polygonus (Fig. 2B).
Fig. 2.
Exine sculpturing and ultrastructure of Potamogeton pollen: P. pusillus in (A, D, E); P. illinoensis in (C); P. polygonus in (B, F, G). (A) Wide pattern of exine sculpturing; (B) intermediary pattern of exine sculpturing; (C) detail of the pollen tectum, columella and foot layer; (D) pollen grain with the lowest density of columella and tectal elements; (E) detail of the pollen wall with a fibrillar intine; (F) pollen grain with intermediary density of columella and tectal elements, and intine thickened on one side (asterisk); (G) detail of the thickened portion of intine. Abbreviations: in, intine; C, columella; FL, foot layer; T, tectum. Scale bars: (A, B) = 10 µm; (C, E) = 0·2 µm; (D, F) = 2 µm; (G) = 1 µm.
Transmission electron microscope observations showed that the exine is composed solely of the ectexine, which is divided in tectum, columella layer and foot layer (Fig. 2C–G). The density of both columella and tectal elements is highest in Potamogeton illinoensis (Fig. 2C), lowest P. pusillus (Fig. 2D, E) and intermediary in P. polygonus (Fig. 2F, G).
The intine is relatively thick and fibrillar in structure in P. pusillus (Fig. 2D, E) and P. polygonus (Fig. 2F, G). In P. polygonus only, this layer eventually appears to be thicker on one side of the pollen grain (Fig. 2F, asterisk, and G). In P. illinoensis it was not possible to observe the intine due to poor fixation.
DISCUSSION
In Potamogeton polygonus and P. illinoensis, cytokinesis during microsporogenesis is successive with centrifugal cell plates. This developmental sequence is widespread in monocots (Furness and Rudall, 1999a; Furness et al., 2002; Penet et al., 2005; Nadot et al., 2006). However, deviations from this pattern have been reported, such as the co-occurrence of both simultaneous and successive cytokinesis in microsporocytes within the same stamen (Sannier et al., 2006), the occurrence of intermediary cytokinesis (Furness et al., 2002; Nadot et al., 2008), centripetal formation of the intersporal wall (Penet et al., 2005; Nadot et al., 2006), as well as the occurrence of various types of microspore tetrads within the same anther (Pettitt, 1981; Hardy and Stevenson, 2000; Hardy et al., 2000; Ressayre, 2001; Penet et al., 2005; Nadot et al., 2006; Sannier et al., 2006).
In P. pusillus, the intersporal wall formation was not observed and it was not possible to characterize the type of cytokinesis. However, the presence of a callose ring was observed in the microsporocytes, and it was noted that, compared with the other species, there is less variability in tetrad types. The presence of a few rhomboidal tetrads suggests that simultaneous cytokinesis takes place at least in some microsporocytes of P. pusillus since it has been shown that this type of tetrad can be formed only through simultaneous cytokinesis (Nadot et al., 2008).
A callose ring has been reported in some Iridaceae with simultaneous cytokinesis and centripetally formed cell plates (Penet et al., 2005). Penet et al. (2005) suggest that such phenomenon can be a way of constraining tetrad shape to that obtained with successive cytokinesis (tetragonal and decussate), thus perhaps canalizing pollen morphology. If the production of inaperturate pollen is dependent on successive cytokinesis (and therefore at least partly conditioned by tetrad shape) in Potamogeton, then the presence of a callose ring in P. pusillus may be a way to constrain the tetrad shape to tetragonal or decussate independently of the type of cytokinesis, and consequently constrain the pollen type. In the present study, various types of tetrads have been observed in P. illinoensis and P. polygonus: tetragonal, decussate, linear and T-shaped. This is the first time that such variation of tetrad types in Potamogeton has been reported. Nadot et al. (2006) have reported variability of tetrad types related to inaperturate pollen grain formation in Strelitzia species (Strelitziaceae); the present data corroborated their hypothesis that in inaperturate pollen there is a release of the developmental constraint on tetrad shape, as no aperture will ever be formed.
In Potamogeton, microsporogenesis is a rapid process, which makes visualizing of all phases very difficult. It seems that wall formation between microspores is a one-step process, although it is not clear whether the cell plates are bare or whether they are covered with additional callose while they are developing. The second hypothesis seems more likely to take place in Potamogeton, since the cell plates are rather thicker than the bare ones already reported for other angiosperms (Ressayre et al., 2005; Nadot et al., 2006). Regardless of that, specific sites on which callose deposition is completed were not observed. This could be a possible explanation for the lack of apertures in Potamogeton pollen grains, since the sites where additional callose deposition is completed coincide with aperture location in some species (Ressayre et al., 2002, 2005). Further studies on other species of Potamogeton as well as other Alismatales may improve our understanding of this process.
The ultrastructure of pollen grains in Potamogeton has up to now been described for only two species, namely P. natans and P. pectinatus (Pettitt and Jermy, 1975). Whereas the pollen grains of P. pusillus and P. illinoensis described here conform to these previous descriptions, an unexpected feature was found in P. polygonus, with the observation of an irregular thickening of the intine. Due to this thickening, pollen grains of Potamogeton polygonus should be classified as functionally monoaperturate, following Furness and Rudall (1999b) citing Cranwell (1953). However, we suggest rather that such a feature might be a processing artefact, which leads the fibrillar intine structure to become unstable, this species being the only one among five to present this feature.
In case such thickening of intine in P. polygonus is not an artefact but genuine, it seems to occur throughout a broad area of the intine and not only at a single site. Thus, the pollen tube could potentially germinate on a broad area. In this case, pollen grains of Potamogeton should be classified neither as functionally monoaperturate nor as omniaperturate, but as intermediate. An alternate hypothesis accounting for the presence of such thickening is the implication of pollen wall ultrastructure in the pollination syndrome in this genus, which needs to be investigated.
The lack of pollen apertures may be due to the reduction or absence of exine (Zavada, 1983; Furness and Rudall, 1999b). Since the formation of apertures might be related to mechanisms that prevent primexine deposition (Heslop-Harrison, 1963; Waterkeyn and Bienfait, 1970; Rowley, 1975), it is probable that such mechanisms are not present during inaperturate pollen formation, as proposed for some eudicots (Furness, 2007). However, as the inaperturate pollen type has evolved independently several times within the monocots clade (Furness and Rudall, 1999b), one can infer that the way it is formed may be different within each clade, if we do not consider iterative evolution, and it may therefore be difficult to discuss the inaperturate character from a general point of view.
Data reported in the current study for Potamogeton illinoensis, P. polygonus and P pusillus strengthens the hypothesis of release of the constraints on tetrad shape in inaperturate pollen. The lack of apertures in Potamogeton pollen may be linked to the way callose is deposited upon microspore walls during microsporogenesis, without specific sites on which callose deposition is completed. We also suggest that inaperturate pollen of Potamogeton should be classified as omniaperturate rather than functionally monoaperturate since the broad area of the intine is a processing artefact. To confirm these observations, transmission electron microscope analyses on germinated pollen grains are needed to verify possible pollen tube germination sites.
ACKNOWLEDGEMENTS
We acknowledge the help of Instituto Ecoplan (PR, Brazil) and Indústrias Pizzatto (PR, Brazil) for logistic support. We thank Sophie Nadot (Université Paris-Sud) for her valuable suggestions and comments on the manuscript. We also thank three anonymous reviewers for their helpful comments and improvement of the manuscript. This work was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) (MSc grant to E.L.P.N.) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (470800/2008-9 to A.I.C.; 47248/2004-0 to C.B. and M.C.C.M.).
LITERATURE CITED
- Angiosperm Phylogeny Group II. An update of the angiosperm phylogeny group classification for the orders and families of flowering plants: APG II. Botanical Journal of the Linnean Society. 2003;141:399–436. [Google Scholar]
- Chase MW. Monocot relationships: an overview. American Journal of Botany. 2004;91:1645–1655. doi: 10.3732/ajb.91.10.1645. [DOI] [PubMed] [Google Scholar]
- Cranwell LM. New Zealand pollen studies: the monocotyledons. Bulletin of the Auckland Institute and Museum. 1953;3:1–91. [Google Scholar]
- Furness CA. Why does some pollen lack apertures? A review of inaperturate pollen in eudicots. Botanical Journal of the Linnean Society. 2007;155:29–48. [Google Scholar]
- Furness CA, Rudall PJ. Microsporogenesis in monocotyledons. Annals of Botany. 1999;a 84:475–499. [Google Scholar]
- Furness CA, Rudall PJ. Inaperturate pollen in monocotyledons. International Journal of Plant Sciences. 1999;b 160:395–414. [Google Scholar]
- Furness CA, Rudall PJ, Sampson FB. Evolution of microsporogenesis in Angiosperms. International Journal of Plant Sciences. 2002;163:235–260. [Google Scholar]
- Hardy CR, Stevenson DW. Development of gametophytes, flower, and floral vasculature in Cochliostema odoratissimum (Commelinaceae) Botanical Journal of the Linnean Society. 2000;134:131–157. [Google Scholar]
- Hardy CR, Stevenson DW, Kiss HG. Development of gametophytes, flower, and floral vasculature in Dichorisandra thyrsiflora (Commelinaceae) American Journal of Botany. 2000;87:1228–1239. [PubMed] [Google Scholar]
- Heslop-Harrison J. An ultrastructural study of pollen wall ontogeny in Silene pendula. Grana Palynologica. 1963;4:7–24. [Google Scholar]
- Karnovsky MJ. A formaldehyde-glutaraldehyde fixative of high osmolality for use in electron microscopy. The Journal of Cell Biology. 1965;27:137A–138A. [Google Scholar]
- Maheshwari P. An introduction to the embryology of angiosperms. New York, NY: McGraw-Hill Co; 1950. [Google Scholar]
- McDowell EM, Trump B. Histological fixatives for diagnostic light and electron microscopy. Archives of Pathology and Laboratory Medicine. 1976;100:405–414. [PubMed] [Google Scholar]
- Nadot S, Forchioni A, Penet L, Sannier J, Ressayre A. Links between early pollen development and aperture pattern in monocots. Protoplasma. 2006;228:55–64. doi: 10.1007/s00709-006-0164-4. [DOI] [PubMed] [Google Scholar]
- Nadot S, Furness CA, Sannier J, et al. Phylogenetic comparative analysis of microsporogenesis in angiosperms with a focus on monocots. American Journal of Botany. 2008;95:1426–1436. doi: 10.3732/ajb.0800110. [DOI] [PubMed] [Google Scholar]
- O'Brien TP, Feder N, McCully ME. Polychromatic staining of plant cell walls by toluidine blue O. Protoplasma. 1965;59:368–373. [Google Scholar]
- Penet L, Nadot S, Ressayre A, Forchioni A, Dreyer L, Gouyon PH. Multiple developmental pathways leading to a single morph: monosulcate pollen (examples from the Asparagales) Annals of Botany. 2005;95:331–343. doi: 10.1093/aob/mci030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettitt JM, Jermy AC. Pollen in hydrophilous angiosperms. Micron. 1975;5:377–405. [Google Scholar]
- Pettitt JM. Reproduction in seagrasses: pollen development in Thalassia hemprichii, Halophila stipulaceae and Thalassodendron ciliatum. Annals of Botany. 1981;48:609–622. [Google Scholar]
- Ressayre A. Equatorial aperture pattern in monocots: same definition rules as in eudicots? The example of two species of Pontederiaceae. International Journal of Plant Sciences. 2001;162:1219–1224. [Google Scholar]
- Ressayre A, Godelle B, Raquin C, Gouyon PH. Aperture pattern ontogeny in angiosperms. 2002;294:122–135. doi: 10.1002/jez.10150. Journal of Experimental Zoology (Molecular and Developmental Evolution) [DOI] [PubMed] [Google Scholar]
- Ressayre A, Dreyer L, Triki-Teurtroy S, Forchioni A, Nadot S. Post-meiotic cytokinesis and pollen aperture pattern ontogeny: comparison of development in four species differing in aperture pattern. American Journal of Botany. 2005;92:576–583. doi: 10.3732/ajb.92.4.576. [DOI] [PubMed] [Google Scholar]
- Reynolds ES. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. The Journal of Cell Biology. 1963;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley JR. Germinal aperture formation in pollen. Taxon. 1975;24:17–25. [Google Scholar]
- Sannier J, Nadot S, Forchioni A, Harley M, Albert B. Variations in the microsporogenesis of monosulcate palm pollen. Botanical Journal of the Linnean Society. 2006;151:93–102. [Google Scholar]
- Schnarf K. Vergleichende Embryologie der Angiospermen. Berlin: Gebrüder Bornträger; 1931. [Google Scholar]
- Sorsa P. Pollen morphology of Potamogeton and Groenlandia (Potamogetonaceae) and its taxonomic significance. Annales Botanici Fennici. 1988;25:179–199. [Google Scholar]
- Spurr AR. A low-viscosity epoxy resin embedding medium for electron microscopy. Journal of Ultrastructure Research. 1969;26:31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Stenar H. Die embryologie der Amaryllideen. Sweden: University of Uppsala; 1925. PhD Thesis. [Google Scholar]
- Thanikaimoni G. Omniaperturate Euphorbiaceae pollen with striate spines. Bulletin du Jardin Botanique National de Belgique. 1984;54:305–325. [Google Scholar]
- Walker JW, Doyle JA. The bases of angiosperm phylogeny, palinology. Annals of the Missouri Botanical Garden. 1975;62:664–723. [Google Scholar]
- Waterkeyn L, Bienfait A. On the possible function of the callosic special wall in Ipomoea purpurea (L.) Roth. Grana. 1970;10:13–20. [Google Scholar]
- Zavada M. Comparative morphology of monocot pollen and evolutionary trends of apertures and wall structure. Botanical Review. 1983;49:331–379. [Google Scholar]