Abstract
Background and Aims
Height gain plays an important role in plant life-history strategies and species coexistence. Here main-stem costs of height gain of saplings across species within a rainforest community are compared.
Methods
Scaling relationships of height to diameter at the sapling stage were compared among 75 woody rainforest plant species in subtropical eastern Australia using standardized major axis regression. Main-stem costs of height gain were then related to other functional traits that reflect aspects of species ecological strategies.
Key Results
Slopes (β) for the height–diameter (H–D) scaling relationship were close to 1·3, in line with previous reports and with theory. Main-stem volume to achieve 5 m in height varied substantially between species, including between species within groups based on adult height and successional status. The variation was largely independent of other species traits, being uncorrelated with mature plant height (Hmax) and with leaf size, and weakly negatively correlated with wood density and seed size. The relationship between volume to reach 5 m and wood density was too weak to be regarded as a trade-off. Estimated main-stem dry mass to achieve 5 m height varied almost three-fold across species, with wood density and stem volume contributing roughly equally to the variation.
Conclusion
The wide range in economy of sapling height gain reported here is presumed to be associated with a trade-off between faster growth and higher mortality rates. It is suggested that wide diameters would have a stronger effect in preventing main-stem breakage in the short term, while high wood density would have a stronger effect in sustaining stem strength over time.
Keywords: Allometry, community ecology, diameter, functional traits, height, saplings, stem volume, trait correlations, wood density
INTRODUCTION
Plant size (measured as mass, height or diameter) can span ten orders of magnitude over the ontogenetic lifetime of an individual tree. Size exerts a powerful influence on plant form, function and life history (Niklas, 1995; Westoby et al., 2002). Coexisting trees can vary considerably in height at similar diameters (diameter at breast height, dbh; Thomas, 1996; King, 1996; Sterck et al., 2001; Kohyama et al., 2003; Poorter et al., 2003, 2006), and diameter–height relationships influence the ‘safety factor’ of species against buckling (Putz et al., 1983; van Gelder et al., 2006).
Two alternative interpretations can be suggested for the smaller diameter at a given height (e.g. dbh) of some species compared with others. One is that narrow-dbh species reflect a fast-growth strategy with a concomitant high risk of stem breakage. The other possibility is that narrow-dbh species compensate for limited basal thickening by having strong, high-density wood. Dense wood has a higher modulus of rupture (Niklas, 1994) and makes trees less vulnerable to breakage. If narrow-dbh species invested more in wood density, then the biomass cost of height gain and the risk of breakage might be similar across different stem-widening strategies.
Thomas (1996) examined the hypothesis that understorey trees have thicker stems (at a common height) than canopy trees in the same assemblage because diameter increment continues even after trees attain their maximum height (King, 1990). Interestingly, he found no correlation between diameter at a common height and maximum height at maturity (Hmax). However, a number of studies have subsequently reported that adult shade-tolerant sub-canopy trees have thicker stems than canopy trees at the same height (King, 1996; Sterck et al., 2001; Kohyama et al., 2003; Poorter et al., 2003, 2006; King et al., 2006a), suggesting that the height–diameter (H–D) relationship does vary in relation to Hmax (Thomas, 1996; King et al., 2006b; Poorter et al., 2006; Osunkoya et al., 2007). Because the diameter–height ratio increases progressively with growth, and species ranking can switch with reference to height, a more general approach to addressing this question is to assess the slope and intercept of log diameter versus log height graphs (Kerkhoff et al., 2006; Poorter and Bongers, 2006; Warton et al., 2006).
The functional basis for the expectation that some traits are influenced by growth form (plant size) is well established (see, for example, Moles et al., 2005). In addition, trait-based approaches to community ecology can potentially link ecological strategy variation and functional diversity (via scaling relationships and performance currencies) to community assembly and species coexistence (Westoby and Wright, 2006; Grime, 2006; McGill et al., 2006). Strong correlations across species have been reported for leaf size, specific leaf area (SLA) and maximum height (e.g. Fonseca et al., 2000); between leaf size, wood density, seed size and maximum height (Ackerly et al., 2002; Cornwell et al., 2006; Cornwell and Ackerly, 2009); and between shade tolerance (reflecting successional status), wood density and maximum height (Falster and Westoby, 2005; discussed in Poorter, 2008; and see Reich et al., 2003; Wright et al., 2007).
This paper compares investment in main stem at the sapling stage across 75 species in Australian subtropical rain forest. The following questions are addressed: (1) how did species vary in the main-stem investment used to attain a given height, and what were the contributions to this variation from allometry (H–D relationships) and from wood density; and (2) were those architectural traits associated with other species traits for which data were available and that are thought to be ecologically significant, namely adult stature (potential maximum height as Hmax), shade tolerance, leaf size and seed size?
MATERIALS AND METHODS
Site and selection of species
Diameter at breast height over bark (dbh) and height for plants >1·0 and <10 cm dbh were collected in 2000–2001 for 18 134 individual understorey trees, small trees and shrubs from 20 previously established permanent plots in sub-tropical rain forest in eastern Australia (Kariuki and Kooyman, 2005; Kariuki et al., 2006). The aim at the time was to supplement data for larger trees (3000 individuals >10 cm dbh, representing 117 species) that had been permanently tagged and monitored for growth over decades. Individual trees were removed from the dataset if they belonged to species represented by low numbers of individuals (predominantly shade-tolerant canopy tree species), if their stems were recorded as broken or had measurements that clearly indicated they were damaged, or if they had dbh <1 cm. The resulting data covered a total of 11 111 individual plants representing 75 species from the total of 138 recorded (Supplementary Data Table S1, available online). The species came from 32 families and 62 genera, and varied substantially in adult stature and successional status.
Of the 75 species included in the study 70 were classed as shade-tolerant and five as shade-intolerant, based on local knowledge at seedling and sapling stages (Kooyman, 1996; Kariuki and Kooyman, 2005; Kariuki et al., 2006). Shade-intolerant species included both pioneers and canopy trees, and shade-tolerant species included shrubs, understorey trees and canopy trees. Rather than using these as categories, the continuous trait of maximum height at maturity as a potential predictor was used, along with shade-tolerance.
Diameter was measured at 1·3 m above ground level using a steel diameter tape or calliper. Height was measured to the topmost foliage using an extendable height pole. Species-level data for leaf size, wood traits (including density) and seed size were taken from published floras and other sources, including Bootle (1983), Stanley and Ross (1983–1989, volumes 1–3), Floyd (1989, pp. 268–270), Harden (1990–1993, volumes 1–4 with revisions), Ilic et al. (2000) and Cornelissen et al. (2003). Minimum and maximum leaf sizes reported in the floras generally reflected the range of variation in leaf size on mature plants, and juvenile leaf sizes were excluded from consideration. Leaf size reported here is for whole simple leaves and for lateral leaflets of compound leaves. Leaf size was estimated using maximum leaf length and width, where area = length × width × 0·70 (cm2), which has been shown to correlate well with photographic area estimates of rainforest tree leaves (e.g. Kraft et al., 2008). Seed size was estimated using maximum dimensions of embryo plus endocarp (length + width/2, in millimetres; reflecting average diameter). Wood density estimates for adults [dry kg m−3; van Gelder et al. (2006) found that adult and juvenile wood density were well correlated across species, r = 0·91, P < 0·001] were extracted from published sources. Estimated maximum height at maturity (Hmax) was based on field information previously collected by R.M.K. that reflects maximum canopy height (m) for species in this location (at largest known diameters, at reproductive maturity). This provides a single value for maximum potential height for each species.
Data analysis
Relationships between height and diameter were described by fitting standardized major axis (SMA) lines, given that the questions were about co-ordination between these two growth measures, rather than about predicting one from the other (Warton et al., 2006). Tests equivalent to analysis of covariance (common slope, different elevations given a common slope, confidence intervals for slopes and elevations) were implemented through SMATR software (Falster et al., 2003, 2006; Warton et al., 2006).
RESULTS
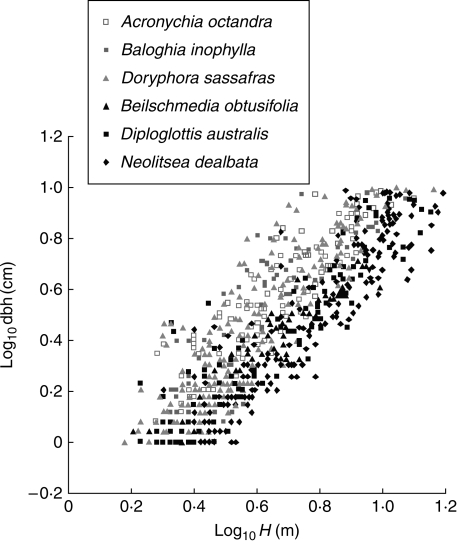
The increase in logD appeared to be linear with logH within this range of size (data shown for six species in Fig. 1; slopes and intercepts included to illustrate the main features). There was substantial scatter in the data, individual saplings within a species having quite a wide range of diameters at a given height. The scatter probably reflected the considerable variation in light and factors associated with forest structure, disturbance, stem density, and interaction effects and competition (Niklas, 1995; King, 1996; Henry and Aarssen, 1999; Niklas et al., 2003; Kariuki et al., 2006; Poorter et al., 2006). Despite the scatter around the height–diameter relationships, some species clearly tended to have greater diameter than others, at a given height (Fig. 1).
Fig. 1.
Scatterplot illustrating the relationships between log10 dbh and log10 height for six species. Each data point is an individual stem in the range 1–9·9 cm dbh for the six species shown in the legend that represent three each with high and low stem volume at a (low) common height. Taxonomic information for all species referred to in this study is provided in the Supplementary Data. SMA slope (β) and intercept (α) values are: Acronychia octandra – β = 1·27, α = –0·29, r2 = 0·81; Baloghia inophyla – β = 1·33, α = –0·35, r2 = 0·75; Doryphora sassafras – β = 1·468, α = –0·44, r2 = 0·8; Beischmeidia obtusifolia – β = 1·10, α = –0·34, r2 = 0·87; Diploglottis australis – β = 1·17, α = –0·37, r2 = 87; Neolitsea delabata – β = 1·31, α = –0·51, r2 = 0·84.
There were no consistent or interpretable differences in slopes between species (Fig. 2A, B, and Supplementary Data Table S1). The common scaling slope of the D–H relationship across the 75 species was β = 1·321, and most slopes fell between the 3/2 expected under elastic similarity and the 1/1 expected under geometric self-similarity models for stem strength and safety (McMahon, 1973; McMahon and Kronauer, 1976; Niklas, 1994, 1995). After Bonferroni correction there were only three species with slopes significantly shallower than β = 1·32, and five species with slopes significantly steeper. Neither group of species had features that stood out from those of other species (see Supplementary Data Table 3a–b). The extreme low slope shown in Fig. 2 was not among those that were significantly different.
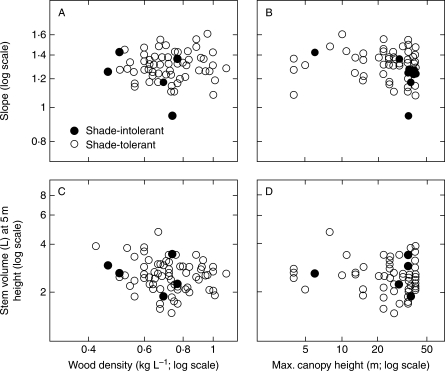
Fig. 2.
Scatterplots relating SMA slopes and volume for saplings at a common height (5 metres) to wood density and estimated maximum height at maturity. Two species groups are shown: shade-intolerant and shade-tolerant, as indicated. The species with the shallowest estimated slope is Alphitonia excelsa, a shade-intolerant tree with medium wood density. SMA slopes represent log10 dbh-diameter (cm) to log10 height (m) for saplings from 75 species in the range 1–10 cm dbh.
SMA fits to the relationships between diameter and height growth differed between species mainly in elevation (intercept of logH–logD graphs) rather than in slope. Diameter (dbh) at a reference height of 5 m was calculated using the SMA line fitted for each species, and varied approx. 1·8-fold (2·5–4·4 cm) across the 75 species.
The range of dbh at 5 m height translated into an approx. three-fold range of stem volume (Fig. 2C, D). Stem volumes were approximated as cones at the common height of 5 m, where V = πr2h)/3, h = 5 m (500 cm) and r was radius at the base of the cone, calculated as r/dbh = 5/(5–1·3). This approximation is not likely to be exact, but we believe it provides a reasonable relative ranking among species. The approx. three-fold range of main-stem volume used to achieve a common height (in this case 5 m) was consistent with the findings of King et al. (2006a).
We considered whether either the logD–logH slope or the estimated main-stem volume to reach 5 m height might be correlated with potential canopy height or with wood density (Fig. 2). Falster and Westoby (2005) and Poorter (2008) had previously noted that species with low potential heights were markedly different depending on whether they were shade-tolerant (understorey species) or shade-intolerant (pioneer species). Accordingly, we tested for interaction, i.e. for response to maximum height or to wood density having different slope depending on shade tolerance. These interaction terms were not significant (Table 1), and nor were any of the main effects except for a weak (r2 = 0·025) tendency towards shallower slope in species with greater maximum heights (Table 1).
Table 1.
Summary data for analyses of covariance corresponding to Fig. 2
| Dependent variable | Source | Type III sum of squares | d.f. | Mean square | F | P-value |
|---|---|---|---|---|---|---|
| Slope | Shade-tolerance | 0·003 | 1 | 0·003 | 1·94 | 0·17 |
| Wood density | 0·003 | 1 | 0·003 | 1·62 | 0·21 | |
| Shade-tolerance × wood density | 0·005 | 1 | 0·005 | 3·00 | 0·09 | |
| Error | 0·114 | 71 | 0·002 | |||
| Slope | Shade-tolerance | 0·003 | 1 | 0·003 | 1·77 | 0·19 |
| Maximum height | 0·008 | 1 | 0·008 | 4·92 | 0·03 | |
| Shade-tolerance × maximum height | 0·005 | 1 | 0·005 | 3·01 | 0·09 | |
| Error | 0·112 | 71 | 0·002 | |||
| Main-stem volume at 5 m | Shade-tolerance | 0·000 | 1 | 0·000 | 0·03 | 0·87 |
| Wood density | 0·005 | 1 | 0·005 | 0·56 | 0·46 | |
| Shade-tolerance × wood density | 0·000 | 1 | 0·000 | 0·03 | 0·85 | |
| Error | 0·666 | 71 | 0·009 | |||
| Main-stem volume at 5 m | Shade-tolerance | 0·000024 | 1 | 0·000024 | 0·003 | 0·96 |
| Maximum height | 0·001 | 1 | 0·001 | 0·07 | 0·79 | |
| Shade-tolerance × maximum height | 0·000067 | 1 | 0·000067 | 0·007 | 0·93 | |
| Error | 0·679 | 71 | 0·010 |
The predicted variable is either slope of the logD–logH relationship (log scaled) or estimated main-stem volume at 5 m height (log scaled). Predictor variables are shade-tolerance (categorical) and either wood density or maximum canopy height (log scaled).
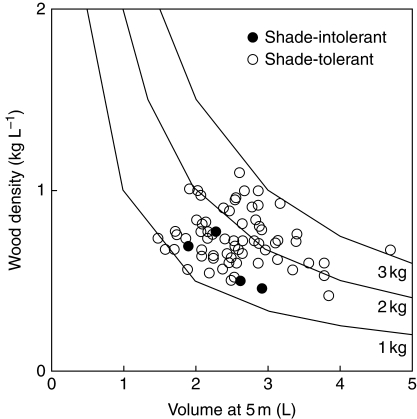
Dry mass investment in the main stem to achieve 5 m sapling height is the product of stem volume and wood density. Consideration was given to whether thicker stemmed species might tend to comprise lighter wood. Larger dbh and hence stem volume at 5 m height was not significantly correlated with lower wood density (r = 0·17, P = 0·14; Fig. 3).
Fig. 3.
Scatterplot of stem volume at a common height and wood density for 75 species. The lines represent the contours of main-stem mass (volume × density) for values of 1, 2 and 3 kg, and provide the link between points of equal cost of height growth in relation to the two measures. Shade-intolerant species (n = 5) and shade-tolerant species (n = 70) are indicated. van Gelder et al. (2006) found that adult and juvenile wood density was well correlated across species (r = 0·91, P < 0·001). Estimates of species sapling strength and safety in the understorey using measures of MR (modulus of rupture) and ME (modulus of elasticity) for adult stems showed strong correlation with (adult) wood density (see Supplementary Data, Appendix S6).
Contours in Fig. 3 indicate main-stem dry mass at 5 m sapling height calculated as estimated volume × wood density. The numbers should be interpreted comparatively between species more than absolutely because the stem volume is approximate, the wood density has been taken from adult stems elsewhere and some of the volume will be bark rather than wood. The more economical main stems were both narrow in dbh and also fairly low in wood density; more expensive main stems were either wide in dbh or had high wood density. Wood density and main-stem volume were almost equally important as sources of variation across species in estimated dry mass of main stem (54 vs. 46 %, multiple regression). Most species lay within about a two-fold range in each dimension (wood density approx. 0·5–1·0, main-stem volume approx. 2·0–4·0 L).
Sapling main-stem volume at 5 m height was only weakly positively correlated with the size of leaves (P < 0·1), and negatively (but not significantly) correlated with seed size (Table 2).
Table 2.
Cross-species correlation coefficients (r) between trait values (including stem volume at 5 m height) using species means; n = 75 except for wood density (n = 72).
| log10 max. height | log10 wood density | log10 leaf area | log10 seed size | |
|---|---|---|---|---|
| log10 max. height | 1 | |||
| log10 wood density | −0·14 | 1 | ||
| log10 leaf area | 0·25** | 0·30*** | 1 | |
| log10 seed size | 0·30*** | 0·22** | 0·18 | 1 |
| log10 stem volume | −0·07 | −0·17 | 0·20* | −0·18 |
Significant relationships are in bold: * P < 0·1, ** P < 0·05, *** P < 0·01.
DISCUSSION
In the saplings examined here, diameter generally increased relatively faster than height (SMA common slope across species β = 1·32, a two-fold increase in height being associated with an approx. 2·5-fold increase in dbh). This happened in a similar way across species, and in shade-intolerant compared with shade-tolerant species. The slope was in line with previously reported results for tropical rain forest species (Kohyama, 1987, 1991; Kohyama and Hotta, 1990; King, 1990; Niklas, 1995) and was consistent with theory (see, for example, Niklas, 1995; Thomas, 1996; Sterck and Bongers, 1998; Sposito and Santos, 2001). Shifting D–H ratios through ontogeny (Sterck and Bongers, 2001; Poorter et al., 2005) are thought to reflect taller stems requiring greater mechanical support, having different respiratory loads and fluid requirements, and eventually in the exposed canopy experiencing different conditions (Givnish, 1988; Niklas, 1994). In addition, as plants approach their maximum height, and with the onset of reproduction and a resultant shift in the allocation of energy, height growth must slow further compared with diameter (Charnov, 1993; Enquist et al., 1999; Koch et al., 2004). Studies including larger size classes have described non-linear diameter–height allometric relationships during ontogeny (Niklas, 1995; Thomas, 1996), and significant differences based on successional status (King, 1981; Alvarez-Buylla and Martinez-Ramos, 1992; but see Sterck and Bongers, 1998).
The increase in the logD–logH relationship (unlike its slope) did differ substantially across species among these saplings, corresponding to an estimated three-fold range in main-stem mass required to achieve a common height. King et al. (2006a) found a similar level of variation in biomass allocation across the 21 rainforest tree species they sampled from Pasoh Forest Reserve in Peninsular Malaysia, although they compared main-stem biomass at 17 m height.
In the present study, among species that required greater mass, some had higher wood density and others had wider stems. However, the trade-off between stem width and wood density was weak at best (negative correlation, r = 0·17, P = 0·14). This is somewhat contrary to the expectation of a trade-off between wood density and stem thickness in relation to safety factors (Enquist et al., 1999; Wright et al., 2003; King et al., 2006a; van Gelder et al., 2006).
Whether this range from high to low main-stem costs of height gain was associated with other aspects of plant ecological strategy was assessed. Indicators available were seed size, maximum height, leaf size, wood density and shade-tolerance. These traits were correlated among each other (Table 2) in patterns similar to those previously reported elsewhere. For example, seed and leaf size were positively correlated with Hmax (Leishman et al., 2000; Moles et al., 2004), and wood density was negatively correlated with leaf size and positively correlated with seed size.
Interestingly, within the species sampled, the range from high to low main-stem costs proved to be unrelated to seed size, to leaf size and to shade-tolerance. The absence of a relationship to shade-tolerance was especially interesting, as it might be expected that shade-intolerant species typically were growing in higher light, and in general, open-grown stems are expected to be shorter and thicker, whereas stems in high-density stands are taller and thinner (Niklas, 1995; Niklas et al., 2003).
In summary, across the species sampled, main-stem expense to reach 5 m did not show any trend in association with Hmax or with wood density. Main-stem expense to achieve 5 m height also varied largely independently of the other ecological traits available to us. Higher main-stem expense implies lower allocation to leaves and roots and hence slower growth than might otherwise have been the case. It seems likely that the benefit of higher main-stem expense lies in improved survival. Although continued height gain in the understorey helps trees to avoid overtopping and suppression, this must be balanced against risks of damage from limb and tree falls in order to maximize survival to reproduction (King, 1990; Kohyama and Hotta, 1990; Niklas, 1995; Thomas, 1996; Poorter et al., 2003; Falster, 2006; Osunkoya et al., 2007; Poorter, 2008). Previous studies have found positive correlations across tree species between lower wood density, higher growth rate and increased mortality rate (Enquist et al., 1999; Wright et al., 2003; King et al., 2006a; van Gelder et al., 2006). Our results show that stem width contributes as much as wood density to variation in main-stem cost, so growth and mortality rates might be more tightly related to overall main-stem cost than to wood density alone.
In theory, a combination of wider diameters with lighter wood should have a stronger effect on mechanical strength and hence on preventing main-stem breakage in the short term. The flexural stiffness of sapling stems (force required to produce a given deflection) is proportional to EI, where E is Young's modulus of elasticity, and I is the second moment of cross-sectional area, which for circular stems is πr4/4. At a given mass per length of stem, flexural stiffness would be substantially greater in sapling species with large diameters, because the fourth-power effect of increasing diameter is expected to outweigh the corresponding decrease in wood density (a strong correlate of modulus of elasticity). On the other hand, stems with high wood density would be expected to suffer less from attack by borers and fungi (e.g. Augspurger, 1984) and to sustain stem strength better over time. Possibly these factors could influence the wide range of alternative ways that a given main-stem cost is incurred.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
We thank Maina Kariuki for his assistance with data collection and for growth and mortality modelling, State Forests of New South Wales (NSW) who originally set up the plots as part of a research effort into the effects of rainforest logging, and NSW DECC for site access. Dan Falster provided assistance with the SMATR program, and with Chris Lusk some useful suggestions. David Warton gave valuable statistical advice. Working groups of the ARC-NZ Research Network for Vegetation Function, funded by the Australian Research Council, provided useful perspectives. The manuscript benefited significantly from the detailed comments on an earlier draft provided by Lourens Poorter and an anonymous reviewer. This work was supported by scholarship and research funding to R.M.K. provided by the Department of Biological Sciences, Macquarie University, through an Australian Postgraduate Award. Rainforest Rescue and Andrew Hall funded the original data collection and research effort, and provided on-going research assistance to R.M.K.
LITERATURE CITED
- Ackerly DD, Knight CA, Weiss SB, Barton K, Starmer KP. Leaf size, specific leaf area and microhabitat distribution of woody plants in a California chaparral: contrasting patterns in species level and community level analyses. Oecologia. 2002;130:449–457. doi: 10.1007/s004420100805. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla ER, Martinez-Ramos M. Demography and allometry of Cecropia obtusifolia, a neo-tropical pioneer tree – an evaluation of the climax–pioneer paradigm for tropical rainforests. Journal of Ecology. 1992;80:275–290. [Google Scholar]
- Augspurger CK. Light requirements of neotropical tree seedlings: a comparative study of growth and survival. Journal of Ecology. 1984;72:777–796. [Google Scholar]
- Bootle KR. Wood in Australia: types, properties and uses. Sydney: McGraw-Hill; 1983. [Google Scholar]
- Charnov EL. Life history invariants: some explanations of symmetry in evolutionary ecology. Oxford: Oxford University Press; 1993. [Google Scholar]
- Cornelissen JHC, Lavorel S, Garnier E, et al. A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Australian Journal of Botany. 2003;51:335–380. [Google Scholar]
- Cornwell WK, Ackerly DD. Community assembly and shifts in plant trait distributions across an environmental gradient in coastal California. Ecological Monographs. 2009;79:109–126. [Google Scholar]
- Cornwell WK, Schwilk DW, Ackerly DD. A trait-based test for habitat filtering: convex hull volume. Ecology. 2006;87:1465–1471. doi: 10.1890/0012-9658(2006)87[1465:attfhf]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Enquist BJ, West GB, Charnov EL, Brown JH. Allometric scaling of production and life history variation in vascular plants. Nature. 1999;401:907–911. [Google Scholar]
- Falster DS. Sapling strength and safety: the importance of wood density in tropical forests. New Phytologist. 2006;171:237–239. doi: 10.1111/j.1469-8137.2006.01809.x. [DOI] [PubMed] [Google Scholar]
- Falster DS, Westoby M. Alternative height strategies among 45 dicot rain forest species from tropical Queensland, Australia. Journal of Ecology. 2005;93:521–535. [Google Scholar]
- Falster DS, Warton DI, Wright IJ. (S)MATR: standardized major axis tests and routines. 2003. http://www.bio.mq.edu.au/ecology/SMATR. [July 2007]
- Falster DS, Warton DI, Wright IJ. Updated user's guide to SMATR: standardised major axis tests and routines. 2006. Version 2.0. http://www.bio.mq.edu.au/ecology/SMATR/ [Google Scholar]
- Floyd AG. Rainforest trees of mainland south-eastern Australia. Sydney: Inkata Press; 1989. [Google Scholar]
- Fonseca CR, Overton JM, Collins B, Westoby M. Shifts in trait-combinations along rainfall and phosphorous gradients. Journal of Ecology. 2000;88:964–977. [Google Scholar]
- van Gelder HA, Poorter L, Sterck FJ. Wood mechanics, allometry, and life-history variation in a tropical rain forest community. New Phytologist. 2006;171:367–378. doi: 10.1111/j.1469-8137.2006.01757.x. [DOI] [PubMed] [Google Scholar]
- Givnish TJ. Adaptations to sun and shade: a whole plant perspective. Australian Journal of Plant Physiology. 1988;15:63–92. [Google Scholar]
- Grime JP. Trait convergence and trait divergence in herbaceous plant communities: mechanisms and consequences. Journal of Vegetation Science. 2006;17:255–260. [Google Scholar]
- Harden GJ. Flora of New South Wales. volumes 1–4. Sydney: UNSW Press; 1990–1993. [Google Scholar]
- Henry H, Aarssen LW. The interpretation of stem diameter–height allometry in trees: biomechanical constraints, neighbour effects, or biased regressions? Ecology Letters. 1999;2:89–97. [Google Scholar]
- Ilic J, Boland D, McDonald M, Downes G, Blakemore P. Wood density phase 1: state of knowledge. 2000. National Carbon Accounting System Technical Report No. 18. Australian Greenhouse Office. http://www.greenhouse.gov.au/ncas/reports/pubs/tr18final.pdf .
- Kariuki M, Kooyman RM. Floristic changes and regeneration patterns for a 12-year period during the 3rd and 4th decades following selection logging in a subtropical rainforest. Austral Ecology. 2005;30:844–855. [Google Scholar]
- Kariuki M, Rolfe M, Smith RGB, Vanclay JK, Kooyman RM. Diameter growth performance varies with species functional-group and habitat characteristics in subtropical rainforests. Forest Ecology and Management. 2006;225:1–14. [Google Scholar]
- Kerkhoff AJ, Fagan WF, Elser JJ, Enquist BJ. Phylogenetic and growth form variation in the scaling of nitrogen and phosphorous in the seed plants. The American Naturalist. 2006;168:E103–E122. doi: 10.1086/507879. [DOI] [PubMed] [Google Scholar]
- King DA. Tree dimensions: maximizing the rate of height growth in dense stands. Oecologia. 1981;51:351–356. doi: 10.1007/BF00540905. [DOI] [PubMed] [Google Scholar]
- King DA. Allometry of saplings and understorey trees of a Panamanian forest. Functional Ecology. 1990;4:27–32. [Google Scholar]
- King DA. Allometry and life history of tropical trees. Journal of Tropical Ecology. 1996;12:25–44. [Google Scholar]
- King DA, Davies SJ, Tan S, Nur NSM. The role of wood density and stem support costs in the growth and mortality of tropical trees. Journal of Ecology. 2006;a 94:670–680. [Google Scholar]
- King DA, Wright SJ, Connell JH. The contribution of interspecific variation in maximum tree height to tropical and temperate diversity. Journal of Tropical Ecology. 2006;b 22:11–24. [Google Scholar]
- Koch GW, Sillett SC, Jennings GM, Davis SD. The limits to tree height. Nature. 2004;428:851–854. doi: 10.1038/nature02417. [DOI] [PubMed] [Google Scholar]
- Kohyama T. Significance of architecture and allometry in saplings. Functional Ecology. 1987;1:399–404. [Google Scholar]
- Kohyama T. A functional model describing sapling growth under a tropical forest canopy. Functional Ecology. 1991;5:83–90. [Google Scholar]
- Kohyama T, Hotta M. Significance of allometry in tropical saplings. Functional Ecology. 1990;4:515–521. [Google Scholar]
- Kohyama T, Suzuki E, Partomihardjo T, Yamada T, Kubo T. Tree species differentiation in growth, recruitment and allometry in relation to maximum height in a Bornean mixed dipterocap forest. Journal of Ecology. 2003;91:797–806. [Google Scholar]
- Kooyman RM. Growing rainforest: rainforest restoration and regeneration – recommendations for the humid sub-tropical region of northern NSW and south-east Qld. Australia: Greening Australia Queensland; 1996. [Google Scholar]
- Kraft NJB, Valencia R, Ackerly DD. Functional traits and niche-based tree community assembly in an Amazonian forest. Science. 2008;322:580–582. doi: 10.1126/science.1160662. [DOI] [PubMed] [Google Scholar]
- Leishman MR, Wright IJ, Moles AT, Westoby M. The evolutionary ecology of seed size. In: Fenner M, editor. Seeds – the ecology of regeneration in plant communities. Wallingford: CAB International; 2000. pp. 31–57. [Google Scholar]
- McGill BJ, Enquist BJ, Weiher E, Westoby M. Rebuilding community ecology from functional traits. Trends in Ecology and Evolution. 2006;21:178–185. doi: 10.1016/j.tree.2006.02.002. [DOI] [PubMed] [Google Scholar]
- McMahon TA. Size and shape in biology. Science. 1973;179:1201–1204. doi: 10.1126/science.179.4079.1201. [DOI] [PubMed] [Google Scholar]
- McMahon TA, Kronauer RE. Tree structures: deducing the principles of mechanical design. Journal of Theoretical Biology. 1976;59:443–466. doi: 10.1016/0022-5193(76)90182-x. [DOI] [PubMed] [Google Scholar]
- Moles AT, Falster DS, Leishman MR, Westoby M. Small-seeded plants produce more seeds per square metre of canopy area per year, but not per individual per lifetime. Journal of Ecology. 2004;92:384–396. [Google Scholar]
- Moles AT, Ackerly DD, Webb CO, et al. Factors that shape seed mass evolution. Proceedings of the National Academy of Sciences. 2005;102:10540–10544. doi: 10.1073/pnas.0501473102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niklas KJ. The allometry of safety factors for plant height. American Journal of Botany. 1994;81:345–351. [Google Scholar]
- Niklas KJ. Size-dependent allometry of tree height, diameter and trunk-taper. Annals of Botany. 1995;75:217–227. [Google Scholar]
- Niklas KJ, Midgley JJ, Rand RH. Tree size frequency distributions, plant density, age and community disturbance. Ecology Letters. 2003;6:405–411. [Google Scholar]
- Osunkoya OO, Omar-Ali K, Amit N, Dayan J, Daud DS, Sheng TK. Comparative height-crown allometry and mechanical design in 22 tree species of Kuala Belalong rainforest, Brunei, Borneo. American Journal of Botany. 2007;94:1951–1962. doi: 10.3732/ajb.94.12.1951. [DOI] [PubMed] [Google Scholar]
- Poorter L. The relationships of wood-, gas- and water fractions of tree stems to performance and life history variation in tropical trees. Annals of Botany. 2008;102:367–375. doi: 10.1093/aob/mcn103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poorter L, Bongers F. Leaf traits are good predictors of plant performance across 53 rain forest species. Ecology. 2006;87:1733–1743. doi: 10.1890/0012-9658(2006)87[1733:ltagpo]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Poorter L, Bongers F, Sterck FJ, Hannsjörg W. Architecture of 53 rainforest trees differing in adult stature and shade tolerance. Ecology. 2003;84:602–608. [Google Scholar]
- Poorter L, Bongers F, Sterck FJ, Woll H. Beyond the regeneration phase: differentiation of height–light trajectories among tropical tree species. Journal of Ecology. 2005;93:256–267. [Google Scholar]
- Poorter L, Bongers L, Bongers F. Architecture of 54 moist forest tree species: traits, tradeoffs, and functional groups. Ecology. 2006;87:1289–1301. doi: 10.1890/0012-9658(2006)87[1289:aomtst]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Putz FE, Coley PD, Lu K, Montalvo A, Aiello A. Uprooting and snapping in trees: structural determinants and ecological consequences. Canadian Journal of Forest Research. 1983;13:1011–1020. [Google Scholar]
- Reich PB, Wright IJ, Cavender-Bares J, et al. The evolution of plant functional variation: traits, spectra and strategies. International Journal of Plant Sciences. 2003;164:143–164. [Google Scholar]
- Sposito TC, Santos FAM. Scaling of stem and crown in eight Cecropia (Cecropiaceae) species of Brazil. American Journal of Botany. 2001;88:939–949. [PubMed] [Google Scholar]
- Stanley TD, Ross EM. Flora of south-eastern Queensland. volumes 1–3. Brisbane: Queensland Department of Primary Industries; pp. 1983–1989. [Google Scholar]
- Sterck FJ, Bongers F. Ontogenetic changes in size, allometry, and mechanical design of tropical rain forest trees. American Journal of Botany. 1998;85:266–272. [PubMed] [Google Scholar]
- Sterck FJ, Bongers F. Crown development in tropical rainforest trees: patterns with tree height and light availability. Journal of Ecology. 2001;89:1–13. [Google Scholar]
- Sterck FJ, Bongers F, Newberry D. Tree architecture in a Bornean lowland rain forest: intra- and inter-specific patterns. Plant Ecology. 2001;153:279–292. [Google Scholar]
- Thomas SC. Asymptotic height as a predictor of growth and allometric characteristics in Malaysian rainforest trees. American Journal of Botany. 1996;83:556–566. [Google Scholar]
- Warton DI, Wright IJ, Falster DS, Westoby M. Bivariate line fitting methods for allometry. Biological Reviews. 2006;81:259–291. doi: 10.1017/S1464793106007007. [DOI] [PubMed] [Google Scholar]
- Westoby M, Wright IJ. Land plant ecology on the basis of functional traits. Trends in Ecology and Evolution. 2006;21:261–268. doi: 10.1016/j.tree.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Westoby M, Falster DS, Moles AT, Vesk PA, Wright IJ. Plant ecological strategies: some leading dimensions of variation between species. Annual Review of Ecology and Systematics. 2002;33:125–159. [Google Scholar]
- Wright IJ, Ackerly DD, Bongers F, et al. Relationships among ecologically important dimensions of plant trait variation in seven neo-tropical forests. Annals of Botany. 2007;99:1003–1015. doi: 10.1093/aob/mcl066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright SJ, Muller-Landau HC, Condit R, Hubbell SP. Gap-dependent recruitment, realized vital rates, and size distributions of tropical trees. Ecology. 2003;84:3174–3185. [Google Scholar]