Abstract
Background and Aims
The Sapindaceae is one of 17 plant families in which seed dormancy is caused by a water-impermeable seed or fruit coat (physical dormancy, PY). However, until now the water gap in Sapindaceae had not been identified. The primary aim of this study was to identify the water gap in Dodonaea petiolaris (Sapindaceae) seeds and to describe its basic morphology and anatomy.
Methods
Seed fill, viability, water-uptake (imbibition) and other characteristics were assessed for D. petiolaris seeds. The location and structure of the water gap were investigated using a blocking experiment, time series photography, scanning electron microscopy and light microscopy. Dodonaea petiolaris seeds with PY also were assessed for loss of PY at four ecologically significant temperatures under moist and dry conditions. Seeds of three other species of Sapindaceae were examined for presence of a water gap.
Key Results
The water gap in D. petiolaris seeds was identified as a small plug in the seed coat adjacent to the hilum and opposite the area where the radicle emerges. The plug was dislodged (i.e. water gap opened = dormancy break) by dipping seeds in boiling water for 2·5 min or by incubating seeds on a moist substrate at 20/35 °C for 24 weeks. Layers of cells in the plug, including palisade and subpalisade, are similar to those in the rest of the seed coat. The same kind of water gap was found in three other species of Sapindaceae, Diplopeltis huegelii, Distichostemon hispidulus and Dodonaea aptera.
Conclusions
Following dormancy break (opening of water gap), initial uptake of water by the seed occurs only through the water gap. Thus, the plug must be dislodged before the otherwise intact seed can germinate. The anatomy of the plug is similar to water gaps in some of the other plant families with PY.
Keywords: Dodonaea petiolaris, physical dormancy, Sapindaceae, seed ecology, seed germination, water gap
INTRODUCTION
Seeds with physical dormancy (PY) have a water-impermeable seed (or fruit) coat, and thus they are unable to imbibe water when placed in a hydrated environment (Baskin and Baskin, 1998, 2004). One monocot and 15 (Baskin and Baskin, 1998; Baskin, 2003; Baskin et al., 2000, 2006) [or 16, assuming Dialyceras (Sphaerosepalaceae, Malvales; Horn, 2004, figures 70, 71 and 72) has PY] eudicot families have been reported to contain species that produce seeds with PY. A common trait amongst species with PY is that imbibition is regulated by a small specialized anatomical structure (water gap) within the seed coat or pericarp that becomes water permeable (i.e. opens) when exposed to specific environmental stimuli such as high or fluctuating temperatures, drying or warm moist conditions (Baskin and Baskin, 1998). In species with PY, the seed or fruit coat remains water impermeable due to a palisade layer of lignified Malphigian cells (Baskin, 2003; Baskin et al., 2000) in the seed or fruit coat, although once the seed has begun to absorb water (after PY has been broken) the whole seed rapidly becomes hydrated and impermeability is lost over the entire seed coat.
The exact structure and location of the water gap varies with the family (only angiosperms), and it differs in origin, morphology and anatomy (Baskin et al., 2000). The water gap functions as an environmental signal detector by opening only when there is a high probability that a proportion of germinating seedlings will become established (Baskin and Baskin, 2000). The water gap has been identified in 13 of the 17 families with PY (Baskin et al., 2000; Baskin and Baskin, 2003; Jayasuriya et al., 2007). However, it has not been identified in the Cucurbitaceae, Rhamnaceae, Surianaceae and, until now, the Sapindaceae.
Of particular interest in this study is the water gap in Sapindaceae, some of which produce seeds with PY (Baskin et al., 2004; Turner et al., 2006; Cook et al., 2008). At the global level, the incidence of PY within Sapindaceae appears to be relatively uncommon with only five genera, Koelreuteria, Diplopeltis, Distichostemon, Dodonaea and Cardiospermum, demonstrated to possess seeds with PY so far (Rehman and Park, 2000; Turner et al., 2006; Baskin et al., 2004; Johnston et al., 1979; Cook et al., 2008). Interestingly, Diplopeltis, Distichostemon and Dodonaea, which are found predominantly within Australia, are taxonomically more closely related to each other than they are to any other Sapindaceae taxa, and all three genera contain species whose seeds have PY (Harrington et al., 2005; Turner et al., 2006; Cook et al., 2008). Of the three Sapindaceae genera in the Australian flora thus far identified to have PY, Dodonaea is by far the largest genus (>70 species), occurring in a vast range of habitats including woodland, forest and shrubland communities (West, 1984; Reynolds, 1985; Shepherd et al., 2007). Baskin et al. (2004) and Cook et al. (2008) found that PY could be easily overcome and germination enhanced by rapidly dipping seeds of five of the six Dodonaea spp. examined in hot water; all five of them germinated readily once PY was removed. However, for D. petiolaris seeds both removal of PY with hot water and germination were more problematic (Cook et al., 2008).
In many cases, seeds with PY can be made water permeable with as little as 1-s exposure to hot water (Baskin et al., 2004), and PY can also be easily removed in some cases with dry heat (Mott et al., 1982), dry storage (Morrison et al., 1992), warm moist incubation (Jayasuriya et al., 2007) and soaking in concentrated H2SO4 (Baskin and Baskin, 1998). Thus, once seeds are exposed to a critical combination of stresses PY is readily lost. Nevertheless, while dipping in hot water, for example, is an effective means of removing PY in many species it is not an ecological condition that seeds experience under natural conditions. Therefore, to understand how the timing of germination is naturally regulated there is a need to understand ecologically significant cues that naturally break dormancy. This can be done by using complementary laboratory and field studies to determine the conditions and time of year when seeds lose PY. For example, Cook et al. (2008) found that PY in seeds of Dodonaea hackettiana could be broken by exposing them to 50 °C for 30 s in a water bath, whereas exposing them to dry heat at 50 °C did not cause them to lose dormancy. In the field, PY was broken in summer when several rainfall events were followed within 24 h by soil temperatures ≥50 °C. Thus, these warm, wet soil conditions are likely to have caused the loss of PY in a proportion of in situ buried seeds.
The primary purpose of this study was to locate and characterize the water gap in seeds of Sapindaceae using Dodonaea petiolaris as the primary study species. Specifically, (a) the percentage seed fill and viability were estimated; (b) the germination of seeds after they had been dipped in hot water and incubated at different temperatures was evaluated; (c) the changes in the seed coat following dipping in hot water and imbibition were described and the location and morphoanatomy of the water gap identified; and (d) the PY break in seeds incubated under ecologically realistic conditions was monitored.
MATERIALS AND METHODS
Seeds
Seeds of Dodonaea petiolaris F.Muell. were collected from Cobra station (24°12′ S; 116°30′E) near Gascoyne Junction, Western Australia in October 1998. A summary of the climatic conditions from the nearest weather station (Gascoyne Junction) are as follows: mean maximum and minimum summer (January) temperatures are 40·7 °C and 23·7 °C, respectively, while the mean maximum and minimum winter (July) temperatures are 23·0 °C and 9·2 °C. Mean annual rainfall is 214 mm with 91 mm falling during the hottest five months (November to March) and 123 mm falling during the remainder of the year (April to October). In addition, there are on average 31 wet days annually (Bureau of Meteorology, 2008). Following collection, seeds were air dried and stored at 5 °C until used in this study. Seeds of Diplopeltis huegelii Endl. were collected in 2003, those of Distichostemon hispidulus Endl. in 1992 and those of Dodonaea aptera Miq. in 2003. After collection seeds were stored at 5, −18 and −18 °C, respectively, until used in this study.
Seed fill and viability
Seeds of D. petiolaris were evaluated for seed fill using a Faxitron X-ray machine, and only fully formed, undamaged and completely filled seeds used for subsequent experimentation. Four replicates of 100 randomly selected seeds were initially assessed using X-ray analysis, and then embryo quality and viability were evaluated on three replicates of 25 seeds. These were initially nicked using a scalpel blade through the seed coat and incubated at 30 °C for 48 h in phosphate-buffered 1 % triphenyl tetrazolium chloride (TTC). Following incubation, embryos were extracted from seeds and scored as viable if the embryonic tissues were stained pink or red and nonviable if they remained non-stained (Paynter and Dixon, 1990).
Seed coat changes following hot water treatment
Three replicates of 25 D. petiolaris seeds were randomly selected, and within each replicate the hilum region of each seed was carefully assessed under a binocular microscope to determine the presence or absence of a small blister adjacent to the hilum (Fig. 1) that had been observed in some seeds in preliminary investigations. Each replicate was then dipped in hot water for 2·5 min and dried with a paper towel. Seeds were then reassessed under a binocular microscope according to the same criteria used prior to hot water treatment.
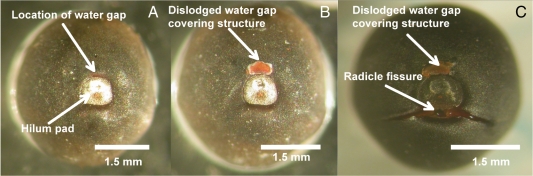
Fig. 1.
The hilum region of Dodonaea petiolaris: (A) before hot water treatment (control); (B) after hot water treatment (2·5 min) only; and (C) after hot water treatment and 24 h hydration. In this example, the reddish-blistered region (dislodged tissue) adjacent to the hilum is visible only after dipping in hot water, and it appears to be the structure covering the water gap (i.e. where imbibition initially occurs; B, C). A fissure formed on the opposite side of the hilum after 24-h hydration is located immediately above the radicle and is the site where the radicle emerges upon germination (C).
In a follow-up trial, ten non-treated seeds of D. petiolaris were individually assessed. Only those seeds (seven in total) in which the water gap had not blistered were selected and photographed under a binocular microscope equipped with a digital camera (Coolpix 4500), focusing on the hilum end of the seed. Each seed was then treated separately with hot water for 2·5 min. Following dipping in hot water, seeds were individually re-photographed. Each seed was placed onto a separate Petri dish lined with a hydrated seed germination paper. Individual seeds were re-photographed after 24 h.
Imbibition of seeds following different treatments
Imbibition tests were undertaken to determine if there was a correlation between the occurrence of blistered D. petiolaris seeds and the amount of water they imbibe. These tests were carried out under ambient room conditions (approx. 23 °C) with each seed individually assessed prior to the experiment to select only those that appeared to be non-damaged and fully formed. From these seeds, five groups were randomly selected: non-treated seeds (control); seeds that were gently scarified using a scalpel to penetrate the water impermeable testa; seeds that were dipped in hot water; non-treated seeds in which all of the blistered ones were removed; and hot water-dipped seeds in which only those that had blistered were selected. Three replicates of 20 seeds from each group were weighed to the nearest 0·1 mg, moistened for 5 min in 90-mm-diameter Petri dishes lined with irrigated seed germination papers, blotted dry and reweighed (time 0). All replicates were reweighed after 1, 2, 3, 4, 6, 8, 24, 48 and 96 h. Percentage increase in seed mass was calculated using the following formula:
where Wi and Wd = mass of imbibed and dry seeds, respectively (Turner et al., 2006). Data from the imbibition test were also used to calculate an imbibition rate index (IRI) (modified from Maguire, 1962) for each treatment according to the following formula:
where t is the imbibition count-hr, Wt the percentage mass increase of seeds at t and Wt−1 the percentage mass increase of seeds from the previous count-hr.
Following completion of the imbibition test, all seeds were dissected to determine whether they were hydrated. Both seed coat and embryo of hydrated seeds were soft, and seeds were filled with liquid. On the other hand, non-hydrated seeds had an intact hard and dry seed coat and a dry embryo with no visible evidence of free water within the seed.
Effect of the blistered region and hot water treatment on imbibition
To determine the importance of the blistered region in facilitating water movement into D. petiolaris seeds, four groups of seeds were randomly selected based on external seed coat morphology: two groups before hot water treatment and two groups following hot water treatment. For the first two groups, the hilum region of each seed was individually assessed and assigned to either (a) seeds in which no blister was present or (b) those in which the blister was clearly evident (Fig. 1A, B). For the second two groups, all seeds were initially hot water treated for 2·5 min and patted dry with paper towel. As with the first two groups, each seed was individually assessed and placed into either (c) those in which no blister was present or (d) those in which the blister was clearly evident. All seeds were then placed into Petri dishes lined with irrigated germination papers and incubated at room temperature (23 °C). After 4 d, these were dissected to determine the number that had imbibed based on the assessment criteria previously described. All seeds that were swollen (i.e. had imbibed) were recorded. There were three replicates of 20 seeds for each treatment.
Seed germination
The effects of constant and alternating incubation temperature were evaluated on nondormant D. petiolaris seeds. Prior to incubation, all seeds were hot water treated and only those that were blistered were selected for testing. These seeds were then surface sterilized in 2 % (w/v) calcium hypochlorite [Ca(OCl)2] solution for 30 min (10 min with vacuum + 10 min normal + 10 min with vacuum), rinsed three times in sterile distilled water and plated onto sterilized hydrated white sand in 90-mm-diameter Petri dishes. Each treatment consisted of three replicates of 25 seeds. Seeds were incubated in constant darkness (except when they were checked for germination in room light) for 8 weeks at 5, 10, 15, 20, 25, 30, 10/20 or 13/26 °C. In addition, another subset of seeds were exposed to one of two stratification treatments, in which case they were started at either 5 or 30 °C and then shifted after 4 weeks to 15 °C and incubated for another 4 weeks at 15 °C.
Dye tracking of imbibition pathway
One per cent fuchsin (acidic) dye solution was used to determine the path by which water moved through the seed coat into the seed interior, following breaking of PY. Seeds were dipped in hot water for 2·5 min, and then 40 seeds where the water gap was blistered were selected and placed into 10 mL of dye solution for 0 (control), 1, 2, 4, 6, 8, 10, 12, 14 and 24 h. After each time period, four seeds were removed and thoroughly rinsed in tap water and dissected transversely. The hilum end of the dissected seed was then examined with the remaining half-embryo carefully removed, and evidence of dye movement (visible as pink/purple staining) into the interior surface of the seed coat was assessed around the area directly beneath the hilum end of the dissected seed.
Scanning electron microscopy (SEM)
Four non-treated D. petiolaris seeds in which the water gap was not blistered and four in which the water gap blistered following hot water-dipping were selected for SEM coating and analysis. Prior to sputter coating, two entire seeds and two half seeds (dissected longitudinally through the hilum region) representing each treatment were mounted on SEM specimen stubs (half seeds with cut surface up) using double-sided carbon tape with contact between the sample and carbon tape improved with the application of carbon paint around the base of each specimen. Samples were sputter coated with gold to a depth of 200 nm. Coated samples were scanned with a Philips XL30 scanning electron microscope using an accelerating voltage of 15 kV and micrographs compared to identify key changes around the area where the proposed water gap is located.
Light microscopy
Anatomical sections were made from seed coat segments derived from non-treated intact seeds (i.e. water gap not blistered) embedded in JB-4 plastic resin (Proscitech Microscopy Supplies, Thuringowa, Qld, Australia). The protocol used for tissue fixation and embedding was modified from O'Brien and McCully (1981) and involved taking small (1–2 mm) segments of the seed coat and hilum region of ten seeds and placing them into a fixative solution (2·5 % glutaraldehyde, pH 6–7 in 0·025 m phosphate buffer) for at least 1 week and then desiccation in an alcohol series comprising methoxyethanol, ethanol, propanol and butanol, for at least 24 h at each step. Once the series was completed sections were incubated for at least 1 month in liquid JB-4 then moved to JB-4 supplemented with catalyst to facilitate polymerization. Seed coat segments were polymerized at 60 °C for 12 h under anoxic conditions. Embedded material was sectioned longitudinally at 2 µm using a Leica Jung RM 2045 microtome and mounted on a glass slide. Prepared sections were stained with a 0·05 % toluidine blue solution (pH 4·4) and observed with a compound microscope. Photographs were taken with a microscope-mounted Coolpix digital camera.
Blocking the water gap
Approximately 300 non-treated D. petiolaris seeds were individually assessed to identify and remove all damaged and water-permeable (blistered) seeds. The remaining seeds were randomly placed in three Petri dishes lined with moistened germination papers for 7 d at 15 °C. Then, all seeds were again individually examined under a binocular microscope and non-imbibed seeds (approx. 90 % of seeds incubated) were removed and air-dried for 3 d. From these remaining seeds, three replicates of 20 seeds were randomly selected and placed into fresh Petri dishes (three) lined with germination papers to be used as control seeds. The remaining seeds were then dipped in hot water for 2·5 min and air-dried. The hilum end of each seed was individually assessed under a binocular microscope to select only those in which the water gap had blistered. Three replicates of 20 blistered seeds were stuck hilum side up onto double-sided tape to prevent movement when blocking was attempted. Using a binocular microscope, the area adjacent to the hilum that had blistered (the water gap) was sealed with a tiny drop of wood sealant (Feast Watson weatherproof polyurethane) administered with a sharpened toothpick. Sealed seeds were left to air-dry for 3 d and then placed into Petri dishes (three) lined with moistened germination papers. An additional three replicates of 20 seeds also were randomly selected from the remaining blistered seeds and placed into Petri dishes (three) lined with moistened germination papers. All seeds were allowed to imbibe for 48 h as previously described. Following imbibition, a cut test was used to determine the percentage of seeds that had imbibed based on hydration criteria previously described. Data from the imbibition test were also used to calculate an imbibition rate index (IRI).
PY break at ecologically relevant temperatures
Seeds of D. petiolaris exposed to eight treatments consisting of four incubation temperatures (15, 10/20, 13/26 and 20/35 °C) and two hydration states (dry or hydrated white sand) were monitored for up to 25 weeks to determine whether PY loss occurs at ecologically relevant temperatures. Three replicates of 25 seeds were used for each treatment combination. Prior to experimental set-up, each seed was assessed, and only intact non-damaged ones with their water gap intact (i.e. not blistered) were selected. Seeds in hydrated treatments were placed onto moistened sand and evaluated weekly with those that were visibly hydrated (larger, darker, and soft when gently pressed) scored and removed. Data from the four hydrated treatments also were used to calculate a dormancy loss index (DLI) (modified from Maguire, 1962) using the formula:
where t is the count-wk, It the percentage of imbibed seeds at t and It−1 the percentage of imbibed seeds from the previous count-wk.
Seeds in the dry treatments were placed onto dry sand and incubated for 24 weeks without weekly scoring. Then, all seeds were removed and reassessed to determine the number of seeds with blistered water gaps. All seeds in the same replicate were then returned onto the white sand, hydrated and incubated at 15 °C for 7 d, after which time those that were visibly hydrated were scored.
Opening of the water gap in other species of Sapindaceae following hot water treatment
Ten non-treated seeds of Diplopeltis huegelii, Distichostemon hispidulus and Dodonaea aptera were individually assessed, and only those seeds that had not blistered were selected and photographed under a binocular microscope equipped with a Coolpix 4500 digital camera, focusing on the hilum end of the seed. Each seed was then treated separately with hot water for 30 s. Following dipping in hot water, seeds were re-examined and re-photographed individually to determine if the same kind of blister observed in D. petiolaris seeds had formed in these three other Sapindaceae species.
Statistical analysis
Imbibition (maximum seed mass and rate of imbibition), seed hydration and germination percentage were analysed by one-way ANOVA using Minitab® version 11. Prior to analysis, percentage data were arcsine-transformed, but non-transformed data appear in all figures. Hartley's test was performed on all data sets to ensure that treatment variances were not significantly different prior to ANOVA. For experiments in which more than two treatments were assessed, Fisher's least significant difference test was used to determine significant differences (P < 0·05) between individual treatments.
RESULTS
Seed fill and viability
Seed fill was 90 ± 1·7 % (mean ± s.e.), but only 48 ± 4·8 % of seed embryos stained pink or red in the presence of TTC.
Seed-coat changes following hot water treatment
Inspection of non-treated D. petiolaris seeds under a binocular microscope (×20 magnification) revealed that 9·3 ± 1·3 % of seeds had a small blister adjacent to the hilum. However, 72·0 ± 4·6 % of the seeds dipped in hot water had the same small-blister structure (P < 0·05) (Fig. 1). Indeed, when seven non-treated/non-blistered seeds were photographed and then dipped in hot water four formed a blister in a small area adjacent to the hilum (Fig. 1B). Following 24 h of hydration, all four seeds imbibed, and a fissure had begun to develop between the seed coat and the hilum, on the side opposite the blister (Fig. 1C). Directly below this fissure is where the radicle is located and emerges (Fig. 1C).
Imbibition of seeds following different treatments
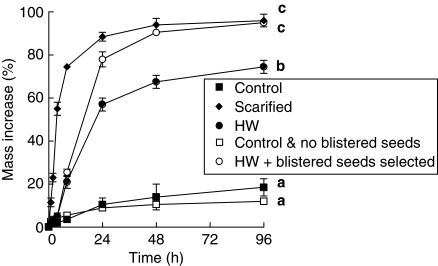
After 96 h imbibition, seed mass had increased 18·5 ± 4·4 % in control seeds and 96·2 ± 2·9 % for scarified seeds (P < 0·05), with 18 % of non-scarified seeds showing signs of imbibition following 4 d moist incubation (Fig. 2). However, for other treatment combinations only the hot water-dipped seeds, all of which were blistered, had an increase in mass (95·0 ± 0·8 %) similar to that of scarified seeds. Seeds that were hot water-dipped only had a lower increase in mass (74·5 ± 3·3 %) than either scarified seeds or hot water dipped-seeds in which all the non-blistered seeds were removed (P < 0·05), while the non-treated seeds in which all the blistered seeds had been removed prior to imbibition had the lowest mass increase (11·9 ± 0·8 %). Scarified seeds also had the highest rate (IRI 28·63 ± 1·83) of imbibition (Fig. 2), while both control treatments (with and without blistered seeds) imbibed water at the lowest rate (IRI 2·22 ± 0·46 and 3·54 ± 0·94, respectively) (P < 0·05). Both hot water treatments (HW only or HW + only blistered seeds) imbibed water at a much lower rate (IRI 7·00 ± 0·46 and 8·24 ± 0·75, respectively) than scarified seeds (P < 0·05).
Fig. 2.
Imbibition (mean % mass increase ± s.e.) of non-treated (control), scarified, hot water dipped (2·5 min), non-treated + not blistered and hot water dipped + blistered seeds over 96 h. Different letters represent significant differences between treatments based on maximum mass increase after 4 d incubation (P < 0·05).
Following 96-h incubation (imbibition), the percentage of seeds that had imbibed was the same for scarified and hot water + blistered treatments (P > 0·05) (100·0 %), while the non-treated + non-blistered seeds had the lowest percentage of imbibed seeds (11·7 ± 1·7; Fig. 2).
Eleven (not HW dipped) to fifteen (HW dipped) per cent of seeds with no blister and 100 % of those with a blister imbibed regardless of whether they had been exposed to hot water or not prior to imbibition (P < 0·05).
Seed germination
The highest germination (28–36 %) occurred at 15, 20, 25 and 13/26 °C (P < 0·05), whereas <25 % of the seeds germinated at 5 °C or 10 °C (data not shown). Neither cold (moving seeds from constant 5 °C to 15 °C) nor warm (moving seeds from constant 30 °C to 15 °C) stratification for 4 weeks improved germination over that observed at the other temperature regimes (data not shown). Most germination occurred during the first 1–2 weeks of incubation, particularly at the warmer temperatures, with minimal additional germination by the end of the 4th week (data not shown).
Dye tracking of imbibition pathway
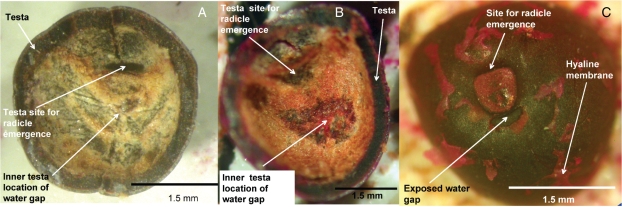
Dye staining was indicated as a small pink/purple stain on the inner surface of the seed coat after 8 h incubation in 1 % acid fuchsin (Fig. 3), but no dye penetrated into the embryo even after 24 h incubation. Indeed, this pink/purple discoloration was not evident until the remaining embryo tissue in the hilum end of the transversely dissected seed was removed (Fig. 3). Once the remaining embryonic tissue was dislodged, fuchsin was clearly evident on the internal surface of the seed coat in the region corresponding to the location of the dislodged palisade cells (blister) on the outer seed coat surface (water gap; Fig. 3).
Fig. 3.
Internal views of the testa of two D. petiolaris seeds dissected transversely and the remaining half of the embryo removed: (A) non-treated seed; (B) seed soaked in 1 % fuchsin (acidic) for 8 h prior to dissection – pink/purple staining is visible in the area that corresponds to the water gap on the external testa surface; (C) corresponding external surface of the same seed in (B) highlighting the location and structure of the exposed water gap.
SEM
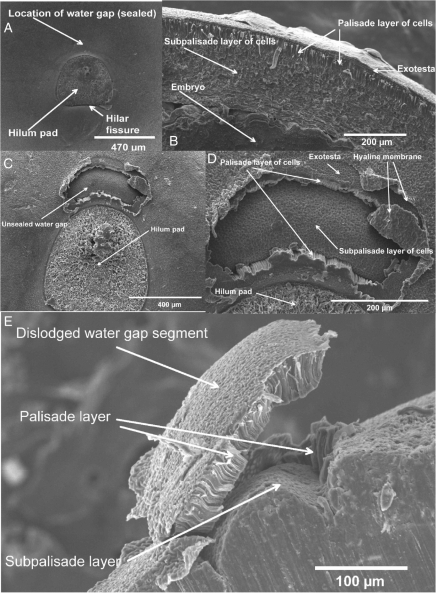
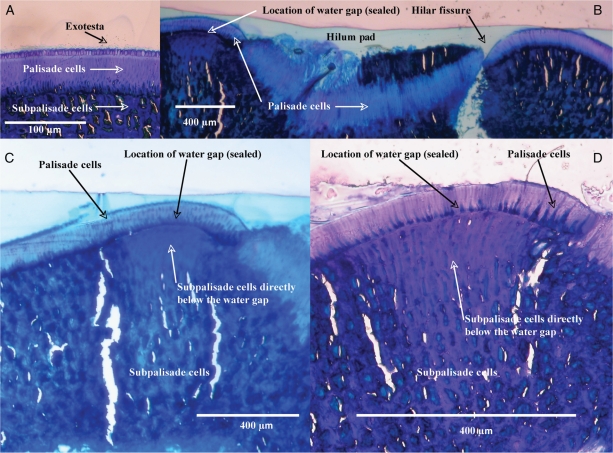
SEM confirmed that the water-impermeable layer in D. petiolaris seeds consists of a continuous single layer of palisade cells with a small plug (forming the blister) of palisade cells adjacent to the hilum that is dislodged from the seed coat after dipping in hot water (Fig. 4E). The layer of palisade cells is approx. 30–50 µm thick, and after rupture a water gap about 400 µm in width is exposed (Fig. 4C, D). After the layer of palisade cells above the water gap has lifted (blistered), the next layer located below the palisade layer, i.e. the water-permeable subpalisade layer is exposed, thus creating a route for water entry into D. petiolaris seeds (Fig. 4D, E).
Fig. 4.
Scanning electron micrographs of Dodonaea petiolaris seeds: (A) close-up of the hilum area on a non-treated D. petiolaris seed; (B) longitudinal section of testa with various cell layers; (C) hilum area on a hot water-treated D. petiolaris seed showing location of the water gap; (D) close-up of the water gap on a hot water-treated D. petiolaris seed showing various cell layers; (E) longitudinal close-up on a hot water-treated D. petiolaris seed showing various cell layers and the ruptured water gap-covering tissue (blister) and the water-gap region below.
Light microscopy
The seed coat of D. petiolaris consists of a thin outer layer of exotestal cells over a much thicker (by ×10) single layer of densely packed water-impermeable palisade cells that cover the entire seed (Fig. 5A). Below the palisade layer are subpalisade cells that are less densely packed than either the exotesta or the palisade layer. The layer of palisade cells is continuous through the hilum region and appears to be considerably thicker there than in other parts of the seed coat (Fig. 5B). The cells in the region of the water gap (in the palisade and subpalisade layers) are well defined relative to the surrounding subpalisade cells, particularly those immediately below the water gap, which appear to be relatively longer and thinner than the surrounding cells (Fig. 5C, D). There is a line of demarcation between the palisade/subpalisade cells in the water gap and those in the rest of the seed coat (Fig. 5C, D). The cells directly below the water gap appear to be less lignified and are more rectangular in shape, while those in the adjacent subpalisade layers appear to be cubical or round and more randomly packed. The region where the palisade and subpalisade layers meet in the water gap is well defined and appears as a line of weakness. In comparison, the demarcation between both cell layers in other parts of the surrounding seed coat is less obvious, and the interface between both cell types appears to be considerably more lignified and the boundary less well defined (Fig. 5A, C, D).
Fig. 5.
Light microscopy photographs of sections of non-treated D. petiolaris seeds: (A) close-up of the testa region; (B) transverse section of testa through the hilum with various cell layers; (C) location of the water gap; (D) close-up of the water-gap region showing the various cell layers. Note: tears and holes are artefacts of the embedding and sectioning process.
Blocking of the water gap
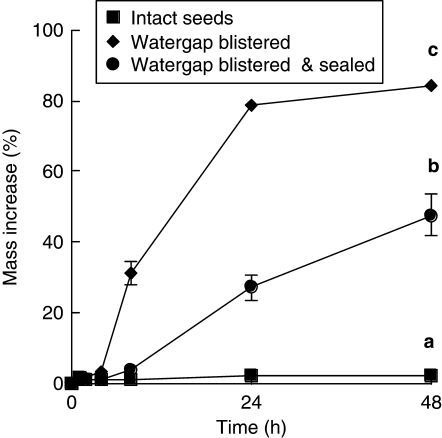
From 0 to 8 h, the rate of water uptake of D. petiolaris seeds with their blistered water gap sealed with polyurethane was similar to that of control seeds (Fig. 6). However, from 8 to 48 h, imbibition of sealed seeds increased, and by 48 h the mass of sealed seeds was significantly higher than that of control seeds (P < 0·05). Nevertheless, it was still less than half the mass of seeds in which the water gap was blistered but not sealed (P < 0·05) (Fig. 6). Blistered seeds had the highest percentage and rate (IRI 7·19 ± 0·29) of imbibition (Fig. 6), while the control treatment (without blistered seeds) and blistered + sealed seeds had much lower rates (IRI 1·25 ± 0·04 and 2·61 ± 0·35, respectively) of imbibition (P < 0·05). Upon completion of the imbibition test, 65·0 ± 7·6 % of sealed seeds were imbibed (based on their hydrated appearance following a cut test), and 35 % remained non-hydrated (hard, dry internally). In comparison, 100 % of non-sealed blistered seeds had imbibed after the same amount of time (48 h).
Fig. 6.
Imbibition during 48 h of non-treated (control), hot water-treated + blistered seeds and hot water-treated + blistered seeds + the water gap sealed with polyeurthane. Different letters represent significant differences between treatments based on maximum mass increase (P < 0·05).
PY break using ecologically relevant temperatures
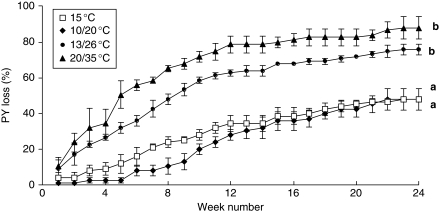
PY was lost gradually for seeds incubated at the four temperatures for 24 weeks under constantly moist conditions (Fig. 7). Rates of dormancy loss at the two warmest temperatures (DLI 18·80 ± 2·93 and 25·14 ± 2·68 at 13/26 °C and 20/35 °C, respectively) were considerably higher than those at the two coolest temperatures (DLI of 7·63 ± 2·02 and 2·67 ± 1·39 at 15 °C and 10/20 °C, respectively; Fig. 7; P < 0·05). Most seeds that lost PY at 13/26 °C and 20/35 °C did so within the first 12 weeks.
Fig. 7.
Cumulative percentage (± s.e.) of seeds that lost PY (i.e. became hydrated) when incubated on moistened sand at four temperature regimes for 24 weeks. Different letters indicate a significance difference in the maximum percentage of seeds with PY loss between incubation temperatures after 24 weeks (P < 0·05).
Following 24 weeks of dry incubation at 15, 10/20, 13/26 and 20/35 °C, ≤5·3 % of seeds had blistered, and <15 % imbibed when subsequently incubated on moistened white sand for 7 d at 15 °C. On the other hand, 48–88 % of seeds moist-incubated for 24 weeks became permeable and hydrated (P < 0·05; Fig. 7).
Opening of the water gap in other species of Sapindaceae following hot water treatment
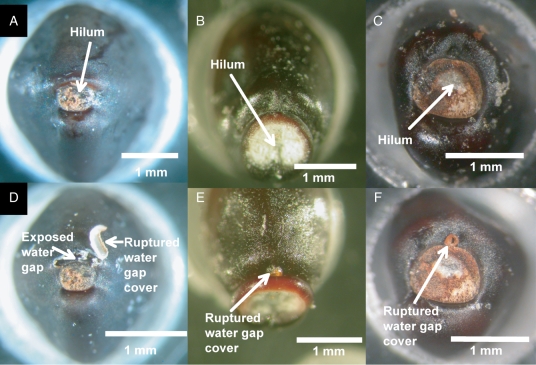
Seeds of Diplopeltis huegelii, Distichostemon hispidulus and Dodonaea aptera behaved in a similar manner to D. petiolaris seeds when exposed to hot water for 30 s. Prior to hot water treatment, none of the seeds assessed was blistered, and there was no evidence of any type of surface testa changes near the hilum. However, following hot water treatment, a blister similar to the one in D. petiolaris seeds was dislodged. The blister was located in a position similar to the one observed on seeds of D. petiolaris, i.e. adjacent to the hilum on the side opposite to the place where the radicle emerges (Fig. 8).
Fig. 8.
Hilum region of (A) Diplopeltis huegelii, (B) Distichostemon hispidulus, (C) Dodonaea aptera before hot water exposure, and (D) D. huegelii, (E) D. hispidulus and (F) D. aptera after dipping in hot water (30 s).
DISCUSSION
The water gap in D. petiolaris seeds is a small region that blisters under stress. It is located adjacent to the hilum and opposite to the side of the seed where the radicle emerges. Macroscopic observation of the hilar region under a binocular microscope clearly showed that the blister (400–600 µm in diameter) is not present prior to hot water treatment but is evident after seeds have been dipped in hot water. Seeds that had blistered subsequently imbibed water much more readily than those without a blister. The maximum mass increase for blistered seeds (95 %) was very similar to that of nicked seeds (96 %). However, the rate of mass increase in blistered seeds was much slower than that of nicked seeds. This was due to the small size of the water gap regulating water uptake in blistered seeds versus the larger size of the opening in nicked seeds. Indeed, a critical finding of this study is that regardless of whether seeds were dipped in hot water (or not), those with clearly visible blisters readily imbibed water, and the presence (or absence) of this blistered region was the key feature for determining water permeability (or not) of D. petiolaris seeds.
Dye-tracking experiments also indicated that the initial route of water into D. petiolaris seeds was via the dislodged palisade layer in the blister and that this was the only point of water entry that was evident after incubation for 8 h in a 1 % fuchsin-dye solution. The use of the dye in this case provided clear and direct evidence that the only initial point of water entry into the seed was via the water gap, with water diffusing throughout the seed once it reached the subpalisade layer. This sequence of events corresponds with the findings of Serrato-Valenti et al. (1992, 1995) for Abelmoschus esculentus (Malvaceae) and Leucaena leucocephala (Fabaceae) and with those of Jayasuriya et al. (2007) for Ipomoea lacunosa (Convolvulaceae). In these three species, aqueous solutions of 0·5 % w/v crystal violet, 0·02 % ruthenium red and a saturated solution of aniline blue, respectively, entered the seed only through the disrupted water gap region during the early phases of imbibition. After dye moved through the water gap it diffused to other parts of the seed.
While the dye-tracking experiment provided visual evidence that water moved only through the water gap during the first stages of imbibition, this was also confirmed by the blocking experiment. During 48 h of moist incubation, the rate of imbibition for sealed blistered seeds was significantly lower than that for non-sealed blistered seeds. However, after 48 h incubation the maximum mass increase for sealed seeds, while still significantly lower than that of non-sealed seeds, was higher than that of intact control seeds. Thus, the sealant on many of the seeds had begun to ‘leak’, which was likely due to incomplete bonding around the edge of the sealant, a commonly experienced problem with blocking experiments of this nature (Egley and Paul, 1981; Hagon and Ballard, 1970; C. C. Baskin, pers. obs.). Nevertheless, 33 % of the sealed seeds had not imbibed after 48 h, which demonstrates that the water gap was indeed blocked – at least temporarily. In comparison, 100 % of the D. petiolaris seeds in which the water gap was ruptured but unsealed had fully imbibed after incubation for 48 h, when <5 % of the non-treated (intact) control seeds had imbibed.
The water gap was open in about 10–15 % of seeds of D. petiolaris prior to any seed treatment, a relatively common phenomenon in collections of seeds of many species with PY, including those of Cistaceae (Thanos and Georghou, 1988), Geraniaceae (Meisert, 2002), Fabaceae (Morrison et al., 1992) and Sapindaceae (Phartyal et al., 2005; Cook et al., 2008). Whether these seeds of D. petiolaris lost PY during storage or PY did not develop during seed maturation is unknown. Eleven to fifteen per cent of intact seeds without an obvious blister also imbibed water, regardless of whether they were dipped in hot water or not. This small portion of seemingly intact seeds that imbibed water may be due to fractures, fissures and other small imperfections in the seed coat that facilitated water entry into the seed. Alternatively, a small proportion of seeds may be naturally shed in a non-dormant state as a bet-hedging mechanism to facilitate rapid germination and recruitment in the event of sustained moisture in the days or weeks following dehiscence.
Previously, eight types of water gap had been identified in the seeds (or fruits) of 13 families (including Sphaerosepalaceae) with PY (Baskin et al., 2000; Baskin, 2003; Jayasuriya et al., 2007, 2008) – carpellary micropyle (Anacardiaceae), imbibition lid (Cannaceae), bixoid chalazal plug [Cistaceae, Malvaceae, Sphaerosepalaceae and other Malvanae families (see Nadi, 1998)], suberized stopper (Geraniaceae), hilar slit (Convolvulaceae, tribe Cuscuteae), lens (Fabaceae, subfamilies Caesalpinoideae, Mimosoideae, Papilionoideae), protuberance (Nelumbonaceae) and ‘bulges’ near the hilum (Convolvulaceae, several tribes). The present study adds the Sapindaceae to the list of families in which the water gap has been characterized. The location and structure of the water gap in D. petiolaris seeds is similar to that described for seeds in some other families with PY. In Sida spinosa (Malvaceae), for example, a kidney-shaped area of palisade cells in the seed coat separates from the underlying subpalisade cells forming a small blister (Egley and Paul, 1981), which appears remarkably similar in location, size and structure to the one observed in D. petiolaris seeds. Separation of the palisade and subpalisade layers in the chalazal area then facilitates imbibition of water when the seeds are placed onto a moistened substrate. Further, Serrato-Valenti et al. (1995) also described water uptake in Leucaena leucocephala (Fabaceae) as occurring in a remarkably similar fashion to that in both Dodonaea petiolaris and S. spinosa. As in seeds of other Fabaceae species, the site of water entry in those of L. leucocephala is the lens (strophiole). However, unlike seeds of species such as Melilotus alba (Hamley, 1932) and Trifolium subterraneum (Hagon and Ballard, 1969), which form a split, fissure or cleft (strophiolar cleft) between adjacent Malpighian (palisade) cells, those of L. leucocephala form a blister-like structure due to the raising of the palisade layer in response to heat. In this opening of the water gap, which is visible as a raised bump, a small section of the palisade layer separates from the lower mesophyll layer, and then water can migrate into the underlying cavity via apoplastic movement (Serrato-Valenti et al., 1995).
Although the water gap was investigated in detail in only one species in this study (Dodonaea petiolaris), the same seed coat structure was also found in three taxa closely related to D. petiolaris: Dodonaea aptera, Diplopeltis huegelii and Distichostemon hispidulus (Sapindaceae; Fig. 8). In these three taxa, the location and size of the water gap was comparable to that in D. petiolaris seeds. Further, as in seeds of D. petiolaris, the water gap in seeds of the other three taxa was not evident prior to hot water treatment but became clearly visible in all species following hot water exposure. Since a structure of similar appearance and position is found in three taxa that are closely related (Harrington et al., 2005) to D. petiolaris indicates that the blister represents the structure most likely regulating water uptake in these species as well.
Worldwide, only five genera of Sapindaceae for which PY has been well documented are known. Three of them (Dodonaea, Distichostemon and Diplopeltis, all in subfamily Dodonaeoideae) share very close taxonomic affinities with each other (Harrington et al., 2005), and they are found predominantly within Australia (Harrington et al., 2005; Cook et al., 2008). The other two genera reported with PY, Cardiospermum and Koelreuteria, are not native to Australia, and belong to subfamily Sapindoideae. Thus, while water-gap anatomy appears to be very similar among Australian genera of Dodonaeoideae with PY, it remains to be determined if the water-gap anatomy in Cardiospermum and Koelreuteria of subfamily Sapindoideae also is similar to that of D. petiolaris.
There may be a number of reasons why the germination percentage of D. petiolaris seeds was never >36 %. While >90 % of the seeds were filled and embryos were white and fully formed, only 48 % of the embryos stained pink or red with 1 % TTC indicating potentially only 48 % of seeds were viable, i.e. metabolically active. However, for seeds of some species with physiological dormancy (PD), TTC can provide false negatives, i.e. not stain when seeds are actually alive (Sukhvibul and Considine, 1994). Thus, this result with TTC should be viewed with some caution. If germination percentage is recalculated after removing those that did not stain with TTC (either dead or physiologically dormant), then the highest germination was around 75 % for the seeds incubated at 13/26 °C and 58–65 % for those incubated at constant 15–25 °C. Other species of Dodonaea evaluated for seed dormancy and germinability (Floyd, 1966; Baskin et al., 2004; Phartyal et al., 2005; Cook et al., 2008) appear to have high seed viability and germinate rapidly once PY is removed. Under optimal conditions, germination is usually >80 % and occurs over a range of temperatures. However, for several other Sapindaceae with a water-impermeable seed coat, PD occurs in combination with PY, i.e. (PY + PD). For example, PD was overcome in Koelreuteria paniculata by cold stratifying seeds that first had been nicked to break PY (Rehman and Park, 2000). In seeds of Diplopeltis huegelii, PD was broken during a short period of (dry) after-ripening following removal of PY by dipping in hot water (Turner et al., 2006). Neither warm nor cold stratification nor 6 months of after-ripening at 30 °C improved germination of D. petiolaris seeds (S. R. Turner, unpubl. res.).
PY break during incubation for 24 weeks was most effective at 20/35 °C, where >80 % of seeds became permeable to water. The loss of PY was also far more effective when seeds were incubated under moist than under dry conditions, with <15 % of dry-incubated seeds losing PY regardless of the incubation temperature. In comparison, moist incubation resulted in at least a 3-fold increase in PY loss. The temperature regime that was most effective for PY loss (20/35 °C) corresponds to that of the two wettest months (February and March) in the Gascoyne Junction area, where D. petiolaris seeds were collected (Bureau of Meteorology, 2008). Wet-heat (defined as ‘incubation under wet, warm-to-hot conditions’; van Klinken et al., 2008) also has been found to be important in release of PY in Parkinsonia aculeata (Fabaceae), a woody plant native to the neotropics but invasive in tropical areas of the world (van Klinken and Flack, 2005; van Klinken et al., 2006, 2008). Further, the two most effective temperatures for germination were 25 °C and 13/26 °C, both of which are more likely to occur during the three wettest months of winter (June to August) in Gascoyne Junction. Thus, PY loss is likely to occur during late summer with the onset of summer rainfall, while germination is likely to occur during the (relatively) cooler winter months, when rainfall is more consistent.
In summary, the role of the water gap in controlling PY is demonstrated in four species from three genera in the Sapindaceae. All species exhibited a morphologically similar mechanism in terms of the production of a blister-like structure following treatment with hot water. The ecological significance of this finding indicates that a common morphological basis exists for regulating PY in many Sapindaceae with this type of dormancy. However, how blister production occurs under natural (in situ) conditions is still uncertain though it is likely due to changes in soil moisture linked to soil warming (as demonstrated here under laboratory conditions), or alternatively, through moist heat penetration following the passage of wild fire. Indeed, further research is still required for improving our understanding of the important roles of temperature and moisture within the soil environment and how these interact to regulate the soil seed bank of D. petiolaris as well as other Sapindaceae with PY. At the global level morphoanatomical studies within representative Koelreuteria and Cardiospermum seeds are also required to determine how these traits relate to phylogenetic affinities within the broader Sapindaceae.
ACKNOWLEDGEMENTS
This research was supported under Australian Research Council's Linkage Projects funding scheme (project number LP0669589). The authors thank Bob Elkins and Luke Sweedman from the Seed Technology Centre, Kings Park and Botanic Garden, for providing seeds; the Australian Microscopy & Microanalysis Research Facility, Centre for Microscopy, Characterisation & Analysis, for use of facilities and for scientific and technical assistance; and Dr John Kuo for generous advice on tissue embedding, sectioning and optical microscopy.
LITERATURE CITED
- Baskin CC. Breaking physical dormancy in seeds: focussing on the lens. New Phytologist. 2003;158:229–232. [Google Scholar]
- Baskin CC, Baskin JM. Seeds: ecology, biogeography and evolution of dormancy and germination. San Diego, CA: Academic Press; 1998. [Google Scholar]
- Baskin JM, Baskin CC. Evolutionary considerations of claims for physical dormancy-break by microbial action and abrasion by soil particles. Seed Science Research. 2000;10:409–413. [Google Scholar]
- Baskin JM, Baskin CC. Classification, biogeography, and phylogenetic relationships of seed dormancy. In: Smith RD, Dickie J, Linington S, Pritchard H, Probert R, editors. Seed conservation: turning science into practice. London: Royal Botanic Gardens, Kew; 2003. pp. 517–544. [Google Scholar]
- Baskin JM, Baskin CC. A classification system for seed dormancy. Seed Science Research. 2004;14:1–16. [Google Scholar]
- Baskin JM, Baskin CC, Li X. Taxonomy, ecology, and evolution of physical dormancy in seeds. Plant Species Biology. 2000;15:139–152. [Google Scholar]
- Baskin JM, Davis BH, Baskin CC, Gleason SM, Cordell S. Physical dormancy in seeds of Dodonaea viscosa (Sapindales, Sapindaceae) from Hawaii. Seed Science Research. 2004;14:81–90. [Google Scholar]
- Baskin JM, Baskin CC, Dixon KW. Physical dormancy in the endemic Australian genus Stylobasium, a first report for the family Surianaceae (Fabales) Seed Science Research. 2006;16:229–232. [Google Scholar]
- Bureau of Meteorology. Australian Government, Bureau of Meteorolgy. 2008. http://www.bom.gov.au/ . Accessed on the 20 October 2008.
- Cook A, Turner SR, Baskin JM, Baskin CC, Steadman KJ, Dixon KW. Occurrence of physical dormancy in seeds of Australian Sapindaceae: a survey of 14 species in nine genera. Annals of Botany. 2008;101:1349–1362. doi: 10.1093/aob/mcn043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egley GH, Paul RN. Morphological observations on the early imbibition of water by Sida spinosa (Malvaceae) seeds. American Journal of Botany. 1981;68:1056–1065. [Google Scholar]
- Floyd AG. Effect of fire upon weed seeds in the wet sclerophyll forests of northern New South Wales. Australian Journal of Botany. 1966;14:243–256. [Google Scholar]
- Hagon MW, Ballard LAT. Reversability of strophiolar permeability to water in seeds of subterranean clover (Trifolium subterraneum L.) Australian Journal of Biological Sciences. 1970;23:519–528. [Google Scholar]
- Hamley DH. Softening of the seeds of Melilotus alba. Botanical Gazette. 1932;93:345–375. [Google Scholar]
- Harrington MG, Edwards KJ, Johnson SA, Chase MW, Gadek PA. Phylogenetic inference in Sapindaceae sensu lato using plastid matK and rbcL DNA sequences. Systematic Botany. 2005;30:366–382. [Google Scholar]
- Horn JW. The morphology and relationships of the Sphaerosepalaceae (Malvales) Botanical Journal of the Linnean Society. 2004;144:1–40. [Google Scholar]
- Jayasuriya KMGG, Baskin JM, Geneve RL, Baskin CC. Morphology and anatomy of physical dormancy in Ipomoea lacunosa: identification of the water gap in seeds of Convolvulaceae (Solanales) Annals of Botany. 2007;100:13–22. doi: 10.1093/aob/mcm070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayasuriya KMGG, Baskin JM, Geneve RL, Baskin CC, Chien C-T. Physical dormancy in seeds of the holoparasitic angiosperm Cuscuta australis (Convolvulaceae, Cuscuteae): dormancy-breaking requirements, anatomy of the water gap and sensitivity cycling. Annals of Botany. 2008;102:39–48. doi: 10.1093/aob/mcn064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston SK, Murray DS, Williams JC. Germination and emergence of balloon-vine (Cardiospermum halicacabum) Weed Science. 1979;27:73–76. [Google Scholar]
- van Klinken RD, Flack L. Wet heat as a mechanism for dormancy release and germination of seeds with physical dormancy. Weed Science. 2005;53:663–669. [Google Scholar]
- van Klinken RD, Flack L, Pettit W. Wet-season dormancy release in seed banks of a tropical leguminous shrub is determined by wet heat. Annals of Botany. 2006;98:875–883. doi: 10.1093/aob/mcl171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Klinken RD, Lukitsch B, Cook C. Interaction between seed dormancy-release mechanisn, environment and seed bank strategy for a widely distributed perennial legume, Parkinsonia aculeate (Caesalpinaceae) Annals of Botany. 2008;102:255–264. doi: 10.1093/aob/mcn087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire JD. Speed of germination: aid in the selection and evaluation for seedling emergence and vigour. Crop Science. 1962;2:176–177. [Google Scholar]
- Meisert A. Physical dormancy in Geraniaceae seeds. Seed Science Research. 2002;12:121–128. [Google Scholar]
- Morrison DA, Auld TD, Rish S, Porter C, McClay K. Patterns of testa imposed dormancy in native Australian legumes. Annals of Botany. 1992;70:157–163. [Google Scholar]
- Mott JJ, Cook SJ, Williams RJ. Influence of short duration, high temperature seed treatment on the germination of some tropical and temperature legumes. Tropical Grasslands. 1982;16:50–55. [Google Scholar]
- Nandi OI. Ovule and seed anatomy of Cistaceae and related Malvanae. Plant Systematics and Evolution. 1998;209:239–264. [Google Scholar]
- O'Brien TP, McCully ME. The study of plant structure: principles and selected methods. Melbourne: Termarcarphi Press; 1981. [Google Scholar]
- Paynter BH, Dixon KW. Seed viability and embryo decline in Geleznowia verrucosa Turcz. (Rutaceae) Scientia Horticulturae. 1990;45:149–157. [Google Scholar]
- Phartyal SS, Baskin JM, Baskin CC, Thapliyal RC. Physical dormancy in seeds of Dodonaea viscosa (Sapindaceae) from India. Seed Science Research. 2005;15:59–61. [Google Scholar]
- Rehman S, Park I. Effect of scarification, GA and chilling on the germination of goldenrain-tree (Koelreuteria paniculata Laxm.) seeds. Scientia Horticulturae. 2000;85:319–324. [Google Scholar]
- Reynolds S. Melianthaceae to Sapindaceae. Canberra: Australian Government Printing Service.; 1985. Flora of Australia. Vol. 25. [Google Scholar]
- Serrato-Valenti G, Cornara L, Lotito S, Quagliotti L. Seed coat structure and histochemistry of Abelmoschus esculentus chalazal region and water entry. Annals of Botany. 1992;69:313–321. [Google Scholar]
- Serrato-Valenti G, de Vries M, Cornara L. The hilar region in Leucaena leucocephala Lam. (De wit) seed: structure, histochemistry and the role of the lens in germination. Annals of Botany. 1995;75:559–574. [Google Scholar]
- Shepherd KA, Rye BL, Meissner RA. Two new Western Australian species of Dodonaea (Sapindaceae) from northern Yilgarn ironstones. Nuytsia. 2007;17:375–383. [Google Scholar]
- Sukhvibul N., Considine JA. Regulation of germination of seed of Anigozanthos manglesii. Australian Journal of Botany. 1994;42:191–203. [Google Scholar]
- Thanos CA, Georghiou K. Ecophysiology of fire-stimulated germination in Cistus incanus ssp. ceticus (L.) Heywood and C. salvifolius L. Plant, Cell & Environment. 1988;11:841–849. [Google Scholar]
- Turner SR, Merritt DJ, Baskin JM, Baskin CC, Dixon KW. Combinational dormancy in seeds of the Western Australian endemic species Diplopeltis huegelii (Sapindaceae) Australian Journal of Botany. 2006;54:565–570. [Google Scholar]
- West JG. A revision of Dodonaea Miller (Sapindaceae) in Australia. Brunonia. 1984;7:1–194. [Google Scholar]