Abstract
Background and Aims
Different populations of the Mediterranean xerohalophyte species Atriplex halimus exhibit different levels of resistance to salt and osmotic stress depending on the nature of the osmocompatible solute they accumulate. There is, however, no conclusive description of the involvement of abscisic acid (ABA) in the plant response to NaCl or osmotic stress in this species.
Methods
Seedlings issued from an inland water-stress-resistant population (Sbikha) and from a coastal salt-resistant one (Monastir) were exposed in nutrient solutions to NaCl (40 or 160 mm) or to 15 % PEG for 1 d and 10 d in the presence or absence of 50 µm ABA.
Key Results
Plants from Sbikha accumulated higher amounts of ABA in response to osmotic stress than those of Monastir, while an opposite trend was recorded for NaCl exposure. Exogenous ABA improved osmotic stress resistance in Monastir through an improvement in the efficiency of stomatal conductance regulation. It also improved NaCl resistance in Sbikha through an increase in sodium excretion through the external bladders. It is suggested that polyamines (spermidine and spermine) are involved in the salt excretion process and that ABA contributes to polyamine synthesis as well as to the conversion from the bound and conjugated to the free soluble forms of polyamine. Proline accumulated in response to osmotic stress and slightly increased in response to ABA treatment while glycinebetaine accumulated in response to salinity and was not influenced by ABA.
Conclusions
It is concluded that ABA is involved in both salt and osmotic stress resistance in the xerohalophyte species Atriplex halimus but that it acts on different physiological cues in response to those distinct environmental constraints.
Keywords: Abscisic acid, Atriplex halimus, bladders, glycinebetaine, halophyte, osmotic stress, polyamines, proline, salinity, saltbush, sodium
INTRODUCTION
Increased salt and drought tolerance of crops are needed to sustain food production in the future since most of suitable land is already used for agriculture production. There is also an urgent need to reduce the amounts of water used for irrigation not only because fresh water will become less available in the future but also because the use of poor quality water is responsible for salinization of irrigated lands in numerous areas of the world (Gerhart et al., 2006). Tolerance of those abiotic stresses is a complex trait from a genetic and a physiological point of view. Most of the cultivated plant species are rather sensitive to both salt and water stress. Hence, there is an obvious interest in studying the physiological basis of stress resistance in xero-halophyte plant species which are well adapted to salinity and drought. Such an approach may help us to identify the key components of stress resistance and, in a second step, to define precisely their genetic basis with the final aim of transferring such properties to cultivated plant species (Colmer et al., 2006). Beside their putative interest for plant breeding, xero-halophyte plants are also important from an ecological point of view: global change will undoubtedly contribute to desertification processes in the next decades and the optimal use of this plant material may be considered as a convenient way to reduce the deleterious impact of climate change in marginal ecosystems. The efficient use of xero-halophyte plant species could also help in the reduction of global warming through CO2 sequestration (Glenn et al., 1992). Because some of these species are also able to tolerate high levels of inorganic pollutants in the soil, they may constitute a valuable material for land rehabilitation in former mining areas, especially under arid environments (Lutts et al., 2004). A better knowledge of the physiological behaviour of those species exposed to water stress and/or salinity is therefore of considerable interest.
Plants exposed to salt stress have to face a double constraint: the first one is directly linked to the accumulation of toxic ions (mainly Na+ and Cl−), while the other one is related to the decrease in the external soil water potential which compromises water absorption, thus inducing a physiological drought within the stressed tissues (Munns, 2002). Abscisic acid (ABA) is frequently overproduced in response to a wide range of environmental constraints. This plant hormone is synthesized in leaves and roots and may move freely through the plant in both the xylem and the phloem. The impact of ABA on stomatal regulation is well established and it may thus contribute to reduce water losses. This hormone has also been reported to be directly responsible for the activation of numerous genes involved in plant response to water stress (Bray, 2002).
Among the known mechanisms associated with stress tolerance, accumulation of osmoprotectants such as glycinebetaine and proline is of particular interest. Those compounds may be involved in both osmotic adjustment and protection of cellular structures. It has been demonstrated that proline and glycinebetaine could reduce the deleterious impact of stress on the generation and/or impact of reactive oxygen species (Hoque et al., 2007), protect enzymes assuming crucial functions in plant metabolism (Rontein et al., 2002) and stabilize the photosystems (Ohnisi and Murata, 2006). Other authors assume that those compounds could also be involved in stress signalling (Ma et al., 2006). Conflicting data are, however, available in the literature concerning the putative influence of ABA on proline and glycinebetaine synthesis (Colmer et al., 2005).
The diamine putrescine (Put) and the polyamines spermidine (Spd) and spermine (Spm) are low molecular weight organic cations that are implicated in various physiological and developmental processes in all living organisms. In plants, these processes include regulation of cell division, rhizogenesis, embryogenesis, senescence, floral development and fruit ripening (Galston et al., 1997; Arena et al., 2005). In addition, polyamines have been shown to afford protection against a large number of environmental biotic and abiotic stresses (Bouchereau et al., 1999; Balestrasse et al., 2005). Under physiological conditions, polyamines may be present in a free soluble form but could also be conjugated to phenolic and hydroxycinnamic acids or bound to macromolecules such as proteins and nucleic acids (Sfichi et al., 2004, Bakhanashvili et al., 2005). ABA has been shown to increase polyamine synthesis in wheat (Aurisano et al., 1993) and rice (Lee et al., 1997). It has also been demonstrated in rice that the ionic component of salt stress may have specific impact on polyamine metabolism and that iso-osmotic concentrations of NaCl on the one hand and polyethylene glycol (PEG) on the other hand do not necessarily have similar effects on polyamine concentrations (Lefèvre et al., 2001). Data concerning polyamine metabolism in xero-halophyte species exposed to drought or to salinity are, however, crucially lacking.
Beside osmotic adjustment and protection of cellular structures, exclusion of the absorbed ions at the leaf surface often appears as an efficient strategy contributing to salinity resistance in some xero-halophte plant species (Batanouny et al., 1992; Ramadan, 1998). This appears to be valid for Atriplex halimus since both abaxial and adaxial leaf surfaces are covered by high density of salt-accumulating bladders, also defined in the literature as vesiculated hairs or trichomes (Mozafar and Goodin, 1970; Breckle et al., 1990). According to Freitas and Breckle (1993), >50 % of the absorbed sodium and chloride may be removed from the mesophyll through this mechanism. As far as is known, however, no exhaustive data are available on the influence of ABA on these salt excretion processes.
It has been demonstrated recently that an inland (Sbikha) and a coastal (Monastir) population of Atriplex halimus widely differ in terms of physiological strategy allowing the plant to cope with NaCl and osmotic stress (Ben Hassine et al., 2008). The coastal population is more tolerant of salinity than the inland population and displays a higher ability to accumulate glycinebetaine in response to this constraint. In contrast, the inland population, exposed in its natural habitat to transient periods of drought, is more resistant to osmotic stress induced by 15 % PEG, and mainly accumulates proline in response to this treatment (Ben Hassine et al., 2008). The coastal population also exhibits a prodigal use of water in respect of a low instantaneous water-use efficiency (WUE), while the inland population is adopting a water-saving strategy and thus exhibits a high WUE. Finally, the higher salt-resistance of the coastal population is not associated with a lower sodium accumulation when plants are maintained in the presence of 160 mm under controlled conditions (Ben Hassine et al., 2008). The endogenous concentration of ABA, the efficiency of the salt excretion process and the putative influence of ABA on salt excretion were never considered until now.
The aims of the present study were (a) to determine the impact of salt and osmotic stress on the endogenous concentration of ABA in plants of A. halimus belonging to these two distinct populations and (b) to analyse the putative physiological influence of this plant growth regulator on seedling response to stress. It is shown that ABA improved the behaviour of coastal population exposed to osmotic stress by improving its stomatal regulation while it helps the inland population to cope with salinity by stimulating Na+ and Cl− exclusion at the bladder level.
MATERIALS AND METHODS
Plant material and growth conditions
Fruits of Atriplex halimus L. were collected from wild plants growing at two different sites in Tunisia. Monastir is a coastal site from East-Tunisia (36°13′N; 10°23°W) while Sbikha is an arid inland site (36°27′N; 9°49′W). The mean annual rainfall (average estimated over the previous 3 years) for Monastir and Sbikha are 346 mm and 253 mm, respectively. The mean relative humidity and the mean annual temperature are 72·9 % and 20·0 °C for Monastir and 60·3 % and 23·2 °C for Sbikha. For both sites, an experimental field of approx. 50 ares spontaneously colonized by shrubs of Atriplex halimus was considered. The soil salinity level was estimated on ten independent soil samples per site. Soil samples were collected in June (which corresponds to a mean precipitation of 27 mm and 35 mm for Sbikha and Monastir, respectively). Electrical conductivity was measured on saturate paste extracts collected at a 20-cm depth using a WTW LF92 conductimeter. The mean electrical conductivity was 2·23 ± 0·43 dS m−1 for Sbikha and 7·18 ± 1·03 dS m−1 for Monastir, thus confirming the saline properties of the latter and the non-saline character of the former site. Seeds were collected from at least ten plants at each site and pooled in order to constitute a mean sample for each population.
After removal of the bracts, seeds were sown in plastic jars containing a sandy textured non-saline soil (50 % sand, 25 % silt, 25 % clay; EC 1·13 dS m−1) and maintained in a growth chamber at 28 °C during the day, 20 °C during the night, under a PAR of 150 µmol m−2 s−1 and a photoperiod of 16 h. After 5 weeks, 320 seedlings per population were distributed among 40 tanks, each one containing 2 L of nutrient solution containing (in mm) 5 KNO3, 1 NH4H2PO4, 0·5 MgSO4, 5·5 Ca(NO3)2 and (in μm) 25 KCl, 10 H3BO3, 1 MnSO4, 1 ZnSO4, 0·25 CuSO4, 10 Na2MoO4 and 50 mg L−1 FeEDTA. Solutions were renewed each week. Plants (eight per tank) were fixed on polystyrene plates at a mean distance of 6 cm. Daytime humidity was maintained at 57 ± 2 % and temperature at 25 °C during the day and 23 °C during the night. The mean PAR was 250 µmol m−2 s−1 provided by Philips lamps (Philips Lighting S.A., Brussels, Belgium).
Treatments were applied 10 d after transfer to the nutrient solution. For salt treatment, NaCl was added to the nutrient solution in order to obtain a final concentration of 40 or 160 mm as previously recommended (Ben Hassine et al., 2008). For osmotic stress, PEG (polyethylene glycol 10000; Sigma Aldrich, Belgium) was added in nutrient solution to reach a final dose of 15 %. Plants maintained in nutrient solution in the absence of NaCl and PEG were used as a control. Osmotic potentials of nutrient solutions were assessed with a vapour pressure osmometer (Wescor 5500): Ψs = –0·12 (control), –0·37 (40 mm NaCl), –1·04 (160 mm NaCl) and –0·97 (15 % PEG) MPa. Solutions were continuously aerated with a stream of air and kept at a constant O2 concentration of 7·5 mg L−1 (87 %) measured with a dissolved oxygen probe (CellOx 325; WTW) and a precision instrument (ProfiLine Dissolved Oxygen Meter Oxi 197-S; WTW). Each of the four treatments was applied on 60 plants per population: 12 of them were harvested after 24 h of exposure and another set of 12 plants after 10 d of treatment. Leaf stomatal conductance (gleaf) was measured daily at 1100 h on the abaxial surface of the leaf located at the middle part of the main stem with an automatic porometer (MK III; Delta-T Devices, UK). For further analysis (ion, ABA, polyamine, proline and glycinebetaine concentration), measurements were performed on a pooled sample of fully expanded leaves collected on a plant, the four basal leaves being discarded to avoid interference with senescing processes. For each treatment, six separated plants were considered.
In another set of experiments, plants were exposed to the same stressing agents in the presence or in the absence of 50 µm ABA (Sigma-Aldrich, Germany). Additions of inhibitors such as 50 µm fluridone (FLU; Olchemlm Ltd, Czech Republic), a known inhibitor of ABA biosynthesis (Kowalczyk-Schröder and Sandmann, 1992) or 0·5 mm methylglyoxal-bis-guanyl hydrazone (MGBG; Sigma Aldrich Belgium), an inhibitor of polyamine biosynthesis, were made when required as stated below.
Ion quantification and bladders analysis
For major cations (K+, Na+, Mg2+ and Ca2+), Pi and microelement (Cu2+, Zn2+, Mn2+, Fe3+) quantification, tissues harvested on five plants per treatment were oven-dried at 80 °C for 48 h and 50 mg dry weight were digested in 35 % (v/v) HNO3. Analyses were conducted by flame atomic absorption spectrophotometry (VARIAN spectra-300). Chloride was determined colorimetrically with feric ammonium sulfate and mercuric thiocyanate according to Guerrier and Patolia (1989).
For the scanning electron microscopy (SEM Phillips XL20), specimens were flash frozen (–212 °C) in liquid nitrogen under vacuum for cryo-SEM (Oxford CT1500 cryo-system), transferred to the preparation chamber, and then to the SEM chamber where the frozen samples were sublimated (–80 °C) to remove ice particles. Samples were sputter coated with gold in the preparation chamber for 75 s under 1·2 kV at –150 to –170 °C. Specimens were viewed under – 5 kV at –170 to –190 °C.
Bladders (also termed ‘trichomes’ or ‘vesiculated hairs’ in the genus Atriplex; Mozafar and Goodin, 1970; Breckle et al., 1990; Freitas and Breckle, 1993) were removed from the leaf surface on samples collected at the mid-photoperiod on eight separated plants per treatment according to Zhang and Oppenheimer (2004) by carefully rubbing the surface of the weighed leaves using a small paintbrush in the presence of 0·05 % Triton X-100 (Sigma Inc; high grade chemical reagent) (the absence of ion contamination in the washing buffer was checked before leaf rinsing by atomic absorption spectrophotometry; Varian SpectrAA-300). The washing solution was retained and stored at –20 °C until ion analysis while the washed leaves were dried in an oven. As far as leaves were concerned, ion quantifications were performed according to Ramadan (1998) on intact leaves, washed leaves and washing solution in order to determine the proportions of ions which were outside (at the leaf surface) and inside (remaining within the leaf tissues).
ABA determination
For ABA quantification, plant tissues were powdered in liquid nitrogen and extracted with 100 % methanol for 2 h at –20 °C in darkness. [3H]ABA (777 TBq mol−1) was added to each sample as a recovery marker at 250 Bq. After centrifugation (24 000 g at 4 °C for 20 min), the supernatant was diluted with water to a final concentration of 80 % methanol and purified on a C18SepPack Cartridge (Waters, Ireland). ABA concentration was determined according to Djilianov et al. (1994). ABA extracts were evaporated to dryness in vacuo and redissolved in phosphate-buffered saline. ABA content was quantified by using ELISA kits (Phytoscience, France) with a monoclonal antibody (Olchemlm Ltd, Czech Republic) specific to free cis-(+)-ABA. The accuracy of the immunoassay was checked by LC-MS. The HPLC was coupled to a VG TRIO 2000 quadrupole mass spectrometer with a VG thermospray probe (Fisons, Manchester, UK) under optimized thermospray conditions (repeller 200 V, capillary temperature 205 °C). Quantitative analyses were performed in Selected Ion Monitoring mode using 265 (MH)+ and 247 (M-H2O)H+ on specific diagnostic ions.
Polyamines, glycinebetaine and proline extraction and quantification
For polyamine extraction, tissues were frozen in liquid nitrogen and ground in a pre-chilled mortar: samples [approx. 500 mg fresh weight (f. wt) for shoots and 250 mg for roots] were then homogenized with 500 µL of cold HCl (1 m), kept on ice for 1 h and then centrifuged at 23100 g at 4 °C for 20 min. Pellets were re-extracted with 500 µL HCl (1 m) and re-centrifuged. The two supernatants were used to determine free polyamines. Dansylation was performed according to Smith and Davies (1985). Samples were re-suspended in 1 mL methanol, centrifuged at 13 000 g for 15 min and filtered through microfilters (Chromafil PES-45/15, 0·45 µm; Macherey-Nagel). Aliquots (20 µL) were injected into a Bio-Rad HPLC system equipped with a Nucleosil 100-5 C18 MN 250/04 column (particle size: 5 µm, 4·6 × 250 mm2). Elution was performed at 35 °C at a flow rate of 1 mL min−1 using a methanol/water stepped gradient programme changing from 60 % to 100 % methanol over 25 min. The column was washed with 100 % methanol for 15 min. Detection of dansylated polyamines was performed with a Shimadzu RF-10Axl fluorimeter, with an excitation wavelength of 320 nm and an emission wavelength of 510 nm. The rate of free polyamine recovery at the end of the procedure was higher than 95 % (Ndayirajige, 2006).
For free and conjugated polyamine analysis, 200 µL of the supernatant was mixed with 200 µL of 12 n HCl and heated at 110 °C for 16 h in tightly capped glass tubes. After acid hydrolysis, HCl was evaporated from the tubes by further heating at 80 °C and the residue was resuspended in 200 µL of 10 % PCA and used for dansylation. To extract PCA, insoluble bound PAs, the pellet was dissolved by vigorous vortexing in 5 mL of 1 n NaOH. The mixture was centrifuged at 23 100 g at 4 °C for 20 min, and the supernatant, including the solubilized bound PAs, was hydrolysed under the same conditions as above. Aliquots of 200 µL of supernatant (free PAs), supernatant hydrolysed (conjugated PAs) and pellet hydrolysed (bound PAs) were dansylated, along with PAs standards, as previously described by Lefèvre et al. (2001). Polyamines were then quantified as described above for free polyamines. For a given treatment, each quantification was performed on three independent samples.
For glycinebetaine determination, collected leaves and roots (approx 200 mg) were mixed with 5 mL distilled water and the crude extracts were applied to a small column (1·6 mL) containing an AG1 X8 resin (200–400 mesh, OH- form Bio-Rad). The column was dried down by centrifugation (3 min, 4 °C, 300 g) and then washed with 875 µL of distilled water. Extracted glycinebetaine was quantified according to Bessieres et al. (1999) after HPLC separation on a Spherisorb 5 ODS2 column (250 × 4·6 mm) preceded by a precolumn (10 × 1 mm) packed with the same phase. The mobile phase contained 13 mm sodium heptane sulfonic acid and 5 mM Na2SO4 in deionized water (pH adjusted to 3·7 with 1 n H2SO4) at a flow rate of 0·8 mL min−1. Quantification was performed by a UV detector (Bio-Rad 1801 UV Monitor). The rate of glycinebetaine recovery at the end of the procedure was at least 94 % (Ndayirajige, 2006).
To quantify free proline, 1 g of tissue was extracted with 5 mL of 5 % salycilic acid; after centrifugation at 5000 g, free proline was specifically quantified according to Bates et al. (1973).
Statistical treatment of the data
The experiment was repeated twice (March and April 2004; April and May 2005) and the results exhibited similar trends. Data presented hereafter are from one single experiment. Percentage data were transformed to arcsine values before statistical analysis. For each duration of stress, data were analysed separately for roots and shoots by one-way analysis of variance (ANOVA, treatment as level of classification) performed with the model procedure of SAS version 9·1 (SAS Institute, 2002). Mean differences were compared according to the Scheffé F test.
RESULTS
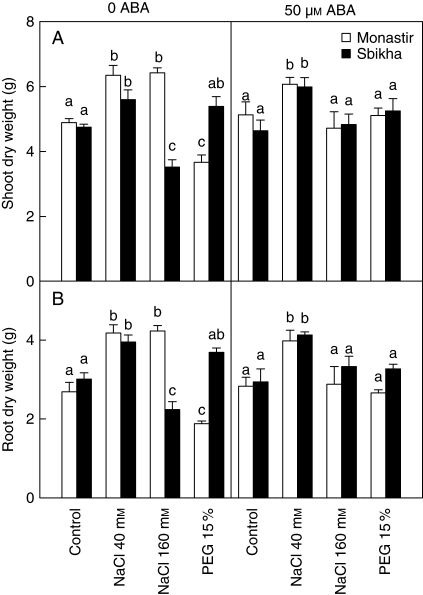
Moderate doses of salt induced a slight increase in shoot and root dry weights (Fig. 1) in seedlings issued from both populations after 10 d of treatment. Shoot and root dry weight decreased in response to 160 mm NaCl in Sbikha compared with controls but not in Monastir, thus confirming the higher salt resistance of the latter compared with the former. Root and shoot dry weight in Sbikha exposed to 15 % PEG remained unaffected compared with controls. In contrast, dry weight of both organs was strongly affected in plants of Monastir maintained for 10 d in the presence of 15 % PEG. Addition of ABA 50 µm improved plant growth in Sbikha exposed to 160 mm NaCl and in Monastir exposed to PEG, suggesting that this plant growth regulator has a positive impact in plant resistance to both type of environmental constraints.
Fig. 1.
Shoot and root dry weights in seedlings of Atriplex halimus issued from a coastal saline site (Monastir) or an inland semi-arid area (Sbikha) and exposed for 10 d to control nutrient solution or to nutrient solution containing 40 or 160 mm NaCl or 15 % PEG in the absence or presence of 50 µm ABA in the nutrient solution. Initial dry weights were 2·53 ± 0·13 g and 1·97 ± 0·11 g for shoots and roots, respectively. Each value is the mean of 12 replicates and vertical bars are s.e. of the means. Values sharing a common letter are not significantly different at P < 0·05.
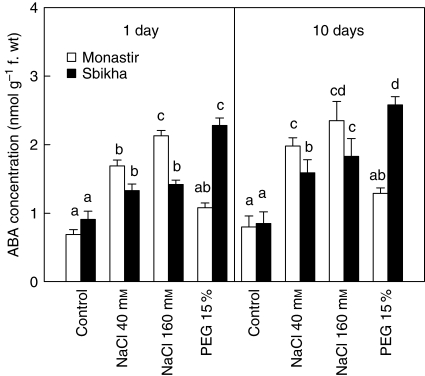
Root water content was hardly affected by salinity or PEG (detailed data not shown). Leaf water content was already reduced by PEG after 24 h of treatment in Monastir (P < 0·05) but not in Sbikha (Table 1). After 10 d of treatment, the leaf WC was reduced in both populations in response to the highest dose of NaCl (160 mm) and to 15 % PEG. Nevertheless, the deleterious impact of NaCl was higher in Sbikha than in Monastir while an inverse trend was recorded for PEG. In the absence of exogenous ABA in the nutrient solution, endogenous ABA had already increased in response to salinity in Monastir and in response to osmotic stress in Sbikha after 24 h of treatment (Fig. 2). The endogenous ABA concentration was always higher in Sbikha than in Monastir in response to osmotic stress (P < 0·01) while an opposite trend was recorded in salt-treated plants (Fig. 2). It might thus be hypothesized that the endogenous concentration of ABA is a limiting factor for acclimation of seedlings from Sbikha exposed to NaCl as well as for those of Monastir exposed to PEG.
Table 1.
Leaf water content (in %; measured on fully expanded leaves) in seedlings of Atriplex halimus issued from a coastal site (Monastir) or an inland area (Sbikha) and exposed for 1 or 10 d to control nutrient solution or to a nutrient solution containing 40 or 160 mm NaCl or 15 % PEG
| Treatment | Population | 1 d | 10 d |
|---|---|---|---|
| Control | Monastir | 84·2 ± 0·7a | 83·9 ± 1·0a |
| Sbikha | 85·3 ± 1·3a | 82·1 ± 0·8a | |
| 40 mm NaCl | Monastir | 83·9 ± 0·9a | 82·8 ± 1·1a |
| Sbikha | 83·4 ± 1·2a | 81·7 ± 0·8b | |
| 160 mm NaCl | Monastir | 81·5 ± 0·2b | 77·9 ± 0·4bc |
| Sbikha | 76·8 ± 0·4c | 72·5 ± 1·2d | |
| 15 % PEG | Monastir | 77·3 ± 0·8c | 70·1 ± 0·4e |
| Sbikha | 84·1 ± 0·4a | 78·2 ± 1·1c |
Each value is the mean of six replicates ± s.e. of the means. For a given time, values sharing a common superscript letter are not significantly different at P < 0·05.
Fig. 2.
Leaf abscisic acid concentration (ABA, in nmol g−1 f. wt) in seedlings of Atriplex halimus issued from a coastal saline site (Monastir) or an inland semi-arid area (Sbikha) and exposed for 1 d and 10 d to control nutrient solution or to nutrient solution containing 40 or 160 mm NaCl or 15 % PEG. Measurements were performed on fully expanded leaves. Each value is the mean of 12 replicates and vertical bars are s.e. of the means. Values sharing a common letter are not significantly different at P < 0·05.
Exogenous application of 50 µm ABA strongly increased total ABA concentration in both roots and shoots, thus suggesting that, when added to the nutrient solution, this plant growth regulator was efficiently absorbed by the roots and translocated to the shoots (Table 2). No difference was detected any more between plants of Sbikha and Monastir, whatever the considered treatment. This confirms that the positive impact of exogenous ABA on treated plants was associated with an increase in the root and shoot total ABA concentrations. Conversely, FLU treatment decreased ABA concentrations in all plant organs including unstressed controls after 10 d of treatment. Exogenous application of ABA increased the leaf WC of Monastir exposed to PEG (Table 2) compared with similar seedlings untreated with ABA (Table 1; P < 0·05) and values of ABA-treated plants were close to those recorded for unstressed controls. In contrast, exogenous ABA had no impact on the leaf WC of salt-treated Sbikha which remained lower for plants exposed to 160 mm NaCl than for controls.
Table 2.
Impact of exogenous ABA (50 µm) and fluridone (FLU) application on total leaf ABA concentration, water content (WC) and leaf stomatal conductance (gleaf) in seedlings of Atriplex halimus issued from a coastal site (Monastir) or an inland area (Sbikha) and exposed for 10 d to control nutrient solution or to a nutrient solution containing 40 or 160 mm NaCl or 15 % PEG
| Total ABA (nmol g−1 f. wt) |
WC (in %) |
gleaf (cm s−1) |
|||||
|---|---|---|---|---|---|---|---|
| Population | 50 µm ABA | FLU | 50 µm ABA | FLU | 50 µM ABA | FLU | |
| Control | Monastir | 5·13 ± 0·12a | 0·21 ± 0·05a | 85·6 ± 1·1a | 77·3 ± 0·8a | 0·27 ± 0·01a | 0·39 ± 0·04a |
| Sbikha | 5·02 ± 0·22a | 0·35 ± 0·02ab | 84·5 ± 0·9a | 78·4 ± 1·1a | 0·24 ± 0·03a | 0·36 ± 0·03a | |
| 40 mm NaCl | Monastir | 5·27 ± 0·14a | 0·29 ± 0·02a | 84·9 ± 1·3a | 73·2 ± 1·0b | 0·25 ± 0·02a | 0·27 ± 0·03b |
| Sbikha | 4·89 ± 0·18a | 0·25 ± 0·08a | 80·5 ± 0·8a | 69·5 ± 0·4c | 0·19 ± 0·01b | 0·23 ± 0·01b | |
| 160 mm NaCl | Monastir | 5·12 ± 0·23a | 0·42 ± 0·04b | 82·3 ± 1·7a | 75·4 ± 1·2ab | 0·30 ± 0·02a | 0·29 ± 0·02b |
| Sbikha | 4·94 ± 0·38a | 0·25 ± 0·03a | 75·3 ± 2·4b | 67·4 ± 1·3c | 0·20 ± 0·03ab | 0·27 ± 0·03b | |
| 15 % PEG | Monastir | 4·98 ± 0·27a | 0·29 ± 0·07a | 84·9 ± 0·7a | 70·2 ± 0·5bc | 0·11 ± 0·02c | 0·49 ± 0·03c |
| Sbikha | 5·05 ± 0·15a | 0·45 ± 0·02b | 83·1 ± 1·1a | 74·5 ± 1·7b | 0·12 ± 0·02c | 0·52 ± 0·05c | |
Measurements were performed on fully expanded leaves. Each value is the mean of six replicates ± s.e. of the means. For a given parameter and a given time, values sharing a common superscript letter are not significantly different at P < 0·05.
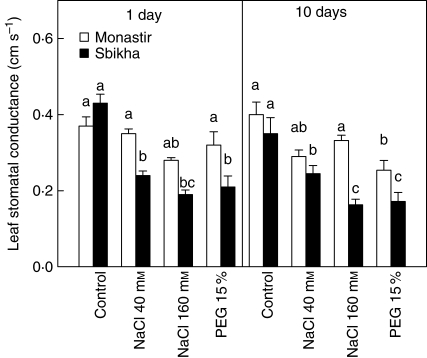
The gleaf was similar in Sbikha and Monastir under control conditions: after 24 h of exposure to all treatments in Sbikha it had already clearly decreased. After 10 d of stress exposure, gleaf was lower in Sbikha than in Monastir, whatever the considered treatment (Fig. 3). Exogenous ABA decreased gleaf in PEG-treated plants issued from Monastir but had no impact on salt-treated plants from both populations (Table 2). Conversely, FLU treatment increased gleaf in water-stressed plants (P < 0·01) but had only a limited impact on gleaf from salt-treated individuals.
Fig. 3.
Leaf stomatal conductance (gleaf, in cm s−1) in seedlings of Atriplex halimus issued from a coastal saline site (Monastir) or an inland semi-arid area (Sbikha) and exposed for 10 d to control nutrient solution or to nutrient solution containing 40 or 160 mm NaCl or 15 % PEG. Measurements were performed at 1100 h on a fully expanded leaf located at the middle part of the stem. Each value is the mean of 12 replicates and vertical bars are s.e. of the means. Values sharing a common letter are not significantly different at P < 0·05.
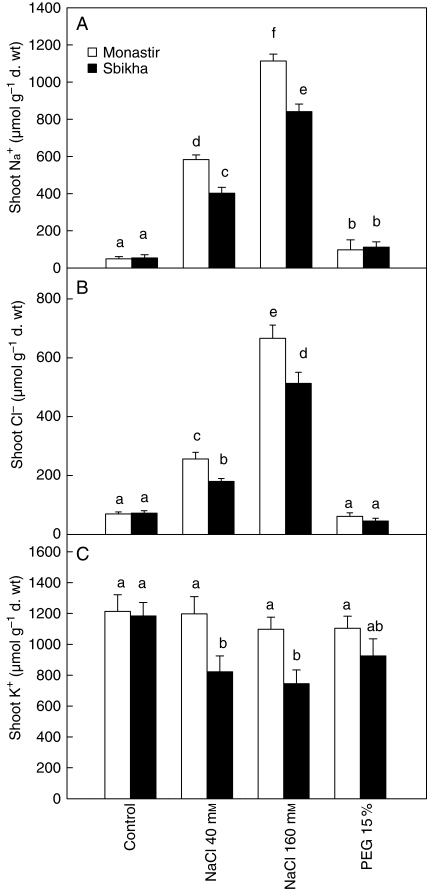
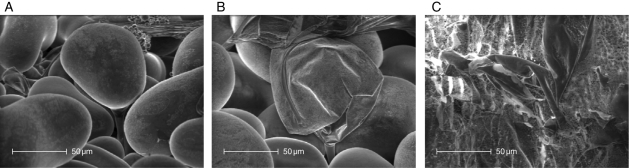
Sodium and chloride concentrations in the shoots of control plants were similar in Sbikha and in Monastir (Fig. 4). Low (40 mm) and high (160 mm) doses of NaCl induced an obvious increase in those elements which accumulated to higher extent in Monastir than in Sbikha (P < 0·05). Sodium concentration also slightly (although significantly) increased in response to PEG in both populations while K+ concentration decreased in salt-treated seedlings from Sbikha only. Other ion concentrations were not modified, whatever the considered treatment or population (data not shown). Exogenous application of ABA had only a marginal impact on total Na+ and Cl− concentrations of stressed seedlings (data not shown) but it drastically affected ions distribution among bladders and lamina. Indeed, a high density of bladders is visible at the leaf surface (Fig. 5). Those vesiculated hairs consist of a stalk cell and a balloon-like tip cell which sequester excess electrolyte and release the salt back in the environment when they are ruptured. Because of the presence of salt crystals at their surface, leaves of Atriplex halimus have a silver reflectance and this layer of bladders is thus an efficient system to remove salt from photostynthetically active tissues and probably to prevent some ultraviolet light reaching leaf tissues. As shown in Table 3, it is noteworthy that salt-treated seedlings of Monastir excreted a higher proportion of Na+ and Cl− at the leaf surface than seedlings issued from Sbikha and that the difference between populations increased with the NaCl dose. As far as K+ is concerned, <15 % was found in the bladders and K+ excretion was not influenced by the treatment and did not differ between populations. It has also to be noticed that exogenous ABA which improved salinity tolerance in Sbikha also clearly improved the sodium excretion process in salt-treated seedlings from this population (detailed data not shown). These data thus suggest that the positive impact of ABA in the salt resistance of Atriplex halimus could be due to its positive action on toxic ions excretion. This hypothesis was reinforced by the observation that the plants from both populations exposed to FLU were clearly less able to excrete sodium compared with non-treated ones (Table 3). Impact of FLU appeared to be similar, although less marked, on Cl− distribution.
Fig. 4.
Sodium (A), chloride (B) and potassium (C) concentrations in the leaves of Atriplex halimus issued from a coastal saline site (Monastir) or an inland semi-arid area (Sbikha) and exposed for 10 d to control nutrient solution or to nutrient solution containing 40 or 160 mm NaCl or 15 % PEG in the absence or presence of 50 µm ABA in the nutrient solution. Measurements were performed on fully expanded leaves. Each value is the mean of 12 replicates and vertical bars are s.e. of the means. Values sharing a common letter are not significantly different at P < 0·05.
Fig. 5.
Scanning electron micrography of the leaf surface of Atriplex halimus showing bladders (A) consisting of vesiculated balloon-like hairs attached to a stalk and playing a significant role in removing salt from the reminder of the leaf thus preventing accumulation of toxic salts in the parenchyma and vascular tissues. Accumulation of electrolytes leads to the bladder shrinking (B) and then bursting, depositing crystals at the leaf surface (C).
Table 3.
Proportions (in %) of Na+ and Cl− excreted at the leaf surface (‘Out’) or remaining inside the lamina (‘In’) in the leaves of Atriplex halimus issued from a coastal site (Monastir) or an inland area (Sbikha) and exposed for 10 d to control nutrient solution or to a nutrient solution containing 40 or 160 mm NaCl or 15 % PEG
| Na+ (distribution in %) |
Cl− (distribution in %) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 ABA |
+ ABA |
+ FLU |
0 ABA |
+ ABA |
+ FLU |
||||||||
| In | Out | In | Out | In | Out | In | Out | In | Out | In | Out | ||
| Control | Monastir | 68·8 | 31·2 | 67·0 | 33·0 | 82·2 | 17·8 | 71·5 | 28·5 | 64·1 | 35·9 | 77·1 | 22·9 |
| Sbikha | 74·7 | 25·3 | 66·2 | 33·8 | 78·7 | 21·3 | 70·6 | 29·4 | 70·8 | 29·2 | 84·4 | 15·6 | |
| 40 mm NaCl | Monastir | 58·6 | 41·4 | 55·8 | 44·2 | 79·8 | 20·2 | 62·8 | 37·2 | 59·7 | 40·3 | 72·7 | 27·3 |
| Sbikha | 70·2 | 29·8 | 59·6 | 40·4 | 84·5 | 15·5 | 79·7 | 20·3 | 61·6 | 38·4 | 82·5 | 17·5 | |
| 160 mm NaCl | Monastir | 49·7 | 50·3 | 48·5 | 51·5 | 71·7 | 28·3 | 54·2 | 45·8 | 50·8 | 49·2 | 73·9 | 26·1 |
| Sbikha | 69·1 | 30·9 | 51·7 | 48·3 | 76·1 | 23·9 | 77·5 | 22·5 | 58·7 | 41·3 | 79·7 | 20·3 | |
Plants were maintained in the absence (0 ABA) or in the presence (+ ABA) of 50 µm ABA or 50 µm fluridone (+ FLU) in the nutrient solution. Measurements were performed on fully expanded leaves. Each value is the mean of six replicates.
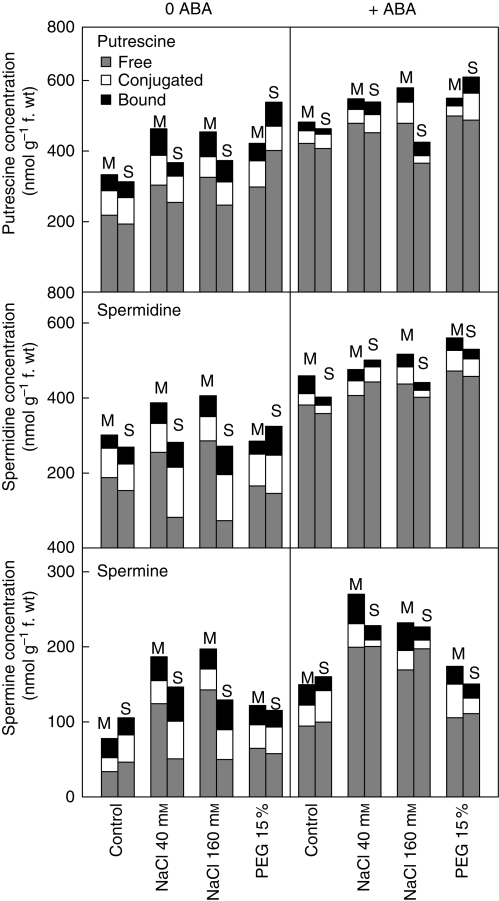
Salt treatment and osmotic stress induced a significant increase in the shoot polyamine concentration (Fig. 6). As far as salt-treated plants are concerned, concentrations of free Spd and Spm were higher in Monastir than in Sbikha while no difference was recorded for PEG-treated plants. Exogenous treatment with 50 µm ABA induced an increase in Spd and Spm, especially in Sbikha exposed to both NaCl doses, and it also induces a conversion from the conjugated and bound forms of polyamine to the free polyamine form. In order to test the hypothesis that the positive effect of exogenous ABA on salt excretion could be related to polyamine metabolism, plants from a separate set of experiments were treated with 0·5 mm MGBG (inhibitor of S-adenosylmethionine decarboxylase; EC 4·1·1·50). As shown in Table 4, MGBG efficiently decreased total Spd and Spm concentration and increased endogenous total Put content. Based on the shoot dry weight, treatment with MGBG decreased the salt resistance of the two considered populations and this effect was directly related to an inhibition of the sodium exclusion process. Treatment with MGBG strongly mitigated the beneficial impact of ABA on the salinity resistance and sodium excretion process in seedlings from the salt-sensitive population Sbikha (Table 4). However, it did not prevent ABA accumulation in the leaf tissue since ABA concentrations in plants simultaneously exposed to ABA and MGBG culminated up to 5·32 and 5·54 nmol g−1 f. wt in Monastir and Sbikha, respectively. These data therefore suggest that Spd and Spm could assume key roles in the sodium excretion process triggered by ABA. In contrast, application of the inhibitor of polyamine synthesis MGBG had no impact on the gleaf of PEG-treated plants and did not prevent stomatal closure in ABA-treated plants (Table 4). Similarly, MGBG had no impact on ABA concentration in plant tissues (detailed data not shown).
Fig. 6.
Leaf polyamine (Putrescine, Spermidine and Spermine) concentration in seedlings of Atriplex halimus issued from a coastal saline site (Monastir; M) and an inland semi-arid area (Sbikha; S) and exposed for 10 d to control nutrient solution or to nutrient solution containing 40 or 160 mm NaCl or 15 % PEG in the absence or presence of 50 µm ABA in the nutrient solution. Measurements were performed on fully expanded leaves. Each value is the mean of 12 replicates. In the columns, concentrations are given for the free, conjugated and bound fractions of each polyamine, as indicated.
Table 4.
Impact of methylglyoxal-bis-guanyl hydrazone 0·5 mm (MGBG) on shoot dry weight, total putrescine (Put), total spermidine (Spd) + spermine (Spm) concentration, percentage of sodium excreted at the leaf surface through the bladders [Na (%)] and stomatal conductance (gleaf) in seedlings of Atriplex halimus issued from a coastal site (Monastir) and an inland area (Sbikha) and exposed for 10 d to control nutrient solution or to a nutrient solution containing 40 or 160 mm NaCl or 15 % PEG
| Dry weight (g) |
Put (nmol g−1 f. wt) |
Spd + Spm (nmol g−1 f. wt) |
Na (%) |
gleaf (cm s−1) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ABA: | – | – | + | – | – | + | – | – | + | – | – | + | – | – | + | |
| MGBG: | – | + | + | – | + | + | – | + | + | – | + | + | – | + | + | |
| Control | Monastir | 4·3 | 3·9 | 4·1 | 351 | 518 | 522 | 418 | 210 | 232 | 30·4 | 20·6 | 17·6 | 0·39 | 0·43 | 0·25 |
| Sbikha | 4·1 | 4·0 | 3·7 | 333 | 507 | 493 | 352 | 173 | 188 | 26·5 | 19·6 | 15·3 | 0·42 | 0·39 | 0·24 | |
| 40 mm NaCl | Monastir | 5·5 | 4·0 | 3·9 | 434 | 559 | 535 | 607 | 183 | 194 | 39·7 | 15·4 | 17·5 | 0·41 | 0·44 | 0·30 |
| Sbikha | 5·2 | 3·3 | 3·4 | 312 | 498 | 548 | 512 | 179 | 203 | 25·8 | 16·2 | 20·2 | 0·25 | 0·30 | 0·20 | |
| 160 mm NaCl | Monastir | 6·0 | 2·8 | 3·2 | 429 | 513 | 555 | 594 | 202 | 188 | 49·9 | 17·3 | 21·4 | 0·32 | 0·35 | 0·19 |
| Sbikha | 3·1 | 2·7 | 3·1 | 322 | 497 | 527 | 488 | 161 | 169 | 35·6 | 12·5 | 18·5 | 0·21 | 0·19 | 0·17 | |
| PEG 15 % | Monastir | 3·9 | 4·2 | 4·0 | 411 | 599 | 603 | 422 | 152 | 225 | 40·2 | 20·6 | 24·6 | 0·40 | 0·38 | 0·14 |
| Sbikha | 5·7 | 5·2 | 5·5 | 421 | 572 | 574 | 434 | 158 | 187 | 34·8 | 22·9 | 17·3 | 0·21 | 0·24 | 0·13 | |
Plants were maintained in the presence or absence of 50 µm ABA. Measurements were performed on fully expanded leaves. Each value is the mean of six replicates.
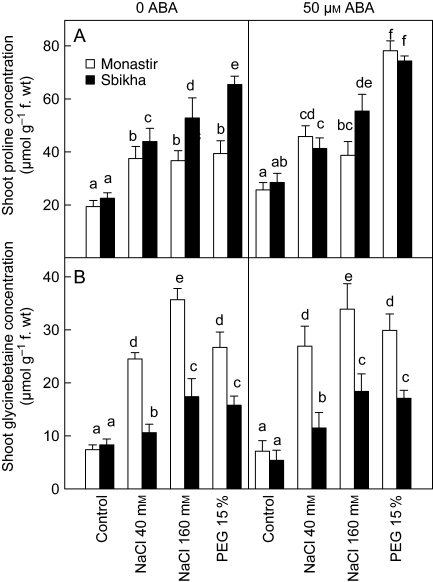
Both proline and glycinebetaine accumulated in response to salt and osmotic stress (Fig. 7). Proline, however, accumulated to a higher extent in PEG-treated plants than in salt-treated ones and reached a higher concentration in Sbikha than in Monastir. Exogenous ABA increased the proline concentration, especially in plants issued from Monastir and exposed to osmotic stress (Fig. 7) while treatment with FLU reduced it in all plants by >40 % (data not shown). In contrast, glycinebetaine reached higher levels in salt-treated plants compared with plants exposed to PEG. At the highest NaCl dose, the leaf glycinebetaine concentration was higher in Monastir than in Sbikha. Exogenous ABA had no impact on glycinebetaine concentration, whatever the treatment or the population considered.
Fig. 7.
Leaf proline and glycinebetaine concentrations in seedlings of Atriplex halimus issued from a coastal saline site (Monastir) and an inland semi-arid area (Sbikha) and exposed for 10 d to control nutrient solution or to nutrient solution containing 40 or 160 mm NaCl or 15 % PEG in the absence or presence of 50 µm ABA in the nutrient solution. Measurements were performed on fully expanded leaves. Each value is the mean of 12 replicates. Values sharing a common letter are not significantly different at P < 0·05.
DISCUSSION
Halophye plant species are well adapted to saline habitats and domestication of those species as new crops could in the future help to sustain food production in many regions of the world (Colmer et al., 2005). Salinity affects almost every aspect of plant physiology and biochemistry. Since the presence of salt in the root medium reduces external osmotic potential and thus compromises water absorption, halophyte plant species are exposed in their natural habitats to both ion toxicities and to physiological drought (Flowers and Colmer, 2008). Some xero-halophyte plant species display a fascinating ability to survive in dry and highly saline habitats.
The present data show that ABA is assuming key functions in Atriplex halimus in relation to NaCl on the one hand and to osmotic stress on the other. Indeed, the plant performance in the harsh environment appeared to be directly limited by their ability to synthesize this plant growth regulator. The present work suggests that ABA synthesis could be differently affected by environmental parameters depending on the considered population: salt-resistant plants originating from a coastal area accumulated lower amounts of ABA in response to drought than plants issued from an inland area. Environmental and developmental signals are operating in the regulation of ABA synthesis in plant tissues. The direct C15 precursor of ABA xanthoxin is produced as a result of 9-cis-violaxanthin cleavage by 9-cis-epoxycarotenoid dioxygenase (NCED) which has been established as the key enzyme in the ABA biosynthesis pathway (Xiong and Zhu, 2003). It has been demonstrated that water stress increased expression of the NCED gene in glycophyte plant species (Qin and Zeevaart, 2002; Xiong and Zhu, 2003). Some aldehyde oxidases may also oxidize abscisic aldehyde to ABA. Some of them were reported to be activated by drought (Bittner et al., 2001) but not by salinity (Zdunek-Zastoca, 2008). Since both NCED and aldehyde oxidases comprise small multigene families, it is possible that different isoforms are regulated differently by environmental cues in distinct populations. Ren et al. (2007) also recently reported that not only ABA synthesis but also catabolism plays a crucial role in the regulation of ABA accumulation by stressed plant tissues. Further studies are therefore required to identify the key limiting factor of ABA synthesis in plants from Monastir exposed to osmotic stress and in those from Sbikha exposed to NaCl. Not only the endogenous concentration of ABA but also the physiological consequences of such an accumulation differed between osmotic stress and salinity. For a given kind of stress, however, the physiological consequences were similar for the two considered populations.
It appears clearly that ABA contributes to osmotic stress resistance through an improvement in stomatal regulation and, hence, to an increased WUE. Plants from Sbikha exposed to water deficit displayed a lower gleaf than those from Monastir and also accumulated higher amounts of ABA in response to PEG. When exogenous ABA was supplied to plants from Monastir, water-stressed plants were able to increase their WUE, thus adopting a water-saving strategy similar to the strategy adopted by the plants from Sbikha and which helps them to cope with water scarcity, even for long periods (Ben Hassine et al., 2008).
Although NaCl implies a lowering of the external water potential and consequently induces a water stress component in the plant tissues, it is noteworthy that WUE did not appear as a key property in salt-treated plants. In contrast, bladders play a significant role. These non-glandular vesiculated hairs present at the leaf surface may accumulate important amounts of Na+ and Cl−, thus helping to preserve the metabolic activity of the photosynthethic tissues (Aslam et al., 1986; Karimi and Ungar, 1989). As far as Atriplex halimus is concerned, bladders may also accumulate other toxic ions such as Cd2+ and Zn2+ and the efficiency of the excretion process is partly controlled by environmental factors (Lefèvre et al., 2009).The present data suggest that ABA could be involved in the signalling components controlling excretion. Indeed, exogenous ABA increased Na+ and Cl− excretion in salt-treated plants from Sbikha. In contrast, the inhibitor of ABA synthesis, FLU, reduced ABA concentration and the efficiency of the excretion process in both populations.
Osmotic stress was shown to increase Na+ absorption in Atriplex halimus, even in the absence of salt (Martínez et al., 2005) and the PEG-induced increase in shoot Na+ concentration was therefore not unexpected, although the physiological role of such an increase remains obscure, especially considering that bladders excrete >30 % of the absorbed Na+. Drought was shown to increase non-glandular bladder density in some species (Gonzáles et al., 2008). Although bladders numbers were not considered in the present study, it has to be mentioned that PEG did not increase the proportion of excreted Na+, even if ABA obviously increased in the PEG-treated plants. Conversely, exogenous ABA decreased gleaf in PEG-treated plants issued from Monastir but had no impact on the gleaf of salt-treated plants from both populations. These data therefore suggest that ABA may be compartmented to specific cell types depending on the nature of the environmental constraint (stomatal guard cells in water-stressed plants and bladders in salt-treated ones) or that the signal transduction initiated by ABA accumulation is differently involved in the regulation of downstream metabolic processes in those cell types. Another intriguing observation is that not only endogenous ABA but also exogenously supplied ABA appears to be transported to the correct cellular target depending on the nature of environmental constraint.
The data obtained also suggest that free polyamines may be involved in ABA-induced increase of excretion processes through epidermal bladders. Indeed, (a) salt-treated seedlings from Monastir contained higher amounts of total polyamines than salt-treated plants from Sbikha, (b) exogenous ABA induced an obvious increase in total polyamine content and (c) the inhibitor of polyamine synthesis (MGBG) reduced both Spd and Spm concentrations as well as the efficiency of excretion processes. ABA has been reported to trigger polyamine synthesis through a transcriptional activation of genes coding for spermidine synthase (Jiménez-Bremont et al., 2007). Although water stress stimulated both spermidine and spermine synthases in Arabidopsis thaliana (Alcázar et al., 2006), no significant PEG-induced increase in Spd and Spm was noticed in Atriplex halimus (Fig. 6) despite the fact that Put accumulated in these water-stressed plants. Conversely, Put was shown to control the ABA level in A. thaliana (Cuevas et al., 2008) but in Atriplex halimus, MGBG-induced increase in Put content did not lead to ABA accumulation, thus suggesting that polyamine metabolism is regulated differently in the Mediterranean xerohalophyte shrub on the one hand and in the model plant species on the other hand.
As far as is known, specific interactions between polyamines and the excretion process at bladder level have never been reported until now. The present data suggest, however, that Spd and Spm rather than Put are involved in this process. Polyamines have been reported to interact with ion fluxes at the tonoplast (Liu et al., 2004; Zhao and Qin, 2004) and plasmamembrane level (Roy et al., 2005). We may thus hypothesize that Spd and Spm could interact with ATPase or ion channels involved in Na+ and Cl− fluxes between epidermis and basal cells of bladders. In most cases, however, data available in the literature suggest that regulation of ion fluxes at the membrane level involve the conjugated and bound forms of polyamines in glycophyte species. The present study, however, suggests that free polyamine are more suitable candidates for such regulation in Atriplex halimus and that ABA which stimulates salt excretion also increases the free to bound and conjugated ratio of polyamines in this species, as was recently reported in Vigna vinifera (Antolín et al., 2008). Shabala et al. (2007) recently reported that externally applied polyamines may block a non-selective cation channel from salt-treated pea mesophyll cells, thus preventing Na+ build-up and K+ efflux. It is thus also possible that elevated polyamine levels in Atriplex halimus leaves reduce Na+ influx into mesophyll cells thus re-directing Na+ flux towards salt-bladders.
The present study confirms that glycinebetaine is mainly involved in the response to salinity while proline is involved in the response to water stress, as previously demonstrated in this species (Ben Hassine et al., 2008). ABA has a dual impact on osmocompatible solute accumulation. Indeed, ABA had no significant impact on glycinebetaine concentration while it increased proline synthesis but in water-stressed plants only (Fig. 7). These observations suggest that a specific component of the signal transduction pathway leading to ABA-dependent proline accumulation should be present mainly in response to osmotic stress but not in response to salinity. This unidentified component should be equally present in the two populations considered since PEG-treated seedlings from Sbikha and Monastir accumulated similar amounts of proline in response to exogenous ABA (Fig. 7). Verslues and Bray (2006) using ABA-insensitive and ABA-deficient mutant suggested that sugar sensing may directly influence the efficiency of ABA-induced proline accumulation in water-stressed plants and further studies are therefore required to analyse salt and water stress impacts on sugar metabolism in different populations of Atriplex halimus. In some halophytes, leaves have been shown to accumulate high amounts of Na+ and Cl−, compartmentalizing these ions to vacuoles which then lower the osmotic potential of cells under saline conditions (Song et al., 2009). Plants issued from the population of Monastir had previously been shown to display a higher ability to adjust osmotically in the presence of salt than those from Sbikha (Ben Hassine et al., 2008). Both Na+ and Cl− accumulated to higher extent in the former than in the latter (Fig. 4) but a consistent proportion of the accumulated ions were excreted to the external salt bladders (Table 3) where they did not assume crucial osmoprotective functions for stressed tissues. The other quantified ions did not vary in response to salt in Monastir while K+ even decreased in salt-treated plants from Sbikha. Similarly, PEG had no impact on ion content, beside a slight increase in Na+ concentrations. It could thus be concluded that organic compounds could assume a key function in the osmotic adjustment of Atriplex halimus, even if NO3− which could also plays a role in this respect (Song et al., 2009), still has to be quantified.
The present work shows that ABA is involved in both salt and osmotic stress response in the halophyte plant species Atriplex halimus but targets different physiological processes in response to the two types of environmental constraints. ABA contributes to osmotic stress resistance through an improvement of stomatal regulation and an increased WUE. In the presence of NaCl, ABA increases excretion of Na+ and Cl− in the external salt-bladders and free polyamine appear to be involved in this process.
ACKNOWLEDGEMENTS
This work was supported by the Fonds national de la Recherche Scientifique (FNRS; Convention no. 1·5·090·08). The authors are grateful to Tunisian authorities for the travel grant of Abir Ben Hassine. This work is dedicated to the memory of Prof. Gilles Guerrier.
LITERATURE CITED
- Alcázar R, Cuevas JC, Patron M, ltabella T, Tiburcio AF. Abscisic acid modulates polyamine metabolism under water stress in Arabidopsis thaliana. Physiologia Plantarum. 2006;128:448–455. [Google Scholar]
- Antolín MC, Santesteban HY, Santa María E, Aguirreolea J, Sánchez-Díaz M. Involvement of abscisic acid and polyamines in berry ripening of Vitis vinifera (L.) subjected to water deficit irrigation. Australian Journal of Grape and Wine Research. 2008;114:123–133. [Google Scholar]
- Arena ME, Pastur GM, Benavides MP, Curvetto N. Polyamines and inhibitors used in successive culture media for in vitro rooting in Berberis buxifolia. New Zealand Journal of Botany. 2005;43:373–380. [Google Scholar]
- Aslam Z, Jeschke WD, Barrettlennard EG, Setter TL, Watkin E, Greenway H. Effect of external NaCl on the growth of Atriplex amnicola and the ion relations and carbohydrate status of the leaves. Plant, Cell & Environment. 1986;9:571–580. [Google Scholar]
- Aurisano N, Bertani A, Mattana M, Reggiani R. Abscisic-acid induced stress-like polyamine pattern in wheat seedlings and its reversal by potassium ions. Physiologia Plantarum. 1993;89:687–692. [Google Scholar]
- Bakhanashvili M, Novitsky E, Levy I, Rahav G. The fidelity of DNA synthesis by human immunodeficiency virus type 1 reverse transcriptase increases in the presence of polyamines. FEBS Letters. 2005;579:1435–1440. doi: 10.1016/j.febslet.2005.01.043. [DOI] [PubMed] [Google Scholar]
- Balestrasse KB, Gallego SM, Benavides MP, Tomaro ML. Polyamines and proline are affected by cadmium stress in nodules and roots of soybean plants. Plant and Soil. 2005;270:343–353. [Google Scholar]
- Batanouny KH, Hassan AH, Fahmy GM. Ecophysiological studies on halophytes in arid and semiarid zonre. 2. Eco-physiology of Limonium delicatulum (Gir) Ktze. Flora. 1992;186:105–116. [Google Scholar]
- Bates LS, Waldren RP, Teare ID. Rapid determination of free proline for water-stress studies. Plant and Soil. 1973;39:205–207. [Google Scholar]
- Ben Hassine A, Ghanem ME, Bouzid S, Lutts S. An inland and a coastal population of the Mediterranean xero-halophyte species Atriplex halimus L. differ in their ability to accumulate proline and glycinebetaine in response to salinity and water stress. Journal of Experimental Botany. 2008;59:1315–1326. doi: 10.1093/jxb/ern040. [DOI] [PubMed] [Google Scholar]
- Bessieres MA, Gibon Y, Lefeuvre JC, Larher F. A single-step purification for glycine betaine determination in plant extracts by isocratic HPLC. Journal of Agricultural and Food Chemistry. 1999;47:3718–3722. doi: 10.1021/jf990031h. [DOI] [PubMed] [Google Scholar]
- Bittner F, Oreb M, Mendel RR. ABA3 is a molybdenum cofactor sulfurase required for activation of aldehyde oxidase and xanthine dehydrogenase in Arabidopsis thaliana. Journal of Biological Chemistry. 2001;276:40381–40384. doi: 10.1074/jbc.C100472200. [DOI] [PubMed] [Google Scholar]
- Bouchereau A, Aziz A, Larher F, Martin-Tanguy J. Polyamines and environmental challenges: recent development. Plant Science. 1999;140:103–125. [Google Scholar]
- Bray EA. Abscisic acid regulation of gene expression during water-deficit stress in the era of Arabidopsis genome. Plant, Cell & Environment. 2002;25:153–161. doi: 10.1046/j.1365-3040.2002.00746.x. [DOI] [PubMed] [Google Scholar]
- Breckle SW, Freitas H, Reimann C. Sampling Atriplex bladders: a comparison of methods. Plant, Cell & Environment. 1990;13:871–873. [Google Scholar]
- Colmer TD, Munns R, Flowers TJ. Improving salt tolerance of wheat and barley: future prospects. Australian Journal of Experimental Agriculture. 2005;45:1425–1443. [Google Scholar]
- Colmer TD, Flowers TJ, Munns R. Use of wild relatives to improve salt tolerance in wheat. Journal of Experimental Botany. 2006;57:1059–1078. doi: 10.1093/jxb/erj124. [DOI] [PubMed] [Google Scholar]
- Cuevas JC, López-Cobollo R, Alcázar R, et al. Putrescine is involved in Arabidopsis freezing tolerance and cold acclimation by regulating abscisic acid levels in response to low temperature. Plant Physiology. 2008;148:1094–1105. doi: 10.1104/pp.108.122945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djilianov D, Gerrits MM, Ivanova A, Vanonckelen HA, Deklerk GJM. ABA content and sensitivity during the development of dormancy in lily bulblets regenerated in vitro. Physiologia Plantarum. 1994;91:639–644. [Google Scholar]
- Flowers TJ, Colmer TD. Salinity tolerance in halophytes. New Phytologist. 2008;179:945–963. doi: 10.1111/j.1469-8137.2008.02531.x. [DOI] [PubMed] [Google Scholar]
- Freitas H, Breckle SW. Accumulation of nitrate in bladder hairs of Atriplex species. Plant Physiology and Biochemistry. 1993;31:887–892. [Google Scholar]
- Galston AW, Kaur-Sawhney R, Altabella T, Tinurcio AF. Plant polyamines in reproductive activity and response to abiotic stress. Botanica Acta. 1997;110:197–207. [Google Scholar]
- Gerhart VJ, Kane R, Glenn EP. Recycling industrial saline wastewater for landscape irrigation in a desert urban area. Journal of Arid Environments. 2006;67:473–476. [Google Scholar]
- Glenn EP, Pitelka LF, Olsen MW. The use of halophyte to sequester carbon. Water, Air and Soil Pollution. 1992;64:251–263. [Google Scholar]
- Gonzáles WL, Negritto MA, Suárez LH, Gianoli E. Induction of glandular and non-glandular bladders by damage in leaves of Madia sativa under contrasting water regimes. Acta Oecologica. 2008;33:128–132. [Google Scholar]
- Guerrier G, Patolia JS. Comparative salt responses of excised cotyledons and seedlings of pea to various osmotic and ionic stresses. Journal of Plant Physiology. 1989;135:330–337. [Google Scholar]
- Hoque MA, Okuma E, Banu MNA, Nakamura Y, Shimoishi Y, Murata Y. Exogenous proline mitigates the detrimental effects of salt stress more than exogenous betaine by increasing antioxidant enzyme activities. Journal of Plant Physiology. 2007;164:553–561. doi: 10.1016/j.jplph.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Jiménez-Bremont JF, Ruiz OA, Rodríguez-Kessler M. Modulation of spermidine and spermine levels in maize seedlings subjected to long term salt-stress. Plant Physiology and Biochemistry. 2007;45:812–821. doi: 10.1016/j.plaphy.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Karimi SH, Ungar IA. Development of epidermal salt hairs in Atriplex triangularis Willd. in response to salinity, light intensity and aeration. Botanical Gazette. 1989;150:68–71. [Google Scholar]
- Kowalczyk-Schröder S, Sandmann G. Interference of fluridone with the desaturation of phytone by membranes of the cyanobacterium Aphanocapsa. Pesticide Biochemistry and Physiology. 1992;42:7–12. [Google Scholar]
- Lee TM, Lur HS, Chu C. Role of abscisic acid in chilling tolerance of rice (Oryza sativa L.) seedlings. 2. Modulation of free polyamine levels. Plant Science. 1997;126:1–10. [Google Scholar]
- Lefèvre I, Gratia E, Lutts S. Discrimination between the ionic and osmotic components of salt stress in relation to free polyamine level in rice (Oryza sativa) Plant Science. 2001;161:943–952. [Google Scholar]
- Lefèvre I, Marchal G, Meerts P, Corréal E, Lutts S. Chloride salinity reduces cadmium accumulation by the Mediterranean halophyte species Atriplex halimus L. Environmental and Experimental Botany. 2009;65:142–152. [Google Scholar]
- Liu HP, Dong BH, Zhang YY, Liu ZP, Liu YL. Relationship between osmotic stress and the levels of free, conjugated and bound polyamines in leaves of wheat seedlings. Plant Science. 2004;166:1261–1267. [Google Scholar]
- Lutts S, Lefèvre I, Delpérée C, et al. Heavy metal accumulation by the halophyte species Mediterranean saltbush. Journal of Environmental Quality. 2004;33:1271–1279. doi: 10.2134/jeq2004.1271. [DOI] [PubMed] [Google Scholar]
- Ma S, Gong Q, Bohnert HJ. Dissecting salt stress pathways. Journal of Experimental Botany. 2006;57:1097–1108. doi: 10.1093/jxb/erj098. [DOI] [PubMed] [Google Scholar]
- Martínez JP, Kinet JM, Bajji M, Lutts S. NaCl alleviates polyethylene glycol-induced water stress in the halophyte species Atriplex halimus L. Journal of Experimental Botany. 2005;56:2421–2431. doi: 10.1093/jxb/eri235. [DOI] [PubMed] [Google Scholar]
- Mozafar A, Goodin JR. Vesiculated hairs: a mechanism for salt tolerance in Atriplex halimus. Plant Physiology. 1970;45:62–65. doi: 10.1104/pp.45.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munns R. Comparative physiology of salt and water stress. Plant, Cell & Environment. 2002;25:239–250. doi: 10.1046/j.0016-8025.2001.00808.x. [DOI] [PubMed] [Google Scholar]
- Ndayirajige A. Belgium: Université catholique de Louvain; 2006. Relations entre métabolisme des polyamines et résistance au stress salin chez le riz (Oryza sativa L.) PhD Thesis. [Google Scholar]
- Ohnishi N, Murata N. Glycinebetaine counteracts the inhibitory effect of salt stress on the degradation and synthesis of the D1 protein during photo inhibition of Synechococus sp. PCC7942. Plant Physiology. 2006;141:758–765. doi: 10.1104/pp.106.076976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin X, Zeevaart JAD. Overexpression of a 9-cis-epoxycarotenoid dioxygenase gene in Nicotiana plumbaginifolia increases abscisic acid and phaseic acid levels and enhances drought tolerance. Plant Physiology. 2002;128:544–551. doi: 10.1104/pp.010663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadan T. Ecophysiology of salt excretion in the xero-halophye Reaumuria hirtella. New Phytologist. 1998;139:273–281. [Google Scholar]
- Ren H, Gao Z, Chen L, et al. Dynamic analysis of ABA accumulation in relation to the rate of ABA catabolism in maize tissues under water deficit. Journal of Experimental Botany. 2007;58:211–219. doi: 10.1093/jxb/erl117. [DOI] [PubMed] [Google Scholar]
- Rontein D, Basset G, Hanson AD. Metabolic engineering of osmoprotectant accumulation in plants. Metabolic Engineering. 2002;4:49–56. doi: 10.1006/mben.2001.0208. [DOI] [PubMed] [Google Scholar]
- Roy P, Niyogi K, SenGupta DN, Ghosh B. Spermidine treatment to rice seedlings recovers salinity stress-induced damage of plasma membrane and PM-bound H+-ATPase in salt-tolerant and salt-sensitive rice cultivars. Plant Science. 2005;168:583–591. [Google Scholar]
- SAS Institute. SAS/GRAPH software. Cary, NC: SAS Institute Inc; 2002. version 9.1. [Google Scholar]
- Sfichi L, Joannidis N, Kotzabasis K. Thylakoid-associated polyamines adjust the UV-B sensitivity of the photosynthetic apparatus by means of light-harvesting complex II changes. Photochemistry and Photobiology. 2004;80:499–506. doi: 10.1562/0031-8655(2004)080<0499:TPATUS>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Shabala S, Cuin TA, Pottosin I. Polyamines prevent NaCl-induced K+ efflux from pea mesophyll by blocking non-selective cation channels. FEBS Letters. 2007;581:1993–1999. doi: 10.1016/j.febslet.2007.04.032. [DOI] [PubMed] [Google Scholar]
- Smith MA, Davies PJ. Separation and quantification of polyamines in plant tissues by high performance liquid chromatography of their dansyl derivaties. Plant Physiology. 1985;78:89–91. doi: 10.1104/pp.78.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Chen M, Feng G, Jia Y, Wang B, Zhang F. Effect of salinity on growth, ion accumulation and the roles of ions in osmotic adjustment of two populations of Suaeda salsa. Plant and Soil. 2009;314:133–141. [Google Scholar]
- Verslues PE, Bray EA. Role of abscisic acid (ABA) and Arabidopsis thaliana ABA-insensitive loci in low water potential-induced ABA and proline accumulation. Journal of Experimental Botany. 2006;57:201–212. doi: 10.1093/jxb/erj026. [DOI] [PubMed] [Google Scholar]
- Xiong L, Zhu J. Regulation of abscisic acid biosynthesis. Plant Physiology. 2003;133:29–36. doi: 10.1104/pp.103.025395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zdunek-Zastocka E. Molecular cloning, characterization and expression analysis of three aldehyde oxidase genes from Pisum sativum L. Plant Physiology and Biochemistry. 2008;46:19–28. doi: 10.1016/j.plaphy.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Zhang X, Oppenheimer DG. A simple and efficient method for isolating trichomes for downstream analyses. Plant Cell Physiology. 2004;45:221–224. doi: 10.1093/pcp/pch016. [DOI] [PubMed] [Google Scholar]
- Zhao FG, Qin P. Protective effect of exogenous polyamines on root tonoplast function against salt stress in barley seedlings. Plant Growth Regulation. 2004;42:97–103. [Google Scholar]