Abstract
The role of TLR2 in modulating experimentally induced asthma is not fully understood. We recently identified that German cockroach (GC) frass contains a TLR2 ligand allowing us to investigate the role of a TLR2 agonist in a complex real world allergen in mediating allergic airway inflammation. GC frass exposure significantly increased airway inflammation, airway hyperresponsiveness and serum IgE levels in wild type mice; however the same exposure in TLR2-deficient mice resulted in greatly exaggerated serum IgE and eosinophilia but diminished airway neutrophilia, suggesting a protective role for TLR2. Since GC frass inhalation usually induces airway neutrophilia, we queried the effect of neutrophil depletion on airway responses. Inhibition of neutrophil recruitment into the airways of naïve wild type mice prior to intratracheal inhalation of GC frass resulted in significantly increased levels of serum IgE and eosinophilia. Neutrophils are a rich source of MMP-9, and we found that MMP-9 levels were significantly increased in the airways of mice following exposure to GC frass. Importantly the levels of MMP-9 were significantly decreased in neutrophil-depleted and TLR2-deficient mice after exposure to GC frass, suggesting that TLR2 regulated MMP-9 release from neutrophils. Functionally, MMP-9-deficient mice had more acute allergic inflammation than wild type mice, suggesting that MMP-9 was protective against experimentally-induced asthma. These data suggest that TLR2 activation of neutrophils leads to release of MMP-9 which decreases allergic responses to GC frass. This suggests a protective role for TLR2 activation and MMP-9 release in the context of experimentally-induced asthma in mice.
Introduction
Asthma is a chronic inflammatory condition of the airways, characterized by airways hyperresponsiveness (AHR), persistent inflammation of the airways, increased serum IgE, excessive production of mucus and airway remodeling. Asthma is also associated with the propensity for allergic T helper type 2 (Th2) cell cytokine responses. While there is a genetic predisposition for asthma, this cannot account for the significant increase in asthma prevalence over the past 20 years. Environmental factors, including house dust mite, cockroach and cat exposure are thought to play a significant role in the increase in asthma (1). German cockroaches (GC; Blattella germanica) are the species which commonly infest homes in the United States. Cockroach allergens are important sensitizing agents, particularly in individuals who live in substandard, multi-family dwellings located in highly populated areas (2). Early life exposure to cockroach allergen was shown to predict allergen-specific responses by 2 years of age (3). Feces (frass) from cockroaches was purported to be the sensitizing agent (4), and in fact our laboratory has recently confirmed that GC frass can induce allergic inflammation in mice (5). Together these studies highlight the importance of cockroach in asthma pathogenesis and substantiate the use of GC frass in the current study.
Activation of the innate immune response is necessary for the induction of adaptive immunity. The innate immune system has evolved to recognize pathogen-associated molecular patterns (PAMPs), which are common to many classes of pathogens. PAMPs are recognized by pathogen recognition receptors, which include toll like receptors (TLRs). TLRs have been shown to play a role in both the early phase of antigen recognition as well as in adaptive immunity (6). We have recently shown that GC frass contains a TLR2 ligand (7); however the role of TLR2 in modulating the asthma phenotype is controversial. Previous studies have suggested that TLR2 may play a role in the initiation of asthma as selective TLR2 ligands administered during sensitization with ovalbumin (OVA) enhanced Th2-mediated allergic inflammation (8, 9). However, other studies found that administration of TLR2 ligands inhibit OVA-induced Th2-mediated allergic responses (10) and inhibit OVA-induced established allergic airway inflammation by increasing the Th1 response (11). A recent report showed that addition of exogenous TLR2 ligands inhibited Th2 cytokine secretion from house dust mite-stimulated human peripheral blood mononuclear cells from atopic individuals (12). The same study also showed increase of the co-stimulatory marker major histocompatibility complex (MHC)II on myeloid dendritic cells following treatment with a TLR2 ligand. Collectively, these studies suggested that TLR2 plays a protective role in allergic asthma. Some of these discrepancies may be explained by the timing of the addition of the TLR2 ligand in the context of sensitization. To date, no one has ever investigated the effect of a TLR2 agonist inherent in a complex allergen.
Neutrophils play a crucial role in the innate host response. Our recent studies showed that the TLR2 agonist in GC frass activated neutrophil cytokine production (7). Other studies have found similar activation of neutrophils by selective TLR2 agonists. Treatment of bone marrow-derived neutrophils with Pam3Cys, a selective TLR2 agonist, significantly increased cytokine expression in a nuclear factor (NF)-κB dependent manner (13). Blocking TLR2 prior to treatment of human neutrophils with Helicobacter pylori resulted in decreased levels of cytokine expression (14). These data suggest that the early inflammatory response may be mediated by TLR2 activation on neutrophils. Numerous studies have identified a population of noneosinophilic asthmatics which appears to be associated with increased airway neutrophilia (reviewed in (15)). Neutrophilic asthma has been associated with increased innate immune responses and increased TLR2 expression (16). While still unclear, neutrophilic inflammation may play an important role in mediating the asthma phenotype, possibly by altering the innate immune response to allergens through the engagement and activation of TLR2.
Neutrophils are the richest source of matrix metalloproteinase (MMP)-9 in the body (roughly 3 μg per million neutrophils) (17). Matrix metalloproteinase (MMP)-9 belongs to a family of proteases that function in the degradation of several extracellular matrix components, and may play a role in airway remodeling. Levels of MMP-9 are significantly increased in the bronchoalveolar lavage (BAL) fluid, blood and sputum of asthmatics (18-21). MMP-9 has been shown to be released from neutrophils following stimulation with the pro-inflammatory cytokine interleukin (IL)-8 (22). MMP-9 can be inhibited by endogenous inhibitor tissue inhibitor of metalloproteinase (TIMP)-1; however neutrophils uniquely release TIMP-free MMP-9 (23). The overall role of MMP-9 in asthma pathogenesis has been investigated using the OVA model of allergic airway inflammation. One group found that MMP-9 deficient mice had impaired cellular infiltration, Th2 cytokine expression and AHR following OVA treatment (24), while another group showed that MMP-9 deficiency resulted in enhanced allergic inflammation in mice also following OVA treatment (25). The differences between these findings were suggested to be due to the level of airway inflammation, and eosinophilia between the two methods of OVA exposure (25). Therefore, the role of MMP-9 in modulating airway responses is still unclear, however the effect of MMP-9 in regulating airway inflammation has never been studied using a real world allergen.
We are in a unique position to query the role of TLR2 activation in modulating the innate and adaptive immune response to a complex real world allergen. In this report, we focus on identifying the role that the TLR2 ligand in GC frass has on regulation of experimentally-induced airway inflammation. We will investigate the role of neutrophil influx following GC frass exposure, and investigate the role of MMP-9 in regulating the asthma phenotype.
Materials and Methods
Cockroach frass and house dust mite
Fecal remnants (frass) were collected from German cockroaches (Blattella germanica) and reconstituted as previously described (26). To cleave lipoproteins from GC frass to remove a potential TLR2 ligand, GC frass or PBS was incubated with lipoprotein lipase (1000 U/mg GC frass for 1 h at 37°C) prior to treatment of cells.
Animals
Six-to-eight week old female Balb/c, C57Bl/6, FVB and MMP-9-deficient mice were obtained from Jackson Laboratory (Bar Harbor, ME) and housed in a laminar hood in a virus-free animal facility. TLR2-deficient mice were obtained from Dr. S. Akira (27). Mice were anesthetized with ketamine (45mg/kg)/xylazine (8 mg/kg) before PBS (40 μl) or GC frass (40 μg/40 μl) exposure by a single intratracheal (i.t.) inhalation as previously described (28). Mice were given a lethal dose of sodium pentobarbital 3 or 18 h later. In some experiments, wild type (Balb/c or FVB) or MMP-9-deficient mice were given three challenges of PBS or GC frass on days 0, 7, and 14 and harvested on day 17. For one experiment, mice were administered an intraperitoneal (i.p) injection with the anti-granulocyte mAb RB6-8C5 antibody (BD Pharmingen, San Jose, CA) or isotype control antibody at a concentration of 100 μg/mouse (7, 29) 24 h before inhalation. In experiments using the C57Bl/6 strain of mice, which are not responsive to sensitization by i.t. inhalation (5), we immunized wild type and TLR2-deficient mice with PBS or GC frass (10 μg/ml) bound to alum (Imject Alum; Pierce Biotechnology, Rockford, IL) on day 0 and 7, followed by i.t. inhalation challenges with PBS or GC frass (40 μg/40 μl) on days 14 and 19. Mice were harvested on day 22 (5). Animal care was provided in accordance with NIH guidelines. These studies were approved by the Cincinnati Children's Hospital Medical Center Institutional Animal Care and Use Committee.
Airway hyperresponsiveness measurements
Allergen-induced AHR was determined as we have previously described (30). Briefly, mice were anesthetized 72 hours after the last GC frass exposure, intubated and ventilated at a rate of 120 breaths per minute with a constant tidal volume of air (0.2 ml), and paralyzed with decamethonium bromide (25 mg/kg). After establishment of a stable airway pressure, 25 μg/kg weight of acetylcholine was injected i.v. and dynamic airway pressure (airway pressure time index [APTI] in cm-H2O × sec−1) was followed for 5 minutes.
Cytokine production
Liberase/DNase I digests of the lung were prepared to obtain single lung cell suspensions. Single cell suspensions (2.5 × 105) were incubated for 72 hours in culture medium (RPMI) treated with Conconavalin A (10 μg/ml) and supernatants were analyzed for IL-5 and IL-13 levels by ELISA (R&D Systems, Minneapolis, MN) (28).
Assessment of airway inflammation
Lungs were lavaged thoroughly with 1 ml of Hanks balanced salt solution (HBSS) without calcium or magnesium. The lavage fluid was centrifuged (1,800 rpm for 10 min), the supernatant was removed for cytokine analysis and immediately stored at -80°C. Total cell numbers were counted on a hemocytometer. Smears of BAL cells prepared with a Cytospin II (Shandon) were stained with Diff-Quick solution for differential cell counting. BAL fluid was analyzed by ELISA according to the manufacturer's specifications (R&D Systems).
Serum IgE and IgG1 levels
Sera was obtained from blood taken during exsanguination of the animals after airway measurements. ELISAs for IgE and IgG1 were performed as previously described (31). For frass-specific IgE, ELISA plates were coated with 50 ml of GC frass (100 mg/ml) in HBSS overnight at room temp. The remainder of the ELISA is performed as previously described (31). Plates were read at 405 nm.
Lung histology
Whole lungs were removed and formalin fixed. Lungs were embedded in paraffin, sectioned and stained with Periodic Acid Schiff (PAS).
MMP-9 analysis
BAL fluid was subject to gelatin zymography on an 8% SDS-PAGE containing 1 mg/ml gelatin under non-reducing conditions as described previously (26). Mature (active) MMP-9 was determined by Western blot under reducing conditions (R&D Systems).
Cell culture
HL-60 promyelocytic leukemia cells (ATCC, Manassas, VA) were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum 50 μg/ml streptomycin, 2 U/mL penicillin, and 2 mM l-glutamine. For differentiation, cells (1 × 106/ml) were incubated in the presence of 1% DMSO for 3 days. Cells were centrifuged, washed and deprived of serum for 6 h. Cells were treated with GC frass (100 ng/ml) or the TLR2-specific agonist Pam3Cys-Ser-(Lys)4 hydrochloride (1 μg/ml; Calbiochem, San Diego, CA) for 16 h. In some cases, cells were pretreated for 1h with DMSO or isohelenin (30 μM; EMD Biosciences, San Diego, CA) prior to treatment with GC frass. Supernatants were clarified and analyzed for MMP-9 by ELISA (R&D Systems).
Mouse bone marrow-derived neutrophils
Femurs and tibias were removed from C57Bl/6 or TLR2 deficient mice. Bone marrow was isolated, rinsed and red blood cells are lysed. Resuspended cells were layered onto a three step Percoll gradient (52%, 64%, 72%) and centrifuged (1,000 rpm for 30 min at RT). The bottom layer (64%-72%) containing neutrophils was collected, counted and plated. Cells were treated with GC frass (300 ng/ml) or TNFα (10 ng/ml; R&D Systems) for 18 h and supernatants analyzed by ELISA.
Isolation of primary human neutrophils
Following approval by the IRB and with informed consent, blood was collected using sterile technique from healthy volunteers for isolation of polymorphonuclear leukocytes. Blood was collected into heparinized vacutainers and then subjected to Dextran T5000 sedimentation, Ficoll-Histopaque density gradient centrifugation, and hypotonic erythrocyte lysis, as previously described (32). Cells were resuspended in serum deprived media and treated with GC frass (300 ng/ml) for 18 h and supernatants analyzed by ELISA.
Statistical analysis
When applicable, statistical significance was assessed by one-way analysis of variance (ANOVA). Differences identified by ANOVA were pinpointed by Student-Newman-Keuls' multiple range test.
Results
TLR2-deficient mice had increased serum IgE and eosinophilia in experimentally-induced asthma
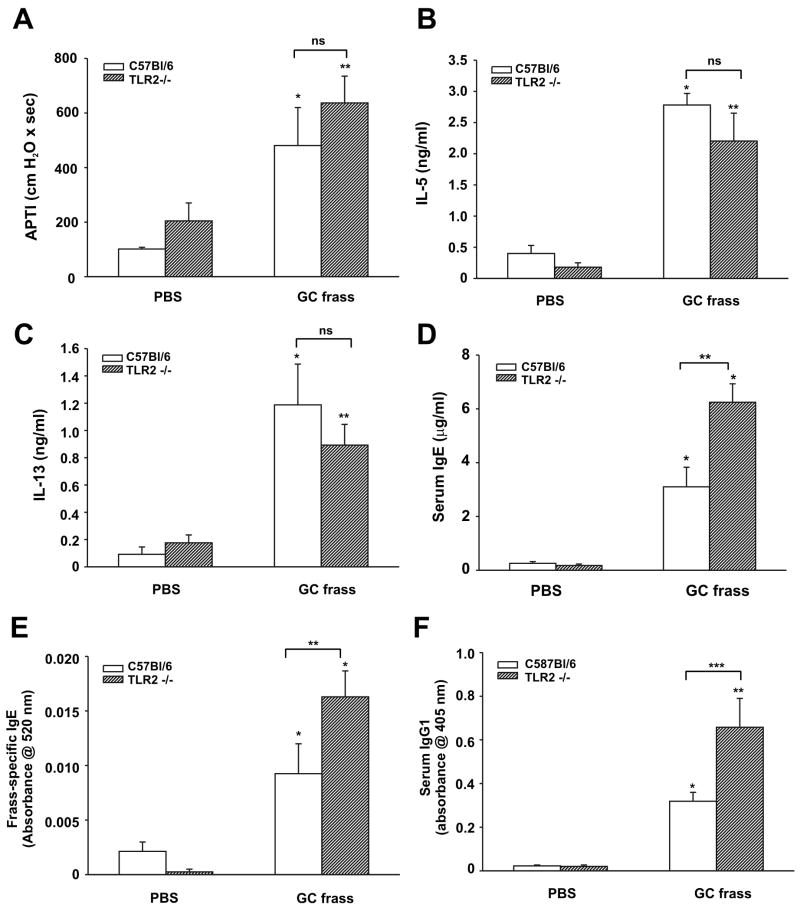
Since GC frass contains a TLR2 ligand (7), we queried the involvement of TLR2 activation in regulating experimentally-induced asthma in our mouse model. We sensitized wild type and TLR2-deficient mice via intraperitoneal injection of GC frass bound to alum and challenged with intratracheal inhalation of GC frass. In line with our previous report (5), sensitization and challenge of GC frass significantly increased the following parameters; airway responsiveness to cholinergic agents (Figure 1A), Th2 cytokine expression IL-5 and IL-13 (Figure 1 B&C), serum IgE levels (Figure 1D), GC frass-specific IgE levels (Figure 1E), serum IgG1 levels (Figure 1F), and cellular infiltration into the BAL fluid (Table 1). Importantly, TLR2-deficiency altered some aspects of experimentally-induced asthma. For example, airway responsiveness (Figure 1A) and levels of Th2 cytokines (Figure 1 B&C) were not significantly altered in TLR2-deficient mice; while levels of serum IgE (Figure 1D), GC frass-specific IgE (Figure 1E), serum IgG1 (Figure 1F), and infiltration of eosinophils (Table 1) were significantly increased. Infiltration of tissues with neutrophils, lymphocytes, and macrophages were significantly decreased in the TLR2-deficient mice (Table 1). These results suggest that TLR2 plays a role in some aspects of allergen-induced allergic inflammation.
Figure 1.
GC frass-induced experimental allergic asthma in TLR2-deficient mice. C57Bl/6 and TLR2-deficient mice were sensitized on day 0 and 7 with an intraperitoneal injection of PBS or GC frass (10 μg/ml) bound to alum. On day 14 and 19, an intratracheal inhalation was performed using PBS (40 μl) or GC frass (40 μg/40 μl). On day 22, mice were anesthetized and acetylcholine was injected after establishment of a stable airway pressure. Blood was collected and BAL fluid was harvested. In all cases, means ± SEM (n=6-8 mice per group) were reported. Statistical significance was determined by ANOVA. A. AHR was measured as airway pressure time index (APTI) in cm-H2O × sec -1 (compared to PBS *p=0.019, **p=0.003). B. IL-5 levels (compared to PBS *p=0.001, **p=0.004). C. IL-13 levels (compared to PBS *p=0.004, **p=0.013). D. Serum IgE levels (compared to PBS *p<0.001; compared to GC frass **p<0.001). E. Frass-specific IgE levels (compared to PBS *p<0.001; compared to GC frass **p=0.019). F. Serum IgG1 levels (compared to PBS *p0.025, ** p<0.001; compared to GC frass ***p=0.007).
Table 1. Differential cell count in BAL fluid of sensitized and challenged wild type (C57Bl/6) and TLR2-deficient (TLR2-/-) mice.
C57Bl/6 mice were given intraperitoneal injections of PBS or GC frass with alum on day 0 and 7. Intratracheal inhalations of PBS or GC frass were performed on days 14 and 19. On day 22, BAL fluid was harvested and differential cell counts performed. These data represent 6-8 mice per group and are expressed as mean ± SEM of cell number × 104. Cell counts were statistically significant between GC frass in the TLR2-deficient mice compared to wild type mice (*p<0.001, **p=0.003, as determined by ANOVA).
| Mac | Epi | Eos | Neut | Lymph | |
|---|---|---|---|---|---|
| C57-PBS | 3.4±1.1 | 3.7±1.4 | 0.03±0.02 | 0.03±0.02 | 0.02±0.006 |
| TLR2-/- PBS | 10.1±2.7 | 6.1±1.7 | 0.014±0.008 | 0.073±0.03 | 0.07±0.06 |
| C57-frass | 95.6±22.7 | 20.5±6.4 | 21.4±8.1 | 55.1±17.3 | 37.25±10.2 |
| TLR2-/- frass | 26.8±2.5* | 3.3±0.3** | 55.8±12.3** | 3.8±1.0* | 5.2±0.7* |
The role of neutrophils in regulating experimentally-induced asthma
We have recently shown that inhalation of GC frass induces neutrophilia. Since inhalation of GC frass normally induces airway neutrophilia (7), and we detect a decrease in neutrophilia in the TLR2-deficient mice, we queried the role of early neutrophil infiltration on mediating allergic inflammation. To do this, we used a protocol we have previously shown to abolish neutrophil infiltration following GC frass inhalation (7). RB6-8C5, an antibody that depletes circulating neutrophils (29) was administered by intraperitoneal injection 24 h before each intratracheal challenge with GC frass. We have previously shown this to completely abolish GC frass-induced neutrophil recruitment into the airways (7). In this experiment, we used Balb/c mice, which we have shown exhibit airway inflammation and hyperresponsiveness to GC frass by inhalation only (5). Mice were given an injection of the isotype control antibody or RB6-8C5 on day -1, 6 and 13, and intratracheal inhalations on day 0, 7, and 14. Due to the fact that PBS-treated mice do not have increased neutrophilia in their airways or elicit experimentally-induced allergic inflammation, and due to the cost of the antibody, we only pretreated mice with the R6B-8C5 antibody that were given intratracheal inhalations of GC frass. On day 17, airway responsiveness, airway inflammation and cellular infiltration into the BAL fluid was measured. As expected, GC frass induced airway hyperresponsiveness, Th2 cytokine production, increased serum IgE levels and cellular infiltration into the airways (data not shown). Inhibiting neutrophilia in the airways following sensitization and challenge with GC frass had no effect on airway hyperresponsiveness to acetylcholine (data not shown), or Th2 cytokines (data not shown). However, there was a significant increase in serum IgE levels (control antibody and PBS 133.3 ±58.9 ng/ml; control antibody and GC frass 654.5 ± 93.8 ng/ml [*p=0.006 compared to control antibody] and PBS; RB6-8C5 antibody and GC frass 1621.6 ± 168 ng/ml [*p<0.001 compared to control antibody and GC frass]) and eosinophilia (Table 2), similar to that found in the TLR2-deficient mice. These data correlate with the TLR2-deficient mouse data in that neither TLR2-deficiency nor depletion of neutrophils altered airway responsiveness or Th2 cytokine production, but both conditions significantly increased serum IgE levels and eosinophilia, and decreased neutrophilia. These data suggest that TLR2 and early neutrophil recruitment into the airways may play a protective role in allergic inflammation.
Table 2. Differential cell count in BAL fluid of Balb/c mice pretreated with a neutrophil-depleting antibody.
Balb/c mice were given an intraperitoneal injection of isotype control antibody or RB6-8C5 antibody on days -1, 6, and 13, while an intratracheal inhalation was performed with PBS or GC frass (40 μg/40 μl) on days 0, 7, and 14. On day 17, BAL fluid was harvested and differential cell counts performed. These data represent 4 mice per group and are expressed as mean ± SEM of cell number × 104. Statistical significance between isotype control Ab-treated mice and RB6-8C5 Ab-treated mice are shown (*p=0.005, **p=0.015, as determined by ANOVA).
| Mac | Epi | Eos | Neut | Lymph | |
|---|---|---|---|---|---|
| Cont Ab/PBS | 5.8±0.8 | 3.1±1.2 | 0 | 0 | 0.23±0.18 |
| Cont Ab/frass | 21.9±3.8 | 1.5±0.7 | 1.5±0.06 | 4.9±0.6 | 5.9±0.8 |
| RB6 Ab/frass | 21.9±3.2 | 2.2±0.6 | 7.2±1.9* | 1.3±0.3** | 7.7±1.8 |
GC frass-induced neutrophil influx is responsible for increased MMP-9 expression
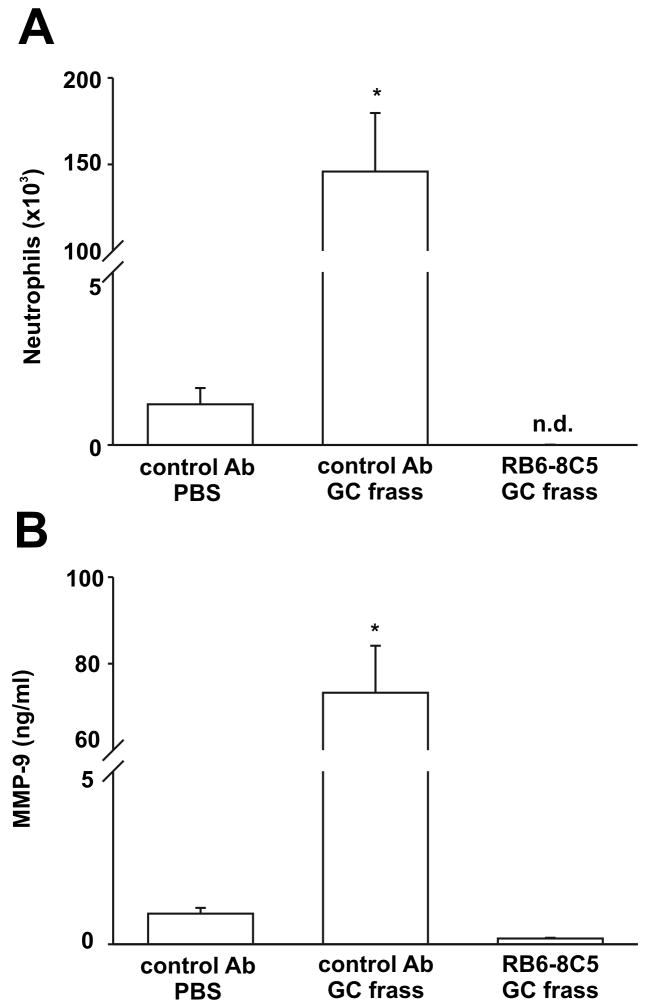
Neutrophils are a rich source of MMP-9, so we queried whether MMP-9 levels were affected by neutrophil depletion. To test this, we first pretreated mice with the RB6-8C5 antibody for 24 hours prior to a single intratracheal inhalation of GC frass. Not surprisingly, GC frass inhalation significantly increased neutrophil numbers in the airways of mice 3 hours post inhalation. Pretreatment with RB6-8C5 totally abolished GC frass-induced neutrophil accumulation in the airways (Figure 2A). Concurrent with the decrease in neutrophils, the concentration of MMP-9 in the BAL fluid was significantly decreased to below baseline levels (Figure 2B). These data suggest that newly recruited airway neutrophils release significant concentrations of MMP-9 in the BAL fluid of mice following an intratracheal inhalation.
Figure 2.
Neutrophil recruitment directly affected MMP-9 release into the airways. Balb/c mice were given a single injection of control Ab or RB65-8C5 (100 μg/mouse) 24h prior to a single intratracheal inhalation of PBS (40μl) or GC frass (40μg/40μl). 3 h later, mice were given a lethal dose of sodium pentobarbital and BAL fluid was harvested. Neutrophils were quantified following differential staining and a MMP-9 ELISA was performed on the BAL fluid. In all cases, means ± SEM (n=6 mice per group) were reported. Statistical significance was determined by ANOVA. A. Neutrophil influx into BAL fluid (*p<0.001) (nd=none detected). B. MMP-9 ELISA of BAL fluid (*p<0.001).
MMP-9 levels in BAL fluid
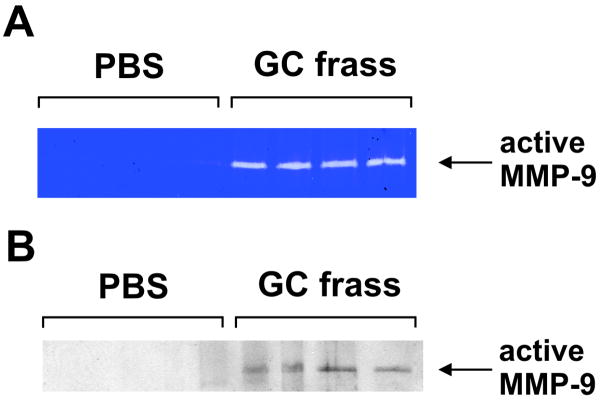
Next, we examined MMP-9 expression in the BAL fluid 3 and 18 hours following a single intratracheal inhalation of PBS or GC frass in naïve Balb/c mice. Three hours post-inhalation, MMP-9 and its natural inhibitor TIMP-1 were significantly upregulated in the GC frass-treated mice as determined by ELISA (Table 3). By 18 hours post inhalation of GC frass MMP-9 levels were lower (25 ± 1.5 ng/ml, n= 7 mice compared to undetectable levels in PBS-treated mice) but were still significantly increased compared to PBS-treated mice. While MMP-9 levels were higher at 3 hours, the next set of experiments was run at 18 hours since active proteases in GC frass also showed up on the gelatin zymogram and made analysis difficult. Gelatin zymography of BAL samples revealed MMP-9 digestion of the gelatin impregnated gel compared to PBS-treated mice (Figure 3A). In addition, active MMP-9 was detected in only the GC frass-treated BAL samples and not BAL from PBS treated mice (Figure 3B). These data show that MMP-9 was significantly increased in the airways of mice following GC frass exposure.
Table 3. Concentration of MMP-9 and TIMP-1 in BAL fluid of naïve Balb/c mice following a single intratracheal inhalation of PBS or GC frass.
Balb/c mice were given a single intratracheal inhalation of PBS (40 μl) or GC frass (40 μg/40 μl). Three hours later, mice were killed and BAL fluid was harvested, clarified and run on ELISA for MMP-9 and TIMP-1. These data represent 9 mice per group and are expressed as mean ± SEM (compared to PBS, GC frass treatment significantly increased MMP-9 and TIMP-1 levels *p<0.001; ANOVA).
| PBS | frass | p value | |
|---|---|---|---|
| MMP-9 (ng/ml) | 1.56±0.3 | 84.6±10.1 | *<0.001 |
| TIMP-1 (ng/ml) | 0.13±0.02 | 0.43±0.06 | *<0.001 |
| ratio M/T | 11.4 | 198.6 |
Figure 3.
MMP-9 levels were increased in BAL fluid of GC frass-treated mice. Balb/c mice were given a single intratracheal inhalation of PBS or GC frass and BAL fluid was harvested 18h later. MMP-9 activity was measured in the BAL fluid as determined by gelatin zymogram (A) or Western blot analysis using an antibody against the mature (active) form of MMP-9 (B).
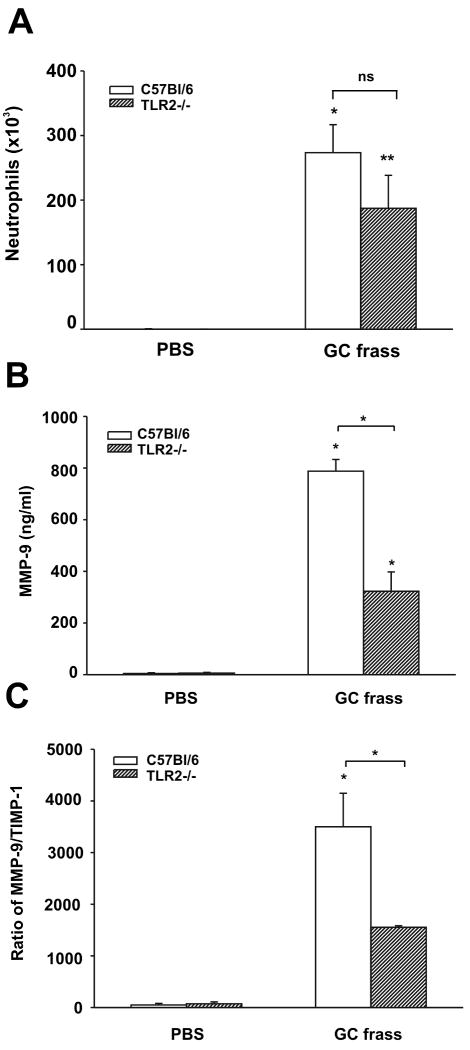
TLR2 partially mediates MMP-9 expression in mouse airways
To test the role of TLR2 in GC frass-induced release of MMP-9 into the BAL fluid, we administered GC frass with a single intratracheal inhalation in wild type and TLR2-/- mice. Neutrophil infiltration into the airways was slightly but not significantly decreased following GC frass treatment in TLR2-deficient mice 3 hours following GC frass inhalation (Figure 4A). GC frass inhalation induced a significant increase in MMP-9 and MMP-9:TIMP levels which was significantly attenuated in the TLR2-/- mice (Figure 4B and 4C). A similar decrease in MMP-9 levels is also seen 18 hours following GC frass inhalation in TLR2 -/- mice compared to wild type controls (data not shown). These data suggest that while TLR2 is not required for neutrophil recruitment into the airways, it is necessary for neutrophil-derived MMP-9 release. Therefore, we asked whether MMP-9 levels were altered in the TLR2-deficient mice sensitized and challenged with GC frass (refer to Figure 1). We found that sensitization to GC frass induced significant levels of MMP-9 (frass exposed 1084.3 ± 386.4 pg/ml compared to PBS 80.3 ± 75.1 pg/ml *p=0.01 n=6-8 mice per group) in wild type mice. TLR2-deficient mice sensitized to GC frass had significantly lower amounts of MMP-9 in their BAL fluid (frass exposed 345.8 ± 163.2 pg/ml compared to PBS 54.6 ± 31pg/ml *p=0.02 n=6-8 mice per group). Together, these data suggest that TLR2 activation resulted in the release of MMP-9 into the BAL fluid of mice.
Figure 4.
Neutrophil accumulation and MMP-9 levels in the airways of TLR2-deficient mice. Wild type (C57Bl/6) and TLR2-deficient mice were given a single intratracheal inhalation of PBS (40 μl) or GC frass (40 μg/40 μl) and 3 h later BAL fluid was harvested and neutrophils were quantified following differential staining or ELISA was performed. In all cases, means ± SEM (n=6 mice per group) were reported. Statistical significance was determined by ANOVA. A. Neutrophil influx into BAL fluid (compared to PBS *p=0.002, **p=0.017; ns= not significant). B. MMP-9 ELISA of BAL fluid (*p<0.001). C. Ratio of MMP-9 to TIMP-1 (*p<0.001).
TLR2 activation directly affects MMP-9 expression/release from neutrophils
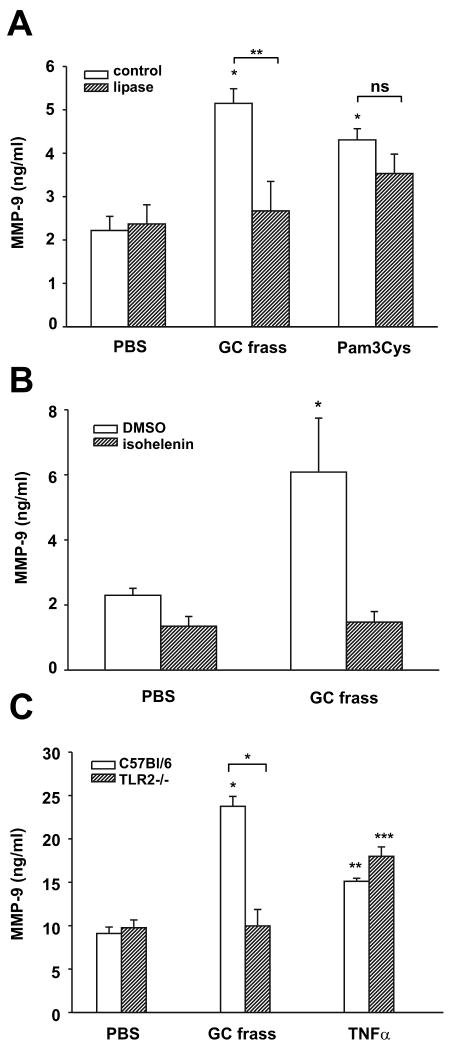
We have recently shown that neutrophils express TLR2 on their surface by flow cytometry (7). To show that GC frass induced the release of MMP-9 from neutrophils, we isolated primary human neutrophils from four normal donors and treated them ex vivo with GC frass for 4 hours. We found that GC frass treatment resulted in the release of MMP-9 (42.3±6.8 ng/ml compared to control 12.7 ±2.5 ng/ml; n=4, *p=0.007). We confirmed these data using DMSO-differentiated human HL-60 cells. Treatment of HL-60 cells with GC frass or the selective TLR2 agonist Pam3Cys both resulted in a significant release of MMP-9 into the media (Figure 5A). Of note, removal of the TLR2 agonist from GC frass using lipoprotein lipase resulted in attenuated GC frass-induced MMP-9 expression (Figure 5A), but had no effect on Pam3Cys-induced MMP-9 expression. Since we have previously shown that GC frass induced NF-κB translocation and activation in human neutrophils, we asked whether GC frass-induced regulation of MMP-9 expression was dependent on activation of NF-κB. To do this, we pretreated HL-60 cells with the NF-κB inhibitor isohelenin, prior to the addition of GC frass. Inhibition of NF-κB activation abolished GC frass-induced MMP-9 expression, suggesting the mechanism by which GC frass increased MMP-9 (Figure 5B). To directly address a difference in the amount of MMP-9 released from wild type and TLR2-deficient neutrophils, bone marrow-derived neutrophils were isolated and treated in the absence or presence of GC frass. GC frass treatment resulted in increased levels of MMP-9 in wild type, but not in TLR2-deficient bone marrow-derived neutrophils (Figure 5C). There was no defect in TLR2-deficient mice synthesizing or releasing MMP-9 as evidenced by the fact that control levels of MMP-9 and MMP-9 levels following TNFα treatment were comparable between wild type and TLR2-deficient mice. Together these data show that GC frass can directly regulate MMP-9 release from neutrophils and shows that the TLR2 ligand in GC frass is an important component for the release of MMP-9.
Figure 5.
GC frass and a TLR2 agonist induced MMP-9 release from neutrophils. A. HL-60 cells were treated with PBS, GC frass, Pam-3-Cys (1 μg/ml) with and without pretreatment with lipoprotein lipase (1000U/mg) for 18 h. Supernatants were harvested, clarified, and run on a MMP-9 ELISA. Data are expressed as means ± SEM for 3 separate experiments (compared to PBS *p<0.001; compared to GC frass **p=0.008, ns= not significant). B. HL-60 cells were pretreated with DMSO or isohelenin (30 μM) for 1 h prior to treatment with GC frass for 18 h. Supernatants were harvested, clarified, and run on a MMP-9 ELISA. Data are expressed as means ± SEM for 3 separate experiments (compared to PBS *p=0.006). C. Bone marrow-derived neutrophils from wild type (C57Bl/6) and TLR2-deficient mice were harvested and treated ex vivo with PBS or GC frass (300 ng/ml) or TNFα (10 ng/ml). 18 h later, supernatants were collected, clarified and analyzed for MMP-9 by ELISA. These data are representative of 3 separate experiments (compared to PBS *p<0.001, **p=0.04, ***p=0.005; compared to wild type *p>0.001).
Induced allergic inflammation is increased in MMP-9-deficient mice
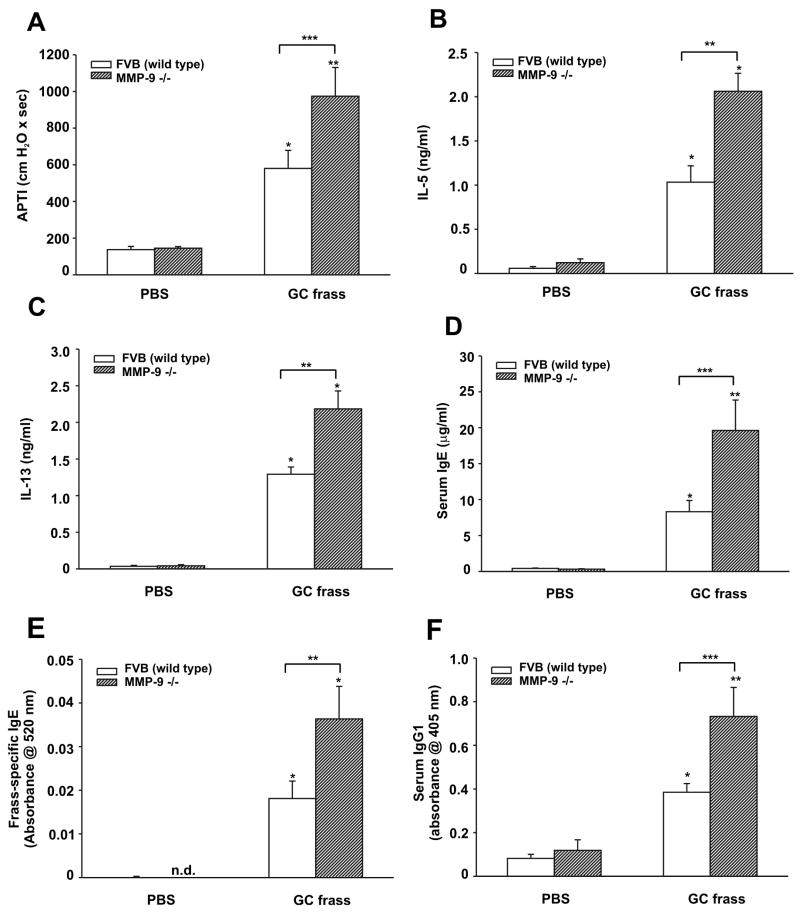
To determine a role for MMP-9 in airway inflammation and the development of airway dysfunction, we sensitized and challenged wild type (FVB) or MMP-9-deficient mice by intratracheal inhalation with GC frass. Sensitization and challenge with GC frass significantly increased airway responsiveness to cholinergic agents in wild type mice; however this was significantly increased in MMP-9-deficient mice (Figure 6A). Levels of the Th2 cytokines IL-5 and IL-13 were increased in wild-type mice following allergen challenge and were higher in MMP-9-deficient mice compared to wild type mice treated with GC frass (Figure 6B+C). Serum IgE levels (Figure 6D), GC frass-specific IgE (Figure 6E) and serum IgG1 levels (Figure 6F) were also increased following sensitization and challenge with GC frass and were higher in MMP-9-deficient mice. Interestingly, MMP-9-deficient mice sensitized to GC frass had significantly more eosinophils and less neutrophils than wild type mice (Table 4), reminiscent of the TLR2-deficient phenotype.
Figure 6.
GC frass-induced experimental allergic asthma in MMP-9-deficient mice. Wild type (FVB) or MMP-9-deficient mice were challenged by intratracheal inhalation on day 0, 7, and 14 with PBS (40 μl) or GC frass (40 μg/40 μl). On day 19, mice were anesthetized and acetylcholine was injected after establishment of a stable airway pressure. In all cases, means ± SEM (n=8-9 mice per group) were reported. Statistical significance was determined by ANOVA. A. AHR was measured as airway pressure time index (APTI) in cm-H2O × sec -1 (compared to PBS *p=0.011, **p<0.001; compared to GC frass ***p=0.008). Lungs from mice were excised; cells dissociated and maintained in a single suspension culture for 3 days in the presence of ConA (10 μg/ml). Supernatants were removed and ELISAs were run. B. IL-5 ELISA (compared to PBS *p<0.001; compared to GC frass **p<0.001). C. IL-13 ELISA (compared to PBS *p<0.001; compared to GC frass **p<0.001). D. Serum IgE levels (compared to PBS *p=0.025, **p<0.001; compared to GC frass **p<0.001). E. Frass-specific IgE levels (compared to PBS *p<0.001; compared to GC frass **p=0.037). F. Serum IgG1 levels (compared to PBS *p=0.04, ** p<0.001; compared to GC frass ***p=0.004).
Table 4. Differential cell count in BAL fluid of sensitized and challenged wild type (FVB) and MMP-9-deficient (MMP-/-) mice.
FVB or MMP-9-deficient mice were given intratracheal inhalations of PBS (40 μl) or GC frass (40 μg/40 μl) on days 0, 7, and 14. On day 17, BAL fluid was harvested and differential cell counts performed. These data represent 6-8 mice per group and are expressed as mean ± SEM of cell number × 104. Cell counts were statistical significance between GC frass in the MMP-9-deficient mice compared to wild type mice (*p=0.002, **p<0.001, as determined by ANOVA).
| Mac | Epi | Eos | Neut | Lymph | |
|---|---|---|---|---|---|
| FVB-PBS | 1.1±0.3 | 1.7±0.4 | 0.01±0.004 | 0.01±0.005 | 0.009±0.005 |
| MMP9-/- PBS | 1.7±0.4 | 1.9±0.5 | 0.04±0.03 | 0.02±0.01 | 0.04±0.02 |
| FVB-frass | 13.7±2.4 | 8.2±2.2 | 53.9±17 | 0.5±0.1 | 3.3±0.5 |
| MMP9-/- frass | 15.3±1.7 | 8.3±2.7 | 139±17* | 0.03±0.02** | 3.5±1 |
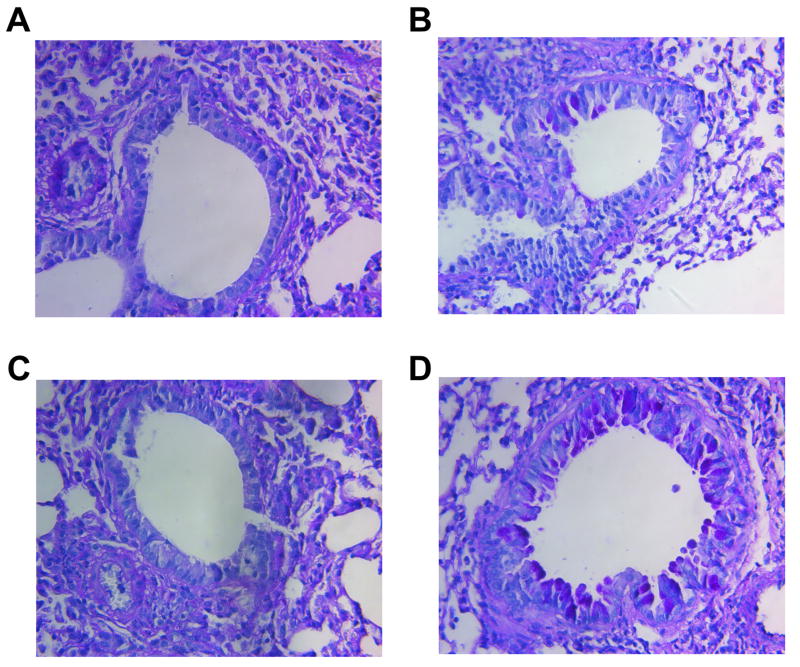
Histological examination of the wild type or MMP-9-deficient mouse lungs following GC frass treatment showed dense perivascular and peribronchiolar infiltrates compared to PBS treatment (data not shown). There was not a significant difference between the levels of infiltrates in wild type or MMP-9-deficient mice. Interestingly, we did not detect any PAS staining in the lungs of the wild type FVB or MMP-9-deficient mice sensitized and challenged with PBS (Figure 7A+7C). Sensitization and challenge with GC frass induced some mucin in the wild type mice (Figure 7B); however there was significantly more PAS staining in the MMP-9-deficient mice (Figure 7D). Therefore, concurrent with the increase in IL-13 and airway hyperresponsiveness, the levels of mucin in the epithelial cells in the MMP-9-deficent mice was significantly worse than in wild-type mouse lungs. Combined, these data suggest that MMP-9 plays a protective role in the asthma phenotype, as we have shown that MMP-9 deficient mice have a significantly increased allergic inflammatory response and airway reactivity.
Figure 7.
Histological assessment of lung sections from PBS or GC frass exposed wild type or MMP-9-deficient mice. Periodic Acid Schiff (PAS) staining of sectioned lungs from wild type (FVB) PBS (A) or GC frass (B) treated mice and MMP-9-deficient PBS (C) or GC frass (D) treated mice. Representative slides are shown of sections from 8-9 mice per group.
Discussion
Herein we show that a real world allergen, among its other constituents, contains a TLR2 agonist which acts to protect against experimentally-induced asthma in mice. Using a real world allergen containing a TLR2 ligand, we found that GC frass activated TLR2 on neutrophils to release MMP-9, and the increased levels of MMP-9 in the airways played a protective role in the generation of experimentally-induced asthma in our murine model. To our knowledge, this is the first report showing that TLR2 regulated MMP-9 release from neutrophils. Activation of TLR2 mediated the release of MMP-9 from neutrophils as evidenced by 1) MMP-9 levels detected in the BAL fluid of mice following GC frass exposure was dependent on neutrophil infiltration into the airways, 2) selective activation of TLR2 increased MMP-9 release from neutrophils in culture, 3) removal of the TLR2 ligand in GC frass or treating TLR2-deficient neutrophils with GC frass attenuated MMP-9 release and 4) TLR2-deficient mice released similar levels of MMP-9 following TNFα treatment. In addition, we found that inhibition of NF-κB signaling attenuated GC frass induced MMP-9 expression. This follows our previous data showing that GC frass induced NF-κB translocation and activation (7). Together these data suggest that TLR2 activation leads to increased MMP-9 release from neutrophils.
We next queried the physiological relevance of altered MMP-9 in the context of experimentally-induced asthma. MMP-9-deficient mice which were sensitized and challenged with GC frass by intratracheal inhalation had significantly more AHR, Th2 cytokine levels in BAL fluid and serum IgE and IgG1 levels compared to wild type mice, suggesting that MMP-9 plays a protective role in allergen-induced experimental asthma. Our study confirms the findings of McMillan et. al. who also showed that MMP-9 is protective in OVA-induced experimental allergic airway inflammation (25). In addition, our study links activation of TLR2 to the regulation of MMP-9 in the airways by showing in TLR2-deficient mice that TLR2 is upstream of MMP-9 release, and that TLR2 also plays a protective role in regulating allergic airway inflammation in mice. The following caveat applies to the TLR2 study; the TLR2-deficient mice are in the C57Bl/6 background which cannot be sensitized by intratracheal inhalation to GC frass (5) or house dust mite (MWK, unpublished observation). Therefore, the direct effects of TLR2 activation on the airways in the sensitization phase cannot be studied using these mice. With that said, TLR2-deficient mice have significantly less MMP-9 in their airways, and many parameters of GC frass-mediated experimentally-induced asthma are consistent with the MMP-9-deficient mice. TLR2 is upstream of a number of mediators, one of which is MMP-9, so the fact that not all parameters tested were altered in TLR2-deficient mice was not surprising. Overall our data suggests that the TLR2 ligand in GC frass activates neutrophils to release MMP-9, the consequence of which is to lessen airway inflammation and airway reactivity in a mouse model of experimentally-induced asthma.
The effect of the innate immune response on activation of adaptive immunity and subsequent allergic inflammation is unclear. In every system tested to date, an immediate consequence of allergen inhalation in rodent and human models is the increase in airway neutrophilia (7, 33-35); however the consequence of this is currently unknown. Our findings that inhibition of neutrophil infiltration following each inhalation of GC frass resulted in increased airway eosinophilia and serum IgE levels suggest that activation of neutrophils play some role in the mediating the allergic asthma phenotype. Intriguingly, it has been shown that transfer of Th1 cells, which induced airway neutrophilia, followed by challenge with OVA was unable to increase airway hyperresponsiveness in mice (36). Importantly, this study may support our hypothesis that it is not neutrophil infiltration per se that regulates airway hyperresponsiveness, but the interaction of the allergen GC frass with the neutrophil. While we believe a direct interaction of GC frass and the newly recruited neutrophil occurs, other possibilities could exist. For example, newly recruited neutrophils may release a mediator which regulates MMP-9 release either from neutrophils or possibly from another cellular source in the airway. We have previously shown that GC frass can regulate MMP-9 release from bronchial epithelial cells (26), however these levels were significantly lower than those from neutrophils. Alveolar macrophages are also capable of releasing MMP-9 (KP, unpublished observation); however these levels are also not comparable to those released by the neutrophil. In addition, we have recently reported that GC frass increased NF-κB activation in neutrophils (7), and now present evidence that TLR2 regulates MMP-9 expression via activation of NF-κB. Overall, our data suggests that the interaction of GC frass with newly recruited neutrophils mediates the release of MMP-9 into the airways.
The fact that MMP-9 depletion increased airway inflammation and airway reactivity were a surprise to us, as we were anticipating that without the degradative effects of MMP-9, the allergic asthma phenotype would be lessened. MMP-9 is known to process and activate pro-IL-1β (37), IL-8 (38), and TNFα (39), all of which are important in inflammation. The question is then, how does the removal of MMP-9 increase inflammation? One possibility is that there is compensation by another MMP in the absence of MMP-9, which perhaps is usually found in lower concentrations in the airways and is upregulated in the MMP-9-deficient mice. We did not investigate the concentrations of the other MMP's in this study, however Yoon et. al. found significant upregulation of MMP-2 in the MMP-9-deficient mice (40). Another possibility is that depletion of MMP-9 results in the loss of activation or degradation of other proteins. For example, it has been shown that MMP-9 proteolytically activates transforming growth factor (TGF)β (41). TGFβ has been shown to inhibit IgE synthesis, mast cell proliferation, and eosinophil survival in a number of studies (42-44). Meade et. al. showed that TGFβ1 inhibited immediate and delayed hypersensitivity in a mouse model (45). It is conceivable then that MMP-9-deficiency may lead to the lack of TGFβ activation and thus an increase in the asthma phenotype. Further studies will investigate the role for TGFβ in GC frass-induced innate immune responses and experimentally-induced asthma in our mouse model.
Our present study is in disagreement with another study which showed that MMP-9-deficient mice had decreased airway inflammation and decreased airway responsiveness to carbachol (24). The main differences in our studies are the mode of sensitization and the choice of allergen. Our model uses a real world allergen and a physiologically-relevant mode of sensitization, namely inhalation. Recent data have shed light on the importance of the airway epithelium in asthma pathogenesis (46, 47). The use of ovalbumin (OVA) bound to aluminum hydroxide (alum) and administered via intraperitoneal injection results in highly enhanced T-cell responses, but does not activate the airway epithelium as inhalation of an allergen would. The fact that the outcome of the two studies is different may shed light on the importance of the airway epithelial involvement in the pathogenesis of asthma.
Overall, our data suggest that activation of TLR2 plays a protective role in experimentally-induced asthma in mice. While GC frass contains the cockroach allergens Bla g1 and Bla g2 (4), it also contains active serine proteases (26, 48), coliform bacteria (49), lipopolysaccharide (50) (a TLR4 agonist) and a TLR2 agonist (7), pheromones, and a number of other uncharacterized components. It is likely that all of these components play important roles in mediating allergic inflammation. It is possible that the degree to which an individual responds to various components contained within the allergen complex may alter the asthma phenotype. Polymorphisms in TLR2 have been suggested to be associated with increased asthma susceptibility. A cross-sectional study performed to identify polymorphisms in TLR2 in children from rural areas in Austria and Germany (51), showed that a polymorphism in TLR2 (A-16934T) allele was found to be a major determinant of the susceptibility to asthma and allergies. A subsequent study found an association between TLR2/+596 and asthma in a case-control and family-based analysis (52). However another study in Japanese children showed no such association (53). Nevertheless, our study using a complex allergen suggests that at least one component, the TLR2 agonist, plays a protective role in determining the susceptibility to asthma by mediating MMP-9 release by neutrophils.
Acknowledgments
We thank Dr. Shizuo Akira for providing the TLR2-/- mice.
This work was supported by the National Institutes of Health Grant HL75568 (KP) and PO1076383 and HL87736-08 (MWK).
References
- 1.Gelber LE, Seltzer LH, Bouzoukis JK, Pollart SM, Chapman MD, Platts-Mills TA. Sensitization and exposure to indoor allergens as risk factors for asthma patients presenting to hospital. Am Rev Resp Dis. 1993;147:573–587. doi: 10.1164/ajrccm/147.3.573. [DOI] [PubMed] [Google Scholar]
- 2.Leaderer BP, Belanger K, Triche E, Holford T, Gold DR, Kim Y, Jankun T, Ren P, McSherry JE, Platts-Mills TAE, Chapman MD, Bracken MB. Dust mite, cockroach, cat and dog allergen concentrations in homes of asthmatic children in the Northeastern United States: impact of socioeconomic factors and population density. Environ Health Perspect. 2002;110:419–425. doi: 10.1289/ehp.02110419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Finn PW, Boudreau JO, He H, Wang Y, Chapman MD, Vincent C, Burge HA, Weiss ST, Perkins DL, Gold DR. Children at risk for asthma: home allergen levels, lymphocyte proliferation, and wheeze. J Allergy Clin Immunol. 2000;105:933–942. doi: 10.1067/mai.2000.106546. [DOI] [PubMed] [Google Scholar]
- 4.Yun YY, Ko SH, Park JW, Lee IY, R HI, Hong CS. Comparison of allergic components between german cockroach whole body and fecal extracts. Annal Allergy Asthma Immunol. 2001;86:551–556. doi: 10.1016/S1081-1206(10)62904-3. [DOI] [PubMed] [Google Scholar]
- 5.Page K, Lierl K, Herman N, Wills-Karp M. Differences in susceptibility to German cockroach frass and its associated proteases in induced allergic inflammation in mice. Resp Res. 2007;8:91. doi: 10.1186/1465-9921-8-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gangloff SC, Guenounou M. Toll-like receptors and immune response in allergic disease. Clin Rev Allergy Immunol. 2004;26:115–125. doi: 10.1007/s12016-004-0006-0. [DOI] [PubMed] [Google Scholar]
- 7.Page K, Lierl KM, Hughes VS, Zhou P, Ledford JR, Wills-Karp M. TLR2-mediated activation of neutrophils in response to German cockroach frass. J Immunol. 2008;180:6317–6324. doi: 10.4049/jimmunol.180.9.6317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Redecke V, Häcker H, Datta SK, Fermin A, Pitha PM, Broide DH, Raz E. Activation of Toll-Like Receptor 2 induces a Th2 immune response and promotes experimental asthma. J Immunol. 2004;172:2739–2743. doi: 10.4049/jimmunol.172.5.2739. [DOI] [PubMed] [Google Scholar]
- 9.Chisholm D, Libet L, Hayashi T, Horner AA. Airway peptidoglycan and immunostimulatory DNA exposures have divergent effects on the development of airway allergen hypersensitivities. J Allergy Clin Immunol. 2004;113:448–454. doi: 10.1016/j.jaci.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 10.Akdis C, Kussebi F, Pulendran B, Akdis M, Lauener RP, Schmidt-Weber CB, Klunker S, I G, Hansjee N, Wynn TA, Dillon S, Erb P, Baschang G, B K, Alkan SS. Inhibition of T helper 2-type responses, IgE production and eosinophilia by synthetic lipopeptides. Eur J Immunol. 2003;33:2717–2726. doi: 10.1002/eji.200323329. [DOI] [PubMed] [Google Scholar]
- 11.Patel M, Xu D, Kewin P, Choo-Kang B, McSharry C, Thomson NC, Liew FY. TLR2 agonist ameliorates established allergic airway inflammation by promoting Th1 response and not via regulatory T cells. J Immunol. 2005;174:7558–7563. doi: 10.4049/jimmunol.174.12.7558. [DOI] [PubMed] [Google Scholar]
- 12.Taylor RC, Richmond P, Upham JW. Toll-like receptor 2 ligands inhibit Th2 responses to mite allergens. J Allergy Clin Immunol. 2006;117:1148–1154. doi: 10.1016/j.jaci.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 13.Lorne E, Zhao X, Zmijewski JW, Liu G, Park YJ, Tsuruta Y, Abraham E. Participation of mTOR complex 1 in TLR2 and TLR4 induced neutrophil activation and acute lung injury. Am J Resp Cell Mol Biol. 2009 doi: 10.1165/rcmb.2008-0290OC. EPub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alvarez-Arellano L, Camorlinga-Ponce M, Maldonado-Bernal C, Torres J. Activation of human neutrophils with Helicobacter pylori and the role of Toll-like receptors 2 and 4 in the response. FEMS Immunol Med Microbiol. 2007;51:473–479. doi: 10.1111/j.1574-695X.2007.00327.x. [DOI] [PubMed] [Google Scholar]
- 15.Douwes J, Gibson PG, Pekkanen J, Pearce N. Non-eosinophilic asthma: importance and possible mechanisms. Thorax. 2002;57:643–648. doi: 10.1136/thorax.57.7.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simpson JL, Grissell TV, Douwes J, Scott RJ, Boyle MJ, Gibson PG. Innate immune activation in neutrophilic asthma and bronchiectasis. Thorax. 2007;62:211–218. doi: 10.1136/thx.2006.061358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Atkinson JJ, Senior RM. Matrix metalloproteinase-9 in lung remodeling. Am J Resp Cell Mol Biol. 2003;28:12–24. doi: 10.1165/rcmb.2002-0166TR. [DOI] [PubMed] [Google Scholar]
- 18.Mautino G, Oliver N, Chanez P, Bousquet J, Capony F. Increased release of matrix metalloproteinase-9 in bronchoalveolar lavage fluid and by alveolar macrophages of asthmatics. Am J Resp Cell Mol Biol. 1997;17:583–591. doi: 10.1165/ajrcmb.17.5.2562. [DOI] [PubMed] [Google Scholar]
- 19.Cataldo DD, Bettiol J, Noel A, Bartsch P, Foidare JM, Louis R. Matrix metalloproteinase-9, but not tissue inhibitor of matrix metalloproteinase-1, increases in the sputum from allergic asthmatic patients after allergen challenge. Chest. 2002;122:1553–1559. doi: 10.1378/chest.122.5.1553. [DOI] [PubMed] [Google Scholar]
- 20.Kelly EAB, Busse WW, Jarjour NN. Increased matrix metalloproteinase-9 in the airway after allergen challenge. Am J Respir Crit Care Med. 2000;162:1157–1161. doi: 10.1164/ajrccm.162.3.9908016. [DOI] [PubMed] [Google Scholar]
- 21.Lee YC, Lee HB, Rhee YK, Song CH. The involvement of matrix metalloproteinase-9 in airway inflammation of patients with acute asthma. Clin Exp Allergy. 2001;31:1623–1630. doi: 10.1046/j.1365-2222.2001.01211.x. [DOI] [PubMed] [Google Scholar]
- 22.Chakrabarti S, Patel KD. Regulation of matrix metalloproteinase-9 release from IL-8-stimulated human neutrophils. Journal of Leukocyte Biology. 2005;78:279–288. doi: 10.1189/jlb.1004612. [DOI] [PubMed] [Google Scholar]
- 23.Ardi VC, Kupriyanova TA, Deryugina EI, Quigley JP. Human neutrophils uniquely release TIMP-free MMP-9 to provide a potent catalytic stimulator of angiogenesis. Proc Nat Acad Sci. 2007;104:20262–20267. doi: 10.1073/pnas.0706438104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cataldo D, Tournoy KG, Vermaelen K, Munaut C, Foidart JM, Louis R, Noel A, Pauwels R. Matrix metalloproteinase-9 deficiency impairs cellular infiltration and bronchial hyperresponsiveness during allergen-induced airway inflammation. Am J Path. 2002;161:491–498. doi: 10.1016/S0002-9440(10)64205-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McMillan SJ, Kearley J, Campbell JD, Zhu XW, Larbi KY, S JM, Senior RM, Nourshargh S, Lloyd CM. Matrix metalloproteinase-9 deficiency results in enhanced allergen-induced airway inflammation. J Immunol. 2004;172:2586–2594. doi: 10.4049/jimmunol.172.4.2586. [DOI] [PubMed] [Google Scholar]
- 26.Page K, Hughes VS, Bennett GW, Wong HR. German cockroach proteases regulate matrix metalloproteinase-9 in human bronchial epithelial cells. Allergy. 2006;61:988–995. doi: 10.1111/j.1398-9995.2006.01103.x. [DOI] [PubMed] [Google Scholar]
- 27.Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, Takeda K, Akira S. Differential roles of TLR2 and TLR4 in recognition of gram-negatice and gram-positive bacterial cell wall components. Immunity. 1999;11:443–451. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- 28.Walters DM, Breysse PN, Wills-Karp M. Ambient urban Baltimore particulate-induced airway hyperresponsiveness and inflammation in mice. Am J Resp Crit Care Med. 2001;164:1438–1443. doi: 10.1164/ajrccm.164.8.2007121. [DOI] [PubMed] [Google Scholar]
- 29.Czuprynski CJ, Brown JF, Maroushek N, Wagner RD, Steinberg H. Administration of anti-granulocyte mAb RB6-8C5 impairs the resistance of mice to Listeria monobytogenes infection. J Immunol. 1994;152:1836–1846. [PubMed] [Google Scholar]
- 30.Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, Donaldson DD. Interleukin-13: Central mediator of allergic asthma. Science. 1988;282:2258–2261. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- 31.Keane-Myers A, Wysocka M, Trinchieri G, Wills-Karp M. Resistance to antigen-induced airway hyperresponsiveness requires endogenous production of IL-12. J Immunol. 1998;161:919–926. [PubMed] [Google Scholar]
- 32.Tennenberg SD, Zemlan FP, Solomkin JS. Characterization of N-formyl-methionyl-leucyl-phenylalanine receptors on human neutrophils. J Immunol. 1988;141:3937–3944. [PubMed] [Google Scholar]
- 33.Boschetto P, Mapp CE, Picotto G, Fabbri LM. Neutrophils and asthma. Eur Resp J. 1989;6:456–459. [PubMed] [Google Scholar]
- 34.Taube C, Dakhama A, Rha YH, Takeda K, Joetham A, Park JW, Balhorn A, Takai T, Poch KR, Nick JA, Gelfand EW. Transient neutrophil infiltration after allergen challenge is dependent on specific antibodies and Fc gamma III receptors. J Immunol. 2003;170:4301–4309. doi: 10.4049/jimmunol.170.8.4301. [DOI] [PubMed] [Google Scholar]
- 35.Lommatzsch M, Julius P, Kuepper M, Garn H, Bratke K, Irmsher S, Luttmann W, Renz H, Braun A, Virchow JC. The course of allergen-induced leukocyte infiltration in human and experimental asthma. J Allergy Clin Immunol. 2006;118:91–97. doi: 10.1016/j.jaci.2006.02.034. [DOI] [PubMed] [Google Scholar]
- 36.Cohn L, Tepper JS, Bottomly K. IL-4-independent induction of airway hyperresponsiveness by Th2, but not Th1 cells. J Immunol. 1998;161:3813–3816. [PubMed] [Google Scholar]
- 37.Schonbeck U, Mach F, Libby P. Generation of biologically active IL-1 beta by matrix metalloproteinases: a novel caspase-1-independent pathway of IL-1 beta processing. J Immunol. 1998;161:3340–3346. [PubMed] [Google Scholar]
- 38.Van den Steen PE, Proost P, Wuyts A, Van Damme J, Opdenakker G. Neutrophil gelatinase B potentiates interleukin-8 tenfold by aminoterminal processing, whereas it degrades CTAP-III, PF-4 and GRO-a and leaves RANTES and MCP-2 intact. Blood. 2000;96:2673–2681. [PubMed] [Google Scholar]
- 39.Mohan MJ, Seaton T, Mitchell J, Howe A, Blackburn K, Burkhart W, Moyer M, Patel I, Waitt GM, Becherer JD, Moss ML, Milla ME. The tumor necrosis factor-alpha converting enzyme (TACE): a unique metalloproteinase with highly defined substrate selectivity. Biochem. 2002;41:9462–9469. doi: 10.1021/bi0260132. [DOI] [PubMed] [Google Scholar]
- 40.Yoon HK, Cho HY, Kleeberger SR. Protective role fo matrix metalloproteinase-9 in ozone-induced airway inflammation. Environ Health Persp. 2007;115:1557–1563. doi: 10.1289/ehp.10289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu Q, Stamenkovic I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes Devel. 2000;14:163–176. [PMC free article] [PubMed] [Google Scholar]
- 42.Kehrl JH, Roberts AB, Wakefield LM, Jakowlew S, Sporn MB, Fauci AS. Transforming growth factor beta is an important immunomodulatory protein for human B lymphocytes. J Immunol. 1986;197:3855–3860. [PubMed] [Google Scholar]
- 43.Alam R, Forsythe P, Stafford S, Fukuda Y. Transforming growth factor beta abrogates the effects of hematopoietins on eosinophils and induces their apoptosis. J Exp Med. 1994;179:1041–1045. doi: 10.1084/jem.179.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Boer WI, van Schadewijk A, Sont JK, Sharma HS, Stolk J, Hiemstra PS, van Krieken JH. Transforming growth factor beta and recruitment of macrophages and mast cells in airways in chronic obstructive pulmonary disease. Am J Resp Crit Care Med. 1998;158:1951–1957. doi: 10.1164/ajrccm.158.6.9803053. [DOI] [PubMed] [Google Scholar]
- 45.Meade R, Askenase PW, Geba GP, Neddermann K, Jacoby RO, Pasternak RD. Transforming growth factor-beta 1 inhibits murine immediate and delayed type hypersensitivity. J Immunol. 1992;149:521–528. [PubMed] [Google Scholar]
- 46.Bousquet J, Jeffrey KL, Busse WW, Johnson M, Vignola AM. Asthma: from bronchoconstriction to airways inflammation and remodeling. Am J Resp Crit Care Med. 2000;161:1720–1745. doi: 10.1164/ajrccm.161.5.9903102. [DOI] [PubMed] [Google Scholar]
- 47.Pantano C, Ather JL, Alcorn JF, Poynter ME, Brown AL, Guala AS, Beuschel SL, Allen GB, Whittaker LA, Bevelander M, Irvin CG, Janssen-Heininger YM. Nuclear factor-kappaB activation in airway epithelium induces inflammation and hyperresponsiveness. Am J Resp Crit Care Med. 2008;177:959–969. doi: 10.1164/rccm.200707-1096OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hughes VS, Page K. German cockroach frass proteases cleave pro-matrix metalloproteinase-9. Exp Lung Res. 2007;33:135–150. doi: 10.1080/01902140701356561. [DOI] [PubMed] [Google Scholar]
- 49.Zurek L, Schal C. Evaluation of the German cockroach (Blattella germanica) as a vector for verotoxigenic Escherichia coli F18 in confined swine production. Vet Microbiol. 2004;101:263–267. doi: 10.1016/j.vetmic.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 50.Bhat RK, Page K, Tan A, Hershenson MB. German cockroach extract increases bronchial epithelial cell interleukin-8 expression. Clin Exp Allergy. 2003;33:35–42. doi: 10.1046/j.1365-2222.2002.01481.x. [DOI] [PubMed] [Google Scholar]
- 51.Eder W, Klimecki W, Yu L, von Mutius E, Riedler J, Braun-Fahrländer C, Nowak D, Martinez FD, Team AS. Toll-like receptor 2 as a major gene for asthma in children of European farmers. J Allergy Clin Immunol. 2004;113:482–488. doi: 10.1016/j.jaci.2003.12.374. [DOI] [PubMed] [Google Scholar]
- 52.Smit LA, Siroux V, Bouzigon E, Oryszczyn MP, Lathrop M, Demenais F, Kauffman F. CD14 and Toll-like Receptor Gene Polymorphisms, Country Living, and Asthma in Adults. Am J Resp Crit Care Med. 2008;179:363–368. doi: 10.1164/rccm.200810-1533OC. [DOI] [PubMed] [Google Scholar]
- 53.Noguchi E, Nishimura F, Fukai H, Kim J, Ichikawa K, Shibasaki M, Arinami T. An association study of asthma and total serum immunoglobulin E levels for Toll-like receptor polymorphisms in a Japanese population. Clin Exp Allergy. 2003;34:177–183. doi: 10.1111/j.1365-2222.2004.01839.x. [DOI] [PubMed] [Google Scholar]