Abstract
We compared two attentional executive processes: updating, which involved attending to a perceptually-present stimulus, and refreshing, which involved attending to a mentally active representation of a stimulus no longer perceptually present. In separate blocks, participants either replaced a word being held in working memory with a different word (update), or they thought back to a just previously seen word that was no longer perceptually present (refresh). Bilateral areas of frontal cortex, supplementary motor area, and parietal cortex were similarly active for both updating and refreshing, suggesting a common network of areas are recruited to bring information to the current focus of attention. In a direct comparison of update and refresh, regions more active for update than refresh included regions primarily in right frontal cortex, as well as bilateral posterior visual processing regions. Regions more active for refresh than update included regions primarily in left dorsolateral frontal and left temporal cortex and bilateral inferior frontal cortex. These findings help account for the similarity in areas activated across different cognitive tasks and may help specify the particular executive processes engaged in more complex tasks.
Keywords: working memory, update, refresh, maintenance
Introduction
A common fronto-parietal network of areas (lateral prefrontal cortex, parietal cortex and supplementary motor area) is active in many cognitive tasks (Cabeza and Nyberg, 2000; Duncan and Owen, 2000) including working memory (WM), visual attention (Kelley et al., 2008; Serences et al., 2005, 2007; Marois et al., 2003), and encoding (Blumenfeld and Ranganath, 2007) and retrieval from long term memory (Cabeza et al., 2008). This similarity of activity is consistent with the idea that common component processes are recruited for diverse cognitive tasks (Duncan and Owen, 2000; Cabeza and Nyberg, 2000; Johnson and Hirst, 1993; see also Awh and Jonides, 2001; Awh, Vogel and Oh 2006). Characterizing these components more specifically is a major challenge for cognitive science.
Two component executive processes that might, in part, account for this common activation across experiments are updating (Roth, Serences and Courtney, 2006) and refreshing (Johnson et al., 2005). Updating, the replacement of an item actively being maintained in working memory with a different item, produces activity in a network including left inferior frontal junction (IFJ, the junction of inferior frontal sulcus and inferior precentral sulcus), dorsolateral prefrontal cortex (DLPFC), supplementary motor area (SMA), and bilateral parietal cortex including intraparietal sulcus (IPS). This network becomes active during updating of different types of visual stimuli (Roth et al., 2006), updating from a sensory stimulus or an item from long term memory (Roth and Courtney, 2007), and when the rule operating on a stimulus is updated (Montojo and Courtney, 2008). Refreshing is the process of attending to information that is not perceptually present but is momentarily active from either recent perception or thought (e.g., thinking back to a just-seen stimulus no longer on the screen; Raye et al., 2002; Johnson et al., 2002, 2005). Refreshing produces activity in a network including DLPFC, anterior cingulate cortex (ACC), supramarginal gyrus (SMG), IPS, and middle temporal gyrus, for a variety of types of stimuli (Johnson et al., 2005; Raye et al, 2002, 2007).
Similarities in activation for updating and refreshing across experiments have been noted before (Courtney et al., 2007), but this is the first experiment to directly compare them. One possibility is that updating and refreshing are the same process investigated in the context of different experimental paradigms and under different names. If so, we would expect refreshing and updating to produce the same patterns of activation in the same participants in procedures using the same materials. On the other hand, Updating has been proposed as a specific mechanism for changing the contents of what is being actively maintained in working memory by prioritizing new information that is concurrently perceived or retrieved. Refreshing has been proposed as a general mechanism for briefly bringing information that is currently active but not perceptually present to the foreground of attention. Update and refresh may involve different areas of cortex. For example, Updating should show relatively greater activity in posterior visual processing regions as a new target is perceptually attended, and Refreshing should show relatively greater activity in frontal regions (e.g., left DLPFC) associated with modulating representations of no longer present perceptual stimuli (M.R. Johnson et al., 2007).
Assuming that there is a distinction between the cognitive operations and neural activity involved in perceptual vs. reflective attention (e.g., Dobbins & Han, 2006; Gilbert, Frith and Burgess, 2005; Johnson, Raye, Mitchell et al., 2005; Johnson & M.R. Johnson, in press), directly comparing the neural correlates of updating working memory from a perceptually present stimulus with refreshing an active representation of a stimulus that is no longer perceptually present should help clarify similarities and differences in neural activity observed in more complex cognitive tasks that recruit these processes.
Methods
Participants
Participants were 22 (12 females) healthy adult (M = 24 yrs, range 19-44) non-smokers, with no history of head injury, psychiatric illness, drug or alcohol abuse, and no current medications that would affect the function of the brain, heart or blood circulation. Participants were compensated and all gave written informed consent. The experiments were undertaken with the approval of Yale University School of Medicine Human Investigation Committee, and in compliance with national legislation and the Code of Ethical Principles for Medical Research Involving Human Subjects of the World Medical Association.
Procedure
Immediately before the fMRI scan, participants had a practice session. They read task instructions, listened to verbal instructions, and performed one practice block of each condition (update, refresh) with experimenter feedback. They then performed, in random order, three blocks of each condition without feedback (performance was monitored to determine that they were doing the task correctly). Each practice block was 64.5 seconds.
There were 6 fMRI experimental runs. Within each run there were 4 blocks of each type (Update and Refresh), presented pseudorandomly and counterbalanced within run and across scan session.
Task blocks were nearly identical in task set-up. Different geometric shapes were used as cues for Update (triangles), Refresh (circles) and Control (squares) conditions. Otherwise the stimulus presentation was the same. In both Update and Refresh blocks, words appeared on the screen one at a time and participants were instructed to read them silently.
Update Blocks
In Update Blocks, there were four event types: read (words that did not match the word currently maintained in WM), update WM, match, and control. Participants were given a word to maintain in WM and sometimes were cued (with a row of triangles) to replace the word currently being held in WM with the next word to appear on the screen (update; see Figure 1). Thus, in Update blocks, participants always maintained one word as the sample stimulus in WM while reading other words, and their task was to determine whether each word they read matched or did not match the word being maintained. If the word on the screen matched the item in WM, participants responded with a button press (match). Occasionally they saw a row of squares, a “null” cue acting as a sensory control event (control), and they were instructed to look at the squares, but continue to maintain the current sample in WM and wait for the next word. Participants were to press a button only when the item on the screen matched the contents of working memory (match).
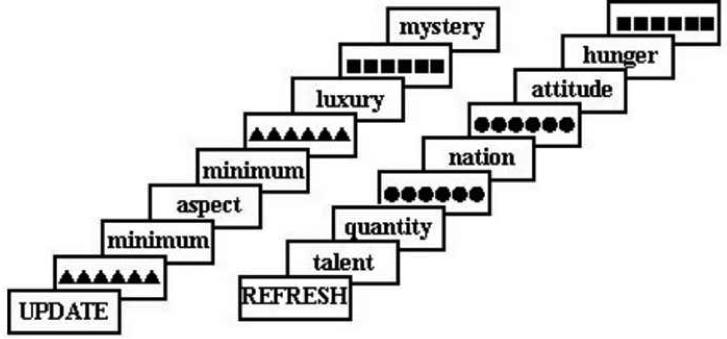
Figure 1.
Task design. First participants were cued with the block type, Update or Refresh. In each block they read each word. In Update Blocks they were to maintain one word in working memory at all times while reading the stream of words. A row of triangles cued them to update working memory by replacing the current contents of working memory with the subsequent word in the stream. (In Update blocks, when they saw a word in the stream that matched the contents of working memory they pressed a button.) All Update blocks began with the update cue so that participants immediately maintained an item in working memory. In Refresh Blocks, a row of dots cued them to think back to the just-previous word. In both block types the control event was a row of squares. Participants were instructed to look at the squares and wait for the next word. Within each block, events occurred pseudorandomly with an Update, Refresh or Control cue every 3 - 20 seconds (mean time between cues was 10.5 seconds). Each word or cue appeared on the screen for 1 second with a 500 ms inter-stimulus interval containing a visual mask (row of plus signs not shown).
Refresh Blocks
As in Update Blocks, single words appeared on the screen one at a time and participants were instructed to read them. Within Refresh Blocks, there were four event types: read, refresh, control, and match. During Refresh blocks participants performed a modified version of a refresh task (see Figure 1; Raye et al, 2002). Occasionally, instead of a word, they saw a row of dots which cued participants to think back to the word that preceded the refresh cue (refresh event). They were instructed to think back to that word once, and not continue to think of it. Occasionally they saw a row of squares that cued them to look at the squares and wait for the next word; this served as a sensory control event in which they saw a row of geometric stimuli, as on Refresh trials, but were not asked to refresh. Occasionally participants read a word they had recently refreshed; these `match' events were included to parallel match events in update trials. Read events corresponded to read trials from the non-match (read) trials in Update Blocks. Participants were not required to make any button press responses during the Refresh Blocks.
Stimulus Presentation
Before each block, a cue (“UPDATE” or “REFRESH”) appeared for 1 second (Figure 1). Within each block, events occurred pseudorandomly with an Update (or Refresh) cue every 3 - 20 seconds (mean time between cues was 10.5 seconds). All events were counterbalanced such that each event type preceded and followed every other event type equally often. Each word or cue appeared on the screen for 1 second with a 500 ms interstimulus interval containing a visual mask (row of plus signs) that appeared between each word or cue. The row of plus signs subtended the same visual angle as the longest word. During the practice session stimuli were presented on a Dell Inspiron 640m laptop running EPrime software Version 1.1 (Psychology Software Tools), which also collected key press responses for match events in the Update blocks. During the fMRI session, stimuli were projected onto a screen located behind the participant's head inside the bore of the scanner. Participants viewed the stimuli via an angled mirror mounted on the head coil. Responses were collected via an MRI-compatible button box.
Stimuli were 24 repeated, 2- and 3-syllable abstract nouns (e.g., method, concept, quality, miracle). Each word appeared in 28-point Arial font centered on the screen in black lower-case font on a white background. In a pilot experiment, 23 participants evaluated words for imagability and emotionality on a 10 point rating scale. Words were eliminated whose scores on either rating were more than two standard deviations from the mean on either measure.
The 6 fMRI experimental runs each lasted 9 minutes 18 seconds, for a total of 55 minutes, 48 seconds of functional data collection. Each block lasted 64.5 seconds with a temporally jittered interblock interval where a row of pluses remained on the screen for 3-7.5 seconds. Across all scans there were 144 events of each type (update, refresh, read during the update task, read during the refresh task, match in the update task, match in the refresh task).
fMRI Data Acquisition
All scans were collected on a Siemens 3T Trio scanner at the Magnetic Resonance Research Center, Yale University School of Medicine. Functional data were collected as T2*-weighted gradient echo, echo planar images (TR = 2000 ms, TE = 25 ms, Flip Angle = 80 degrees, voxel size 3.438 × 3.438 mm, 3 mm axial slice, 1 mm gap, FOV = 22cm, matrix = 64 × 64) during the experimental task. A high-resolution T1-weighted anatomical scan was collected between the 3rd and 4th functional scan runs (TR = 2530 ms, TE = 3.34, Flip Angle = 7 degrees; FOV = 256 × 256).
fMRI Analysis
Data were slicetime corrected and motion corrected with SPM5 as a standard lab preprocessing step. SPM software was not utilized after this point. Functional data were filtered with a 3rd order polynomial orthogonal function to correct for mean, linear drift and any third order nuisance variable. The frequency of change between block types is more frequent than the periodicity of a third order polynomial function, therefore this filter should not reduce statistical power. Anatomical and functional images were coregistered then morphed into MNI space using BioimageSuite software (http://www.bioimagesuite.org). After preprocessing, fMRI data were analyzed using Analysis of Functional NeuroImages (AFNI) software (Cox, 1996). Data were normalized to have a mean of 100. Data for each voxel were analyzed via a simultaneous regression for all critical and control events (Update, Read in Update, Match in Update, errors detecting match events, Control in Update, Refresh, Read in Refresh, Match in Refresh, Control in Refresh, Update and Refresh Block cues) as well as block regressors for each block type (Update Block and Refresh Block). This was a mixed block/event-related design. Each event included two stimuli. For example, an Update event included the cue to update and the subsequent word to be maintained in working memory. For each event, the convolution of event regressors began with the onset of the first stimulus. Event regressors and block regressors both were convolved with a standard hemodynamic response function using the AFNI software waver program (delay time = 2 seconds, rise time = 3 seconds, fall time = 5 seconds, no undershoot; Cox et al., 1996). Block regressors modeled sustained activity within the two block types. Simultaneous regression with the block regressors and the event regressors allows for both sustained and transient components of the activity to be identified (Visscher et al., 2003). Given the temporal jitter of events within blocks, the correlation between block and each event regressor did not exceed .3, allowing for an efficient estimation of sustained and event related activity. Interblock intervals were not modeled and contributed to the baseline.
For the purpose of creating regressors for the different event types, all events modeled in the fMRI analysis included two successive stimuli. An update event included the update cue and the subsequent word to be encoded into memory. Refresh events included the word to be refreshed (the just-previous item) and the refresh cue. Control events were modeled separately within update and refresh blocks. In each case, an “event” included two stimuli. In the Update blocks control events consisted of the control cue and subsequent word to be read, and in Refresh blocks, control events consisted of the control cue and the word preceding it. Read events, modeled separately within update and refresh blocks, included two words in a row to be read, that neither matched the contents of WM, each other, or the word recently refreshed. Match events included the item that matched the contents of WM and the subsequent word to be read. Each of these events included two successive stimuli for the purpose of making particular direct comparisons. Within the update blocks direct comparisons were made between Update events and control events. An Update event included the cue to update and the subsequent word, which was to be brought to the current focus of attention and maintained in working memory; the control event included a null cue and a word to be read. The refresh event included a word to be read followed by the cue to refresh; thus, its control event included a word to be read followed by a “null” cue. For direct comparisons between update and refresh, in both cases, the item to be brought into the current focus of attention was the second stimulus of a two stimulus event. Block cue events included the instructional cue marking the beginning of the block and the subsequent fixation cue. The block cue event only included one stimulus and was included simply to account for the variance in signal associated with this cue.
Individual participant data were smoothed with a three dimensional 4mm Gaussian kernel, then entered into a series of t-tests to compare events of interest, with participant as a random factor. To find regions of the brain differentially active for each condition, t-tests compared the following events: Update versus Control event within the Update blocks, Refresh versus Control event within the Refresh blocks, Update versus Refresh events, and Update Block versus Refresh Block. This last comparison of activity for the Update Block versus Refresh Block identified areas associated with sustained activity associated with WM maintenance since maintenance was required in Update, but not Refresh, blocks.
For each resulting t-map, individual voxel thresholds were set at p < 0.01. Voxelwise data were corrected for multiple comparisons by spatial extent of contiguous suprathreshold individual voxels (experiment-wise p < 0.01 for a cluster). A Monte Carlo simulation was run in the AFNI software package on a brain mask with 4x4x4 mm voxels, a smoothing kernel of 4 mm, connection radius of 5.2 mm, to determine that a 704 microlitre cluster satisfied a threshold of p<0.01 (Forman et al, 1995).
To find regions of the brain similarly active in Update events, Refresh events, and sustained maintenance, we performed an overlap analysis of three Voxelwise t-maps: Update versus Control Events, Refresh versus Control Events, and Update blocks versus Refresh Blocks. An overlap map was generated using AFNI software by weighting equally the active voxels from each input map and summing the inputs to yield a map where different sums corresponded to different combinations of active conditions from the input maps. The threshold for each of the three t-maps contributing to this overlap map was p < .01. Therefore, this is a conservative method for determining areas of overlap as each region of overlap must pass the threshold of two or more t-tests.
Results
Behavioral results
The percent correct (87.3%, standard error 2.5%) for the response to match to sample, and false alarm rate (0.17%, standard error 0.03%), in the Update task indicated that participants effectively updated WM.
fMRI results
Overlap in executive function areas
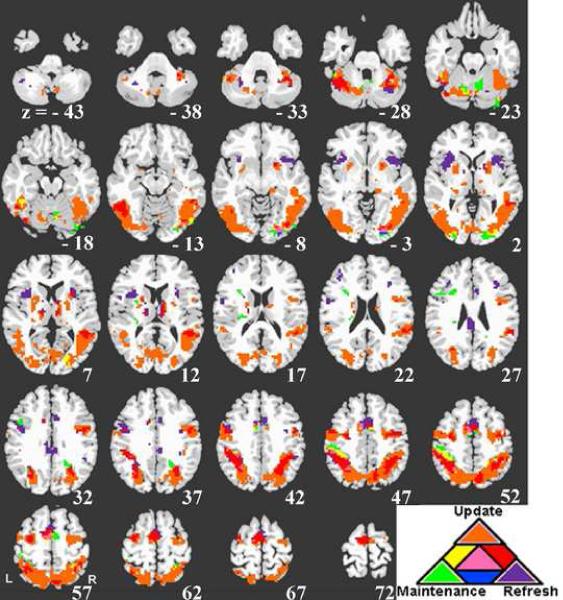
Figure 2 shows areas of activation associated with transient activity associated with update and refresh, sustained activity associated with working memory maintenance (identified with a sustained block regressor for the Update blocks) and their overlap. As is clear from Figure 2, update and refresh both (red areas) showed activity in bilateral areas of precentral gyrus and superior frontal junction (SFJ) including left IFJ, right middle frontal gyrus, SMA, bilateral inferior parietal lobule (IPL) and intraparietal sulcus (IPS), and an area of left posterior fusiform gyrus. Regions of left superior parietal cortex and right SMA were active for both update and maintenance (green areas). (See Table 1; supplementary Table 1 includes a complete list of regions for different combinations of overlap.)
Figure 2.
Overlap analysis of Update, Refresh, and Maintenance (see text). Regions significantly responsive to update are shown in orange, refresh in purple and maintenance in green. Regions responsive both to update and refresh are shown in red. Regions responsive both to update and maintenance are shown in yellow. Regions responsive to refresh and maintenance are shown in blue. Regions responsive to update, refresh and maintenance are shown in pink. The left side of the image represents the left side of the brain.
Table 1.
Regions of overlap for Update and Refresh and regions active in the direct comparison of Update and Refresh.
| MNI Center of Mass (mm) | |||||
|---|---|---|---|---|---|
| Region | BA | Volume (microlitres) | X | Y | Z |
| Overlap of Update and Refresh | |||||
| Prefrontal | |||||
| L Precentral Gyrus / IFJ | 6 | 1216 | -50 | -2 | 42 |
| R MFG / Precentral Gyrus / SFJ | 6 | 1730 | 44 | -3 | 39 |
| L SFJ | 6 | 1568 | -32 | -8 | 55 |
| Medial | |||||
| Bilateral SMA | 6 | 3757 | -4 | -4 | 62 |
| Parietal | |||||
| L Inferior Parietal / Intraparietal Sulcus | 40 | 7838 | -38 | -48 | 44 |
| R Inferior Parietal / Intraparietal Sulcus | 40 | 5514 | 33 | -52 | 44 |
| R Precuneus | 7 | 1568 | 10 | -70 | 52 |
| Temporal | |||||
| L Posterior Fusiform / IT | 19, 37 | 4811 | -44 | -57 | -22 |
| Update > Refresh | |||||
| Prefrontal | |||||
| R SFJ / Superior Frontal Sulcus | 6 | 1000 | 32 | -1 | 56 |
| R SFG / MFG | 10 | 568 | 27 | 42 | -5 |
| Occipital | |||||
| Bilateral Primary and Secondary Visual Cortex, Cuneus | 7, 17, 18, 19 | 42379 | 2 | -72 | 9 |
| Refresh > Update | |||||
| Prefrontal | |||||
| L SFG / Superior Frontal Sulcus | 8, 9, 10 | ** | -22 | 21 | 54 |
| L MFG | 8, 9, 46 | ** | -30 | 16 | 39 |
| L SFG | 6 | ** | -15 | 4 | 56 |
| L IFG | 47 | ** | -46 | 37 | -8 |
| R STG / IFG | 38, 47 | 757 | 24 | 22 | -23 |
| R Precentral Gyrus, Precentral Sulcus, IFG | 4, 6, 44 | 757 | 63 | -3 | 19 |
| L IFJ / IFG | 44, 45 | ** | -55 | 12 | 27 |
| L Anterior Insula | 13 | ** | -45 | 9 | -2 |
| R Anterior Insula | 13 | ** | 41 | 4 | -2 |
| Medial | |||||
| Bilateral SMA | 6, 8 | ** | -7 | 18 | 57 |
| Bilateral preSMA | 6, 8 | 3027 | -2 | -8 | 69 |
| Bilateral ACC | 32 | ** | -3 | 18 | 38 |
| L ACC | 24, 32 | ** | -7 | 43 | 1 |
| Parietal | |||||
| L Inferior Parietal, Postcentral Gyrus | 2, 40 | ** | -51 | -29 | 47 |
| L Supramarginal Gyrus | 40 | ** | -55 | -40 | 28 |
| Temporal | |||||
| L MTG / STG | 21, 22 | 3703 | -60 | -36 | -8 |
Notes: R = Right; L = Left; ACC = Anterior Cingulate Cortex; IFG = Inferior Frontal Gyrus; IFJ = Inferior Frontal Junction (Junction of the Precentral Sulcus and Inferior Frontal Sulcus); IT = Inferior Temporal Gyrus; MFG = Middle Frontal Gyrus; MTG = Middle Temporal Gyrus; SFG = Superior Frontal Gyrus; SFJ = Superior Frontal Junction (Junction of the Precentral Sulcus and Superior Frontal Sulcus); SMA = Supplementary Motor Area; STG = Superior Temporal Gyrus. Brodmann areas are reported not only for the peak active voxel but for the entire active region. Only areas of overlap with volumes of of 200 microlitres or greater are included in this table. For additional regions active in this contrast see Supplementary Materials Table 1.
For the Refresh > Update contrast, there was a large cluster (85,191 microlitres) and coordinates for local maxima for subregions of this large cluster are reported instead of centers of mass.
Direct comparison of Update and Refresh
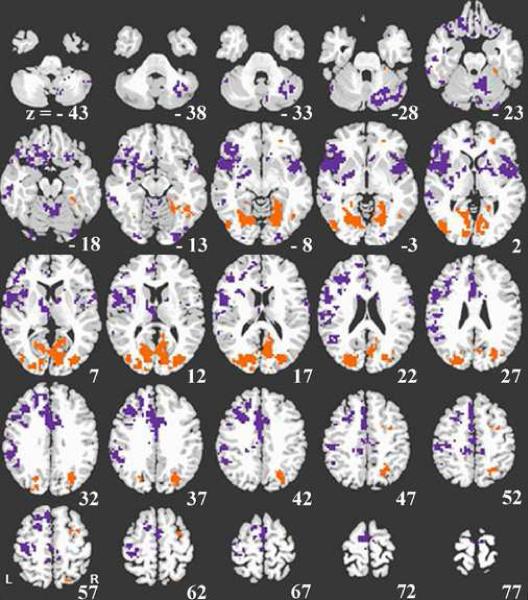
Areas more active for Update than Refresh events included right SFJ and superior frontal sulcus, right superior and middle frontal gyrus, and bilateral primary and secondary visual cortex, and cuneus (see Figure 3, orange areas, and Table 1).
Figure 3.
Direct comparison of update and refresh. Refresh activity greater than update activity is shown in purple. Update activity greater than refresh activity is shown in orange. The left side of the image represents the left side of the brain.
Regions more active for Refresh than Update events included left DLPFC (superior and middle frontal gyrus), bilateral inferior frontal gyrus (including left IFJ), anterior insula, SMA, and ACC, left IPL and left supramarginal gyrus, and left middle and superior temporal gyrus, (see Figure 3, purple areas, and Table 1).
Discussion
This study investigated two types of executive function: attending to a perceptual stimulus in order to replace a representation in WM (update) and attending to an active representation that is no longer perceptually present (refresh). We identified areas that were commonly active across these two types of executive function and areas that differed between these two executive functions.
Common regions of activation across types of executive function
In the present experiment, both updating and refreshing brought information into the focus of attention, either perceptually (update) or reflectively (refresh). For both processes, we found anatomical areas similar to those found in other experiments involving attending to information in various ways. For example, some areas in the current study that showed activity in both update and refresh, including left precentral gyrus, IFJ, SMA, and bilateral IPS and IPL, are similar to those involved in visual attention switching (Serences et al, 2004), task switching (Derrfuss et al., 2005), episodic and semantic long-term memory retrieval (Shannon and Buckner, 2004; Nyberg et al, 2003), and to cues signaling the start of a task block (Dosenbach et al, 2006).
A recent study also examined regions of activation common across tasks involving bringing information into the focus of attention (Marklund et al, 2007). They directly compared transient activity during episodic and semantic long term memory and working memory to the transient activity during an attention task. Aside from ACC/preSMA (SMA in the current study), the regions common to Update and Refresh in our study, as would be expected, were not differentially active across the memory and attention conditions in the Marklund et al. study. If, as we argue, Update and Refresh are both involved in bringing information to the focus of attention, then we might expect that these common regions will be absent from comparisons where both conditions include such attentional processes.
Distinguishing elements of executive function
Although the commonality of activity across refreshing and updating was striking, equally important are the differences we observed. First, Figure 2 shows that some areas exhibit a functional topography where immediately adjacent regions were responsive to different processes. For example in left superior parietal cortex, a region with diverse cell types, functional activity, and extensive connectivity to diverse regions (Rushworth, Behrens and Johansen-Berg, 2005; Nickel and Seitz, 2005; Scheperjans et al. 2008), there were adjacent regions showing activity for maintenance, both update and maintenance, update and refresh, and update. Similarly, different subregions were responsive to different elements of executive function in left IFJ and SMA.
A recent study by Dosenbach et al. (2006) also was directed at identifying common and distinct regions associated with executive functions. They compared 10 studies all conducted on the same scanner, and all using a mixed blocked/event-related design, and various types of cognitive tasks and materials. Comparing activations across studies, Dorsenbach et al. (2006) identified regions of dorsal anterior cingulate cortex/medial superior frontal cortex (Talairach coordinates -1, 10, 46), and bilateral anterior insula (-35, 14, 5; 36, 16, 4) common across tasks and phases within tasks. They suggested that these regions form a core task-set system. Consistent with this idea of a general purpose task-set system, we found an area of SMA activation (see Supplementarly Table 1) in the overlap of Update, Refresh, and Maintenance within 2.5 voxels of their dorsal anterior cingulate cortex/medial superior frontal cortex area. Interestingly, in the Refresh condition, three areas (bilateral SMA/ACC, and left and right anterior insula, see Supplementary Table 1) were within 7.3, 3 and 4.6 millimeters, respectively, of the three regions in Dosenbach et al.'s proposed core task set system. These findings suggest that refreshing may be a component of the kind of core task set system suggested by Dosenbach et al. In addition, both studies suggest there may be more functional specificity within areas commonly reported in meta-analyses across diverse tasks (Cabeza and Nyberg, 2000; Duncan and Owen, 2000), and finer-grained information about such functional specificity should improve the accuracy of “reverse inferences” (Poldrack, 2006) about executive processes.
In the present study, the best evidence for differences in the neural substrates of the executive functions of updating and refreshing comes from a direct contrast between them under conditions where we held other factors (e.g., type of stimuli, control conditions, cue conditions) as similar as possible (Figure 3). It is not surprising that regions more active for updating than refreshing included SFJ (often called the frontal eye fields), and areas of bilateral primary and secondary visual cortex and cuneus. These are regions commonly active in studies of perception and perceptual attention, and a primary difference between Update and Refresh conditions was that the critical item was perceptually present at the time of Update and not Refresh. Furthermore, SFJ has been associated with maintenance in WM (Courtney et al., 1998; Roth et al, 2006), further supporting the notion that SFJ activity in the Update condition in the present study reflects a change in the state of the WM maintenance network (Roth et al, 2006). In addition, a region of right anterior frontal cortex (BA 10) was more active in Update than Refresh. It has been suggested that anterior frontal cortex (sometimes called frontopolar cortex) is recruited for complex tasks such as those involving monitoring and integrating subgoals (Braver & Bongiolatti, 2002), or contingent task switching or “branching” (Koechlin et al., 1999; Koechlin & Hyafil, 2007). Compared to Refresh, the update task likely involved more coordination of subgoals given that the task has several components -- reading words while maintaining an item in working memory, assessing whether a word matches the maintained item, and occasionally replacing the maintained item. Greater activity in right BA 10 could reflect the simultaneous engagement of multiple, non-superordinate operations, and/or suspending one operation while another is performed (see Koechlin and Hyafil, 2007).
In the Refresh task participants must reflectively attend to a representation of a word just seen but no longer present. Prior refresh studies found a network that includes regions similar to those in the present study that were significantly more active in Refresh than Update: left MFG, left SFG, ACC/MdPFC, and left SMG (Raye et al., 2002; Johnson et al., 2003; Johnson et al., 2004; Johnson et al., 2005; Raye et al., 2007; Raye et al., 2008). Furthermore, studies suggest that DLPFC and parietal cortex are involved in modulating posterior representational areas (e.g., Johnson and Johnson, 2007), while anterior PFC (BA 10/SFG) is involved in initiating the refresh process (Raye et al., 2007, Experiment 1). We found an area of left superior frontal sulcus (largely BA 8, extending into BA 10) in the Refresh > Update contrast. This area was somewhat posterior and superior to the right BA 10 area where Update was greater than Refresh. Although these are not exactly homologous areas, these findings may suggest some lateralization of perceptual attention (right PFC) and reflective attention (left PFC). Activity in BA 10 has been hypothesized to be involved in the evaluation of internally generated information (Christoff, Ream, Geddes and Gabrieli, 2003) relative to the evaluation of information in the perceptual environment, and switching between stimulus-oriented and stimulus-independent thought (Gilbert, Frith and Burgess, 2005). In a refresh event in the present experiment the participant first sees a cue (stimulus-oriented thought) then must reactivate a representation of information no longer present (stimulus-independent thought).
Although our focus is on update and refresh, we should note that there were regions of left ventrolateral PFC that were active for maintenance (i.e., maintaining an item in working memory in the update task), as would be expected (Smith and Jonides, 1999; see green regions in Figure 2 and supplementary Table 1). There were very few regions where update, refresh, or both overlapped with areas associated with maintenance, as noted (see supplementary Table 1). These findings are consistent with the idea that refresh and update are involved in brief attentional processes that are different from process(es) typically involved in sustaining information over intervals of several seconds (see Raye et al., 2007, Exp 2, for additional evidence differentiating refreshing and rehearsing and Roth et al., 2006, for additional evidence differentiating updating from maintenance). Similarly there is a dissociation of regions involved in the sustained vs. transient control of task switching (Braver, Reynolds and Donaldson, 2003). Only a few small regions were responsive to update, refresh and maintenance. Interestingly, these areas (SMA, middle occipital, and parietal, see Figure 2 and supplementary Table1) were located directly adjacent to regions active for both update and maintenance, providing further support that updating involves a change in the state of the maintenance system.
Additional studies involving direct comparison of putatively different executive functions are necessary to identify the common and distinct regions involved when information enters the current focus of attention, as in studies of perceptual attention (Yantis and Serences, 2003), selection of information via priority changes (Courtney, Roth and Sala, 2007), task updating (Derrfuss et al., 2005), component processes of executive function (e.g., initiating, noting, Johnson et al., 2003; Raye et al., 2007), or shifts in context (Miller and Cohen, 2001; MacDonald et al., 2000). Such studies would help disambiguate the functional relevance of similarity and differences in areas activated across experiments, which reflect unspecified combinations of perceptual and reflective attentional demands of complex tasks. That is, such studies would clarify the component processes of executive function as discussed in several models (Cowan, 2008; Johnson & Hirst, 1993; Kane and Engle, 2000; Miyake et al, 2001).
Conclusions
The current results indicate that it is possible to separate executive functioning into regions commonly active across different executive functions and regions more specific to particular functions such as attending to an incoming stimulus to replace a maintained stimulus (update) or attending to an already active representation (refresh). Updating and refreshing are two mechanisms for bringing information into the focus of conscious attention. We would expect the network of commonly active regions found for updating and refreshing to be found also in within-participant comparisons of update or refresh with other tasks requiring changes in which information is currently attended (e.g., visual attention switching, monitoring information in short term memory or retrieved from long term memory), providing further evidence of a similarity of function across diverse executive processes. Such studies would also clarify differences in executive functions by identifying differences in their neural signatures.
Supplementary Material
Acknowledgements
This research was funded in part by the National Institutes of Health (NS051622 to RTC and AG09253 to MKJ). The authors wish to thank Karen Mitchell for comments on the manuscript and the staff of the Yale Magnetic Resonance Research Center, especially Hedy Sarofin, Karen Martin and Terry Hickey, for their help with fMRI data acquisition.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Awh E, Jonides J. Overlapping mechanisms of attention and spatial working memory. Trends in Cognitive Science. 2001;5:119–126. doi: 10.1016/s1364-6613(00)01593-x. [DOI] [PubMed] [Google Scholar]
- Awh E, Vogel EK, Oh SH. Interactions between attention and working memory. Neuroscience. 2006;139:201–208. doi: 10.1016/j.neuroscience.2005.08.023. [DOI] [PubMed] [Google Scholar]
- Blumenfeld RS, Ranganath C. Prefrontal cortex and long-term memory encoding: an integrative review of findings from neuropsychology and neuroimaging. Neuroscientist. 2007;13:280–291. doi: 10.1177/1073858407299290. [DOI] [PubMed] [Google Scholar]
- Braver TS, Bongiolatti SR. The role of frontopolar cortex in subgoal processing during working memory. NeuroImage. 2002;15:523–536. doi: 10.1006/nimg.2001.1019. [DOI] [PubMed] [Google Scholar]
- Braver TS, Reynolds JR, Donaldson DI. Neural mechanisms of transient and sustained cognitive control during task switching. Neuron. 2003;39:713–726. doi: 10.1016/s0896-6273(03)00466-5. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L. Imaging cognition II: An empirical review of 275 PET and fMRI studies. Journal of Cognitive Neuroscience. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Ciaramelli E, Olson IR, Moscovitch M. The parietal cortex and episodic memory: an attentional account. Nature Reviews Neuroscience. 2008;9:613–625. doi: 10.1038/nrn2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoff K, Ream JM, Geddes LP, Gabrieli JD. Evaluating self-generated information: anterior prefrontal contributions to human cognition. Behavioral Neuroscience. 2003;117:1161–8. doi: 10.1037/0735-7044.117.6.1161. [DOI] [PubMed] [Google Scholar]
- Courtney SM, Petit L, Maisog JM, Ungerleider LG, Haxby JV. An area specialized for spatial working memory in human frontal cortex. Science. 1998;279:1347–1351. doi: 10.1126/science.279.5355.1347. [DOI] [PubMed] [Google Scholar]
- Courtney SM, Roth JK, Sala JB. A hierarchical biased-competition model of domain-dependent working memory maintenance and executive control. In: Osaka N, Logie R, D'Esposito M, editors. Working memory - behavioural and neural correlates. Oxford University Press; Oxford, UK: 2007. pp. 369–383. [Google Scholar]
- Cowan N. What are the differences between long-term, short-term, and working memory? Progress in Brain Research. 2008;169:323–338. doi: 10.1016/S0079-6123(07)00020-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–163. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Derrfuss J, Brass M, Neurmann J, von Cramon Y. Involvement of the inferior frontal junction in cognitive control: Meta-analyses of switching and stroop studies. Human Brain Mapping. 2005;25:22–34. doi: 10.1002/hbm.20127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbins IG, Han S. Cue- versus probe-dependent prefrontal cortex activity during contextual remembering. Journal of Cognitive Neuroscience. 2006;18:1439–1452. doi: 10.1162/jocn.2006.18.9.1439. [DOI] [PubMed] [Google Scholar]
- Dosenbach NU, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, Burgund ED, Grimes AL, Schlaggar BL, Petersen SE. A core system for the implementation of task sets. Neuron. 2006;50:799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends in Neuroscience. 2000;23:475–483. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magnetic Resonance in Medicine. 1995;33:636–47. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Gilbert SJ, Frith CD, Burgess PW. Involvement of rostral prefrontal cortex in selection between stimulus-oriented and stimulus-independent thought. European Journal of Neuroscience. 2005;21:1423–1431. doi: 10.1111/j.1460-9568.2005.03981.x. [DOI] [PubMed] [Google Scholar]
- Johnson MK, Hirst W. MEM: Memory subsystems as processes. In: Collins AF, Gathercole SE, Conway MA, Morris PE, editors. Theories of memory. Psychology Press; London: 1993. pp. 241–281. [Google Scholar]
- Johnson MK, Raye CL, Mitchell KJ, Greene EJ, Anderson AW. fMRI evidence for an organization of prefrontal cortex by both type of process and type of information. Cerebral Cortex. 2003;13:265–273. doi: 10.1093/cercor/13.3.265. [DOI] [PubMed] [Google Scholar]
- Johnson MK, Mitchell KJ, Raye CL, Greene EJ. An age-related deficit in prefrontal cortical function associated with refreshing information. Psychological Science. 2004;15:127–132. doi: 10.1111/j.0963-7214.2004.01502009.x. [DOI] [PubMed] [Google Scholar]
- Johnson MK, Raye CL, Mitchell KJ, Greene EJ, Cunningham WA, Sanislow CA. Using fMRI to investigate a component process of reflection: Prefrontal correlates of refreshing a just-activated representation. Cognitive Affective and Behavioral Neuroscience. 2005;5:339–361. doi: 10.3758/cabn.5.3.339. [DOI] [PubMed] [Google Scholar]
- Johnson MR, Mitchell KJ, Raye CL, D'Esposito M, Johnson MK. A brief thought can modulate activity in extrastriate visual areas: top-down effects of refreshing just-seen visual stimuli. Neuroimage. 2007;37:290–299. doi: 10.1016/j.neuroimage.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane MJ, Engle RW. Working-memory capacity, proactive interference, and divided attention: limits on long-term memory retrieval. Journal of Experimental Psychology Learning Memory and Cognition. 2000;26:336–358. doi: 10.1037//0278-7393.26.2.336. [DOI] [PubMed] [Google Scholar]
- Kelley TA, Serences JT, Giesbrecht B, Yantis S. Cortical mechanisms for shifting and holding visuospatial attention. Cerebral Cortex. 2007;18:114–125. doi: 10.1093/cercor/bhm036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koechlin E, Basso G, Pietrini P, Panzer S, Grafman J. The role of the anterior prefrontal cortex in human cognition. Nature. 1999;399:148–151. doi: 10.1038/20178. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Hyafil A. Anterior Prefrontal Function and the Limits of Human Decision-Making. Science. 2007;318:594–598. doi: 10.1126/science.1142995. [DOI] [PubMed] [Google Scholar]
- Lacadie CM, Fulbright RK, Rajeevan N, Constable RT, Papademetris X. More accurate Talairach coordinates for neuroimaging using nonlinear registration. Neuroimage. 2008;42:717–725. doi: 10.1016/j.neuroimage.2008.04.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald AW, III, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- Marklund P, Fransson P, Cabeza R, Petersson KM, Ingvar M, Nyberg L. Sustained and transient neural modulations in prefrontal cortex related to declarative long-term memory, working memory, and attention. Cortex. 2007;43:22–37. doi: 10.1016/s0010-9452(08)70443-x. [DOI] [PubMed] [Google Scholar]
- Marois R, Chun MM, Gore JC. A common parieto-frontal network is recruited under both low visibility and high perceptual interference conditions. Journal of Neurophysiology. 2004;92:2985–2992. doi: 10.1152/jn.01061.2003. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Rettinger DA, Shah P, Hegarty M. How are visuospatial working memory, executive functioning, and spatial abilities related? A latent variable analysis. Journal of experimental psychology: General. 2001;4:621–640. doi: 10.1037//0096-3445.130.4.621. [DOI] [PubMed] [Google Scholar]
- Montojo CA, Courtney SM. Differential neural activation for updating rule versus stimulus information in working memory. Neuron. 2008;59:173–182. doi: 10.1016/j.neuron.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickel J, Seitz RJ. Functional clusters in the human parietal cortex as revealed by an observer-independent meta-analysis of functional activation studies. Anatomy and Embryology. 2005;210:463–472. doi: 10.1007/s00429-005-0037-1. [DOI] [PubMed] [Google Scholar]
- Nyberg L, Marklund P, Persson J, Cabeza R, Forkstam C, Petersson KM, Ingvar M. Common prefrontal activations during working memory, episodic memory, and semantic memory. Neuropsychologia. 2003;41:371–377. doi: 10.1016/s0028-3932(02)00168-9. [DOI] [PubMed] [Google Scholar]
- Papademetris X, Jackowski M, Rajeevan N, Okuda H, Constable RT, Staib LH. BioImage Suite: An integrated medical image analysis suite. Section of Bioimaging Sciences, Dept. of Diagnostic Radiology, Yale School of Medicine; http://www.bioimagesuite.org. [Google Scholar]
- Poldrack RA. Can cognitive processes be inferred from neuroimaging data? Trends in Cognitive Science. 2006;10:59–63. doi: 10.1016/j.tics.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Raye CL, Johnson MK, Mitchell KJ, Reeder JA, Greene EJ. Neuroimaging a single thought: Dorsolateral PFC activity associated with refreshing just-activated information. NeuroImage. 2002;15:447–453. doi: 10.1006/nimg.2001.0983. [DOI] [PubMed] [Google Scholar]
- Raye CL, Johnson MK, Mitchell KJ, Greene EJ. Refreshing: A minimal executive function. Cortex. 2007;43:135–145. doi: 10.1016/s0010-9452(08)70451-9. [DOI] [PubMed] [Google Scholar]
- Raye CL, Mitchell KJ, Reeder JA, Greene EJ, Johnson MK. Refreshing one of several active representations: Behavioral and functional magnetic resonance imaging differences between young and older adults. Journal of Cognitive Neuroscience. 2008;20:852–862. doi: 10.1162/jocn.2008.20508. [DOI] [PubMed] [Google Scholar]
- Roth JK, Serences JT, Courtney SM. Neural system for controlling the contents of object working memory in humans. Cereb Cortex. 2006;16:1595–1603. doi: 10.1093/cercor/bhj096. [DOI] [PubMed] [Google Scholar]
- Roth JK, Courtney SM. Neural system for updating object working memory from different sources: sensory stimuli or long-term memory. NeuroImage. 2007;38:617–630. doi: 10.1016/j.neuroimage.2007.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth MF, Behrens TE, Johansen-Berg H. Connection patterns distinguish 3 regions of human parietal cortex. Cerebral Cortex. 2006;16:1418–30. doi: 10.1093/cercor/bhj079. [DOI] [PubMed] [Google Scholar]
- Scheperjans F, Eickhoff SB, Hömke L, Mohlberg H, Hermann K, Amunts K, Zilles K. Probabilistic maps, morphometry, and variability of cytoarchitectonic areas in the human superior parietal cortex. Cereb Cortex. 2008;18:2141–2157. doi: 10.1093/cercor/bhm241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serences JT, Schwarzbach J, Courtney SM, Golay X, Yantis S. Control of object-based attention in human cortex. Cerebral Cortex. 2004;14:1346–1357. doi: 10.1093/cercor/bhh095. [DOI] [PubMed] [Google Scholar]
- Serences JT, Shomstein S, Leber AB, Golay X, Egeth HE, Yantis S. Coordination of voluntary and stimulus-driven attentional control in human cortex. Psychological Science. 2005;16:114–122. doi: 10.1111/j.0956-7976.2005.00791.x. [DOI] [PubMed] [Google Scholar]
- Serences JT, Yantis S. Spatially selective representations of voluntary and stimulus-driven attentional priority in human occipital, parietal, and frontal cortex. Cerebral Cortex. 2007;17:284–293. doi: 10.1093/cercor/bhj146. [DOI] [PubMed] [Google Scholar]
- Shannon BJ, Buckner RL. Functional-anatomic correlates of memory retrieval that suggest nontraditional processing roles for multiple distinct regions within posterior parietal cortex. Journal of Neuroscience. 2004;24:10084–10092. doi: 10.1523/JNEUROSCI.2625-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EE, Jonides J. Storage and executive processes in the frontal lobes. Science. 1999;283:1657–1661. doi: 10.1126/science.283.5408.1657. [DOI] [PubMed] [Google Scholar]
- Visscher KM, Miezin FM, Kelly JE, Buckner RL, Donaldson DI, McAvoy MP, Bhalodia VM, Petersen SE. Mixed blocked/event-related designs separate transient and sustained activity in fMRI. NeuroImage. 2003;19:1694–1708. doi: 10.1016/s1053-8119(03)00178-2. [DOI] [PubMed] [Google Scholar]
- Yantis S, Serences JT. Cortical mechanisms of space-based and object-based attentional control. Current Opinions in Neurobiology. 2003;13:187–193. doi: 10.1016/s0959-4388(03)00033-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.