Abstract
Opioid agonists and antagonists respectively increase and decrease food intake. That selective mu opioid antagonists are more effective than antisense probes directed against the mu opioid receptor (MOR-1) gene in reducing deprivation-induced feeding suggests a role for isoforms. Both food restriction and deprivation alter protein and mRNA levels of opioid peptides and receptors. Antisera directed against exon 4 of the MOR-1-like immunoreactivity (LI) (Exon 4) clone or directed against mouse exons 7/8 (mE7/8-LI) revealed high levels of immunoreactivity in brain nuclei related to feeding behavior. Therefore, the present study assessed MOR-1LI and mE7/8-LI in hypothalamic and extra-hypothalamic sites in rats exposed to ad libitum feeding, food restriction (2, 7, 14 days) or food deprivation (24, 48 h). MOR-1-LI displayed robust reactivity, but was insensitive to food restriction or deprivation. mE7/8-LI, both in terms of cell counts and relative optical density, was significantly and selectively increased in the dorsal and ventral parvocellular subdivisions of the hypothalamic paraventricular nucleus in food-restricted (14 days) rats, but all other restriction or deprivation regimens were ineffective in other hypothalamic nuclei. In contrast, significant and site-specific decreases in relative optical density in the rostral part of the nucleus tractus solitarius were observed in food-restricted (2, 7 days) or food-deprived (24, 48 h) animals, but these regimens were ineffective in the other extrahypothalamic sites. This study indicates the sensitivity of this mE7/8-LI probe in the hypothalamic parvocellular paraventricular nucleus and rostral nucleus tractus solitarius to food restriction and deprivation in rats.
Keywords: Paraventricular Hypothalamic Nucleus, Nucleus Tractus Solitarius, Opioids
It is well established that manipulations of the endogenous opioid system significantly alter feeding behavior in that opioid agonists typically stimulate food intake, and opioid antagonists typically inhibit food intake (see reviews: Bodnar, 2004; Cooper et al., 1988; Gosnell and Levine, 1996; Levine et al., 1985; Morley et al., 1983). In turn, levels of opioid peptides, receptors and genes are altered by such behavioral states as food restriction (Aravich et al., 1993; Berman et al., 1994, 1997; Brady et al., 1990; Carr et al., 1998, 1999; Kim et al., 1996; Kotz et al., 1996; Tsujii et al., 1986a, 1986b; Wolinsky et al., 1994, 1996b), streptozotocin-induced diabetes (Berman et al., 1995, 1997; Kim et al., 1999; Locatelli et al., 1986; Wolinsky et al., 1996a), glucoprivation (Giraudo et al., 1998c), and food deprivation (Rodi et al., 2002), as well as exposure to palatable diets or animal models of obesity (Barnes et al., 2003; Kelley et al., 2003; Kim et al., 2000; Margules et al., 1978; Park and Carr, 1998; Pomonis et al., 2000; Roane et al., 1988; Tanda et al., 1998; Welch et al., 1996). General opioid antagonists decrease both food and water intake in deprived and non-deprived rats and mice following systemic administration (Brown and Holtzman, 1979; Cooper, 1980; Frenk and Rogers, 1979; Holtzman, 1974, 1975; Levine et al., 1990a; Maickel et al., 1977) as well as direct injections into the ventromedial (VMH), lateral (LH) and paraventricular (PVN) hypothalamic nuclei as well as the nucleus accumbens (NAC) and ventral tegmental area (VTA) (Bodnar et al., 1995; Kelley et al., 1996; Koch et al., 1995; Ragnauth et al., 1997; Thornhill and Saunders, 1984). Deprivation-induced feeding is decreased markedly by ventricular mu opioid antagonists, moderately by kappa opioid antagonists, and weakly by delta and mu1 opioid antagonists (Arjune and Bodnar, 1990; Arjune et al., 1990, 1991; Koch and Bodnar, 1994; Levine et al., 1990b, 1991; Simone et al., 1985; Ukai and Holtzman, 1988) with intracerebral studies indicating mu opioid antagonist effectiveness in the PVN and NAC, but not the VTA (Bodnar et al., 1995; Kelley et al., 1996; Koch et al., 1995; Ragnauth et al., 1997).
The identification of the mu (MOR-1), kappa (KOR-1), delta (DOR-1) and orphan (ORL1, orphanin FQ/nociceptin; OFQ/N) opioid receptor genes (see reviews: Pasternak, 2001; Uhl et al., 1994) and the subsequent development of antisense oligodeoxynucleotide (AS ODN) probes with sequences complementary to specific regions of mRNA to presumably down-regulate receptor proteins (see review: Pasternak and Standifer, 1995) allowed the study of the relationship of cloned opioid receptors to opioid-mediated actions in vivo. The use of highly selective AS ODN probes directed against individual exons of opioid receptor genes revealed unique exon-specific profiles of sensitivity of these probes to opioid agonists in analgesic studies (see review: Rossi et al. 1997) that could also be observed for opioid agonist-induced feeding responses. Feeding elicited by morphine and D-Ala2-Glyol4-enkephalin (DAMGO) were blocked by MOR-1 AS ODN probes directed against exons 1 and 4, but not exons 2 or 3, whereas feeding elicited by the morphine metabolite, morphine-6ß-glucuronide displayed the opposite pattern (Leventhal et al., 1997, 1998). Feeding elicited by beta-endorphin (BEND) was most potently blocked by mu, secondarily by kappa and minimally by delta opioid antagonists, and was most potently blocked by AS ODN probes directed against MOR-1 (exons 1, 3 and 4), and minimally by probes directed against the exons of the DOR-1, KOR-1 and ORL1 genes (Silva et al., 2001). In contrast, feeding elicited by dynorphin A(1-17) (DYN) was most potently blocked by kappa, secondarily by mu and minimally by delta opioid antagonists, and was most potently blocked by AS ODN probes directed against KOR-1 and ORL1 (exons 1 and 2) and minimally by probes against DOR-1 and MOR-1 (Silva et al., 2002).
MOR-1 AS ODN probes effectively reduced feeding and body weight under spontaneous intake conditions (exons 1, 2, 3 and 4: Leventhal et al., 1996), and markedly reduced intake following either glucoprivation (exons 1 and 2: Burdick et al., 1998) or lipoprivation (exons 1, 2 and 3: Stein et al., 2000). In contrast to the rank-order potency of mu > kappa > delta opioid antagonist effects upon deprivation-induced feeding, potent reductions in deprivation-induced feeding were only observed in the rat following a KOR-1 AS ODN probe (exon 2). Significant though modest reductions were noted for deprivation-induced feeding following MOR-1 AS ODN probes (exons 2, 3 and 4: Hadjimarkou et al., 2003). Further, significant reductions in deprivation-induced feeding following AS probes directed against either exons 2, 4, 7, 8, or 13 of MOR-1 in mice also were modest when compared with mu antagonists (Hadjimarkou et al., 2004). The differential actions of MOR-1 AS ODN probes upon agonist-induced and environmentally-induced ingestive responses in general, and upon deprivation-induced intake in particular, thereby suggested that MOR-1 itself may not be fully responsible for all mu-mediated effects, but rather these effects might be mediated by recently-identified MOR-1 isoforms (Bare et al., 1994; Pan et al., 1999, 2000, 2001, 2005a, 2005b; Pasternak and Pan, 2000; Pasternak et al., 2004; Zhang et al., 2006; Zimprich et al., 1995). Anatomical localization of immunoreactivity of some of these MOR-1 selective probes (MOR-1, MOR-1C, MOR-1D, MOR-1G, MOR-1M, MOR-1N) indicated important differences in density and distribution in mouse brain (Abbadie and Pasternak, 2001; Abbadie et al., 2000a, 2000b; 2001 2004; Ding et al., 1996). Importantly, immunoreactivity elicited by the MOR-1 and particularly, the mMOR-1C (characterized by mE7/8-LI) probes, were differentially localized in sites intimately implicated in the opioid mediation of ingestive behavior (see reviews: Bodnar, 2004; Glass et al., 1999; Gosnell and Levine, 1996), including the PVN, periventricular, VMH, and arcuate hypothalamic nuclei as well as extra-hypothalamic areas such as the amygdala, BNST, NAC, lateral septum, parabrachial nucleus (PBN) and nucleus tractus solitarius (NTS).
Therefore, the goal of this study was to examine central adaptive changes and opioid receptor plasticity in MOR-1-LI and mE7/8-LI in rats exposed to different levels of food restriction (2, 7, 14 days, or 14 days followed by a 7-day recovery period) or food deprivation (24, 48 h or 48 h followed by a 7-day recovery period).
MATERIALS AND METHODS
Subjects and Experimental Procedures
All experimental procedures were approved by the Queens College Institutional Animal Care and Use Committee. Fifty-two young (~80 days of age) male adult Sprague-Dawley rats (~325 g, Charles River Laboratories) were housed individually in wire mesh cages and maintained on a 12 h light: 12 h dark cycle with water available ad libitum. The groups were matched for body weight and subdivided into the following eight experimental conditions over a 21-day paradigm: ad libitum access to food over the time course (group 1, n=12), food restriction for either two (group 2, n=5), seven (group 3, n=5) or fourteen (group 4, n=12) days prior to sacrifice, fourteen days of food restriction followed by seven days of ad libitum feeding prior to sacrifice (group 5, n=5), food deprivation for either 24 h (group 6, n=10) or 48 h prior to sacrifice (group 7, n=5), and 48 h of food deprivation followed by seven days of ad libitum feeding prior to sacrifice (group 8, n=4). Thus, ad libitum control animals (Group 1) had water and food available at all times, whereas food-deprived animals (Groups 6-8) had no food available. Animals in the food-restriction paradigm (Groups 2-5) had food provided to them in restricted amounts (~15g/day). The restricted group typically consumed their entire food allotment each day. Food intake and body weight were monitored on Days 1, 5, 10, 15 and 21 of the experimental procedure with fresh food provided to the ad libitum and restriction groups immediately after determining body weight.
Immunohistochemistry
On the morning of the last day of each experimental condition, rats were deeply anesthetized with euthasol (Delmarva, Henry Schein, NY) and transcardially perfused with 0.9% saline in 0.1 M phosphate buffer (PB: pH=7.4; 50 ml) followed by 4% formaldehyde (in 0.1 M PB, 300 ml). The brains were removed and postfixed in the same fixative solution overnight, and then cryoprotected in 30% sucrose (in 0.1 M PB). Coronal sectioning (40 μm) was performed on a freezing microtome (Leica) and sections from brainstem, hypothalamic and forebrain structures were collected. The brains were coded so that the person sectioning, processing and evaluating the tissue was uninformed about the animals' experimental condition.
Immunostaining was performed according to the avidin-biotin peroxidase method (Hsu et al., 1981). Sections were incubated for 1 h with a solution of 0.1 M PB with 0.9% saline, 3% normal goat serum and 0.3% Triton-X100 before being left to incubate overnight at room temperature in the primary antiserum. The sections were washed and then incubated in biotinylated goat anti-rabbit IgG (1:200; Vector Labs, Burlingame, CA) and avidin-biotin-peroxidase complex (1:100; Vector Labs). To localize the horseradish peroxidase (HRP) immunoreaction product, an adapted nickel-intensified diaminobenzidine protocol with glucose oxidase (Llewellyn-Smith and Minson, 1992) was used. Finally, the sections were washed in phosphate buffer, mounted on gelatin-coated slides, dried and coverslipped with DPX (Aldrich, Milwaukee, WI).
The antibodies used for these experiments, which recognize an epitope in the carboxy terminus of MOR-1 and mE7/8 respectively, were previously characterized (Arvidsson et al., 1995; Abbadie et al., 2000a, 2000b). The MOR-1 has an amino acid carboxy terminus coded by exon 4 (LENLEAETAPLP), and its protein sequence and epitope is the same in mouse, rat and human, so the epitope is identical in all three species. The mouse mE7/8 isoform has an amino acid carboxy terminus coded by exons 7 and 8 (PTLAVSVAQIFTGYPSPTHVEKPCKSCMDRGMRNLLPDDGPRQESGEGQLGR) (see: Abbadie et al., 2000a for details). The Exon 4-LI (MOR-1-LI) antiserum from guinea pig (Chemicon, Temucula, CA) was generated from the terminal 15 amino acids comprising the intracellular carboxy tail, corresponding to the complete exon 4 and the adjacent three amino acids encoded by exon 3. The mE7/8-LI antisera was generated against a 20-residue peptide (underlined above) present in the mouse mMOR-1C (Multiple Peptide Systems, San Diego, CA). There is strong homology between exon 7 in the mouse and rat, but exon 8 diverges between the two species. This is likely why this antiserum does not recognize the cloned rMOR-1C1 and rMOR-1C2 isoforms previously reported in the rat (PCKSYRDRPRPCGRTWSLKSRAESNVEHFHCGAALIYNNVNFI; see review: Pan, 2005; Pasternak et al., 2004). However, the mE7/8-LI antisera employed in the present study yields a regional distribution in the rat homologous to that previously reported in the mouse (Abbadie et al., 2000a, 2000b, 2001), suggesting that it labels a rat variant homologous to the mouse mMOR-1C (see review: Pan, 2005). The mE7/8-LI antiserum was used in a dilution of 1:500 for peroxidase labeling. While the antibodies used in the present study have been well characterized, it is always possible that a portion of the labeling obtained with each antibody may reflect detection of similar or identical peptides in a different protein. Thus, for all data and discussion, the terms “labeling” or “staining” should be understood to mean antigen-like immunoreactivity.
Quantification of Immunoreactivity and Statistical Analysis
For each anatomical region, immunoreactivity was measured in 2 sections per nucleus of interest from each rat using a computer-assisted image analysis system (Spot, Mac, NIH Image). First, a 10X objective and a CCD camera were used to capture an image of the region of interest, and relative optical density was then measured. The sections were selected based on the degree of staining and were matched at their anatomical level. In regions where cell bodies were clearly defined, cell profile counts were assessed in addition to optical density, using stereological controls by only counting all of the cells that were in focus in each region. In the case of the PVN, for which subdivisions are at the same level, the counting was performed separately and separate analyses were performed for each one of the subdivisions (see Results). In assessing density, the mean optical density of the labeling was measured in a defined area and, in order to account for background, this value was subtracted from a same-size area of a control zone in the same section. In order to reduce variability to the immunohistochemistry reaction, a single rat from a number of the experimental groups was reacted at the same time. The investigators responsible for measuring the optical density and cell counts displayed very strong (over 90%) inter-rater reliability, and were uninformed about the specific experimental condition for each animal.
The following hypothalamic regions were examined: PVN (separate dorsal and ventral parvocellular and magnocellular zones), periventricular nucleus, arcuate nucleus and VMH. It should be noted that each of these sites have been implicated in the opioid control of ingestive behavior, and that previous studies indicate weak expression of rMOR-1-LI in hypothalamic nuclei (Ding et al., 1996) and strong expression of rMOR-1C-LI in hypothalamic nuclei (Abbadie et al., 2000a). The extra-hypothalamic regions examined were the following: lateral septum, bed nucleus of the stria terminalis (BNST), amygdala, PBN and the rostral NTS. They were chosen either for their direct opioid mediation of ingestive behavior (see review: Bodnar, 2004), and/or because of opioid peptide/receptor or c-fos changes following food deprivation or food restriction (e.g., Berman et al., 1994, 1997; Carr et al., 1998, 1999; Tsujii et al., 1986a, 1986b; Wolinsky et al., 1994, 1996b). Although the NTS can also be subdivided into rostral and caudal portions, only the rostral part of the NTS was used for quantification based on its level of staining.
All values are expressed as mean (±SEM). One-way analyses of variance were performed to assess significant effects across conditions for each measure in each site, and Tukey comparisons (P<0.05) to ascertain individual significant effects.
RESULTS
Body Weight Changes Across Experimental Conditions
Animals in the eight groups displayed matched weights on the first experimental day (F(7,48)= 0.019, n.s.; Mean ~325 g). Significant differences were observed among groups (F(7,70)= 41.13, P<0.0001), between the pre-treatment and post-treatment conditions (F(1,10)= 55.42, P<0.0001) and for the interaction between groups and treatments (F(7,70)= 85.83, P<0.0001). Thus, whereas control (ad libitum) rats systematically gained weight over the experimental regimen (79 g over 21 days: ~4 g/day), the two deprivation groups of rats displayed significant reductions in weight at 24 (pre: 370 g; post: 358 g) and 48 (pre: 385g; post: 360 g) h of deprivation respectively. In contrast, rats deprived of food for 48 h (341 g) and sacrificed 7 days later (7 days ad lib) recovered their body weight (411 g). Time-dependent and significant reductions in weight were noted for animals that were restricted for 2 (pre: 398 g; post: 376 g), 7 (pre: 375 g; post: 342 g) and 14 (pre: 325 g; post: 307 g) days. In contrast, rats that were food-restricted for 14 days (276 g) and sacrificed 7 days later recovered their body weight (365 g).
Food Restriction, Food Deprivation and MOR-1-LI
Neither food restriction nor food deprivation significantly altered either the density or cell number of MOR-1LI in any of the nuclei examined (data not shown), suggesting that these manipulations failed to change MOR-1 gene expression. In contrast, site-specific and condition-specific changes in mE7/8-LI were observed across the sites.
Food Restriction and mE7/8-LI in the PVN
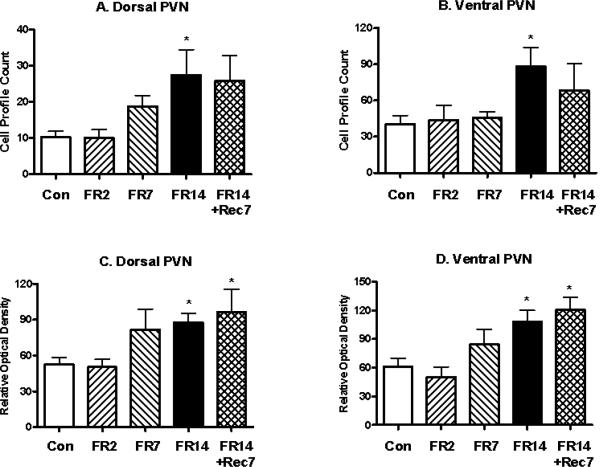
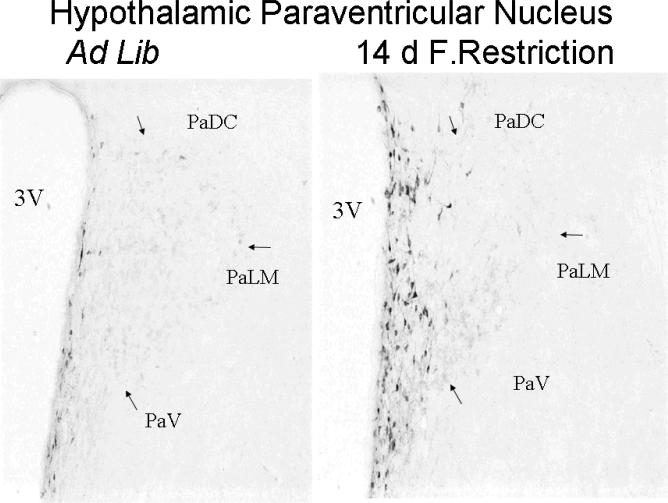
The PVN was examined in three different parts: the magnocellular division of the PVN and the dorsal and ventral parvocellular subdivisions of the PVN. Whereas mE7/8-LI was present in the two parvocellular PVN subdivisions (see below), the magnocellular division of the PVN was essentially devoid of mE7/8-LI in either control or restricted animals (data not shown). In contrast, significant differences in cell number were observed among the control and restriction conditions in the dorsal (F(4,32)= 2.58, p<0.05) and ventral (F(4,32)= 2.76, p<0.05) parvocellular PVN subdivisions. As the length of restriction increased, a systematic corresponding increase in the number of mE7/8-LI immunopositive cells was observed for the dorsal (Figure 1A) and ventral (Figure 1B) parvocellular PVN subdivisions with 14 days of food restriction producing significant increases in both sites. Interestingly, animals restricted for 14 days and then allowed ad libitum access to food for seven days (recovery) persistently displayed comparable increases in the number of mE7/8-LI immuopositive cells in both parvocellular subdivisions despite the return of the animals in this group to normal body weight. Correspondingly, significant differences in optical density were observed among the control and restriction conditions in the dorsal (F(4,32)= 4.14, p<0.01) and ventral (F(4,32)= 5.54, p<0.01) parvocellular PVN subdivisions. Again, as the length of restriction increased, a systematic corresponding increase in mE7/8-LI optical density was observed for the dorsal (Figure 1C) and ventral (Figure 1D) parvocellular PVN subdivisions with 14 days of food restriction producing significant results. Accordingly, 14-day food-restricted rats allowed 7 days of ad libitum recovery also displayed significant increases in mE7/8-LI in the dorsal (Figure 1C) and ventral (Figure 1D) parvocellular PVN. Figure 2 illustrates the greater intensity of mE7/8-LI in the dorsal and ventral parvocellular PVN subdivisions in a representative animal exposed to 14 days of food restriction (Figure 2B) relative to a representative control animal under ad libitum feeding conditions (Figure 2A).
Figure 1.
Alterations (Mean, ±SEM) in the number of hypothalamic paraventricular nucleus (PVN) cells (Panels A and B) and in the optical density of these cells (Panels C and D) with mE7/8-LI in the dorsal (Panels A and C) and ventral (Panels B and D) parvocellular subdivisions of the PVN in animals food restricted (FR) for 2 (FR2), 7 (FR7) or 14 (FR14) days relative to either ad libitum fed control (Con) animals or animals restricted for 14 days and then allowed ad libitum access to food for 7 days (FR14+Rec7). It should be noted that the magnocellular subdivision of the PVN had minimal mE7/8-LI in either ad libitum-fed or restricted animals. The asterisks (*) in this and subsequent figures denote significant alterations in a measure relative to ad libitum-fed controls.
Figure 2.
Representative photomicrographs of mE7/8-LI in the PVN in animals that were either fed ad libitum (left panel) or food restricted for 14 days (right panel).
Food Deprivation and mE7/8-LI in the PVN
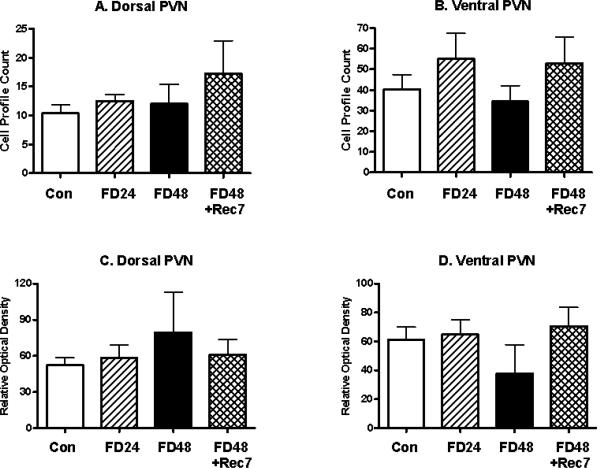
The effects of food restriction in the parvocellular PVN upon mE7/8-LI appeared to be condition-specific and limited to restriction-induced weight loss. First, the magnocellular division of the PVN was again devoid of mE7/8-LI both in terms of optical density or cell counts in control or animals deprived for 24 or 48 h. However, significant differences in the number of cells failed to be observed among the control and any of the deprivation conditions in the dorsal (F(3,22)= 1.12, ns; Figure 3A) and ventral (F(3,22)= 0.91, ns; Figure 3B) parvocellular PVN subdivisions. Moreover, significant differences in optical density failed to be observed among the control and any of the deprivation conditions in the dorsal (F(3,22)= 0.59, ns; Figure 3C) and ventral (F(3,22)= 1.07 ns; Figure 3D) parvocellular PVN subdivisions.
Figure 3.
Alterations (Mean, ±SEM) in the number of cells (Panels A and B) and in the optical density of cells (Panels C and D) with mE7/8-LI in the dorsal (Panels A and C) and ventral (Panels B and D) parvocellular subdivisions of the PVN in animals food deprived (FD) for 24 (FD24) or 48 (FD48) h relative to either ad libitum fed control (Con) animals or animals deprived for 48 h and then allowed ad libitum access to food for 7 days (FD48+Rec7).
Food Restriction, Food Deprivation and mE7/8-LI in Other Hypothalamic Sites
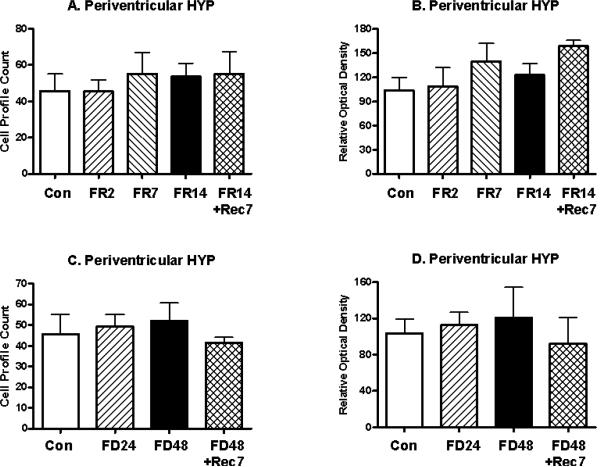
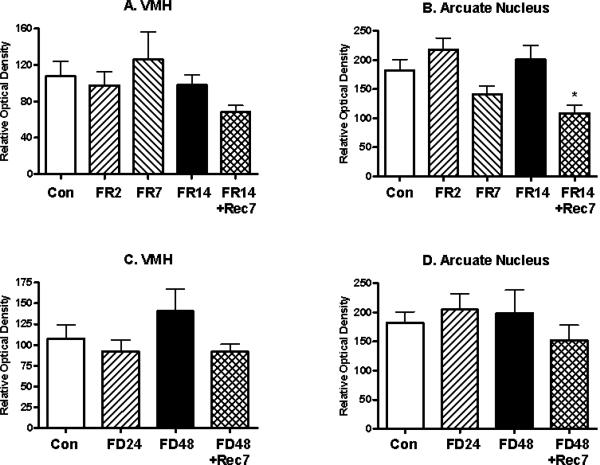
The effects of food restriction in the parvocellular PVN upon mE7/8-LI also appeared to be site-specific within the hypothalamus. Thus, in the adjacent periventricular hypothalamus, mE7/8-LI failed to differ for food-restricted animals relative to controls in either cell number (F(4,32)= 0.24, ns; Figure 4A) or optical density (F(4,32)= 1.36, ns; Figure 4B) or for food-deprived animals relative to controls in either cell number (F(3,22)= 0.17, ns; Figure 4C) or optical density (F(3,22)= 0.24, ns; Figure 4D). Due to the fact that the cells in the VMH and the arcuate nuclei were not clearly defined, only the optical density measures were used to assess changes in immunoreactivity. The optical density of mE7/8-LI in the VMH failed to differ for food-restricted animals (F(4,32)= 1.07, ns; Figure 5A) or for food-deprived animals (F(4,32)= 1.14, ns; Figure 5C) relative to controls. Although the optical density of mE7/8-LI in the arcuate nucleus significantly differed among food restriction conditions (F(4,32)= 2.97, p<0.05; Figure 5B), this was entirely due to a significantly reduced density observed only in animals restricted for 14 days and then allowed ad libitum access to food for seven days. In contrast, the optical density of mE7/8-LI in the arcuate nucleus failed to differ for food-deprived animals relative to controls (F(3,32)= 0.57, ns; Figure 5D).
Figure 4.
Alterations (Mean, ±SEM) in the number of cells (Panels A and C) and in the optical density (Panels B and D) of mE7/8-LI in the periventricular hypothalamus in animals food restricted (FR) for 2 (FR2), 7 (FR7) or 14 (FR14) days relative to either ad libitum fed control (Con) animals or animals allowed 7 days to recover (FR14+Rec7) (Panels A and B) as well as animals food deprived (FD) for 24 (FD24) or 48 (FD48) h relative to either ad libitum fed control (Con) animals or animals allowed 7 days to recover (FD48+Rec7) (Panels C and D).
Figure 5.
Alterations (Mean, ±SEM) in the optical density of mE7/8-LI in either the hypothalamic ventromedial (VMH: Panels A and C) or arcuate (Panels B and D) nuclei in animals either food restricted (FR) for 2(FR2), 7 (FR7) or 14 (FR14) days (Panels A and B) or food deprived (FD) for 24 (FD24) or 48 (FD48) h (Panels C and D) relative to either ad libitum fed control (Con) animals or animals allowed 7 days to recover (FR14+Rec7 and FD48+Rec7 respectively).
Food Restriction, Food Deprivation and mE7/8-LI in Extra-hypothalamic Sites
Site-specific, but not condition-specific effects were observed for mE7/8-LI in extra-hypothalamic sites. Again, the cells identified using mE7/8-LI in the rostral NTS, PBN, lateral septum, BNST and amygdala were poorly defined, thus cell counts were not formally analyzed. Furthermore, optical density measures of mE7/8-LI in the BNST and amygdala were quite variable, and therefore not formally analyzed.
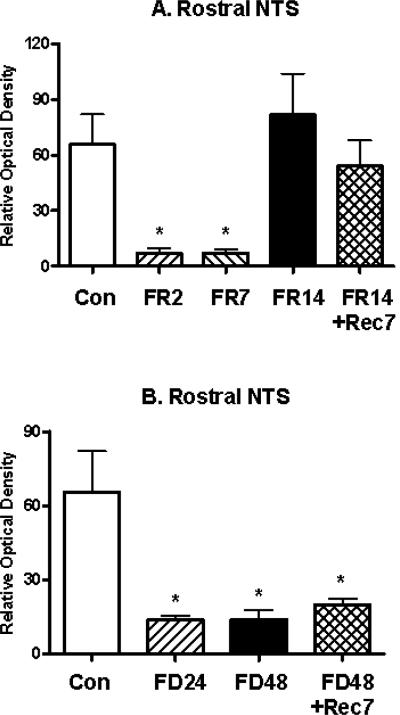
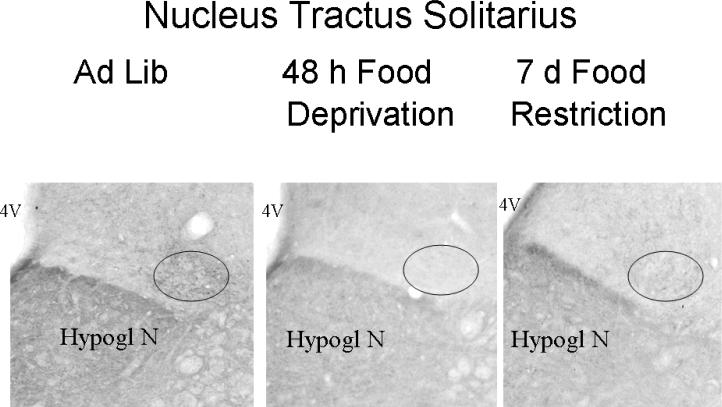
In the rostral NTS, significant differences in the optical density of mE7/8-LI were observed in food-restricted animals (F(4,32)= 3.04, p<0.05; Figure 6A) and in food-deprived animals (F(3,32)= 3.90, p<0.05; Figure 6B) relative to ad libitum-fed controls. Interestingly, the direction and pattern of effects differed from that observed in the parvocellular PVN. Thus, animals restricted for either 2 or 7 days displayed significant reductions in the density of rostral NTS mE7/8-LI relative to controls, whereas normal density measures were observed in animals restricted for 14 days and in animals restricted for 14 days followed by seven days of recovery (Figure 6A). However, animals deprived of food for 24 or 48 h displayed significant reductions in the density of rostral NTS mE7/8-LI relative to controls; this effect persisted in animals deprived for 48 h followed by a 7-day recovery period (Figure 6B). Figure 7 illustrates the reduction in intensity in mE7/8-LI in the rostral NTS in representative animals exposed to either 7 days of food restriction (Figure 7B) or 48 h of food deprivation (Figure 7C) relative to a representative control animal under ad libitum feeding conditions (Figure 7A). As indicated earlier, we only observed consistent mE7/8-LI staining in the rostral NTS, and not in its more caudal subdivisions. Therefore, the caudal extent of the NTS was not formally examined for deprivation and restriction effects.
Figure 6.
Alterations (Mean, ±SEM) in the optical density of mE7/8-LI in the nucleus tractus solitarius (NTS) in animals either food restricted (FR) for 2 (FR2), 7 (FR7) or 14 (FR14) days (Panel A) or food deprived (FD) for 24 (FD24) or 48 (FD48) h (Panel B) relative to either ad libitum fed control (Con) animals or animals allowed 7 days to recover (FR14+Rec7 and FD48Rec7 respectively).
Figure 7.
Representative photomicrographs of mE7/8-LI in the NTS in animals that were either fed ad libitum (Panel A), food restricted for 7 (Panel B) or food deprived for 48 h (Panel C).
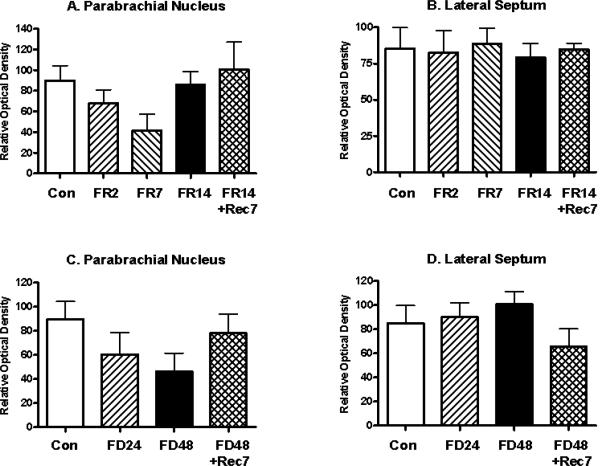
In contrast, the optical density of mE7/81C-LI in the PBN failed to differ for food-restricted animals (F(4,32)= 1.44, ns; Figure 8A) or for food-deprived animals (F(3,32)= 1.42, ns; Figure 8C) relative to controls. Moreover, the optical density of mE7/8-LI in the lateral septum failed to differ for food-restricted animals (F(4,32)= 0.78, ns; Figure 8B) or for food-deprived animals (F(3,32)= 0.72, ns; Figure 8D) relative to controls.
Figure 8.
Alterations (Mean, ±SEM) in the optical density of mE7/8-LI in the parabrachial nucleus (Panels A and C) or lateral septum (Panels B and D) in animals either food restricted (FR) for 2 (FR2), 7 (FR7) or 14 (FR14) days (Panels A and B) or food deprived (FD) for 24 (FD24) or 48 (FD48) h (Panels C and D) relative to either ad libitum fed control (Con) animals or animals allowed 7 days to recover (FR14+Rec7 and FD48+Rec7 respectively).
DISCUSSION
This study reports the following five major findings: a) neither food restriction nor food deprivation appreciably altered MOR-1-LI; b) the parvocellular subdivisions, but not the magnocellular subdivision of the PVN displayed significant time-dependent increases in the number and density of mE7/8-LI following extended food restriction, but not following extended food deprivation, an effect that persisted in animals despite a subsequent seven-day ad libitum recovery period; c) hypothalamic changes in mE7/8-LI following food restriction were limited to the PVN, and not the periventricular, ventromedial or arcuate nuclei; d) the rostral NTS displayed significant time-dependent reductions in the density of mE7/8-LI following brief exposure to either food restriction (2 and 7 days) or deprivation (24 and 48 h); and e) these effects were not observed in other extra-hypothalamic sites (PBN, lateral septum, BNST, amygdala) associated with opioid mediation of feeding.
Differential MOR-1-LI and mE7/8-LI Distribution in Feeding-Sensitive Sites
Previous anatomical studies in mice and rats have indicated differential distributions of the MOR-1 and mE7/8 isoforms (Abbadie et al., 2000a, 2000b, 2001, 2004; Ding et al., 1996). In examining sites either directly related to the intracerebral ingestive actions of opioid agonists and antagonists, and/or sites in which opioid peptides, receptors or mRNA is changed as a function of ingestive state, the present study indicated quite striking site-specific differences in immunoreactivity. Thus, the MOR-1-LI was greater than mE7/8-LI in two brainstem sites, the rostral NTS and PBN, and in two of the forebrain sites, the NAC (core and shell) and the central nucleus of the amygdala (Table I). In contrast, mE7/8-LI was greater than MOR-1-LI in all four hypothalamic nuclei (PVN, periventricular, arcuate and VMH) as well as the BNST and lateral septum (Table I). It is important to note that these differences are matters of degree, not a situation in which one isoform is present and the other is absent. It is also important to note that the intensity of immunoreactivity may not have anything to do with the abundance of the proteins because different antibodies can yield different intensities. This caveat is also extended to the antisera probes used in the present study which were derived an epitope from the C-terminus of the mE7/8-LI isoform (PTLAVSVAQIFTGYPSPTHVEKPCKSCMDRGMRNLLPDDGPRQESGEGQLGR) (see: Abbadie et al., 2000a for details). Although exon 7 in the mouse is highly homologous to the rat, the sequences of the mouse and rat exon 8 diverge, explaining why this antiserum does not recognize the cloned rMOR-1C1 and rMOR-1C2 isoforms previously reported in the rat (PCKSYRDRPRPCGRTWSLKSRAESNVEHFHCGAALIYNNVNFI; see review: Pan, 2005). Thus, the mE7/8-LI antisera employed in the present study may label a rat variant containing an exon homologous to the mouse exon 8 which underscores the complexity of the opioid receptor system with respect to its function in a yet another complex system of food intake homeostasis (see review: Pan, 2005; Pasternak et al., 2004).
TABLE I.
Anatomical distribution of MOR-1-LI and mE7/8-LI Immunoreactivity
| Site | MOR-1-LI | mE7/8-LI |
|---|---|---|
| Exon 4-LI | exons 7/8/9-LI | |
| Lateral Septum | + | +++ |
| Nucleus Accumbens, Core and Shell | ++ | -/+ |
| Bed Nucleus of the Stria Terminalis | -/+ | ++ |
| Amygdala, Central Nucleus | ++ | -/+ |
| Paraventricular Hypothalamic Nucleus | -/+ | ++ |
| Periventricular Hypothalamic Nucleus | -/+ | ++ |
| Arcuate Nucleus and Median Eminence | +/++ | +++/++++ |
| Ventromedial Hypothalamic Nucleus | -/+ | + |
| Parabrachial Nucleus, Lateral and Medial Divisions | ++/+++ | +/++ |
| Nucleus of the Solitary Tract | +++ | + |
Intensity of immunoreactivity: -/+, absent to minimal; +, low; ++, moderate; +++, strong; ++++, intense (Abbadie et al, 2000a,b).
Minimal Sensitivity of MOR-1-LI to Food Restriction or Food Deprivation
Neither food restriction nor food deprivation significantly altered either the density or number of MOR-1-LI in any of the nuclei examined, suggesting that these manipulations failed to change MOR-1 gene expression in these opioid-sensitive ingestion-related sites. Deprivation-induced feeding is reduced to the same degree by general and mu-selective opioid antagonists (Arjune et al., 1990; Bodnar et al., 1995; Brown and Holtzman, 1979; Cooper, 1980; Frenk and Rogers, 1979; Holtzman, 1974, 1975; Kelley et al., 1996; Koch and Bodnar, 1994; Koch et al., 1995; Levine et al., 1990a, 1991; Maickel et al., 1977; Ragnauth et al., 1997; Simone et al., 1985; Thornhill and Saunders, 1984; Ukai and Holtzman, 1988). Yet only modest, but significant reductions in deprivation-induced feeding occurred following administration of MOR-1 AS ODN probes in the rat and mouse (exons 2, 3 and 4: Hadjimarkou et al., 2003, 2004). This stands in contrast to the ability of MOR-1 AS ODN probes directed against each of its four exons to reduce spontaneous intake and body weight (Leventhal et al., 1996), and the ability of mu-selective antagonists and MOR-1 AS ODN probes to produce comparable levels of inhibition of feeding elicited by glucoprivation (Arjune et al., 1990; Burdick et al., 1998; Koch and Bodnar, 1994), lipoprivation (Stein et al., 2000) and by mu-selective opioid agonists (morphine, DAMGO, morphine-6β-glucuronide, BEND: Leventhal et al., 1997, 1998; Silva et al., 2001). The fact that MOR-1-LI is not appreciably altered by either food restriction or food deprivation complements the modest actions of MOR-1 AS ODN probes upon deprivation-induced feeding. Combined with the modest effects of other AS probes targeting exons 2, 4, 7, 8, or 13 in both the rat and mouse, these observations together suggest that the effects are mediated by combinations of variants, explaining why the antagonists, which block all the isoforms, are more effective than antisense, which targets a limited number (Hadjimarkou et al., 2004).
mE7/8-LI is Increased in the Parvocellular PVN Subdivisions following Food Restriction
The PVN is divided into magnocellular and parvocellular subdivisions with the former providing major axonal output to the neurohypophysis, and the latter providing output to the zona externa of the median eminence, to limbic and other forebrain areas, and to midbrain, hindbrain and spinal areas (e.g., Sawchenko and Swanson, 1982; Swanson and Kuypers, 1980; Swanson et al., 1980). The present study clearly demonstrated that mE7/8-LI was far more pronounced in the dorsal and ventral parvocellular subdivisions of the PVN relative to the magnocellular subdivision in control ad libitum-fed animals, and that the low levels of mE7/8-LI in the magnocellular PVN subdivision failed to be affected by either food restriction or food deprivation. In contrast, both the number of cells and the optical density of mE7/8-LI were significantly increased in animals placed on a food restriction schedule for 14 days, and even for animals that were food restricted for 14 days followed by 7 days of a return to ad libitum feeding. This effect was time-dependent in that two and seven days of food restriction monotonically though non-significantly increased mE7/8-LI. It should be noted that absolute food deprivation over the previous 24 or 48 h failed to alter mE7/8-LI, a lack of an effect that is not readily explained. Yet it should be noted that peptide levels of DYN A1-17 in the PVN are increased by either chronic food restriction (Berman et al., 1994, 1997; Tsujii et al., 1986b) or by palatability-induced hyperphagia in the absence of changes in met-enkephalin or BEND (Welch et al., 1996). Further, increased hypothalamic DYN levels are associated with corresponding increases in nocturnal intake (Przewlocki and Lason, 1982; Takahashi et al., 1986), and peripheral butorphanol, a mu/kappa opioid receptor agonist, stimulated PVN c-fos activity (Kim et al., 2001). Finally, food deprivation lowers PVN ORL-1 receptor mRNA (Rodi et al., 2002). Therefore, the parvocellular divisions of the PVN appear quite sensitive to several longer-term central adaptive changes in the opioid system by increasing the opioid peptide (e.g., DYN) or opioid receptor (Exon 7/8/9-LI) signals, following food restriction and even following a short-term (7 days) recovery from the restriction regimen. This effect is not surprising given the ability of opioid agonists and peptides to stimulate feeding following PVN microinjections (Gosnell et al., 1986; Kim et al., 2002; Leibowitz and Hor, 1982; McLean and Hoebel, 1982, 1983; Pomonis et al., 1996; Stanley et al., 1989; Tepperman and Hirst, 1983; Woods and Leibowitz, 1985). Further, naltrexone pretreatment in the PVN blocked DAMGO-induced feeding elicited from either the central nucleus of the amygdala (Giraudo et al., 1998a) or the VTA (Quinn et al., 2003). Moreover, general opioid antagonist pretreatment in the PVN modestly reduced neuropeptide Y (NPY)-induced feeding from the same site without affecting NPY-induced reductions in brown fat thermogenesis (Kotz et al., 1995). Importantly, deprivation-induced feeding is markedly reduced by PVN pretreatment with mu and kappa, but not delta opioid antagonists, effects greater than those observed for glucoprivic and palatable intake (Koch et al., 1995).
Site-Specificity of Hypothalamic Changes in mE7/8-LI following Food Restriction
The ability of food restriction to increase cell numbers and optical density of mE7/8-LI was limited to the parvocellular PVN, as negligible effects of either food restriction or food deprivation were noted for mE7/8-LI in the neighboring periventricular, ventromedial or arcuate hypothalamic nuclei. This is not to say that these sites are impervious to such central adaptive changes in the opioid system, since DYN A1-17 is increased in the VMH following either chronic food restriction (Berman et al., 1994, 1997; Tsujii et al., 1986b) or streptozotocin-induced diabetes (Berman et al., 1995, 1997). Moreover, whereas chronic food restriction decreases hypothalamic arcuate BEND and DYN (Brady et al., 1990; Kim et al., 1996), its combination with exercise increases hypothalamic BEND and DYN (Aravich et al., 1993). Therefore, it appears that separate hypothalamic components of the opioid system may be differentially responding to these homeostatic challenges.
mE7/8-LI in Extra-hypothalamic Sites Respond Differentially to Food Restriction and Food Deprivation
In contrast to increased mE7/8-LI in the parvocellular PVN following long-term food restriction, the rostral NTS displayed significant time-dependent reductions in the density of mE7/8-LI following brief exposure to either food restriction (2 and 7 days) or food deprivation (24 and 48 h). Moreover, these effects were specific to the rostral NTS, and not observed in the other extra-hypothalamic sites (PBN, lateral septum, BNST, amygdala) associated with opioid mediation of feeding. The immunohistochemical results were only derived from the rostral part of the NTS because the rostral NTS was the only area that displayed the most consistent mE7/8-LI staining in control animals. Interestingly, it is the rostral NTS that has been previously implicated in opioid-mediated feeding. Although microinjection of mu and delta opioid agonists into the rostro-caudal extent of the NTS elicits feeding (Kotz et al., 1997), a bidirectional opioid-opioid signaling pathway was identified between the rostral NTS and the central nucleus of the amygdala in that DAMGO-induced feeding elicited from one site (e.g., the rostral NTS) was blocked by naltrexone pretreatment in the other site (e.g., the central nucleus of the amygdala) and vice-versa (Giraudo et al., 1998b). Moreover, whereas DAMGO-induced feeding elicited from the NAC was blocked by rostro-caudal NTS treatment with muscimol (Will et al., 2003), rostral NTS c-fos activity was stimulated by butorphanol (Kim et al., 2001) and OFQ/N (Olszewski et al., 2000), and met-enkephalin suppressed neuronal activity induced by the sweet taste of sucrose only in the rostral NTS in a naltrexone-reversible manner (Li et al., 2003). This finding is consistent with the proposition (Glass et al., 1999) that opioids in the hindbrain, including the rostral NTS, are presumably involved in the sensory (taste) integration regulating food intake. Therefore, these factors would come very much into play following shorter periods of food restriction or food deprivation, and may provide a schema for the present findings that the optical density of mE7/8-LI is reduced in the rostral NTS following short food restriction or deprivation.
Conclusions
Thus, a mE7/8-LI antiserum that likely labels a rat variant homologous to the mouse MOR-1C receptor, is selectively altered by different durations of the regulatory challenges of food restriction and food deprivation. Thus, mE7/8-LI is selectively increased after 14 days of food restriction but not food deprivation, in the dorsal and ventral parvocellular subdivisions of the hypothalamic PVN, but not in other adjacent hypothalamic nuclei. In contrast, mE7/8-LI is selectively decreased after short exposure to restriction (2-7 days) or deprivation (24-48 h) in the NTS, but not in other extra-hypothalamic sites. Given the critical role of the PVN and NTS in pharmacological analysis of the opioid mediation of food intake, these anatomical and pharmacological findings taken together implicate these structures in the ability of isoforms or splice variants of the MOR-1 gene to regulate homeostatically driven ingestive behaviors.
Acknowledgements
This research was supported in part by a National Science Foundation Grant (IBN98-16699 to RJB), National Institute of Drug Abuse Grants (DA07242, DA02165 and DA00220 to GWP, and DA13997 to YXP), PSC/CUNY Grants (63278, 64298 and 65285 to RJB), CUNY Collaborative Grant (80209 to RJB) and CUNY Science Fellowships (MMH, LK).
REFERENCES
- Abbadie C, Pan YX, Drake CT, Pasternak GW. Comparative immunohistochemical distributions of carboxy terminus epitopes from the mu-opioid receptor splice variants MOR-1D, MOR-1 and MOR-1C in the mouse and rat CNS. Neurosci. 2000b;100:141–153. doi: 10.1016/s0306-4522(00)00248-7. [DOI] [PubMed] [Google Scholar]
- Abbadie C, Pan YX, Pasternak GW. Differential distribution in rat brain of mu opioid receptor carboxy terminal splice variants MOR-1C-like and MOR-1-like immunoreactivity: Evidence for region-specific processing. J Comp Neurol. 2000a;419:244–256. doi: 10.1002/(sici)1096-9861(20000403)419:2<244::aid-cne8>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Abbadie C, Pan YX, Pasternak GW. Immunohistochemical study of the expression of exon11-containing mu opioid receptor variants in mouse brain. Neurosci. 2004;127:419–430. doi: 10.1016/j.neuroscience.2004.03.033. [DOI] [PubMed] [Google Scholar]
- Abbadie C, Pasternak GW. Differential in vivo internalization of MOR-1 and MOR-1C by morphine. Neuroreport. 2001;12:3069–3072. doi: 10.1097/00001756-200110080-00017. [DOI] [PubMed] [Google Scholar]
- Abbadie C, Pasternak GW, Aicher SA. Presynaptic localization of the carboxy terminus epitopes of the mu opioid receptor splice variants MOR-1C and MOR-1D in the superficial laminae of the rat spinal cord. Neurosci. 2001;106:833–842. doi: 10.1016/s0306-4522(01)00317-7. [DOI] [PubMed] [Google Scholar]
- Aravich PF, Rieg TS, Lauterio TJ, Doerries LE. Beta-endorphin and dynorphin abnormalities in rats subjected to exercise and restricted feeding: relationship to anorexia nervosa? Brain Res. 1993;622:1–8. doi: 10.1016/0006-8993(93)90794-n. [DOI] [PubMed] [Google Scholar]
- Arjune D, Bodnar RJ. Suppression of nocturnal, palatable and glucoprivic intake in rats by the kappa opioid antagonist, nor-binaltorphamine. Brain Res. 1990;534:313–316. doi: 10.1016/0006-8993(90)90147-4. [DOI] [PubMed] [Google Scholar]
- Arjune D, Bowen WD, Bodnar RJ. Ingestive behavior following central [D-Ala2,Leu5,Cys6]-enkephalin (DALCE), a short-acting agonist and long-acting antagonist at the delta opioid receptor. Pharmacol Biochem Behav. 1991;39:429–436. doi: 10.1016/0091-3057(91)90203-e. [DOI] [PubMed] [Google Scholar]
- Arjune D, Standifer KM, Pasternak GW, Bodnar RJ. Reduction by central beta-funaltrexamine of food intake in rats under freely-feeding, deprivation and glucoprivic conditions. Brain Res. 1990;535:101–109. doi: 10.1016/0006-8993(90)91828-5. [DOI] [PubMed] [Google Scholar]
- Arvidsson U, Riedl M, Chakrabarti S, Lee J-H, Nakano AH, Dado RJ, Loh HH, Law P-Y, Wessendorf MW, Elde R. Distribution and targeting of a mu-opioid receptor (MOR1) in brain and spinal cord. J Neurosci. 1995;15:3328–3341. doi: 10.1523/JNEUROSCI.15-05-03328.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bare LA, Mansson E, Yang D. Expression of two variants of the human mu opioid receptor mRNA in SK-N-SH cells and human brain. FEBS Letters. 1994;354:213–216. doi: 10.1016/0014-5793(94)01129-x. [DOI] [PubMed] [Google Scholar]
- Barnes MJ, Lapanowski K, Conley A, Rafols JA, Jen KL, Dunbar JC. High fat feeding is associated with increased blood pressure, sympathetic nerve activity and hypothalamic mu opioid receptors. Brain Res Bull. 2003;61:511–519. doi: 10.1016/s0361-9230(03)00188-6. [DOI] [PubMed] [Google Scholar]
- Berman Y, Devi L, Carr KD. Effects of chronic food restriction on prodynorphin-derived peptides in rat brain regions. Brain Res. 1994;664:49–53. doi: 10.1016/0006-8993(94)91952-6. [DOI] [PubMed] [Google Scholar]
- Berman Y, Devi L, Carr KD. Effects of streptozotocin-induced diabetes on prodynorphin-derived peptides in rat brain regions. Brain Res. 1995;685:129–134. doi: 10.1016/0006-8993(95)00419-q. [DOI] [PubMed] [Google Scholar]
- Berman Y, Devi L, Spangler R, Kreek MJ, Carr KD. Chronic food restriction and streptozotocin-induced diabetes differentially alter prodynorphin mRNA levels in rat brain regions. Molec Brain Res. 1997;46:25–30. doi: 10.1016/s0169-328x(96)00175-1. [DOI] [PubMed] [Google Scholar]
- Bodnar RJ. Endogenous opioids and feeding behavior: a thirty-year historical perspective. Peptides. 2004;25:697–725. doi: 10.1016/j.peptides.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Bodnar RJ, Glass MJ, Ragnauth A, Cooper ML. General, mu and kappa opioid antagonists in the nucleus accumbens alter food intake under deprivation, glucoprivic and palatable conditions. Brain Res. 1995;700:205–212. doi: 10.1016/0006-8993(95)00957-r. [DOI] [PubMed] [Google Scholar]
- Brady LS, Smith MA, Gold PW, Herkenham M. Altered expression of hypothalamic neuropeptide mRNAs in food-restricted and food-deprived rats. Neuroend. 1990;52:441–447. doi: 10.1159/000125626. [DOI] [PubMed] [Google Scholar]
- Brown DR, Holtzman SJ. Suppression of deprivation induced food and water intake in rats and mice by naloxone. Pharmacol Biochem Behav. 1979;11:567–583. doi: 10.1016/0091-3057(79)90043-1. [DOI] [PubMed] [Google Scholar]
- Burdick K, Yu W-Z, Ragnauth A, Moroz M, Pan YX, Rossi GC, Pasternak GW, Bodnar RJ. Antisense mapping of opioid receptor clones: effects upon 2-deoxy-D-glucose-induced hyperphagia. Brain Res. 1998;794:359–363. doi: 10.1016/s0006-8993(98)00331-x. [DOI] [PubMed] [Google Scholar]
- Carr KD, Kutchukhidze N, Park TH. Differential effects of mu and kappa opioid antagonists on Fos-like immunoreactivity in extended amygdala. Brain Res. 1999;822:34–42. doi: 10.1016/s0006-8993(99)01088-4. [DOI] [PubMed] [Google Scholar]
- Carr KD, Park TH, Stone EA. Neuroanatomical patterns of Fos-like immunoreactivity induced by naltrexone in food-restricted and ad libitum fed rats. Brain Res. 1998;779:26–32. doi: 10.1016/s0006-8993(97)01074-3. [DOI] [PubMed] [Google Scholar]
- Cooper SJ. Naloxone: effects on food and water consumption in the non-deprived and deprived rat. Psychopharm. 1980;71:1–6. doi: 10.1007/BF00433244. [DOI] [PubMed] [Google Scholar]
- Cooper SJ, Jackson A, Kirkham TC, Turkish S. Endorphins, opiates and food intake. In: Rodgers RJ, Cooper SJ, editors. Endorphins, opiates and behavioral processes. John Wiley and Sons; New York: 1988. pp. 43–186. [Google Scholar]
- Ding YQ, Kaneko K, Nomura S, Mizuno N. Immunohistochemical localization of mu-opioid receptors in the central nervous system of the rat. J Comp Neurol. 1996;367:375–402. doi: 10.1002/(SICI)1096-9861(19960408)367:3<375::AID-CNE5>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Frenk H, Rogers GH. The suppressant effects of naloxone on food and water intake in the rat. Behav Neur Biol. 1979;26:23–40. doi: 10.1016/s0163-1047(79)92855-3. [DOI] [PubMed] [Google Scholar]
- Giraudo SQ, Kim EM, Grace MK, Billington DJ, Levine AS. Effect of peripheral 2-DG on opioid and neuropeptide Y gene expression. Brain Res. 1998c;792:136–40. doi: 10.1016/s0006-8993(98)00197-8. [DOI] [PubMed] [Google Scholar]
- Giraudo SQ, Billington CJ, Levine AS. Effects of the opioid antagonist naltrexone on feeding induced by DAMGO in the central nucleus of the amygdala and in the paraventricular nucleus in the rat. Brain Res. 1998a;782:18–23. doi: 10.1016/s0006-8993(97)01140-2. [DOI] [PubMed] [Google Scholar]
- Giraudo SQ, Kotz CM, Billington CJ, Levine AS. Association between the amygdala and the nucleus of the solitary tract in mu opioid induced feeding in the rat. Brain Res. 1998b;802:184–188. doi: 10.1016/s0006-8993(98)00602-7. [DOI] [PubMed] [Google Scholar]
- Glass MJ, Billington CJ, Levine AS. Opioids and food intake: distributed functional neural pathways? Neuropep. 1999;33:360–368. doi: 10.1054/npep.1999.0050. [DOI] [PubMed] [Google Scholar]
- Gosnell BA, Levine AS. Stimulation of ingestive behavior by preferential and selective opioid agonists. In: Cooper SJ, Clifton PG, editors. Drug receptor subtypes and ingestive behavior. Academic Press; London: 1996. pp. 147–166. [Google Scholar]
- Gosnell BA, Morley JE, Levine AS. Opioid-induced feeding: localization of sensitive brain sites. Brain Res. 1986;369:177–184. doi: 10.1016/0006-8993(86)90526-3. [DOI] [PubMed] [Google Scholar]
- Hadjimarkou MM, Khaimova E, Pan Y-X, Rossi GC, Pasternak GW, Bodnar RJ. Feeding induced by food deprivation is differentially reduced by opioid receptor antisense oligodeoxynucleotide probes in rats. Brain Res. 2003;987:223–232. doi: 10.1016/s0006-8993(03)03342-0. [DOI] [PubMed] [Google Scholar]
- Hadjimarkou MM, Singh A, Kandov Y, Israel Y, Pan Y-X, Rossi GC, Pasternak GW, Bodnar RJ. Opioid receptor involvement in food deprivation-induced feeding: evaluation of selective antagonist and antisense oligodeoxynucleotide probe effects in mice and rats. J Pharmacol Exp Ther. 2004;311:1188–1202. doi: 10.1124/jpet.104.071761. [DOI] [PubMed] [Google Scholar]
- Holtzman SG. Behavioral effects of separate and combined administration of naloxone and d-amphetamine. J Pharmacol Exp Ther. 1974;189:51–60. [PubMed] [Google Scholar]
- Holtzman SG. Effects of narcotic antagonists on fluid intake in the rat. Life Sci. 1975;16:1465–1470. doi: 10.1016/0024-3205(75)90043-0. [DOI] [PubMed] [Google Scholar]
- Hsu SM, Raine L, Fanger H. A comparative study of the peroxidase-antiperoxidase method and an avidin-biotin complex method for studying polypeptide hormones with radioimmunoassay antibodies. Am J Clin Pathol. 1981;75:734–738. doi: 10.1093/ajcp/75.5.734. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Bless EP, Swanson CJ. Investigation of the effects of opiate antagonists infused into the nucleus accumbens on feeding and sucrose drinking in rats. J Pharmacol Exp Ther. 1996;278:1499–1507. [PubMed] [Google Scholar]
- Kelley AE, Will MJ, Steininger TL, Zhang M, Haber SN. Restricted daily consumption of a highly palatable food (chocolate Ensure(R)) alters striatal enkepahlin gene expression. Eur J Neurosci. 2003;18:2592–2598. doi: 10.1046/j.1460-9568.2003.02991.x. [DOI] [PubMed] [Google Scholar]
- Kim EM, Grace MK, O'Hare E, Billington CJ, Levine AS. Injection of alpha-MSH, but not beta-endorphin, into the PVN decreases POMC gene expression in the ARC. Neuroreport. 2002;13:497–500. doi: 10.1097/00001756-200203250-00028. [DOI] [PubMed] [Google Scholar]
- Kim E-M, Grace MK, Welch CC, Billington CJ, Levine AS. STZ-induced diabetes decreases and insulin normalizes POMC mRNA in arcuate nucleus and pituitary in rats. Am J Physiol. 1999;276:R1320–R1326. doi: 10.1152/ajpregu.1999.276.5.R1320. [DOI] [PubMed] [Google Scholar]
- Kim EM, O'Hare E, Grace MK, Welch CC, Billington CJ, Levine AS. ARC POMC mRNA and PVN alpha-MSH are lower in obese relative to lean Zucker rats. Brain Res. 2000;862:11–16. doi: 10.1016/s0006-8993(00)02060-6. [DOI] [PubMed] [Google Scholar]
- Kim EM, Shi Q, Olszewski PK, Grace MK, O'Hare E, Billington CJ, Levine AS. Identification of central sites involved in butorphanol-induced feeding in rats. Brain Res. 2001;907:125–129. doi: 10.1016/s0006-8993(01)02322-8. [DOI] [PubMed] [Google Scholar]
- Kim E-M, Welch CC, Grace MK, Billington CJ, Levine AS. Chronic food restriction and acute food deprivation decrease mRNA levels of opioid peptides in the arcuate nucleus. Am J Physiol. 1996;270:R1019–R1024. doi: 10.1152/ajpregu.1996.270.5.R1019. [DOI] [PubMed] [Google Scholar]
- Koch JE, Bodnar RJ. Selective alterations in macronutrient intake of food-deprived or glucoprivic rats by centrally-administered opioid receptor subtype antagonists in rats. Brain Res. 1994;657:191–201. doi: 10.1016/0006-8993(94)90967-9. [DOI] [PubMed] [Google Scholar]
- Koch JE, Glass MJ, Cooper ML, Bodnar RJ. Alterations in deprivation, glucoprivic and sucrose intake following general, mu and kappa opioid antagonists in the hypothalamic paraventricular nucleus of rats. Neurosci. 1995;66:951–957. doi: 10.1016/0306-4522(95)00001-y. [DOI] [PubMed] [Google Scholar]
- Kotz CM, Billington CJ, Levine AS. Opioids in the nucleus of the solitary tract are involved in feeding in the rat. Am J Physiol. 1997;272:R1028–R1032. doi: 10.1152/ajpregu.1997.272.4.R1028. [DOI] [PubMed] [Google Scholar]
- Kotz CM, Grace MK, Briggs JE, Billington CJ, Levine AS. Naltrexone induces arcuate nucleus neuropeptide Y gene expression in the rat. Am J Physiol. 1996;271:R289–R294. doi: 10.1152/ajpregu.1996.271.1.R289. [DOI] [PubMed] [Google Scholar]
- Kotz CM, Grace MK, Briggs J, Levine AS, Billington CJ. Effects of opioid antagonists naloxone and naltrexone on neuropeptide Y-induced feeding and brown fat thermogenesis in the rat. J Clin Invest. 1995;96:163–170. doi: 10.1172/JCI118017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibowitz SF, Hor L. Endorphinergic and alpha-noradrenergic systems in the paraventricular nucleus: effects on eating behavior. Peptides. 1982;3:421–428. doi: 10.1016/0196-9781(82)90102-4. [DOI] [PubMed] [Google Scholar]
- Leventhal L, Cole JL, Rossi GC, Pan YX, Pasternak GW, Bodnar RJ. Antisense oligodeoxynucleotides against the MOR-1 clone alter weight and ingestive responses in rats. Brain Res. 1996;719:78–84. doi: 10.1016/0006-8993(96)00089-3. [DOI] [PubMed] [Google Scholar]
- Leventhal L, Silva RM, Rossi GC, Pasternak GW, Bodnar RJ. Morphine-6beta-glucuronide-induced hyperphagia: characterization of opioid action by selective antagonists and antisense mapping in rats. J Pharmacol Exp Ther. 1998;287:538–544. [PubMed] [Google Scholar]
- Leventhal L, Stevens LB, Rossi GC, Pasternak GW, Bodnar RJ. Antisense mapping of the MOR-1 opioid receptor clone: modulation of hyperphagia induced by DAMGO. J Pharmacol Exp Ther. 1997;282:1402–1407. [PubMed] [Google Scholar]
- Levine AS, Grace M, Billington CJ. The effect of centrally administered naloxone on deprivation and drug-induced feeding. Pharmacol Biochem Behav. 1990a;36:409–412. doi: 10.1016/0091-3057(90)90424-g. [DOI] [PubMed] [Google Scholar]
- Levine AS, Grace M, Billington CJ. B-funaltrexamine (B-FNA) decreases deprivation and opioid-induced feeding. Brain Res. 1991;562:281–284. doi: 10.1016/0006-8993(91)90632-6. [DOI] [PubMed] [Google Scholar]
- Levine AS, Grace M, Billington CJ, Portoghese PS. Nor-binaltorphamine decreases deprivation and opioid-induced feeding. Brain Res. 1990b;534:60–64. doi: 10.1016/0006-8993(90)90112-o. [DOI] [PubMed] [Google Scholar]
- Levine AS, Morley JE, Gosnell BA, Billington CJ, Bartness TJ. Opioids and consummatory behavior. Brain Res Bull. 1985;14:663–672. doi: 10.1016/0361-9230(85)90116-9. [DOI] [PubMed] [Google Scholar]
- Li CS, Davis BJ, Smith DV. Opioid modulation of taste responses in the nucleus of the solitary tract. Brain Res. 2003;965:21–34. doi: 10.1016/s0006-8993(02)03973-2. [DOI] [PubMed] [Google Scholar]
- Llewellyn-Smith IJ, Minson JB. Complete penetration of antibodies into Vibratome sections after glutaraaldehyde fixation and ethanol treatment: light and electron microscopy for neuropeptides. J Histochem Cytochem. 1992;40:1741–1749. doi: 10.1177/40.11.1431060. [DOI] [PubMed] [Google Scholar]
- Locatelli V, Petraglia F, Tirloni N, Muller EE. Beta-endorphin concentrations in the hypothalamus, pituitary and plasma of streptozotocin-diabetic rats with and without insulin substitution therapy. Life Sci. 1986;38:379–386. doi: 10.1016/0024-3205(86)90086-x. [DOI] [PubMed] [Google Scholar]
- Maickel RP, Braude MC, Zabik JE. The effects of various narcotic agonists and antagonists on deprivation-induced fluid consumption. Neuropharm. 1977;16:863–866. [Google Scholar]
- Margules DL, Moisset B, Lewis MJ, Shibuya H, Pert CB. Beta-endorphin is associated with overeating in genetically-obese mice (ob/ob) and rats (fa/fa) Science. 1978;202:988–991. doi: 10.1126/science.715455. [DOI] [PubMed] [Google Scholar]
- McLean S, Hoebel BG. Opiate and norepinephrine-induced feeding from the paraventricular nucleus of the hypothalamus are dissociable. Life Sci. 1982;31:2379–2382. doi: 10.1016/0024-3205(82)90161-8. [DOI] [PubMed] [Google Scholar]
- McLean S, Hoebel BG. Feeding induced by opiates injected into the paraventricular hypothalamus. Peptides. 1983;4:287–292. doi: 10.1016/0196-9781(83)90134-1. [DOI] [PubMed] [Google Scholar]
- Morley JE, Levine AS, Yim GKW, Lowy MT. Opioid modulation of appetite. Neurosci Biobehav Rev. 1983;7:281–305. doi: 10.1016/0149-7634(83)90020-9. [DOI] [PubMed] [Google Scholar]
- Olszewski PK, Billington CJ, Levine AS. Fos expression in feeding-related brain areas following intracerebroventricular administration of orphanin FQ in rats. Brain Res. 2000;855:171–175. doi: 10.1016/s0006-8993(99)02239-8. [DOI] [PubMed] [Google Scholar]
- Pan L, Xu J, Xu MM, Pan YX, Pasternak GW. Identification and characterization of six new alternatively spliced variuants of the human mu opioid receptor gene, Oprm. Neurosci. 2005a;133:209–220. doi: 10.1016/j.neuroscience.2004.12.033. [DOI] [PubMed] [Google Scholar]
- Pan YX. Diversity and complexity of the mu opioid receptor gene: alternative pre-mRNA splicing and promoters. DNA Cell Biol. 2005;24:736–750. doi: 10.1089/dna.2005.24.736. [DOI] [PubMed] [Google Scholar]
- Pan YX, Xu J, Bolan E, Abbadie C, Chang A, Zuckerman A, Rossi GC, Pasternak GW. Identification and characterization of three new alternatively spliced MOR-1 opioid receptor isoforms. Mol Pharmacol. 1999;56:396–403. doi: 10.1124/mol.56.2.396. [DOI] [PubMed] [Google Scholar]
- Pan YX, Xu J, Bolan E, Chang A, Mahurter L, Rossi GC, Pasternak GW. Isolation and expression of a novel alternatively spliced mu receptor isoform, MOR-1F. FEBS Lett. 2000;466:337–340. doi: 10.1016/s0014-5793(00)01095-4. [DOI] [PubMed] [Google Scholar]
- Pan YX, Xu J, Bolan E, Moskowitz HS, Xu M, Pasternak GW. Identification of four novel exon 5 splice variants of the mouse mu-opioid receptor gene: functional consequences of C-terminal splicing. Mol Pharmacol. 2005b;68:866–875. doi: 10.1124/mol.105.011858. [DOI] [PubMed] [Google Scholar]
- Pan YX, Xu J, Mahurter L, Bolan E, Xu M, Pasternak GW. Generation of the mu opioid receptor (MOR-1) protein by three new splice variants of the Oprm gene. Proc Natl Acad Sci (USA) 2001;98:14084–14089. doi: 10.1073/pnas.241296098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park TH, Carr KD. Neuroanatomical patterns of fos-like immunoreactivity induced by palatable meal and meal-paired environment in saline- and naltrexone-treated rats. Brain Res. 1998;805:169–180. doi: 10.1016/s0006-8993(98)00719-7. [DOI] [PubMed] [Google Scholar]
- Pasternak D, Pan L, Xu J, Yu R, Xu MM, Pasternak GW, Pan Y-X. Identification of three new alternative spliced variants of the rat mu opioid receptor gene: dissociation of affinity and efficacy. J Neurochem. 2004;91:881–890. doi: 10.1111/j.1471-4159.2004.02767.x. [DOI] [PubMed] [Google Scholar]
- Pasternak GW. Incomplete cross tolerance and multiple mu opioid peptide receptors. Trends Pharmacol Sci. 2001;22:67–70. doi: 10.1016/s0165-6147(00)01616-3. [DOI] [PubMed] [Google Scholar]
- Pasternak GW, Pan YX. Antisense mapping: Assessing functional significance of genes and splice variants. Meth Enzym. 2000;314:51–60. doi: 10.1016/s0076-6879(99)14094-1. [DOI] [PubMed] [Google Scholar]
- Pasternak GW, Standifer KM. Mapping of opioid receptors using antisense oligodeoxynucleotides: Correlating their molecular biology and pharmacology. Trends Pharmacol Sci. 1995;16:344–350. doi: 10.1016/s0165-6147(00)89068-9. [DOI] [PubMed] [Google Scholar]
- Pomonis JD, Billington CJ, Levine AS. Orphanin FQ, agonist of orphan opioid receptor ORL1, stimulates feeding in rats. Neuroreport. 1996;8:369–371. doi: 10.1097/00001756-199612200-00072. [DOI] [PubMed] [Google Scholar]
- Pomonis JD, Jewett DC, Kotz CM, Briggs JE, Billington CJ, Levine AS. Sucrose consumption increases naloxone-induced c-fos immunoreactivity in limbic forebrain. Am J Physiol. 2000;278:R712–R719. doi: 10.1152/ajpregu.2000.278.3.R712. [DOI] [PubMed] [Google Scholar]
- Przewlocki R, Lason W. The opioid peptide dynorphin, circadian rhythms and starvation. Science. 1982;219:71–73. doi: 10.1126/science.6129699. [DOI] [PubMed] [Google Scholar]
- Quinn JG, O'Hare E, Levine AS, Kim EM. Evidence for a mu-opioid-opioid connection between the paraventricular nucleus and ventral tegmental area in the rat. Brain Res. 2003;991:206–211. doi: 10.1016/j.brainres.2003.08.020. [DOI] [PubMed] [Google Scholar]
- Ragnauth A, Ruegg H, Bodnar RJ. Evaluation of opioid receptor subtype antagonist effects in the ventral tegmental area upon food intake under deprivation, glucoprivic and palatable conditions. Brain Res. 1997;767:8–16. doi: 10.1016/s0006-8993(97)00539-8. [DOI] [PubMed] [Google Scholar]
- Roane DS, Iadarola MJ, Porter JR. Decreased [3H]-naloxone binding and elevated dynorphin-A(1-8) content in the Zucker rat brain. Physiol Behav. 1988;43:371–374. doi: 10.1016/0031-9384(88)90201-6. [DOI] [PubMed] [Google Scholar]
- Rodi D, Polidori C, Bregola G, Zucchini S, Simonato M, Massi M. Pro-nociceptin/orphanin FQ and NOP receptor mRNA levels in the forebrain of food deprived rats. Brain Res. 2002;957:354–361. doi: 10.1016/s0006-8993(02)03678-8. [DOI] [PubMed] [Google Scholar]
- Rossi GC, Pasternak GW. Establishing the molecular biology of opioid behavior through antisense approaches. In: Weiss B, editor. Antisense Oligodeoxynucleotides and Antisense RNA. CRC Press; Boca Raton, FL: 1997. pp. 115–130. [Google Scholar]
- Sawchenko PE, Swanson LW. Immunohistochemical identification of neurons in the paraventricular nucleus of the hypothalamus that project to the medulla or to the spinal cord in the rat. J Comp Neurol. 1982;205:260–272. doi: 10.1002/cne.902050306. [DOI] [PubMed] [Google Scholar]
- Silva RM, Grossman HC, Hadjimarkou MM, Rossi GC, Pasternak GW, Bodnar RJ. Dynorphin A1-17-induced feeding: Pharmacological characterization using selective opioid antagonists and antisense probes in rats. J Pharmacol Exp Ther. 2002;301:513–518. doi: 10.1124/jpet.301.2.513. [DOI] [PubMed] [Google Scholar]
- Silva RM, Hadjimarkou MM, Rossi GC, Pasternak GW, Bodnar RJ. Beta-endorphin-induced feeding: pharmacological characterization using selective opioid antagonists and antisense probes in rats. J Pharmacol Exp Ther. 2001;297:590–596. [PubMed] [Google Scholar]
- Simone DA, Bodnar RJ, Goldman EJ, Pasternak GW. Involvement of opioid receptor subtypes in rat feeding behavior. Life Sci. 1985;36:829–833. doi: 10.1016/0024-3205(85)90206-1. [DOI] [PubMed] [Google Scholar]
- Stanley BG, Lanthier D, Leibowitz SF. Multiple brain sites sensitive to feeding stimulation by opioid agonists: a cannula-mapping study. Pharmacol Biochem Behav. 1989;31:825–832. doi: 10.1016/0091-3057(88)90391-7. [DOI] [PubMed] [Google Scholar]
- Stein JA, Znamensky V, Baumer F, Rossi GC, Pasternak GW, Bodnar RJ. Mercaptoacetate induces feeding through central opioid-mediated mechanisms in rats. Brain Res. 2000;864:240–251. doi: 10.1016/s0006-8993(00)02162-4. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Kuypers HG. The paraventricular nucleus of the hypothalamus: cytoarchitectural subdivisions and organization of projections to the pituitary, dorsal vagal complex, and spinal cord as demonstrated by retrograde fluorescence double-labeling methods. J Comp Neurol. 1980;194:555–570. doi: 10.1002/cne.901940306. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE, Wiegand SJ, Price JL. Separate neurons in the paraventricular nucleus project to the median eminence and to the medulla or spinal cord. Brain Res. 1980;198:190–195. doi: 10.1016/0006-8993(80)90354-6. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Motomatsu T, Nobunaga M. Influences of water deprivation and fasting on hypothalamic, pituitary and plasma opioid peptides and prolactin in rats. Physiol Behav. 1986;37:603–608. doi: 10.1016/0031-9384(86)90293-3. [DOI] [PubMed] [Google Scholar]
- Tanda G, DiChiara G. A dopamine-mu1 opioid link in the rat ventral tegmentum shared by palatable food (Fonzies) and the non-psychostimulant drugs of abuse. Eur J Neurosci. 1998;10:1179–1187. doi: 10.1046/j.1460-9568.1998.00135.x. [DOI] [PubMed] [Google Scholar]
- Tepperman FS, Hirst M. Effects of intrahypothalamic injection of D-Ala-2,D-Leu- 5-enkephalin on feeding and temperature in the rat. Eur J Pharmacol. 1983;96:243–249. doi: 10.1016/0014-2999(83)90313-8. [DOI] [PubMed] [Google Scholar]
- Thornhill JA, Saunders W. Ventromedial and lateral hypothalamic injections of naloxone or naltrexone suppress the acute food intake of food-deprived rats. Appetite. 1984;5:25–30. doi: 10.1016/s0195-6663(84)80046-x. [DOI] [PubMed] [Google Scholar]
- Tsujii S, Nakai Y, Fukata J, Koh T, Takahashi H, Usui T, Imura H. Effects of food deprivation and high fat diet on opioid receptor binding in rat brain. Neurosci Lett. 1986a;72:169–173. doi: 10.1016/0304-3940(86)90074-1. [DOI] [PubMed] [Google Scholar]
- Tsujii S, Nakai Y, Koh T, Takahashe H, Usui T, Ikeda H, Matsuo T, Imura H. Effects of food deprivation on opioid receptor binding in the brain of lean and fatty Zucker rats. Brain Res. 1986b;399:200–203. doi: 10.1016/0006-8993(86)90620-7. [DOI] [PubMed] [Google Scholar]
- Uhl GR, Childers SR, Pasternak GW. An opiate receptor gene family reunion. Trends Neurosci. 1994;17:89–93. doi: 10.1016/0166-2236(94)90110-4. [DOI] [PubMed] [Google Scholar]
- Ukai M, Holtzman SG. Effects of beta-funaltrexamine on ingestive behaviors in the rat. Eur J Pharmacol. 1988;153:161–165. doi: 10.1016/0014-2999(88)90602-4. [DOI] [PubMed] [Google Scholar]
- Welch CC, Kim E-M, Grace MK, Billington CJ, Levine AS. Palatability-induced hyperphagia increases hypothalamic dynorphin peptide and mRNA levels. Brain Res. 1996;721:126–131. doi: 10.1016/0006-8993(96)00151-5. [DOI] [PubMed] [Google Scholar]
- Will MJ, Franzblau EB, Kelley AE. Nucleus accumbens mu-opioids regulate intake of a high-fat diet via activation of a distributed brain network. J Neurosci. 2003;23:2882–2888. doi: 10.1523/JNEUROSCI.23-07-02882.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolinsky TD, Abrahamsen GC, Carr KD. Diabetes alters mu and kappa opioid binding in rat brain: comparison with effects of food restriction. Brain Res. 1996a;738:167–171. doi: 10.1016/0006-8993(96)00994-8. [DOI] [PubMed] [Google Scholar]
- Wolinsky TD, Carr KD, Hiller JM, Simon EJ. Effects of chronic food restriction on mu and kappa opioid binding in rat forebrain: a quantitative autoradiographic study. Brain Res. 1994;656:274–280. doi: 10.1016/0006-8993(94)91470-2. [DOI] [PubMed] [Google Scholar]
- Wolinsky TD, Carr KD, Hiller JM, Simon EJ. Chronic food restriction alters mu and kappa opioid receptor binding in the parabrachial nucleus of the rat: a quantitative autoradiographic study. Brain Res. 1996b;706:333–336. doi: 10.1016/0006-8993(95)01337-7. [DOI] [PubMed] [Google Scholar]
- Woods JS, Leibowitz SF. Hypothalamic sites sensitive to morphine and naloxone: effects on feeding behavior. Pharmacol Biochem Behav. 1985;23:431–438. doi: 10.1016/0091-3057(85)90017-6. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Pan YX, Kolesnikov Y, Pasternak GW. Immunohistochemical labeling of the mu opioid receptor carboxy terminal splice variant mMOR-1B4 in the mouse central nervous system. Brain Res. 2006;1099:33–43. doi: 10.1016/j.brainres.2006.04.133. [DOI] [PubMed] [Google Scholar]
- Zimprich A, Simon T, Hollt V. Cloning and expression of an isoform of the rat mu opioid receptor (rMOR1B) which differs in agonist-induced desensitization from rMOR1. FEBS Lett. 1995;359:142–146. doi: 10.1016/0014-5793(95)00028-8. [DOI] [PubMed] [Google Scholar]