Abstract
Gap junctions (GJs) provide a low-resistance pathway for cardiac electrical propagation. The role of GJ regulation in arrhythmia is unclear, partly due to limited availability of pharmacological tools. Recently, we showed that a peptide called “RXP-E” binds to the carboxyl terminal of connexin43 (Cx43) and prevents chemically-induced uncoupling in Cx43-expressing N2a cells. Here, pull-down experiments show RXP-E binding to adult cardiac Cx43. Patch-clamp studies revealed that RXP-E prevented heptanol-induced and acidification-induced uncoupling in pairs of neonatal rat ventricular myocytes (NRVM’s). Separately, RXP-E was concatenated to a cytoplasmic transduction peptide for cytoplasmic translocation (CTP-RXP-E). The effect of RXP-E on action potential (AP) propagation was assessed by high resolution optical mapping in monolayers of NRVM’s, containing ~20% of randomly distributed myofibroblasts. In contrast to control experiments, when heptanol (2 mmol/L) was added to the superfusate of monolayers loaded with CTP-RXP-E, AP propagation was maintained, albeit at a slower velocity. Similarly, intracellular acidification (pHi=6.2) caused a loss of AP propagation in control monolayers; however, propagation was maintained in CTP-RXP-E treated cells, though at a slower rate. Patch clamp experiments revealed that RXP-E did not prevent heptanol-induced block of sodium currents, nor did it alter voltage dependence or amplitude of Kir2.1/Kir2.3 currents. RXP-E is the first synthetic molecule known to: (1) bind cardiac Cx43; (2) prevent heptanol and acidification-induced uncoupling of cardiac GJ’s and 3) preserve AP propagation among cardiac myocytes. RXP-E can be used to characterize the role of GJs in the function of multicellular systems, including the heart.
Keywords: Cx43CT, particle-receptor interaction, gap junctions, connexin43, rotigaptide
INTRODUCTION
Connexins are integral membrane proteins that oligomerize to form intercellular channels called gap junctions. The most abundant gap junction (GJ) protein in a number of mammalian systems is connexin43 (Cx43). Gap junctions allow passage of ions and small molecules between cells and are regulated by a variety of chemical interactions between the connexin molecule and the microenvironment. As such, gap junctions act as active filters to control passage of intercellular messages and modulate function.
Our previous work has suggested that regulation of Cx43 results from the association of the carboxyl terminal (CT) domain, acting as a gating particle, and a separate region of the connexin molecule acting as a receptor for the gating particle.1, 2 Additional studies have shown that this intra-molecular particle-receptor interaction can be modulated by other inter-molecular interactions in the microenvironment of the gap junction plaque.3–5 Based on the particle-receptor model, we reasoned that regulation of Cx43 could be disrupted by the binding of exogenous molecules to regions of the gating particle required for its interaction with the receptor. The latter rationale led us to the identification of a 34-amino acid peptide dubbed “RXP-E.”6 This peptide bound in vitro to Cx43CT with an apparent KD of 3.9μmol/L, modified the structure of Cx43CT, partially prevented octanol-induced and acidification-induced uncoupling of N2a cells transfected with exogenous Cx43, and caused a significant prolongation of the single channel open time.6 Accordingly, we proposed that RXP-E could be developed as a tool to address the relevance of preserved intercellular communication in the maintenance of electrical synchrony in the heart. Here, we show that RXP-E binds to native cardiac Cx43, prevents the closure of cardiac gap junctions, can be introduced into multicellular preparations, and can prevent heptanol- and acidification-induced action potential propagation block in monolayers of neonatal cardiac myocytes. Our results suggest that RXP-E can be used as a platform for development of a pharmacophore that could interfere with Cx43 regulation. The potential of gap junction modifiers as antiarrhythmic agents has been described before.7–10 Yet, the identity of the molecular target for existing compounds is unknown, thus making them unsuitable for optimization by targeted drug design. Our results identify RXP-E as the first molecule known to bind Cx43 and in doing so, preserve action potential propagation in cardiac myocytes under conditions otherwise expected to induce propagation block.
MATERIALS AND METHODS
All details for methods are provided in the online supplement at http://circres.ahajournals.org. Brief descriptions are presented below.
Recombinant Protein Production and Immunochemical Assays
All immunochemical protocols, as well as production of recombinant glutathione S-transferase (GST) fusion proteins and the GST pull-down assays, followed standard techniques.1, 11 Details are provided in the online supplement.
Myocyte Isolation and Culture
Primary cultures of NRVM’s for patch clamping, immunofluorescence, and monolayers were obtained using established procedures.12–14 After dissociation, cells were resuspended in supplemented M199 media and preplated for 2 hours to reduce the presence of noncardiomyocytes. Cells were plated on 35-mm tissue culture dishes at a density of 1.2×106 cells/dish for monolayers and at low density on 22-mm coverslips for patch clamp experiments. For immunofluorescence, cells were seeded onto 22-mm coverslips at a density of 5.0×105 cells/coverslip.
Translocation of RXP-E into NRVM’s
Peptides were concatenated with a cytoplasmic transfer peptide (CTP15) sequence for translocation into the cytoplasm of NRVM’s. Peptide synthesis was carried out by a commercial supplier (Anaspec, Inc). Purity of peptide products was >85%.
Electrophysiological Analysis
Determination of gap junction currents
Experiments were performed in NRVM pairs endogenously expressing Cx43. The dual-whole-cell voltage clamp technique was used to record gap junction currents, as previously described.6, 16, 17
Determination of Nav1.5 currents
Voltage clamp experiments were conducted in HEK293 cells (ATCC, CRL1573) transfected (Effectene Transfection Kit, Qiagen) with human SCN5A cDNA subcloned in a pcDNA3.1 vector. The protocol and solutions used for recording sodium current from HEK293 cells stably expressing the SCN5A gene are described in the online supplement.
Determination of Kir2.1-Kir2.3 currents
Kir2.1 and Kir2.3 currents were recorded from HEK293 cells (ATCC, CRL1573) using established procedures.18, 19
Effect of heptanol superfusion on ionic currents
The effect of heptanol on cardiac gap junction currents, as well as in the amplitude of Nav1.5 and Kir2.1–2.3 currents was assessed in the presence and absence of RXP-E in the internal pipette solution using methods previously described.6
Effect of intracellular acidification on junctional currents
Experiments were conducted to assess the effect of RXP-E on acidification-induced uncoupling of NRVM’s. Intracellular acidification was induced by filling the patch pipettes with a solution buffered to pH 6.2 (see online supplement). Junctional conductance (Gj) was measured immediately after patch break and for the following 10 minutes.
Optical Mapping
Optical mapping was used to study action potential propagation in monolayers of NRVM’s. Measurements were performed in cells kept for 3–4 days in culture. Preparations were stained for 15 minutes with a voltage sensitive dye (di-8-ANEPPS (40μmol/L; Molecular Probes) to optically determine the characteristics of impulse propagation. A custom-made setup was used to assess changes in fluorescence that correspond to transmembrane voltage changes. Details are described elsewhere.20
Heptanol experiments
Heptanol superfusion was initiated after 3 minutes of successful pacing. Heptanol (1–2mmol/L, as specified), was prepared in HBSS solution. Heptanol perfusion was continued for 5 minutes, followed by washout. All monolayers demonstrated complete washout of heptanol, as established by return to paced propagation.
Intracellular acidification in monolayers of NRVM’s
Intracellular acidification was induced by modifying the proton concentration of a Na-acetate superfusing solution. Intracellular pH calibration was carried out as described in the online supplement (Online Figure I).
RESULTS
RXP-E Pulls Down Native Cardiac Cx43
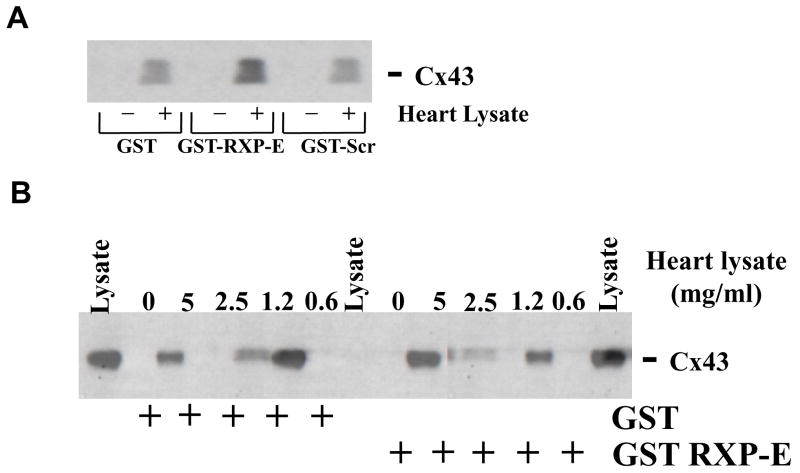
We have previously shown RXP-E binding to recombinant Cx43CT.6 Here, we explored whether RXP-E is able to interact with cardiac Cx43. A GST-RXP-E recombinant protein was bound to glutathione beads and used as a bait to pull-down Cx43 from rat heart lysate. Parallel negative control experiments were conducted using either a GST protein (without RXP-E), or a construct coding for a “scrambled” version of RXP-E (“GST-Scr;” see “Methods;” see also6). The presence or absence of Cx43 in the lysate or in the precipitate was assessed by Western blot using an antibody against the N-terminal domain of Cx43. Results are shown in Figure 1A. A Cx43-immunoreactive band of the appropriate size was recovered from the precipitate of beads coated with GST-RXP-E and exposed to heart lysate (fourth lane from the left). On the other hand, no signal was obtained from samples obtained after exposure of heart lysate to either GST, or GST-Scr, or from coated beads that were not exposed to heart lysate. Additionally, the density of the Cx43-immunoreactive band was found to be dependent on the concentration of the heart lysate, as demonstrated in Figure 1B. Overall, the results indicate that RXP-E can interact with native cardiac Cx43.
Figure 1.
Co-immunoprecipitation of RXP-E with Cx43 from rat heart lysates. A, GST-fused RXP-E was bound to glutathione beads and incubated either in the absence (lanes marked “minus”) or presence of cell lysate from adult rat heart (lanes marked “plus”). GST alone or GST-Scr-RXP-E failed to pull down Cx43; whereas, as expected, GST-RXP-E brought down cardiac Cx43. B, A sample of lysate was run in first, seventh, and last lanes as positive control. The protein concentration in the lysate is noted at the top of the figure.
Effect of RXP-E on heptanol-induced uncoupling of NRVM’s
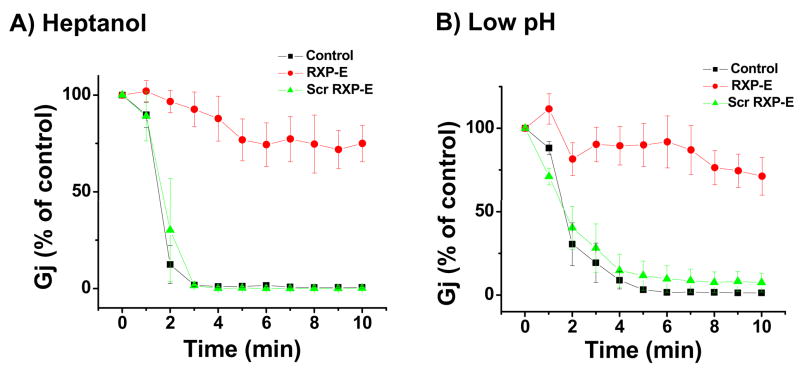
The ability of RXP-E to bind cardiac Cx43 led us to propose that this peptide may also alter the behavior of cardiac gap junctions. Whole cell gap junction currents were recorded from NRVM’s via dual-whole-cell voltage clamp. Junctional current amplitude was measured every 20 seconds. All cell pairs showed an initial Gj value of <35 nS. Ten minutes after patch break, heptanol superfusion (1mmol/L) was initiated. The time course of heptanol-induced changes in junctional conductance is shown in Figure 2A. Data were obtained from pairs recorded in control conditions (black squares; n=10) or when the internal pipette solution contained either RXP-E (red circles; n=10), or the “scrambled” version of the peptide (green triangles; n=10). Peptide concentration in all cases was 0.05mmol/L. The plot correlates percentage of junctional conductance (relative to control) as a function of time after onset of heptanol superfusion. Clearly, heptanol exposure led to a rapid drop in electrical coupling either in control, or in the presence of the scrambled RXP-E peptide. However, all cell pairs recorded in the presence of RXP-E remained electrically coupled (ie, Gj did not reach 0) throughout continuous heptanol superfusion. The average Gj decreased only to 71.8% of control, and average Gj measured 10 minutes after onset of heptanol superfusion from cells kept in control was significantly different from that measured from cells dialyzed with RXP-E (p<0.05)
Figure 2.
Effect of RXP-E on heptanol- and acidification-induced uncoupling of endogenous Cx43-expressing neonatal myocytes. Peptides were diluted in internal pipette solution (0.05 mmol/L). Junctional conductance (Gj) was measured using dual patch clamp (see18 and online supplement). Bars represent SEM. A, Time course of heptanol-induced uncoupling in NRVM’s. Time zero indicates onset of heptanol superfusion. Gj in cells exposed to RXP-E decreased to 71.8% of control at 10 minutes after heptanol superfusion (p<0.001 when compared to control). Data were compared with those obtained in the presence of “scrambled RXP-E” (green triangles). B, Time course of acidification-induced uncoupling. Ordinates represent time elapsed after patch break. Internal pipette solution was buffered to pH 6.2. Ten minutes after patch break, Gj in control decreased to 1.3% of the initial value. Scrambled RXP-E peptide did not disrupt acidification-induced uncoupling (green triangles; n=10; P>0.05 when compared to control). In contrast, in the presence of RXP-E, Gj decreased only to 71.2% of maximum (p<0.05).
RXP-E Partially Prevented Acidification-Induced Uncoupling in NRVM’s
Next, we assessed whether RXP-E can interfere with the extent and time course of uncoupling induced by reduced intracellular pH (pHi). Patch pipettes were filled with a 2(N-morpholino) ethanesulfonic acid (MES)-containing solution, buffered to a pH of 6.2. Junctional current (Ij) was measured immediately after patch break and every 20 seconds thereafter. Figure 2B shows the results. In the absence of RXP-E, Gj decreased progressively, reaching 1.3% of control within 10 minutes after patch break (black squares; n=10). In the presence of RXP-E (red circles; n=10), a decrease in Gj was also observed, but it was significantly dampened; after 10 minutes, average Gj decreased only to 71.2% of the initial value. This value was significantly different from that recorded in control (p<0.05). Interestingly, scrambled RXP-E did not disrupt acidification-induced uncoupling (green triangles; n=10; p>0.05 when compared to control). Overall, the data show that RXP-E partially prevented closure of Cx43 channels consequent to a reduction in pHi.
Does RXP-E prevent the effect of heptanol on Nav1.5 currents?
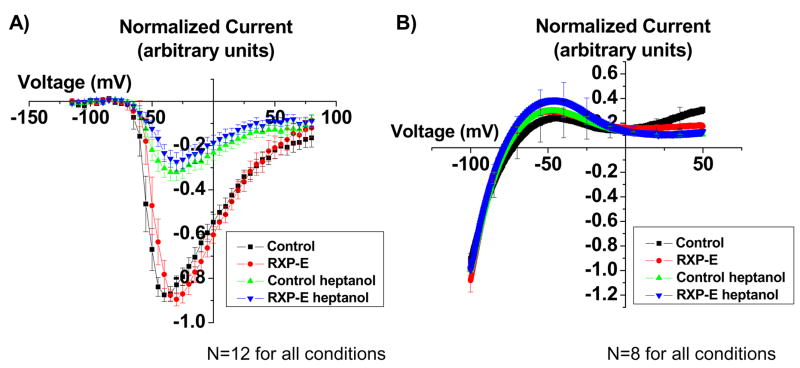
Our results show that RXP-E prevents heptanol-induced closure of gap junction channels. Heptanol is known to affect sodium currents as well.21 Here, we asked whether RXP-E can interfere with the effect of heptanol on the ionic current obtained after expression of the SCN5A gene in transfected HEK293 cells. Figure 3A shows the results. Peak current amplitude was plotted as a function of the test voltage (see “Methods” for details). From each cell, a complete current-voltage relation was obtained before and 10 minutes after onset of superfusion with heptanol (2mmol/L). To minimize variability between experiments, the amplitude of the peak current recorded at each voltage step was normalized to maximum peak current amplitude obtained from the same cell prior to heptanol superfusion. As expected, heptanol caused a drastic reduction in the amplitude of the Nav1.5 current. Yet, as opposed to what we observed in the case of gap junctions, the effect of heptanol was the same regardless of whether or not RXP-E was present in the pipette solution. Peak current amplitude was observed at a command potential of −35+/− 5 mV for control (black symbols; n=12), and −30 +/− 5 mV in the presence of RXP-E (red symbols; n=12; p>0.05). After heptanol, the command voltage that elicited maximum peak current amplitude was −30 +/−5 mV for control and −30 +/− 5mV in the presence of RXP-E (p>0.05 when values before and after heptanol were compared). Moreover, the relative reduction of maximum peak current amplitude was 36.6+/−4.8% for control and 30.5+/−5.1% in the presence of RXP-E (green and blue symbols, respectively; p>0.05). Overall, the data show that RXP-E does not interfere with heptanol-induced reduction of sodium current amplitude.
Figure 3.
Effect of RXP-E on ionic currents. Peptides were diluted in internal pipette solution (0.1 mmol/L). Peak current amplitude was plotted as a function of test voltage (see “Methods” for details). From each cell, a complete current-voltage relation was obtained before and 10 minutes after onset of heptanol superfusion (2mmol/L; see online supplement). Bars represent SEM. A, Current-voltage plot of heptanol-induced alterations in maximal Na+ current in SCN5a-expressing HEK cells. Black symbols, control (n=12). Red symbols, data collected in the presence of RXP-E (n=12). The data show that RXP-E does not interfere with heptanol-induced reduction of sodium current amplitude. B, Current-voltage plot of heptanol-induced alterations in maximal K+ current in Kir2.1 and Kir2.3-expressing HEK cells. Current-voltage relations were generated as detailed in “Methods.” Kir2.1 and Kir2.3 currents were not affected by heptanol, and no significant shifts in the current-voltage relation were recorded in the presence of RXP-E.
RXP-E and inward rectifier currents
Further assessment of RXP-E specificity involved measurements of Kir2.1 and Kir2.3 currents recorded from double stably transfected HEK293 cells. Figure 3B shows the results. Current-voltage relations were generated as detailed in “Methods.” In this case, currents were normalized to the amplitude measured at −100 mV. Kir2.1 and Kir2.3 currents were not affected by heptanol, and no significant shifts in the current-voltage relation were recorded in the presence of RXP-E.
From Cell Pairs to Multicellular Preparations Transfer of RXP-E to the Intracellular Space in Multicellular Preparations
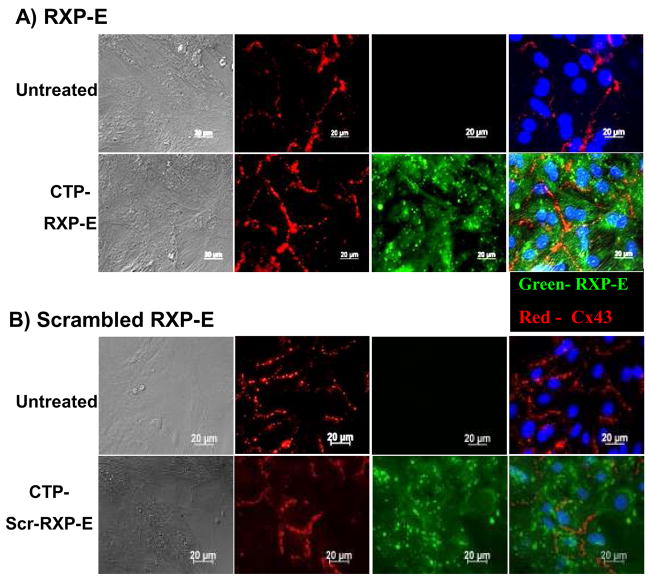
Our results show that RXP-E can prevent closure of gap junctions in cardiac cell pairs. As a next step, we developed an assay for introducing RXP-E into multicellular preparations. The latter was necessary for assessment of the effect of RXP-E on action potential propagation in cardiac cells. Monolayers of NRVM’s were exposed to synthetic fusion peptides containing a cytoplasmic transduction peptide (CTP512 domain; sequence YGRRARRRRRR15). A cysteine residue at the C-terminus allowed us to conjugate FITC to the peptides for detection by fluorescence microscopy. Synthetic peptides were created for both RXP-E, and its scrambled version. Figure 4A shows an example of peptide transfer into NRVM’s. Monolayers were prepared from the hearts of neonates (1 day old); cells were cultured for 6 days. The image in the top panel was obtained from cells maintained in control conditions (no CTP-RXP-E). The image in the bottom was obtained from cells previously incubated with CTP-RXP-E (0.1 mmol/L) for 2 hours. Cx43 was immunolabeled (red) and nuclei were stained (Hoechst33528; blue) for easier identification of individual cells. No FITC signal was present in control; however, intense signal was recorded from all cells that had been pre-incubated with CTP-RXP-E. These experiments were repeated, this time with the scrambled CTP-RXP-E, as shown in Figure 4B. Top images correspond to cells not presented with the peptide. Bottom panels were recorded after cells were incubated for 30 minutes with a solution containing 0.1 mmol/L of scrambled CTP-RXP-E. Right panels show the overlay of the blue (nuclei) green (fluorescein-labeled RXP-E) and red (Cx43) signals. Left panels show the DIC image of the myocyte monolayers. Clearly, a bright green fluorescein signal was detected from all cells within the field, indicating successful peptide translocation. Signal was detected up to 18 hours after peptide incubation (not shown). Further confirmation of successful transfer into near-100% of the cells was obtained by flow-cytometric detection of the FITC signal from NRK cells (not shown). Similarly timed transduction experiments were repeated in NRK cells (Online Figure II). Overall, the data show that CTP allows for efficient transfer of FITC-labeled peptides into the cytoplasm of rat neonatal cardiac myocytes. We used this translocation protocol to test for the effect of RXP-E on action potential propagation in monolayers of NRVM’s.
Figure 4.
Immunolocalization of Cx43 and RXP-E in NRVM’s kept in control conditions (top panels), treated with fluoresceinated CTP-RXP-E (green in bottom panels A) or with a “scrambled” construct (bottom panels B). Column on the left displays DIC images; column on the right corresponds to merged images from the co-localization experiments. Intracellular distribution of fluorescein-labeled constructs after CTP-mediated transfer into rat neonatal cardiac myocytes is shown in green in the third columns. Cx43 (red) was immunolocalized in the same preparations (second columns). Nuclei were labeled with Hoechst33528 (blue).
Effect of RXP-E on action potential propagation
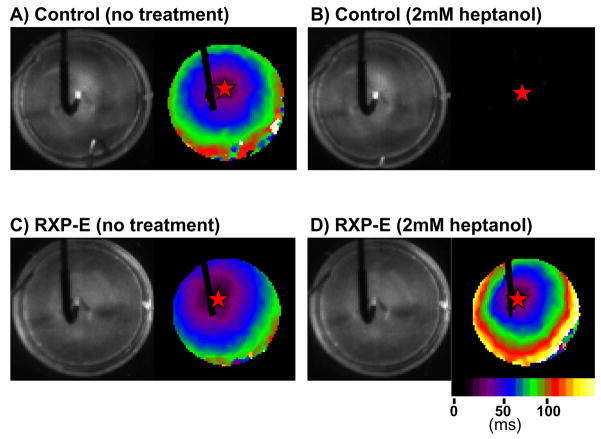
The effect of RXP-E on action potential propagation was assessed by high resolution optical mapping. Monolayers of NRVM’s were labeled with a voltage-sensitive dye (di-8-ANEPPS, 40μmol/L) and a high resolution, high speed camera was used to record the electrical activity in the 35mm monolayer (see20; see also “Methods” for details). A stimulating electrode was placed in the center of the preparation (see left panels in Figure 5). The electrical activity propagated from the center toward the periphery of the preparation, as shown by the activation map displayed on the right panel of Figure 5A. (Notice however the “shadow” of the stimulus electrode, preventing view of activity at the site of stimulation.) Overall, conduction velocity in normal saline solution was unaffected by the presence or absence of the RXP-E peptide. Indeed, the average conduction velocity of all experiments in monolayers not treated with CTP peptides was 164 ± 8mm/sec (n = 12). This value was not statistically different from those obtained from monolayers in which either CTP-Scr-RXP-E or CTP-RXP-E had been translocated into cells (CV=158 ± 10mm/sec n= 6 and 180 ± 7mm/sec n=10, respectively). Exposure of non-treated monolayers to 2 mmol/L (n=4) or even 1 mmol/L (n=4) of heptanol, caused a total loss of propagated activity (see, e.g., Figure 5B) that was restored upon washout. Similar results were obtained from monolayers treated with CTP-Scr-RXP-E (two experiments at each heptanol concentration; complete conduction block in all four cases). However, a different result was obtained from CTP-RXP-E treated monolayers. In that case, the propagated activity observed in normal saline solution (Figure 5C) was not interrupted by exposure to the uncoupler (Figure 5D). Indeed,, when the higher dose of heptanol (2 mmol/L) was added to the superfusate,, action potential propagation was maintained, albeit at a slower velocity.. In average, conduction velocity in CTP-RXP-E treated monolayers exposed to 2 mmol/L of heptanol was 87 ± 5mm/sec (n=4; p<0.001 by paired t-test when compared to conduction velocity prior to heptanol superfusion in the same preparation). The result was consistent with the ability of RXP-E to preserve gap junction communication, but not to prevent the effect of heptanol on sodium currents.
Figure 5.
Effect of RXP-E on action potential propagation in heptanol-induced uncoupling. A stimulating electrode was placed in the center of the preparation (left panels). Activation maps are shown on the right (calibration bar represents activation times, in ms). Exposure of the monolayer to heptanol caused total loss of propagated activity (Panel B). Propagation in a monolayer loaded with CTP-RXP-E is shown in panel C. In this case, action potential propagation was maintained in the presence of heptanol, albeit at a slower velocity (panel D).
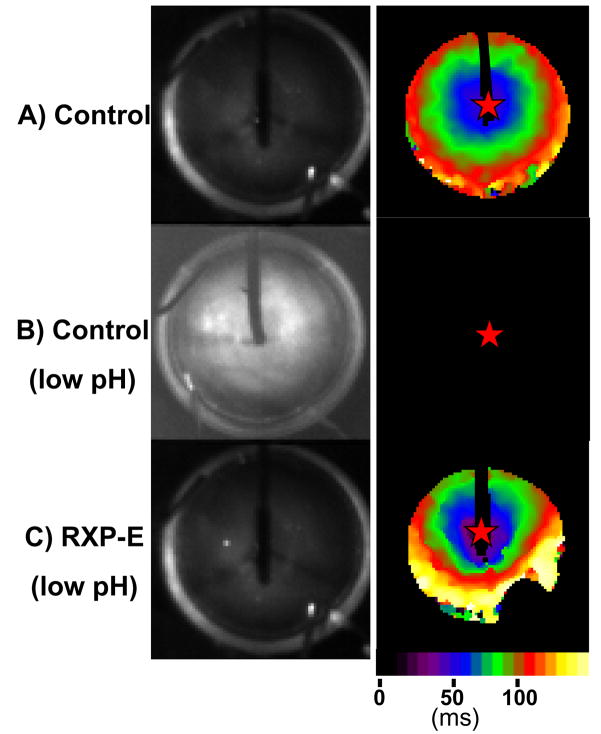
RXP-E also prevented action potential propagation failure due to acidification of the intracellular space. The recordings shown in Figure 6, panel A, were obtained from a preparation not treated with peptide. When the bathing solution was kept at normal pH (7.4), action potentials propagated through the preparation with a conduction velocity of 180 ± 18mm/sec. When the same preparation was exposed to a low pH solution (intracellular pH 6.2 as estimated by the protocol described in “Methods”) propagation failure was observed (n=6; see, e.g., Figure 6B). Propagation failure was also observed in two monolayers pre-treated with CTP-Scr-RXP-E. Yet, in preparations pre-treated with CTP-RXP-E, propagation was maintained despite acidification of the intracellular space (see Figure 6C), though conduction velocity was significantly decreased (average CV=93 ± 28mm/sec; n=4, p<0.05 when compared by paired t test with values at normal pH measured from the same preparation). Overall, these results demonstrate that RXP-E can preserve action potential propagation under conditions which consistently induce propagation block.
Figure 6.
Effect of RXP-E on action potential propagation in cells exposed to low pH. Recordings shown in panel A were obtained from a preparation in control conditions. When the same monolayer was exposed to a low pH solution (intracellular pH 6.2 as estimated by protocol described in “Methods”) propagation failure was observed (B). Yet, in the presence of CTP-RXP-E, propagation was maintained despite intracellular acidification (C).
Discussion
Previous work from our laboratory, using high-throughput phage display analysis, led to the identification of the “RXP” sequence as a Cx43CT binding motif.6 Of the sequences identified, a particular 34 amino acid peptide ( “RXP-E”) was shown to prevent heptanol- and acidification-induced uncoupling of N2a cells exogenously expressing Cx43 (Shibayama et al, 2006).6 Here, we show that RXP-E: (1) binds to endogenous cardiac Cx43; (2) prevents heptanol and acidification-induced uncoupling of cardiac gap junctions; 3) can be introduced into monolayers of cardiac myocytes using a peptide translocator and 4) preserves action potential propagation among cardiac myocytes. These results strongly suggest that RXP-E can be used as a platform for the development of a pharmacophore that could interfere with Cx43 regulation. Overall, the results identify RXP-E as the first Cx43-binding molecule capable of preserving action potential propagation in cardiac myocytes under conditions otherwise expected to induce propagation block.
Cellular delivery
The utility of peptidic molecules targeting intracellular domains is limited by the fact that, in general, peptides are not membrane-permeable molecules. Recent advances, however, have made it possible to translocate peptides into the intracellular space. A particular strategy consists of fusing the sequence of interest with a “cell-penetrating peptide” (CPP22–24). For our studies, we concatenated RXP-E with a cytoplasmic transduction peptide (CTP), as described by Kim et al.15 Our results show that a fluorescein-labeled molecule abundantly localized to the cytoplasm, though showed no preference for junctional membranes. Whether the CTP-RXPE-FITC complex remained as one concatenant inside the cells, remains to be determined. If peptide cleavage occurred, the FITC signal would not pinpoint the exact location of RXP-E but rather, of the cleaved FITC molecule. If, on the other hand, the complex remained intact, our results suggest that RXP-E distributes diffusely within the cell. This may be a reflection of the limited concentration capacity of the peptide at the site of cell-cell apposition, consistent with its low binding affinity to Cx43.6 What is evident from our data is that the peptide modified gap junction function and prevented propagation block, suggesting that, though its affinity for Cx43 may be low (see Shibayama et al6) its efficacy to modify GJ function was preserved. Overall, the CTP system allowed for pharmacological manipulation of a molecular target previously “protected” by the cell membrane. These and other techniques will be useful to determine whether modulation of the pH regulatory mechanism of Cx43 can modify the likelihood of cardiac arrhythmias under conditions that cause acidification of the intracellular space.
Conduction velocity and effect of RXP-E
It is worth noting that the propagation velocities measured in our experiments were lower than those reported by other authors.20, 25 A likely explanation is that our pre-plating and culture methods did not completely remove fibroblasts from the cell preparation.26 In fact, ancillary experiments where we immunolocalized non-myocyte cells using a α-smooth muscle actin antibody (Anaspec, Inc, data not shown) indicated that ~25% of the cells were non-myocytes. Our conduction velocities, therefore, correspond well with those measured by Miragoli et al.25 The presence of fibroblasts is unlikely to affect the outcome of our results. In fact, it is interesting to note that a number of cardiac pathologies associate with deposition of fibroid tissue and, likely, fibroblasts electrically coupled to the cardiac myocytes.26, 27 It is also of interest that RXP-E by itself did not modify propagation. Conduction velocities measured from samples kept in control conditions were not different from those pre-loaded with RXP-E. This is similar to results obtained with other GJ openers28, 29 and supports the notion that, in a well-coupled system of cells, GJ modification minimally affects propagation.30, 31 Yet, once the system was challenged, RXP-E was capable of preserving action potential propagation, albeit at a slower velocity than in control. The latter is consistent with the observation that RXP-E did not prevent heptanol-induced decrease in sodium current amplitude (see Figure 3). In addition, since other connexin isoforms also express in NRVM’s, it is possible that the observed decreases in junctional conductance (and conduction velocity) could reflect, at least in part, closure of those channels by heptanol or acidification. The effect (or lack thereof) of RXP-E on other connexins remains to be determined. Or data do show that RXP-E preserves propagation albeit with a slower conduction velocity. Whether preservation of conduction at a slower velocity is an effective antiarrhythmic strategy, or instead, a pro-arrhythmic event, remains to be determined.
Pharmacology of Gap Junctions
It is unclear whether holding gap junctions open under pathological conditions such as ischemia would be beneficial or deleterious to the heart, though initial data seem to indicate that preservation of GJ communication may have a powerful antiarrhythmic effect (see studies with “rotigaptide,” or “ZP123”8, 9). However, one of the reasons as to why such a gap in knowledge exists is because we lack pharmacological agents that can, with at least some partial selectivity, interfere with GJ regulation. (Much has been learned about the functional role of sodium channels, for example, from results obtained in the presence and absence of tetrodotoxin.) Our previous studies show that RXP-E can interfere with chemically-induced closure of gap junctions. In the present study, we sought to determine the extent of selectivity of RXP-E action and its effect on propagation velocity and susceptibility to block.
While other molecules (e.g., cAMP) have been described that increase GJ coupling,28, 32–36 their target remains elusive, and studies have not identified the connexin molecule itself as the target.37 Previous studies have determined that a small peptide (AAP10) can facilitate intercellular communication in heart preparations.38 Additional chemical modifications on AAP10 led to a compound initially identified as “ZP123” (now “rotigaptide”). Experiments have suggested a potential therapeutic value to this molecule10 and, in general, to the approach of modifying GJ conductance to prevent or treat cardiac arrhythmias.8 Yet, while rotigaptide-based experiments suggest that GJ openers represent a promising strategy for antiarrhythmic therapy, this particular molecule lacks an identifiable target. (The molecule -or molecules- acting as receptor for rotigaptide is -are- not yet known.)8 The latter limits the possibility of conducting structure-activity studies to optimize the mechanisms of action of rotigaptide through target-based drug design. Moreover, recent studies suggest that rotigaptide may modify the catalytic activity of some kinases.39 Given the broad range of substrates and intracellular signaling pathways that can be affected by kinase modification, secondary effects on other cellular functions are likely. Here, we have used a different approach, that is, to select the target molecule (Cx43CT) as the bait for identification of the ligand (RXP-E). With this knowledge, we now demonstrate that RXP-E acts as a ligand for cardiac Cx43, and prevents the action of at least two well-known chemical uncouplers.
In conclusion, we have presented evidence indicating that a molecule derived from the “RXP” series of Cx43-binding molecules6 is capable of binding to cardiac Cx43, modifying cardiac gap junctions and preventing action potential propagation block. This is the first Cx43-targetted molecule capable of preserving electrical conduction in a preparation of cardiac cells. Much remains to be done to determine the arrhythmogenic vs antiarrhythmic effect of gap junction closure/opening under conditions of metabolic stress. Although pharmacological optimization and minimization is necessary, the results presented here suggest that RXP-E can serve as a starting point for the development of a target-based class of drugs centered on the objective of modifying cardiac gap junction function.
Acknowledgments
We thank Viviana Munoz, David Auerbach, and Sergey Mironov for assistance with optical mapping experiments and analysis.
Sources of Funding
Work was supported by NIH grants HL39707 and GM057691. R.L. was supported by a Predoctoral Fellowship from the American Heart Association. K.P. and M.S.N. were funded by the Danish National Research Foundation and the Danish Cardiovascular Research Academy.
Footnotes
Disclosures: None.
References
- 1.Morley GE, Taffet SM, Delmar M. Intramolecular interactions mediate pH regulation of connexin43 channels. Biophys J. 1996;70:1294–1302. doi: 10.1016/S0006-3495(96)79686-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ek-Vitorin JF, Calero G, Morley GE, Coombs W, Taffet SM, Delmar M. PH regulation of connexin43: Molecular analysis of the gating particle. Biophys J. 1996;71:1273–1284. doi: 10.1016/S0006-3495(96)79328-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maass K, Ghanem A, Kim JS, et al. Defective epidermal barrier in neonatal mice lacking the C-terminal region of connexin43. Mol Biol Cell. 2004;15:4597–4608. doi: 10.1091/mbc.E04-04-0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calero G, Kanemitsu M, Taffet SM, Lau AF, Delmar M. A 17mer peptide interferes with acidification-induced uncoupling of connexin43. Circ Res. 1998;82:929–935. doi: 10.1161/01.res.82.9.929. [DOI] [PubMed] [Google Scholar]
- 5.Seki A, Coombs W, Taffet SM, Delmar M. Loss of electrical communication, but not plaque formation, after mutations in the cytoplasmic loop of connexin43.see comment. Heart Rhythm. 2004;1:227–233. doi: 10.1016/j.hrthm.2004.03.066. [DOI] [PubMed] [Google Scholar]
- 6.Shibayama J, Lewandowski R, Kieken F, et al. Identification of a novel peptide that interferes with the chemical regulation of connexin43. Circ Res. 2006;98:1365–1372. doi: 10.1161/01.RES.0000225911.24228.9c. [DOI] [PubMed] [Google Scholar]
- 7.Srinivas M, Duffy HS, Delmar M, Spray DC. Prospects for pharmacological targeting of gap junction channels. In: Zipes DZ, Jalife J, editors. Cardiac Electrophysiology: From Cell to Bedside. 2004. Philadelphia: Saunders; 2004. pp. 158–167. [Google Scholar]
- 8.Herve JC, Dhein S. Pharmacology of cardiovascular gap junctions. Adv Cardiol. 2006;42:107–131. doi: 10.1159/000092565. [DOI] [PubMed] [Google Scholar]
- 9.Eloff BC, Gilat E, Wan X, Rosenbaum DS. Pharmacological modulation of cardiac gap junctions to enhance cardiac conduction: Evidence supporting a novel target for antiarrhythmic therapy. Circulation. 2003;108:3157–3163. doi: 10.1161/01.CIR.0000101926.43759.10. [DOI] [PubMed] [Google Scholar]
- 10.Haugan K, Petersen JS. Gap junction modifying antiarrhythmic peptides: Therapeutic potential in atrial fibrillation. Drugs Fut. 2007;32:245–260. [Google Scholar]
- 11.Oxford EM, Musa H, Maass K, Coombs W, Taffet SM, Delmar M. Connexin43 remodeling caused by inhibition of plakophilin-2 expression in cardiac cells. Circ Res. 2007;101:703–711. doi: 10.1161/CIRCRESAHA.107.154252. [DOI] [PubMed] [Google Scholar]
- 12.Gaudesius G, Miragoli M, Thomas SP, Rohr S. Coupling of cardiac electrical activity over extended distances by fibroblasts of cardiac origin. Circ Res. 2003;93:421–428. doi: 10.1161/01.RES.0000089258.40661.0C. [DOI] [PubMed] [Google Scholar]
- 13.Rohr S, Fluckiger-Labrada R, Kucera JP. Photolithographically defined deposition of attachment factors as a versatile method for patterning the growth of different cell types in culture. Pflugers Arch. 2003;446:125–132. doi: 10.1007/s00424-002-1000-0. [DOI] [PubMed] [Google Scholar]
- 14.Kucera JP, Heuschkel MO, Renaud P, Rohr S. Power-law behavior of beat-rate variability in monolayer cultures of neonatal rat ventricular myocytes. Circ Res. 2000;86:1140–1145. doi: 10.1161/01.res.86.11.1140. [DOI] [PubMed] [Google Scholar]
- 15.Kim D, Jeon C, Kim JH, et al. Cytoplasmic transduction peptide (CTP): New approach for the delivery of biomolecules into cytoplasm in vitro and in vivo. Exp Cell Res. 2006;312:1277–1288. doi: 10.1016/j.yexcr.2005.12.029. [DOI] [PubMed] [Google Scholar]
- 16.Moreno AP. Biophysical properties of homomeric and heteromultimeric channels formed by cardiac connexins. Cardiovasc Res. 2004;62:276–286. doi: 10.1016/j.cardiores.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 17.Seki A, Duffy HS, Coombs W, Spray DC, Taffet SM, Delmar M. Modifications in the biophysical properties of connexin43 channels by a peptide of the cytoplasmic loop region. Circ Res. 2004;95:e22–8. doi: 10.1161/01.RES.0000140737.62245.c5. [DOI] [PubMed] [Google Scholar]
- 18.Munoz V, Vaidyanathan R, Tolkacheva EG, Dhamoon AS, Taffet SM, Anumonwo JM. Kir2.3 isoform confers pH sensitivity to heteromeric Kir2.1/Kir2.3 channels in HEK293 cells. Heart Rhythm. 2007;4:487–496. doi: 10.1016/j.hrthm.2006.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dhamoon AS, Pandit SV, Sarmast F, et al. Unique Kir2.x properties determine regional and species differences in the cardiac inward rectifier K+ current. Circ Res. 2004;94:1332–1339. doi: 10.1161/01.RES.0000128408.66946.67. [DOI] [PubMed] [Google Scholar]
- 20.Munoz V, Grzeda KR, Desplantez T, et al. Adenoviral expression of IKs contributes to wavebreak and fibrillatory conduction in neonatal rat ventricular cardiomyocyte monolayers. Circ Res. 2007;101:475–483. doi: 10.1161/CIRCRESAHA.107.149617. [DOI] [PubMed] [Google Scholar]
- 21.Nelson WL, Makielski JC. Block of sodium current by heptanol in voltage-clamped canine cardiac purkinje cells. Circ Res. 1991;68:977–983. doi: 10.1161/01.res.68.4.977. [DOI] [PubMed] [Google Scholar]
- 22.Frankel AD, Pabo CO. Cellular uptake of the tat protein from human immunodeficiency virus. Cell. 1988;55:1189–1193. doi: 10.1016/0092-8674(88)90263-2. [DOI] [PubMed] [Google Scholar]
- 23.Green M, Loewenstein PM. Autonomous functional domains of chemically synthesized human immunodeficiency virus tat trans-activator protein. Cell. 1988;55:1179–1188. doi: 10.1016/0092-8674(88)90262-0. [DOI] [PubMed] [Google Scholar]
- 24.Wadia JS, Stan RV, Dowdy SF. Transducible TAT-HA fusogenic peptide enhances escape of TAT-fusion proteins after lipid raft macropinocytosis. Nat Med. 2004;10:310–315. doi: 10.1038/nm996. [DOI] [PubMed] [Google Scholar]
- 25.Miragoli M, Gaudesius G, Rohr S. Electrotonic modulation of cardiac impulse conduction by myofibroblasts. Circ Res. 2006;98:801–810. doi: 10.1161/01.RES.0000214537.44195.a3. [DOI] [PubMed] [Google Scholar]
- 26.Miragoli M, Salvarani N, Rohr S. Myofibroblasts induce ectopic activity in cardiac tissue. Circ Res. 2007;101:755–758. doi: 10.1161/CIRCRESAHA.107.160549. [DOI] [PubMed] [Google Scholar]
- 27.Kohl P, Camelliti P. Cardiac myocyte-nonmyocyte electrotonic coupling: Implications for ventricular arrhythmogenesis. Heart Rhythm. 2007;4:233–235. doi: 10.1016/j.hrthm.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 28.Darrow BJ, Fast VG, Kleber AG, Beyer EC, Saffitz JE. Functional and structural assessment of intercellular communication. increased conduction velocity and enhanced connexin expression in dibutyryl cAMP-treated cultured cardiac myocytes. Circ Res. 1996;79:174–183. doi: 10.1161/01.res.79.2.174. [DOI] [PubMed] [Google Scholar]
- 29.Rohr S, Kucera JP, Fast VG, Kleber AG. Paradoxical improvement of impulse conduction in cardiac tissue by partial cellular uncoupling. Science. 1997;275:841–844. doi: 10.1126/science.275.5301.841. [DOI] [PubMed] [Google Scholar]
- 30.Kleber AG, Rudy Y. Basic mechanisms of cardiac impulse propagation and associated arrhythmias. Physiol Rev. 2004;84:431–488. doi: 10.1152/physrev.00025.2003. [DOI] [PubMed] [Google Scholar]
- 31.Severs NJ, Coppen SR, Dupont E, Yeh HI, Ko YS, Matsushita T. Gap junction alterations in human cardiac disease. Cardiovasc Res. 2004;62:368–377. doi: 10.1016/j.cardiores.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 32.De Mello WC. Cyclic AMP and junctional communication viewed through a multibiophysical approach. In: Peracchia C, editor. Biophysics of Gap Junction Channels. Boca Raton, USA: CRC Press; 1991. pp. 229–239. [Google Scholar]
- 33.De Mello WC. Effect of intracellular injection of calcium and strontium on cell communication in heart. J Physiol (Lond) 1975;250:231–245. doi: 10.1113/jphysiol.1975.sp011051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burt JM, Spray DC. Inotropic agents modulate gap junctional conductance between cardiac myocytes. Am J Physiol. 1988;254:H1206–10. doi: 10.1152/ajpheart.1988.254.6.H1206. [DOI] [PubMed] [Google Scholar]
- 35.Doble BW, Chen Y, Bosc DG, Litchfield DW, Kardami E. Fibroblast growth factor-2 decreases metabolic coupling and stimulates phosphorylation as well as masking of connexin43 epitopes in cardiac myocytes. Circ Res. 1996;79:647–658. doi: 10.1161/01.res.79.4.647. [DOI] [PubMed] [Google Scholar]
- 36.Pimentel RC, Yamada KA, Kleber AG, Saffitz JE. Autocrine regulation of myocyte Cx43 expression by VEGF. Circ Res. 2002;90:671–677. doi: 10.1161/01.res.0000014823.75393.4d. [DOI] [PubMed] [Google Scholar]
- 37.Dhein S, Polontchouk L, Salameh A, Haefliger JA. Pharmacological modulation and differential regulation of the cardiac gap junction proteins connexin 43 and connexin 40. Biol Cell. 2002;94:409–422. doi: 10.1016/s0248-4900(02)00018-7. [DOI] [PubMed] [Google Scholar]
- 38.Muller DJ, Hand GM, Engel A, Sosinsky GE. Conformational changes in surface structures of isolated connexin 26 gap junctions. EMBO J. 2002;21:3598–3607. doi: 10.1093/emboj/cdf365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dhein S, Weng S, Grover R, et al. Protein kinase C alpha mediates the effect of antiarrhythmic peptide on gap junction conductance. Cell Commun Adhes. 2001;8:257–264. doi: 10.3109/15419060109080734. [DOI] [PubMed] [Google Scholar]