Abstract
We performed intravenous sedation with dexmedetomidine hydrochloride during minor oral surgery and compared this agent with propofol. Patients were randomly divided into 2 groups: dexmedetomidine hydrochloride (D) and propofol (P) groups. In Group D, systolic blood pressure (SBP) increased immediately after the start of initial loading, although no significant differences were noted. Both SBP and diastolic blood pressure (DBP) gradually decreased during maintenance administration and were significantly lower than pretreatment values. The heart rate decreased immediately after the start of administration and was significantly lower during both initial loading and maintenance administration; the heart rate was also significantly lower than that in Group P. In Group D, arterial blood oxygen saturation (SpO2) significantly decreased after the sedation level reached an optimum level until the end of administration. The bispectral index (BIS) value gradually decreased during initial loading. At the optimal sedation level, it decreased to 80 to 85. During maintenance administration, marked changes were observed in this parameter. No marked differences in amnestic effects and comfort were noted between the 2 groups. If the sedation level can be evaluated accurately via another objective method, intravenous sedation with dexmedetomidine hydrochloride may be useful in dental treatment.
Keywords: Intravenous sedation, Dexmedetomidine, Hydrocholoide, Minor oral surgery
Indications for dexmedetomidine hydrochloride include sedation during artificial respiration and after extubation in patients in whom early extubation is possible under intensive care.1 Its sedative actions resemble physiologic sleep,2 and respiratory depression is less marked; this differs from the features of benzodiazepines and propofol, which have been used in the dental field for psychosedation. It has been reported that this agent causes cardiovascular and psychiatric/neurologic side effects such as hypertension and bradycardia.1 In this study, we performed intravenous sedation with dexmedetomidine hydrochloride during minor oral surgery and compared this agent with propofol.
METHODS
Subjects were 14 patients for whom the status was evaluated as 1 according to the American Society of Anesthesiologists (ASA) classification; minor surgery was scheduled in the Department of Oral Surgery, Matsumoto Dental University Hospital. When dexmedetomidine hydrochloride was administered, the drug name, effects, and side effects were explained, and informed consent was obtained. Patients were randomly divided into 2 groups: dexmedetomidine hydrochloride (D) and propofol (P) groups.
We measured blood pressure, heart rate, and arterial blood oxygen saturation (Moneo BP-88 Si, Corin, Japan). The bispectral index (BIS) was measured using an A-2000 monitor (Aspect Medical Systems Inc, Newton, Mass). Control values were measured 5 minutes after each monitor was applied in a dental chair. Each parameter was measured at 2-minute intervals during initial loading, and at 5-minute intervals during maintenance administration until the end of treatment. With respect to the presence or absence of memory on injection of local anesthesia and at the start of treatment, as well as the comfortableness of sedation, subjects were requested to complete a questionnaire the day after treatment. Comfortableness was evaluated using a visual analog scale (VAS) (0: very uncomfortable, 100: very comfortable, want to undergo the sedation procedure for additional surgery). All values were expressed as the mean ± standard deviation.
Immediately after the control values were measured, the venous route was obtained. In Group D, for initial loading, 6 µg/kg/h of dexmedetomidine hydrochloride was injected intravenously for 10 minutes at maximum until the score reached 3 (awakening responses to calling despite closed eyes), according to Mackenzie's sedation assessment scoring. The rate of maintenance administration was established as 0.4 µg/kg/h and was increased or decreased if necessary. In Group P, 20 mg of lidocaine hydrochloride was administered intravenously. For initial loading, 0.5 mg/kg of propofol was administered. The rate of maintenance administration was established as 4 mg/kg/h and was increased or decreased if necessary, so that Mackenzie's score was 3. In Groups D and P, we used a Telfusion TCI pump TE-371 (Termo, Japan) for drug administration.
For statistical analysis, 1-way analysis of variance was performed, as was the Dunnett multiple comparison test. Furthermore, values at each measurement point were compared between the 2 groups using the Mann-Whitney U test. P < .05 was regarded as significant.
RESULTS
Age, height, body weight, and duration of treatment in Groups D and P are shown in Table 1. No significant differences in any parameters were noted between the 2 groups.
Table 1.
Demographic Characteristics of Subjects
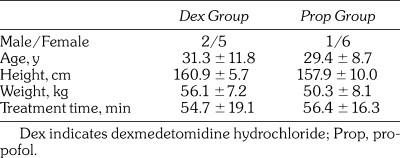
1. Changes in blood pressure (Figure 1)
Figure 1.
Changes in blood pressure. In group D, systolic blood pressure (SBP) was significantly higher than that in group P. SBP and diastolic blood pressure (DBP) significantly decreased during maintenance administration compared with pretreatment value in groups D.
In Group D, the systolic blood pressure (SBP) increased from 2 minutes after the start until the end of initial loading. However, no significant differences were seen in comparison with the pretreatment value. In Group P, SBP decreased from 2 minutes until 4 minutes (end of initial loading) after the start of initial loading. The value 4 minutes after the start of initial loading was significantly lower than the pretreatment value. Furthermore, the SBP value 4 minutes after the start of initial loading in Group D was significantly higher than that in Group P.
In Group D, the diastolic blood pressure (DBP) significantly increased compared with the pretreatment value 2 minutes after the start of initial loading, but then it gradually decreased to the pretreatment value 8 minutes after the start of initial loading. In Group P, DBP continued to decrease from the start until the end of initial loading. However, no significant differences were seen in comparison with the pretreatment value.
During maintenance administration, SBP gradually decreased in the 2 groups. This parameter significantly decreased compared with the pretreatment value from 20 and 15 minutes after the start of maintenance administration until the end of administration in Groups D and P, respectively. Immediately after the start of maintenance administration, the value in Group D was significantly higher than that in Group P, but then no marked differences were noted.
During maintenance administration, DBP also gradually decreased in the 2 groups. This parameter significantly decreased compared with the pretreatment value from 20 and 10 minutes after the start of maintenance administration until the end of administration in Groups D and P, respectively. No marked differences between the 2 groups were noted.
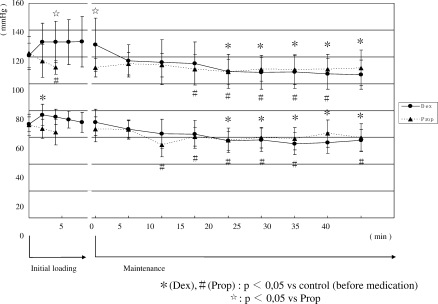
2. Changes in heart rate (Figure 2)
Figure 2.
Changes in heart rate. In group D, heart rate significantly decreased from 6 minutes after the start of initial loading until the start of maintenance administration. Heart rate in group D was lower than that in group P.
In Group D, the heart rate gradually decreased after the start of initial loading. It significantly decreased from 6 minutes after the start of initial loading until the start of maintenance administration. Thereafter, it increased to the pretreatment value and did not change until the end of administration. In Group P, the heart rate slightly increased during maintenance administration. However, no significant differences were observed during administration. The value in Group D was significantly lower than that in Group P from 4 minutes after the start of initial loading until 25 minutes after the start of maintenance administration.
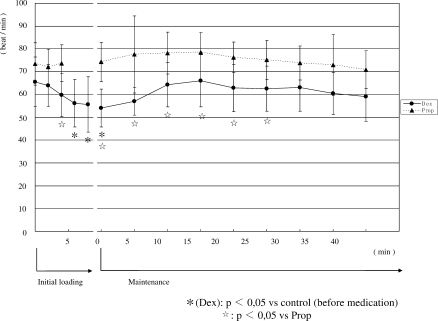
3. Changes in percutaneous arterial blood oxygen saturation (SpO2) (Figure 3)
Figure 3.
Changes in percutaneous arterial blood oxygen saturation (SpO2). In group D, SpO2 significantly decreased from 6 minutes after the start of administration until the end of maintenance administration. SpO2 in Group D was significantly lower than that in Group P.
In Group D, SpO2 decreased after the start of initial loading. It significantly decreased compared with the pretreatment value from 6 minutes after the start of administration until the end of maintenance administration, at a minimum of 95.7%. In Group P, SpO2 significantly decreased 4 minutes after the start of administration but did not change thereafter. During maintenance administration, the value was 97 to 98%. Four minutes after the start of initial loading and 25 minutes or more after the start of maintenance administration, SpO2 in Group D was significantly lower than that in Group P, ranging from 95.7 to 96.6%.
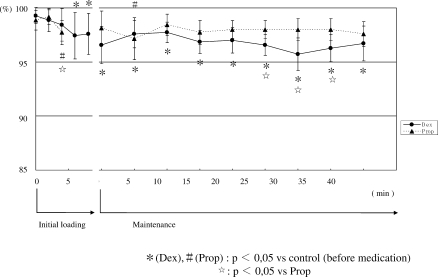
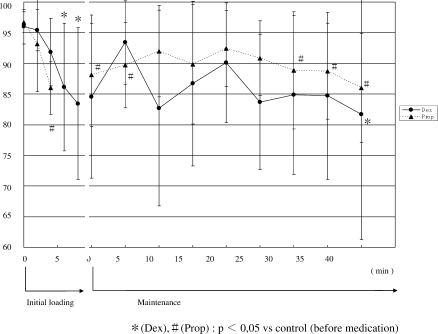
4. Changes in BIS (Figure 4)
Figure 4.
Changes in bispectral index (BIS). At the optimum sedation level, BIS was significantly lower than pretreatment values in the 2 groups. During maintenance administration, the fluctuation of BIS in Group D was greater than that in Group P.
During initial loading, BIS gradually decreased in the 2 groups. At the optimum sedation level, values were significantly lower than pretreatment values. In Group D, the BIS value remained low during maintenance administration, although it varied. In Group P, BIS slightly increased after the start of maintenance administration but significantly decreased after 25 minutes or longer. During administration, no significant differences were seen between the 2 groups.
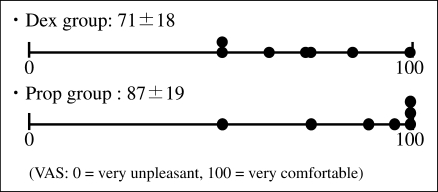
5. Memory on injection of local anesthesia and at the start of treatment (Table 2) and comfortableness (Figure 5)
Table 2.
Presence of Memory
Figure 5.
Comfortableness of sedation. No significant differences were noted between the 2 groups.
Four patients in each of Groups D and P remembered the injection of local anesthesia. Two patients in Group D and 3 patients in Group P remembered the start of treatment. No marked differences were observed between the 2 groups. In Group D, the score for comfortableness (VAS) was 71.4 ± 18.0—lower than that in Group P. However, no significant differences were noted between the 2 groups.
DISCUSSION
1. Hemodynamic changes
Dexmedetomidine hydrochloride induces peripheral vasoconstriction due to α2B receptor stimulation and increases blood pressure.3 In this study, both SBP and DBP gradually decreased after the start of administration in Group P. In Group D, these values increased immediately after the start of initial loading. SBP remained higher than the pretreatment value during initial loading, although no significant differences were observed. In particular, SBP increased in 6 patients until 4 minutes after the start of administration. In contrast, dexmedetomidine hydrochloride has been reported to decrease blood pressure and cause bradycardia via inhibition of the sympathetic nervous system and activation of the parasympathetic nervous system in the presence of α2A receptor stimulation.4,5 In this study, in Group D, both SBP and DBP gradually decreased during maintenance administration to values significantly lower than pretreatment values. However, the degree of decrease in blood pressure was similar to that in Group P. In Group D, the heart rate decreased immediately after the start of administration and was significantly lower until the start of treatment. In 6 patients, bradycardia was noted. During maintenance administration, the heart rate was also significantly lower than that in Group P, although it gradually increased to the pretreatment value.
Because dexmedetomidine hydrochloride has no selectivity for α2A/α2B receptors, blood pressure changes are not constant. In this study, an increase in blood pressure and bradycardia were seen on initial loading, during which the blood concentration of this agent rapidly increased. During maintenance administration, circulatory depression was seen frequently. We previously encountered a patient in whom intravenous sedation was performed for the extraction of impacted lower wisdom teeth. A decrease in blood pressure and bradycardia were observed on mandibular foramen conduction anesthesia following initial loading despite the standard administration method, and atropine sulfate was administered to this patient.6 Thus, hemodynamic changes are marked during initial loading, and close monitoring is needed. So this procedure can be applied to intravenous sedation for dental treatment, the rate and duration of initial loading should be investigated with the goal of reducing changes in circulation.
2. Respiratory changes
In this study, we measured SpO2 alone. In Group D, SpO2 significantly decreased after the sedation level reached an optimum until the end of administration. This was consistent with findings reported by Yamashita et al.7 but contrary to the slight respiratory depression that is characteristic of dexmedetomidine hydrochloride. SpO2 in Group D was 95% or greater in the absence of oxygen inhalation, although it was slightly lower than that in Group P. Therefore, this change may not result in clinical problems. The results of this study did not show that dexmedetomidine hydrochloride–related respiratory depression was less marked than that related to propofol, which has been used for intravenous sedation. However, according to Yamashita et al.,7 administration of dexmedetomidine hydrochloride did not influence the respiratory rate. Changes in tidal volume and arterial blood carbon dioxide tension should be investigated.
3. Changes in BIS
The BIS value gradually decreased during initial loading. At the optimal sedation level, it decreased to 80 to 85. During maintenance administration, marked changes were seen in this parameter, and there was no constant tendency. Yamashita et al.7 reported that BIS was useful as an index of relative changes in optimum sedation level in comparison with the clinical assessment using Ramsay's sedation score, although the algorithm for calculating BIS was not matched to dexmedetomidine hydrochloride–related electroencephalographic changes. In this study, sedation was stable during initial loading because there were no external stimuli prior to the start of treatment. The BIS value may have been matched to the clinical assessment of optimum sedation; there was a constant tendency. However, when treatment was started, various stimuli were added, which affected patients' sedation levels. Dexmedetomidine hydrochloride–related sedation resembles physiologic sleep,2 and stimulation facilitates awakening even during continuous administration. Therefore, even when the BIS value is high, the sedation level is not always light. The quality of dexmedetomidine hydrochloride–related sedation may be a weak point in dental treatment. Furthermore, it may be difficult to evaluate sedation based on BIS during treatment with stimuli.
4. Amnestic effects and comfortableness
Concerning amnestic effects, we examined the presence or absence of memory on local anesthesia injection and the start of treatment. No marked differences were noted between the 2 groups. When treatment-related stimuli are less marked, amnestic effects may be similar to those of propofol. However, in this study, we did not investigate these effects during surgery. Boku et al.8 administered pethidine hydrochloride prior to oral surgery with intravenous sedation, and reported that combination therapy with dexmedetomidine hydrochloride and midazolam achieved favorable amnestic effects. However, they indicated that the effects of this combination were less marked in the presence of strong invasiveness. Intravenous sedation with dexmedetomidine hydrochloride alone for dental treatment may show less potent amnestic effects compared with the conventional method.
No significant difference in comfortableness was observed between the 2 groups. However, in Group D, the VAS score on comfortableness varied among patients, possibly because the sedation level was light owing to the difficulty in evaluation, and because awakening associated with treatment-related stimuli reduced amnestic effects. Amnestic effects are not the main purpose of psychosedation. As reported by Boku et al.,8 intravenous sedation with dexmedetomidine hydrochloride may exhibit less potent amnestic effects, which may have contributed to differences in comfortableness in comparison with propofol. Thus, accurate evaluation of the sedation level may increase the quality of intravenous sedation with dexmedetomidine hydrochloride. Furthermore, another method of using dexmedetomidine hydrochloride, such as basing it on the rate of administration and combination with other agents, should be investigated to obtain the optimum sedation level during dental treatment even in the presence of treatment-related stimuli.
CONCLUSION
We performed intravenous sedation with dexmedetomidine hydrochloride or propofol for minor oral surgery and compared blood pressure, heart rate, SpO2, BIS, memory on local anesthesia injection and the start of treatment, and comfortableness. No marked difference in respiratory depression was noted between intravenous sedation with dexmedetomidine hydrochloride and that with propofol. However, hemodynamic changes were marked in the former group, and close monitoring is needed. Furthermore, it is difficult to evaluate intraoperative sedation level based on BIS in many cases, and other evaluation methods must be developed. If the sedation level can be evaluated accurately via further investigation of administration methods, intravenous sedation with dexmedetomidine hydrochloride may be useful in dental treatment.
REFERENCES
- Taiji K. Dexmedetomidine hydrochloride. Med Drug J. 2005;41:306–314. [Google Scholar]
- Nelson L.E. The alpha 2-adrenoceptor agonist dexmedetomidine converges on an endogenous sleep-promoting pathway to exert its sedative effects. Anesthesiology. 2003;98:428–436. doi: 10.1097/00000542-200302000-00024. [DOI] [PubMed] [Google Scholar]
- Talke P, Lobo E, Brown R. Systemically administered alpha 2-agonist-induced peripheral vasoconstriction in humans. Anesthesiology. 2003;99:65–70. doi: 10.1097/00000542-200307000-00014. [DOI] [PubMed] [Google Scholar]
- Doze V.A, Chen B.X, Tinklenberg J.A, Segal I.S, Maze M. Pertussis toxin and 4-aminopyridine differentially affect the hypnotic-anesthetic action of dexmedetomidine and pentobarbital. Anesthesiology. 1990;73:304–307. doi: 10.1097/00000542-199008000-00019. [DOI] [PubMed] [Google Scholar]
- Sullivan A.F, Kalso E.A, McQuay H.J, Dickenson A.H. The antinociceptive actions of dexmedetomidine on dorsal horn neuronal responses in the anaesthetized rat. Eur J Pharmacol. 1992;215:127–133. doi: 10.1016/0014-2999(92)90617-d. [DOI] [PubMed] [Google Scholar]
- Taniyama K, Shibutani T, Oda H, Okawa K, Himeno K, Hirose I. Intravenous sedation with dexmedetomidine for dental treatment—report of two cases with side effects. Jpn Dent Soc Anesthesiol. 2007;35:64–67. [Google Scholar]
- Yamashita A, Kunimatsu T, Itsumura K, et al. Usefulness of dexmedetomidine for intravenous conscious sedation. J Jpn Dent Soc Anesthesiol. 2005;33:687–692. [Google Scholar]
- Boku A, Morimoto Y, Hanamoto H, Kagamiuchi H, Niwa H. The efficacy of intravenous sedation in combination with dexmedetomidine and midazolam in oral and maxillofacial surgery. J Jpn Dent Soc Anesthesiol. 2006;34:485–491. [Google Scholar]