Abstract
Sodium channel-blocker insecticides (SCBIs), such as indoxacarb and metaflumizone, are a new class of insecticides with a mechanism of action different from those of other insecticides that target sodium channels. SCBIs block sodium channels in a manner similar to local anesthetics (LA) such as lidocaine. Several residues, particularly F1579 and Y1586, in the sixth transmembrane segment (S6) of domain IV (IV) of rat Nav1.4 sodium channels are required for the action of LAs and SCBIs and may form part of overlapping receptor sites. However, the binding site for SCBIs in insect sodium channels remains undefined. We used site-directed mutagenesis, the Xenopus laevis oocyte expression system, and the two-electrode voltage clamp technique to study the effects on SCBI activity of mutating F1817 and Y1824 (analogous to those residues identified in mammalian sodium channels) to alanine in the voltage-sensitive sodium channel of the German cockroach, Blattella germanica. The mutant channels showed no effect or a marked increase in channel sensitivity to both DCJW (the active metabolite of indoxacarb) and metaflumizone. Thus, it appeared that although the F1817 residue plays a role in the action of SCBIs and both residues are involved in LA activity in mammalian sodium channels, neither F1817 nor Y1824 are integral determinants of SCBI binding on insect sodium channels. Our results suggest that the receptor site of SCBIs on insect sodium channels may be significantly different from that on mammalian sodium channels.
Introduction
Voltage-sensitive sodium channels are large integral membrane proteins that consist of four homologous domains (I-IV) each containing six transmembrane segments (S1-S6) connected by extra- and intracellular loops (Catterall, 2000; Goldin, 2001). Sodium channels are commonly found in excitable cells, including nervous and cardiac tissues, and are largely responsible for the upstroke in membrane voltage during an action potential. Thus, the importance of voltage-sensitive sodium channels in cellular excitability makes them ideal targets for natural and synthetic toxins, such as pesticides.
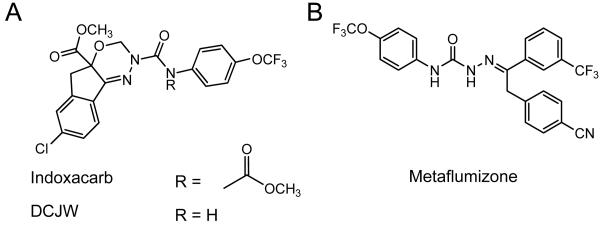
Sodium channel blocking insecticides (SCBIs) are a relatively new group of insecticides. Indoxacarb (Fig. 1A), the first registered insecticide of this class, causes cessation of feeding, poor coordination, paralysis, and death (Harder, et al., 1996; Narahashi, 2001; Silver and Soderlund, 2005; Wing, et al., 2005) in a wide range of agriculturally important pests. Indoxacarb, a proinsecticide, is activated by insects to its more potent, N-decarbomethoxylated metabolite, DCJW (Fig. 1A) (Wing, et al., 2005; Wing, et al., 2000; Wing, et al., 1998). Metaflumizone (Fig. 1B), the second SCBI to be commercialized, causes poisoning symptoms that are similar to those produced by other SCBIs (Salgado and Hayashi, 2007). SCBIs inhibit voltage-sensitive sodium channels in both insects and mammals (Salgado and Hayashi, 2007; Silver and Soderlund, 2005; Silver and Soderlund, 2006; Wing, et al., 2000; Wing, et al., 1998; Zhao, et al., 2005; Zhao, et al., 2003). These compounds have no effect at hyperpolarized membrane potentials, but cause a voltage-dependent, nearly irreversible block as the membrane is depolarized (Salgado and Hayashi, 2007; Silver and Soderlund, 2005; Silver and Soderlund, 2006; Zhao, et al., 2005; Zhao, et al., 2003).
Figure 1.
Structures of A. indoxacarb and DCJW, and B. metaflumizone.
Block of sodium channels by SCBIs is very similar to inhibition of sodium channels by local anesthetics (LA), class I anticonvulsants, and class I antiarrhythmics (Catterall, 1987; Wang and Wang, 2003). Salgado (1992) first documented the similarity between the mode of action of LAs and the pyrazoline SCBI RH3421, a progenitor of the indoxacarb/DCJW insecticide family, in crayfish giant axons. Both groups of compounds are voltage-dependent inhibitors of sodium channels, and both therapeutic sodium channel blockers and SCBIs cause hyperpolarizing shifts in the voltage-dependence of inactivation (Salgado, 1992; Silver and Soderlund, 2005; Silver and Soderlund, 2006; Zhao, et al., 2003). Studies in mouse brain synaptosomes document similar mechanisms for inhibiting veratradine-stimulated uptake of radiosodium ions by LAs and RH3421 (Catterall, 1981; Deecher and Soderlund, 1991; Payne, et al., 1998). Furthermore, RH3421 and dibucaine are mutually competitive inhibitors of veratradine-stimulated uptake into mouse brain synaptosomes (Payne, et al., 1998), and phenytoin interferes with the ability of DCJW and RH3421 to inhibit rat Nav1.4 sodium channels expressed in Xenopus laevis oocytes (Silver and Soderlund, 2005). Also, studies in mammalian brain show that RH3421 is an allosteric competitive inhibitor of [3H]-batrachotoxinin-A-20-α-benzoate (BTX-B) binding in a manner similar to therapeutic sodium channel blockers (Creveling, et al., 1983; Deecher, et al., 1991).
In recent years, site-directed mutagenesis studies to map the LA receptor in sodium channels have identified a number of residues in the S6 transmembrane segments of domains I, III, and IV that affect therapeutic drug activity in rat Nav1.2 sodium channels (Ragsdale, et al., 1994; Ragsdale, et al., 1996; Yarov-Yarovoy, et al., 2001; Yarov-Yarovoy, et al., 2002), and similar results have been found in other mammalian sodium channel isoforms, including the Nav1.4 sodium channel (Nau, et al., 1999; Wang, et al., 1998; Wang, et al., 2004; Wang, et al., 2000). In particular, two residues in IVS6, F1764 and Y1771 (numbered according to their location in the rat Nav1.2 sodium channel) are critical for the binding and action of a variety of therapeutic sodium channel blockers (Liu, et al., 2003; Ragsdale, et al., 1994). Interestingly, a recent study shows that the action of indoxacarb and DCJW on mammalian sodium channels is also affected by these mutations. Alanine substitution at F1579 (corresponding to F1764 in Nav1.2) resulted in a significant reduction in the ability of DCJW and RH3421 to inhibit Nav1.4 sodium channels expressed in Xenopus oocytes (Silver and Soderlund, 2007). In contrast, mutation of the tyrosine residue at position 1586 (corresponding to Y1771 in Nav1.2) in Nav1.4 channels resulted in a significant increase in the potency of indoxacarb, DCJW, and RH3421 (Silver and Soderlund, 2007).
A previous study has shown that DCJW blocks sodium channels of the German cockroach, Blattella germanica, (BgNav) in a state-dependent manner; and BgNav variants with different voltage dependences of fast and slow inactivation exhibit differential sensitivities to DCJW (Song, et al., 2006). However, so far no efforts have been made to map the SCBI receptors in insect sodium channels. Here we report the first attempt to locate the receptor site for SCBIs on a DCJW-sensitive BgNav variant, BgNav1-1a (Song, et al., 2004; Song, et al., 2006) by examining the role of F1817 and Y1824 (homologous to F1764 and Y1771 in rat Nav1.2) in IVS6 of the BgNav1-1a sodium channel in the interactions of insect sodium channels with SCBIs.
Materials and Methods
Site-directed mutagenesis
Site-directed mutagenesis was performed by PCR using mutant primers and Pfu Turbo DNA polymerase (Stratagene, La Jolla, CA). All mutagenesis results were verified by DNA sequencing.
Expression of BgNav Sodium Channels in Xenopus laevis Oocytes
The procedures for oocyte preparation and cRNA injection are identical to those described previously (Tan, et al., 2002). For robust expression of the BgNav sodium channel, cRNA was co-injected into oocytes with Drosophila melanogaster tipE cRNA (2:1 ratio), which enhances the expression of insect sodium channels in oocytes (Feng, et al., 1995; Warmke, et al., 1997).
Electrophysiological Recording and Analysis
Sodium currents were recorded using the two-electrode voltage clamp technique. Electrodes were pulled from borosilicate glass and filled with 3 M KCl and 0.5% agarose. Resistances ranged between 0.5 and 1.5 MΩ. Currents were measured with an oocyte clamp amplifier OC725C (Warner Instrument Corp., Hamden, CT), Digidata 1200A (Axon Instruments, Foster City, CA), and pClamp 8.2 software (Axon Instruments). Capacitive transient leak currents were subtracted using the P/N (N = 2) subtraction method. Specific voltage protocols are detailed in the figure legends.
The extremely slow blocking kinetics of SCBIs and the voltage-dependence of their action make quantification of blocking potency difficult. The most straightforward method for quantifying block is the onset of block at or near the potential of 50% steady-state inactivation. After establishing a stable voltage clamp near the half-inactivation potential specific to a channel variant, insecticide-containing solution was perfused into the bath at a rate of 3 ml/min over the first 5 min whereas the time course of onset of block was recorded for 30 min. This protocol was followed by fast inactivation and slow inactivation protocols in succession, with 2 min pauses between protocols.
The above method for measuring block at the half-inactivation potential is strongly affected by slight potential drift and rundown, so a more quantitative method was developed, based on differences in time course of recovery from inactivation between blocked and unblocked channels. Oocytes were incubated with or without insecticide for at least an hour at resting membrane potential (~-25 to -35 mV) in ND-96 to allow complete equilibration of inactivated sodium channels with DCJW or metaflumizone. Recovery from block and inactivation was initiated by clamping the membrane at -120 mV and simultaneously beginning perfusion of insecticide-free ND-96. The time course of recovery of the peak current at any given drug concentration is the algebraic sum of two time courses: recovery of unblocked channels from slow inactivation and recovery of blocked channels from block/slow inactivation, weighted by the fractions of blocked (B) and unblocked (U) channels, respectively. If we let U(t) be the recovery time course of SCBI-free channels and B(t) be the time course of recovery of SCBI-blocked channels, then the recovery time course y(t,c) at a given SCBI concentration, c, is
| (1) |
Where f = 1/(1+cp)/Kd, the fraction of channels that are SCBI-free, p is the Hill coefficient and Kd the dissociation constant.
For each channel variant, we measured families of recovery curves for various concentrations of blockers, and determined the values of Kd and p that gave the best global fit of the theoretical family of curves calculated from Equation 1 to the measured curve family. The Solver add-in for Microsoft Excel was used to find the values of Kd and p that resulted in the minimum sum of squares of all residuals between actual and theoretical time courses in a family of data. Unfortunately, no method was available to estimate the uncertainty in these parameter estimates.
All experiments were performed at room temperature. Indoxacarb and DCJW were provided by K. D. Wing (DuPont Agrochemicals) and metaflumizone was provided by BASF Agricultural Products. Drugs and insecticides were perfused onto oocytes in a manner similar to that previously described (Tatebayashi and Narahashi, 1994).
Results
Modification of BgNav1-1a channel gating by F1817A and Y1824A substitutions
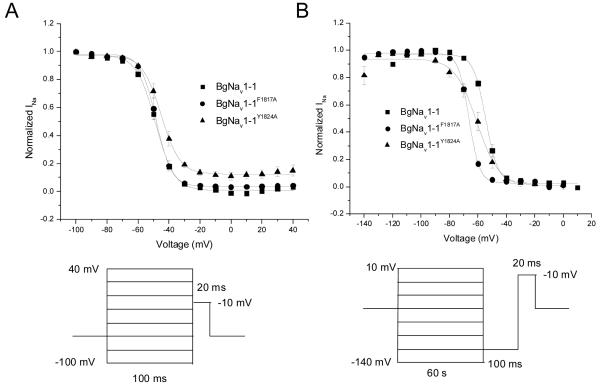
Figure 2 displays the effects of the alanine mutations on the voltage dependence of fast (A) and slow (B) inactivation of BgNav1-1a sodium channels, at depolarized holding potentials. When measured at depolarized holding potentials, neither the F1817A nor the Y1824A mutation caused any significant changes to the voltage dependence of fast inactivation of BgNav1-1a sodium channels (Fig. 2A, p > 0.05, Table 1). In contrast, both mutations resulted in significant hyperpolarizing shifts in the voltage dependences of slow inactivation of BgNav1-1a sodium channels (Fig. 2B, p < 0.05, Table 1) and the slope of the slow inactivation curve for BgNav1-1aY1824A sodium channels was significantly greater (p < 0.05) than that of wild-type channels.
Figure 2.
Effect of alanine substitutions of F1817 and Y1824 in IVS6 on the voltage-dependence of fast (A) and slow (B) inactivation of BgNav1-1a channels. Fast inactivation was measured with 200-ms conditioning pulses to potentials ranging from -100 to 40 mV from a holding potential of -60 mV (BgNav1-1a and BgNav1-1aY1824A) or -65 mV (BgNav1-1aF1817A) followed immediately by 20-ms test pulses to -10 mV. Slow inactivation was measured using 60-s conditioning pulses to potentials ranging from -140 to 10 mV followed by hyperpolarizing pulses to -120 mV (100 ms) to remove any fast inactivation and 20-ms test pulses to -10 mV. Conditioning and test pulses were applied from holding potentials of -60 mV (BgNav1-1a and BgNa 1-1aY1824A) or -65 mV (BgNav1-1aF1817A v). Currents were normalized to the amplitude of the largest recorded current.
Table 1.
Voltages of half inactivation (V0.5) and slopes (k) of fast and slow inactivation curves for BgNav1-1a BgNav1-1aF1817A, and BgNav1-1aY1824A sodium channels treated with indoxacarb or DCJW at depolarized holding potentials
| Control | Indoxacarb | DCJW | Metaflumizone | |||||
|---|---|---|---|---|---|---|---|---|
| V0.5 (mV) | k | V0.5 (mV) | k | V0.5 (mV) | k | V0.5 (mV) | k | |
| Fast Inact | ||||||||
| BgNav1-1a | -48.9 ± 0.8 | 5.9 ± 0.4 | -50.8 ± 1.5 | 6.4 ± 0.7 | -48.3 ± 0.9 | 5.2 ± 0.1 | -45.2 ± 1.3 | 4.0 ± 0.4 |
| BgNav1-1aF1817A | -48.4 ± 1.6 | 4.8 ± 0.2 | -52.2 ± 1.2 | 6.0 ± 0.3 | -49.9 ± 1.7 | 9.1 ± 2.0b | -46.3 ± 1.0 | 4.8 ± 0.2 |
| BgNav1-1aY1824A | -45.2 ± 1.6 | 5.5 ± 0.4 | -41.1 ± 1.2 | 4.9 ± 0.2 | -40.4 ± 1.0 | 5.0 ± 0.2 | -40.0 ± 0.9 | 5.0 ± 0.1 |
| Slow Inact | ||||||||
|---|---|---|---|---|---|---|---|---|
| BgNav1-1a | -54.6 ± 0.9 | 4.2 ± 0.2 | -64.6 ± 1.6 | 6.78 ± 0.4 | -71.8 ± 3.8b | 13.1 ± 1.0b | -51.1 ± 1.4 | 8.2 ± 0.2b |
| BgNav1-1aF1817A | -66.4 ± 0.4a | 3.8 ± 0.2 | -71.9 ± 0.9b | 5.2 ± 0.3 | -99.5 ± 1.0b | 10.1 ± 0.1b | -81.0 ± 1.6b | 13.5 ± 0.6b |
| BgNav1-1aY1824A | -61.4 ± 2.0a | 7.8 ± 0.8a | -57.0 ± 4.2 | 7.1 ± 1.4 | -57.8 ± 4.1 | 8.9 ± 2.1 | -54.8 ± 0.9 | 6.9 ± 0.6 |
significantly different from wild-type (BgNav1-1a, p < 0.05, n ≥ 3)
significantly different from control (p < 0.05, n ≥ 3)
Time course of inhibition by DCJW and metaflumizone of wild-type and mutant BgNav1-1a channels
It is well-known that SCBI insecticides preferably block slow-inactivated states of sodium channels (Salgado 1992; Zhao, et al., 2003; Silver and Soderlund, 2005; Zhao, et al., 2005; Silver and Soderlund, 2006; Song et al., 2006; Salgado and Hayashi, 2007). We performed our experiments with SCBIs at depolarized holding potentials that resulted in similar levels of inactivation in each channel variant, in order to compare the state-dependent inhibition of sodium channels by the insecticides among wild-type and mutant channels. Thus, all further experiments, except as noted below, were carried out at holding potentials of -60 mV for BgNav1-1 and BgNav1-1Y1824A, and -65 mV for BgNav1-1F1817A sodium channels, to adjust for the effect of the F1817 mutation on slow inactivation of BgNav1-1a channels.
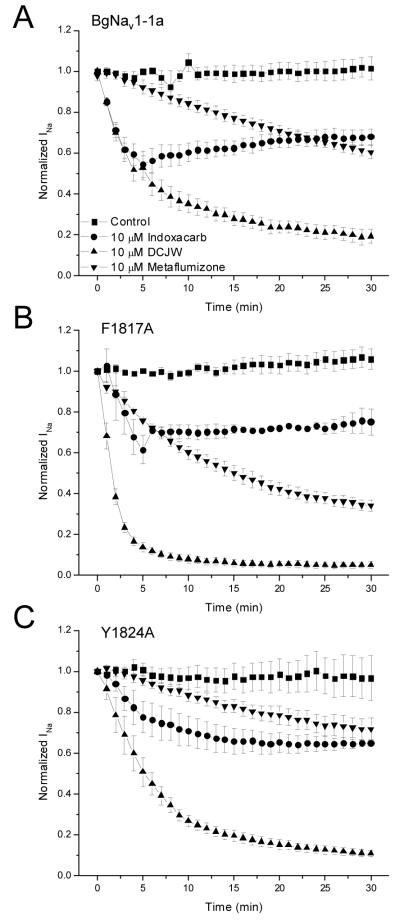
Figure 3A shows the time course of inhibition of wild-type BgNav1-1a sodium channels by 10 μM indoxacarb, 10 μM DCJW, and 10 μM metaflumizone. Inhibition of sodium current increased steadily over the entire 30-min period for metaflumizone, whereas inhibition by DCJW increased steadily but then reached a steady-state after about 25 min of exposure. In contrast, inhibition by indoxacarb increased for the first 5 min, after which sodium current appeared to recover slightly. After 30 min of exposure to insecticide, BgNav1-1a sodium currents were reduced to 67.9 ± 3.7%, 19.2 ± 3.4%, and 60.3 ± 3.0% of the initial current by indoxacarb, DCJW, and metaflumizone, respectively.
Figure 3.
Time course of inhibition of A. BgNav1-1a, B. BgNav1-1aF1817A, or C. BgNav1-1Y1824A channels by indoxacarb, DCJW, or metaflumizone in depolarized oocytes. Currents were elicited once every minute for 30 min with 20-ms test pulses to -10 mV from holding potentials of -60 mV (BgNav1-1a and BgNav1-1aY1824A) or -65 mV (BgNav1-1aF1817A). Insecticides were applied during the first 5 min of recording and remained in the bath throughout the rest of the recording period. Currents were normalized to the first current recorded just prior to insecticide application.
Inhibition of BgNav1-1aF1817A sodium channels by metaflumizone also increased over the entire 30 min recording period, without reaching a steady-state (Fig. 3B), but inhibition of this mutant channel was significantly greater than that of either wild-type or BgNav1-1aY1824A channels (p < 0.05, n = 4). Block of BgNav1-1aF1817A sodium channels by DCJW was also greater compared to that of BgNav1-1a or BgNav1-1aY1824A channels, but the difference was not statistically significant (p > 0.05, n = 4). Inhibition of BgNav1-1aF1817A sodium channels by DCJW also appeared to increase more rapidly, reaching a maximum after only 20 min of treatment as compared to 25 min in wild-type channels. Indoxacarb followed a similar pattern to that described above for BgNav1-1a channels, with an initial increase in inhibition followed by slight recovery of current. Following 30 min of exposure, 10 μM indoxacarb, 10 μM DCJW, and 10 μM metaflumizone reduced sodium currents to 75.3 ± 6.3%, 5.2 ± 1.7%, and 33.0 ± 2.3% of control, respectively.
Indoxacarb, DCJW, and metaflumizone inhibited BgNav1-1aY1824A sodium channels to a similar degree as wild-type channels (Fig. 3C, differences were not significant, p > 0.05, n = 4). For both indoxacarb (10 μM) and DCJW (10 μM), inhibition increased gradually, reaching a steady-state after about 25 min of exposure. Inhibition of sodium channels by metaflumizone (10 μM), however, increased over the entire 30 min recording period without reaching a steady-state. BgNav1-1aY1824A sodium currents were 65.0 ± 1.8%, 10.9 ± 1.4%, and 71.8 ± 5.5% of control following treatment with indoxacarb, DCJW, and metaflumizone, respectively. Comparison of the degree of inhibition induced by indoxacarb or DCJW in wild-type and mutant channels revealed no significant differences (p > 0.05) in inhibition between channel variants, but metaflumizone caused significantly greater inhibition of BgNav1-1aF1817A sodium channels than wild-type or BgNav1-1aY1824A sodium channels (p < 0.05, n = 4). However, metaflumizone did not reach a steady-state of inhibition in either of these channels, therefore it is difficult to determine if the sensitivity of BgNav1-1aF1817A sodium channels is greater than wild-type channels.
Effects of DCJW and metaflumizone on gating of wild-type and mutant BgNav1-1a channels
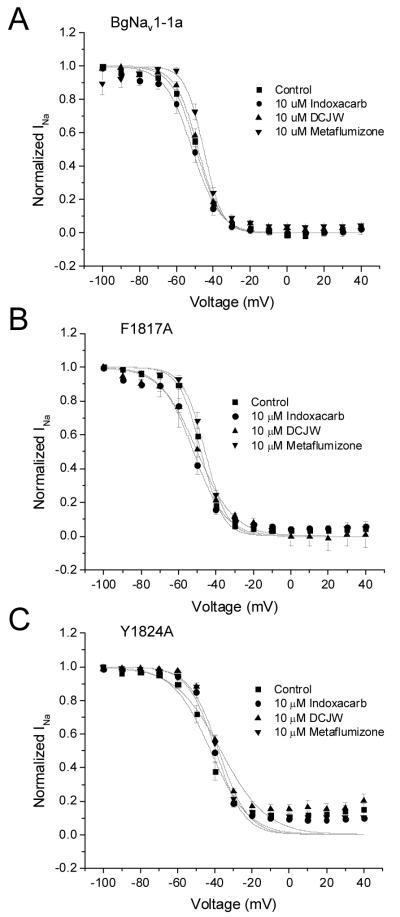
Figures 4 and 5 display the effects of SCBIs on the voltage dependence of fast and slow inactivation, respectively, of wild-type and mutant sodium channels, at depolarized holding potentials. SCBIs (10 μM each) had no effect on the half inactivation potential (V0.5) of fast inactivation of BgNav1-1a, BgNav1-1aF1817A or BgNav1-1aY1824A sodium channels (Fig. 4, Table 1), but the slope of the fast inactivation curve for BgNav1-1aF1817A channels treated with DCJW was significantly greater than control (p < 0.05, n = 3). In contrast, DCJW caused significant (p < 0.05, n = 4 and 7, respectively) hyperpolarizing shifts in the voltage dependence of the slow inactivation curves of both BgNav1-1a and BgNav1-1aF1817A sodium channels (Figs. 5A and 5B, Table 1). Metaflumizone and indoxacarb significantly (p < 0.05, n = 6 and 3, respectively) shifted the voltage dependence of the slow inactivation curve only in BgNav1-1aF1817A sodium channels (Fig. 5B, Table 1). Metaflumizone appeared to have little effect on the voltage dependence of slow inactivation during strong depolarizing pulses, however. Interestingly, despite the sensitivity of BgNav1-1aY1824A channels to inhibition by indoxacarb, DCJW, and metaflumizone, none of these compounds had any significant effect on the voltage dependence of slow inactivation of this mutant channel (Fig. 5C, Table 1).
Figure 4.
Effects of indoxacarb, DCJW, or metaflumizone on the voltage-dependence of fast inactivation of BgNav1-1a (A), BgNav1-1aF1817A (B) and BgNav1-1aY1824A (C) sodium channels. Fast inactivation was measured as in Fig. 2A.
Figure 5.
Effects of indoxacarb, DCJW, or metaflumizone on the voltage-dependence of slow inactivation of BgNav1-1a (A), BgNav1-1aF1817A (B), and BgNav1-1aY1824A (C) sodium channels. Slow inactivation was measured as in Fig. 2B.
Effects of F1817A and Y1824A substitutions on the recovery of BgNav1-1a from SCBI block
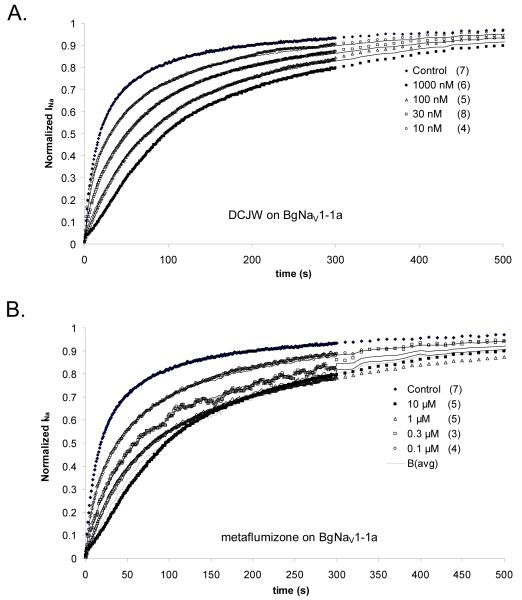
To more accurately quantify the effects of the F1817A and Y1824A mutations on the sensitivity of insect sodium channels to SCBIs, we determined the binding parameters from the time course of recovery from inactivation, in the presence and absence of insecticide, as described in the Materials and Methods. Figure 6A shows the effect of increasing concentrations of DCJW from 10 nM to 1 μM, on the recovery from depolarization of BgNav1-1a channels. The time course of recovery from slow inactivation in the absence of drug, U(t), could be fit with the sum of two exponentials with time constants of 10.5 and 108 s. The recovery from depolarization in the presence of 1 μM DCJW, taken to be the time course of recovery of blocked channels, B(t), proceeded much more slowly, and could be fit with the sum of two exponentials with time constants of 64 and 264 seconds. The recovery time courses at the lower concentrations of DCJW shown in Figure 6A could be fit with the sum of these components in the proportions calculated from equation 1, with a Hill coefficient of 0.96 and a Kd of 25.5 nM. The calculated curves at 10, 30 and 100 nM are shown by the solid lines superimposed on the curves.
Figure 6.
A. Effect of SCBIs on the recovery of BgNav1-1a sodium channels from prolonged depolarization. The first test pulse (20 ms) was elicited to -10 mV from a holding potential of -30 mV. Thereafter, test pulses (20 ms) to -10 mV were applied from a holding potential of -120 mV at 1 Hz for the first 5 min, then at 0.1 Hz for up to 90 min. Currents were normalized to the largest current recorded during recovery. Averaged data for various concentrations are shown with different symbols, with the number of replicates shown in parentheses. Solid lines superimposed on the graph are calculated from equation 1, with a Kd for DCJW of 25 nM and a Hill coefficient, p, of 0.96. Data at 0 and 1000 nM were taken to represent the time courses of recovery of unblocked (U) and blocked (B) channels, respectively, and values of Kd and p were found by global minimization of the sum of the squares of the point-by-point differences between data at the three lower DCJW concentrations and the time course calculated from Equation 1, as shown by the solid lines superimposed upon the data. B. Similar data and analysis for metaflumizone, except that the time course of B was not directly measurable but was calculated as described in the text and shown by the dashed curve (Bavg).
The analysis of similar data for metaflumizone on BgNav1-1a channels is shown in Figure 6B. In this case, however, the recovery time course at 10 μM metaflumizone exhibited a distinct delay that could not be accounted for in the model and was assumed to be a measurement artifact due to block of channels from intracellular metaflumizone, whose concentration would be expected to decrease more slowly than extracellular metaflumizone upon washout. Because of this artifact, the curve for 10 μM metaflumizone could not be used as B(t) for the calculations. Instead, equation 1 was solved for B(t) and the resulting equation was used to estimate B(t) from each curve in the presence of metaflumizone. The average value so calculated, Bavg, shown by the dashed curve in Fig. 6B, was then used to optimize Kd and p, as described above for DCJW. The values for Kd and p obtained by this method were 112 nM and 1.13, respectively (Table 2).
Table 2.
Dissociation constants, Kd and Hill coefficients, p, for binding of DCJW and metaflumizone to BgNav1-1a BgNav1-1aF1817A and BgNav1-1aY1824A sodium channels, derived from fits of the time course of recovery at -120 mV from equilibration with the SCBIs under strong depolarization (Figs. 6-8)
| DCJW | Metaflumizone | |||
|---|---|---|---|---|
| Kd (nM) | p | Kd (nM) | p | |
| BgNav1-1a | 25.5 | 0.96 | 112 | 1.13 |
| BgNav1-1aF1817A | 33.5 | 0.70 | - | - |
| BgNav1-1aY1824A | 1.9 | 0.6 | 10 | 0.47 |
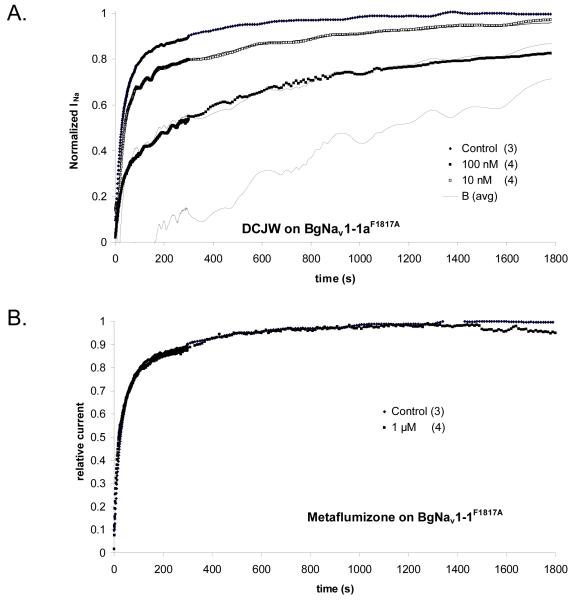
Figure 7 shows the analysis of the time courses of recovery from depolarization of BgNav1-1aF1817A channels in varying concentrations of DCJW or metaflumizone. For this mutant, the control time course of recovery could be fit by a dual exponential curve with time constants of 14.3 and 123 seconds. Recovery from depolarization was slowed in 10 and 100 nM DCJW treatments, and these curves were used to estimate the time course of recovery from block, as described above for Fig. 6B. Bavg calculated in this way is shown by the dashed curve, and was used to find the optimum values of Kd and p, as described earlier (Table 2). 10 μM metaflumizone did not slow the recovery time course (Fig. 7B), although it did block the channels (Fig. 3).
Figure 7.
A. Effect of DCJW on the recovery of BgNav1-1aF1817A sodium channels from prolonged depolarization, using the protocol described in the legend of Fig. 6. Analysis and curve fitting were done as in Fig. 6A, where estimation of the average value of B ((Bavg, dashed curve) was used. B. Metaflumizone at 1 μM did not slow the time course of recovery from depolarization. The numbers of replicates for each time course are shown in parentheses.
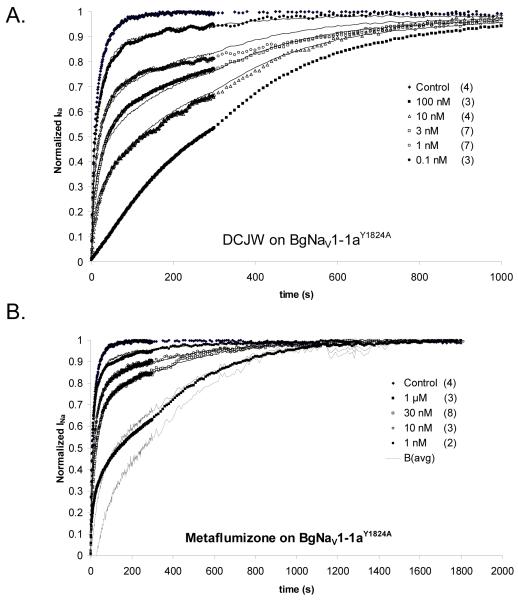
Figure 8 shows the analysis of the time courses of recovery from depolarization of BgNav1-1aY1824A channels in the presence of varying concentrations of DCJW (A) or metaflumizone (B). For this mutant, the control time course of recovery was significantly faster than that of wild-type channels, and could be fit by a dual exponential curve with time constants of 3.7 and 19.8 seconds. Recovery from depolarization was greatly slowed by DCJW. Application of the analysis method used in Figure 6A, with the curve for 100 nM being taken as the time course for blocked channels, B(t), gave values of 1.9 nM and 0.6 for Kd and p, respectively. Recovery was also greatly slowed by metaflumizone, but again, as for wild-type channels, the recovery time course, B(t), of blocked channels had to be estimated as in Figure 6B. Again, Bavg calculated in this way is shown by the dashed curve, and was used to find the optimum values of Kd and p of 10 nM and 0.47, respectively (Table 2).
Figure 8.
A. Effect of DCJW on the recovery of BgNav1-1aY1824A sodium channels from prolonged depolarization, using the protocol described for Fig. 6. Data analysis and curve fitting were as in Fig. 6A. B. Effect of metaflumizone on the recovery of BgNav1-1aY1824A sodium channels from prolonged depolarization. Analysis and curve fitting were done as in Fig. 6B, where estimation of the average value of B ((Bavg, dashed curve) was needed. The numbers of replicates for each time course are shown in parentheses.
In summary, the Y1824A substitution increased the sensitivity of BgNav1-1a sodium channels to DCJW and metaflumizone by 13-fold and 11-fold, respectively. As for the F1817A substitution, it caused a 1.3-fold increase in BgNav1-1a sodium channel sensitivity to DCJW. In contrast, metaflumizone did not have any effect, even at the highest concentrations (10 μM), on the recovery of BgNav1-1aF1817A channels from slow inactivation.
Discussion
Previous studies have established several amino acid residues that interact with therapeutic drugs in mammalian voltage-sensitive sodium channels (Liu, et al., 2003; Nau, et al., 1999; Ragsdale, et al., 1994; Ragsdale, et al., 1996; Wang, et al., 1998; Wang, et al., 2004; Wang, et al., 2000; Yarov-Yarovoy, et al., 2001; Yarov-Yarovoy, et al., 2002) and conceptual frameworks have been developed to model the interaction of LAs with mammalian sodium channels (Kondratiev and Tomaselli, 2003; Lipkind and Fozzard, 2005; Yarov-Yarovoy, et al., 2002). Likewise, an effort has been made to identify potential determinants of SCBI binding in rat Nav1.4 sodium channels (Silver and Soderlund, 2007) using the LA receptor as a model, in light of biochemical, pharmacological, and electrophysiological evidence indicating overlapping binding sites (Catterall, 1981; Creveling, et al., 1983; Deecher, et al., 1991; Deecher and Soderlund, 1991; Payne, et al., 1998; Salgado, 1992; Silver and Soderlund, 2005; Silver and Soderlund, 2006; Zhao, et al., 2003). Silver and Soderlund (2007) demonstrated that a LA-interacting residue, F1579, was also important for SCBI activity, indicating that SCBIs and LAs share at least one molecular determinant of binding.
This study represents the first effort to characterize the binding site of SCBIs on an insect sodium channel. The effects of the F1817A mutation on the cockroach BgNav channel sensitivity to SCBIs are strikingly different from those observed in mammalian sodium channels. Rat Nav1.4 sodium channels bearing the F1579A mutation were significantly less sensitive to inhibition and showed smaller hyperpolarizing shifts in the voltage-dependence of slow inactivation in the presence of DCJW and RH3421 (Silver and Soderlund, 2007). In BgNav sodium channels, the F1817A mutation had no effect on inhibition by indoxacarb or DCJW, as measured by onset of block at half inactivation (Fig. 3) or by recovery from block (Fig. 7A). On the other hand, this mutation appeared to enhance the inhibition by metaflumizone as measured by the onset of block at half inactivation. Surprisingly, the recovery of F1817A channels from metaflumizone block appeared to be faster than recovery from slow inactivation (Fig. 7B). Also, the hyperpolarizing shifts in the voltage-dependence of slow inactivation were greater for DCJW and metaflumizone than in wild-type. These results indicate that, in contrast to mammalian sodium channels where the phenylalanine residue in DIVS6 provides a crucial aromatic-aromatic interaction with the drug/insecticide molecule (Lipkind and Fozzard, 2005; Silver and Soderlund, 2007), F1817 in BgNav1-1a sodium channels does not play a major role in the binding of SCBIs. Nevertheless, removal of the aromatic side chain at this position seems to allow metaflumizone to interact more strongly and to get into and out of the site more rapidly.
Previous studies show that mutation of the tyrosine residue analogous to Y1824 in mammalian sodium channels results in an increase in sensitivity to SCBIs (Silver and Soderlund, 2007). Similarly, our results show that BgNav1-1aY1824A channels are more sensitive to both DCJW and metaflumizone (Fig. 8, Table 2). However, our results also showed no increase in inhibition of mutant channels at their inactivation midpoint potentials (Fig. 3) and no hyperpolarizing shift of the voltage dependence of slow inactivation in the presence of DCJW or metaflumizone. Metaflumizone did not cause a hyperpolarizing shift in the slow inactivation curve of wild type channels, but DCJW certainly did. In contrast, the corresponding mutation in rat Nav1.4 sodium channels resulted in significant (58-fold) increase in inhibition by DCJW in sodium channels that were completely inactivated (Silver and Soderlund, 2007).
We found that mutation of F1817 and Y1824 to alanine in BgNav1-1a caused significant hyperpolarizing shifts in the voltage dependence of slow inactivation (Fig. 6A), but we attempted to negate those factors through experimental design. First, time course of inhibition, fast inactivation, and slow inactivation protocols were all performed at the inactivation midpoint potential in order to minimize any effects on our measurements. Also, oocytes used to record the recovery from slow inactivation were left to incubate with SCBIs at resting membrane potential or about -20 to -35 mV, potentials at which all, mutant or wild-type, sodium channels are inactive. Thus, our protocols minimized the effects of the changes in sodium channel gating that resulted from alanine substitution.
The modulated receptor hypothesis (Hille, 1977; Hondeghem and Katzung, 1984) suggests that the LA receptor in sodium channels exists in two states: a high-affinity state available during channel opening and inactivation, and a low-affinity state available in resting channels. LAs, as part of their therapeutic effect, inhibit and stabilize open or inactivated sodium channels while having a smaller effect on resting channels. Our results show that SCBIs have no effect on resting, fast inactivated, or open channels and stabilize only the slow inactivated state. The slow kinetics of SCBIs are a likely cause for the lack of interaction with the highly transient fast inactivated and open states, as suggested previously (Salgado, 1992). Our results, therefore, are consistent with a modulated receptor model for both LA and SCBI activity in insect sodium channels.
The mutations examined in this study appear to affect the interaction of SCBIs and LAs in a site-specific and compound-specific manner. The F1817A mutation enhanced the ability of DCJW and metaflumizone to interact with inactivated channels, but provided an easier access and escape pathway for metaflumizone to leave its receptor site during recovery from inactivation. In contrast, the Y1824A mutation retarded the escape of DCJW and metaflumizone from the receptor site (slower recovery from inactivation), yet eliminated the ability of SCBIs to cause hyperpolarizing shifts in the voltage dependence of slow inactivation.
In summary, although residues corresponding to F1817 and/or Y1824 in the cockroach BgNav sodium channels are important determinants of binding of LA and SCBI activity in mammalian sodium channels, they are not of primary importance to the binding of SCBIs in insect sodium channels, and indeed may hinder access of SCBIs to the receptor site. Thus, our results raise an intriguing possibility that the receptor for SCBIs will be determined by a different subset of amino acid residues in insect sodium channels. Future site-directed mutagenesis of other regions of insect sodium channels is necessary to identify key molecular determinants of the SCBI binding site on insect sodium channels.
Acknowledgements
The study was supported by BASF Corporation and National Institutes of Health (GM057440 to K. D). We thank anonymous reviewers for valuable suggestions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Catterall WA. Inhibition of voltage-sensitive sodium channels in neuroblastoma cells by antiarrhythmic drugs. Mol Pharmacol. 1981;20:356–62. [PubMed] [Google Scholar]
- Catterall WA. Common modes of drug action of Na+ channels: local anesthetics, antiarrhythmics, and anticonvulsants. Trends Pharmacol Sci. 1987;8:57–65. [Google Scholar]
- Catterall WA. From ionic currents to molecular mechanisms: The structure and function of voltage-gated sodium channels. Neuron. 2000;26:13–25. doi: 10.1016/s0896-6273(00)81133-2. [DOI] [PubMed] [Google Scholar]
- Creveling CR, McNeal ET, Daly JW, Brown GB. Batrachotoxin-induced depolarization and [3H]batrachotoxinin-A 20α-benzoate binding in a vesicular preparation from Guinea pig cerebral cortex. Mol Pharmacol. 1983;23:350–58. [PubMed] [Google Scholar]
- Deecher DC, Payne GT, Soderlund DM. Inhibition of [3H]batrachotoxinin A 20-α-benzoate binding to mouse brain sodium channels by the dihydropyrazole insecticide RH 3421. Pestic Biochem Physiol. 1991;41:265–73. [Google Scholar]
- Deecher DC, Soderlund DM. RH 3421, an insecticidal dihydropyrazole, inhibits sodium channel-dependent sodium uptake in mouse brain preparations. Pestic Biochem Physiol. 1991;39:130–37. [Google Scholar]
- Feng G, Deak P, Chopra M, Hall LM. Cloning and functional analysis of TipE, a novel membrane protein that enhances Drosophila para sodium channel function. Cell. 1995;82:1001–11. doi: 10.1016/0092-8674(95)90279-1. [DOI] [PubMed] [Google Scholar]
- Goldin AL. Resurgence of sodium channel research. Annu Rev Physiol. 2001;63:871–94. doi: 10.1146/annurev.physiol.63.1.871. [DOI] [PubMed] [Google Scholar]
- Harder HH, Riley SL, McCann SF, Irving SN. DPX-MP062: a novel, broad-spectrum, environmentally-soft, insect control compound; Brighton Crop Protection Conference - Pests and Diseases; Farnham, Surrey, UK. 1996.pp. 449–54. [Google Scholar]
- Hille B. Local anesthetics: hydrophilic and hydrophobic pathways for the drug-receptor reaction. J Gen Physiol. 1977;69:497–515. doi: 10.1085/jgp.69.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hondeghem LM, Katzung BG. Antiarrhythmic agents: the modulated receptor mechanism of action of sodium and calcium channel-blocking drugs. Annual Review of Pharmacology and Toxicology. 1984;24:387–423. doi: 10.1146/annurev.pa.24.040184.002131. [DOI] [PubMed] [Google Scholar]
- Kondratiev A, Tomaselli GF. Altered gating and local anesthetic block mediated by residues in the I-S6 and II-S6 transmembrane segments of voltage-dependent Na+ channels. Mol Pharmacol. 2003;64:741–52. doi: 10.1124/mol.64.3.741. [DOI] [PubMed] [Google Scholar]
- Lipkind GM, Fozzard HA. Molecular modeling of local anesthetic drug binding by voltage-gated sodium channels. Mol Pharmacol. 2005;68:1611–22. doi: 10.1124/mol.105.014803. [DOI] [PubMed] [Google Scholar]
- Liu G, Yarov-Yarovoy V, Nobbs M, Clare JJ, Scheuer T, Catterall WA. Differential interactions of lamotrigine and related drugs with transmembrane segment IVS6 of voltage-gated sodium channels. Neuropharmacology. 2003;44:413–22. doi: 10.1016/s0028-3908(02)00400-8. [DOI] [PubMed] [Google Scholar]
- Narahashi T. Recent Progress in the Mechanism of Action of Insecticides: pyrethroids, fipronil, and indoxacarb. J Pestic Sci. 2001;26:277–85. [Google Scholar]
- Nau C, Wang S-Y, Strichartz GR, Wang GK. Point mutations at N434 in D1-S6 of μ1 Na+ channels modulate binding affinity and stereoselectivity of local anesthetic enantiomers. Mol Pharmacol. 1999;56:404–13. doi: 10.1124/mol.56.2.404. [DOI] [PubMed] [Google Scholar]
- Payne GT, Deecher DC, Soderlund DM. Structure-activity relationships for the action of dihydropyrazole insecticides on mouse brain sodium channels. Pestic Biochem Physiol. 1998;60:177–85. [Google Scholar]
- Pugsley MK, Goldin AL. Effects of bisaramil, a novel class I antiarrhythmic agent, on heart, skeletal muscle and brain Na channels. Eur J Pharmacol. 1998;342:93–104. doi: 10.1016/s0014-2999(97)01420-9. [DOI] [PubMed] [Google Scholar]
- Ragsdale DS, McPhee JC, Scheuer T, Catterall WA. Molecular determinants of state-dependent block of Na+ channels by local anesthetics. Science. 1994;265:1724–28. doi: 10.1126/science.8085162. [DOI] [PubMed] [Google Scholar]
- Ragsdale DS, McPhee JC, Scheuer T, Catterall WA. Common molecular determinants of local anesthetic, antiarrhythmic, and anticonvulsant block of voltage-gated Na+ channels. Proc Natl Acad Sci. 1996;93:9270–75. doi: 10.1073/pnas.93.17.9270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salgado VL. Slow voltage-dependent block of sodium channels in crayfish nerve by dihydropyrazole insecticides. Mol Pharmacol. 1992;41:120–26. [PubMed] [Google Scholar]
- Salgado VL, Hayashi JH. Metaflumizone is a novel sodium channel blocker insecticide. Veterinary Parasitology. 2007;150:182–89. doi: 10.1016/j.vetpar.2007.08.032. [DOI] [PubMed] [Google Scholar]
- Silver K, Soderlund DM. Action of pyrazoline-type insecticides at neuronal target sites. Pestic Biochem Physiol. 2005;81:136–43. [Google Scholar]
- Silver K, Soderlund DM. State-dependent block of rat Nav1.4 sodium channels expressed in Xenopus oocytes by pyrazoline-type insecticides. Neurotoxicology. 2005;26:397–406. doi: 10.1016/j.neuro.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Silver K, Soderlund DM. Differential sensitivity of rat voltage-sensitive sodium channel isoforms to pyrazoline-type insecticides. Toxicology and Applied Pharmacology. 2006;214:209–17. doi: 10.1016/j.taap.2005.12.018. [DOI] [PubMed] [Google Scholar]
- Silver K, Soderlund DM. Point Mutations at the Local Anesthetic Receptor Site Modulate the State-Dependent Block of Rat Nav1.4 Sodium Channels by Pyrazoline-type Insecticides. Neurotoxicology. 2007;28:655–63. doi: 10.1016/j.neuro.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Song W, Liu Z, Tan J, Nomura Y, Dong K. RNA editing generates tissue-specific sodium channels with distinct gating properties. J Biol Chem. 2004;279:32554–61. doi: 10.1074/jbc.M402392200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song WZ, Liu ZQ, Dong K. Molecular basis of differential sensitivity of insect sodium channels to DCJW, a bioactive metabolite of the oxadiazine insecticide indoxacarb. Neurotoxicology. 2006;27:237–44. doi: 10.1016/j.neuro.2005.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J, Liu Z, Nomura Y, Goldin AL, Dong K. Alternative splicing of an insect sodium channel gene generates pharmacologically distinct sodium channels. J Neurosci. 2002;22:5300–09. doi: 10.1523/JNEUROSCI.22-13-05300.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatebayashi H, Narahashi T. Differential mechanism of action of the pyrethroid tetramethrin on tetrodotoxin-sensitive and tetrodotoxin-resistant sodium channels. J Pharmacol Exp Ther. 1994;270:595–603. [PubMed] [Google Scholar]
- Wang GK, Quan C, Wang S-Y. A common local anesthetic receptor for benzocaine and etidocaine in voltage-gated μ1 Na+ channels. Eur J Physiol. 1998;435:293–302. doi: 10.1007/s004240050515. [DOI] [PubMed] [Google Scholar]
- Wang GK, Quan C, Wang S-Y. Local anesthetic block of batrachotoxin-resistant muscle Na+ channels. Mol Pharmacol. 1998;54:389–96. doi: 10.1124/mol.54.2.389. [DOI] [PubMed] [Google Scholar]
- Wang GK, Russell C, Wang S-Y. State-dependent block of voltage-gated Na+ channels by amitriptyline via the local anesthetic receptor and its implication for neuropathic pain. Pain. 2004;110:166–74. doi: 10.1016/j.pain.2004.03.018. [DOI] [PubMed] [Google Scholar]
- Wang S-Y, Nau C, Wang GK. Residues in Na+ channel D3-S6 segment modulate batrachotoxin and local anesthetic affinities. Biophys J. 2000;79:1379–87. doi: 10.1016/S0006-3495(00)76390-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S-Y, Wang GK. Voltage-gated sodium channels as primary targets of diverse lipid-soluble neurotoxins. Cell Signal. 2003;15:151–59. doi: 10.1016/s0898-6568(02)00085-2. [DOI] [PubMed] [Google Scholar]
- Warmke JW, Reenan RAG, Wang P, Qian S, Arena JP, Wang J, Wunderler D, Liu K, Kaczorowski GJ, Van Der Ploeg LHT, Ganetzky B, Cohen CJ. Functional expression of Drosophila para sodium channels: modulation by the membrane protein tipE and toxin pharmacology. J Gen Physiol. 1997;110:119–33. doi: 10.1085/jgp.110.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing KD, Andaloro JT, McCann SF, Salgado VL. Indoxacarb and the sodium channel blocker insecticides: chemistry, physiology, and biology in insects. In: Gilbert LI, Iatrou K, Gill SS, Gilbert LI, Iatrou K, Gill SSs, editors. Comprehensive Molecular Insect Science. Elsevier; New York: 2005. pp. 31–53. [Google Scholar]
- Wing KD, Sacher M, Kagaya Y, Tsurubuchi Y, Mulderig L, Connair M, Schnee ME. Bioactivation and mode of action of the oxadiazine indoxacarb in insects. Crop Protect. 2000;19:537–45. [Google Scholar]
- Wing KD, Schnee ME, Sacher M, Connair M. A novel oxadiazine insecticide is bioactivated in lepidopteran larvae. Arch Insect Biochem Physiol. 1998;37:91–103. [Google Scholar]
- Yarov-Yarovoy V, Brown J, Sharp E, Clare JJ, Scheuer T, Catterall WA. Molecular determinants of voltage-dependent gating and binding of poreblocking drugs in transmembrane segment IIIS6 of the Na+ channel α subunit. J Biol Chem. 2001;276:20–27. doi: 10.1074/jbc.M006992200. [DOI] [PubMed] [Google Scholar]
- Yarov-Yarovoy V, McPhee JC, Idsvoog D, Pate C, Scheuer T, Catterall WA. Role of amino acid residues in transmembrane segments IS6 and IIS6 of the Na+ channel α subunit in voltage-dependent gating and drug block. J Biol Chem. 2002;277:35393–401. doi: 10.1074/jbc.M206126200. [DOI] [PubMed] [Google Scholar]
- Zhao X, Ikeda T, Salgado VL, Yeh JZ, Narahashi T. Block of two subtypes of sodium channels in cockroach neurons by indoxacarb insecticides. Neurotoxicology. 2005;26:455–65. doi: 10.1016/j.neuro.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Zhao X, Ikeda T, Yeh JZ, Narahashi T. Voltage-dependent block of sodium channels in mammalian neurons by the oxadiazine insecticide indoxacarb and its metabolite DCJW. Neurotoxicology. 2003;24:83–96. doi: 10.1016/s0161-813x(02)00112-2. [DOI] [PubMed] [Google Scholar]