Abstract
B lymphopoiesis in aged mice is characterized by reduced B cell precursors and an altered antibody repertoire. This likely results, in part, from reduced surrogate light chains in senescent B cell precursors and compromised preBCR checkpoints. Herein, we show that aged mice maintain an ordinarily minor pool of early c-kit+ pre-B cells, indicative of poor preBCR expression, even as preBCR competent early pre-B cells are significantly reduced. Therefore, in aged mice, B2 B lymphopoiesis shifts from dependency on preBCR expansion and selection to more preBCR deficient pathways. B2 c-kit+ B cell precursors, from either young or aged mice, generate new B cells in vitro that are biased to larger size, higher levels of CD43, and decreased κ light chain expression. Notably, immature B cells in aged bone marrow exhibit a similar phenotype in vivo. We hypothesize that reduced surrogate light chain expression contributes to decreased pre-B cells in aged mice. The B2 pathway is partially blocked with limited B cell development and reduced preBCR expression and signaling. In old age, B2 pathways have limited surrogate light chain and increasingly generate new B cells with altered phenotype and light chain expression.
Keywords: pre-B cells, immature B cells, surrogate light chain, light chain, senescence, pre-B cell receptor
INTRODUCTION
Bone marrow B lymphopoiesis is compromised, to variable extents, in aged mice of several inbred strains (1-6). Multiple developmental stages show deficits, including pre-B cells, pro-B cells, and CLPs (1-6). While the mechanisms responsible remain to be fully characterized, diminished responses of B cell precursors and progenitors to IL-7, increased apoptosis, and reduced capacity for V gene recombination have been described in aged mice (5,7-9). We have shown that the transcriptional program underlying B lineage specification and commitment is altered in aged mice (10-13). Aged B cell precursors are variably deficient in E2A expression; this results from increased turnover of E2A encoded proteins (13,14). E2A, together with EBF, regulates transcription of the surrogate light chain genes λ5 and VpreB (15). Given the reductions in E2A, it is not surprising that B cell precursors from aged mice are often deficient in surrogate light chains (3,11). We hypothesize that reductions in surrogate light chain level may limit formation and function of the preBCR in nascent pre-B cells in aged mice (10,16). This would be expected to affect the capacities for pre-B cell expansion and further maturation in senescence.
As shown by Kawano et. al., different μ heavy chain V regions show varying capacities for binding surrogate light chains and this correlates well with preBCR signaling and further differentiation (17). Therefore, each pre-B cell, individually, undergoes further proliferation and differentiation dictated by its particular capacity for expressing the preBCR. In previous studies, lack of preBCR expression in surrogate light chain knockout mice does not preclude μ heavy chain rearrangements, but does dampen pre-B cell proliferation (18-21). In λ5 gene knockout mice, where preBCR expression can not occur, the remaining pre-B cells have a unique phenotype and retain surface expression of the tyrosine kinase c-kit and fail to up-regulate CD25 and CD2 (19,20). In studies by Kawano et. al., the extent of preBCR signaling coincided with down-regulation of c-kit in pre-B cells (17). As shown by ten Boekel et. al. (22), c-kit+ pre-B cells, from either λ5 knockout or wild-type mice, differ from c-kit- pre-B cells in their Vh repertoires, the former being enriched in rearranged 3' Vh gene families. Notably, the majority of μ chains cloned from c-kit+ pre-B cells failed to associate with surrogate light chains while μ chains derived from c-kit- pre-B cells bound surrogate light chains and were competent to form the preBCR (22). Therefore, the expression (or lack thereof) of c-kit serves as a phenotypic marker of two distinct pre-B subsets that likely differ in their capacities for preBCR expression and signaling, and consequently, expansion and differentiation to the B cell stage.
B cell development in the bone marrow is dominated by the B2 B cell lineage, the precursors of typical follicular B cells. In aged mice, we would anticipate that poor surrogate light chain expression, by compromising the preBCR checkpoint, would diminish the numbers of pre-B cells generated in the bone marrow, but would allow continued, albeit less effective, B cell differentiation along a more “preBCR deficient” path. Since preBCR mediated selection, which is dependent upon Vh sequence, would be minimized, newly generated B cells in aged mice would be expected to have altered specificity and possibly function.
Consistent with this hypothesis, we have previously reported that aged mice show an increased proportion of immature B cells within the bone marrow that appear to have undergone activation as evidenced by altered surface phenotype (e.g., expression of CD43, CD5, CD11b, PD-1 antigens), dependence on the BCR pathway kinase Btk, increased size, and an altered Vh repertoire as evidenced by increased usage of the VhS107 family (23,24). In this report, we show that B cells with a comparable phenotype are preferentially derived from preBCR deficient c-kit+ pre-B cells within the B2 lineage. Consequently, we hypothesize that the quality and quantity of new B cells derived from senescent bone marrow is impacted by reduced preBCR dependent B2 B lymphopoiesis, subsequent to low surrogate light chain expression.
MATERIALS AND METHODS
Mice
Young (2-4 months) and aged (21-26 months) BALB/c mice were purchased from the National Institutes of Aging colony at Harlan Sprague Dawley, Indianapolis, IN. Mice with obvious abdominal tumors and/or splenomegaly in the thoracic or abdominal cavities were eliminated from the studies. The λ5 gene knockout mice were originally constructed by Kitamura et al. (18), and provided by W. Haas and K. Rajewsky, Cologne, Germany, and they and their normal littermate controls were bred in our colony.
Cell cultures
Femur and tibia pairs were flushed to harvest cells from the bone marrow as previously described (1). Red blood cells were removed by treatment with ACK (0.15 M NH4CL, 1mM KHCO3, 0.1 mM EDTA) for 5 minutes at room temperature followed by centrifugation to remove red cell debris. Bone marrow cells were counted and used for cell sorting, flow cytometry, or cell culture. For unfractionated bone marrow culture, cells were resuspended at 1×106/ml in RPMI-1640 (Gibco Life Technologies, Grand Island, NY), supplemented with 10% FCS (Sigma Aldrich, St. Louis, MO) plus 1% penicillin-streptomycin, 1% L-glutamine and 2-mercaptoethanol at 2×10-5M. Purified recombinant mouse IL-7 (rmIL-7, BioSource International, Camarillo, CA) was added at 5ng/ml and remained in culture for 5-7 days after which non-adherent cells were harvested and used for cell sorting and further analysis. For B cell precursors used in in vitro studies, the different subsets were sorted by fluorescence flow cytometry or magnetic bead sorting and cultured as above with rmIL-7 (5ng/ml) and recombinant mouse stem cell factor (SCF) (BioSource International, Camarillo, CA) at 50ng/ml for 4 days.
Fluorescence Flow Cytometry
Mouse bone marrow cells freshly harvested or cultured were stained with the following antibodies that were labeled with an appropriate fluorochrome: IgM (II/41), CD43 (S7), B220 (RA3-6B2), CD19 (1D3), κ (187.1), λ(1-3) (R26-46) (BD Bioscience, San Diego, CA), and CD93 (AA4.1) (e-Bioscience, San Diego, CA). For the cytoplasmic staining of μ chain, goat anti-mouse μ (Jackson ImmunoResearch) and, for λ5, LM34 (BD Bioscience) antibodies were employed. Cells were initially stained for surface markers, permeablized using BD Cytofix/Cytoperm (BD Bioscience), washed with PermWash (BD Bioscience), and followed by cytoplasmic stain addition. Cells were analyzed within 30 minutes of staining. Analysis was performed on an LSR II fluorescence flow cytometer (BD Bioscience), and at least 5×105 events were acquired.
Cell Sorting
B cell precursors (surface IgM-CD19+) from C57BL/6 (B6) and λ5 knockout mice were isolated as follows: mouse bone marrow cells were first surface stained with anti-IgM APC (II/41) followed by addition of anti-APC magnetic microbeads according to the MiniMACS protocol (Miltenyi Biotec, Auburn, CA). IgM negative cells were separated from IgM positive cells and post sorts revealed 90-95% purity. From the IgM- cells, CD19+ cells were further magnetically sorted first using anti-CD19 PE (1D3) and then anti-PE magnetic beads with a purity of >90%.
In some experiments, IL-7 cultured B cell precursors were sorted for CD2- pro-B cells by magnetic bead separation prior to analysis. Cells were first surface stained with anti-CD2 PE (BD Bioscience) and then stained using anti-PE microbeads according to the MiniMACS protocol. Purity of CD2- cells was >98%.
Bone marrow cells isolated from tibia and femur pairs of young and aged mice were sorted for c-kit+ and c-kit- precursors. Cells were stained for surface IgM (II/41), CD43 (S7), B220 (RA3-6B2), CD19 (1D3) (BD Bioscience), CD93 (AA4.1) and c-kit (2B8) (e-Bioscience, San Diego, CA). C-kit+ precursors were defined as IgM-B220+CD43+CD19+AA4.1+c-kit+; c-kit- precursors were defined as IgM-B220+CD43+CD19+AA4.1+c-kit-. Cells were sorted with a FACS Aria (BD ImmunoCytometry, San Jose, CA) with purity ranging between 94-99%.
Western Blotting
Cells were lysed with Mammalian Protein Extraction Reagent (Pierce, Rockford, IL) at 1×106 cells/10μl. Protein Extraction reagent was supplemented with Halt Protease Inhibitor Cocktail (Pierce) at 10μl/ml. Samples were denatured by boiling for 4 minutes in sample buffer, subjected to reducing conditions, and electrophoresed using SDS-PAGE 4-12% polyacrylamide gels for 50 minutes at 200V. Proteins were run out on gels and then transferred onto nitrocellulose membranes for 90 minutes at 100V. Nonspecific sites were blocked by incubation of the membranes with PBS-Tween 20 (1x PBS/0.05% Tween 20) containing 10% milk for 2 hours at room temperature. Membranes were incubated as required with mouse monoclonal anti-actin (C-2, Santa Cruz, Santa Cruz, CA), or hamster anti-λ5 monoclonal antibody FS1 (3,11,25). Following overnight incubation with the primary antibody, immunoblots were incubated with the appropriate HRP-labeled secondary antibodies for 2 hours at room temperature, developed by enzyme chemiluminescence, and analyzed via an Alpha Innotech FluorChem Gel-doc system (San Leandro, CA).
Statistical analysis
Student's t-test (two-tailed), either paired or unpaired, or the non-parametric Mann-Whitney U-test were utilized as appropriate.
RESULTS
Subsets of pre-B cells are differentially affected by senescence
Individual pre-B cells within the bone marrow differ in their capacities to assemble the preBCR and signal at the preBCR checkpoint. This is dependent upon the unique abilities of each μ heavy chain, inherent in its variable region, to associate with the surrogate light chain. The assembly, expression, and signaling via the preBCR results in a phenotypic change in newly formed pre-B cells in which the c-kit surface protein is down-regulated progressively in proportion to preBCR levels (17,22,26). This provides a well-characterized means to identify early pre-B cells within the bone marrow that are preBCR competent (e.g., c-kit-) and progress through the preBCR checkpoint and pre-B cells that likely are less effective in preBCR signaling (e.g., c-kitlow/-). Consistent with this, early pre-B cells present in the bone marrow of λ5 knockout mice, which are deficient in the preBCR, are uniformly c-kit+ (Figure 1 and reference 19).
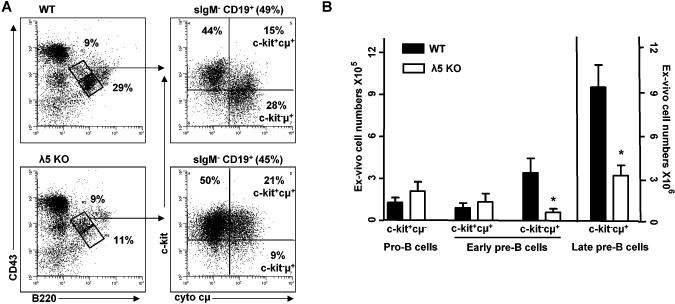
FIGURE 1. λ5 knockout mice show loss of c-kit-, but not c-kit+ early pre-B cells in vivo.
(A) Representative dot plots of c-kit+ and c-kit- precursors in wild-type (B6) (WT) and λ5 knockout (KO) mice. B220+CD43+ are pro-B/early pre-B cells and late pre-B cells are B220+CD43- with percentages out of total bone marrow shown. Pro-B/early pre-B cells were further gated as surface IgM-CD19+ with percent cells present in total bone marrow indicated. Surface IgM-CD19+B220+CD43+ B cell precursors were gated based on c-kit and cytoplasmic μ (cμ) with proportion indicated for c-kit+cμ- pro-B, c-kit+cμ+ early pre-B, and c-kit-cμ+ early pre-B subsets. (B) Analysis of c-kit+ pro-B, c-kit+ and c-kit- early pre-B, and late pre-B cell numbers for 7 pairs of WT and λ5 KO mice. *p<0.02.
We and others (1-6) have previously reported that pre-B cells are reduced in senescence; however, it is not clear whether this applies to all or only some of the identifiable subsets of pre-B cells within aged bone marrow. That aged mice might be anticipated to lose c-kit- pre-B cells, which are dependent upon preBCR signaling, but possibly retain c-kit+ pre-B cells is suggested by our previous observations that B cell precursors from aged mice often express lower levels of both λ5 and VpreB components of the surrogate light chain (3,11,13). This is confirmed and extended in Figure 3 as discussed below.
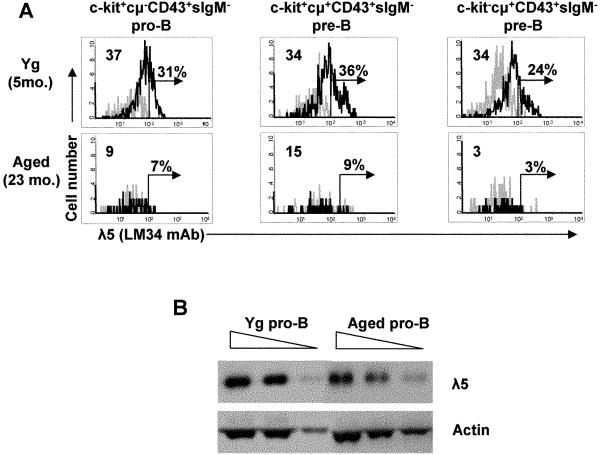
FIGURE 3. Loss of λ5 protein in aged mice occurs at pro-B and pre-B cell stages.
(A) Cytoplasmic λ5 protein was stained and analyzed by flow cytometry in bone marrow c-kit+cμ- pro-B, c-kit+cμ+ early pre-B, and c-kit-cμ+ early pre-B cells in young and old BALB/c mice. Data shown are representative of 3 mice. Grey line=isotype control. Percent positive cells was determined with a cut-off as <2% positives in isotype controls. The change in mean fluorescent intensity (ΔMFI) was defined as (MFI experimental-MFI isotype control) and is shown in the upper left. (B) λ5 protein expression was determined in CD2-cμ- pro-B cells generated from young and old BALB/c mice upon bone marrow cell culture with IL-7 for 5-7 days. CD2- pro-B cells were separated by MACS sorting prior to lysate preparation. Proteins were assayed by Western blot at 2.5, 5, and 10 ×105 cell equivalents. Data are representative of 3 experiments.
As shown in Figure 2A,B, ~30-40% of IgM-CD19+B220+CD43+ cμ+ early pre-B cells in young adult BALB/c mice show expression of c-kit. In contrast, also as shown in Figure 2A,B, in BALB/c mice approximately 20-22 months of age, late-stage (Hardy Fraction D [27]; CD43-B220+) pre-B cells are markedly reduced (~33% of young controls). IgM-CD19+B220+CD43+c-kit-cμ+ (“c-kit- ”) early pre-B cells are also reduced (Figure 2A,B); however, the numbers of IgM-CD19+B220+CD43+c-kit+cμ+ (“c-kit+”) pre-B cells are better maintained in aged bone marrow. These experiments indicate that, while late stage pre-B cells and c-kit- early pre-B cells are decreased in aged mice compared to young adult mice, c-kit+ early pre-B cells are less affected in aged bone marrow. In addition to pre-B cells, pro-B cells (IgM-CD19+B220+CD43+c-kit+cμ-) are variably reduced in aged mice (Figure 2B) in accordance with previous reports (3-5).
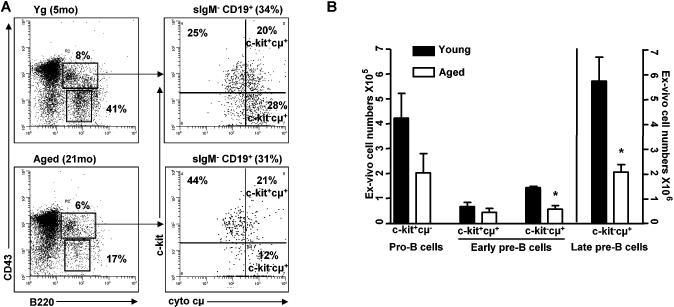
FIGURE 2. Aged mice show loss of c-kit-, but not c-kit+ early pre-B cells in vivo.
(A) Representative dot plots of c-kit+ and c-kit- precursors in young (Yg) (2-4mo) and aged BALB/c mice (21-26mo). B220+CD43+ are pro-B/early pre-B cells and late pre-B cells are B220+CD43- with percentages out of total bone marrow shown. Pro-B/early pre-B cells were further gated as surface IgM-CD19+ with percent cells present in total bone marrow indicated. Surface IgM-CD19+B220+CD43+ B cell precursors were gated based on c-kit and cytoplasmic μ (cμ) with proportion indicated for c-kit+cμ- pro-B, c-kit+cμ+ early pre-B, and c-kit-cμ+ early pre-B subsets. (B) Analysis of c-kit+ pro-B, c-kit+ and c-kit- early pre-B, and late pre-B cell numbers for 6 pairs of young and old mice. *p<0.02.
In this regard, aged bone marrow, depleted of preBCR dependent c-kit- pre-B cells, begins to resemble young adult λ knockout bone marrow where c-kit+ pre-B cells are predominant within the early pre-B pool. This is shown diagrammatically in Figure 8 where pro-B cells in aged mice, low in surrogate light chain, generate more frequently new pre-B cells bearing c-kit at the expense of normal preBCR driven c-kit- pre-B cell development.
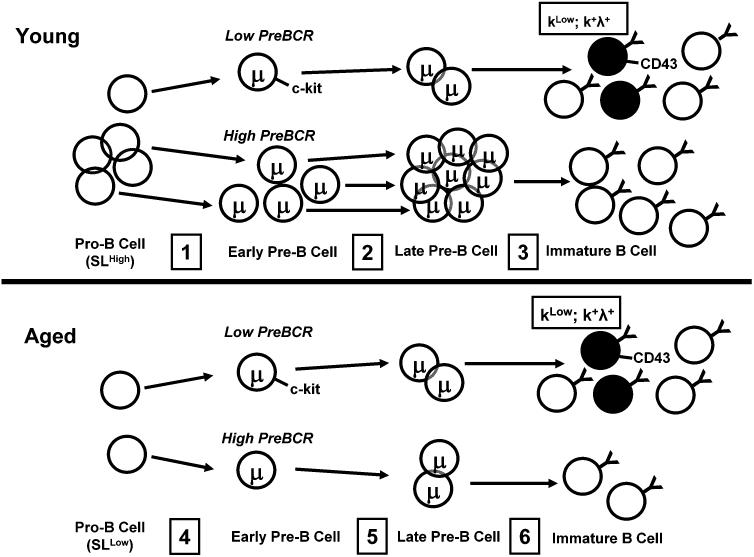
FIGURE 8. Surrogate light chain down-regulation in aged B cell precursors alters B2 B lymphopoiesis and promotes development of new B cells with distinct surface phenotype and light chain isotypes.
Young Mice: Pro-B cells undergo Vh-Dh-Jh recombination (step 1); preBCR competency is dictated by Vh-Dh-Jh sequence. PreBCR signaling results in down-regulation of c-kit and expansion and population at the late pre-B cell stage (step 2). A minor proportion of early pre-B cells retains c-kit and express μ heavy chains that assemble poorly with surrogate light chains (SL). (Step 3) Late pre-B cells differentiate into immature B cells with most derived from preBCR competent precursors. Immature B cells with increased CD43 and altered light chain expression derived from c-kit+ pre-B cells are a minor contributor to the immature B cell pool. Aged Mice: SL and preBCR expression is low and overall pre-B cell development is reduced (step 4). C-kit+ pre-B cells (step 5) generate immature B cells with increased CD43+ and altered light chain patterns at increased frequencies (step 6).
B cell precursors in aged bone marrow exhibit reduced levels of surrogate light chain protein
As shown in λ5 knockout mice, deficiencies in surrogate light chain expression led to loss of c-kit- pre-B cells with retention of c-kit+ pre-B cells. We hypothesized that aged mice with increased representation of c-kit+ early pre-B cells and reduced numbers of c-kit- pre-B cells would have reduced levels of surrogate light chain. We have previously reported that B cell precursors in aged mice often show a decline in surrogate light chain expression and that this coincides with reductions in the E47 transcription factor, a product of the E2A gene necessary for optimal surrogate light chain transcription (11-14). Moreover, the level of λ5 surrogate light chain expression in aged B cell precursors was significantly correlated with the extent of late stage pre-B cell loss in the bone marrow (11).
It was confirmed in the present study that reductions in surrogate light chain λ5 protein were observed, by fluorescent staining, in aged B cell precursors as early as the sIgM-CD19+B220+CD43+cμ- pro-B cell stage in vivo (Figure 3A). The reduced λ5 protein expression was observed in both c-kit+ and c-kit- early pre-B cells from aged mice (Figure 3A). These in vivo results were replicated in pro-B cell precursors grown from aged mice in the presence of IL-7 in vitro (Figure 3B). Therefore, abnormal decline in surrogate light chain expression is likely a relatively early event during B cell development in aged mice, occurring at or prior to the pro-B cell stage and extending through the pre-B cell stages.
Both c-kit- and c-kit+ early pre-B cells generate immature B cells in vitro, but with different efficiencies
The above results indicate that low surrogate light chain levels, as are apparent in young adult λ5 gene knockout mice and in wild-type senescent mice, are associated with altered representation of c-kit+ vs. c-kit- early pre-B cells. In our previous studies, we have noted that those individual aged mice whose pre-B cell numbers are severely depleted (e.g., <20% that of young adults) also have reduced numbers of immature B cells (6,24). Therefore, we next determined the relative capabilities of c-kit+ and c-kit- B cell precursors to generate immature B cells in vitro in order to determine whether differences in immature B cell production might also result from changes in pre-B cell composition in old age.
The λ5 knockout mouse, with highly enriched c-kit+ pre-B cells, provides a model to determine the behavior of this pre-B subset. B cells are capable of being generated in vivo from the limited pool of predominantly c-kit+ pre-B cells available in surrogate light chain knockout mice, albeit the efficiency of B cell population in the periphery is considerably reduced when compared to wild-type mice (18). Therefore, it was expected that the c-kit+, surrogate light chain deficient pre-B cells in λ5 gene knockout mice would generate B cells in vitro.
Surface IgM-CD19+ bone marrow cells were isolated from wild-type mice (~70% of early pre-B cells were c-kit-) and from λ5 gene knockout mice (>70% of early pre-B cells were c-kit+). These wild-type and λ5 knockout precursors were cultured in the presence of IL-7 and stem cell factor (SCF; c-kit ligand) for four days; during culture, proliferation of the B cell precursors was greater with wild-type as opposed to λ5 knockout precursor cells (Figure 4A). Cultures of wild-type precursors yielded a greater percentage of B cells than did those from λ5 knockout precursors (Figure 4B). Upon taking differences in cell growth into account, production of new B cells from wild-type B cell precursors was ~20-fold more effective than from λ5 gene knockout B cell precursors (Figure 4C). The B cells produced from both wild-type and λ5 gene knockout precursors were of recent origin as shown by similar levels of the AA4.1 antigen, a marker of immature B cells and present on >80% of B cells in our cultures (data not shown). In culture, the IgM-CD19+ cells grown from both wild-type and λ5 gene knockout mice had similar proportions of cμ+ pre-B cells (~12-15%; data not shown). As shown by Milne et. al. (28), pro-B cells proliferate extensively generating pre-B and new B cell formation continuously in IL-7 containing cultures. Therefore, it is unlikely that the different efficacies of B cell generation resulted from any disparities in the number of pre-B cells available for further differentiation in vitro.
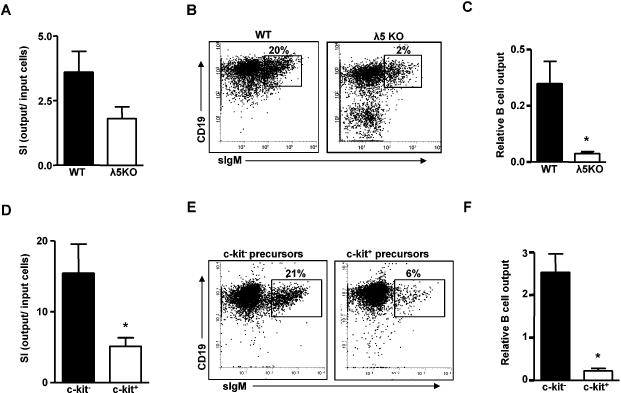
FIGURE 4. C-kit+ and c-kit- precursors from λ5 knock-out and wild-type mice proliferate and differentiate into B cells in vitro.
Panels A-C: Bone marrow cells from WT (B6) and λ5 KO mice were MACS sorted as IgM-CD19+ B cell precursors and cultured for 4 days in the presence of IL-7 (5ng/ml) and stem cell factor (SCF) (50ng/ml). Panels E-F: Bone marrow from young BALB/c mice were pooled and IgM-CD19+AA4.1+B220+CD43+c-kit+ and IgM-CD19+AA4.1+B220+CD43+c-kit- B cell precursors were FACS sorted and cultured for 4 days in the presence of IL-7 (5ng/ml) and SCF (50ng/ml). (A,D) Stimulation index (SI) (output CD19+ cells/input CD19+ cells) of cultures initiated with precursors from either WT or λ5 KO mice (A) or BALB/c precursor populations (D). (B,E) Representative dot plot of B cells generated from either WT or λ5 KO precursors (B) or BALB/c precursor populations (E). (C,F) Relative B cell output from precursors of WT and λ5 KO mice (C) or BALB/c precursor populations (F). Relative B cell output was determined as SI x %B cells in culture. Data are summarized for 6-8 experiments. *p<0.02.
Similar results were seen when surface IgM-CD19+B220+AA4.1+CD43+c-kit+ and IgM-CD19+B220+AA4.1+CD43+c-kit- precursors were isolated by cell sorting from BALB/c mice and cultured with IL-7 and SCF for four days. Again, c-kit- precursors demonstrated more robust growth in vitro compared to c-kit+ precursors (Figure 4D). While both c-kit+ and c-kit- precursors generated AA4.1+ immature B cells in vitro, c-kit- precursors were considerably more effective in producing new B cells (Figure 4E). When differences in growth were also taken into account, the relative efficacy of B cell production from c-kit- precursors was ~10-fold greater than for c-kit+ precursors (Figure 4F). In vitro, both c-kit+ and c-kit- precursor cells that expanded in response to cytokine showed similar composition with ~30% of cells at day four expressing cμ chain, but not surface IgM, and therefore were pre-B cells (data not shown).
Immature B cells derived from c-kit+ B cell precursors in young and aged mice have altered CD43 expression
We have previously reported that, in vivo, aged mice often have an increased frequency of immature bone marrow B cells characterized by higher surface expression of CD43 (recognized by the S7 monoclonal antibody) and increased cell size (23,24). In order to determine if this phenotype was associated with the origin of the B cell precursors (e.g., c-kit- vs. c-kit+), and noting the increased proportion of c-kit+ vs. c-kit- pre-B cells in aged mice, we compared immature B cells derived from c-kit+ wild-type and λ5 knockout precursor cells with those derived from c-kit- B cell precursors in vitro.
Immature B cells derived from either c-kit+ B cell precursors isolated from young adult BALB/c mice or present in λ5 knockout bone marrow exhibited altered surface phenotype with higher levels of CD43 than were seen on B cells generated from c-kit- B cell precursors in vitro (Figure 5). In addition, immature B cells from c-kit+ precursors were generally larger in size, as assessed by forward angle scatter (FSC), than was seen for B cells derived from c-kit- precursors (data not shown). Although CD43 levels were increased on B cells generated from c-kit+ precursors, little or no detectable CD23, CD5, or CD11b was seen on these B cells during the four day culture period (data not shown).
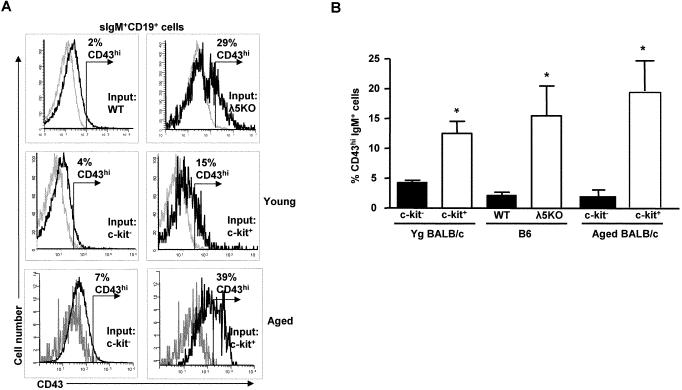
FIGURE 5. B cells derived from young and aged c-kit+ precursors show increased CD43 in vitro.
(A) Representative histograms of CD43 expression on B cells derived from WT (B6) and λ5 KO precursors (upper panels), as described in Fig. 4, and from young and aged BALB/c c-kit+ and c-kit- precursors (middle and lower panels), on day 4 of IL-7/SCF culture (grey line=isotype control). (B) Comparison of percent CD43hi B cells derived from WT (B6) and λ5 KO precursors and isolated young and aged BALB/c c-kit+ and c-kit- precursors. CD43 “high” B cells (CD43hi) were determined as those with fluorescence greater than isotype controls as indicated in (A) * p<0.05 for each pair.
Like young adult c-kit+ precursors, c-kit+ precursors in aged mice also yielded new B cells with altered phenotype in culture exemplified by increased CD43 expression (Figure 5). In addition, immature B cells from c-kit+ precursors from aged mice were also larger in size compared to B cells derived from c-kit- precursors (data not shown). These studies indicated that generation of new B cells characterized by increased cell size and enhanced CD43 expression was a property of c-kit+ B cell precursors in both young and aged mice.
B cells derived from c-kit- and c-kit+ precursors from both young and aged mice display different expression of Ig light chain isotypes
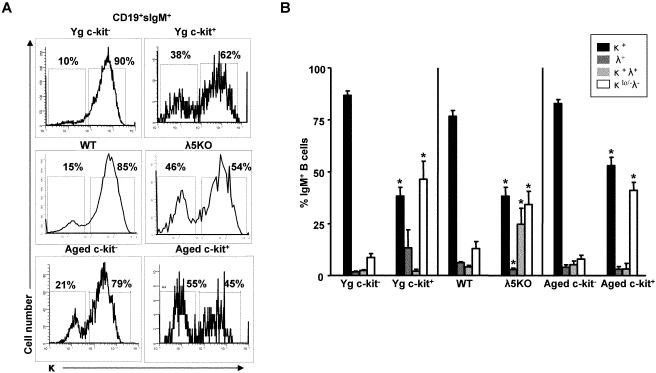
B cells derived in vitro from either c-kit- young adult BALB/c precursors or from IgM-CD19+ cells from wild-type (B6) mice showed predominant usage of the κ light chain (~80-90%) (Figure 6A). In contrast, in vitro derived B cells from both c-kit+ young BALB/c precursors and IgM-CD19+ cells from λ5 knockout mice showed significant decreases in levels of κ expression (~40-60%) (Figure 6A). The reduction in κ light chains among B cells generated in these instances reflected, in part, increases in B cells expressing the λ1, λ2, and/or λ3 isotypes (λ[1-3]); B cells with κ light chains in addition to λ(1-3) expression (κ+λ[1-3]+); but most importantly, B cells that were low in κ or negative for both κ and λ(1-3) light chains (κlo/-λ[1-3]-) (Figure 6B). Isolated populations of c-kit- and c-kit+ precursors from aged BALB/c mice yielded new B cells with different expression of the κ/λ isotypes similar to that seen with the same populations obtained from young adult mice (Figure 6A,B).
FIGURE 6. B cells derived from c-kit+ precursors from young, aged, or λ5 knockout mice show altered light chain expression in vitro.
(A) Representative histograms show κ light chain expressing B cells derived in culture from WT (B6) and λ5 KO sIgM-CD19+ bone marrow, and young (Yg) and aged BALB/c isolated c-kit+ and c-kit- precursors cultured as indicated in Fig. 4. Data are representative of 3-6 experiments. (B) Summary of light chain expression on B cells derived in culture for 3-6 mice per group. *p<0.05.
In vivo, aged wild-type mice and young λ5 gene knockout mice exhibit alterations in immature B cell light chain expression
Comparable to the results obtained in analysis of B cell development from λ5 knockout precursors in vitro, IgM+CD19+AA4.1+ immature B cells from λ5 knockout mice in vivo showed a lower proportion expressing normal levels of κ light chains (Figure 7A,B). In λ5 knockout mice, increased λ(1-3) usage on immature B cells was observed, often with co-expression of κ light chain; additionally, a greater incidence of immature B cells without detectable λ(1-3) and low to negligible κ light chains was also observed. In addition, the immature B cells characterized as κlo/-λ(1-3)- had lower levels of surface IgM than did either κ+ or κ+λ(1-3)+ immature B cells (data not shown). Hence, in the absence of surrogate light chain and preBCR signaling where c-kit+ pre-B cells are prevalent, immature B cells show skewed usage of light chain isotypes in vivo similar to that seen in vitro.
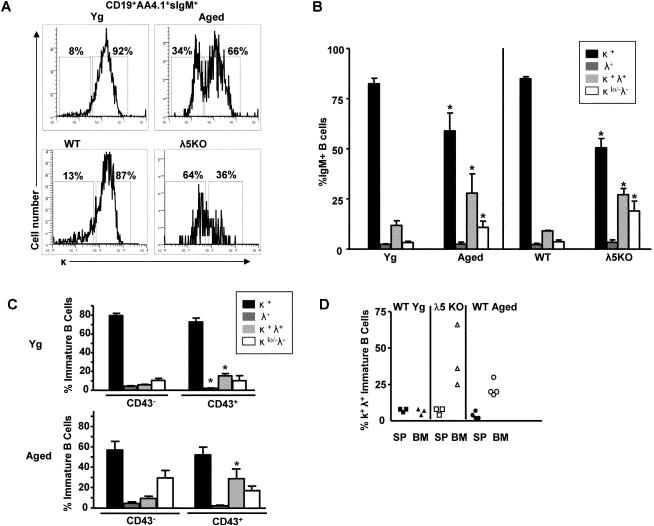
FIGURE 7. Immature B cells in aged BALB/c and λ5 knockout bone marrow exhibit altered light chain expression in vivo.
(A) Representative histograms show κ light chain expressing immature (AA4.1+) bone marrow B cells from young and old BALB/c mice and WT (B6) and λ5 KO mice in vivo. (B) Summary of light chain expression on immature bone marrow B cells from young and aged BALB/c mice and WT (B6) and λ5 KO mice. (C) Relative incidences of light chain expression among CD43- and CD43+ immature B cells in young and aged BALB/c bone marrow. (D) Bone marrow (BM) immature B cells (sIgM+ AA4.1+ CD19+) and splenic (SP) transitional B cells (sIgM+ AA4.1+ CD19+) from λ5 KO and aged mice were further evaluated for κ and λ dual light chain expression. Aged mice were chosen that had substantial increases in dual light chain isotype expressing immature B cells in their bone marrow for comparison with those in spleen. Data are cumulative for 3-8 mice. *, p<0.05.
In aged bone marrow, alterations in light chain usage were also readily detected among AA4.1+ immature B cells, in particular among aged mice that were highly depleted (>90%) in c-kit- pre-B cells. These “severely pre-B cell depleted” (6) aged mice had reductions in κ light chain expression compared to young controls (Figure 7A,B). This occurred coincident with both increased κ+λ(1-3)+ and κlo/-λ(1-3)- immature B cells in aged bone marrow (Figure 7B).
Since our previous results have shown that B cells expressing CD43 increased in proportion within the immature B cell pool of aged bone marrow (24), we next asked if CD43+ immature B cells preferentially exhibited altered light chain isotype expression. In both young adult and aged bone marrow, CD43+ immature B cells were enriched in κ/λ(1-3) dual expression (20-40% κ/λ dual expression in CD43+ compared to 5-10% in CD43- immature B cells) (Figure 7C). In contrast, immature B cells with low κ expression (κlow/- λ-) were roughly equivalent within the immature B cell pools; however, their levels increased in both CD43- and CD43+ immature B cell compartments of aged mice (Figure 7C). Therefore, altered light chain isotype expression was seen in both CD43- and CD43+ immature B cell subsets within aged bone marrow.
It remains to be seen whether those immature B cells with unusual phenotype (e.g., those with high CD43, dual light chain isotypes, and/or low κ light chain levels) are capable of migration to the spleen for further development and function. Initial experiments indicate that ~10-30% of immature/transitional splenic B cells (T2/T3; IgM+AA4.1+ CD23+) express CD43 while CD23- T1 transitional B cells do not (data not shown). While B cell maturation has been regarded as a progression from T1→T2→T3 in spleen, it has been demonstrated that this process is asynchronous and CD23 expression and progression into T2/T3 subsets occurs in bone marrow (29). These bone marrow T2/T3 cells may then transit to the spleen. While CD43+ immature B cells may transit from bone marrow to the spleen, experiments shown in Figure 7D indicate that κ+λ+ dual isotype B cells, enriched in the CD43+ subset (Fig. 7C), are increased among immature B cells in bone marrow of both young adult λ5 KO mice and in aged BALB/c mice, but are only rarely observed in the transitional AA4.1+ immature B cell compartments of the spleen. Therefore, phenotypically defined subsets of immature bone marrow B cells exhibit different capacities to migrate to and/or populate the spleen.
DISCUSSION
It is clear that senescent mice, of various inbred strains, have reduced numbers of bone marrow B cell precursors (1-6). This is readily observed as a reduction in late stage pre-B cells (e.g., Hardy Fraction D), although Miller and Allman (4); Labrie et. al. (5); and we (3,6) have reported variable losses of pro-B cells and earlier B lineage progenitors in old mice as well. The loss of late stage pre-B cells may be due to multiple mechanisms, including increased apoptotic death (8); poor proliferation to IL-7 (7); decreased Ig heavy chain rearrangements (5,9); and, as we have suggested, diminished expression of the preBCR surrogate light chains and reduced preBCR signaling at the pro-B to pre-B cell transition (3,10-14).
In aged mice, dampening of the preBCR checkpoint leads to partial arrest of the B2 B cell pathway, but allows for residual preBCR deficient B cell development
The B2 pathway of B lymphopoiesis involves the sequential development of pro-B and pre-B cells with expansion at the early pre-B cell stage due to preBCR signaling. The preBCR-mediated transition of pro-B to pre-B cells is characterized by changes in several surface antigens, including down-regulation of c-kit (20,22). Diminished expression of surrogate light chains in aged B cell precursors, as shown herein and in (3,11), likely reduces production of pre-B cells in aged bone marrow. This is seen in our studies as a loss of early and late stage pre-B cells, in particular those that down-regulate c-kit expression. About 20% of early pre-B cells in young adult bone marrow retain c-kit and, as shown by others (20,22,26), fail to express the preBCR; comparable numbers are also detected in aged bone marrow, even as total pre-B cell numbers are reduced. This indicates that, overall, pre-B cell generation is partially blocked in aged mice with an increasing proportion of early pre-B cells in aged bone marrow that likely have not transited the preBCR checkpoint. These c-kit+ B cell precursors retain the capacity to generate new B cells, albeit with reduced efficiency. This is not surprising since preBCR signaling has been implicated in the induction of light chain rearrangements (30).
We propose that, in aged mice, the well-described B2 pathway reliant on preBCR assembly and signaling, is constrained by loss of surrogate light chain and diminished preBCR expression at the pro-B to pre-B cell transition. Although generally a minor component of normal B lymphopoiesis, pre-B cell maturation and B cell formation in a manner less dependent on preBCR signaling can occur and this deviation of normal B lymphopoiesis is more apparent in aged bone marrow as summarized in Figure 8.
Alterations in immature B cell phenotypes in aged bone marrow
The immature B cell pools of aged mice differ significantly in lifespan, surface markers, and antibody repertoire from that of young adults (24,31-34). In our previous studies, focusing mainly on aged mice with severe deficits in B cell precursors, newly formed B cells were often decreased, consistent with their extensive loss of pre-B cells (6,24). However, these studies revealed that at least two subsets of immature B cells were present in the bone marrow: those characterized by relatively high CD43 levels and those with low or absent CD43 (23,24). Those B cells expressing CD43 also often expressed other antigens associated with activation including CD5, CD11b, and/or PD-1 (23,24).
Moreover, those immature B cells characterized by CD43 expression were maintained in aged bone marrow and comprised an increased percentage of the total immature B cell pool (24). As shown previously, optimal generation of CD43+ immature B cells required Btk and likely required BCR signaling (23). This is analogous to requisites for CD23 expression reported for a subset of bone marrow immature B cells as described by Lindsley, et. al. (29). Indeed, our findings suggest that the CD43+ immature B cells comprise a subset of the CD23+ immature B cells in bone marrow (Williamson-Leon, T. and Riley, R.L., unpublished results). As we have previously shown, immature B cells with this activation phenotype are not undergoing significant levels of proliferation in vivo (23,24).
Of importance, as shown herein, generation of B cells that express relatively high levels of CD43 is associated with differentiation from c-kit+ B2 lineage precursors rather than from c-kit- B2 pre-B cells. Taken together with our previous results, this suggests that c-kit+ pre-B cells may preferentially develop into B cells that more readily undergo activation. We speculate that such B cells have undergone partial activation in response to self-antigens within the bone marrow microenvironment. This is supported by the increased incidences of CD43+ immature B cells that co-express dual κ and λ light chains. This may reflect receptor editing/dilution as seen in both normal immature B cells and in transgenic mice with anti-phosphorylcholine or anti-DNA specific B cells (35-38). The increase in such immature B cells as well as increased levels of newly produced B cells, both CD43+ and CD43-, with reduced levels of κ light chain/surface Ig, may also reflect increased tolerance (receptor editing/anergy) in aged bone marrow (38). Whether immature B cells with these unusual phenotypes are capable of exiting the bone marrow and populating the periphery as functional mature B lymphocytes or instead remain in the bone marrow, possibly contributing to the increasingly “stagnant” pool of immature B cells described by Johnson, et. al. (33), is not known. However, since increased proportions of dual light chain isotype expressing immature B cells in the bone marrow of both λ5 KO and aged mice are not seen among splenic transitional B cells, this suggests that these B cells may not undergo typical patterns of migration to the spleen and further maturation along normal pathways in the periphery.
However, the question remains as to whether these alterations in B lymphopoiesis affect B cell development in old age. We speculate, based on our in vitro experiments, that in aged mice with highly reduced B2 B lymphopoiesis (<10-20% of normal), preBCR compromised pre-B cells (e.g., c-kit+), could account for up to 20% of immature B cell generation in the bone marrow. As discussed below, such a shift in relative usage of B lymphopoietic pathways has significant importance in determining the phenotype and light chain usage of immature B cells in aged mice.
We suspect that the alterations in B cell repertoire previously reported in aged mice (24,31), particularly within the bone marrow, have their basis, in part, in the relative use of distinct B cell developmental pathways characterized by differences in preBCR signaling. While a variety of deficits may contribute to changes in B cell precursor numbers and B cell functions in aged mice, it is remarkable that particular changes in B cell precursor phenotype (e.g., loss of c-kit-, but retention of c-kit+ pre-B cells) as well as alterations seen in the properties of new B cells (e.g., CD43 and light chain expression) are mimicked in the λ5 gene knockout mouse. This suggests that some, but likely not all, defects seen in B lymphopoiesis in aged mice, particularly at the preBCR selection checkpoint, may be derived from reduced surrogate light chain expression in B cell precursors. In aged mice, down-regulation of surrogate light chain (likely resulting from compromised E2A expression [3,11]), results in truncation of the B2 pathway and its diversion along a preBCR-compromised track.
That differences in development of B cell precursors underlie changes in new B cell formation and antibody repertoire in old age is consistent with the results obtained by Klinman and colleagues (31,34) indicating that alterations in the emerging B cell repertoire in aged bone marrow and spleen were dictated by the developmental potential of bone marrow surface Ig- precursor cells (e.g., pre-B cells). Our findings indicate that alterations in the pre-B cell compartment in aged mice, likely due to poor preBCR function, result in changes in phenotype and light chain expression by immature B cells (summarized in Fig. 8). The functions of these distinct B cell subsets, their roles in peripheral immunity, and consequences of their alterations in old age remain issues to be further explored.
Acknowledgements
We gratefully acknowledge the assistance of Jim Phillips and the Flow Cytometry Core Facility at the Sylvester Comprehensive Cancer Center. We thank Ana Marie Landon for assistance in maintaining and characterizing the λ5 knockout mice. We thank all members of the Riley and Blomberg laboratories for their support in the performance of these studies.
Supported by NIH grants to RLR and to BBB.
Footnotes
Publisher's Disclaimer: This is an author-produced version of a manuscript accepted for publication in The Journal of Immunology (The JI). The American Association of Immunologists, Inc. (AAI), publisher of The JI, holds the copyright to this manuscript. This version of the manuscript has not yet been copyedited or subjected to editorial proofreading by The JI; hence, it may differ from the final version published in The JI (online and in print). AAI (The JI) is not liable for errors or omissions in this author-produced version of the manuscript or in any version derived from it by the U.S. National Institutes of Health or any other third party. The final, citable version of record can be found at www.jimmunol.org.
REFERENCES
- 1.Riley RL, Kruger MG, Elia J. B cell precursors are decreased in senescent BALB/c mice, but retain normal mitotic activity in vivo and in vitro. Clin. Immunol. Immunopathol. 1991;59:301–313. doi: 10.1016/0090-1229(91)90026-7. [DOI] [PubMed] [Google Scholar]
- 2.Stephan RP, Sanders VM, Witte PL. Stage-specific alterations in murine B lymphopoiesis with age. Int. Immunol. 1996;8:509–518. doi: 10.1093/intimm/8.4.509. [DOI] [PubMed] [Google Scholar]
- 3.Sherwood EM, Blomberg BB, Xu W, Warner CA, Riley RL. Cutting edge: Senescent BALB/c mice exhibit decreased expression of λ5 surrogate light chains and reduced development within the pre-B cell compartment. J. Immunol. 1998;161:4472–4475. [PubMed] [Google Scholar]
- 4.Miller JP, Allman D. The decline in B lymphopoiesis in aged mice reflects loss of very early B-lineage precursors. J. Immunol. 2003;171:2326–2330. doi: 10.4049/jimmunol.171.5.2326. [DOI] [PubMed] [Google Scholar]
- 5.Labrie JE, Borghesi L, Gerstein R. Bone marrow microenvironmental changes in aged mice compromise V(D)J recombinase activity and B cell generation in aged mice. J. Exp. Med. 2005;200:411–423. doi: 10.1016/j.smim.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 6.Van der Put E, Sherwood EM, Blomberg BB, Riley RL. Aged mice exhibit distinct B cell precursor phenotypes differing in activation, proliferation and apoptosis. Exp. Gerontol. 2003;38:1137–1147. doi: 10.1016/j.exger.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 7.Stephan RP, Lill-Elghanian DA, Witte PL. Development of B cells in aged mice: decline in the ability of pro-B cells to respond to IL-7 but not to other growth factors. J. Immunol. 1997;158:1598–1609. [PubMed] [Google Scholar]
- 8.Kirman I, Zhao K, Wang Y, Szabo P, Telford W, Weksler ME. Increased apoptosis of bone marrow pre-B cells in old mice associated with their low number. Int. Immunol. 1998;10:1385–1392. doi: 10.1093/intimm/10.9.1385. [DOI] [PubMed] [Google Scholar]
- 9.Szabo P, Shen S, Telford W, Weksler ME. Impaired rearrangement of IgH V to DJ segments in bone marrow pro-B cells from old mice. Cell. Immunol. 2003;222:78–87. doi: 10.1016/s0008-8749(03)00084-4. [DOI] [PubMed] [Google Scholar]
- 10.Riley RL, Van der Put E, King AM, Frasca D, Blomberg BB. Deficient B lymphopoiesis in murine senescence: potential roles for dysregulation of E2A, Pax-5, and STAT5. Semin Immunol. 2005;17:330–336. doi: 10.1016/j.smim.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 11.Sherwood EM, Xu W, King AM, Blomberg BB, Riley RL. The reduced expression of surrogate light chains in B cell precursors from senescent BALB/c mice is associated with decreased E2A proteins. Mech. Ageing. Dev. 2000;118:45–59. doi: 10.1016/s0047-6374(00)00157-3. [DOI] [PubMed] [Google Scholar]
- 12.Frasca D, Nguyyen D, Riley RL, Blomberg BB. Decreased E12 and/or E47 transcription factor activity in the bone marrow as well as in the spleen of aged mice. J. Immunol. 2003;170:719–726. doi: 10.4049/jimmunol.170.2.719. [DOI] [PubMed] [Google Scholar]
- 13.Van der Put ED, Frasca, King AM, Blomberg BB, Riley RL. Decreased E47 in senescent B cell precursors is stage specific and regulated posttranslationally by protein turnover. J. Immunol. 2004;173:818–827. doi: 10.4049/jimmunol.173.2.818. [DOI] [PubMed] [Google Scholar]
- 14.King AM, Van der Put E, Blomberg BB, Riley RL. Accelerated Notch-dependent degradation of E47 proteins in aged B cell precursors is associated with increased ERK MAPK activation. J. Immunol. 2007;178:3521–3529. doi: 10.4049/jimmunol.178.6.3521. [DOI] [PubMed] [Google Scholar]
- 15.Sigvardsson M, O'Riordan M, Grosschedl R. EBF and E47 collaborate to induce expression of the endogenous immunoglobulin surrogate light chain genes. Immunity. 1997;7:25–36. doi: 10.1016/s1074-7613(00)80507-5. [DOI] [PubMed] [Google Scholar]
- 16.Riley RL, Blomberg BB, Frasca D. B cells, E2A, and aging. Immunol. Rev. 2005;205:30–47. doi: 10.1111/j.0105-2896.2005.00268.x. [DOI] [PubMed] [Google Scholar]
- 17.Kawano Y, Yoshikawa S, Minegishi Y, Karasuyama H. Pre-B cell receptor assesses the quality of IgH chains and tunes the pre-B cell repertoire by delivering differential signals. J Immunol. 2006;177:2242–2249. doi: 10.4049/jimmunol.177.4.2242. [DOI] [PubMed] [Google Scholar]
- 18.Kitamura D, Kudo A, Schaal S, Muller W, Melchers F, Rajewsky K. A critical role of λ5 protein in B cell development. Cell. 1992;69:823–831. doi: 10.1016/0092-8674(92)90293-l. [DOI] [PubMed] [Google Scholar]
- 19.Karasuyama H, Rolink A, Shinkai Y, Young F, Alt FW, Melchers F. The expression of Vpre-B/lambda 5 surrogate light chain in early bone marrow precursor B cells of normal and B cell-deficient mutant mice. Cell. 1994;77:133–143. doi: 10.1016/0092-8674(94)90241-0. [DOI] [PubMed] [Google Scholar]
- 20.Rolink A, Grawunder U, Winkler TH, Karasuyama H, Melchers F. IL-2 receptor alpha chain (CD25, TAC) expression defines a crucial stage in pre-B cell development. Int Immunol. 1994;6:1257–1264. doi: 10.1093/intimm/6.8.1257. [DOI] [PubMed] [Google Scholar]
- 21.Rolink AG, Winkler T, Melchers F, Andersson J. Precursor B cell receptor-dependent B cell proliferation and differentiation does not require the bone marrow or fetal liver environment. J Exp Med. 2000;191:23–32. doi: 10.1084/jem.191.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.ten Boekel E, Melchers F, Rolink AG. Changes in the V(H) gene repertoire of developing precursor B lymphocytes in mouse bone marrow mediated by the pre-B cell receptor. Immunity. 1997;7:357–368. doi: 10.1016/s1074-7613(00)80357-x. [DOI] [PubMed] [Google Scholar]
- 23.Wilson EL, Sherwood EM, King AM, Riley RL. A phenotyically distinct subset of immature B cells exhibits partial activation, increased survival, and preferential expression of VhS107. Eur. J. Immunol. 2003;33:3398–3408. doi: 10.1002/eji.200324324. [DOI] [PubMed] [Google Scholar]
- 24.Wilson EL, King AM, Sherwood EM, Riley RL. Pre-B cell loss in senescence coincides with preferential development of immature B cells characterized by partial activation and altered Vh repertoire. Exp. Gerontol. 2005;40:67–79. doi: 10.1016/j.exger.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 25.Shinjo F, Hardy RR, Jongstra J. Monoclonal anti-λ5 antibody FS1 identifies a 130kDa protein associated with λ5 and VpreB on the surface of early pre-B cell lines. Int. Immunol. 1994;6:393–399. doi: 10.1093/intimm/6.3.393. [DOI] [PubMed] [Google Scholar]
- 26.Kawano Y, Yoshikawa S, Minegishi Y, Karsuyama H. Selection of stereotyped VH81X-μH chains via pre-B cell receptor early in ontogeny and their conservation in adults by marginal zone B cells. Int Immunol. 2005;17:857–867. doi: 10.1093/intimm/dxh265. [DOI] [PubMed] [Google Scholar]
- 27.Hardy RR, Carmack CE, Shinton SA, Kemp JD, Hayakawa K. Resolution and characterization of pro-B and pre-pro-B stages in normal mouse bone marrow. J. Exp. Med. 1991;173:1213–1225. doi: 10.1084/jem.173.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Milne CD, Fleming HE, Paige CJ. IL-7 does not prevent pro-B/pre-B cell maturation to the immature/sIgM(+) stage. Eur. J. Immunol. 2004;34:2647–2655. doi: 10.1002/eji.200425400. [DOI] [PubMed] [Google Scholar]
- 29.Lindsley RC, Thomas M, Srivastava B, Allman D. Generation of peripheral B cells occurs via two spatially and temporally distinct pathways. Blood. 2007;109:2521–2528. doi: 10.1182/blood-2006-04-018085. [DOI] [PubMed] [Google Scholar]
- 30.Yamamoto M, Hayashi K, Nojima T, Matsuzaki Y, Kawano Y, Karasuyama H, Goitsuka R, Kitamura D. BASH-novel PKC-Raf-1 pathway of pre-BCR signaling induces kappa gene rearrangement. Blood. 2006;108:2703–2711. doi: 10.1182/blood-2006-05-024968. [DOI] [PubMed] [Google Scholar]
- 31.Riley SC, Froscher BG, Linton PJ, Zharhary D, Marcu K, Klinman NR. Altered VH gene segment utilization in the response to phosphorylcholine by aged mice. J. Immunol. 1989;143:3798–3805. [PubMed] [Google Scholar]
- 32.Kline GH, Hayden TA, Klinman NR. B cell maintenance in aged mice reflects both increased B cell longevity and decreased B cell generation. J Immunol. 1999;162:3342–3349. [PubMed] [Google Scholar]
- 33.Johnson KM, Owen K, Witte PL. Aging and developmental transitions in the B cell lineage. Int. Immunol. 2002;14:1313–1323. doi: 10.1093/intimm/dxf092. [DOI] [PubMed] [Google Scholar]
- 34.Klinman NR, Kline GH. The B-cell biology of aging. Immunol Rev. 1997;160:103–114. doi: 10.1111/j.1600-065x.1997.tb01031.x. [DOI] [PubMed] [Google Scholar]
- 35.Kenny JJ, Rezanka LJ, Lustig A, Fischer RT, Yoder J, Marshall S, Longo DL. Autoreactive B cells escape clonal deletion by expressing multiple antigen receptors. J Immunol. 2000;164:4111–4119. doi: 10.4049/jimmunol.164.8.4111. [DOI] [PubMed] [Google Scholar]
- 36.Li Y, Li H, Ni D, Weigert M. Anti-DNA B cells in MRL/lpr mice show altered differentiation and editing pattern. J. Exp. Med. 2002;196:1543–1552. doi: 10.1084/jem.20021560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rezanka LJ, Kenny JJ, Longo DL. Dual isotype expressing B cells [κ(+)/λ(+)] arise during the ontogeny of B cells in the bone marrow of normal nontransgenic mice. Cell Immunol. 2005;238:38–48. doi: 10.1016/j.cellimm.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 38.Merrell KT, Benschop RJ, Gauld SB, Aviszus K, Decote-Ricardo D, Wysocki LJ, Cambier JC. Identification of anergic B cells within a wild-type repertoire. Immunity. 2006;25:953–962. doi: 10.1016/j.immuni.2006.10.017. [DOI] [PubMed] [Google Scholar]