Abstract
While cytomegalovirus (CMV) infects and replicates in a multitude of cell types, the ability of the virus to replicate in antigen presenting cells (APCs) is believed to play a critical role in the viral dissemination and latency. CMV infection of APCs and manipulation of their function is an important area of investigation. CMV down regulation of MHC II is reportedly mediated by the HCMV proteins US2, US3, UL83, UL111a (vIL10) or through the induction of cellular IL10. In this study, we demonstrate that rat CMV (RCMV) significantly reduces MHC II expression by mechanisms that do not involve orthologues of the known HCMV genes nor by an increase in cellular IL10. Rat bone marrow derived dendritic cells (BMDC) were highly susceptible to infection with RCMV and a recombinant RCMV expressing eGFP. RCMV infection of BMDCs depleted both surface and intracellular MHC II to nearly undetectable levels as well as reduced surface expression of MHC I. The effect on MHC II only occurred in the infected GFP positive cells and is mediated by an immediate early or early viral gene product. Furthermore, treatment of uninfected immature DCs with virus-free conditioned supernatants from infected cells failed to down regulate MHC II. RCMV depletion of MHC II was sensitve to treatment with lysosomal inhibitors but not proteasomal inhibitors suggesting that the mechanism of RCMV mediated down-regulation of MHC II occurs through endocytic degradation. Since RCMV does not encode homologues of US2, US3, UL83 or UL111a, these data indicate a novel mechanism for RCMV depletion of MHC II.
INTRODUCTION
Cytomegaloviruses (CMV) are ubiquitous, species-specific β-herpesviruses. Between fifty and ninety percent of adults worldwide are infected with Human Cytomegalovirus (HCMV) (Mocarski, 2001). While infection with HCMV is normally asymptomatic in the immunocompetent host, immunosuppressed individuals and neonates infected with the virus may develop severe complications including pneumonia, retinitis, gastrointestinal disease and death. Primary HCMV infection typically results in life long persistence characterized by a latent phase with intermittent reactivations and secondary infections (Mocarski, 2001). The establishment of this persistent infection provides a unique challenge to the virus, which is under constant surveillance by the host immune system. CMVs derive their success in maintaining their prevalence from their ability to evade immune recognition and clearance through the acquisition of genes specifically targeting both the adaptive and innate immune response. With a coding capacity of approximately 180 predicted open reading frames, only 40–60 of which have been demonstrated to be essential for in vitro viral replication, it is estimated that nearly two thirds of the CMV genome is devoted to sustaining infection in vivo, a prime component of which is subversion of host immune surveillance (Dunn et al., 2003; Mocarski, 2002).
The adaptive strategies employed by CMV to counteract the host immune system fall into two broad categories: immunomodulation and immune evasion. Examples of immunomodulation can be found in viral recruitment of leukocytes to the site of primary infection through the expression of virus-encoded chemokines and through virus-induced expression of host cell cytokines and growth factors. Dampening of the inflammatory response and virus-induced functional paralysis of antigen presenting cells (APCs) such as dendritic cells (DC) and macrophages occurs through the altered cytokine profiles of infected cells as well as by the down regulation of host cell receptors and ligands (Mocarski, 2002). While evasion strategies implemented by CMV include the ability of the virus to maintain a quiescent infection in APCs, the prevailing paradigm of immune evasion is typified by CMV manipulation of both the classical and nonclassical major histocompatability complexes.
The capacity of APCs to present antigen in the context of MHC I and II is central to the ability of the host to activate the adaptive immune response to viral infection. Like other members of the herpesvirus family, CMV has evolved to avoid host immune detection by manipulating MHC processing and presentation (reviewed in (Lilley and Ploegh, 2005)). Several ORFs have been identified in both mouse CMV (MCMV) and HCMV that mediate MHC I down regulation at various stages of MHC processing. Three MCMV ORFs, m4, m6 and m152, encode glycoproteins with nonredundant and complementary function in MHC I interference (Kavanagh et al., 2001). M4 binds MHC I in the ER and is transported to the cell surface where it interferes with CD8+ T cell recognition of MHC I (Kavanagh, Koszinowski, and Hill, 2001; Kleijnen et al., 1997). M6 redirects assembled MHC I:peptide complex from the endoplasmic reticulum (ER) to the lysosome where it is subsequently degraded (Reusch et al., 1999). M152 retains MHC I in the ER/Golgi compartment, thus blocking plasma membrane localization (Ziegler et al., 2000; Ziegler et al., 1997). The glycoproteins encoded by the four HCMV ORFs, US2, US3, US6 and US11 have all been associated with post translational blockade at several critical steps in MHC I processing (Ahn et al., 1996). US2 and US11 inhibit cell surface expression of MHC I and US3 and US6 both block peptide loading by inhibiting tapasin and Transporter associated with Antigen Processing (TAP).
A critical component of the CMV life cycle is the ability of the virus to replicate in MHC II positive cells, including endothelial cells, epithelial cells, monocytes, macrophages and DCs (Fish et al., 1998; Lockridge et al., 1999; Michelson, 1997). Infection of macrophage progenitors is believed to play a pivotal role in viral dissemination and reactivation from latent or low level persistent infections. While a variety of mechanisms and virus-encoded ORFs that mediate MHC I down regulation have been extensively studied, relatively little is known about CMV induced MHC II down regulation. Four HCMV ORFs have been identified as modulators of MHC II cell surface expression: US2, US3, UL83 and UL111A (vIL10) (Chang et al., 2004; Hegde et al., 2002; Tomazin et al., 1999). US2 modulates MHC II cell surface expression by directly causing the degradation of HLA-DRα and destabilizes newly formed MHC II α/β complex (17). US3 blocks efficient sorting of MHC II molecules to the peptide loading compartment by binding class II α/β complexes, which compromises the stability of those complexes (Hegde et al., 2002). In fibroblasts induced to express MHC II, UL83 and UV inactivated virus was shown to down regulate cell surface HLA-DRα by an unknown mechanism (Odeberg et al., 2003). Studies investigating vIL10 have demonstrated that it is both a structural and functional homologue of cellular IL10, however there are conflicting reports on the effectiveness of the viral cytokine to down regulate cell surface MHC II (Kotenko et al., 2000). For example, Raftery et al. have shown that vIL10 blocked LPS induced MHC II cell surface expression in immature peripheral blood derived dendritic cells (PBDC); while Chang et al. reported that vIL10 has no effect on MHC II expression in mature PBDC (Chang et al., 2004; Raftery et al., 2004). MCMV lacks an IL10 homologue, however, the virus down regulates surface MHC II on primary macrophages and the macrophage cell line, IC21 by inducing secretion of cellular IL10 (Redpath et al., 1999).
Several laboratories have outlined the impact of direct infection of APCs on their ability to initiate both innate and adaptive immune responses to CMV in vitro. Likewise, human DC function in the context of a natural HCMV infection has also been analyzed (Frascaroli et al., 2006; Varani et al., 2005a; Varani et al., 2005b). Circulating DC obtained from patients undergoing active CMV infection were both phenotypically and functionally impaired with a 50% reduction in MHC II (Frascaroli et al., 2006). Furthermore, DC derived from heart transplant recipients with acute CMV disease have reduced MHC II and diminished ability to stimulate T cell proliferation (Varani et al., 2005a). The current study utilizes a direct infectivity model of rat bone marrow derived DC (BMDC) to investigate RCMV induced MHC manipulation. DC progenitors are highly abundant in the bone marrow, easily accessible and an important cell type in CMV biology. While RCMV has previously been shown to transiently down regulate MHC I in fibroblasts, there are no published reports describing RCMV infection of DC or the ability of the virus to manipulate MHC II. Here we demonstrate that immature rat DCs are highly susceptible to RCMV infection, and that infection of these cells, results in a greater than 90% reduction in the levels of intracellular and cell surface MHC II. The virus-induced depletion of MHC II is the result of direct infection, requiring immediate early and/or early viral gene expression, is not mediated by a secreted factor and appears to occur by at least two mechanisms: one affecting synthesis of nascent MHC II alpha, the other degrading mature MHC II by acid dependent proteases.
MATERIALS & METHODS
Generation of Bone Marrow Derived DCs
Rodents were housed in the AAALAC accredited Portland VA Medical Center animal facility in a specific-pathogen-free room in compliance with USDA/HHS guidelines. Bone marrow was flushed from the femurs and tibias of Lewis and F344 adult male rats and strained through a 70μm filter. Bone marrow cells were washed twice in complete RPMI-1640 medium supplemented with 10% v/v fetal bovine serum, 100 U/ml penicillin, 100μg/ml streptomycin and 2mM L-glutamine. Bone marrow cells were then cultured at a cell density of 1×106 cells per ml in complete RPMI supplemented with 10ng/ml recombinant rat GM-CSF (R&D Systems, 518-GM) and 5ng/ml recombinant rat IL4 (R&D Systems), in a final volume of 10ml. Five days after plating, the cells were given fresh media plus GM-CSF and IL-4. Nine days after initial plating, the non-adherent cells were removed from the culture; adherent cells were washed three times with DPBS, once with RPMI and then cultured in complete RPMI without cytokine. In order to stimulate the immature BMDCs to mature, the cells were treated with 1μg/ml LPS. After an additional 24-hour incubation, the LPS-matured or immature BMDC were washed twice with DPBS and once with RPMI, and then cultured in complete RPMI.
Rat cytomegalovirus
Tissue culture-derived stocks of the Maastricht strain of RCMV were amplified and titered in rat lung fibroblasts (RFL6). Cell free virus was obtained from the cultured supernatants of infected cells, which were spun at 2k RPM for 20 min to remove cell debris, and then pelleted at 22k RPM (Beckman SW28) through a sorbitol cushion (20% D-sorbitol, 0.05M Tris, 1mM MgCl2). The virus pellet was resuspended in Minimal Essential Medium (MEM) culture media. Plaque assays were performed in confluent 24-well plates by infection with an appropriate serial virus dilution in 0.2 ml of media and then incubated at 37°C for three hours. Following incubation, the infected cells were overlaid with 1 ml MEM supplemented with 10% fetal bovine serum (FBS), non-essential amino acids, pen/strep and 20 mM L-Glutamine with carboxymethylcellulose (Sigma, St. Louis, MO). After 7 days, the cells were fixed in 3.7% formalin in PBS and stained with 0.05% aqueous methylene blue (Sigma). The plaques were counted by light microscopy. To inactivate RCMV replication, the required pfu of infectious virus was diluted in serum-free medium in 10cm dish and exposed for 4 min at 999 mJ/cm2 in a UV-Crosslinker (Fisherbrand-UVXL-1000), which we have previously determined is sufficient to inactivate 100% of RCMV replication in fibroblasts using immunostaining at 24hrs with antibody to IE gene product. In addition, the RCMV DNA polymerase inhibitor, Foscarnet (0.5mM) was used to treat infected cell cultures at 8 hours post infection in order to determine whether the effect of the RCMV down regulation of MHC was due to the expression of a late viral gene product.
Construction of RCMV-GFP
To mark infected cells, we constructed a recombinant RCMV strain (RCMV-Δ145-7GFP), in which the ORFs r145, r146 and part of r147 were deleted and replaced by homologous recombination with a GFP expression cassette. The recombination plasmid pΔR145-7 was constructed as follows. A 1kb region of RCMV from 196,976 to 197,998 (RB1) was produced by PCR with the primers r144Fwd: ata aag ctt atc gcg gac gcg gac agc gag ata t and r144Rev: ata gat ctt taa tta acg gga ttg aga tat acg tac acc gtg and cloned into pCDNA-3 using the restriction enzymes HindIII and NotI. The primers for the second PCR fragment from 200,771 to 201,803 were r147Fwd: ata tta att aat gta tca ggc acc gtg tac tcg ata acg and r147Rev: ata gcg gcc gca agc ttg agg tag aag tag aat aaa gcg tta tga. The second fragment (RB2) was cloned into pCDNA-3Δr144 using NotI and XhoI. The GFP cassette was blunt end inserted into the Not1 site found in pCDNA-3Δr144-7. The EF-1α/eGFP cassette from the vector pQ100 (a gift from Dr. J. Vieira, University of Washington) was cut with EcoRV/XhoI and blunt ended for subsequent cloning into the recombination vector to make pΔR145-7. Since the primer r147 Rev also contained an internal HindIII site, this enzyme was used to release a 3.5kb fragment containing the RCMV flanking regions and GFP cassette.
The virus was constructed by transfecting HindIII cut pΔR145-7 into 1×106 RFL-6 cells (260kV, 975μFarrads) with a GenePulser (BioRad). The cells were plated in complete DMEM for 6 hours and then infected with 1×105 PFU of salivary gland-derived RCMV. After two days the cells were scraped, and cells and supernatants were frozen at −80°C. The mix was thawed and added to fresh RFL-6 cells. On the second pass, GFP+ plaques were isolated and subcloned by limiting dilution on RFL-6 cells in 96-well plates (4 times). The integrity and clonal purity of the recombinant virus were confirmed by restriction endonuclease digestions in combination with Southern blot analysis (data not shown).
Antibodies
The following primary antibodies were used for phenotypic analysis of BMDCs and splenocytes: MHC II [RT1.B] (Serotec, MRC OX-6), (Serotec, OX-17), MHC I [RT1.A] (Serotec, OX-18), CD80 (Serotec, 3H5), CD86 (Serotec, MRC OX-48), CD44H (BD Pharmingen, OX-49), CD8α (BD Pharmingen, OX-8), CD11b (Serotec, OX-42), CD11c (Serotech, 8A2), CD45R (BD Pharmingen, HIS24), CD172a (Serotec, OX-41), OX-62 (Serotec, MRC OX-62), IL10 (PeproTech Inc., 500-P139), RhoA (Santa Cruz, sc-179) and GAPDH (AbCAM, ab8245). The following secondary antibodies were used: anti-mouse IgG APC (eBiosciences, 17401282), donkey anti-rabbit HRP (Amersham, NA934V), swine anti-goat HRP (Biosource, ACI 3404), anti-mouse HRP (Amersham, NXA-931). Isotype Controls: IgG2a (BD Pharmingen, 553454), IgG1 (BD Pharmingen, 550615), IgG1 (BD Pharmingen, 557273).
Flow Cytometry
BMDCs were removed from plastic dishes with gentle scraping, and spun at 2k RPM for 5min to pellet. The pelleted cells were washed in DPBS one time, resuspended at a cell density of 1×107 cells per ml in FACS Block Buffer (20% Normal Goat Serum in Dulbelcco’s Phosphate Buffered Saline, 0.1% NaN3) and incubated on ice for 20 min. Blocked cells were spun at 2k RPM for 5 min and resuspended in FACS Wash Buffer (1% NGS in DPBS, 0.01% NaN3) at 1×107 cells per ml. A total of 1×106 cells were stained with each appropriate primary antibody for 30 min on ice. Following primary antibody incubation, cells were washed three times with FACS Wash Buffer and resuspended in 200μl FACS Wash Buffer and incubated with the appropriate secondary antibody or streptavidin-allophycocyanin (BD Pharmingen, 554067) for 30 min on ice. The stained cells were washed twice with FACS Wash Buffer and analyzed on a FACS Calibur (Becton Dickinson) utilizing Cell Quest software. Final data analysis was performed using FlowJo software (Tree Star, Inc.).
Immunofluorescence Microscopy
BMDC samples were cytospun (Shandon) onto glass slides, which were subsequently washed with PBS and fixed with 2% PFA in PBS. For intracellular staining, the samples were permeablized and blocked in intracellular staining buffer (ISB: 1g BSA, sodium azide, 0.5% Triton-X100, and 500ml PBS) with 10% normal goat serum (NGS) for 20 minutes. The primary antibodies (diluted in ISB) directed against MHC II, CD44H, and RCMV IE were incubated for 2 hrs at room temperature. IgG isotype antibodies were used as a negative staining control. Samples were washed with ISB and incubated with fluorescein isothiocyanate (FITC) or L-rhodamine conjugated secondary anti-mouse or anti-rabbit antibodies (BioSource International, Camarillo, CA), diluted in ISB for 1hr. Samples were washed with ISB, mounted and visualized using a Delta Vision RT microscope by Applied Precision. Photomicrographs were obtained at 60× magnification.
Western Blot
Total cell lysates from mock infected iDC or iDC infected at an MOI=1 treated with and without epoxomicin (100nM) or bafilomycin (10μM) were run on 12% polyacrylamide SDS PAGE gels. Proteins were transferred to PVDF membrane (Millipore). The blots were blocked with 5% milk in DPBS, and then incubated with the indicated primary antibody. Membranes were washed with Tris Buffered Saline plus 0.2% Tween 20 (TBS-T), incubated with the corresponding HRP conjugated secondary antibody, and washed with TBS-T. The proteins were visualized with the Advance ECLWestern Blot Detection Kit (Amersham, RPN2135) and autoradiography. Protein levels were normalized to GAPDH or RhoA.
Mixed Leukocyte Reaction
Lewis rat BMDCs were prepared as described above and mock-infected or infected at an MOI equal to 1.0 with WT RCMV. Allogeneic splenocytes were prepared from macerated whole spleens harvested from ACI strain rats, washed three times in sterile DPBS and labeled with CFSE (CellTrace CFSE Cell Proliferation Kit, Molecular Probes) per manufacturer’s instructions. Labeled splenocytes were cocultured with mock- or RCMV-infected BMDCs (72hpi) at a 1:1 ratio for five days and then stained for CD4. T cell proliferation was measured as a function of CFSE dilution in the CD4+ cell population by flow cytometry.
Metabolic Labeling, Proteasome Inhibition
BMDCs were gently scraped from the plates at 24hpi, washed twice in DPBS and incubated for 1hr at 37°C in starvation media (Hepes buffered RPMI without cysteine or methionine). Following starvation, cells were pulsed with 35S-methionine/cysteine (Perkin Elmer, Easy Tag 35S protein labeling mix) at 0.2mCi/ml for 20 min. After the pulse, cells were diluted in chase media containing an excess of cold cysteine and methionine and then washed two times with ice cold PBS. Cells were then chased for the indicated times at 37°C. Samples treated with inhibitor were incubated with either 100nM epoxomicin (Peptides International, IEP-4381-V) or 50μM lactacystin (Sigma, L6785) throughout the starvation, pulse, and chase periods.
Immunoprecipitation
Cell pellets were lysed on ice in lysis buffer (0.5% NP-40, 50mM Tris HCl pH 7.4, 5mM MgCl2, 1mM PMSF, 150mM NaCl plus protease inhibitors). Lysates were preabsorbed with Protein A/G agarose beads preincubated with 2μg IgG isotype control Ab for 30 min at 4°C. After the 30 min incubation the bead/lysate mixture was spun at 10k RPM for 5 min and the cleared supernatant transferred to fresh Eppendorf tube containing 2μg of the indicated antibody and rotated for 1hr at 4°C, followed by a 2hr incubation with Protein A/G beads. Beads were washed four times with PBS, pelleted and resuspended in Laemelli’s buffer and analyzed by SDS-PAGE and visualized by autoradiography.
Quantitative RT-PCR detection of MHC I and MHC II
Total RNA was prepared from uninfected and infected BMDC using the Trizol method (Gibco BRL)at 12, 24 and 36 hpi. cDNA was generated using Superscript III RT (Invitrogen) and analyzed by real-time PCR techniques using primer sets recognizing MHC I RT1A (fwd: ACAGATCACCCGGAACAAGTG, rev: ATCTGCGGAGCGACTCCAC), MHC II RT1B (fwd: ACAACCTGCTGGTCTGCTCA, rev: TCCCCGTTCCTAATAAGCTGTG), and L32 (fwd: GAAGATTCAAGGGCCAGATCC, rev: GTGGACCAGAAACTTCCGGA). Primers sets were identified using Primer Express software (Applied Biosystems). RT-PCR reactions were performed using the SYBR Green PCR Master Mix (Applied Biosystems). Following thermal activation of AmpliTaq Gold (10 min. at 95°C), a total of 40 cycles were performed (15 sec. at 95°C and 1 min. at 58°C) using the ABI Prism 7700 Sequence Detection System (Applied Biosystems). Plasmid clones containing each gene fragment were used as positive controls and quantification standards. PCR results were analyzed using ABI Prism 7700 Sequence Detection Software. The sensitivity of detection of this assay was <100 plasmid copies for the tested genes. The ribosomal subunit, L32, was used to normalize the expression data. Quantitative PCR data were analyzed by ANOVA and student’s t-test.
RESULTS
RCMV infection of rat bone marrow derived dendritic cells
Dendritic cells and macrophages play an important role in immune surveillance and CMV dissemination throughout the infected host, as well as development and maintenance of latency and reactivation. Previous reports utilizing peripheral blood derived DC and DC/APC cell lines have demonstrated a limited susceptibility of in vitro cultured murine and human DC to MCMV and HCMV infections, respectively. Currently there are no published reports demonstrating rat CMV (RCMV) infection of rat DCs. Therefore, we developed an in vitro model using RCMV infected BMDCs to examine the effects of RCMV on DC immunobiology. In order to derive dendritic cell cultures, bone marrow cells were cultured for 9 days in RPMI containing GM-CSF (10ng/ml) and IL4 (5ng/ml) (Muthana et al., 2004). At 9 days, the nonadherent cells were removed by washing, leaving only those cells that were tightly adherent. The adherent BMDCs were fed fresh media without cytokine and incubated for 24 hours (hrs) in the presence or absence of LPS.
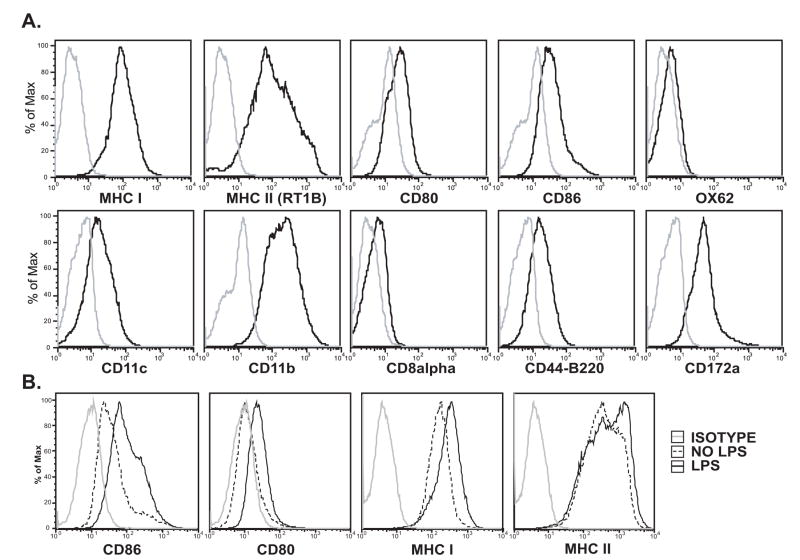
BMDCs were analyzed by flow cytometry for cell surface markers, including CD8α, CD11b, CD11c, CD45R-B220, CD86 (B7.2), CD80 (B7.1), CD172a, OX42, OX62, and major histocompatibility marker complexes, MHC I (RT1A), and MHC II (RT1B). BMC cultured under the conditions described above express classical dendritic cell markers, including inherently high levels of MHC I and II (Figure 1A). BMDC cultured in the absence of LPS were defined as immature (iDC) due to their low levels of costimulatory molecules, CD80 and CD86. BMC incubated in the presence of LPS for 24 hrs had increased cell surface expression of CD86, CD80, MHC I and MHC II relative to mock treated BMC (Figure 1B), which is a characteristic of DC maturation (Muthana et al., 2004).
Figure 1. Rat Bone Marrow Derived DC Cell Surface Marker Expression.
Immature DC cultures were generated from total bone marrow cells isolated from the femurs of adult male Lewis rats and cultured for 9 days in complete media plus GM-CSF and IL-4. Nonadherent cells were removed by washing, and the remaining adherent DCs were incubated for 24 hours in fresh media without (A) and with (B) LPS.
A) Cells were analyzed by flow cytometry for cell surface expression of CD8α, CD11b, CD11c, CD45R-B220, CD86 (B7.2), CD80 (B7.1), CD172a, OX42, OX62, and major histocompatibility marker complexes, MHC I (RT1A), and MHC II (RT1B). Isotype control (grey).
B) Cells were analyzed at 24 hours post treatment by flow cytometry for cell surface expression of CD86, CD80, MHC I and II (RT1B). Isotype control (grey), LPS treated cells (solid black), Mock treated-no LPS (dashed).
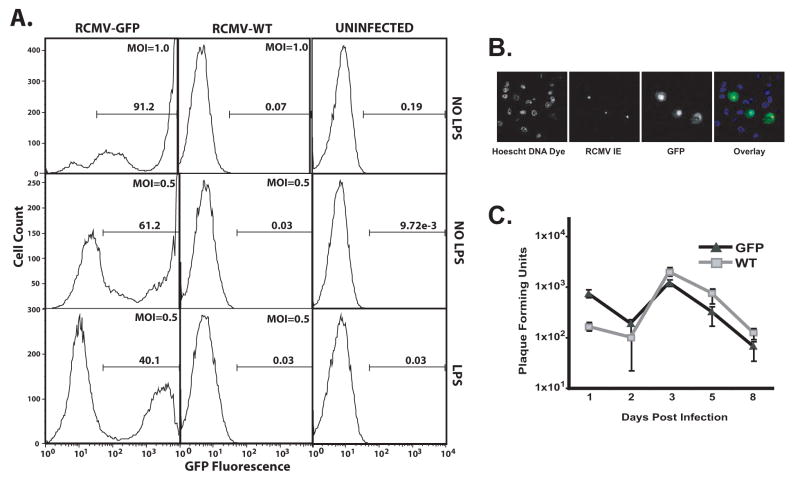
BMDCs were infected with either wild type RCMV (WT) or a recombinant RCMV expressing GFP under an eF1α promoter in order to mark the infected DC and compare the direct and indirect effects of viral infection in infected versus uninfected cells from the same culture. Immature and mature (LPS treated) BMDC were mock-infected or infected with RCMV-WT or RCMV-GFP at a multiplicity of infection (MOI) equal to 0.5 or 1.0. Infection was assessed by flow cytometry for GFP expression 48hrs post infection (hpi) (Figure 2A). Uninfected cells (mock) and those cells infected with a non-GFP expressing RCMV (RCMV-WT) served as negative controls for autofluorescence. We found that rat BMDCs are highly susceptible to RCMV infection. Greater than 90% of iDC were GFP positive when infected at an MOI of 1.0 with RCMV-GFP. Figure 2B demonstrates that all of the GFP positive cells also stained positive by immunofluorescence for RCMV-Immediate Early proteins. In general, the infected cells displayed high levels (mean fluorescent intensities; MFI) of GFP expression, however there were cells that displayed low but detectable levels of GFP. Approximately 61% of iDC infected at an MOI of 0.5 were GFP positive at 48hpi. Infection of mDC at an MOI of 0.5 yielded a lower infectivity rate compared to iDC (40% GFP+). The reduction in the total number of GFP positive cells coupled with the 44% reduction in MFI in infected mDC cultures compared to iDC cultured in parallel suggests a reduced susceptibility of mDC to RCMV infection relative to iDC. In the remainder of this study we further examined RCMV infection of iDC.
Figure 2. Rat BMDCs Are Highly Susceptible To RCMV Infection.
A) BMDCs were infected 24h post LPS treatment (mDC) or mock (iDC) at an MOI=1.0 (top panel) or 0.5 (middle and bottom panels) for 48h and then assessed by flow cytometry for GFP expression. Gate numbers represent the percent of the population positive for GFP.
B) BMDC were infected with RCMV-GFP at an MOI=0.1. At 48 hours post-infection, the cells were fixed with 1% paraformaldehyde, permeabilized and stained with anti-RCMV IE antibody (red) and with Hoechst (blue), GFP (green). Mag=60x.
C) Growth kinetics of RCMV-WT (squares) and RCMV-GFP (triangles) in BMDCs. At the indicated time points after infection (days post-infection) the presence of virus (combined intracellular and extracellular) in the cultures was determined by standard plaque assays on rat RFL6 fibroblasts. Viral titers are expressed as plaque forming units per ml and represent the average of three replicates.
To determine whether RCMV productively infects BMDCs, we analyzed growth of the virus in these cells. For this experiment, DCs were infected with RCMV-WT or RCMV-GFP at an MOI equal to 1 and harvested at 1, 2, 3, 5, and 8 dpi. The samples were stored at −80°C until analysis. Titration of the combined cellular and supernatant fractions was performed by standard plaque assays. Viral replication was minimal in these cells, which mimics the growth of HCMV in DCs. The amount of infectious virus from the infected DCs was similar for both the RCMV-WT and RCMV-GFP viruses and the levels peaked at 3 dpi (Figure 2C).
RCMV reduces expression of DC surface markers critical to antigen presentation
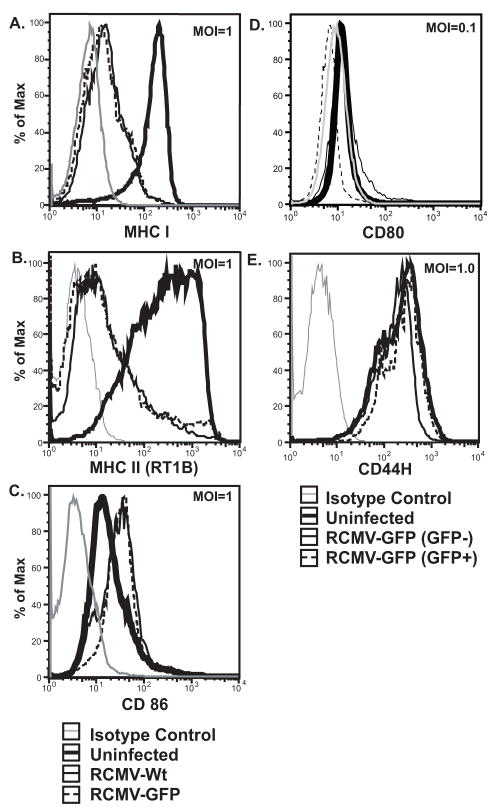
DC induced activation of T cells depends upon antigen presentation in the context of both MHC and costimulatory molecules. To investigate the effects of RCMV infection on antigen presentation and DC activation, iDCs were infected at an MOI of 1.0 or 0.1 with either RCMV-WT or RCMV-GFP. As shown in Figure 3, cell surface expression of the costimulatory molecules CD80 and CD86 as well as MHC I and II was analyzed at 48hpi by flow cytometry. Infection of iDCs resulted in a 94% and 89% drop in both MHC II and I (respectively) MFIs relative to uninfected cells (Figure 3A&B). DC infected with RCMV-WT and -GFP at an MOI equal to 1.0 displayed equivalent levels of MHC on the cell surface, suggesting that the deletion of ORFs r145-147 or the expression of eGFP does not affect RCMV-mediated down regulation of MHC. CD44H, hyaluronic acid receptor, expression was analyzed as a control for a possible viral induced global down regulation of host cell surface markers (Figure 3E). The levels of CD44H were unchanged in infected versus uninfected cells. This finding suggests that the RCMV-induced reduction in MHC expression is a specific event and not the result of a global down regulation of cell surface molecules.
Figure 3. RCMV Down Regulates MHC I And II Cell Surface Expression.
A–C. iDCs were infected at an MOI equal to 1.0 with either RCMV Wt (thin black line), RCMV GFP (dashed black line) or mock (thick black line) for 48hrs and surface stained for MHC I (A), MHC II (RT1B) (B) or CD86 (C). Isotype controls were depicted with a thin grey line. In panels D and E, iDCs infected with RCMV-GFP at an MOI=0.1 were stained for surface expression of CD80 (D), or CD44H (E). Infected cultures were gated for GFP expression [GFP positive cells (thin dashed line), GFP negative cells (thin black line), mock treated cells (thick black line), and isotype controls (thin grey line)].
Expression levels of the costimulatory molecules, CD80 and CD86, were also examined as possible RCMV targets of the host cell antigen presentation pathway. While uninfected iDCs maintained low level CD86 expression, cells infected at an MOI=1.0 with either RCMV-WT or -GFP up regulated surface expression of CD86 (Figure 3C). Cell surface expression of CD80 was examined in the context of RCMV-GFP infection (MOI=0.1); iDCs from the infected culture were gated on GFP expression. CD80 expression in infected (GFP positive) cells was reduced to isotype control levels, while uninfected (GFP negative) cells had slightly elevated levels of the costimulatory molecule compared to cells from uninfected cultures (Figure 3D). The dramatic reduction in cell surface expression of MHC I, II and the costimulatory molecule CD80 to isotype control levels in RCMV infected BMDC suggests a specific targeting of the host cell antigen presentation pathway. The increased expression of CD86 and static level of CD44H in infected BMDC further suggests that the loss of MHC and CD80 is not the result of a universal down regulation of cell surface marker expression, but rather a specific viral strategy aimed at subversion of the host cell adaptive immune response.
RCMV infection of iDC blocks CD4 T cell proliferation in an allogeneic mixed leukocyte reaction
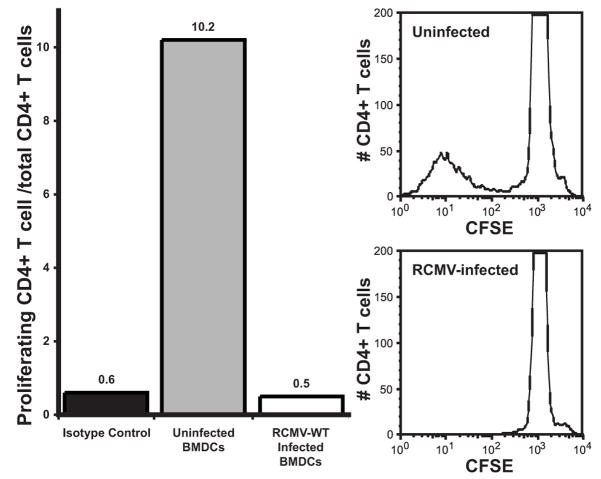
To determine the impact of RCMV-mediated down regulation of cell surface MHC II on DC function as antigen presenting cells, mixed leukocyte reactions were performed on infected versus uninfected iDCs. CD4 T cell proliferation (as monitored by CFSE dilution) was used as a measure of CD4 T cell activation. BMDC were generated, as described above, from Lewis strain rats. These cells were mock infected or infected with RCMV-WT for 72h at MOI=1.0, and then incubated with CFSE labeled splenocytes isolated from the allogeneic rat strain ACI. BMDC and splenocytes were cocultured for 5 days before analysis by flow cytometry. Nonadherent cells were taken from the culture and stained for CD4. T cell proliferation was measured as a loss of CFSE intensity. Data is represented as the percent of total CD4+ cells that proliferated. A greater than 20-fold reduction in T-cell proliferation was observed in the allogeneic T-cells added to the infected iDC cultures compared to the uninfected cells (Figure 4). This finding suggests that iDCs infected with RCMV are functionally impaired and lack the ability to induce T-cell proliferation.
Figure 4. RCMV Infected BMDC Are Unable To Induce Allogeneic CD4 T Cell Proliferation In A Mixed Leukocyte Reaction.
BMDC isolated from Lewis strain rats were infected with RCMV-Wt for 72h, and cocultured with CFSE labeled splenocytes taken from ACI strain rats for 5 days. Non-adherent cells were stained with anti-CD4 Ab and analyzed by FACS. CD4+ T cell proliferation was measured by CFSE dilution. T cell proliferation is represented as the percent of total CD4+ T cells that have divided. Isotype control (black fill), Uninfected (dark grey fill), RCMV infected (white fill). Shown in the right panels are histograms demonstrating CFSE fluorescence in CD4+ T cells mixed with infected or uninfected DCs.
RCMV depletes both cell surface expression and intracellular MHC II in DCs
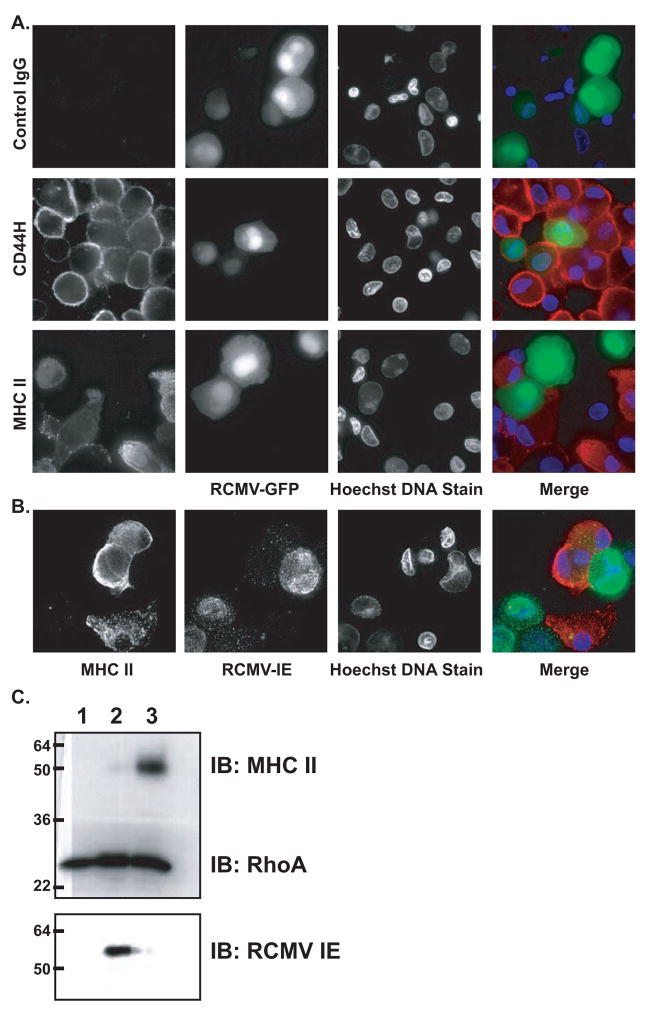
To determine whether the RCMV-induced down regulation of MHC II surface levels was due retention of these molecules in intracellular compartments, we assessed the total levels of MHC II in infected iDCs by immunofluorescence staining and western blotting. BMDC infected with RCMV-GFP at an MOI=1.0 were fixed and permeabilized at 48 hpi. Cells were stained for MHC II and visualized by epifluorescence using a deconvolution microscope. The infected, GFP positive, iDC had undetectable levels of MHC II staining, while GFP negative cells in the same field of view showed both a diffuse cytoplasmic and concentrated plasma membrane staining for MHC II (Figure 5A). The GFP positive cells stained similarly for CD44H compared to uninfected cells. Immunofluorescence images shown in Figure 5B of iDC infected with RCMV-WT further confirm the RCMV-GFP IF staining. BMDC were infected at an MOI=0.1. Infected cells were stained for IE (green) and MHC II (red). The absence of MHC II in IE positive cells is in agreement with the reduction of detectable MHC II in RCMV-GFP positive cells. The mutually exclusive staining patterns of MHC II and RCMV IE/GFP is consistent with the flow cytometry data in which cell surface levels of MHC II were nearly undetectable in infected iDC. Similarly, DCs infected with RCMV were completely devoid of MHC II as determined by western blot analysis (Figure 5C).
Figure 5. Intracellular MHC II Is Undetectable In RCMV Infected BMDC.
A. BMDCs were infected with RCMV-GFP. Cells were fixed, permeabilized and stained 48hpi with either an IgG isotype control antibody or antibodies directed against CD44H or MHC II (red). Deconvolution microscopy was used to visualize the stained cells. Mag=60x.
B. BMDCs were infected with RCMV-Wt at an MOI=0.5. Cells were permeabilized and stained 48hpi with Hoechst (blue), MHC II (green) and anti-RCMV IE antibody (red). Deconvolution microscopy was used to visualize the stained cells. Mag=60x.
C. BMDCs were infected with RCMV-WT at an MOI=1 and the cells were harvested in Laemmli’s sample buffer at 48 hours post-infection (lane 2). Uninfected rat RFL6 cells (lane 1) and uninfected BMDCs (lane 3) served as controls. Samples were analyzed by Western blot analysis for the presence of MHC II, RCMV-IE, or RhoA.
RCMV-mediated MHC down regulation occurs at a post-transcriptional step
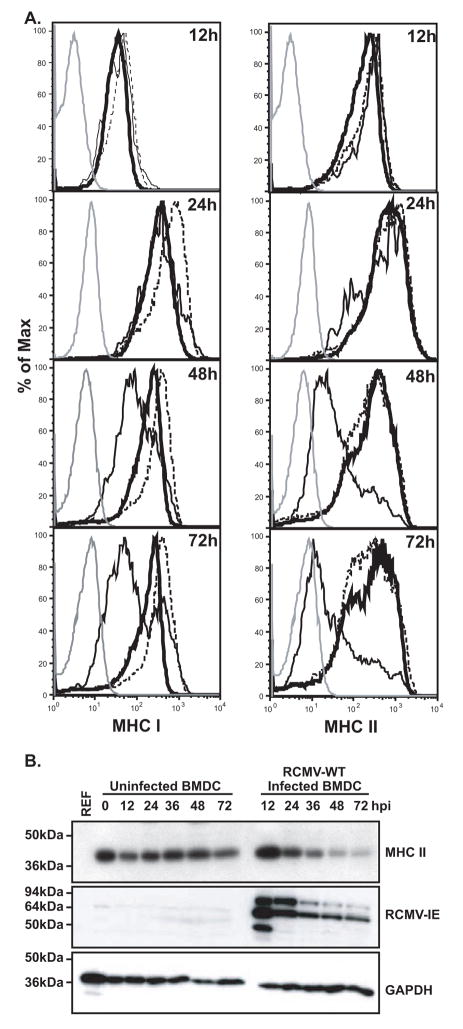
To determine the kinetics of the RCMV-mediated down regulation of MHC I and II, a time course analysis of MHC staining following virus infection was undertaken. For this experiment, in order to identify infected vs. uninfected cells within the same culture, RCMV-GFP was used to infect iDC at an MOI=0.1. Cells were analyzed for surface expression levels of MHC I and II at 12, 24, 48, and 72 hpi, and gated on GFP expression to examine direct effects of RCMV infection and GFP negative cells to study indirect effects. The virus-mediated MHC II down regulation becomes detectable between 12 and 24hpi (Figure 6A). However, the most striking drop in MHC II cell surface expression occurs at 24hpi (83% drop in MFI of infected versus uninfected cells), which corresponds to the timing of early viral gene expression. By 72hpi cell surface levels approach isotype control levels indicating that RCMV infection stably removes MHC II to background levels. The kinetics of MHC I down regulation is delayed in comparison to MHC II. Cell surface levels drop in the infected cells between 24 and 48hpi, further declining (greater than 40% reduction) by 72hpi (Figure 6A). Importantly, MHC I and II are only down regulated in the GFP positive (infected) cells suggesting that this effect is actively mediated by virus infection and not a soluble factor found within the inoculum or secreted as a result of infection. We also analyzed total levels of MHC II by western blotting at 12, 24, 36, 48, and 72 hpi in order to determine whether the kinetics of the observed degradation of total MHC II corresponds to the decrease in surface expression. As shown in Figure 6B, initially at 12 hpi MHC II is slightly up regulated in the infected cultures. However, MHC II degradation begins between 24 and 36 hpi occuring at the time of the reduction in surface expression. By 48 hpi the levels have dropped by 90%. This data demonstrates that the reduction in MHC II surface expression corresponds to a similar reduction in total cellular MHC II levels.
Figure 6. RCMV Induces A Stable Depletion Of Cell Surface MHC I And II.
A. BMDC were infected with RCMV-GFP at an MOI=0.1 and harvested at the times indicated for analysis via FACS. Uninfected cells were harvested in parallel at the indicated times. Isotype control (grey line), Uninfected cells (thick black line), RCMV infected, GFP positive cells (thin black line), BMDC from the same infection culture but uninfected, GFP negative (dashed black line).
B. Western blot analysis of MHC II depletion in RCMV-infected BMDC. BMDC were infected with RCMV-WT at an MOI=1 and the cells were harvested in Laemmli’s sample buffer at the indicated time points after infection. Uninfected rat RFL6 cells and uninfected BMDCs served as controls. Blots were stained for MHC II, RCMV-IE or GAPDH.
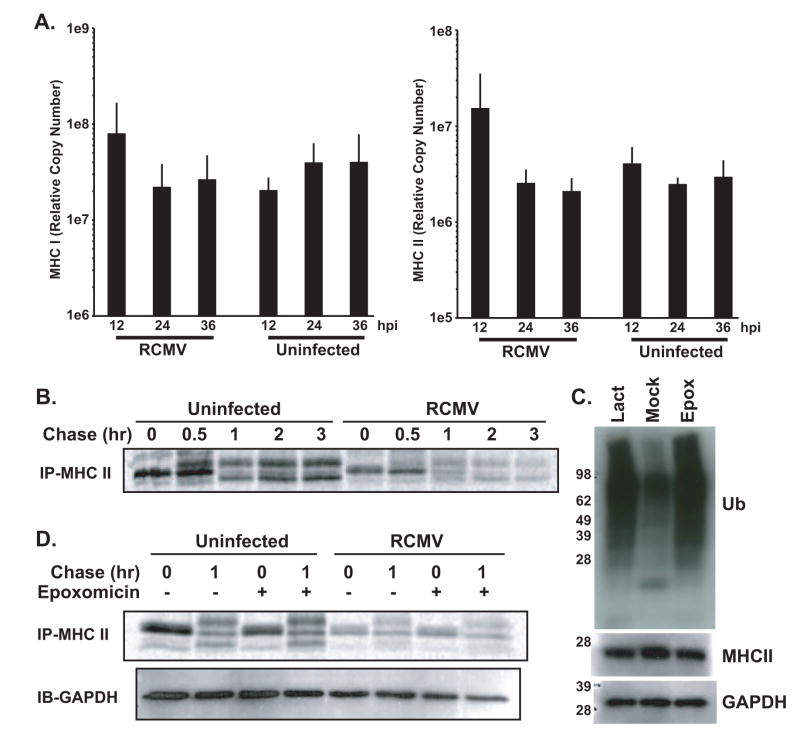
To elucidate the mechanisms of RCMV-mediated down regulation of MHC, we determined the effects of RCMV infection on MHC transcription and translation. To determine whether RCMV infection affects transcription of MHC in BMDC, we used quantitative RT-PCR TaqMan to compare the mRNA levels of MHC I and II at 12, 24, and 36 hpi. The expression of both MHC I and II was slightly elevated at 12 hpi. However, by 24 hpi the levels of gene expression returned to those levels observed in uninfected cells (Figure 7A). This finding indicates that RMCV infection does not affect the transcription of MHC. Therefore, since a massive depletion of MHC II occurs in the infected cells beginning at 24–36 hpi, we next explored whether RCMV modulated translation of MHC II. For this assay, BMDC were infected with RCMV for 24 hrs and then metabolically labeled in a pulse-chase experiment and the cellular lysates were immunoprecipitated with antibodies directed against MHC II and analyzed by SDS-PAGE. The pre-glycosylated form of MHC II was present in the pulse samples for both the uninfected and infected cell lysates (Figure 7B). However, there was a reduction in the accumulation of labeled MHC II in the RCMV infected BMDCs. This was accompanied by a similar reduced level of MHC II in the chase of the infected cells. In order to establish whether this down regulation was mediated by the proteasome, epoxomicin was used to treat the cells prior to and during the pulse-chase. Western blots to measure the increase in poly-ubiquinated species upon proteasome inhibition was used to confirm the effective concentration of lactacystin and epoxomicin in the rat BMDCs (Figure 7C). Epoxomicin treatment did not affect MHC II protein levels observed in both the pulse and chase samples from the uninfected or infected cells (Figure 7D). This suggests that while there is a post-transcriptional reduction in MHC II it is not mediated by proteasomal degradation.
Figure 7. RCMV Infection Does Not Alter Gene Expression Of MHC I And II In Rat BMDCs.
A. RT-PCR TaqMan was used to detect expression of MHC I and MHC II in uninfected and RCMV-infected rat BMDC at 12, 24, and 36 hpi (n=3). RT-PCR TaqMan detection mRNA from the gene for the ribosomal protein L32 was used to normalize the expression data (n=4).
B. Pulse chase analysis was performed in mock- and RCMV-infected BMDC at 24hpi. Cells were starved and then labeled with 35S-methionine/cysteine at 0.2mCi/ml for 10 min. After the pulse, cells were diluted in chase media containing an excess of cold cysteine and methionine and then washed. Cells were then chased for 0.5, 1, 2 or 3 hrs at 37°C and then harvested by lysing the cell pellets in lysis buffer. MHC II was immunoprecipitated using the indicated antibody for 1hr at 4°C, followed by a 2hr incubation with Protein A/G beads. Beads were washed and resuspended in sample buffer and analyzed by SDS-PAGE and visualized by autoradiography.
C. Western blot analysis of Ub in BMDC treated with epoxomicin (100nM) or lactacystin (50μM) for 36hrs. Cells were harvested in sample buffer at 36hrs post treatment. Untreated BMDCs (mock) served as controls. Blots were stained for Ubiquitinated proteins, MHC II RT1B and GAPDH.
D. Pulse chase analysis was performed in mock- and RCMV-infected BMDC cultures treated with or without the proteosomal inhibitor epoxomicin (100nM). Cells were starved and then labeled with 35S-methionine/cysteine at 0.2mCi/ml for 10min. After the pulse, cells were diluted in chase media containing an excess of cold cysteine and methionine and then washed. Cells were then chased for 1hr at 37°C and then harvested by lysing the cell pellets in lysis buffer. MHC II was immunoprecipitated using the indicated antibody for 1hr at 4°C, followed by a 2hr incubation with Protein A/G beads. Beads were washed and resuspended in sample buffer and analyzed by SDS-PAGE and visualized by autoradiography. A fraction of the cellular lysate for each sample was also probed by western blotting for GAPDH to ensure equal loading.
RCMV Degradation Of MHC II Is Inhibited By Increasing Endosome/Lysosomal pH
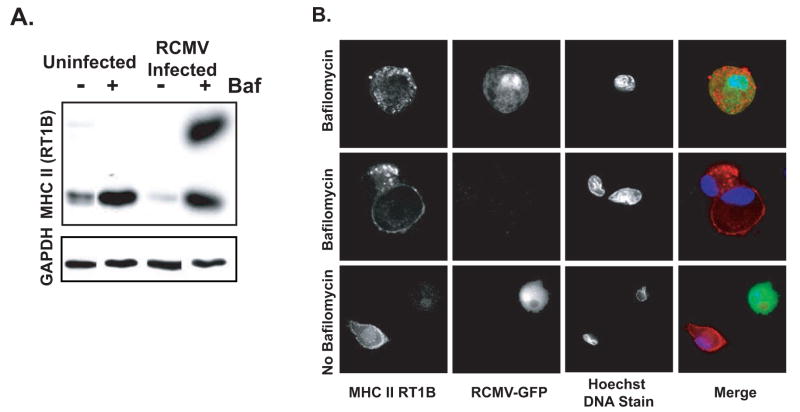
While the post-transcriptional effect on MHC II synthesis in RCMV infected cells at 36 hpi suggests a mechanism for the sustained reduction, this mechanism would not be sufficient for the rapid degradation of MHC II occurring by 24 hpi. We reasoned that one possible mechanism for this massive depletion of cell surface MHC II is through increased recycling and degradation in the infected cells. To test this hypothesis, we performed western blot analysis for MHC II in cellular lysates from infected and uninfected BMDCs treated at 12hpi with bafilomycin for an additional 36hpi. Bafilomycin inhibits the proton pump associated with endosomes and lysosomes ultimately inhibiting the pH sensitive proteinases found in these vesicles. Bafilomycin inhibited the degradation of MHC II observed in the infected cells (Figure 8A). Bafilomycin also increased accumulation of MHC II in uninfected cells. Interestingly, a SDS-stable form of MHC II was only detected in the bafilomycin treated RCMV infected BMDCs. To confirm the effect of bafilomycin on preventing RCMV-mediated MHC II degradation and to visualize the cellular location of MHC II accumulation, we visualized immunofluorescence stained uninfected and RCMV-infected BMDCs with and without bafilomycin treatment by deconvolution microscopy. For this assay, BMDCs were infected with an MOI=0.5 to ensure that at least 50% of the cells were infected. As demonstrated above, MHC II was present in the uninfected cells but not in the GFP+ infected BMDCs in the same culture (Figure 8-lower panel). However, MHC II was observed in the infected cells when treated for 24 hrs with bafilomycin. In these cells MHC II was predominantly in large vesicles suggesting that RCMV promotes the recycling of MHC II, which is then degraded in an endocytic or lysosomal compartment.
Figure 8. RCMV Down Regulation Of MHC II Is Sensitive To Treatment With Bafilomycin.
A. Western blot analysis of MHC II depletion in RCMV-infected BMDC treated with bafilomicin (100nM). BMDC were infected with RCMV-WT at an MOI=1 for 12 hours, treated with drug for an additional 36hrs and then harvested in sample buffer at 48hpi. Uninfected BMDCs served as controls. Blots were stained for MHC II RT1B and then the blot was stripped and probed for GAPDH.
B. BMDCs were infected with RCMV-GFP for 12hrs and then treated with bafilomicin (100nM) for an additional 36 hrs. Cells were fixed at 48hpi, permeabilized and stained with antibodies directed against MHC II RT1B (red) and Hoechst DNA dye (blue). Infection was detected by GFP expression (green). Deconvolution microscopy was used to visualize the stained cells. Mag=60x.
RCMV down regulation of MHC I & II requires IE/E gene expression
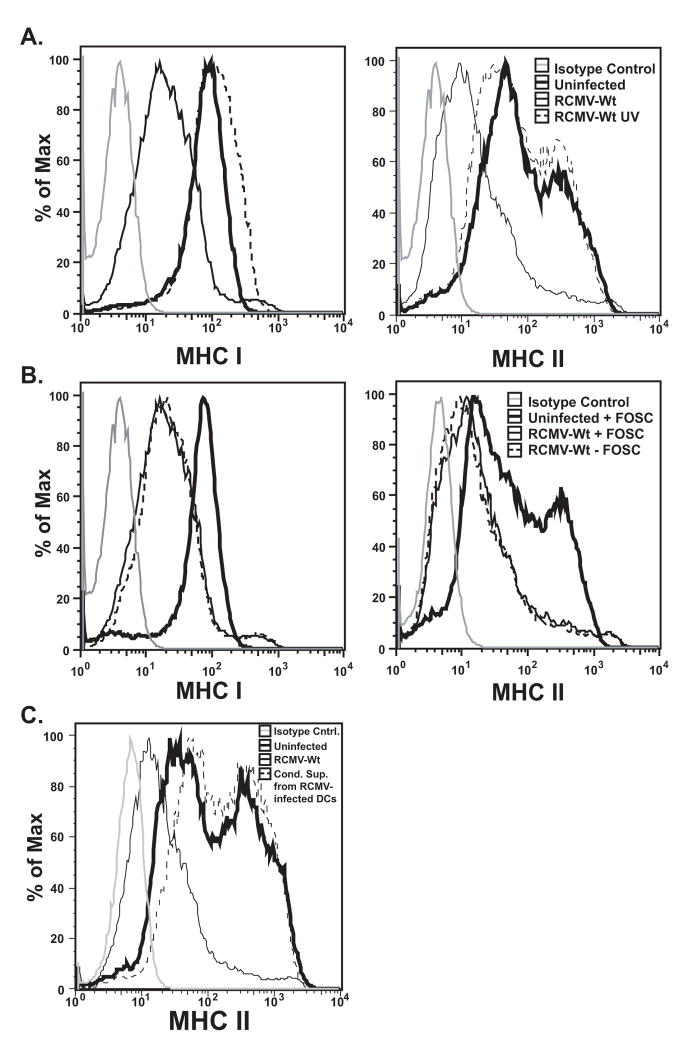
While we show above that RCMV mediated MHC down-modulation only occurs in the infected cell, this process could be mediated by a number of factors including a cellular response to RCMV binding and entry, a component of the virion itself used in a post-entry step, or a protein encoded in the viral genome requiring viral replication for expression. To determine whether virus replication was necessary for MHC down regulation, BMDC were infected at an MOI=1.0 with either RCMV-WT or UV inactivated RCMV-WT from the same viral preparation. Infected and mock treated cells were examined 48hpi by flow cytometry for the presence of MHC I and II (Figure 9A). MHC I cell surface expression was slightly increased (MFI equal to 103 versus uninfected MFI equal to 71.1) in cells infected with UV inactivated virus, while MHC II levels were relatively unchanged (MFI=70.1) compared to uninfected cells (MFI=79.8). These data demonstrate that neither a component of the viral inoculum, a protein component of the virion itself, nor viral binding/entry mediate the effect observed on MHC down regulation induced by RCMV infection.
Figure 9. RCMV Induced Reduction In MHC I and II Expression Is Not Mediated By Viral Binding and Entry, Late Gene Expression Or By A Secreted Factor.
A. BMDC were infected at an MOI=1 with RCMV-Wt (thin black line) or Wt-UV inactivated (dashed black line) or mock infected (thick black line) for 48h and analyzed by FACS for cell surface expression of MHC I and II. Isotype control (grey).
B. BMDC were pretreated with Foscarnet or mock, and then infected at an MOI equal to one with RCMV-Wt. Isotype control (grey), Uninfected + Foscarnet (thick black line), Infected + Foscavir (thin black line), Infected − Foscarnet (dashed black line).
C. BMDC were infected with RCMV-Wt (thin black line) or mock (thick black line), or conditioned supernatants from RCMV infected BMDCs (dashed black line), for 48hrs and then assessed for cell surface expression of MHC II. Isotype control (grey line), RCMV-Wt infected (thin black line), uninfected (thick black line), conditioned supernatant treatment (dashed black line).
Next, we determined whether late viral gene expression was required to induce MHC down regulation. RCMV infected iDCs were treated with Foscarnet, an inhibitor of RCMV DNA synthesis, which blocks virus late gene expression and viral replication. MHC I and II cell surface levels were reduced regardless of Foscarnet treatment in the infected cells and were unaffected in mock-infected cells (Figure 9B). Although basal levels of MHC II (on uninfected iDC) were reduced in cells treated with Foscarnet, versus uninfected untreated iDC, infection in the presence or absence of the inhibitor continued to reduce the level of cell surface MHC II (by greater than 65%). The continued loss of MHC expression in the presence of Foscarnet suggests that RCMV-induced MHC depletion is not mediated by late viral gene transcription. Together, these results support the hypothesis that the RCMV-induced depletion of MHC I and II is mediated by a viral immediate early or early gene product.
Soluble factors do not mediate RCMV induced MHC down regulation
Previous studies investigating HCMV and MCMV induced MHC II manipulation have implicated both a viral homologue of IL10 (UL111a) and the induction of cellular IL10, respectively (Chang et al., 2004; Redpath et al., 1999; Spencer et al., 2002). Since the RCMV genome lacks an UL111a homologue, a possible role for cellular IL10 or other secreted factors in the modulation of MHC II surface expression was explored. For this experiment, supernatants collected from RCMV-WT infected iDC were clarified by ultracentrifugation and these cell- and virus-free supernatants were incubated with uninfected iDC for 48hrs. Surface levels of MHC II were slightly elevated in cells treated with supernatants from infected cells compared to those cells treated with supernatants from uninfected DC (Figure 9C). One could argue that a soluble factor released by an infected cell may have a limited stability in culture. If this were true, one would predict that the uninfected cells within the same infected DC culture would also down regulate MHC II from the cell surface. However, that is not the case since uninfected cells from low MOI infected cell cultures, wherein not all of the cells are infected, do not down regulate MHC molecules from their surface (shown in Figures 3E&6A). As an added control for whether virus-derived or -induced cellular secreted IL10 mediated the effect on MHC down regulation, infected and uninfected cells were incubated with saturating concentrations of a neutralizing antibody to rat IL10. Both infected and uninfected cells treated with anti-IL10 neutralizing antibody failed to elicit any significant changes in their MHC levels compared to antibody-untreated cultures. These findings in combination with our low MOI experiments presented in Figures 3E&6A argue against a soluble factor causing the depletion of MHC molecules from RCMV-infected DC.
DISCUSSION
In the current report an RCMV/DC infection system was utilized to explore the effects of CMV infection on DC function. We demonstrate for the first time that rat BMDC are highly susceptible to RCMV and that infection significantly down regulates surface levels of CD80, MHC I and MHC II, while other cell surface markers such as CD44H are not down regulated suggesting that the effect is specific for a subset of cell surface markers. A greater than 90% reduction of MHC II from both the cell surface and intracellular pools in RCMV infected rat DCs was observed. The significance of the loss of MHC and the costimulatory molecule, CD80, was underscored when a functional impairment in DC mediated antigen presentation was confirmed in a mixed leukocyte reaction. RCMV does not affect MHC transcription but may have a small effect on translation at a post-transcriptional step. The RCMV-mediated degradation of MHC II was inhibited by the ATPase/proton pump inhibitor bafilomycin, suggesting that the virus promotes destruction of MHC II in endosomes/lysosomes by cellular or viral pH-dependent proteases. Unlike MCMV, the RCMV-mediated reduction of MHC was not caused by secreted factors since supernatants from infected cells failed to induce a reduction in MHC surface expression. Furthermore, when cells infected at low MOI (equal to 0.1) with RCMV-GFP were gated for GFP expression, only the GFP positive cells were depleted of MHC II. In the same infected DC culture the uninfected GFP negative cells had high levels of MHC II. Viral replication (and not a viral structural protein) was required for the down regulation of MHC I and II as DCs infected with UV-inactivated RCMV displayed MHC levels that paralleled uninfected cells. The effect on MHC I and II surface expression was mediated by an immediate early or early gene, other than R145-147, since both the treatment with Foscarnet (inhibits late viral gene expression) and infection with the RCMV-GFP(ΔR145-147) virus failed to block the down regulation. The high susceptibility of the DC to infection coupled with the stable and near complete loss of MHC all highlight the RCMV-BMDC infection model as an extremely useful tool to study the role of CMV infection in the down regulation of MHC II.
MHC I down regulation is likely the most widely used means of viral immune evasion occurring in both RNA and DNA viruses. Down regulation of MHC I in CMV infected cells eliminates CTL recognition of infected fibroblasts in vitro (Pinto et al., 2006). Although a dramatic enhancement in viral fitness in vivo remains to be linked directly to MHC I depletion, blockade of CD8+ T cell surveillance is likely to assist in viral persistence or reactivation from latency in a particular cell type, as seen during HSV reactivation from latency (Liu et al., 2000). In this report we demonstrate that RCMV dramatically reduces MHC I surface expression to near background levels. A previous study by Hassink et. al. demonstrated that RCMV down regulates MHC I expression on the surface of RCMV infected fibroblasts (Hassink et al., 2005). However, the effects of RCMV infection on MHC I expression as reported by Hassink et. al. were modest and transient, occurring only at 12 hpi. Here we demonstrate that RCMV infection causes a massive down regulation of MHC I surface expression in infected dendritic cells. While this is not the first report to demonstrate that RCMV infection affects MHC I surface levels, the data outlined in this study are consistent with the effects observed in HCMV, RhCMV, and MCMV infected cells. It is unclear why such a dramatic surface down regulation of MHC I occurs in RCMV-infected DCs but not in fibroblasts, however the distinct effects may reflect the differences in the cell types used for these assays.
While viral interference with the MHC I antigen presentation pathway has long been recognized as an adaptive strategy to evade CD8+ T cell recognition, the advantage of viral subversion of the MHC II pathway has only recently been the subject of investigation. MHC II is typically associated with the exclusive presentation of extracellular antigen, and presentation of viral antigen is more commonly associated with intracellular MHC I presentation, however, manipulation of MHC II could provide at least two advantages to a long-term persistent virus such as CMV. First, blockade of MHC II would inhibit APC triggering of the adaptive arm of the immune system in response to phagocytosed infected cells (extracellular antigen). Second, it has been demonstrated that intracellular CMV antigen may be presented in the context of MHC II during an active infection of class II expressing cells (Hegde et al., 2005). The build up of viral glycoproteins within the trans golgi network or acidic compartments in preparation for viral assembly may lead to a leakage of endogenous viral antigen into the MHC II compartment. CMV induced MHC II blockade is likely to be advantageous to establishing a persistent infection in both its ability to evade detection during a primary infection of an APC and the ability to suppress the CD4+ T cell responses including the downstream production of antibodies.
Interestingly, RCMV differentially affected expression of the co-stimulatory molecules, CD80 and CD86. RCMV down regulated surface expression of CD80 while CD86 surface expression was elevated in the infected cells. MCMV and HCMV infection of macrophages or DCs causes the down-regulation of both of these molecules (Loewendorf et al., 2004; Mintern et al., 2006; Moutaftsi et al., 2002). Although ligation of CD28 on T cells by either CD80 or CD86 can provide the needed “second stimulus” required to activate naïve T cells, recent reports support a functional duality in CD80 versus CD86 ligation. A role for CD80 in the development of a Th1 response has been identified, particularly enhancing CTL effector function, whereas CD86 ligation stimulates naïve T cells to differentiate into Th2 cells (enhancing among other things antibody and IL10 production) (Kuchroo et al., 1995; Lang et al., 2002). Differential regulation of CD80/86 expression in RCMV infected APCs, may further shift the balance in favor of viral persistence by inducing a tolerizing state in the host immune response.
One of the most striking features of the RCMV-BMDC model system is the degree of MHC II depletion in infected dendritic cells. At 72 hpi, RCMV infected BMDC have a greater than 84% reduction in MHC II cell surface MFI compared to uninfected BMDC. Furthermore, intracellular MHC II was below the limit of detection by immunostaining in cells infected for 48h, indicating that in addition to the lack of MHC II on the cell surface it is either rapidly destroyed in the infected DC and/or RCMV blocks the synthesis of MHC II, however, the mechanisms remain to be elucidated. Reductions in MHC II cell surface expression ranging from 15–50% have been reported for HCMV-infected human monocyte derived DC and MCMV-infected mouse DC (Andrews et al., 2001; Mathys et al., 2003; Moutaftsi et al., 2002; Raftery et al., 2001; Varani et al., 2005b). Whether the variation in the degree of MHC depletion is due to specific differences in the origin of the cells, the differentiation state of the cells, the distinction between HCMV and MCMV lab-adapted strains compared to a more wild type strain of RCMV, or simply due to an RCMV-specific phenomena, remains to be elucidated. However, CMV may have a more dramatic effect on MHC II down regulation in DC derived from bone marrow versus DC derived from peripheral blood monocytes, which underscores the importance of these cells in CMV pathogenesis. In any case, the RCMV model system provides a unique opportunity to explore the mechanisms and impact of CMV induced MHC II manipulation in the context of infection, which has previously proven problematic using the HCMV in vitro model systems. In fact, most of our knowledge about HCMV-mediated MHC II manipulation comes from studies that employed U373 cells transfected with the MHC constitutive transactivator, MIITA, to produce high levels of MHC II. Similarly, the study identifying UL83 as a modulator of MHC II utilized a system in which MHC II negative fibroblasts were induced to express the molecule upon stimulation with IFNγ (Odeberg et al., 2003). In these studies, isolated expression of UL83, US2 and/or US3 mediated the down regulation of MHC II in these induced expression systems (Hegde et al., 2002; Odeberg et al., 2003; Tomazin et al., 1999). Similarly, HCMV infection of these cells also caused a significant decrease in MHC II expression. However, the degradation of MHC II was only decreased by about one-third when the MIITA transfected U373 cells were infected with a recombinant strain of HCMV (RV7186) lacking the IRS1-US11 region of the genome, suggesting that other regions of the genome were also involved in down regulating MHC II (Cebulla et al., 2002). These findings highlight the importance of developing direct infection-based systems for the identification of the full complement of CMV genes involved in the modulation of MHC II.
A specific MCMV ORF that mediates MHC II down modulation has yet to be identified, however, both MCMV and HCMV have been shown to down regulate MHC II surface expression by inducing cellular IL10 (Nordoy et al., 2003; Redpath et al., 1999). HCMV also encodes a viral homologue of cellular IL10, cmvIL10 (UL111A), which is a structural and functional homologue of the cellular version (Chang et al., 2004; Kotenko et al., 2000; Lockridge et al., 2000; Raftery et al., 2004). Due to the inefficient infection rate of monocyte derived DC with HCMV, studies examining the effects of both cellular and viral IL10 have been performed with recombinant proteins and by transferring infected fibroblast conditioned supernatants onto DCs (Chang et al., 2004; Raftery et al., 2004). The effects of neither cellular nor cmvIL10 on MHC II expression in DCs has been explored in the context of an actual HCMV infection. In contrast to the ability of conditioned supernatants from HCMV and MCMV infected cells to transfer the effect of MHC II down regulation, we demonstrate in this report that RCMV conditioned supernatants alone do not deplete MHC II in uninfected DCs. The failure of RCMV cultured supernatants to modify MHC II was further substantiated when using the RCMV-GFP virus. In these experiments, GFP positive cells were low in MHC II expression, whereas GFP negative cells from the same infection culture expressed high levels of MHC II. Taken together, these results suggest the absence of a paracrine effect in RCMV induced MHC II down regulation. Studies investigating MHC II surface expression in macrophages shortly following HCMV infection found that infection with UV-inactivated virus depleted MHC II surface expression (Odeberg et al., 2003; Odeberg and Soderberg-Naucler, 2001). Further examination of this effect in IFN-γ stimulated fibroblasts identified UL83 (pp65), the ORF encoding a prominent virion component, as a mediator of MHC II surface depletion in fibroblasts (Odeberg et al., 2003). While RCMV encodes a UL83 homologue, we show that the effect of MHC II down regulation in rat BMDC did not occur in cells infected with UV-inactivated virus. In addition, RCMV is not known to encode sequence homologues to the other three ORFs identified as MHC II modulators in HCMV, US2 US3, and UL111a suggesting an additional mechanism by which RCMV infection of DCs blocks MHC II surface expression and facilitates the degradation of intracellular MHC II (Hegde et al., 2002; Tomazin et al., 1999).
While the MHC II presentation pathway is likely a prime target of CMV manipulation due to its importance in coordinating the adaptive antiviral response, MHC II down regulation may have additional consequences in CMV induced disease. For example, the fact that CMV remains latent in myeloid lineage cells destined to become professional antigen presenting cells suggests that the effect on MHC II could have an important role in reactivation from latency. In addition, in vascularized solid organ and bone marrow transplantation HCMV seropositivity has long been identified as a major contributing factor in graft rejection (McLaughlin et al., 2002). Our previous work showing that recipient immune response to allogeneic donor tissue is required for RCMV acceleration of transplant vascular sclerosis, the primary indicator of vascularized graft rejection, further highlights the interplay between the host immune system and CMV infection during transplant rejection (Orloff et al., 2002). Of particular interest is a recent study demonstrating that a subset of dendritic cells presenting alloantigen in the context of MHC II mediates allograft tolerance (Ochando et al., 2006). The presentation of alloantigen by a subset of DC facilitated the generation of alloantigen specific regulatory T cells, which mediate graft tolerance (Ochando et al., 2006). Human DC derived from patients undergoing active CMV infections are functionally impaired at inducing T-cell responses and express higher levels of proinflammatory cytokines (Frascaroli et al., 2006; Varani et al., 2005a). Our finding that RCMV infection of BMDC nearly eliminates MHC II from the cell surface, taken together with the work of other groups suggests a possible mechanism by which RCMV accelerates allograft rejection by elimination of MHC II from the surface of infected DC diminishing their capacity to stimulate the development of regulatory T cells critical to maintaining tolerance to the donor tissue (Frascaroli et al., 2006; Ochando et al., 2006; Varani et al., 2005a). The alteration of suppressor T cell development could tip the immune system from a state of tolerance to a state that enhances allograft rejection.
In summary, bone marrow derived DCs are highly susceptible to RCMV infection, and the process of active viral infection modulates the ability of these infected cells to mediate an effective immune response. As demonstrated in this report, the RCMV-BMDC infection model provides three advantages to the study of CMV infection of DC and the effect on CMV disease. First, the infection model will facilitate the identification of novel RCMV ORFs mediating CMV induced MHC II manipulation. The finding of previously unrecognized RCMV gene products affecting MHC II may lead to the discovery of both functional and sequence homologues in HCMV and MCMV. Second, the high rate of infection of BMDCs with RCMV will facilitate mechanistic studies of CMV induced depletion of MHC II in infected DC. Third, the RCMV-BMDC model system will permit the investigation of CMV infection of DC and their possible role in graft rejection. A thorough analysis of the RCMV genes and mechanisms of MHC II down regulation will be imperative to dissect the role of Class II depletion in HCMV infected individuals.
Acknowledgments
We would like to thank Drs. Nagendra Hedge and Klaus Frueh for critically reviewing this manuscript and Drs. Bill Britt and David Johnson for thoughtful discussions. This work was supported by research grants to S.L. Orloff from the Department of Veterans Affairs and from the National Institutes of Health (HL 66238-01) and grants from the National Institutes of Health to C.C. Baca Jones (AI07472), D.N. Streblow (HL083194) and J.A. Nelson (AI21640, HL65754, and HL71695). D.N. Streblow is also supported by an AHA Scientist Development Grant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahn K, Angulo A, Ghazal P, Peterson PA, Yang Y, Fruh K. Human cytomegalovirus inhibits antigen presentation by a sequential multistep process. Proc Natl Acad Sci U S A. 1996;93(20):10990–5. doi: 10.1073/pnas.93.20.10990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews DM, Andoniou CE, Granucci F, Ricciardi-Castagnoli P, Degli-Esposti MA. Infection of dendritic cells by murine cytomegalovirus induces functional paralysis. Nat Immunol. 2001;2(11):1077–84. doi: 10.1038/ni724. [DOI] [PubMed] [Google Scholar]
- Cebulla CM, Miller DM, Zhang Y, Rahill BM, Zimmerman P, Robinson JM, Sedmak DD. Human cytomegalovirus disrupts constitutive MHC class II expression. J Immunol. 2002;169(1):167–76. doi: 10.4049/jimmunol.169.1.167. [DOI] [PubMed] [Google Scholar]
- Chang WL, Baumgarth N, Yu D, Barry PA. Human cytomegalovirus-encoded interleukin-10 homolog inhibits maturation of dendritic cells and alters their functionality. J Virol. 2004;78(16):8720–31. doi: 10.1128/JVI.78.16.8720-8731.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn W, Chou C, Li H, Hai R, Patterson D, Stolc V, Zhu H, Liu F. Functional profiling of a human cytomegalovirus genome. Proc Natl Acad Sci U S A. 2003;100(24):14223–8. doi: 10.1073/pnas.2334032100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish KN, Soderberg-Naucler C, Mills LK, Stenglein S, Nelson JA. Human cytomegalovirus persistently infects aortic endothelial cells. J Virol. 1998;72(7):5661–8. doi: 10.1128/jvi.72.7.5661-5668.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frascaroli G, Varani S, Mastroianni A, Britton S, Gibellini D, Rossini G, Landini MP, Soderberg-Naucler C. Dendritic cell function in cytomegalovirus-infected patients with mononucleosis. J Leukoc Biol. 2006;79(5):932–40. doi: 10.1189/jlb.0905499. [DOI] [PubMed] [Google Scholar]
- Hassink GC, Duijvestijn-van Dam JG, Koppers-Lalic D, van Gaans-van den Brink J, van Leeuwen D, Vink C, Bruggeman CA, Wiertz EJ. Rat cytomegalovirus induces a temporal downregulation of major histocompatibility complex class I cell surface expression. Viral Immunol. 2005;18(4):607–15. doi: 10.1089/vim.2005.18.607. [DOI] [PubMed] [Google Scholar]
- Hegde NR, Dunn C, Lewinsohn DM, Jarvis MA, Nelson JA, Johnson DC. Endogenous human cytomegalovirus gB is presented efficiently by MHC class II molecules to CD4+ CTL. J Exp Med. 2005;202(8):1109–19. doi: 10.1084/jem.20050162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegde NR, Tomazin RA, Wisner TW, Dunn C, Boname JM, Lewinsohn DM, Johnson DC. Inhibition of HLA-DR assembly, transport, and loading by human cytomegalovirus glycoprotein US3: a novel mechanism for evading major histocompatibility complex class II antigen presentation. J Virol. 2002;76(21):10929–41. doi: 10.1128/JVI.76.21.10929-10941.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanagh DG, Gold MC, Wagner M, Koszinowski UH, Hill AB. The multiple immune-evasion genes of murine cytomegalovirus are not redundant: m4 and m152 inhibit antigen presentation in a complementary and cooperative fashion. J Exp Med. 2001;194(7):967–78. doi: 10.1084/jem.194.7.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanagh DG, Koszinowski UH, Hill AB. The murine cytomegalovirus immune evasion protein m4/gp34 forms biochemically distinct complexes with class I MHC at the cell surface and in a pre-Golgi compartment. J Immunol. 2001;167(7):3894–902. doi: 10.4049/jimmunol.167.7.3894. [DOI] [PubMed] [Google Scholar]
- Kleijnen MF, Huppa JB, Lucin P, Mukherjee S, Farrell H, Campbell AE, Koszinowski UH, Hill AB, Ploegh HL. A mouse cytomegalovirus glycoprotein, gp34, forms a complex with folded class I MHC molecules in the ER which is not retained but is transported to the cell surface. Embo J. 1997;16(4):685–94. doi: 10.1093/emboj/16.4.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotenko SV, Saccani S, Izotova LS, Mirochnitchenko OV, Pestka S. Human cytomegalovirus harbors its own unique IL-10 homolog (cmvIL-10) Proc Natl Acad Sci U S A. 2000;97(4):1695–700. doi: 10.1073/pnas.97.4.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchroo VK, Das MP, Brown JA, Ranger AM, Zamvil SS, Sobel RA, Weiner HL, Nabavi N, Glimcher LH. B7-1 and B7-2 costimulatory molecules activate differentially the Th1/Th2 developmental pathways: application to autoimmune disease therapy. Cell. 1995;80(5):707–18. doi: 10.1016/0092-8674(95)90349-6. [DOI] [PubMed] [Google Scholar]
- Lang TJ, Nguyen P, Peach R, Gause WC, Via CS. In vivo CD86 blockade inhibits CD4+ T cell activation, whereas CD80 blockade potentiates CD8+ T cell activation and CTL effector function. J Immunol. 2002;168(8):3786–92. doi: 10.4049/jimmunol.168.8.3786. [DOI] [PubMed] [Google Scholar]
- Lilley BN, Ploegh HL. Viral modulation of antigen presentation: manipulation of cellular targets in the ER and beyond. Immunol Rev. 2005;207:126–44. doi: 10.1111/j.0105-2896.2005.00318.x. [DOI] [PubMed] [Google Scholar]
- Liu T, Khanna KM, Chen X, Fink DJ, Hendricks RL. CD8(+) T cells can block herpes simplex virus type 1 (HSV-1) reactivation from latency in sensory neurons. J Exp Med. 2000;191(9):1459–66. doi: 10.1084/jem.191.9.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockridge KM, Sequar G, Zhou SS, Yue Y, Mandell CP, Barry PA. Pathogenesis of experimental rhesus cytomegalovirus infection. J Virol. 1999;73(11):9576–83. doi: 10.1128/jvi.73.11.9576-9583.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockridge KM, Zhou SS, Kravitz RH, Johnson JL, Sawai ET, Blewett EL, Barry PA. Primate cytomegaloviruses encode and express an IL-10-like protein. Virology. 2000;268(2):272–80. doi: 10.1006/viro.2000.0195. [DOI] [PubMed] [Google Scholar]
- Loewendorf A, Kruger C, Borst EM, Wagner M, Just U, Messerle M. Identification of a mouse cytomegalovirus gene selectively targeting CD86 expression on antigen-presenting cells. J Virol. 2004;78(23):13062–71. doi: 10.1128/JVI.78.23.13062-13071.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathys S, Schroeder T, Ellwart J, Koszinowski UH, Messerle M, Just U. Dendritic cells under influence of mouse cytomegalovirus have a physiologic dual role: to initiate and to restrict T cell activation. J Infect Dis. 2003;187(6):988–99. doi: 10.1086/368094. [DOI] [PubMed] [Google Scholar]
- McLaughlin K, Wu C, Fick G, Muirhead N, Hollomby D, Jevnikar A. Cytomegalovirus seromismatching increases the risk of acute renal allograft rejection. Transplantation. 2002;74(6):813–6. doi: 10.1097/00007890-200209270-00014. [DOI] [PubMed] [Google Scholar]
- Michelson S. Interaction of human cytomegalovirus with monocytes/macrophages: a love-hate relationship. Pathol Biol (Paris) 1997;45(2):146–58. [PubMed] [Google Scholar]
- Mintern JD, Klemm EJ, Wagner M, Paquet ME, Napier MD, Kim YM, Koszinowski UH, Ploegh HL. Viral interference with B7-1 costimulation: a new role for murine cytomegalovirus fc receptor-1. J Immunol. 2006;177(12):8422–31. doi: 10.4049/jimmunol.177.12.8422. [DOI] [PubMed] [Google Scholar]
- Mocarski ES. Cytomegaloviruses and their replication. In: Fields BN, editor. Virology. 4. Vol. 2. Lippincott-Raven; Philadelphia: 2001. pp. 2629–2673. [Google Scholar]
- Mocarski ES., Jr Immunomodulation by cytomegaloviruses: manipulative strategies beyond evasion. Trends Microbiol. 2002;10(7):332–9. doi: 10.1016/s0966-842x(02)02393-4. [DOI] [PubMed] [Google Scholar]
- Moutaftsi M, Mehl AM, Borysiewicz LK, Tabi Z. Human cytomegalovirus inhibits maturation and impairs function of monocyte-derived dendritic cells. Blood. 2002;99(8):2913–21. doi: 10.1182/blood.v99.8.2913. [DOI] [PubMed] [Google Scholar]
- Muthana M, Fairburn B, Mirza S, Slack LK, Pockley AG. Systematic evaluation of the conditions required for the generation of immature rat bone marrow-derived dendritic cells and their phenotypic and functional characterization. J Immunol Methods. 2004;294(1–2):165–79. doi: 10.1016/j.jim.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Nordoy I, Rollag H, Lien E, Sindre H, Degre M, Aukrust P, Froland SS, Muller F. Cytomegalovirus infection induces production of human interleukin-10 in macrophages. Eur J Clin Microbiol Infect Dis. 2003;22(12):737–41. doi: 10.1007/s10096-003-1028-x. [DOI] [PubMed] [Google Scholar]
- Ochando JC, Homma C, Yang Y, Hidalgo A, Garin A, Tacke F, Angeli V, Li Y, Boros P, Ding Y, Jessberger R, Trinchieri G, Lira SA, Randolph GJ, Bromberg JS. Alloantigen-presenting plasmacytoid dendritic cells mediate tolerance to vascularized grafts. Nat Immunol. 2006;7(6):652–62. doi: 10.1038/ni1333. [DOI] [PubMed] [Google Scholar]
- Odeberg J, Plachter B, Branden L, Soderberg-Naucler C. Human cytomegalovirus protein pp65 mediates accumulation of HLA-DR in lysosomes and destruction of the HLA-DR alpha-chain. Blood. 2003;101(12):4870–7. doi: 10.1182/blood-2002-05-1504. [DOI] [PubMed] [Google Scholar]
- Odeberg J, Soderberg-Naucler C. Reduced expression of HLA class II molecules and Iinterleukin-10- and transforming growth factor beta1-independent suppression of T-cell proliferation in human cytomegalovirus-infected macrophage cultures. J Virol. 2001;75(11):5174–81. doi: 10.1128/JVI.75.11.5174-5181.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orloff SL, Streblow DN, Soderberg-Naucler C, Yin Q, Kreklywich C, Corless CL, Smith PA, Loomis CB, Mills LK, Cook JW, Bruggeman CA, Nelson JA, Wagner CR. Elimination of donor-specific alloreactivity prevents cytomegalovirus-accelerated chronic rejection in rat small bowel and heart transplants. Transplantation. 2002;73(5):679–88. doi: 10.1097/00007890-200203150-00005. [DOI] [PubMed] [Google Scholar]
- Pinto AK, Munks MW, Koszinowski UH, Hill AB. Coordinated function of murine cytomegalovirus genes completely inhibits CTL lysis. J Immunol. 2006;177(5):3225–34. doi: 10.4049/jimmunol.177.5.3225. [DOI] [PubMed] [Google Scholar]
- Raftery MJ, Schwab M, Eibert SM, Samstag Y, Walczak H, Schonrich G. Targeting the function of mature dendritic cells by human cytomegalovirus: a multilayered viral defense strategy. Immunity. 2001;15(6):997–1009. doi: 10.1016/s1074-7613(01)00239-4. [DOI] [PubMed] [Google Scholar]
- Raftery MJ, Wieland D, Gronewald S, Kraus AA, Giese T, Schonrich G. Shaping phenotype, function, and survival of dendritic cells by cytomegalovirus-encoded IL-10. J Immunol. 2004;173(5):3383–91. doi: 10.4049/jimmunol.173.5.3383. [DOI] [PubMed] [Google Scholar]
- Redpath S, Angulo A, Gascoigne NR, Ghazal P. Murine cytomegalovirus infection down-regulates MHC class II expression on macrophages by induction of IL-10. J Immunol. 1999;162(11):6701–7. [PubMed] [Google Scholar]
- Reusch U, Muranyi W, Lucin P, Burgert HG, Hengel H, Koszinowski UH. A cytomegalovirus glycoprotein re-routes MHC class I complexes to lysosomes for degradation. Embo J. 1999;18(4):1081–91. doi: 10.1093/emboj/18.4.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer JV, Lockridge KM, Barry PA, Lin G, Tsang M, Penfold ME, Schall TJ. Potent immunosuppressive activities of cytomegalovirus-encoded interleukin-10. J Virol. 2002;76(3):1285–92. doi: 10.1128/JVI.76.3.1285-1292.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomazin R, Boname J, Hegde NR, Lewinsohn DM, Altschuler Y, Jones TR, Cresswell P, Nelson JA, Riddell SR, Johnson DC. Cytomegalovirus US2 destroys two components of the MHC class II pathway, preventing recognition by CD4+ T cells. Nat Med. 1999;5(9):1039–43. doi: 10.1038/12478. [DOI] [PubMed] [Google Scholar]
- Varani S, Frascaroli G, Gibellini D, Potena L, Lazzarotto T, Lemoli RM, Magelli C, Soderberg-Naucler C, Landini MP. Impaired dendritic cell immunophenotype and function in heart transplant patients undergoing active cytomegalovirus infection. Transplantation. 2005a;79(2):219–27. doi: 10.1097/01.tp.0000147359.63158.29. [DOI] [PubMed] [Google Scholar]
- Varani S, Frascaroli G, Homman-Loudiyi M, Feld S, Landini MP, Soderberg-Naucler C. Human cytomegalovirus inhibits the migration of immature dendritic cells by down-regulating cell-surface CCR1 and CCR5. J Leukoc Biol. 2005b;77(2):219–28. doi: 10.1189/jlb.0504301. [DOI] [PubMed] [Google Scholar]
- Ziegler H, Muranyi W, Burgert HG, Kremmer E, Koszinowski UH. The luminal part of the murine cytomegalovirus glycoprotein gp40 catalyzes the retention of MHC class I molecules. Embo J. 2000;19(5):870–81. doi: 10.1093/emboj/19.5.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler H, Thale R, Lucin P, Muranyi W, Flohr T, Hengel H, Farrell H, Rawlinson W, Koszinowski UH. A mouse cytomegalovirus glycoprotein retains MHC class I complexes in the ERGIC/cis-Golgi compartments. Immunity. 1997;6(1):57–66. doi: 10.1016/s1074-7613(00)80242-3. [DOI] [PubMed] [Google Scholar]