Abstract
Herpes Simplex Virus type 2 causes genital herpes but is frequently transmitted asymptomatically; therefore, a prophylactic vaccine is desirable. A candidate vaccine in clinical trials has only shown efficacy in preventing disease in women. Using this subunit vaccine candidate, we were able to demonstrate infection prophylaxis, improved disease prevention and modulated antibody production by complimenting vaccination with estradiol in our murine model. Findings of estradiol-enhanced vaccine efficacy are the first of their kind using a vaccine of this type and have potential clinical relevance to the development of other vaccines and our understanding of gender differences in vaccine efficacy.
Keywords: estradiol, herpesvirus 2, human, Herpes Simplex Virus Vaccines
1. INTRODUCTION
Herpes Simplex Virus type 2 (HSV-2) is a pathogen of significant public health importance. Data from the US National Health and Nutrition Examination Surveys (NHANES) report 17% of Americans are seropositive for HSV-2 and there are published reports that HSV-2 prevalence in parts of the developing world is as high as 80% [1, 2]. HSV-2 causes genital herpes and, as with most sexually transmitted infections, women bear a disproportionate burden of HSV-2 infection [2]. HSV-2 is commonly transmitted to women via the reproductive tract where it infects and passes through the vaginal mucoepithelial layer to establish a lifelong infection in the dorsal root ganglia (DRG) [3]. Between 63% and 87% of HSV-2 infections are asymptomatic, leaving many people unaware of their HSV-2 infection [2, 3]. Virus shed in the absence of symptoms is believed to be the major source of genital herpes transmission to susceptible sex partners. Much effort has gone into developing a prophylactic vaccine [4, 5]. HSV-2 is also known to be a synergistic co-pathogen to HIV and it is believed that resolution of the HSV-2 pandemic could provide progress towards resolving the HIV pandemic [6].
Clinical trials indicate that the most effective candidate vaccine thus far is a glycoprotein D vaccine formulated in AS04 because it provided women significant protection against genital herpes disease, although it failed to provide sterilizing immunity to HSV-2 infection; additional Phase III trials are currently underway[4]. Notably, this protection was only seen in women, which is surprising since women are more susceptible to infection.
Many vaccines have shown gender-specific differences in the protection they afford [7]. Others have already begun to explore the role that estradiol might have in susceptibility to HSV-2 infection and vaccine efficacy and they have shown that estradiol impacted vaccine-elicited protection using a live attenuated virus vaccine [8–13]. Extending their previous work, here we used the nonreplicating gD-antigen vaccine in the AS04 formulation that is currently in clinical trials, rather than a live attenuated vaccine, in order to completely separate the issues of vaccine-elicited protection from both vaccine- and pathogenic-viral susceptibility and replication. This enabled us to isolate and observe the impact of estradiol on the efficacy of a clinically relevant vaccine in a small animal model of a pathogen of major public health importance. The results of our studies clearly demonstrate that estradiol enhanced vaccine-elicited protection, even in the context of already-robust protection and even in exquisitely sensitive models, using a subunit vaccine in clinical trials.
2. MATERIALS AND METHODS
2.1 Virus
HSV-2 strain 186 was prepared on Vero cell monolayers and stored frozen (−80°C) until used, as previously described [14].
2.2 Animals and hormone treatments
Ovary-intact and ovariectomized female Swiss Webster mice (Jackson Laboratories, Bar Harbor, ME) approximately eight-weeks-old were housed in Association for Assessment and Accreditation of Laboratory Animal Care-approved quarters for these studies and all procedures were approved by the University of Texas Medical Branch Intuitional Animal Use and Care Committee. Animals were allowed to acclimate to the vivarium for seven days prior to use.
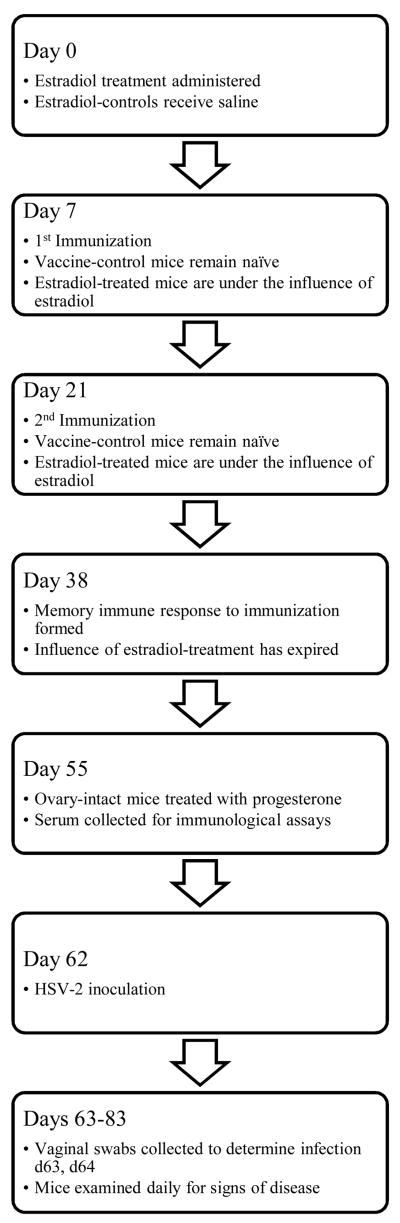
Mice in estradiol-treated groups were intramuscularly injected with a 50 μL mineral oil solution(heavy paraffin oil, Fisher-Scientific, Fair Lawn, NJ) containing 6.23 μg estradiol valerate (Monarch Pharmaceuticals, Bristol, TN) in the right hind leg 7 days prior to vaccination. The dose of estradiol was, based on pharmacologic data for rodents, was sufficient to produce an effect that would last 38 days, so as to span the immunization regimen, but not to overlap with the virus challenge later in the study nor be so large that it was physiologically irrelevant [15 and unpublished data]. Control mice were injected with 50 μL of saline. For challenge studies using ovary-intact mice, the animals were treated 2 mg medroxyprogesterone acetate (The Upjohn Company, Kalamazoo, MI) one-week prior to HSV-2 inoculation to make the epithelium permissive to viral infection, as previously described [16, 17]. Ovariectomized mice did not receive progesterone. See Figure 1 for a timeline of the main experimental design.
FIG. 1.
Timeline of the experimental design.
2.3 Cellvizio Confocal Imaging System
In vivo confocal images of the vaginal surface were collected in situ using a confocal microendoscope imaging system (Cellvizio Lab Mauna Kea Technologies, Paris, France). The system provides 488 nm illumination via an optical fiber bundle to the distal imaging probe and collects fluorescence emission in the 505–700 nm range. The imaging probe was 1.5mm in diameter and provided a field of view of 600×500 mm with an axial and lateral resolution of 15 and 3.5 μm, respectively. Images provided by this system are single plane en face images of the sample surface. The system was used to collect real time videos at an acquisition rate of 12 frames/sec.
2.4 Mouse Imaging Experiments
Mice were anesthetized with an intraperitoneal injection (50 mg/kg) of Sodium Pentobarbital. The vaginal tract was gently flushed with 1.5 mL saline, and then approximately 0.1 mL of 0.2% W/V Acridine Orange (Product #31,833-7, Aldrich Chemical Company) was administered. Five minutes later, the vaginal tract was again flushed with saline to remove excess dye prior to imaging. The confocal microprobe was then gently inserted into the vaginal tract and a 30-second video was collected of the vaginal tract wall, from which representative still images were selected. Animals were then euthanized and the reproductive tract excised and fixed in neutral-buffered formalin fixative for a minimum of 24 hours.
2.5 Histological Processing and Analysis
Samples were submitted for routine histology processing. Several, 4–5 micron transverse sections of the vaginal tract were collected at 100μm intervals. Slides were stained with Hematoxylin and Eosin (H&E) and reviewed under a light microscope (Olympus IX71, Olympus America, Center Valley, PA). For each sample, one histology cross-section near the cervix was chosen for epithelial thickness measurements. From that section 1 to 3 micrographs of the cervicovaginal epithelium were collected using a color digital camera (Spot RT Slider, Diagnostic Instruments, Sterling Heights, MI) at a magnification of 200×. Spot Advanced software (Diagnostic Instruments, Sterling Heights, MI) was used to measure the epithelial thickness from the digital images using a calibrated measuring tool within the software program. Twenty randomly chosen epithelial sites were measured from the microphotographs in order to obtain a mean cervicovaginal epithelial thickness value for each animal.
2.6 Vaccine
The gD/AS04 vaccine formulation, used in recent and ongoing clinical trials, was kindly provided by GlaxoSmithKline Biologicals (Rixensart, Belgium) [5]. Each mouse was vaccinated intramuscularly in the left hind leg with 50 μL of the vaccine, which contained 2 μg of HSV-2 surface glycoprotein D. Animals received a second vaccination two weeks later, while estradiol-treated animals were still under the influence of the estradiol dose.
2.7 Mouse Model of Genital Herpes
Four weeks after the second vaccination, and one week after progesterone treatment in ovary-intact mice, all vaccinated animals and age-matched naïve control animals were intravaginally inoculated with HSV-2. The inocula used ranged from 1 × 101 to 1 × 106 PFU in 15 μL, depending on the study, as previously described [14]. On days 1 and 2 postinoculation, vaginal swab samples were collected from all mice. Samples were plated on Vero cell monolayers and incubated for 5 days at 37°C to determine infection. Animals were defined as infected if viral cytopathic effects of HSV-2 were observed from either swab sample. Mice were examined daily for 21 days postinoculation for clinical signs of genital herpes disease and were defined as having such if they showed pathological signs of cutaneous disease (hair loss and erythema on the perineum) or signs of more severe, neurological disease (urinary inconstance and hind-limb paralysis). Mice progressing to severe neurological involvement either quickly succumbed to encephalitis or were euthanized.
2.8 ELISA for HSV-specific Antibodies
One week prior to viral inoculation, blood samples were collected from the retro-orbital plexus of each mouse. ELISA assays were performed as previously described [18, 19]. Briefly, serum samples were plated in duplicate wells coated with HSV-2 glycoprotein as the antigen (or glycoprotein from uninfected cells as the control mock antigen). Plates were developed using biotinylated anti-mouse IgG antibody (Southern Biotech, Birmingham, AL), streptavidin peroxidase (Sigma, St. Louis, MO) and o-phenylenediamine dihydrochloride with hydrogen peroxide. The OD490 values were obtained using a VersaMax plate reader (Molecular Devices, Sunnyvale, CA), compared to the linear portion of the standard curve, and HSV-2 gD-specific antibody concentrations were calculated using SoftMax Pro software (Molecular Devices).
2.9 Neutralization Assays
Neutralizing serum antibody titres were described by a modification of our previously described technique [20]. Briefly, serum from vaccinated and naïve control mice was heat inactivated at 56°C for 15 min. A series of two-fold dilutions was then made in 2% titration media and Low-Tox H Rabbit Complement (Cedarlane Laboratories, Burlington NC) at a final concentration of 1/20480. Approximately 100 PFU HSV-2 strain 186 was added to each tube in the dilution sequence. Following incubation at 37°C for 1 hour, the dilution sequence was plated on Vero cell monolayers for PFU quantification, as above. After incubation for 3 days, the plates were stained with crystal violet and the number of viral plaques was counted. The end-point neutralizing antibody titre was defined as the log10 of the final serum dilution that produced a >50% reduction in the number of viral plaques compared to the number of plaques in control serum wells [21].
2.10 Statistics
Fisher’s exact tests were used to compare all infection, disease and outcome data. The Student’s unpaired t-test was used to compare group mean values. All reported P values are two-tailed and values <0.05 were considered to indicate statistical significance. All statistical analysis was performed using SPSS 16.0 for Mac OS.
3. RESULTS
3.1 Impact of estradiol on the vaginal epithelium of ovary-intact mice
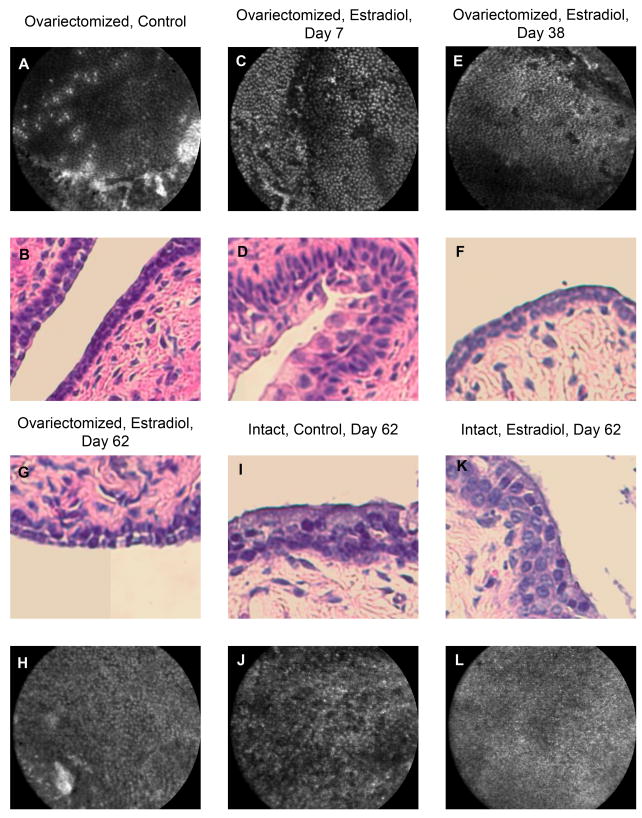
The aim of these studies was to observe the impact of estradiol on vaccine-elicited protection. In order to do so, we wanted to deliver a single, biologically significant dose of estradiol that would last for the duration of the vaccination regimen but would have lapsed by the time of viral inoculation. Estradiol is known to cause the vaginal epithelium to thicken; we wanted to be certain the estradiol treatment had lapsed prior to inoculation because otherwise it would be impossible to determine whether a reduced incidence of infection was the result of an estradiol-enhanced vaccine response or a physiological artifact of an estradiol-thickened vaginal epithelium. We selected estradiol valerate as our estrogen treatment because it is available by prescription, therefore its sustained-release kinetics have been established in both rodents and humans [22]. We calculated a single dose that we believed would be potent for the duration of the two-injection vaccination regimen and would lapse prior to viral inoculation [15, 22]. To confirm this, we used two imaging modalities, Cellvizio and traditional H&E histology, that served two objectives: verify that the dose was in fact biologically potent and verify that its duration of action was short enough to not pose a physiologic barrier to infection. This was done by measuring the thickness of the vaginal epithelium in histological cross-sections and the characteristics of the epithelium surface with Cellvizio. Groups of ovary-intact mice were sacrificed and imaged on days 0, 7 and 62 post-estradiol treatment along with age-matched saline-treated controls (N=5 per group). Somewhat variable results were seen, as would be expected, because the ovaries continued to be a source of estrogen during estradiol treatment and thus they were in various phases of their estrous cycles. On day 0, the mean thickness was 62 μm ± 13; on day 7, when the estradiol would be expected to be thickening the vaginal epithelium and the vaccination regimen would begin, the mean had increased to 82 μm ± 13. By day 62, long after the estradiol treatment should have lapsed and one week after the progesterone, when the viral challenge would occur in the following studies, the mean thickness had decreased to 39 μm ± 4 (Figure 2K). We are able to verify that the impact of estradiol on the epithelium has lapsed and that progesterone has primed (i.e., thinned) the vaginal epithelium for viral challenge because the saline-control mice also had a mean thickness of 39 μm ± 5 (Figure 2I). The Cellvizio images (black & white) show the brightly stained nuclei of the uppermost cell-layer of the epithelium, which provide supplementary information depicting the nuclear size and density as an overall in vivo qualification of epithelial health and an indicator of the influence of estradiol; the crossectional histological images (color) enable an actual measurement of epithelial thickness, also an indicator of the influence of estradiol as well an indicator to physiological infection permissivity. Progesterone-priming is required for viral challenge in ovary-intact mice [9, 14]. Therefore, as an indicator of potency and bioavailability, the estradiol did thicken the vaginal epithelium; and this affect had lapsed, as anticipated, thus posing no physiologic barrier to infection on day 62.
FIG. 2.
Images of mouse epithelium 62 days after estradiol treatment. Panels A–H are representative images of the vaginal epithelium of ovariectomized mice; A, B were not treated with estradiol; C–H were taken on the indicated number of days after estradiol treatment. Panels I, J are from an ovary-intact mouse 62 days after saline treatment and 7 days after progesterone treatment; K, L are from ovary-intact mice 62 days after estradiol treatment and 7 days after progesterone. Black and white images are Cellvizio confocal micrographs showing the nature of the vaginal epithelial surface; color images are H&E crossectional histology micrographs showing epithelial layers and thickness. Panels A, B (controls) show the thin, simple, squamous epithelium of the vaginal tract in an ovariectomized mouse; approx 13 μm. Panels C, D show the estradiol-thickened, stratified epithelium with large, proliferating nuclei seven days after estradiol treatment; approx 37 μm. Panels E, F show the epithelium has returned to a thin, simple, squamous layer of cells 38 days after estradiol treatment; approx 16 μm. Panels G, I, K (day 62) show the epithelial thickness to be approx 16 μm, 39 μm and 39 μm, respectively; the effects of estradiol treatment have lapsed. The ovary-intact mice have been progesterone-primed to make them infection-permissive and the ovariectomized mouse’s thin vaginal epithelium is already very susceptible to infection. (N=5/group).
3.2 Impact of estradiol on vaccine-elicited protection in ovary-intact mice
We next examined the effect of estradiol using a high titre inoculum of HSV-2 (1 × 105 PFU) in ovary-intact mice. All naïve-control animals in these studies became infected and developed disease, as expected. Vaccination, with and without estradiol treatment, resulted in a significant reduction of both infection and disease in these studies; however, the protection against infection and disease was greater in the estradiol-treated animals (Table 1). Adding estradiol reduced the incidence of infection by 50% and disease by 75%, compared to vaccination alone. Even when only examining infected animals, we observed that both vaccination alone and with estradiol still provided significant reduction in the incidence of disease compared to the naïve controls (raw data not shown, p<0.01), but the difference between the two former groups was not significant.
TABLE 1.
Impact of estradiol on vaccine efficacy in ovary-intact mice
| Group | N | Infection | Clinical Disease | Mortality | |||
|---|---|---|---|---|---|---|---|
| # | Pa | # | Pa | # | Pa | ||
| Naïve | 19 | 19 | - | 19 | - | 19 | - |
| Vaccinated | 20 | 12 | 0.003 | 4 | ≪0.001 | 0 | ≪0.001 |
| Estradiol + Vaccinated | 20 | 6 | ≪0.001 | 1 | ≪0.001 | 0 | ≪0.001 |
P values were calculated to test for a statistically significant difference in the observed incidence compared to naïve controls.
We next examined whether adding estradiol to the vaccination regimen would impact the inoculum required to establish infection at the genital mucosa and/or the inoculum required to cause genital herpes disease in ovary-intact mice. Groups of mice (N=15/group) were challenged with a range of HSV-2 inocula (10 thru 106 PFU) to determine if the inoculum required to infect half of all animals in a group (ID50) or cause disease in half of all animals in a group (DD50) would be changed. The ID50 for mice in the naïve group was approximately 1.6 × 102 PFU; the ID50 in the vaccinated groups, both with and without estradiol, were approximately 4 × 103 PFU. The DD50 for the naïve group was approximately 2 × 102 PFU; the DD50 for the group receiving vaccination alone was approximately 3.16 × 105 PFU; and the DD50 for the group with estradiol added to the vaccination regimen was >106 PFU. Both these benchmark inocula were increased by vaccination and disease protection was improved further by the addition of estradiol to vaccination regimen. This, combined with the high dose challenges above, suggested a trend of estradiol-enhanced vaccine-elicited protection, however the robust protection afforded by vaccination alone made quantification of the enhancement difficult. This prompted us to examine the impact of estradiol in ovariectomized mice, which are known to be more susceptible to HSV-2 infection [11].
3.3 Impact of estradiol on the vaginal epithelium of ovariectomized mice
Ovariectomized mice also provide a cleaner hormonal background since their primary source of endogenous estradiol has been removed. Therefore, we examined the impact of estradiol on the vaginal epithelium of ovariectomized mice to ensure our dosing schedule was appropriate. Groups of ovariectomized mice were sacrificed and imaged on days 0, 7, 38 and 62 post-estradiol treatment along with age-matched saline-treated controls (N=5 per group). Figure 2 shows representative images from the ovariectomized, estradiol-treated groups. On day 0 (Figure 2, A and B), the mean vaginal epithelial thickness was 13 μm ± 3 in the ovariectomized group. On day 7 (Figure 2, C and D), when the estradiol would be expected to be thickening the vaginal epithelium and the vaccination regimen would begin, the mean thickness was 37 μm ± 9. On day 38 (Figure 2, E and F), when the vaccination regimen is over and the estradiol was pharmacologically expected to have lapsed, the mean thickness had in fact dropped back to 17 μm ± 4 (day 38 controls had a mean thickness of 16 μm ± 3, not shown). And on day 62 (Figure 2, G and H), when viral challenge would occur, the mean epithelial thickness was 16 μm ± 3. Therefore, as an indicator of potency and bioavailability, the estradiol did thicken the vaginal epithelium; and this affect had lapsed, as anticipated, 38 days later, thus posing no physiologic barrier to infection on day 62. This was a significant thickening from day 0 to day 7 (P<0.05), a significant thinning between day 7 and day 38 (P<0.05), and no significant difference at the time points of days 0, 38 and 62, with respect to each other.
3.4 Impact of estradiol on vaccine-elicited protection in ovariectomized mice
Having shown that the estradiol treatment was appropriate for the ovariectomized mice, we next examined the impact of estradiol on vaccine efficacy in this model and Table 2 shows the results of those studies. All but one of the naïve-control mice challenged with the large 1 × 105 PFU inoculum became infected and developed disease. Vaccination alone reduced incidence of infection, but not significantly. However, estradiol-treated vaccinated animals had a significantly reduced incidence of disease compared to naïve-controls (P<0.01). This mirrors what was reported from the gD/AS04 vaccine trials in humans, where the vaccine significantly reduced the incidence of genital herpes disease but did not significantly reduce the number of women acquiring HSV-2 infection [5]. In addition, these results demonstrated the increased stringency of the ovariectomized mouse model. Mice that were treated with estradiol during vaccination regimen showed a significant reduction in the incidence of infection compared to naïve control mice (P<0.01). Mice receiving estradiol treatment also experienced significantly reduced incidence of disease when compared to both the naïve controls (P<0.01) as well as the vaccine-only group (P<0.05). Among infected mice, although both vaccination alone and vaccination with estradiol significantly reduced the incidence of disease compared to naïve controls (raw data not shown, p<0.05), the difference between the two former groups was not significant. However, the difference in the severity of the disease was significant. Among the 32 vaccinated mice that became infected, 13 (40%) progressed to severe neurological involvement that resulted in mortality. In contrast, 0 out of 25 infected mice that had also been estradiol-treated developed signs of neurological involvement and all of them survived until the end of the study.
TABLE 2.
Impact of estradiol on vaccine efficacy in ovariectomized mice
| Group | N | Infection | Clinical Disease | Mortality | |||||
|---|---|---|---|---|---|---|---|---|---|
| # | Pa | # | Pb | Pa | # | Pb | Pa | ||
| Naïve | 30 | 29 | - | 29 | - | - | 29 | - | - |
| Vaccinated | 40 | 32 | NS | 25 | - | <0.01 | 13 | - | <0.01 |
| Estradiol + Vaccinated | 40 | 25 | <0.01 | 14 | <0.05 | <0.01 | 0 | <0.01 | <0.01 |
P values were calculated to test for a statistically significant difference in the observed incidence compared to naïve controls. NS indicates that the difference was not significant.
P values were calculated to test for a statistically significant difference in the observed incidence compared to mice receiving vaccine only.
3.5 Impact of estradiol on HSV-2 antibody production
Serum was collected from mice prior to viral inoculation and the HSV-2 gD-specific IgG antibody was quantified by ELISA. The estradiol-treated group had slightly more vaccine-specific antibody (1.62 × 105 ng/mL ± 1.58 × 105 SD; N=21) than the group receiving vaccine alone (1.24 × 105 ng/mL ± 9.54 × 104 SD; N=21), however the difference was not statistically significant.
The functionality of the vaccine-elicited antibody was assessed by neutralization assay. The neutralizing antibody titre of the estradiol-treated vaccinated mice was 3.15 log10 (± 0.023 SD), which was significantly greater than that of mice vaccinated alone, 2.99 log10 (± 0.015 SD, P<0.05; N=21/group).
4. DISCUSSION
Our data shows that estradiol improves vaccine-elicited protection against genital herpes infection and disease and results in an enhanced antibody response. Others have previously examined the impact of estradiol on vaccine efficacy against genital herpes in mouse models using a thymidine kinase deficient virus as an attenuated vaccine [8, 11, 12, 23]. The results presented here are consistent with and extend those studies. If estradiol was going to modulate susceptiblity to and/or protection against HSV-2 infection, as we and they hypothesized, then the potential existed that it could be modulating susceptibility to the vaccine virus as well as or instead of the pathogenic virus challenge. We avoided this potential problem by using a subunit vaccine, so that replication of the immunogen is not a factor. Further, this vaccine candidate showed efficacy in initial phase III clinical trials, lending additional translational relevance. Another major concern we had was in the dosing schedule of the estradiol. In establishing whether estradiol could enhance vaccine-elicited protection, we wanted to be certain that an estradiol-thickened vaginal epithelium did not confound our ability to detect alterations in vaccine efficacy by leaving the mice physiologically impermeable to virus, especially since the magnitude of this effect is much greater in mice; at no time in the menstrual cycle is a woman’s vagina impermeable to HSV-2 infection. We confirmed that our results were not contaminated by any physiological impact of estradiol by directly imaging it, rather than trying to gauge or time the estrous cycles of the mice or serologically quantify the estradiol. The imaging studies presented here enabled us to visually verify that our administration of a sustained-release estradiol into the mouse model posed no physiological barrier to viral infection. Because we are able to demonstrate in Figure 2 that the vaginal epithelial thickness had returned to pre-estradiol values (or to progesterone-regulated values in the case of intact mice), it is then supportable to accept that the differences observed in the subsequent studies (incidence of infection, disease, circulating antibody, etc.) represented an estradiol-mediated impact on the immune system. Secondarily, the imaging studies confirmed the bioavailability and metabolic potency of the estradiol valerate preparation and dosage used.
It was also interesting to note, from the imaging studies, that the ovariectomized mouse vaginal epithelium, before estradiol treatment (Figure 2B) and after estradiol treatment has lapsed (Figure 2F&G), is thinner than the progesterone-treated epithelium of an ovary-intact mouse (Figure 2I&K), which explains why ovariectomized are exquisitely sensitive to HSV-2 infection and are thus an ideal model to examine the impact of estradiol on vaccine efficacy. The gD/AS04 vaccine alone was able to reduce the incidence of infection by 20%, a trend towards protection against infection but falling short of statistical significance (Table 2). It should be noted that, in this regard, the ovariectomized mouse model parallels the clinical observations using the same vaccine candidate with which additional expanded phase III clinical trials are currently underway [4]. In ovariectomized mice treated with estradiol valerate one week prior to the first vaccination, an additional gain of 20% vaccine efficacy was observed in comparison to vaccination alone, significantly reducing the incidence of infection by almost 40% compared to naïve controls (P<0.01) (Table 2). This is an exciting observation. Sterilizing immunity has not been widely believed to be a feasible goal of an HSV-2 vaccination in clinical application. It is anticipated that an HSV-2 vaccine brought to market would provide immunity sufficient to prevent disease and might reduce the burden of latent virus in the ganglia, which could in turn reduce the shedding into the genital shedding and transmission; modeling indicates that, in this way, a vaccine that provides nonsterilizing immunity can still significantly reduce the incidence of HSV-2 infection [24, 25]. However, adding estradiol to the vaccination regimen in ovariectomized mice directly enhanced protection against infection as well as enhancing the protection against disease. Vaccination alone significantly reduced the incidence of clinical disease overall by 34% (P<0.01) and among infected mice by almost 22% (P<0.01) (Table 2). The estradiol-treated groups had reduced incidence of disease by an additional 27.5% overall compared to vaccination (61.7% compared to naïve). Among infected mice, estradiol treated mice had an additional 22% reduction in incidence of disease compared to vaccination alone (not significant) and a 44 % reduction in incidence of disease compared to naïve controls (P<0.01). Thus the rigorous ovariectomized mouse model shows that estradiol treatment not only boosts vaccine efficacy to provide enhanced protection against disease but it also boosts it up to a threshold adequate to prevent infection.
Key effector and regulatory cells of the immune system express estrogen receptors and a mechanism involving an interfacing of the endocrine and reproductive systems with the immune system is not a novel idea [26, 27]. It has long been understood that the immune system of the female reproductive tract must be modulated to not attack and reject a fetus that is 50% foreign. In contrast, the idea that estradiol could be systemically modulating the formation of adaptive immune memory responses is much newer and is supported by our data [8, 28]. While the gD/AS04 vaccine is designed to stimulate both Th1 and Th2 responses, our HSV-2-specific IgG ELISA data and neutralization assay suggest estradiol-treated mice produce additional HSV-2 gD-specific antibody. This explains, at least in part, why the estradiol-treated vaccinated mice had a better outcome than their vaccine-only counterparts.
The ovary-intact studies were consistent with the principles demonstrated in the ovariectomized mice and the literature. A complicating variable in intact studies is endogenous estrogen. This is particularly true when the studies are designed to test the modulation of vaccine-elicited immunity. In non-hormone studies this is controlled for by the administration of progesterone prior to viral challenge, which synchronizes and halts, at least temporarily, the estrous cycles of the mice. However, this is too late for the purposes of our studies because the endogenous estrogen may or may not have been significantly contributing the sum estradiol levels in the mouse. Moreover, such contributions, if significant, would have come at unsynchronized times in the development of the memory immune response to the vaccine. Also, progesterone is known to play an antagonistic role to estradiol in many endocrine pathways; some have suggested it does so in immune ones as well and this is an area of ongoing investigation [11]. Therefore, we thought it important to examine and report the impact of estradiol on vaccine efficacy in intact animals because it provides an additional perspective on the translational relevance of our data (noting the ovariectomized mice performed more like the human vaccine trials than the intact mice) and because it then enables us to put this data in the context of all the other published HSV-2 mouse model data. Also noteworthy, there was a reduction in the incidence of disease across all viral challenge titres in the estradiol-treated groups compared to the groups receiving vaccination alone (data not shown), again suggesting an enhancement of efficacy against the background of already-robust protection, as is more fully explored in the ovariectomized studies.
While there are still additional questions to be explored regarding the impact of estradiol on infection and immunity, the studies described here present an important demonstration that estradiol’s effects go far beyond physiology and can shape the host response to vaccination. The conclusions drawn from these studies have implications that need to be explored using other animal models, other candidate vaccines and other infectious organisms so that, once the mechanism of action of estradiol on the host-response is clearly understood, it may be exploited in a wide range of scenarios to elicit optimal protection from otherwise suboptimal vaccines.
Acknowledgments
This research was funded by Ro1 A1052372 (NB) and R21 AI076062 (MM). JWP was supported by the Sealy Center for Vaccine Development Predoctoral Fellowship.
We would like to thank Chin-Fun Chu, Alison Johnson, Rebecca Johnston for their excellent technical assistance and GlaxoSmithKline for the generous gift of the vaccine.
Footnotes
The authors have no financial conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Weiss H. Epidemiology of herpes simplex virus type 2 infection in the developing world. Herpes. 2004 Apr Apr;11(Suppl 1):24A–35A. [PubMed] [Google Scholar]
- 2.Xu F, Sternberg MR, Kottiri BJ, McQuillan GM, Lee FK, Nahmias AJ, et al. Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. Jama. 2006 Aug 23;296(8):964–73. doi: 10.1001/jama.296.8.964. [DOI] [PubMed] [Google Scholar]
- 3.Corey L. Herpes Simplex Virus. In: Mandell, Bennett, Dolin, editors. Principles and Practices of Infectious Diseases. 6. Philadelphia: Elsevir; 2005. pp. 1762–80. [Google Scholar]
- 4.Stanberry LR. Clinical trials of prophylactic and therapeutic herpes simplex virus vaccines. Herpes. 2004 Aug;11( Suppl 3):161A–9A. [PubMed] [Google Scholar]
- 5.Stanberry LR, Spruance SL, Cunningham AL, Bernstein DI, Mindel A, Sacks S, et al. Glycoprotein-D-adjuvant vaccine to prevent genital herpes. The New England journal of medicine. 2002 Nov 21;347(21):1652–61. doi: 10.1056/NEJMoa011915. [DOI] [PubMed] [Google Scholar]
- 6.Corey L. Synergistic copathogens--HIV-1 and HSV-2. The New England journal of medicine. 2007 Feb 22;356(8):854–6. doi: 10.1056/NEJMe068302. [DOI] [PubMed] [Google Scholar]
- 7.Cook IF. Sexual dimorphism of humoral immunity with human vaccines. Vaccine. 2008 Jul 4;26(29–30):3551–5. doi: 10.1016/j.vaccine.2008.04.054. [DOI] [PubMed] [Google Scholar]
- 8.Bhavanam S, Snider DP, Kaushic C. Intranasal and subcutaneous immunization under the effect of estradiol leads to better protection against genital HSV-2 challenge compared to progesterone. Vaccine. 2008 Nov 11;26(48):6165–72. doi: 10.1016/j.vaccine.2008.08.045. [DOI] [PubMed] [Google Scholar]
- 9.Kaushic C, Ashkar AA, Reid LA, Rosenthal KL. Progesterone increases susceptibility and decreases immune responses to genital herpes infection. Journal of virology. 2003 Apr;77(8):4558–65. doi: 10.1128/JVI.77.8.4558-4565.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gallichan WS, Rosenthal KL. Effects of the estrous cycle on local humoral immune responses and protection of intranasally immunized female mice against herpes simplex virus type 2 infection in the genital tract. Virology. 1996 Oct 15;224(2):487–97. doi: 10.1006/viro.1996.0555. [DOI] [PubMed] [Google Scholar]
- 11.Gillgrass AE, Fernandez SA, Rosenthal KL, Kaushic C. Estradiol regulates susceptibility following primary exposure to genital herpes simplex virus type 2, while progesterone induces inflammation. Journal of virology. 2005 Mar;79(5):3107–16. doi: 10.1128/JVI.79.5.3107-3116.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gillgrass AE, Tang VA, Towarnicki KM, Rosenthal KL, Kaushic C. Protection against genital herpes infection in mice immunized under different hormonal conditions correlates with induction of vagina-associated lymphoid tissue. Journal of virology. 2005 Mar;79(5):3117–26. doi: 10.1128/JVI.79.5.3117-3126.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yao XD, Fernandez S, Kelly MM, Kaushic C, Rosenthal KL. Expression of Toll-like receptors in murine vaginal epithelium is affected by the estrous cycle and stromal cells. Journal of reproductive immunology. 2007 Jun 15; doi: 10.1016/j.jri.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 14.Bourne N, Milligan GN, Schleiss MR, Bernstein DI, Stanberry LR. DNA immunization confers protective immunity on mice challenged intravaginally with herpes simplex virus type 2. Vaccine. 1996 Sep;14(13):1230–4. doi: 10.1016/s0264-410x(96)00027-8. [DOI] [PubMed] [Google Scholar]
- 15.Kuhnz W, Putz B. Pharmacokinetic interpretation of toxicity tests in rats treated with oestradiol valerate in the diet. Pharmacology & toxicology. 1989 Sep;65(3):217–22. doi: 10.1111/j.1600-0773.1989.tb01160.x. [DOI] [PubMed] [Google Scholar]
- 16.Teepe AG, Allen LB, Wordinger RJ, Harris EF. Effect of the estrous cycle on susceptibility of female mice to intravaginal inoculation of herpes simplex virus type 2 (HSV-2) Antiviral research. 1990 Oct–Nov;14(4–5):227–35. doi: 10.1016/0166-3542(90)90004-q. [DOI] [PubMed] [Google Scholar]
- 17.Bourne N, Stegall R, Montano R, Meador M, Stanberry LR, Milligan GN. Efficacy and toxicity of zinc salts as candidate topical microbicides against vaginal herpes simplex virus type 2 infection. Antimicrobial agents and chemotherapy. 2005 Mar;49(3):1181–3. doi: 10.1128/AAC.49.3.1181-1183.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Milligan GN, Bernstein DI, Bourne N. T lymphocytes are required for protection of the vaginal mucosae and sensory ganglia of immune mice against reinfection with herpes simplex virus type 2. J Immunol. 1998 Jun 15;160(12):6093–100. [PubMed] [Google Scholar]
- 19.Bernstein DI, Stanberry LR, Harrison CJ, Shukla R, Kappes JC, Myers MG. Antibody response to herpes simplex virus glycoprotein D: effects of acyclovir and relation to recurrence. The Journal of infectious diseases. 1987 Sep;156(3):423–9. doi: 10.1093/infdis/156.3.423. [DOI] [PubMed] [Google Scholar]
- 20.Bourne N, Stanberry LR, Bernstein DI, Lew D. DNA immunization against experimental genital herpes simplex virus infection. The Journal of infectious diseases. 1996 Apr;173(4):800–7. doi: 10.1093/infdis/173.4.800. [DOI] [PubMed] [Google Scholar]
- 21.Bourne N, Milligan GN, Stanberry LR, Stegall R, Pyles RB. Impact of immunization with glycoprotein D2/AS04 on herpes simplex virus type 2 shedding into the genital tract in guinea pigs that become infected. The Journal of infectious diseases. 2005 Dec 15;192(12):2117–23. doi: 10.1086/498247. [DOI] [PubMed] [Google Scholar]
- 22.Dusterberg B, Nishino Y. Pharmacokinetic and pharmacological features of oestradiol valerate. Maturitas. 1982 Dec;4(4):315–24. doi: 10.1016/0378-5122(82)90064-0. [DOI] [PubMed] [Google Scholar]
- 23.Gillgrass AE, Ashkar AA, Rosenthal KL, Kaushic C. Prolonged exposure to progesterone prevents induction of protective mucosal responses following intravaginal immunization with attenuated herpes simplex virus type 2. Journal of virology. 2003 Sep;77(18):9845–51. doi: 10.1128/JVI.77.18.9845-9851.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garnett GP. Role of herd immunity in determining the effect of vaccines against sexually transmitted disease. Journal of Infectious Diseases. 2005 Feb 1;191(Suppl 1):S97–106. doi: 10.1086/425271. Feb 1. [DOI] [PubMed] [Google Scholar]
- 25.Garnett GP, Dubin G, Slaoui M, Darcis T. The potential epidemiological impact of a genital herpes vaccine for women. Sexually transmitted infections. 2004 Feb;80(1):24–9. doi: 10.1136/sti.2002.003848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bouman A, Heineman MJ, Faas MM. Sex hormones and the immune response in humans. Human Reproduction Update. 2005 Jul–Aug;11(4):411–23. doi: 10.1093/humupd/dmi008. [DOI] [PubMed] [Google Scholar]
- 27.Pessina MA, Hoyt RF, Jr, Goldstein I, Traish AM. Differential regulation of the expression of estrogen, progesterone, and androgen receptors by sex steroid hormones in the vagina: immunohistochemical studies. The journal of sexual medicine. 2006 Sep;3(5):804–14. doi: 10.1111/j.1743-6109.2006.00290.x. [DOI] [PubMed] [Google Scholar]
- 28.Parr MB, Parr EL. Vaginal immunity in the HSV-2 mouse model. International reviews of immunology. 2003 Jan–Feb;22(1):43–63. doi: 10.1080/08830180305228. [DOI] [PubMed] [Google Scholar]