Abstract
Background and Aims
Morphological mutants have been useful in elucidating the phytomeric structure of plants. Recently described mutants have shed new light on the ontogeny (development of plant structures) and the phytomeric system of barley (Hordeum vulgare). Since the current model for barley phytomers was not adequate to explain the nature of some mutants, a new model is proposed.
Methods
New phytomer mutants were detected by visual assessment of mutant families in the Optic barley mutation grid population. This was done at various growth stages using laboratory, glasshouse and field screens. Simple explanations were adopted to account for aberrant phytomer phenotypes and a thesis for a new phytomer model was developed.
Key Results and Conclusions
A barley phytomer model is presented, in which the origins of vegetative and generative structures can be explained by a single repeating phytomer unit. Organs on the barley plant are divided into two classes, single or paired, depending on their origin. Paired structures are often fused together to create specific organs. The model can be applied to wheat (Triticum aestivum) and related grasses.
Key words: Barley, Hordeum vulgare, phytomer, floral structure, mutants
INTRODUCTION
The concept that organisms are composed of repeated building blocks containing fixed regions of cell division and origins of differentiation can be traced back to Bateson (1894). In higher plants the repeated unit is referred to as a phytomer and the most basic form is the ‘stem and leaf’ model proposed by Rutishauser and Sattler (1985). The phytomer unit contains a specific spatial arrangement of meristematic regions that give rise to an ordered development of organs. Different organs, e.g. floral structures, can arise by variation, but the phytomer structure provides a degree of rigidity and predictability to the morphological development of the plant. The phytomer is therefore of fundamental importance in plant development and systematics (evolution, speciation and taxonomy) as well as pure and applied aspects of botany.
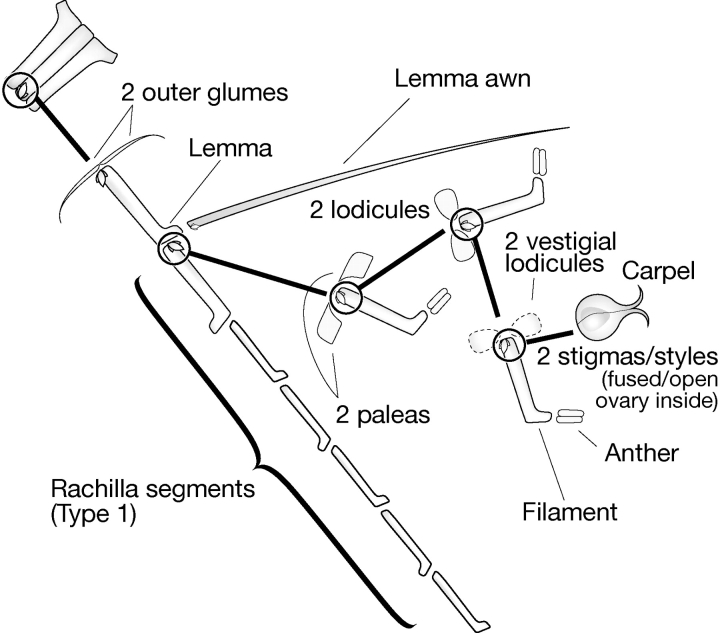
Barley (Hordeum vulgare) was chosen by Bossinger et al. (1992) to develop the phytomer concept in higher plants because it has a relatively simple, two-rowed (distichous) arrangement of leaves and spikelets produced on opposite and alternating sides of vegetative stems and floral axes, respectively (for morphology and anatomy, see Reid, 1985). Barley also has an advantage in that several developmental mutants were available for study from which Bossinger et al. (1992) developed an elaborate phytomeric system for the vegetative and generative structures. Two phytomer types were proposed: type 1, which are produced in series (one on top of the other), and type 2, which is produced as a branch in axillary buds. Various combinations of these provided the architectural structures of the barley stem, tillers (stem branching), rachis, rachilla and floret (Fig. 1). The phytomeric structure of barley given in the model of Bossinger et al. (1992) has a number of weaknesses: embryo structure is not considered, suppression of phytomer regions is not clearly explained and the origins of floral units and reproductive organs are not explained (connections between these structures are undefined black boxes, Fig. 1). Phenotyping of new induced mutant families (B. P. Forster et al., unpubl. res.) has provided additional and novel morphological mutants that shed new light on the structure of the barley phytomer and stimulated a review of previous ontogeny studies.
Fig. 1.
The phytomeric structure of barley, re-drawn from Bossinger et al., 1992.
The nodal structure of barley (and other grasses) is critical with respect to grass architecture as all other structures are derived from it. Nodes are structures that link phytomer units together to form axes for vegetative and reproductive structures, as outlined by Bossinger et al. (1992) (Fig. 1). The node is composed of two segments or half nodes. Internodes of the stem (culm), rachis and rachilla phytomers are connected together by a half node at each end. Buds and root initials arise from the half node at the base of the phytomer, the upper half of the node. The half node at the top of the phytomer, the lower half node, gives rise to a single side arm. The nodal concept brings together contributions from two phytomers, thus the developing components of one phytomer are protected by the sheath produced by the phytomer below it. This concept is also important in the inflorescence where internode elongation of the rachis and rachilla is restricted and floral organs arise from branching phytomers.
Taxonomically, barley belongs to the section Hordeum of the genus Hordeum, which forms part of the tribe Triticeae of the grass family Poaceae. The Triticeae is composed of over 350 species. In addition to barley, the Triticeae is home to other important small grain cereals, bread and durum wheats (Triticum spp.), rye (Secale cereale) and the wheat/rye hybrid crop, triticale (×Triticosecale), as well as several forage grass species (von Bothmer, 1992; von Bothmer et al., 1995). The phytomer model developed for barley has implications for these related species and other monocots.
THE THESIS
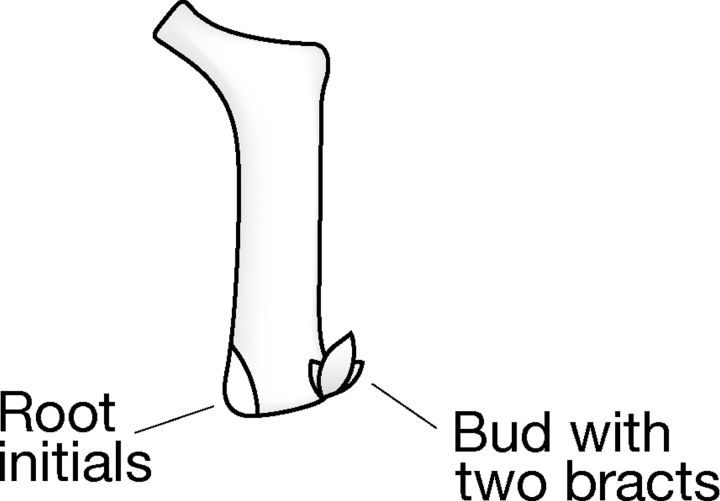
The development of a modified phytomer model has been progressive and based on recent novel morphological mutants induced and detected in the barley ‘Optic’ (Caldwell et al., 2004; B. P. Forster et al., unpubl. res., see also http://germinate.scri.sari.ac.uk/barley/mutants/) as well as mutants described in the Barley Genetics Stocks Database (http://ace.untamo.net). As a guide, the simplest explanation for each new phytomer mutant detected was adopted. Patterns in organ development became apparent from which the revised phytomer structures were deduced (Fig. 2).
Fig. 2.
The basic barley phytomer.
Fused paired bracts
A morphological mutant of the palea was amongst the first of a set of new unfused organ mutants that brought into question the earlier phytomeric model of Bossinger et al. (1992). In barley, the palea is a bifid structure (von Bothmer and Jacobsen, 1985) which, along with the lemma, protects floral parts (Fig. 3B) and, after pollination, the caryopsis (one-seeded fruit) develops. A split palea mutant was detected in the Optic mutant population (Fig. 3B). Our explanation for a split palea is that the normal palea in barley represents two paired structures that are fused together at a common edge. The normal fusion mechanism was dysfunctional in the mutant. This assumption is supported by the fact that the normal palea has two keels and the awned palea (adp1) mutant possesses two awns (see Table 1 for references to all quoted phytomer mutants of barley). Also, split and divided paleas are well known in other members of the Triticeae and are botanical descriptors of species such as Triticum timopheevi and T. diccocoides (Peterson, 1965). The palea was therefore considered to have a similar origin as other paired structures of the barley plant, e.g. coleoptile, outer glumes, lodicules, styles, etc. (Tables 2 and 3).
Fig. 3.

Split organ phytomer mutants in barley: (A) split coleoptile (right, wild type to left): (B) split palea (abaxial and adaxial surfaces of the caryopsis, mutant to right, wild type to left).
Table 1.
Mutants of barley that alter phytomer development
| Gene symbol | Gene or locus name/description | Type specimen (where available) |
|---|---|---|
| abr1 | Accordion basal rachis internode 1 | BGS 472 |
| acr1 | Accordion rachis 1 | BGS 097 and others |
| adp1 | Awned palea 1 | BGS 593 |
| als1 | Absent lower laterals 1 | BGS 101 |
| ant4 | Anthocyanin-deficient 4 | BGS 595 |
| ari | Breviaristatum/short awn | BGS 132 and others |
| ari-e.156 | Breviaristatum-e.156/short awn | BGS 328 |
| asp1 | Aborted spike | BGS 649 |
| blf1 | Broad leaf 1 | BGS 326 |
| bra | Bracteatum/leafy bracts on rachis | BGS 586, 619 |
| brc1 | Branched 1/branched spike | BGS 613 |
| brh | Brachytic/dwarf | BGS 001 and others |
| brh13 | Brachytic 13 | BGS 656 |
| brh16 | Brachytic 16 | BGS 044 |
| btr | Non-brittle rachis | BGS 115 and others |
| cal | Calcaroides/hooded lemma | BGS 146 and others |
| com1.a | Compositum/branched spikelets | BGS 473 and others |
| crl1 | Curly laterals 1/curled awns on lateral spikelets | BGS 325 |
| cud | Curly dwarf/leaves short and twisted | BGS 229, 324 |
| cul | Uniculm | BGS 253 and others |
| cur | Curly/all plant parts appear twisted | BGS 262 and others |
| dsp | Dense spike/reduced rachis internode length | BGS 009 and others |
| dub1 | Double seeds 1 | BGS 661 |
| Dwf2 | Dominant dwarf 2/insensitive to gibberellic acid | BGS 542 |
| eam | Early maturity/photoperiod responses | BGS 065 and others |
| eli-a | Eligulum-a/liguleless | BGS 623 |
| eog1 | Elongated outer glume 1 | BGS 057 |
| ert | Erectoides/short rachis internodes | BGS 028 and others |
| flo | Extra floret | BGS 074, 182, 327 |
| fol | Angustifolium/organs reduced in size | BGS 073, 548 |
| gig | Gigas/culm and other organs greater in size | BGS 463, 612 |
| glo | Globosum/short spikelets, round grain | BGS 168 and others |
| gra1 | Granum 1/numerous tillers with thin leaves | BGS 131 |
| hcm1 | Short culm 1 | BGS 077 |
| int | Intermedium spike/extra spikelets | BGS 320 and others |
| int-c | Intermedium spike-c/six-rowed spike modifier | BGS 178 |
| int-h | Intermedium spike-h | BGS 544 |
| int-i | Intermedium spike-i | BGS 545 |
| int-m | Intermedium spike-m | BGS 547 |
| Kap1 | Hooded lemma 1/deformed floret on lemma | BGS 152 |
| lax | Laxatum/lax spike | BGS 475 and others |
| lax-a | Laxatum-a | BGS 474 |
| lbi | Long basal rachis internode | BGS 308 and others |
| lel1 | Leafy lemma 1 | BGS 235 |
| Lfb1 | Leafy bract 1/leafy collar | BGS 343 |
| lfs | Leaf-less/variable leaf blade development | |
| Lga1 | Long glume awn 1 | BGS 549 |
| lig1 | Liguleless 1 | BGS 060 |
| lin1 | Lesser internode number 1 | BGS 099 |
| Lin2 | Lesser internode number 2 | BGS 636 |
| lks | Short awn | BGS 010 and 172 |
| Lks1 | Awn-less 1 | BGS 075 |
| lnt1 | Low number of tillers | BGS 118 |
| mac3 | Maculosus/necrotic fleck plus a broad leaf | |
| min | Semi-minute dwarf | BGS 160, 161 |
| mnd | Many-noded dwarf | BGS 633 and others |
| mov1 | Multi-ovary 1 | BGS 043 |
| mov2 | Multi-ovary 2 | BGS 147 |
| msg | Male sterile genetic | BGS 357 and others |
| mul1 | Multiflorus 1/supernumerary florets = vrs4 | BGS 124 |
| mul2 | Multiflorus 2/rachilla florets formed | BGS 251 |
| nld1 | Narrow leaf dwarf 1/also degenerate auricles | BGS 323 |
| ops | Opposite spikelets/variable rachis internodes | BGS 624 and others |
| ovl | Ovary-less | BGS 176, 646 |
| pyr | Pyramidatum/pyramide spike shape | |
| raw | Smooth awn | BGS 312 and others |
| rtt1 | Rattail spike | BGS 051 |
| sbk1 | Subjacent hood 1/Calcaroides | BGS 062 |
| sci | Scirpoides/narrow leafed dwarf | BGS 525 and others |
| scl | Scirpoides leaf/narrow folded leaf blade | BGS 526 and others |
| sdw | Semi-dwarf | BGS 518 and others |
| sdw2 | Semi-dwarf 2 | BGS 133 |
| seg8 | Shrunken endosperm 8 | BGS 455 |
| sid1 | Single elongated internode dwarf 1 | BGS 180 |
| Sil1 | Subcrown internode length 1 | BGS 228 |
| sld | Slender dwarf | BGS 126 and others |
| sls1 | Small lateral spikelet 1 | BGS 227 |
| snb1 | Subnodal bract/additional bract on spikelet 1 | BGS 026 |
| srh1 | Short rachilla hair 1 | BGS 321 |
| tfl | Thick filament | |
| trd1 | Third outer glume 1/additional bract on spikelet | BGS 202 |
| trp1 | Triple awned lemma 1 | BGS 061 |
| tst | Tip sterile | BGS 636, 647 |
| ubs4 | Unbranched style 4 | BGS 011 |
| uzu1 | Semi-brachytic/shortened organs | BGS 102 |
| viv | Viviparoides/plantlets on the spike | BGS 627 and others |
| vrs1.a | Six-rowed spike 1 | BGS 006 and others |
| vrs3 | Six-rowed spike 3 | BGS 315 |
| vrs4 | Six-rowed spike 4 = Multiflorus 1 | BGS 124 |
| Vrs1.t | Deficiens 1/extremely reduced lateral spikelet | BGS 067 |
| wnd1 | Winding dwarf 1/coiled peduncle | BGS 023 |
| Zeo | Zeocriton, short culms, dense spike | BGS 082 and others |
These mutants are described in greater detail in the Barley Genetics Stocks AceDB Database (http://ace.untamo.net), in Barley Genetics Newsletter Vol. 35 and by personal communication. Gene nomenclature is based on rules given at the 9th International Barley Genetics Symposium (2004).
Table 2.
Single and paired organs of barley
| Single organs | Paired organs |
|---|---|
| Plumule | Coleoptile |
| Leaf sheath | Prophyll |
| Leaf blade | Auricles |
| Ligule | Outer glumes |
| Lemma | Palea |
| Awn | Lodicules |
| Anther | Style/stigma |
| Pistil | Integuments |
| Ovule | Lateral lobes of the endosperm |
| Nucellus | |
| Central lobe of the endosperm |
Table 3.
Organs classed by phytomeric origin
| Paired basal buds | Side arm | Phytomer on side arm |
|---|---|---|
| Coleoptile | Leaf sheath | Leaf blade |
| Prophyll | Collar | Awn |
| Outer glumes | Lemma | Stigma |
| Palea | Stamen anther | |
| Lodicules | Carpel | |
| Stigma/styles | ||
| Auricles | ||
| Styles |
A second split organ mutant observed in seedlings of the Optic mutant population was a split coleoptile (Fig. 3A). The coleoptile is a sheath that protects the terminal bud of the embryo (plumule); it grows out as a tube which protects the shoot during germination. The coleoptile has two mid-ribs and two vascular bundles opposite each other (Arber, 1934) and is thought to originate from two fused structures (Bossinger et al., 1992). This conclusion is supported by the finding of a split coleoptile mutant, which is assumed to be dysfunctional in the fusing process. The uzu1 semi-dwarf mutant produces two notches at the tip of the coleoptile and may represent a partial split of the coleoptile into two parts. The coleoptile is also regarded as having a structure similar to that of the prophyll (Jacques-Felix, 1957; Guignard, 1962; Gould and Shaw, 1983). The prophyll is the organ that protects the developing axillary shoots (tillers); however, to date no split prophyll mutant has been reported.
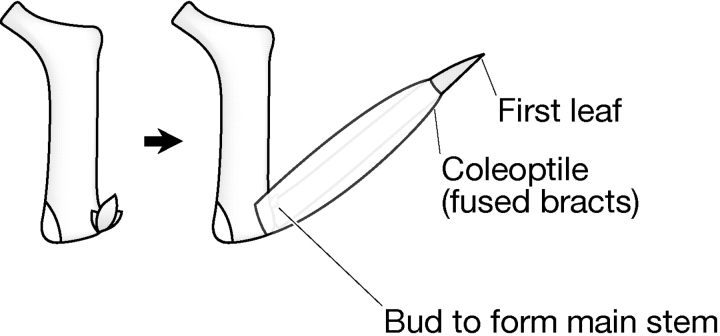
It became apparent that vegetative organs could be classed as either paired (fused or unfused) or as single structures (Table 2). Furthermore, paired organs were always associated with a single-structured organ, e.g. coleoptile (paired) and the first seedling leaf (single); auricles (paired) and leaf blade (single), prophyll (paired) and the first tiller leaf (single) (Figs 4–7).
Fig. 4.
Phytomeric structure of the first leaf.
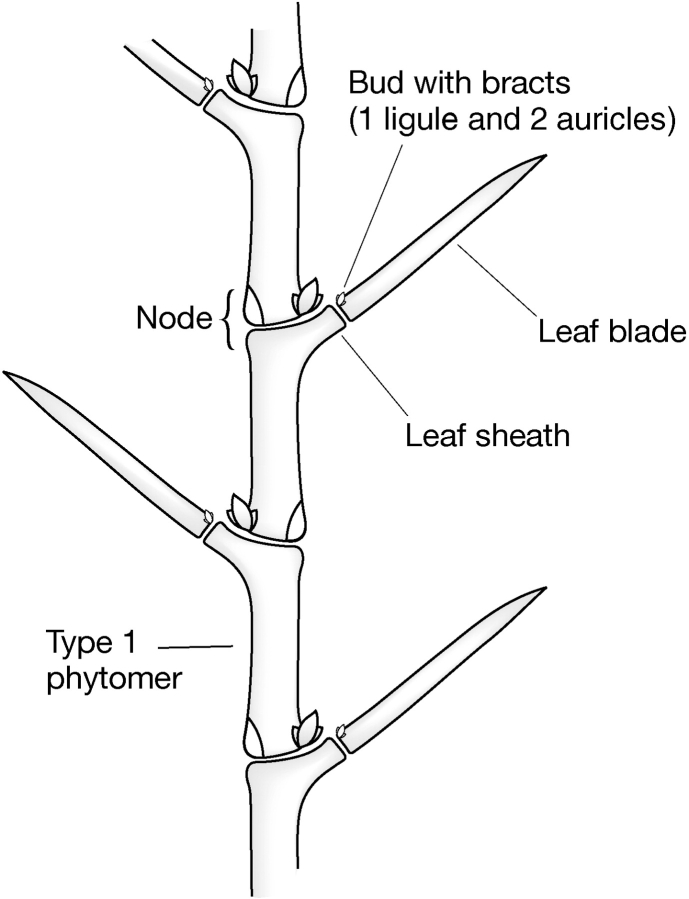
Fig. 5.
Phytomeric structure of the culm.
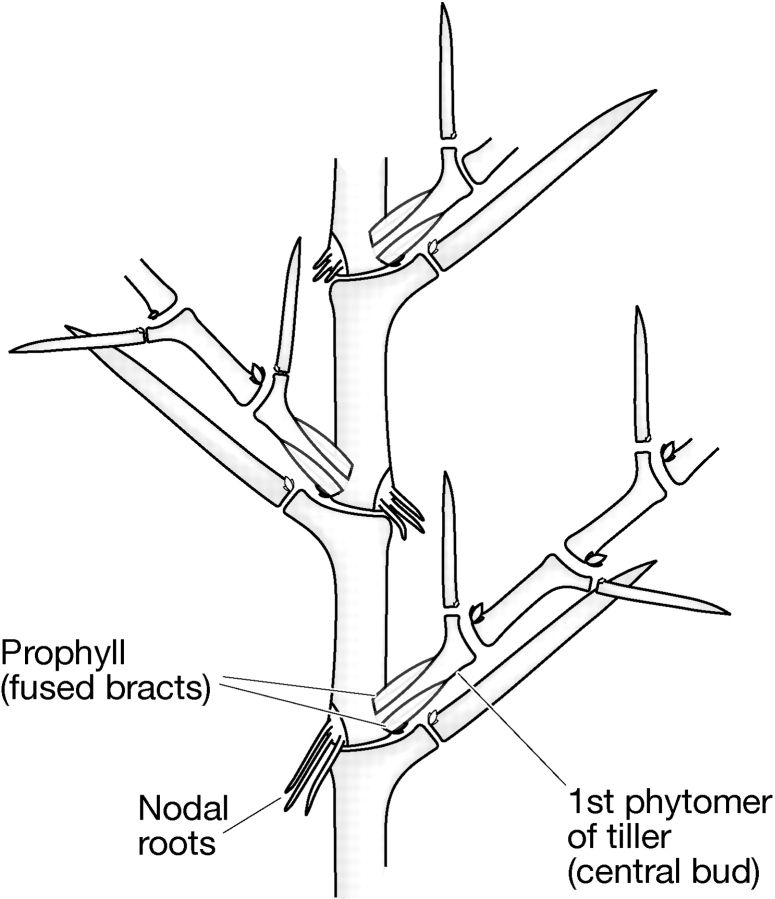
Fig. 6.
Phytomeric structure of tiller production.
Fig. 7.
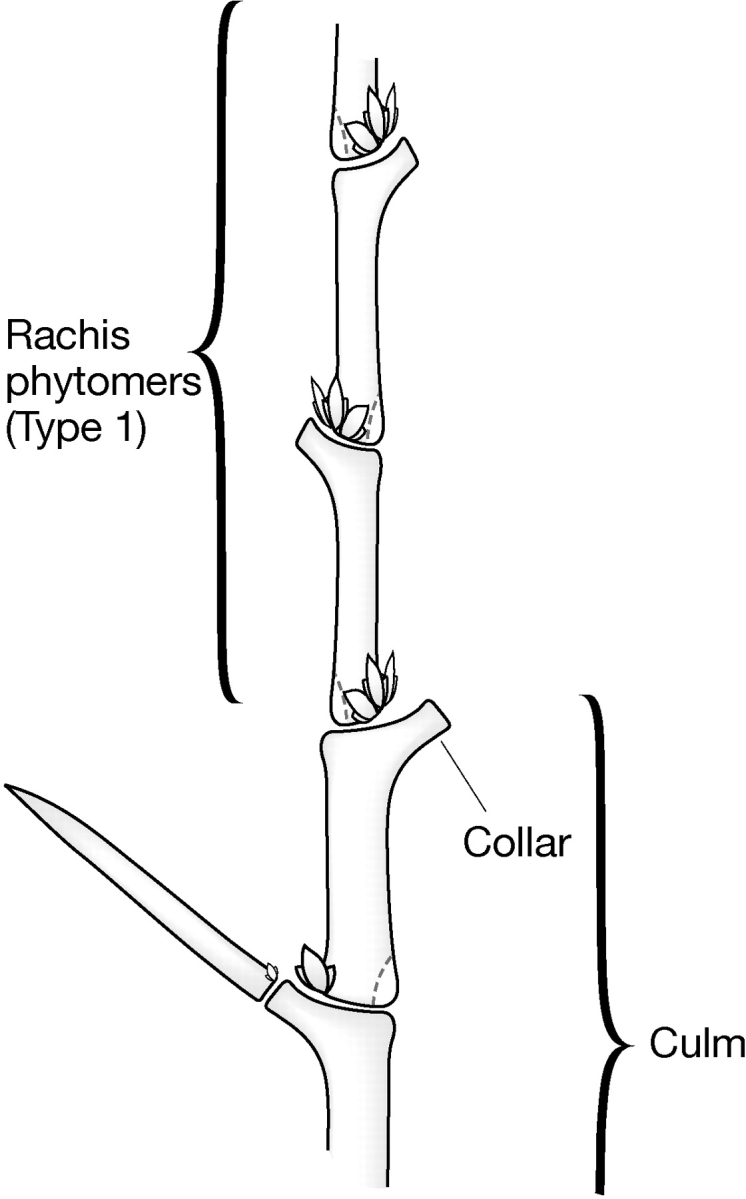
Phytomeric structure of the transition from culm to rachis.
Paired bracts in reproductive structure
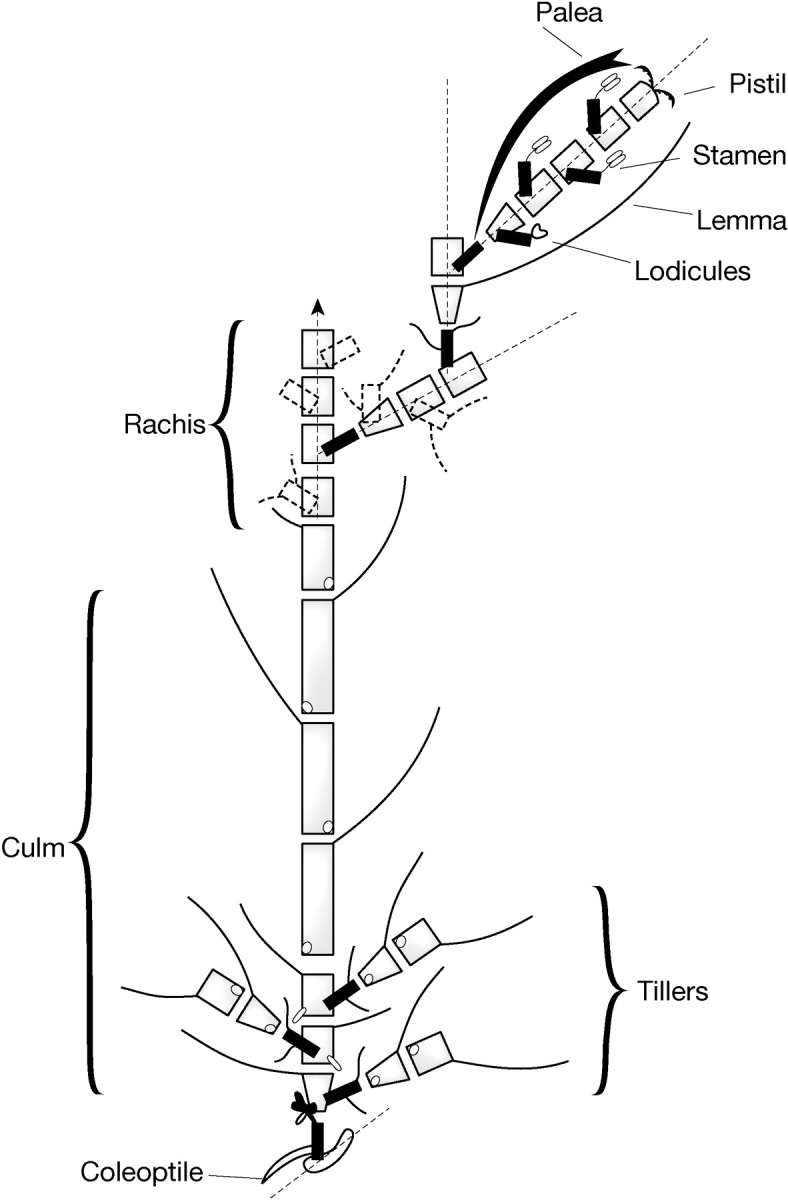
The paired-single-paired-single sequence was also apparent in generative structures, thus the spikelet is subtended by a pair of outer glumes with a single lemma inside followed by a palea (fused pair), etc. (Fig. 8). The logical extension of the 2 + 1 series of spikelet organs is: palea (paired), then the first stamen (single), followed by lodicules (paired), the second stamen (single), followed by an unknown paired structure, the third stamen and finally the pistil complex (Fig. 8). A missing piece in the series is a paired structure following the second anther. The mature barley floret shows no obvious twin structure at this point. However, several grass species are known to possess two pairs of lodicules, a dorsal and an anterior pair, with the dorsal pair being absent in some species (Guedes and Dupuy, 1976; Dahlgren et al., 1985). It is therefore reasonable to assume that formation of the second pair of lodicules is suppressed in barley (see below for further discussion on abortive paired structures during transitional phases).
Fig. 8.
Phytomeric structure of the spikelet.
Phytomer models
The branch or type-2 phytomer model was developed by Bossinger et al. (1992) to take into account the 2 + 1 association of leaf-like organs in buds. It is the modification of the ‘stem and leaf’ type-1 phytomer proposed by Bossinger et al. (1992). In the original model (type 1) contiguous phytomer units are added on top of each other by the activity of the apical meristem. This one-dimensional activity is responsible for the build-up of vegetative stem units, and, after transition to generative growth, to those of the rachis. The leaf sheath is placed at the side and top of the phytomer and is attached to the upper half node. The next type-1 phytomer is orientated 180° to protect the bud initials and expose the root initials. New type-1 phytomer units can also be added onto the branch or type-2 phytomer. A key difference in the model proposed here is that all structures can be derived from one phytomer design. Some structures may be simple (e.g. bracts) whereas others require specific combinations of phytomers produced by branching, series and side arm development.
Bud numbers and positions
Normally one axillary bud (with a pair of bracts) forms at the base of the phytomer; it is likely that the development of other buds is suppressed in stems. The exception in barley is that three buds generally develop on the rachis phytomers and produce three spikelets at each rachis internode. The number of floral buds is partially unrestricted, however, in multiflorous mutants such as mul1 or vrs4. Two additional adventitious buds develop in pairs near the base and on opposite sides of the previous adventitious bud, giving a bud number series of 1, 3, 7, and possibly 15. Further proliferation of floral buds produces the rattail-like spike (rtt) mutants. In addition, a single adventitious floral bud (spikelet) occasionally can arise below the central bud (spikelet) in the case of the extra floret (flo) mutants. Lateral spikelets can have a short pedicel in some intermedium and six-rowed spike mutants (int-c, vrs3 and vrs4). Seminal root number has not been studied in detail, but it is likely that these are also produced in the same (2n − 1) series as adventitious buds.
Figure 5 shows the phytomeric composition of a barley culm (stem) and leaf development. In the model, the leaf sheath develops from a broad meristematic region on the upper half node of the phytomer. The meristematic region at the tip of the sheath forms a bud which develops into a phytomer at the tip of the sheath, which produces the leaf blade and two auricles and the leaf blade in a 2 + 1 association. The leaf blade forms on top of the side arm of the phytomer below. The ligule may represent an aborted segment of the phytomer that is below the blade attachment point.
Bud development
Tillering proceeds by an axillary bud developing into the first phytomer of the side shoot. The growing point of the bud is protected by the prophyll (a fused organ produced by two lateral bracts of the phytomer). Root buds are assumed to be present at the base of the phytomer opposite the bud position, but they are only active in stem sections near the base of the plant (Fig. 2). The number of buds that develop into tillers can be restricted by environmental and genetic factors. The uniculm 2 (cul2) mutant has only a primary tiller. Low tiller number (lnt1 or int-l) mutants produce only two to four well-formed tillers. Other cul mutants produce a primary tiller plus several weak secondary tillers. Besides drastically reduced tillering, the cul, als1 and lnt1 mutants cause spike malformations. Excess tillering is observed in multi-noded dwarf (mnd), granum (gra1) and leaf-less (lfs) mutants.
Internode and leaf elongation
Elongation of internodes can occur in the sub-crown region, the culm and reproductive structures such as the rachis, rachilla, etc. A number of genes control stem growth or plant height producing either tall (gig and ant4) or semi-dwarf (ari, brh, dwf, ert, hcm, lzd, mnd, sdw, sid, sld, uzu and Zeo) mutants. The rachis internodes can be lengthened in accordion rachis (acr) and laxatum (lax) mutants or shortened (ari, brh, ert, dsp, pyr and Zeo mutants). The opposite spikelet (ops) mutants have irregular rachis internode lengths. The length of the sheath, leaf blade, awns and coleoptiles can be altered by a number of the same genes. Some ari and lks mutants only have shortened awns. Elongation of the sub-crown internode is suppressed by the Sil1 gene in some winter barley cultivars. Elongation of internodes is suppressed in numerous barley organs and the presence of phytomers can be deduced only by development of buds or branches.
The width of leaf blades and related organs such as the lemma is also under genetic control. Wide-leafed phenotypes include six-rowed barley and some mutants (blf1, gig2 and mac3) while narrow-leafed phenotypes include mutants of the blf2, gra1, mnd, nld, sci and sci series. Excessive twisting of leaves and other organs is caused by curly (cur) and curly dwarf (cud) mutants. The winding dwarf (wnd1) mutant appears to only affect coiling of the peduncle.
Transition to reproductive structures
Spike development is preceded by the final culm phytomer unit (called the peduncle) in which the leaf is reduced to a collar (Fig. 7). A number of photoperiod response or early maturity (eam, mat and ppd) genes determine the timing of this transition. Physical transition to the collar and spike is delayed in the many-noded dwarf (mnd) mutants and nearly stopped in some tillers of the viviparoides (viv) mutants, which remain vegetative. The collar is a leaf-like structure in the leafy bract (Lfb1) and semi-dwarf 2 (sdw2) mutants. The leaf-like structure can persist for several rachis nodes in the third outer glume (trd1) and bracteatum (bra) mutants. An extreme example of transition failure is the aborted spike (asp1) mutant in which spike formation partially or completely fails.
The rachis (spike axis) like the vegetative stem is composed of a single file of phytomers and produces spikelets in a strict distichous fashion, but with a much reduced internode length. Elongation of the first rachis internode is not adequately suppressed in the long basal internode (lbi) mutants and in some intermedium (int-h and int-i) mutants. In the accordion basal rachis internode (abr1) mutant, several basal rachis internodes are elongated and secondary or tertiary vegetative branches may form at the lower rachis nodes. The number of fertile rachis nodes on the rachis can be reduced by lesser internode number (lin) and tip sterile (tst) mutants.
Spikelet development
A diagnostic feature of the Hordeum genus is the possession of three one-flowered spikelets at each rachis node (von Bothmer et al., 1995). Since alternating triplets appear opposite each other in two ranks, six files of spikelets can be observed. Rachis phytomers therefore have three buds at their base, which develop into three separate spikelets. If the three spikelets at each rachis internode are fertile the plant is identified as a six-rowed barley. In two-rowed forms only the central spikelet of the triplet is fertile. Positioning of extra spikelets is considered above.
The outer glumes are the first visible structure of the spikelet, but do not necessarily define a spikelet, as supernumerary and branched spikes can form in the case of some many-noded dwarf (mnd) mutants and the branched spike (brc1) mutant, respectively. Bonnett (1966) provides a detailed description of the development of supernumerary spikelets. A series of mutants can cause fasciation at the tip of the spike (ari-e.156, brh13, brh16, int-i and int-m) and com1.a caused fasciations at the base. Fasciations of the floret (wide lemma trait) results in the formation of double- (dub1) and triple-kernel (flower) mutants.
From the lower half node of each rachis node, side branches can develop into spikelets, each having a rachilla axis made up of phytomers in series. Flowering in barley is determinate with the production of a single floret per spikelet. The main axis of the spikelet continues to develop, forming a vestigial rachilla with greatly reduced internode lengths (Fig. 8). In the multiflorous 2 (mul2) mutant, the rachilla of lateral spikelets develops a small lemma-like structure, which can develop into a floret and set a seed in some six-rowed cultivars. Spike-like branches arise from the rachilla in the branched inflorescence rachilla (com) mutants. Several sterile basal rachis nodes are observed in some accordion rachis (acr) and laxatum (lax) mutants and in the absent lower laterals (als1) and the slender (sln1) mutants.
Floral components
The floret is composed of a series of organs derived from branch phytomers linked to two other phytomers (Fig. 8). In the 2 + 1 sequence of the model, the two outer glumes are followed by an awned lemma. A fused palea (paired structure) develops from the pair of bracts of a bud phytomer above the lemma and is protected by the lemma. The palea has the same reverse orientation of the bracts that form the prophyll. The second phytomer of the bud produces a stamen on its side arm; its internode elongates to produce a filament on top of which an anther is formed. The palea and associated stamen is another 2 + 1 organ series. Continuing this repeating pattern, the bud of the second phytomer of the palea branch develops into a pair of lodicules (bracts) followed by the second stamen. The third stamen is associated with a pair of vestigial lodicules. Finally the pistil complex is formed (Fig. 8).
Mutants that can cause a conversion of floral parts into other floral components are common in barley. The outer glumes can range in size from normal to lemma-like as determined by alleles of the elongated outer glume (eog) locus. The lodicules of the multi-ovary 1 (mov1) mutant become somewhat leafy or sepal-like. Conversion of the lodicules into anthers occurs in the laxatum-a (lax-a) mutants, but the extra anthers are deficient in having two rather than four microsporangia (Bossinger et al., 1992), their incomplete development being symptomatic of their aberrant origins. In the multi-ovary (mov) mutants, stamens are partially or completely converted into pistils. Failure of a functional ovary to develop occurs in ovary-less (ovl) mutants, while anther development fails in many male-sterile genetic (msg) mutants. Reversion of the floral structures to plantlets occurs in the viviparoides (viv) mutants. The Optic population contained sterile mutants where the palea–lemma association was repeated numerous times and other floral organs were not present (G. Simpson, pers. comm., recorded in http://germinate.scri.sari.ac.uk/barley/mutants/).
Differentiation of pistil structures
Assuming the repeated pattern of the spikelet continues, the next set of 2 + 1 structures should be the styles and the ovule. Based on drawings of the vascular structure of the wheat ovary by Batygina (see Lersten, 1987), a pair of styles arise below the ovule, i.e. formed from paired bracts. The next repeated structural combination is the integuments that surround the nucellus. The 2 + 1 association can be extended further to the three lobes of the endosperm. In the shrunken endosperm genetic 8 (seg8) mutant, only the central lobe fails to enlarge and results in the formation of very thin kernels with a distinct dorsal crease in the caryopsis (Felker et al., 1985).
Phytomers in awns and leaves
According to our model the lemma-awn is a distinct phytomeric unit (Fig. 8). Several mutants can be expressed at the lemma/lemma-awn junction. For example, in the hooded lemma (Kap1) mutant floral-like structures are produced at the tip of the lemma instead of an awn (Bonnett, 1966). The gene causing this is similar to the Knotted 1 mutant in maize (Müller et al., 1995). The leafy lemma (lel1) mutant converts the lemma and lemma-awn into a leaf-like structure that is divided into distinct sheath and blade segments. Positioning of the meristematic region is lower on the lemma in the subjacent hood (sbk1) or calcaroides (cal) mutants. Bonnett (1966) published a detailed morphological description of the hood produced by the Kap1 mutant and showed that the tip of the lemma of the normal floret serves as the lemma of the accessory spikelet (Bonnett, 1966). There are therefore phytomeric similarities between leaf (sheath and blade) and lemma structures (lemma and lemma-awn). Morphological similarities between the lemma-awn and the leaf blade include the possession of multiple veins. Awn growth is prevented at the awn-less (Lks1) mutant, while the leaf-less (lfs) mutants partially prevent leaf blade development. The placement of the auricles is variable in some uniculm (cul) mutants while development of awn branches is variable in the triple-awned lemma (trp1) mutant. The base of the lemma-awn is malformed in the curly lateral (crl1) and is easily broken in a number of barley cultivars and in ari-k mutants.
Multifloreted spikelets
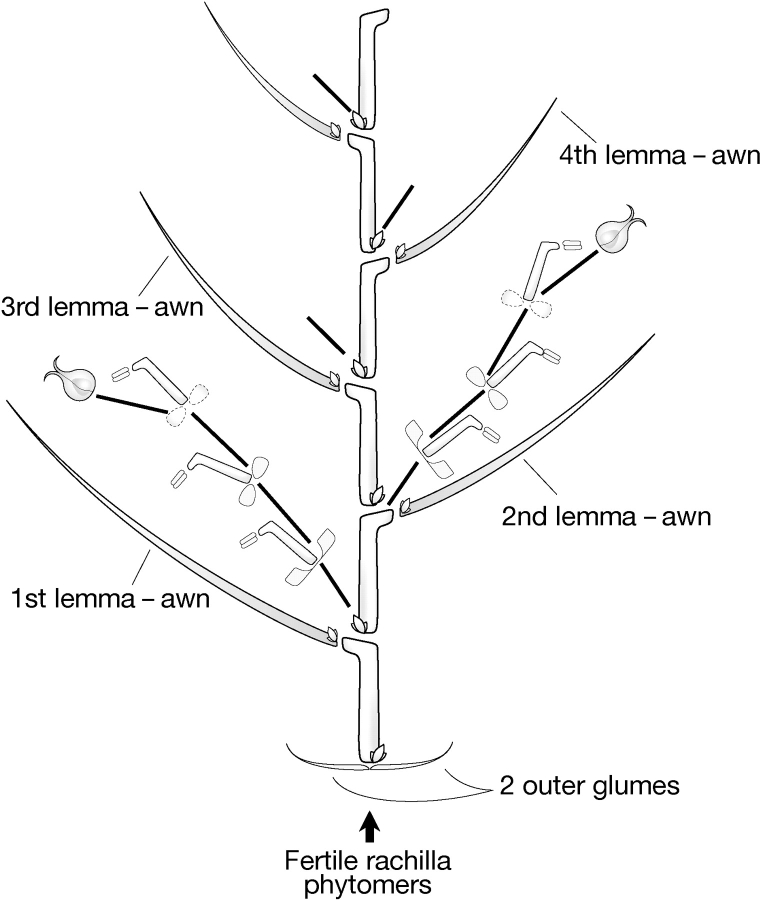
A spikelet of an indeterminate flowering mutant is shown in Fig. 9. In this mutant, two or more florets are produced within a spikelet, the alternating florets face each other and the multi-floreted structure is contained within a pair of glumes. The phytomeric structure of this mutant spikelet is similar to that of wheat and can be created simply by the production of fertile (rather than infertile) rachilla phytomers (Fig. 10). This mutant is of major significance because it not only has the floral structure of another genus, but challenges the taxonomic distinction of the Hordeum genus, which is characterized by having one-flowered spikelets.
Fig. 9.
Indeterminate floral mutant of barley (right) compared with a single floreted spikelet of wild-type barley (left).
Fig. 10.
Phytomeric structure of wheat spikelet.
Nodal structure
Barley mutants also can alter the fusion of nodal segments and this suggests that the node is not simply half nodes attached to each other. The phytomer model assumes that half nodes are cemented together and their separation does not occur. This is not the case in wild barley where disarticulation at the rachis nodes occurs. The brittle rachis mutants (btr) prevent shattering of the spike. Disarticulation in two-rowed barley cultivars occurs at the base of the spikelet. The nodal structure, therefore, may be complex. Some support for this concept comes from eligulum-a (eli-a) mutants where the ligule develops poorly and the stem breaks easily at the junction of the two half nodes.
FEATURES OF THE MODEL, FURTHER ASSUMPTIONS AND CONSEQUENCES
Similarities to the model of Bossinger et al.
As in the model of Bossinger et al. (1992), phytomer units in structures such as the culm, rachis and rachilla are added in single file with a 180° orientation between contiguous phytomers. Tillers and roots emerge from buds arising from the lower regions of the phytomer in half nodes near the base of the plant. These phytomers have relatively short internode lengths and are close to the soil surface. Buds of the elongated internodes that make up the culm are normally suppressed. The rachis is a continuation of the shoot with reduced internode lengths. No leaves or roots are produced on the rachis. Each rachis section produces one or more spikelets from the upper half of each node (Fig. 1).
Differences from the model of Bossinger et al.
The main difference with the previous model is that one phytomer unit can explain the entire architecture of the plant. The phytomer can arise in series from an apical meristem or as a branch (meristematic side bud) or from the top of a side arm. Key elements in the new model are (a) the activity and suppression of various regions of the phytomer, and (b) their associations with linked phytomers. Paired bracts, for example, arise during branching and are essentially dead-end organs, whereas floral structures are associated with three phytomers expressing different activities. The importance of branching, especially in floral structures, is underestimated by Bossinger et al. (1992) and, as a consequence, there is some disagreement in the origins of some organs.
The 2 + 1 organ series
The model divides the organs of the barley plant into paired and single structures arising from either bracts or buds, respectively. All paired structures have a common origin, as outgrowths (possibly modified bracts) at the base of the phytomer unit. Single structures arise as side arms of the phytomer from the lower half of a node. From this, structures can be classed as having paired or single origins (Tables 2 and 3). The relatedness of these structures can then be investigated. Support for this model comes from other phytomer mutants (Table 1). For example, the lemma is in the single origin category and is turned into a leaf-like structure in the leafy lemma (lel1) mutant. The junction between the lemma and awn is similar to the sheath–blade juncture because it can be converted into an organ with multiple parts, as in the hooded (Kap1) mutant. The model also predicts that buds at the end of side arms produce leaf blades and lemma-awns and stigmas. Smooth awn (raw) mutants cause reduced awn barbing and stigma hair numbers on these modified leaf blades. The unbranched style (ubs4) mutants reduce both awn length and stigma length.
Bud suppression during transitional developmental phases
Various transitional/end phases can be identified during developmental stages of a plant's life cycle. In barley and other grasses, these include germination, crown formation and tillering, spike development, fertilization and grain growth and caryopsis maturation. Bud suppression and cessation of organ growth appear to occur during and at the end of these phases. For example, stem elongation is often associated with the suppression and cessation of root development and lateral bud development. Leaf numbers are limited by spike development. The basal bud and bracts of the first rachilla phytomer are suppressed during floret development. The lack of a second pair of lodicules in the barley floret may be a consequence of a transition from the production of male to female reproductive organs. Determinate flowering is also brought about by a suppression of bud activity in producing floret structures from rachilla phytomers once the first floret is formed. In the model presented, axillary buds fail to develop in branch phytomers of vegetative organs such as the tiller and rachilla, which is in marked contrast to floral structures where phytomer branching is the basis of floral structures. It is likely that bud dominance and suppression are also effective during embryo development, germination and establishment of the first culm phytomer.
Phytomer units of the culm carry leaf phytomers, but leaves are missing on the rachis and rachilla. In this respect, the lemma can be considered as a modified leaf. Leaf-like structures can be produced from the lower half of the rachis node at which spikelets are attached, in such mutants as bracteatum (bra) and third outer glume (trd1) (Table 1) in the precise location at which a leaf unit would be expected if it was present.
Developmental associations can be inferred from mutants that have similar effects on related structures. For example, the smooth awn (raw) mutants reduce the number and size of barbs on the lemma-awn, but also reduce the number of hairs on the stigma (as a result raw mutants are often associated with reduced female fertility). Branching and shortening of the rachilla hairs is caused by the short rachilla hair (srh1) mutant, but the rachis margin and the outer glumes also have shortened hairs. The unbranched style (ubs4) mutants reduce both stigma length and awn length. Conversely, mutants that affect plant height, awn length, dense and lax spike, grain shape, leaf width and coiling of plant parts often show pleiotropic effects on phytomers in other parts of the plant.
Taxonomic problems and evolutionary aspects
Morphological and anatomical descriptors are traditional tools used to distinguish all levels of taxonomy in the plant kingdom, and these are inextricably linked to phytomer activity. For example, morphological and anatomical descriptors are used to divide the Hordeum genus into four sections, Hordeum, Anisolepis, Critesion and Stenostachys; the section Anisolepsis is characterized as lacking auricles, which can be accounted for by phytomer bract suppression. The identification of a barley mutant with fertile multiple florets is a taxonomic non sequitur as Hordeum, by definition, is a genus with a one-floreted spikelet. Indeterminate/determinate floret production is a result of bud growth regulation in rachilla phytomers. Clearly, genus and species borders can be transgressed by mutation. The ability to produce mutants that transgress long-established taxonomic boundaries emphasizes the relatedness between species and genera, especially since differences which may be caused by single genes. A benefit is that the barley phytomer model can be applied to other related species, such as wheat, to study phytomer evolutionary differences. The barley model is easily adapted to produce the wheat spikelet architecture by assuming indeterminacy of the rachilla. Species variation can be of interest to plant breeders as it may be possible to manipulate one species to attain a trait present in another, thus the development of indeterminate flowering in barley may have the potential to increase barley yields to levels of wheat. Subtleties in phytomer orientation/angle may also give rise to morphological differences between species.
Applications
The structure of phytomers is of basic interest to botany and has the potential to elucidate the evolutionary development of organs, species and genera. The patterns of organ development are determined by the positioning and switching on and off the development of phytomeric units, and the suppression of various phytomeric components can be studied in a more predictive manner.
ACKNOWLEDGEMENTS
SCRI receives grant-in-aid from the Scottish Executive Environment and Rural Affairs Department (SEERAD).
LITERATURE CITED
- Arber A. The Graminae. A study of cereal, bamboo and grasses. Cambridge: Cambridge University Press; 1934. [Google Scholar]
- Bateson W. Materials for the study of variation treated with especial regard to discontinuity in the origin of species. London: MacMillan; 1894. [Google Scholar]
- Bonnett OT. Hood and supernumerary spike development in barley. Illinois Agricultural Experiment Station Bulletin No. 721. 1966:78–91. [Google Scholar]
- Bossinger G, Rohde W, Lundqvist U, Salamini F. Genetics of barley development: mutant phenotypes and molecular aspects. In: Shewry PR, editor. Barley: genetics, biochemistry, molecular biology and biotechnology. Wallingford: CAB International; 1992. pp. 231–264. [Google Scholar]
- von Bothmer R. The wild species of Hordeum: relationships and potential use for improvement of cultivated barley. In: Shewry PR, editor. Barley: genetics, biochemistry, molecular biology and biotechnology. Wallingford: CAB International; 1992. pp. 3–18. [Google Scholar]
- von Bothmer, Jacobsen N. Origin, taxonomy and related species. In: Rasmussen DC, editor. Barley. Monographs in Agronomy. Vol. 26. Madison, WI: American Society of Agronomy; 1985. pp. 19–56. [Google Scholar]
- von Bothmer R, Jacobsen N, Baden C, Jørgensen RB, Linde-Laursen I. Hordeum: an ecogeographical study of the genus. 2nd edn. Rome: International Plant Genetic Resources Institute; 1995. [Google Scholar]
- Caldwell DG, McCallum N, Shaw P, Muehlbauer GJ, Marshall DF, Waugh R. A structured mutation population for forward and reverse genetics in barley (Hordeum vulgare L.) The Plant Journal. 2004;40:143–150. doi: 10.1111/j.1365-313X.2004.02190.x. [DOI] [PubMed] [Google Scholar]
- Dahlgren R, Clifford HT, Yeo PF. The families of the monocotyledons. Structure, evolution and taxonomy. New York, NY: Springer-Verlag; 1985. [Google Scholar]
- Felker FC, Peterson DM, Nelson OE. Anatomy of immature grains of eight shrunken endosperm barley mutants. American Journal of Botany. 1985;72:248–256. [Google Scholar]
- Gould FW, Shaw RB. Grass systematics. 2nd edn. College Station: Texas A&M University Press; 1983. [Google Scholar]
- Guedes M, Dupuy P. Comparative morphology of lodicules in grasses. Botanical Journal of the Linnean Society. 1976;73:317–331. [Google Scholar]
- Guignard JL. Recherche sur d'embryogenie des Graminees: rapports des Graminees avec les autres monocotyledones. Annales des Sciences Naturelles, Botanique, Series. 1962;12:491–610. [Google Scholar]
- Jacques-Felix H. Sur une interpretation nouvelle de l'embryon des Graminees. La nature axillaire de la gemmule. Competes Rendus Hebdomadoires de l'Academie des Sciences. 1957;245:1260–1263. [Google Scholar]
- Lersten NR. Morphology and anatomy of the wheat plant. In: Heyne EG, editor. Wheat and wheat improvement. 2nd edn. Madison, WI: American Society of Agronomy; 1987. pp. 33–75. [Google Scholar]
- Müller KJ, Romano N, Gerstner O, Gracia-Maroto F, Pozzi C, Salamimi F, et al. The barley Hooded mutation caused by a duplication in a homeobox gene intron. Nature. 1995;374:727–730. doi: 10.1038/374727a0. [DOI] [PubMed] [Google Scholar]
- Peterson RF. Wheat; botany, cultivation and utilzation. New York, NY: Interscience Publishers; 1965. [Google Scholar]
- Reid DA. Morphology and anatomy of the barley plant. In: Rasmussen DC, editor. Barley. Monographs in Agronomy. Vol. 26. Madison, WI: American Society of Agronomy; 1985. pp. 73–101. [Google Scholar]
- Rutishauser R, Sattler R. Complementarity and heuristic value of contrasting models in structural botany. Botanische Jahrbücher für Systematik, Pflanzengeschichte und Planzengeographie. 1985;107:415–455. [Google Scholar]