Abstract
Background and Aims
The thin cell layer (TCL) technique is based on the use of very small explants and has allowed enhanced in vitro morphogenesis in several plant species. The present study evaluated the TCL technique as a procedure for somatic embryo production and plantlet regeneration of peach palm.
Methods
TCL explants from different positions in the shoot apex and leaf sheath of peach palm were cultivated in MS culture medium supplemented with 0–600 µm Picloram in the presence of activated charcoal. The production of primary calli and embryogenic calli was evaluated in these different conditions. Histological and amplified fragment length polymorphism (AFLP) analyses were conducted to study in vitro morphogenetic responses and genetic stability, respectively, of the regenerated plantlets.
Key Results
Abundant primary callus induction was observed from TCLs of the shoot meristem in culture media supplemented with 150–600 µm Picloram (83–97 %, respectively). The production of embryogenic calli depends on Picloram concentration and explant position. The best response observed was 43 % embryogenic callus production from shoot meristem TCL on 300 µm Picloram. In maturation conditions, 34 ± 4 somatic embryos per embryogenic callus were obtained, and 45·0 ± 3·4 % of these fully developed somatic embryos were converted, resulting in plantlets ready for acclimatization, of which 80 % survived. Histological studies revealed that the first cellular division events occurred in cells adjacent to vascular tissue, resulting in primary calli, whose growth was ensured by a meristematic zone. A multicellular origin of the resulting somatic embryos arising from the meristematic zone is suggested. During maturation, histological analyses revealed bipolarization of the somatic embryos, as well as the development of new somatic embryos. AFLP analyses revealed that 92 % of the regenerated plantlets were true to type. The use of TCL explants considerably improves the number of calli and somatic embryos produced in comparison with previously described protocols for in vitro regeneration of peach palm.
Conclusions
The present study suggests that the TCL somatic embryogenesis protocol developed is feasible, although it still requires further optimization for in vitro multiplication of peach palm, especially the use of similar explants obtained from adult palm trees.
Key words: Bactris gasipaes, tissue culture, clonal propagation, Picloram
INTRODUCTION
The development of in vitro regenerative protocols in peach palm (Bactris gasipaes) has direct applications for clonal mass propagation of selected plants and may be integrated into breeding and conservation programmes (Mora-Urpí et al., 1997). Today this species is important for its fruit, popular throughout its traditional distribution, and for its heart-of-palm, a gourmet vegetable extracted from the shoot apex. Various Latin American institutions have breeding programmes for one or both uses, where in vitro techniques for plantlet regeneration could be important tools. In vitro plantlet regeneration of peach palm was described for the first time by indirect organogenesis (Arias and Huete, 1983), later by somatic embryogenesis (Valverde et al., 1987; Stein and Stephens, 1991) and by direct organogenesis (Almeida and Kerbauy, 1996). However, plantlet regeneration was often difficult, and to date there is no efficient protocol describing in detail the in vitro regeneration of peach palm from leaf sheaths or shoot meristems, which would be the perfect explant source for this caespitose palm. Valverde et al. (1987) described a protocol for in vitro regeneration of peach palm, although only ten embryogenic calli were obtained (out of 100 shoot apexes – where one explant could produce more than one callus) and each callus produced 2–8 somatic embryos. In the protocol described by Stein and Stephens (1991), only four calli cultures (out of 18 explants) producing > 10 plantlets per culture were established. Both success rates are clearly insufficient for large-scale plantlet production. Thus, the in vitro regeneration of peach palm still requires the optimization of the protocols.
In the study of Valverde and co-workers, the production of somatic embryos was obtained with the use of Picloram. This auxin analogue has been successfully used for somatic embryogenesis induction in numerous plant species (Barro et al., 1999; Groll et al., 2001; Kaur and Kothari, 2004), and in peach palm our results confirm it as a reliable auxin source for inducing somatic embryogenesis from zygotic embryos and immature inflorescences (Steinmacher et al., 2007 a, b). During the development of a protocol for plantlet regeneration, morphological and histological aspects should be evaluated to elucidate the in vitro response, such as the origin and development of the calli and somatic embryos, as well as the characterization of the morphogenetic route. No histological observations of peach palm in vitro plantlet regeneration from leaf sheaths or shoot meristems have been reported. Sané et al. (2006) recently described a detailed protocol for Phoenix dactylifera regeneration through somatic embryogenesis with all the steps analysed histologically. They described the origin and development of the calli, as well as the histochemical alterations during in vitro culture, which may serve as markers for somatic embryogenesis competence.
Somatic embryogenesis is the preferred in vitro regenerative route for palms, as this morphogenetic pathway may increase the number of regenerated plantlets in comparison with organogenesis. Among other advantages, somatic embryogenesis permits creation of cycling cultures through the use of cell suspensions (Teixeira et al., 1995; Sané et al., 2006) or through secondary somatic embryogenesis (Perez-Nunez et al., 2006). The production of somatic embryos capitalizes upon the totipotency of plant cells and involves the development of bipolar structures resembling zygotic embryos (Dodeman et al., 1997). This morphogenetic route is influenced by several factors imposed by in vitro conditions (Feher et al., 2003). Among these, the explant source and the developmental stage are considered key elements that alter cellular competence (Merkle et al., 1995). In addition, the size of the explants greatly influences their morphogenetic capacity (Benkirane et al., 2000; Delporte et al., 2001), probably due to the establishment of a symplast domain (Bouget et al., 1998; Haywood et al., 2002) that maintains the coordinated development of the cells and tissues (Wu et al., 2002; Roberts and Oparka, 2003). Roberts and Oparka (2003) define the symplast domain as a continuum between the cells of a specific tissue domain, allowing molecular movement through the plasmodesmata, and maintaining and coordinating morphogenetic activity in the tissue.
The thin cell layer (TCL) technique utilizes very small explants and was first described in Nicotiana tabacum (Tran Thanh Van et al., 1974). It could be an alternative method to disrupt symplast domains, as well as to modulate in vitro response. This procedure gave enhanced results for numerous in vitro culture systems (Tran Thanh Van and Bui, 2000), including Digitaria sanguinalis (Van Le et al., 1997), Oryza sativa (Nhut et al., 2000), Elaeis guineensis (Teixeira et al., 1994) and Cocos nucifera (Samosir et al., 1998).
In vitro culture may cause disturbances to the genome organization of regenerated plantlets, resulting in somaclonal variation (Larkin and Scowcroft, 1981). To evaluate possible somaclonal variation in regenerated plants, DNA should be randomly and evenly analysed (Polanco and Ruiz, 2002). Molecular markers, such as amplified fragment length polymorphisms (AFLPs) (Vos et al., 1995), allow the analysis of genomic DNA using a multilocus approach with high sensitivity. The AFLP method combines the reproducibility of restriction fragment analysis and the power of the polymerase chain reaction (PCR) in a straightforward technique (Mueller and Wolfenbarger, 1999). AFLP markers have been used to detect somaclonal variation in plants (e.g. Carolan et al., 2002; Polanco and Ruiz, 2002; Pontaroli and Camadro, 2005). The sensitivity of the restriction analysis and the use of stringently controlled PCRs often allow the detection of point mutations (insertions/deletions), and are proving useful in detecting somaclonal variation during somatic embryogenesis (e.g. Cervera et al., 2002). In P. dactylifera, AFLPs were more sensitive than random amplification of polymorphic DNA (RAPD) markers to detect genomic alterations in regenerated plantlets (Saker et al., 2006); in E. guineensis, for example, the RAPD technique did not detect differences between groups of somaclonal variants (Rival et al., 1998). Also in E. guineensis the AFLP technique was used successfully with methylation-sensitive restriction enzymes to detect variants from in vitro culture (Matthes et al., 2001). The AFLP technique has already been used for distinguishing peach palm landraces (Clement et al., 2002).
The TCL technique associated with different Picloram concentrations was evaluated to develop an efficient procedure for somatic embryogenesis and plantlet regeneration of peach palm. In addition, histological and AFLP analyses were conducted in order to verify the in vitro morphogenetic responses, as well as the genetic stability of the regenerated plantlets.
MATERIALS AND METHODS
Plant material
Nearly mature fruits, about 12 weeks after pollination, were collected from one selected open-pollinated plant of peach palm of the Yurimaguas population of the Pampa Hermosa landrace kept at the Instituto Nacional de Pesquisas da Amazônia (INPA) germplasm collection, Manaus, Amazonas, Brazil. The hard endocarps were removed and the kernels (i.e. zygotic embryo enclosed by endosperm) were surface-sterilized during 1 min immersion in 70 % ethanol, followed by a 40 min immersion in sodium hypochlorite solution, provided by a solution of 60 % commercial bleach (2·0–2·5 % active chlorine), plus one drop of Tween-20® in each 100 mL. Thereafter, in aseptic conditions, the kernels were rinsed three times in sterile distilled water. Zygotic embryos were aseptically removed from the kernels under a stereoscopic microscope and transferred to test tubes containing 10 mL of basal culture medium [MS salts (Murashige and Skoog, 1962) plus Morel's vitamins (Morel and Wetmore, 1951), 3 % sucrose, 1·5 g L−1 activated charcoal and gelled with 7·0 g L−1 Agar (Merse)]. The culture medium was adjusted to pH 5·8 prior to adding the gelling agent and was autoclaved for 15 min at 1 kgf cm−2. The cultures were kept at 26 ± 1 °C in a 16 h light period, with 50–60 μmol m−2 s−1 intensity provided by cool-white fluorescent lamps (Sylvania), until the plantlets reached 5–8 cm in height.
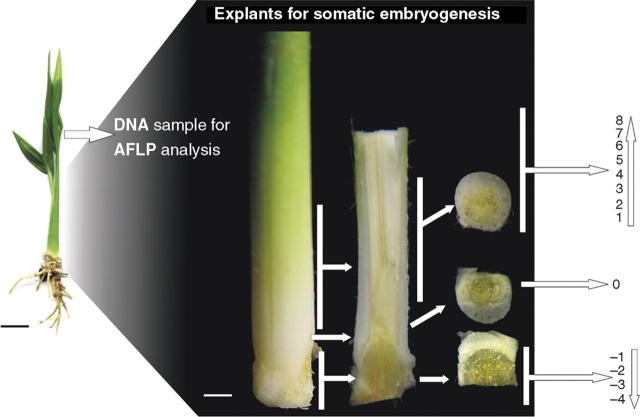
The leaves, roots, haustorial tissue and the most external green leaf sheath of the plantlets were removed. The leaves were individually identified and frozen for subsequent AFLP analysis. The remaining tissue was transversely sectioned in 0·7–1 mm slices to obtain different histogenic layers. Some of these explants were composed only of sub-apical tissues or of leaf sheath and apical meristem, as well as different leaf developmental stages. A similar procedure was described for D. sanguinalis (Van Le et al., 1997), although in the present study the explants were individually evaluated. The sub-apical tissues were numbered − 4 to − 1, the apical meristem 0, and the upper sections 1–8 (Fig. 1) to evaluate the effect of the explant's position on the production of embryogenic calli and somatic embryos. Thus, 13 transverse sections were obtained from one plantlet and inoculated in one Petri dish. The original orientation of the explant was maintained.
Fig. 1.
Schematic diagram showing the origin of the explants utilized in the present study (dark scale bar = 1·75 cm, white scale bar = 3 mm).
Culture media and conditions
The explants were inoculated in Petri dishes containing the basal culture medium described above, supplemented with 500 mg L−1 glutamine, and the gelling agent was substituted by 2·5 g L−1 Phytagel®. Picloram was added at different concentrations (0, 150, 300 or 600 μM) to evaluate its effect on the production of embryogenic callus. No sub-culture was applied until embryogenic calli were obtained. Further analyses were carried out only with calli produced on 300 or 600 µm Picloram-enriched media. The embryogenic calli obtained were maintained on a maturation medium composed of basal medium enriched with 40 µm 2,4-D (2,4-dichlorophenoxyacetic acid), 10 μm 2-iP [2-isopentyladenine (6-dimethylaminopurine)], 1 g L−1 glutamine and 0·5 g L−1 hydrolysed casein, with sub-cultures at 4-week intervals. The somatic embryos were selected on the basis of their shape (well formed, globular to oblong) and colour (white to yellowish). Fully developed somatic embryos were transferred to conversion medium, constituted by basal medium without activated charcoal and containing 24·6 µm 2-iP and 0·44 µm NAA (naphthalene acetic acid), and cultured for 30 d. The somatic embryos were then transferred to basal culture medium until conversion, and plantlets >6 cm tall were then acclimatized. The culture medium was adjusted to pH 5·8 before the addition of the gelling agent; it was then autoclaved for 15 min at 1 kgf cm−2, and 30 mL were transferred to Petri dishes or flasks. During the induction and maturation of somatic embryos, the cultures were kept in the dark at 26 ± 1 °C. For somatic embryo conversion and plantlet growth, the cultures were kept in a growth chamber at 26 ± 1 °C under a 16 h light period with 50–60 µmol m−2 s−1 intensity provided by cool-white fluorescent lamps (Sylvania).
Histological procedure
Samples were collected at critical periods of in vitro culture and fixed for 48 h at 4 °C in 0·2 m phosphate buffer (pH 7·3) containing 2·5 % paraformaldehyde. Thereafter, the samples were washed with the same buffer without fixative and dehydrated in a graded alcohol series (30–100 %), twice for 15 min at each step. The samples were embedded in Leica Historesin® and alcohol (1 : 1, v/v) overnight, followed with pure resin for 24 h. Gelatin capsules with the samples were filled with resin and allowed to polymerize following the manufacturer's instructions. Afterwards, 5–7 µm sections were obtained with a manual microtome, mounted on a slide with a drop of water and stained with 0·5 % toluidine blue O in 0·1 m phosphate buffer (pH 6·8).
AFLP analyses
Eight ‘mother-plants’ (F1–8; the seedlings used to extract explants) and three regenerated plantlets (a–c) from each mother-plant were randomly sampled; four sets (mother-plant and their regenerated plantlets) were regenerated using 300 µm Picloram (F1–4) and four using 600 µm Picloram (F5–8). DNA was extracted according to Doyle and Doyle (1990) with slight modifications. Leaves were collected and about 200 mg of plant material were ground with a mortar and pestle in liquid nitrogen, transferred to a 1·5 mL microtube containing 1·0 mL of DNA extraction solution [2 % cetyltrimethylammonium bromide (CTAB); 0·7 m NaCl; 50 mm Tris–HCl; 10 mm EDTA pH 8·0; and 0·1 % β-mercaptoethanol] and incubated at 60 °C for 1 h with occasional swirling. An organic extraction was carried out by adding 0·6 mL of chloroform–isoamyl alcohol (24 : 1) to the mixture. The tubes were centrifuged at 8000 g for 5 min, the supernatant phase transferred to a new microtube and 10 µL of a 10 % CTAB solution (10 % CTAB; 1·2 m NaCl) was added to each sample, followed by 1·0 mL of cold 100 % ethanol. The samples were then incubated at –4 °C overnight. After centrifugation, the ethanol was discarded and the DNA pellet was washed with 1·0 mL of 70 % ethanol during 1 min. After discarding the ethanol, the DNA was dried at room temperature, diluted in 70 µL of TE buffer with RNase A (10 mm Tris–HCl pH 8·0; 1 mm EDTA; 10 µg mL−1 RNase A), quantified in a 0·8 % agarose gel and stored at −20 °C until use.
The AFLP reactions were performed as described by Vos et al. (1995), with slight modifications as described by Samuel Hazen of Michigan State University (available at http://www.msu.edu/user/hazensam/aflp/AFLPprotocolMSU.html). Six selective primer combinations (M-CTG/E-AAC, M-CTG/E-AAG, M-CAG/E-AGC, M-CAG/E-ACT, M-CAG/E-AAG and M-CAC/E-ACA) were used, based on the screening by Clement et al. (2002). All PCRs were carried out in a Peltier Thermal Cycler PTC-100 (MJ Research). The fragments were separated in a 6 % denaturing polyacrylamide gel at 75 W for 3 h. Silver nitrate was used for staining (Caetano-Anollés and Gresshoff, 1994) and the bands were visualized under white light. AFLP products were manually scored as present or absent, and transformed into a binary matrix for data analysis. Only reliable bands between 50 and 800 bp were scored.
Statistical procedure
Four Picloram concentrations were evaluated (0, 150, 300 or 600 mm), as was the effect of initial explant position, on primary callus induction and embryogenic callus production. The experiment was a factorial in a completely randomized design, with five repetitions. Each repetition was composed of at least four Petri dishes and each Petri dish had all 13 histogenic layers from one plant. After 3 and 5 months of culture, the primary callus and somatic embryogenesis induction rates, respectively, were evaluated. The data were transformed by √(x + 5) and submitted to analysis of variance (ANOVA); means were compared with Duncan's multiple range test at 95 % significance.
For the AFLP analysis, the plantlets regenerated on 300 or 600 µm Picloram were compared with their mother-plant. The relationship among individuals was assessed by computing Dice's similarity coefficient (Dice, 1945). The similarity matrix was used as input for a principal coordinate analysis (PCO), implemented with NTSYS-pc 2·0 (Rohlf, 1998). The differences between the genetic similarities and the number of fragments lost or gained in the two Picloram concentrations were tested. The significance of the difference was measured using an exact probability test (Raymond and Rousset, 1995), based on Fisher's test for RxC contingency tables. The analysis was performed with RxC (Miller, 1997), using 1000 batches of 10 000 replicates and 1000 de-memorization steps for the Markov chain method.
RESULTS
Callus and somatic embryo production and plantlet regeneration
The induction of primary calli was strongly influenced by the interaction between the Picloram concentration and the explant position (P < 0·01). On culture medium devoid of Picloram, some explants presented the development of a meristem and root growth from sub-apical tissue, but no callus was observed and most of the explants showed intense oxidation after 4 weeks of culture. The highest primary callus induction was observed in explants from the apical meristem region on culture media supplemented with Picloram, ranging from 83 to 97 % induction with 150–600 µm Picloram, without significant differences among these treatments, but differing from the results in the absence of Picloram (Fig. 2). Explants from sub-apical tissues (−1 and –2) showed significantly higher induction of primary calli on 300 and 600 µm Picloram culture media, with 74 and 71 %, respectively, not differing from the explants from the apical meristem (Fig. 2). Explants from the upper layers (1–8) showed induction of primary calli with 600 µm Picloram, ranging from 50 to 10 %. For those explants composed of leaf sheath (1–8), a horizontal response gradient was also observed, as the outer leaf sheath showed low response and eventual oxidation, while the inner leaf sheaths or leaf primordia showed the development of primary callus. The most responsive leaf sheath explants were 1–3 (Fig. 2; see also figures available online at http://www.cca.ufsc.br/lfdgv/AoBs), which coincide with the region containing leaf primordia.
Fig. 2.
Influence of explant position and Picloram concentration (0, 150, 300 or 600 µm) on primary callus induction of peach palm. Upper case letters represent statistical differences among the Picloram concentrations (at each explant position) and lower case letters represent differences among the explant positions (at each Picloram concentration) according to Duncan's test.
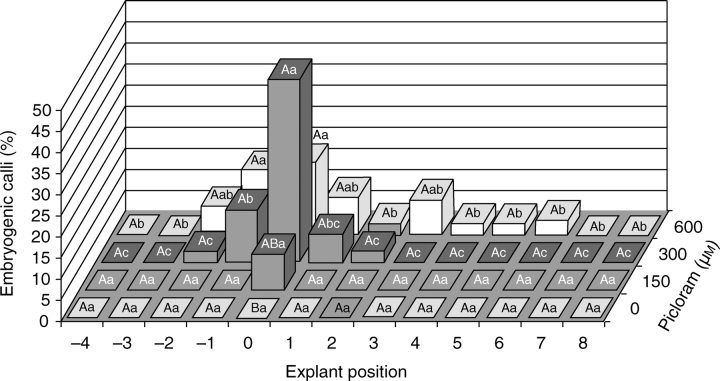
The production of embryogenic calli was evaluated 5 months after inoculation and followed the same response pattern as primary callus induction (compare Figs 2 and 3), with interaction between Picloram concentration and explant position (P < 0·05). On culture medium with 300 µm Picloram, explants from the apical meristem region showed 43 % embryogenic callus production, while on 150 or 600 µm Picloram this was 8 and 17 %, respectively (Fig. 3), without significant differences among these values. The production of calli with embryogenic characteristics in explants from the upper layers (3–8) was observed only on 600 µm Picloram-supplemented culture medium, ranging from 3 to 9 % (Fig. 3).
Fig. 3.
Influence of explant position and Picloram concentration (0, 150, 300 or 600 µm) on embryogenic callus production of peach palm. Upper case letters represent statistical differences among the Picloram concentrations (at each explant position) and lower case letters represent differences among the explant positions (at each Picloram concentration) according to Duncan's test.
On the maturation culture medium, an average of 34 ± 4 somatic embryos was obtained per embryogenic callus, without differences between the 300 and 600 µm Picloram treatments (36 ± 5 and 32 ± 5 somatic embryos per embryogenic callus, respectively). Fully developed somatic embryos were obtained, and those selected had a 45·0 ± 3·4 % conversion rate, again without differences between the two Picloram concentrations. Plantlets were obtained (Fig. 4F) and were transferred to culture flasks and later acclimatized (Fig. 4G). During the acclimatization step, approximately 80 % of the plantlets survived and were transferred to the greenhouse, where all survived after 2 months.
Fig. 4.
Somatic embryogenesis and plantlet regeneration from thin cell layers of peach palm. (A) Primary callus (arrow). (B) Primary callus induction from shoot meristem TCL: note the development of primary callus (arrow) in tissue surrounding the shoot meristem. (C) Embryogenic callus from shoot meristem TCL: note the development of the shoot meristem (thick arrow) and embryogenic callus (thin arrow). (D) Somatic embryo development (arrow) from primary callus. (E) Embryogenic callus on maturation culture medium. (F) Plantlets obtained from converted somatic embryos. (G) Acclimatized plantlets. Scale bars: A = 1 mm; B–D = 2·5 mm; E = 1 cm; F, G = 2·5 cm.
Morpho-histological aspects
After 1 week of culture, swelling and growth of the explant were observed, resulting in the development of primary callus. The primary calli were yellow in colour, compact, with radial but non-organized growth (Fig. 4A) from which somatic embryos arose (Fig. 4D). Interestingly, while the meristem region (explant 0) was the most responsive, morphological analyses revealed that embryonic calli also arose from tissues adjacent to the apical meristem (i.e. leaf primordia), while the apical meristem itself showed only an initial growth and occasionally primary callus development (Fig. 4B, C). The embryogenic calli were transferred to maturation culture medium, where growth of somatic embryos as well as signs of polarization were observed (Fig. 4E). In addition, in maturation conditions, several developmental stages could be observed, revealing non-synchronized somatic embryo production.
Histological analyses of fresh green tissue showed that peach palm has mesophyll composed of parenchymatic isodiametric cells with collateral vascular bundles distributed along the tissue and intercalated with fibre bundles (Fig. 5A). About 4–5 weeks after inoculation on culture medium supplemented with Picloram, the first cellular division events were observed to occur simultaneously in several cells adjacent to the vascular tissue, while the remaining parenchymatic cells degenerated (Fig. 5B); these actively dividing cells progressed to burst through the epidermis of the explants, resulting in primary callus (Fig. 5C). Additionally, primary callus induction did not occur simultaneously, as some calli were observed to develop later on the explant, without an apparent relationship to their proximity to vascular tissue or meristems. The radial growth of the primary calli was ensured by the presence of a meristematic zone composed of small meristematic isodiametric cells (Fig. 5D).
Fig. 5.
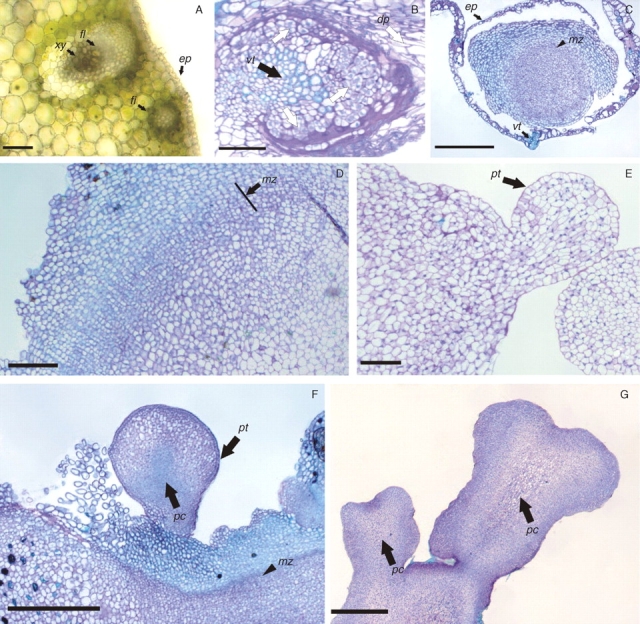
Histological analyses of peach palm somatic embryogenesis. (A) Histological section of fresh tissue to illustrate the organization of a mature tissue evidencing a collateral vascular bundle distributed along the tissue and intercalated with a fibre bundle. (B) First cell division events (arrow) observed in cells adjacent to the vascular tissue and the degenerating parenchyma (dp, arrow). (C) Primary callus induction: note the formation of a meristematic zone (arrowhead). (D) Details from the meristematic zone (arrow). (E) Globular somatic embryo, showing polarization signals and a well-defined protodermis. (F) Globular somatic embryos observed in maturation culture conditions. (G) Mature somatic embryos showing a well-developed procambium and without vascular connection with the matrix tissue. Abbreviations: xy, xylem; fl, phloem; fi, fibre bundle; ep, epidermis; dp, degenerating parenchyma; vt, vascular tissue; pt, protodermis; pc, procambium; mz, meristematic zone. Scale bars: A, B, D, E = 200 µm; C, F, G = 500 µm.
Subsequently, development of globular structures arising from the primary callus was observed. Histological analyses revealed that these structures arose from the meristematic zone, and presented a well-delimited protoderm and signs of polarization (Fig. 5E), characteristics of somatic embryos at the globular stage. After transfer of these embryogenic calli to the maturation culture medium, some cells of the peripheral zone of the primary callus also exhibited embryogenic competence, resulting in the development of additional globular somatic embryos (Fig. 5F). Most of the well-formed somatic embryos developed and showed complete polarization, illustrated by the presence of procambium (Fig. 5G), and could be converted into plantlets (Fig. 4F).
AFLP analyses of the regenerated plantlets
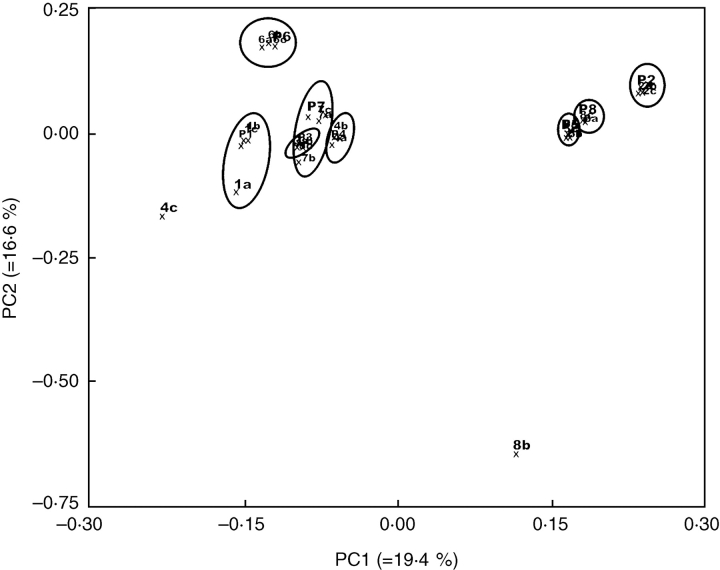
The AFLP primer combinations used in this study generated a total of 252 fragments, ranging from 30 to 59, with a mean of 41·7 fragments per primer combination (Table 1). The PCO analysis based on Dice's similarity index explained 36 % of the total variation in the first two axes. Six out of eight plant sets grouped in clusters formed by the mother-plant and its respective clones (Fig. 6). The two clones with lowest genetic similarity diverged significantly from their plant sets: clone 4c displayed 81 % identity to its mother-plant and clone 8b displayed 69 % identity. However, clone 8b also had a relatively high level of missing data, so the results on this plantlet may be inconclusive. A fragment was considered to be missing data when it was present but weakly stained compared with an adjacent strongly stained band.
Table 1.
Mean number of fragments scored and number of fragments lost and gained for each AFLP primer pair combination from peach palm clones regenerated through somatic embryogenesis in two concentrations of Picloram
| 300 µm Picloram | 600 µm Picloram | ||||
|---|---|---|---|---|---|
| Primer combination | No. of fragments | Fragment loss | Fragment gain | Fragment loss | Fragment gain |
| AAC/CTG | 30 | 13 | 9 | 10 | 0 |
| AAG/CTG | 44 | 12 | 12 | 7 | 9 |
| AGC/CAG | 40 | 16 | 10 | 25 | 4 |
| ACT/CAG | 49 | 25 | 6 | 23 | 3 |
| AAG/CAG | 30 | 4 | 0 | 13 | 0 |
| ACA/CAC | 59 | 20 | 7 | 46 | 11 |
| Overall | 252 | 90 | 44 | 124 | 27 |
The exact test (Raymond and Rousset, 1995) revealed a significant difference between Picloram treatments for fragment loss (P = 0·017) and fragment gain (P = 0·0002).
Fig. 6.
Principal coordinate analysis (PCO) based on Dice's similarities, revealing the genetic relatedness of eight seedling–clone sets of peach palm analysed using 262 AFLP markers.
The two Picloram concentrations (300 or 600 µm) had no effect on the mean similarities among clones and their respective mother-plant: 96 and 95 %, respectively. Ten of 12 plantlets with genetic similarities to their respective mother-plant of ≥ 97 % were produced with 300 µm Picloram, while the 600 µm Picloram yielded nine of 12 plantlets with genetic similarities in this range (Table 2). Considering all AFLP primer combinations, a higher frequency of fragment loss (90 fragments for 300 µm and 124 fragments for 600 µm) than of fragment gain (44 fragments for 300 µm and 27 fragments for 600 µm) was observed. The comparison between Picloram treatments revealed significant differences between treatments regarding loss (P = 0·017) or gain (P = 0·0002) of AFLP fragments.
Table 2.
Summary of the AFLP analysis of somaclonal variation in regenerated peach palm, based on Dice's coefficient of similarity between the mother-plant and its clone
| 300 µm Picloram | 600 µm Picloram | ||||
|---|---|---|---|---|---|
| Mother-plant | Clone | Coefficient of similarity | Mother-plant | Clone | Coefficient of similarity |
| F1 | 1a | 0·89 | F5 | 5a | 0·99 |
| 1b | 0·99 | 5b | 0·99 | ||
| 1c | 0·99 | 5c | 0·99 | ||
| F2 | 2a | 0·99 | F6 | 6a | 0·97 |
| 2b | 0·98 | 6b | 0·94 | ||
| 2c | 0·99 | 6c | 0·98 | ||
| F3 | 3a | 0·99 | F7 | 7a | 0·99 |
| 3b | 0·99 | 7b | 0·92 | ||
| 3c | 0·97 | 7c | 0·99 | ||
| F4 | 4a | 0·98 | F8 | 8a | 0·99 |
| 4b | 0·98 | 8b | 0·69 | ||
| 4c | 0·81 | 8c | 0·99 | ||
| Mean | 0·96 | Mean | 0·95 | ||
The exact test (Raymond and Rousset, 1995) revealed no significant difference (P = 0·75) between Picloram treatments for Dice's coefficient of similarity.
DISCUSSION
The use of the TCL technique offers promise for peach palm in vitro plant regeneration. Explant size has an important role in peach palm in vitro response, as in our preliminary experiments low callus induction was observed from explants that were 1 cm thick (data not shown). Small explants also presented higher morphogenetic capacity in Triticum durum (Benkirane et al., 2000) and in T. aestivum (Delporte et al., 2001). Benkirane et al. (2000) postulated that larger explants maintain normal tissue interactions, and such interactions may inhibit cell division through maintenance of symplast domains. Additionally, explants with reduced size showed synthesis of new cell wall components, such as oligosaccharides, that can act as signals to the cell to re-enter the mitotic cycle (Tran Thanh Van and Bui, 2000). Small explants also present higher surface contact with the culture medium, and can be considered to be more stressed, increasing the cell's metabolism (Feher et al., 2003).
The present study confirms the use of Picloram as a reliable auxin analogue to trigger somatic embryogenesis in peach palm, as observed by Valverde et al. (1987) and Steinmacher et al. (2007a, b). In interaction with the Picloram concentration, explant position influenced in vitro response, revealing a response gradient for primary callus production from layers 1 to 8 cultivated on 600 µm Picloram. In addition, a horizontal gradient was observed as outer leaf sheaths presented a low morphogenetic response and greater oxidation compared with inner leaf sheaths. The original explant position also had a fundamental role in the acquisition of somatic embryogenesis competence in Coffea arabica (Quiroz-Figueroa et al., 2002), Dactylis glomerata (Somleva et al., 2000; Alexandrova and Conger, 2002) and Avena sativa (Nuutila et al., 2002), and both vertical and horizontal gradients were also observed in D. sanguinalis (Van Le et al., 1997).
In the present study, the production of embryogenic calli from explants of the apical meristem (explant 0) was highest on culture medium with 300 µm Picloram. The induction of embryogenic callus followed the same tendency as primary callus development, suggesting that the morphogenetic competence of the explants was governed by the capacity of the explant cells to re-enter the mitotic cycle, as suggested by Tran Thanh Van and Bui (2000). Although somatic embryogenesis induction might not be simply related to the cell cycle or to a certain level of mitotic activity of the cells in the explants (Doleželová et al., 1992), it has been shown that the formation of callus is correlated with cell cycling and that the subsequent origin of somatic embryos initially involves cycling of cells at the margins of the callus of Arabidopsis (Raghavan, 2004). In the present study, most initiation of somatic embryos was also associated with cycling of cells, but from a meristematic zone formed within the callus. Also, cells able to produce somatic embryos were mitotically more active than non-embryogenic cells (Pasternak et al., 2002). In contrast, the in vitro recalcitrance of C. nucifera was linked to a block in the cell cycle (Sandoval et al., 2003). This suggests that the TCL technique may be an interesting strategy for the induction and control of in vitro morphogenesis in other palm species, as is the case with peach palm.
Histological analyses showed that the first cell divisions occurred in cells adjacent to vascular tissue, resulting in primary calli. In palms, cells adjacent to the vascular tissue apparently have higher morphogenetic capacity. Studies with C. nucifera (Fernando et al., 2003), E. guineensis (Schwendiman et al., 1988), Euterpe edulis (Guerra and Handro, 1998) and P. dactylifera (Sané et al., 2006) showed that the first events of cell division were always observed in cells adjacent to the vascular tissue, resulting in primary calli or meristematic nodules similar to those observed in the present study.
Somatic embryos were observed arising from the meristematic zone of primary calli 5 months after inoculation, characterizing the indirect pattern of somatic embryogenesis with possible multiple cellular origins (Williams and Maheswaran, 1986). These somatic embryos were observed at the first developmental stage, and presented a well-delimited protoderm and polarization signals. In C. nucifera, the same pattern was observed, although in this species a fragmentation of the meristematic zone was observed prior to the development of the somatic embryos, which could therefore have uni- or multicellular origins (Verdeil et al., 1994; Dussert et al., 1995; Fernando et al., 2003).
Considering that in the present study relatively high concentrations of Picloram were used to stimulate somatic embryo production and that in vitro culture conditions affect the organization of plant genomes (Larkin and Scowcroft, 1981), AFLP analyses were conducted to determine to what extent the present conditions could generate divergent plantlets. Although most of the clones revealed a similarity greater than 97 % to the mother-plant (see Table 2 and Fig. 6), some degree of somaclonal variation appears to exist in regenerated peach palm plantlets as some minor changes of the banding patterns were observed (see Supplementary figures available online at http://www.cca.ufsc.br/lfdgv/AoB) and two plantlets were off type (Fig. 6). The two clones with similarities lower than 81 % to the mother-plant showed normal morphology. In Codonopsis lanceolata, normal phenotypes often had genotypic alterations to some degree (Guo et al., 2006), and the authors postulated that such differences imply that most genomic changes occurred in non-coding regions with little effect on gene expression, and/or that the change could be a recessive mutation, without changes in the phenotype. In the present study, independently of the Picloram concentration, the most common mutation observed in the more divergent plantlets was the loss of fragments, implying that most mutations were in the restriction sites of the enzymes.
Summarizing, the present study suggests that the TCL somatic embryogenesis protocol developed is feasible, although it still requires further optimization for in vitro multiplication of peach palm. Additionally, in the present study, the explants were obtained from in vitro grown plantlets; however, similar explants can be obtained easily from adult palms since peach palm produces off-shoots that will allow the cloning of selected adult plants. More detailed studies are being conducted to evaluate the genotype effect of the donor plant on in vitro responses, as well as the incidence of somaclonal variation. Further studies will also elucidate to what extent the genomic alterations detected by AFLP might affect the phenotype of adult plants. Additionally, an embryogenic cell suspension was recently obtained in P. dactylifera (Sané et al., 2006) from calli similar to those described in the present study, and the adaptation of the present protocol to conform with that from P. dactylifera may create an embryogenic cell suspension of peach palm.
ACKNOWLEDGEMENTS
The authors thank Dr Saare-Surminski and Professor Dr Lieberei for critical reading of the manuscript. D.A.S. thanks the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – CAPES – Brasília/Brazil. and V.M.S. thanks the Conselho Nacional de Desenvolvimento Científico e Tecnológico – CNPq – Brasília/Brazil, for financial support for the development of this study. We thank two anonymous reviewers for their numerous useful criticisms and suggestions, which greatly improved the quality of this article.
LITERATURE CITED
- Alexandrova KS, Conger BV. Isolation of two somatic embryogenesis-related genes from orchardgrass (Dactylis glomerata) Plant Science. 2002;162:301–307. [Google Scholar]
- Almeida M, Kerbauy GB. Micropropagation of Bactris gasipaes (Palmae) through flower bud culture. Revista Brasileira de Fisiologia Brasileira. 1996;8:215–217. [Google Scholar]
- Arias O, Huete F. Vegetative propagation in vitro of pejibaye (Bactris gasipaes HBK) Turrialba. 1983;33:103–108. [Google Scholar]
- Barro F, Martin A, Lazzeri PA, Barcelo P. Medium optimization for efficient somatic embryogenesis and plant regeneration from immature inflorescences and immature scutella of elite cultivars of wheat, barley and tritordeum. Euphytica. 1999;108:161–167. [Google Scholar]
- Benkirane H, Sabounji K, Chlyah A, Chlyah H. Somatic embryogenesis and plant regeneration from fragments of immature inflorescences and coleoptiles of durum wheat. Plant Cell, Tissue and Organ Culture. 2000;61:107–113. [Google Scholar]
- Bouget FY, Berger F, Brownlee C. Position dependent control of cell fate in the Fucus embryo: role of intercellular communication. Development. 1998;125:1999–2008. doi: 10.1242/dev.125.11.1999. [DOI] [PubMed] [Google Scholar]
- Caetano-Anollés G, Gresshoff P. Staining nucleic acids with silver: an alternative to radioisotopic and fluorescent labeling. Promega Notes Magazine. 1994;45:13–18. [Google Scholar]
- Carolan JC, Hook ILI, Walsh JJ, Hodkinson TR. Using aflp markers for species differentiation and assessment of genetic variability of in vitro-cultured Papaver bracteatum (section Oxytona) In Vitro Cellular and Development Biology – Plant. 2002;38:300–307. [Google Scholar]
- Cervera MT, Ruiz-Garcia L, Martinez-Zapater JM. Analysis of DNA methylation in Arabidopsis thaliana based on methylation-sensitive AFLP markers. Molecular Genetics and Genomics. 2002;268:543–552. doi: 10.1007/s00438-002-0772-4. [DOI] [PubMed] [Google Scholar]
- Clement CR, Sousa NR, Rodrigues DP, Astolfi-Filho S, Núñez Moreno Y, Pascual VT, Gallego Rodríguez FJ. Use of AFLPS to distinguish landraces of pejibaye (Bactris gasipaes) in brazilian Amazonia. Scientia Agricola. 2002;59:743–753. [Google Scholar]
- Delporte F, Mostade O, Jacquemin JM. Plant regeneration through callus initiation from thin mature embryo fragments of wheat. Plant Cell, Tissue and Organ Culture. 2001;67:73–80. [Google Scholar]
- Dice L. Measures of the amount of ecologic association between species. Ecology. 1945;26:297–302. [Google Scholar]
- Dodeman VL, Ducreux G, Kreis M. Zygotic embryogenesis versus somatic embryogenesis. Journal of Experimental Botany. 1997;48:1493–1509. [Google Scholar]
- Doleželová M, Doležel J, Neštický M. Relationship of embryogenic competence in maize (Zea mays L.) leaves to mitotic activity, cell cycle and nuclear DNA content. Plant Cell, Tissue and Organ Culture. 1992;31:215–221. [Google Scholar]
- Doyle J, Doyle J. Isolation of plant DNA from fesh tissue. Focus. 1990;12:13–15. [Google Scholar]
- Dussert S, Verdeil JL, Buffardmorel J. Specific nutrient-uptake during initiation of somatic embryogenesis in coconut calluses. Plant Science. 1995;111:229–236. [Google Scholar]
- Feher A, Pasternak TP, Dudits D. Transition of somatic plant cells to an embryogenic state. Plant Cell, Tissue and Organ Culture. 2003;74:201–228. [Google Scholar]
- Fernando SC, Verdeil JL, Hocher V, Weerakoon LK, Hirimburegama K. Histological analysis of plant regeneration from plumule explants of Cocos nucifera. Plant Cell, Tissue and Organ Culture. 2003;72:281–284. [Google Scholar]
- Groll J, Mycock DJ, Gray VM, Laminski S. Secondary somatic embryogenesis of cassava on picloram supplemented media. Plant Cell, Tissue and Organ Culture. 2001;65:201–210. [Google Scholar]
- Guerra MP, Handro W. Somatic embryogenesis and plant regeneration in different organs of Euterpe edulis Mart. (Palmae): control and structural features. Journal of Plant Research. 1998;111:65–71. [Google Scholar]
- Guo WL, Gong L, Ding ZF, Li YD, Li FX, Zhao SP, Liu B. Genomic instability in phenotypically normal regenerants of medicinal plant Codonopsis lanceolata Benth. et Hook. f., as revealed by ISSR and RAPD markers. Plant Cell Reports. 2006;25:896–906. doi: 10.1007/s00299-006-0131-8. [DOI] [PubMed] [Google Scholar]
- Haywood V, Kragler F, Lucas WJ. Plasmodesmata: pathways for protein and ribonucleoprotein signaling. Plant Cell. 2002;14:S303–S325. doi: 10.1105/tpc.000778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur P, Kothari SL. Plant Cell, Tissue and Organ Culture. 2004;77:73–79. In vitro culture of kodo millet: influence of 2,4-D and picloram in combination with kinetin on callus initiation and regeneration. [Google Scholar]
- Larkin PJ, Scowcroft WR. Somaclonal variation – a novel source of variability from cell cultures for plant improvement. Theoretical and Applied Genetics. 1981;60:197–214. doi: 10.1007/BF02342540. [DOI] [PubMed] [Google Scholar]
- Matthes M, Singh R, Cheah SC, Karp A. Variation in oil palm (Elaeis guineensis Jacq.) tissue culture-derived regenerants revealed by AFLPs with methylation-sensitive enzymes. Theoretical and Applied Genetics. 2001;102:971–979. [Google Scholar]
- Merkle SA, Parrott WA, Flinn BS. Morphogenic aspects of somatic embryogenesis. In: Thorpe TA, editor. In vitro embryogenesis in plants. Dordrecht: Kluwer Academic Press; 1995. pp. 155–203. [Google Scholar]
- Miller M. RxC, a program for the analysis of contingency tables. Department of Biological Sciences, Northern Arizona University; 1997. [Google Scholar]
- Mora-Urpí J, Weber JC, Clement CR. Peach-Palm (Bactris gasipaes Kunth) 1997 Rome. Available at http://www.ipgri.cgiar.org/publications/pdf/155.pdf :Institute of Plant Genetics and Crop Plant Research and International Plant Genetic Resources Institute. [Google Scholar]
- Morel G, Wetmore RH. Tissue culture of monocotyledons. American Journal of Botany. 1951;38:138–140. [Google Scholar]
- Mueller UG, Wolfenbarger LL. AFLP genotyping and fingerprinting. Trends in Ecology and Evolution. 1999;14:389–394. doi: 10.1016/s0169-5347(99)01659-6. [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiologia Plantarum. 1962;15:473–497. [Google Scholar]
- Nhut DT, Le BV, Tran Thanh Van K. Somatic embryogenesis and direct shoot regeneration of rice (Oryza sativa L.) using thin cell layer culture of apical meristems. Journal of Plant Physiology. 2000;157:559–565. [Google Scholar]
- Nuutila AM, Villiger C, Oksman C. Embryogenesis and regeneration of green plantlets from oat (Avena sativa L.) leaf-base segments: influence of nitrogen balance, sugar and auxin. Plant Cell Reports. 2002;20:1156–1161. [Google Scholar]
- Pasternak TP, Prinsen E, Ayaydin F, Miskolczi P, Potters G, Asard H, Van Onckelen HA, Dudits D, Feher A. The role of auxin, pH, and stress in the activation of embryogenic cell division in leaf protoplast-derived cells of alfalfa. Plant Physiology. 2002.;129:1807–1819. doi: 10.1104/pp.000810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Nunez MT, Chan JL, Saenz L, Gonzalez T, Verdeil JL, Oropeza C. Improved somatic embryogenesis from Cocos nucifera (L.) plumule explants. In Vitro Cellular and Developmental Biology – Plant. 2006;42:37–43. [Google Scholar]
- Polanco C, Ruiz ML. AFLP analysis of somaclonal variation in Arabidopsis thaliana regenerated plants. Plant Science. 2002;162:817–824. [Google Scholar]
- Pontaroli AC, Camadro EL. Plant regeneration after long-term callus culture in clones of Asparagus officinalis L. Biocell. 2005;29:313–317. [PubMed] [Google Scholar]
- Quiroz-Figueroa FR, Fuentes-Cerda CFJ, Rojas-Herrera R, Loyola-Vargas VM. Histological studies on the developmental stages and differentiation of two different somatic embryogenesis systems of Coffea arabica. Plant Cell Reports. 2002;20:1141–1149. [Google Scholar]
- Raghavan V. Role of 2,4-dichlorophenoxyacetic acid (2,4-D) in somatic embryogenesis on cultured zygotic embryos of Arabidopsis: cell expansion, cell cycling, and morphogenesis during continuous exposure of embryos to 2,4-D. American Journal of Botany. 2004;91:1743–1756. doi: 10.3732/ajb.91.11.1743. [DOI] [PubMed] [Google Scholar]
- Raymond M, Rousset F. An exact test for population differentiation. Evolution. 1995;49:1280–1283. doi: 10.1111/j.1558-5646.1995.tb04456.x. [DOI] [PubMed] [Google Scholar]
- Rival A, Bertrand L, Beule T, Combes MC, Trouslot P, Lashermes P. Suitability of RAPD analysis for the detection of somaclonal variants in oil palm (Elaeis guineensis Jacq) Plant Breeding. 1998;117:73–76. [Google Scholar]
- Roberts AG, Oparka KJ. Plasmodesmata and the control of symplastic transport. Plant, Cell and Environment. 2003;26:103–124. [Google Scholar]
- Rohlf F. NTSYS-pc: numerical taxonomy and multivariate analysis system (Version 2·0) State University of New York; 1998. [Google Scholar]
- Saker M, Adawy S, Mohamed A, El-Itriby H. Monitoring of cultivar identity in tissue culture-derived date palms using RAPD and AFLP analysis. Biologia Plantarum. 2006;50:198–204. [Google Scholar]
- Samosir YMS, Godwin ID, Adkins SW. An improved protocol for somatic embryogenesis in coconut (Cocos nucifera L.) Acta Horticulturae. 1998;461:467–476. [Google Scholar]
- Sandoval A, Hocher V, Verdeil JL. Flow cytometric analysis of the cell cycle in different coconut palm (Cocos nucifera L.) tissues cultured in vitro. Plant Cell Reports. 2003;22:25–31. doi: 10.1007/s00299-003-0651-4. [DOI] [PubMed] [Google Scholar]
- Sané D, Aberlenc-Bertossi F, Gassama-Dia YK, Sagna M, Trouslot MF, Duval Y, Borgel A. Histocytological analysis of callogenesis and somatic embryogenesis from cell suspensions of date palm (Phoenix dactylifera) Annals of Botany. 2006;98:301–308. doi: 10.1093/aob/mcl104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwendiman J, Pannetier C, Michaux-Ferriere N. Histology of somatic embryogenesis from leaf explants of the oil palm Elaeis guineensis. Annals of Botany. 1988;62:43–52. [Google Scholar]
- Somleva MN, Schmidt EDL, de Vries SC. Embryogenic cells in Dactylis glomerata L. (Poaceae) explants identified by cell tracking and by SERK expression. Plant Cell Reports. 2000;19:718–726. doi: 10.1007/s002999900169. [DOI] [PubMed] [Google Scholar]
- Stein M, Stephens C. Effect of 2,4-dichlorophenoxyacetic acid and activated-charcoal on somatic embryogenesis of Bactris gasipaes Hbk. Turrialba. 1991;41:196–201. [Google Scholar]
- Steinmacher DA, Cangahuala-Inocente GC, Clement CR, Guerra MP. Somatic embryogenesis from peach palm zygotic embryos. In Vitro Cellular and Development Biology – Plant. 2007;a 43:124–132. [Google Scholar]
- Steinmacher DA, Clement CR, Guerra MP. Somatic embryogenesis from immature peach palm inflorescence explants: towards development of an efficient protocol. Plant Cell, Tissue and Organ Culture. 2007;b 89:15–22. [Google Scholar]
- Teixeira JB, Söndahl MR, Kirby EG. Somatic embryogenesis from immature inflorescences of oil palm. Plant Cell Reports. 1994;13:247–250. doi: 10.1007/BF00233313. [DOI] [PubMed] [Google Scholar]
- Teixeira JB, Söndahl MR, Nakamura T, Kirby EG. Establishment of oil palm cell suspensions and plant regeneration. Plant Cell, Tissue and Organ Culture. 1995;40:105–111. [Google Scholar]
- Tran Thanh Van K, Bui VL. Curent status of thin cell layer method for the induction of organogenesis or somatic embryogenesis. In: Mohan SJ, Gupta PK, Newton RJ, editors. Somatic embryogenesis in woody plants. Vol. 6. Dordrecht: Kluwer Academic; 2000. pp. 51–92. [Google Scholar]
- Tran Thanh Van M, Dien NT, Chlyah A. Regulation of organogenesis in small explants of superficial tissue of Nicotiana tabacum L. Planta. 1974;119:149–159. doi: 10.1007/BF00390888. [DOI] [PubMed] [Google Scholar]
- Valverde R, Arias O, Thorpe TA. Picloram-induced somatic embryogenesis in pejibaye palm (Bactris gasipaes HBK) Plant Cell Tissue and Organ Culture. 1987;10:149–156. [Google Scholar]
- Van Le B, My Nghieng Thao D, Gendy C, Vidal J, Tran Thanh Van K. Somatic embryogenesis on thin cell layers of a C4 species, Digitaria sanguinalis (L.) Scop. Plant Cell, Tissue and Organ Culture. 1997;49:201–208. [Google Scholar]
- Verdeil JL, Huet C, Grosdemange F, Buffard-Morel J. Plant regeneration from cultured immature inflorescences of coconut (Cocos nucifera L): evidence for somatic embryogenesis. Plant Cell Reports. 1994;13:218–221. doi: 10.1007/BF00239896. [DOI] [PubMed] [Google Scholar]
- Vos P, Hogers R, Bleeker M, Reijans M, van de Lee T, Hornes M, et al. AFLP: a new technique for DNA fingerprinting. Nucleic Acids Research. 1995.;23:4407–4414. doi: 10.1093/nar/23.21.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams EG, Maheswaran G. Somatic embryogenesis: factors influencing coordinated behaviour of cells as an embryogenic group. Annals of Botany. 1986;57:443–462. [Google Scholar]
- Wu X, Weigel D, Wigge PA. Signaling in plants by intercellular RNA and protein movement. Genes and Development. 2002;16:151–158. doi: 10.1101/gad.952002. [DOI] [PubMed] [Google Scholar]