Abstract
Background and Aims
Gross vegetative and floral morphology, as well as modern molecular techniques, indicate that Cryptocentrum Benth. and Sepalosaccus Schltr. are related to Maxillaria Ruiz & Pav. However, they differ from Maxillaria in their possession of floral spurs and, in this respect, are atypical of Maxillariinae. The labellar micromorphology of Maxillaria, unlike that of the other two genera, has been extensively studied. In the present report, the labellar micromorphology of Cryptocentrum and Sepalosaccus is compared with that of Maxillaria and, for the first time, the micromorphology of the floral spur as found in Maxillariinae is described.
Methods
Labella and dissected floral spurs of Cryptocentrum and Sepalosaccus were examined using light microscopy (LM) and scanning electron microscopy (SEM).
Key Results
In each case, the labellum consists of a papillose mid-lobe (epichile), a cymbiform region (hypochile) and, proximally, a spur, which is pronounced in Cryptocentrum but short and blunt in Sepalosaccus. The inner epidermal surface of the spur of Cryptocentrum is glabrous or pubescent, and the bicellular hairs, where present, are unlike any hitherto described for Maxillariinae. Similar but unicellular hairs also occur in the floral spur of Sepalosaccus, whereas the glabrous epidermis lining the spur of C. peruvianum contains putative nectar pores.
Conclusions
The labellar micromorphology of Cryptocentrum and Sepalosaccus generally resembles that of Maxillaria. The floral spur of Cryptocentrum displays two types of organization in that the epidermal lining may be glabrous (possibly with nectar pores) or pubescent. This may have taxonomic significance and perhaps reflects physiological differences relating to nectar secretion. The trichomes found within the spurs of Cryptocentrum and Sepalosaccus more closely resemble the hairs of certain unrelated, nectariferous orchid taxa than those found in the largely nectarless genus Maxillaria, and this further supports the case for parallelism.
Key words: Labellum, Maxillariinae, micromorphology, nectar pore, nectary, spur, trichome
INTRODUCTION
Sub-tribe Maxillariinae, as currently circumscribed, is a Neotropical, species-rich assemblage displaying diverse vegetative morphology and growth patterns, yet retaining a relatively conservative floral morphology (Dressler, 1993; Atwood and Mora de Retana, 1999; Ryan et al., 2000; Whitten et al., 2000; Koehler et al., 2002; Chase et al., 2003; Chase, 2005). It includes Maxillariinae Benth., the former sub-tribes Bifrenariinae Dressler and Lycastinae Schltr., as well as the genus Xylobium Lindl. (Davies and Stpiczyńska, 2006, and references therein). Of those genera that constitute Maxillariinae sensu stricto, Cryptocentrum Benth. is surely one of the most remarkable since it possesses a pronounced sepaline spur. According to Brieger (1977), sepaline spurs also occur in other members of Maxillariinae sensu stricto, such as Anthosiphon Schltr., Pseudomaxillaria Hoehne and Sepalosaccus Schltr., but here they are much shorter and often resemble a mentum. As a result, Senghas (1993) states that of the genera that comprise the Maxillariinae, Cryptocentrum alone possesses a spur. However, Brieger (1977) states that in these four genera ‘the column-foot is not at right angles to the ovary, but curves downward and is more or less parallel to the ovary, while the two lateral sepals are united in their lower part with each other forming the spur, and the labellum is inserted again at the end of the column-foot with its lower part included within the spur’. Sepaline spurs are not restricted to Maxillariinae and also occur in Spiranthinae Lindl., Glomerinae Schltr., Podochilinae Benth. & Hook. and Comparettiinae Schltr. (Oncidiinae Benth.) (Brieger, 1977).
The genus Cryptocentrum was erected to accommodate an Ecuadorian orchid ‘in which the very long, slender spur… is closely appressed to the ovary and enclosed with it in the sheathing bract’ (Bentham, 1881). This feature, in combination with the absence of pseudobulbs, distinguishes Cryptocentrum from other members assigned to Maxillariinae sensu stricto and eventually precipitated its removal to a newly established sub-tribe, much nearer to Comparettiinae than to Maxillariinae, namely Cryptocentrinae Garay (Garay, 1958; also cited in Senghas, 1994). Dressler (1961), however, felt that this move was not justified, and Brieger (1977), who had reached the same conclusion, believed that Cryptocentrum, Anthosiphon, Sepalosaccus and Pseudomaxillaria represent a generic series best referred to as Cryptocentra.
In Cryptocentrum, the floral spur is a double structure comprising a labellar spur formed by the extension of the somewhat straight, lateral lobes of the labellum and sheathed by a second spur formed by fusion of the lateral sepals; the whole partially concealed by a sheathing bract. Senghas (1994) states that a long floral spur is atypical of Maxillariinae sensu stricto and differs from the typical floral spur of Orchidaceae (e.g. Angraecum Bory) in that, when examined in transverse section, it is attached dorsally to the sepaline spur, yet remains free both laterally and ventrally. Senghas, thus, considered the dorsal surface of the spur homologous with the column-foot and, since a prominent mentum is commonly present in Maxillariinae, that the floral spur of Cryptocentrum is derived ontogenetically and possibly phylogenetically from that structure. Similarly, Carnevali (1996, 1999, 2005) proposed that Cryptocentrum is closely related to Anthosiphon and may have evolved from that genus by elongation of the sepaline spur and proportional reduction of the sepaline cup. The delicate nature of the floral spur, however, makes interpretation of its morphology very difficult (Brieger, 1977). Various earlier attempts are outlined by Brieger (1977) whose interpretation conforms to that of Schlechter (cited in Brieger, 1977). This states that the lateral sepals are connate and form a cup- or funnel-like tube. The lateral sepals are adnate to a long column-foot, the labellum inserted at its end having a long claw which is free from the sepals.
That Cryptocentrum and Maxillaria Ruiz & Pav. are related is further supported by studies of their respective seeds. Senghas (1993, 1994) claims that although the seeds of Cryptocentrum differ from those of Maxillaria, they are clearly derived from them.
The genus Cryptocentrum is also unusual amongst members of Maxillariinae sensu stricto in that, with the exception of two species, namely C. pseudobulbosum C. Schweinf. and C. roseans (Schltr.) A.D. Hawkes, it lacks pseudobulbs. Instead, it has branched shoots with distichous or spirally arranged, linear leaves. As a result, it was generally considered by Dressler (1961), Brieger (1977) and Carnevali (1996, 1999, 2001, 2005), amongst others, to have a monopodial habit. Senghas (1994), however, argued that the absence of pseudobulbs alone does not necessarily constitute a monopodium and that Cryptocentrum is sympodial.
Senghas (1994) recognized some 16 species of Cryptocentrum distributed from Costa Rica to Venezuela and south to Peru, whereas Carnevali (2001, 2005), whose infrageneric classification is based upon growth habit, phyllotaxis, foliar anatomy and relative length of the floral bract and spur, lists 17 species for the genus. Of these, only six or so occur in cultivation, and then only infrequently. Consequently, Cryptocentrum has seldom been investigated and, with the exception of the authoritative work of Carnevali (1996, 1999, 2001, 2005), information about the genus is scant.
Likewise, according to Senghas (1993), almost no one has had the opportunity to study a living specimen of the related, Costa Rican genus Sepalosaccus (Schlechter, 1923). This genus contains only two species (Senghas, 1993) and differs from Cryptocentrum in its possession of unifoliate pseudobulbs and its lack of a prominent floral spur. Instead, the lateral sepals are partially fused, forming a globose, saccate perianth, and the labellum is fixed, as in certain species of Maxillaria formerly assigned to Ornithidium R. Br. (Brieger, 1977; Senghas, 1993). The labellum is obscurely three-lobed and the side lobes are turned towards the column. It has two longitudinal ridges united by a transverse ridge at their upper end between the margins of the lateral lobes. The foot of the column is relatively long, runs parallel to the ovary and turns sharply upward. The lateral sepals are adnate to the foot and connate, forming a blunt spur (Brieger, 1977). Dressler (1990) grouped Anthosiphon, Chrysocycnis Linden & Rchb.f., Cryptocentrum, Cyrtidiorchis Rauschert, Maxillaria, Mormolyca Fenzl, Pityphyllum Schltr., Scuticaria Lindl. and Trigonidium Lindl. within Maxillariinae. In species of Maxillaria formerly assigned to Ornithidium, the lip is, to a greater or lesser degree, immovable or fixed as in Pseudomaxillaria, and it would seem that Sepalosaccus is but one genus within this complex. This view is supported by Brieger (1977) who considered both Sepalosaccus and Pseudomaxillaria valid genera. Dressler (1993), in a more recent revision, recognized a more broadly defined Maxillaria comprising Camaridium Lindl., Heterotaxis Lindl., Ornithidium, Marsupiaria Hoehne, Neourbania Fawc. & Rendle, Pseudomaxillaria and Sepalosaccus, as well as seven minor genera, namely Anthosiphon, Chrysocycnis, Cryptocentrum, Cyrtidiorchis, Mormolyca, Pityphyllum and Trigonidium. Furthermore, the Phylogenetics of Maxillariinae (Orchidaceae) website (http://www.flmnh.ufl.edu/herbarium/max/default.htm) shows that Sepalosaccus strumatus (Endres & Rchb.f.) Garay (syn. S. humilis Schltr.), referred to as Maxillaria strumata (Endres & Rchb.f.) Ames & Correll, is nested within the ‘Maxillaria core’. Conversely, Singer et al. (2007) showed that the Cryptocentrum/Anthosiphon clade is most closely related to the Maxillaria picta Hook. alliance, a taxon whose species they assigned to a new genus, Brasiliorchis R. Singer, S. Koehler & Carnevali on the basis of ‘multiple sequence data and a combination of distinctive vegetative and floral features’. In contrast to Sepalosaccus, both these clades fall outside the ‘Maxillaria core’.
Stern et al. (2004) have shown that Maxillariinae and Lycastinae can be distinguished on anatomical grounds from other sub-tribes of Maxillarieae (sans Oncidiinae) in that they possess tilosomes, foliar glands and foliar fibre bundles. These features were also found to be present in Cryptocentrum. Unfortunately, insufficient material precluded the investigators from determining whether all these characters are present in any one of the species of Cryptocentrum studied.
Modern molecular data have generally supported the inclusion of Cryptocentrum and Sepalosaccus in Maxillariinae. For example, the cladistic parsimony analyses of rbcL nucleotide sequences have vindicated the placement of Cryptocentrum within Maxillariinae (including Bifrenariinae but excluding Lycastinae) (Cameron et al., 1999). Whitten et al. (2000), who investigated the monophyly of Maxillariinae using parsimony analyses of combined nuclear ribosomal and plastid DNA sequence data of internal transcribed spacer (ITS) 1 and 2, matK, the trnL intron and the trnL–F intergenic spacer, showed that Maxillariinae sensu lato is strongly supported by SW bootstrap analysis and contains several strongly supported clades including Maxillariinae sensu stricto containing Maxillaria, Trigonidium and Cryptocentrum. Dathe and Dietrich (2006) investigated the phylogenetic relationships of Maxillariinae sensu stricto using maximum parsimony and Bayesian analyses of nuclear ribosomal ITS1 and ITS2 DNA sequences. Their results showed that Maxillariinae is monophyletic but that Maxillaria, in its current, narrower circumscription, is paraphyletic with the currently accepted Chrysocycnis, Cryptocentrum, Mormolyca and Trigonidium, as well as the former segregates Camaridium, Heterotaxis, Marsupiaria, Neourbania, Ornithidium and Pseudomaxillaria, nested within it. Since then, Whitten et al. (in prep.), by means of combined molecular data, have shown that Maxillaria sensu stricto and Camaridium are distinct clades and that Sepalosaccus is embedded within Camaridium.
Recently, the labellar micromorphology of Maxillariinae sensu stricto and Bifrenariinae has been extensively studied (Davies and Winters, 1998; Davies et al., 2000, 2003a, b; Davies and Turner, 2004a; Davies and Stpiczyńska, 2006). The present report compares the labellar micromorphology of the seldom encountered and enigmatic genera Cryptocentrum and Sepalosaccus with other members of Maxillariinae sensu lato as part of an ongoing programme to characterize the micromorphological features of this sub-tribe. The authors had also intended to compare the floral micromorphology of other related genera such as Anthosiphon, Pityphyllum, Chrysocycnis and Cyrtidiorchis. Unfortunately, this proved impossible owing to a lack of suitable fresh and spirit-preserved material.
MATERIALS AND METHODS
Spirit-preserved material of four species of Cryptocentrum and one species of Sepalosaccus was obtained from the Royal Botanic Garden, Kew, UK (Table 1). Their accession numbers are prefixed ‘K’. The names by which these specimens were originally collected have been retained, but recent changes in nomenclature have been noted. The authorities for plant names follow Brummit and Powell (1992). Preserved material, whilst at R.B.G. Kew, was stored in Kew mix [53 % ethanol (industrial methylated spirit), 37 % water, 5 % formaldehyde solution, 5 % glycerol]. However, it was transferred to, and kept in, Copenhagen mix [70 % ethanol (industrial methylated spirit), 28 % water, 2 % glycerol] for the duration of this study.
Table 1.
Taxa examined and their provenance
| Taxon | Accession no. | Collector | Collector no. | Provenance | Date | Taxonomic notes |
|---|---|---|---|---|---|---|
| Cryptocentrum calcaratum (Schltr.) Schltr. | K13598 | Costa Rica | ||||
| C. gracillimum Ames & C. Schweinf. | K37139 | Dunsterville | Venezuela | |||
| C. peruvianum (Cogn.) C. Schweinf. | K48260 | H. Pfennig | 1558 | Ecuador | 1984 | |
| C. standleyi Ames | K48525 | Panama | ||||
| Sepalosaccus humilis Schltr. | K12636 | L.H. Lankester | Costa Rica | 1935 | syn. Maxillaria strumata (Endres & Rchb.f.) Ames & Correll, syn. Sepalosaccus strumatus (Endres & Rchb.f.) Garay |
Following preliminary examination by means of light microscopy, labella and dissected floral spurs were prepared for scanning electron microscopy (Stpiczyńska et al., 2004; Davies and Turner, 2004b) and examined by means of a TESLA BS-300 at an accelerating voltage of 20–25 kV.
The floral spurs of a number of unrelated orchid taxa were obtained from Swansea Botanical Complex, UK (accession numbers prefixed ‘S’) and compared with the above.
RESULTS
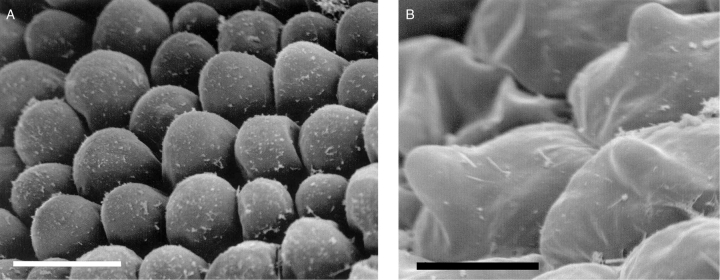
The labella of all four species of Cryptocentrum studied are obscurely tri-lobed and comprise three distinct regions: a linguiform mid-lobe (epichile), a cymbiform region (hypochile) formed by the upturned margins of the lateral lobes of the labellum and, proximally, a tubular spur (Fig. 1A). Three prominent veins run the length of the labellum, but only the central vein extends almost to the tip of the mid-lobe. The mid-lobe is papillose (Figs 1B and 2A), the papillae being obpyriform (Figs 1C and 2B), whereas the cymbiform region is usually glabrous, and in some species, such as C. standleyi, composed of thin-walled cells that are longer than wide (Fig. 1D). In others, such as C. gracillimum (Fig. 2C) and C. calcaratum, these cells are more or less isodiametric. In the latter species, the cymbiform region becomes increasingly pubescent proximally (Figs 3B, C). Similarly, epidermal cells lining the spur may be elongate (Figs 1E and 3E, F) or isodiametric (Fig. 2D). In C. standleyi and C. peruvianum, the epidermis lining the spur is glabrous (Fig. 1E), whereas that of C. gracillimum (Fig. 2D) and C. calcaratum (Figs 3E, F) is pubescent. Although hairs, at low magnifications, appear unicellular, they are in fact bicellular, comprising a short basal cell and a longer, gradually tapering, terminal cell (Figs 2D, E and 3A–F). In C. calcaratum, trichomes, whether within the spur or upon the proximal surface of the cymbiform region, have very similar features (Fig. 3A–F). The spur of C. peruvianum, however, lacks trichomes, but elliptic perforations in the epidermis lining the spur were occasionally observed (Fig. 1F), measuring approx. 31·3 × 5·0 µm.
Fig. 1.
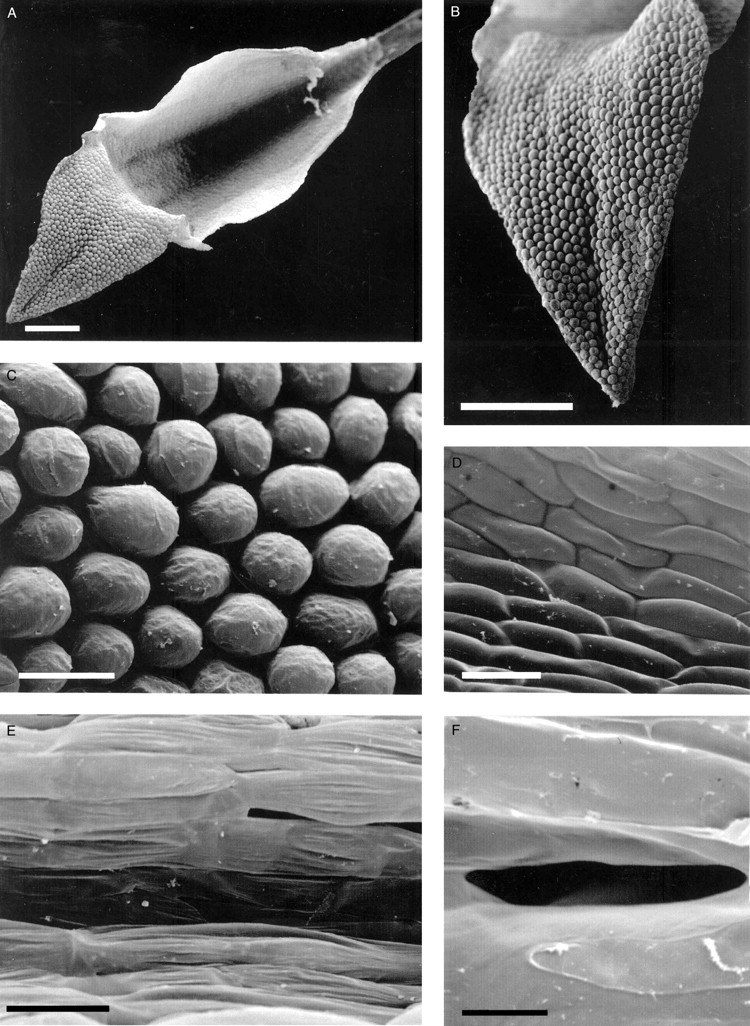
Cryptocentrum standleyi (accession no. K48525). (A) Labellum showing papillose, linguiform mid-lobe, cymbiform median region and part of a spur arising proximally. Scale bar = 500 µm. (B) Detail of a papillose mid-lobe. Scale bar = 500 µm. (C) Detail of obpyriform papillae of the mid-lobe. Scale bar = 50 µm. (D) Detail of glabrous, cymbiform region of the labellum with rectangular epidermal cells. Scale bar = 50 µm. (E, F) Cryptocentrum peruvianum (accession no. K48260). (E) Glabrous, internal surface of spur. Scale bar = 50 µm. (F) Detail of a putative nectar pore. Scale bar = 10 µm.
Fig. 2.
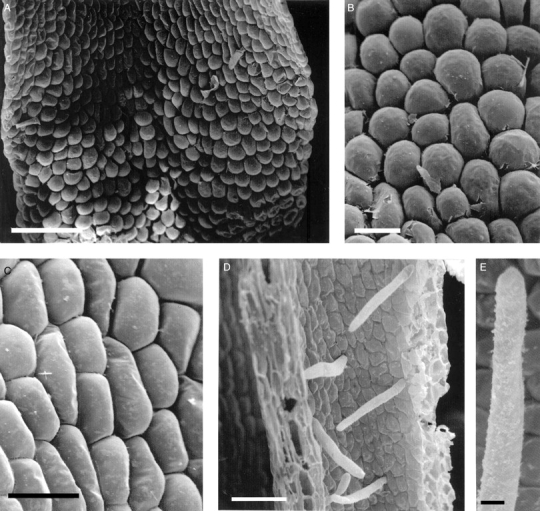
Cryptocentrum gracillimum (accession no. K37139). (A) Papillose, mid-lobe of labellum similar to that of C. standleyi. Scale bar = 200 µm. (B) Detail of obpyriform, mid-lobe papillae. Scale bar = 50 µm. (C) Detail of glabrous, cymbiform region of labellum with isodiametric to rectangular epidermal cells. Scale bar = 50 µm. (D) Internal surface of a spur with bicellular trichomes. Scale bar = 100 µm. (E) Detail of a single spur trichome. Scale bar = 10 µm.
Fig. 3.
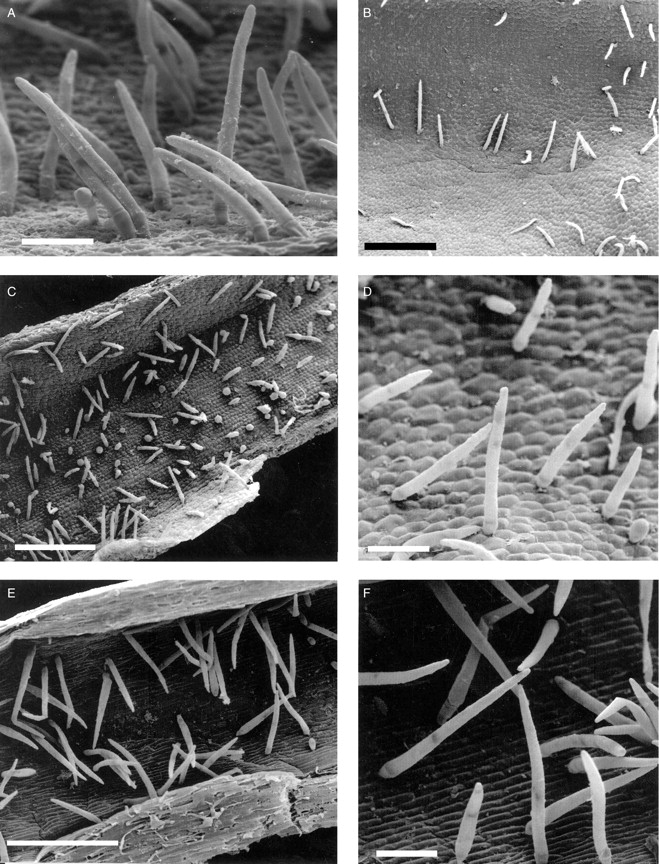
Cryptocentrum calcaratum (accession no. K13598). (A) Bicellular hairs of C. calcaratum comprising a basal cell and a longer, tapering terminal cell with rounded tip. Scale bar = 100 µm. (B) Pubescent, proximal part of cymbiform region of labellum with isodiametric, epidermal cells. Scale bar = 500 µm. (C) Increasingly pubescent region of labellum close to the origin of the spur. Note that isodiametric, epidermal cells are still present here. Scale bar = 500 µm. (D) Detail of trichomes from the proximal part of the cymbiform region of a labellum. Scale bar = 100 µm. (E) Pubescent, internal surface of a spur. Note the elongate epidermal cells. Scale bar = 500 µm. (F) Detail of the internal surface of a spur showing trichomes and elongate epidermal cells. Scale bar = 100 µm.
In many ways, the labellum of Sepalosaccus resembles that of Cryptocentrum. The mid-lobe of the labellum, although more rounded than that of Cryptocentrum, is again papillose, and the papillae, like those of Cryptocentrum, are obpyriform (Fig. 4A). Marginally, the mid-lobe bears nipple-like, conical papillae. The upturned margins of the lateral lobes of the labellum, as in Cryptocentrum, form a cymbiform region which is composed of thin-walled, more or less isodiametric cells. Proximally, the labellum forms a blunt spur whose cells are isodiametric and shortly papillose (Fig. 4B). Unicellular trichomes arise from these papillae.
Fig. 4.
Sepalosaccus humilis (accession no. K12636). (A) Detail of papillose mid-lobe of labellum. Scale bar = 50 µm. (B) Detail of the proximal region of a labellum showing short papillae which develop into unicellular trichomes. Scale bar = 50 µm.
DISCUSSION
Cryptocentrum is atypical for Maxillariinae in its possession of a floral spur. Spur length is usually correlated with that of the proboscis of the insect pollinator (Johnson, 1997, 2006; Nilsson, 1998, and references therein; Benitez-Vieyra et al., 2006). Thus, the relatively long spur of Cryptocentrum, together with flower coloration and sweet nocturnal scent (Carnevali, 1999, 2001), is probably an adaptation for moth pollination (Dressler, 1990; Carnevali, 2005), unlike most species of Maxillaria which are thought to be pollinated by Meliponini (stingless bees) (Singer and Cocucci, 1999; Roubik, 2000). The floral spur of Cryptocentrum is a double structure consisting of a labellar spur enclosed within an outer, sepaline spur, and is reported to contain nectar at its extreme tip (Carnevali, 2005). Floral spurs also occur in a number of unrelated genera native to other continents. They arise from the proximal part of the labellum and are formed by the partial fusion of the lateral sepals (Figueiredo and Pais, 1992; Galetto et al., 1997; Stpiczyńska, 1997, 2003a, 2004; Singer and Sazima, 1999; Stpiczyńska and Matusiewicz, 2001). Some flowers, such as those of Satyrium hallackii Bolus ssp. hallackii, have two spurs (Johnson, 1997). However, the possession of a floral spur does not necessarily indicate that a flower produces nectar (Roy and Widmer, 1999) since flowers of some rewardless orchids mimic unrelated species that have nectar-laden flowers. For example, Disa ferruginea (Thunb.) Sw. mimics Tritoniopsis triticea (Burm. F.) Goldbl. (Iridaceae) and Kniphofia uvaria (L.) Hook. (Asphodelaceae) (Johnson, 1994).
The epidermis lining the floral spur of orchids may be glabrous as in Thunia × veitchiana [T. alba (Lindl.) Rchb.f. × T. bensoniae Hook.f.] (S00004322) and Cribbia cf. confusa P.J. Cribb (S19970064), but is frequently pubescent as in Angraecum scottianum Rchb.f. (S19950021), A. germinyanum Hook.f. (S1997002), Mystacidium braybonae Summerh. (S19960027) (K.L. Davies, unpublished data), Aeranthes arachnites (Thouars) Lindl., A. grandiflora Lindl. (Roberts, 2001), Platanthera bifolia (L.) Rich. (Stpiczyńska, 1997), P. chlorantha (Custer) Rchb. (Stpiczyńska, 2003a, 2004) and Gymnadenia conopsea (L.) R. Br. (Stpiczyńska and Matusiewicz, 2001). These hairs are unicellular and, especially in the case of P. bifolia (Stpiczyńska, 1997), resemble to varying degrees the spur trichomes of Cryptocentrum spp. However, the spur hairs of A. germinyanum, unlike those of Cryptocentrum, have wide bases and longitudinal, cuticular striations. Hairs can significantly enlarge the surface area for nectar secretion and nectar resorption (Stpiczyńska 2003a, b, 2004; Nepi and Stpiczyńska, 2007), thereby enabling the conservation of valuable material and energy resources. The resemblance of the spur trichomes of the Neotropical genus Cryptocentrum to those of the unrelated and largely European P. bifolia (Stpiczyńska, 1997), as well as other unrelated orchid species native to other continents, is perhaps indicative of parallelism, especially since such hairs have not been described for the closely related genera Maxillaria and Mormolyca (Maxillariinae) (Davies and Winters, 1998; Davies et al. 2000, 2003a, b; Davies and Turner, 2004a; Davies and Stpiczyńska, 2006). Indeed, it has been estimated that only some 8 % of Maxillaria spp. produce nectar (Davies et al., 2005), and trichomes in this genus tend to be multicellular. The presence of bicellular hairs in Anthosiphon would support the proposal that it is nested within Cryptocentrum (Singer et al., 2007) but, unfortunately, specimens of Anthosiphon were not available for study. Unicellular hairs, however, occur in Mormolyca. These are much longer than wide, with narrow points of insertion and pointed tips, much like those of Ophrys L. (Servettaz et al., 1994; Ascensão et al., 2005), and this may be due to the fact that pseudocopulation occurs in both genera (Singer et al., 2004; Flach et al., 2006). Trichomes similar to those found in Cryptocentrum and Sepalosaccus do not occur in Bifrenaria, Rudolfiella, Teuscheria or Xylobium (genera formerly assigned to Bifrenariinae but currently assigned to Maxillariinae sensu lato). Nevertheless, the genus Bifrenaria, which is nectarless and is pollinated by Eufriesia violacea (Euglossini) and Bombus brasiliensis (Bombini) (Singer and Koehler, 2004), has unicellular, labellar hairs, and certain species of Xylobium, a genus pollinated by Meliponini, have bicellular trichomes (Davies and Stpiczyńska, 2006). In both cases, however, these hairs differ in their relative proportions from those found in Cryptocentrum and Sepalosaccus.
The spurs of certain species of Cryptocentrum, such as C. standleyi and C. peruvianum, lack hairs. Instead, elliptical perforations were found on the inner epidermal surface of the spur of C. peruvianum, and these resemble the nectar pores found in Chamaecytisus ruthenicus A. Klaskova and Retama monosperma (L.) Boiss. (Fabaceae: Genistinae) (Vogel, 1997). The nectar pores of Chamaecytisus are non-stomatal in origin and are thought to arise from an initially intact epidermis by the selective dissolution of epidermal cells. Moreover, they have similar dimensions to the putative nectar pores of C. peruvianum. Senghas (1994) and Carnevali (2001, 2005) placed C. peruvianum and C. standleyi, together with C. flavum Schltr., in a separate sub-genus (Caulescentes K. Senghas) since they differ from other species of Cryptocentrum in that they display polystichous phyllotaxis and relatively short floral bracts. Thus, the absence or presence of hairs within the floral spur of Cryptocentrum may be taxonomically significant and requires further investigation.
The labellum of Sepalosaccus strumatus (syn. S. humilis) resembles that of Cryptocentrum. Hairs occur within the short, blunt spur of this species, and although these are unicellular, they otherwise resemble spur hairs of Cryptocentrum. They are derived from small, conical papillae similar to those lining the floral spur of Gymnadenia conopsea (Stpiczyńska and Matusiewicz, 2001). Conical, floral papillae are ubiquitous amongst angiosperms (Kay et al., 1981), including the genus Maxillaria (Davies and Turner, 2004a). Even so, the presence of unicellular hairs in Sepalosaccus is atypical of Maxillariinae sensu stricto. Their presence within the short, blunt spur and their similarity to those found within the spurs of unrelated, nectariferous orchids supports the possibility that nectar occurs in this species and also parallelism. Indeed, on the basis of phylogenetic analyses [Phylogenetics of Maxillariinae (Orchidaceae) website], S. strumatus (listed as M. strumata) is thought to be closely related to other nectariferous species of Maxillaria such as M. parviflora (Poepp. & Endl.) Garay, although there nectar occurs in a shallow depression of the lip (Singer and Koehler, 2004). Moreover, combined molecular data indicate that Sepalosaccus is embedded within Camaridium, a taxon that contains several nectariferous species (Whitten et al., in prep.). However, Stern et al. (2004) and Dathe and Dietrich (2006) concur with Benzing (1986) that, owing to convergence, anatomical characters alone, and in particular those related to pollination, are of limited use in determining relationships within Maxillariinae.
Finally, the relative rarity of Cryptocentrum and Sepalosaccus spp. in European collections precluded examination of live flowers and, until this is done, neither the presence and composition of nectar nor the role of spur hairs and putative nectar pores in its secretion can be established. However, it is significant that the flowers of nectarless members of Maxillariinae sensu stricto, whose micromorphology otherwise resembles that of Cryptocentrum and Sepalosaccus, lack such hairs, even though they are present in unrelated, nectariferous species. On the basis of this evidence, it is speculated that representatives of both genera produce nectar and that spur hairs are modified for this purpose.
ACKNOWLEDGEMENTS
K.L.D. is grateful to the Stanley Smith (UK) Trust for their generous grant. The authors also thank the staff of the Royal Botanic Gardens, Kew, UK, and Alan Gregg, Swansea Botanical Complex, Swansea, UK for plant material.
LITERATURE CITED
- Ascensão L, Francisco A, Cotrim H, Pais MS. Comparative structure of the labellum in Ophrys fusca and O. lutea (Orchidaceae) American Journal of Botany. 2005;92:1059–1067. doi: 10.3732/ajb.92.7.1059. [DOI] [PubMed] [Google Scholar]
- Atwood JT, Mora de Retana DE. Orchidaceae: tribe Maxillarieae: subtribes Maxillariinae and Oncidiinae. Fieldiana. 1999;40:1–182. [Google Scholar]
- Benitez-Veira S, Medina AM, Glinos E, Cocucci AA. Pollinator mediated selection on floral traits and size of floral display in Cyclopogon elatus, a sweat bee-pollinated orchid. Functional Ecology. 2006;20:948–957. [Google Scholar]
- Bentham G. Notes on Orchidaceae. Journal of the Linnean Society, London. 1881;18:325. [Google Scholar]
- Benzing DH. The genesis of orchid diversity: emphasis on floral biology leads to misconceptions. Lindleyana. 1986;1:73–89. [Google Scholar]
- Brieger FG. On the Maxillariinae (Orchidaceae) with sepaline spur. Botanische Jahrbucher fur Systematik Pflanzengeschichte und Pflanzengeographie. 1977;97:548–574. [Google Scholar]
- Brummitt RK, Powell CE. Authors of plant names. Kew: Royal Botanic Gardens; 1992. [Google Scholar]
- Cameron KM, Chase MW, Whitten WM, Kores PJ, Jarrell DC, Albert VA, et al. A phylogenetic analysis of the Orchidaceae: evidence from RBCL nucleotide sequences. American Journal of Botany. 1999;86:208–224. [PubMed] [Google Scholar]
- Carnevali G. St Louis-Missouri Botanical Garden; 1996. Systematics, phylogeny, and twig epiphytism in Cryptocentrum (Orchidaceae) PhD dissertation, University of Missouri. [Google Scholar]
- Carnevali G. Atwood JT, Mora de Retana DE, editors. Flora Costaricensis; Orchidaceae. Fieldiana: Botany. 1999;(No. 40):2. Cryptocentrum. (New Series) 29–32. [Google Scholar]
- Carnevali G. A synoptical view of the classification of Cryptocentrum Benth. (Orchidaceae), new taxa, and a key to the genus. Harvard Papers in Botany. 2001;5:467–486. [Google Scholar]
- Carnevali G. Cryptocentrum Benth. In: Pupúlin F, editor. Vanishing beauty: native Costa Rican orchids. Vol. 1. San José, Costa Rica: Costa Rica University Press; 2005. pp. 167–171. [Google Scholar]
- Chase MW. Classification of Orchidaceae in the age of DNA data. Curtis's Botanical Magazine. 2005;22:2–7. [Google Scholar]
- Chase MW, Barret RL, Cameron KN, Freudenstein JV. DNA data and Orchidaceae systematics: a new phylogenetic classification. In: Dixon KM, editor. Orchid conservation. Sabah, Malaysia: Natural History Publications; 2003. pp. 69–89. Kota Kinabalu. [Google Scholar]
- Dathe S, Dietrich H. Comparative molecular and morphological studies of selected Maxillariinae orchids. Wildenowia. 2006;36:89–102. [Google Scholar]
- Davies KL, Stpiczyńska M. Labellar micromorphology of Bifrenariinae Dressler (Orchidaceae) Annals of Botany. 2006;98:1215–1231. doi: 10.1093/aob/mcl204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies KL, Turner MP. Morphology of floral papillae in Maxillaria Ruiz & Pav. (Orchidaceae) Annals of Botany. (a) 2004;93:75–86. doi: 10.1093/aob/mch007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies KL, Turner MP. Pseudopollen in Eria Lindl. Section Mycaranthes Rchb.f. (Orchidaceae) Annals of Botany. (b) 2004;94:707–715. doi: 10.1093/aob/mch195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies KL, Winters C. Ultrastructure of the labellar epidermis in selected Maxillaria species (Orchidaceae) Botanical Journal of the Linnean Society. 1998;126:349–361. [Google Scholar]
- Davies KL, Winters C, Turner MP. Pseudopollen: its structure and development in Maxillaria (Orchidaceae) Annals of Botany. 2000;85:887–895. [Google Scholar]
- Davies KL, Turner MP, Gregg A. Atypical pseudopollen-forming hairs in Maxillaria Ruiz & Pav. (Orchidaceae) Botanical Journal of the Linnean Society. (a) 2003;143:151–158. [Google Scholar]
- Davies KL, Turner MP, Gregg A. Lipoidal labellar secretions in Maxillaria Ruiz & Pav. (Orchidaceae) Annals of Botany. (b) 2003;91:439–446. doi: 10.1093/aob/mcg038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies KL, Stpiczyńska M, Gregg A. Nectar-secreting floral stomata in Maxillaria anceps Ames & C. Schweinf. (Orchidaceae) Annals of Botany. 2005;96:217–227. doi: 10.1093/aob/mci182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dressler RL. The systematic position of Cryptocentrum (Orchidaceae) Brittonia. 1961;13:266–270. [Google Scholar]
- Dressler RL. The orchids – natural history and classification. London: Harvard University Press; 1990. [Google Scholar]
- Dressler RL. Phylogeny and classification of the orchid family. Cambridge: Cambridge University Press; 1993. [Google Scholar]
- Figueiredo ACS, Pais MS. Ultrastructural aspects of nectary spur of Limodorum abortivum (L) Sw. (Orchidaceae) Annals of Botany. 1992;70:325–331. [Google Scholar]
- Flach A, Marsaioli AJ, Singer RB, Amaral MCE, Menezes C, Kerr WE, et al. Pollination by sexual mimicry in Mormolyca ringens: a floral chemistry that remarkably matches the pheromones of virgin queens of Scaptotrigona sp. Journal of Chemical Ecology. 2006;32:59–70. doi: 10.1007/s10886-006-9351-1. [DOI] [PubMed] [Google Scholar]
- Galetto L, Bernardello G, Riveira GL. Nectar, nectaries, flower visitors, and breeding systems in five terrestrial Orchidaceae from Central Argentina. Journal of Plant Research. 1997;110:393–403. [Google Scholar]
- Garay L. Studies in American orchids IV. Botanical Museum Leaflets Harvard University. 1958;18:186–218. [Google Scholar]
- Johnson SD. Evidence for Batesian mimicry in a butterfly-pollinated orchid. Botanical Journal of the Linnean Society. 1994;53:91–104. [Google Scholar]
- Johnson SD. Pollination ecotypes of Satyrium hallackii (Orchidaceae) in South Africa. Botanical Journal of the Linnean Society. 1997;123:225–235. [Google Scholar]
- Johnson SD. Pollination by long-proboscid flies in the endangered African orchid Disa scullyi. South African Journal of Botany. 2006;72:24–27. [Google Scholar]
- Kay QON, Daoud HS, Stirton CH. Pigment distribution, light reflection and cell structure in petals. Botanical Journal of the Linnean Society. 1981;83:57–84. [Google Scholar]
- Koehler S, Williams NH, Whitten WM, Amaral MCE. Phylogeny of the Bifrenaria (Orchidaceae) complex based on morphology and sequence data from nuclear rDNA internal transcribed spaces (ITS) and chloroplast trnL–trnF region. International Journal of Plant Sciences. 2002;163:1055–1066. [Google Scholar]
- Nepi M, Stpiczyńska M. Nectar resorption and translocation in Cucurbita pepo L. and Platanthera chlorantha (Custer) Rchb. Plant Biology. 2007;9:93–100. doi: 10.1055/s-2006-924287. [DOI] [PubMed] [Google Scholar]
- Nilsson A. Deep flowers for long tongues. Trends in Ecology and Evolution. 1998;13:259–260. doi: 10.1016/s0169-5347(98)01359-7. [DOI] [PubMed] [Google Scholar]
- Roberts DL. Reproductive biology and conservation of the orchids of Mauritius. University of Aberdeen; 2001. PhD thesis. [Google Scholar]
- Roubik DW. Deceptive orchids with Meliponini as pollinators. Plant Systematics and Evolution. 2000;222:271–279. [Google Scholar]
- Roy BA, Widmer A. Floral mimicry: a fascinating yet poorly understood phenomenon. Trends in Plant Science. 1999;4:325–330. doi: 10.1016/s1360-1385(99)01445-4. [DOI] [PubMed] [Google Scholar]
- Ryan A, Whitten WM, Johnson MAT, Chase MW. A phylogenetic assessment of Lycaste and Anguloa (Orchidaceae: Maxillarieae) Lindleyana. 2000;15:33–45. [Google Scholar]
- Schlechter R. Beiträge zur Orchideenkunde von Zentralamerika. Fedde. Repert. Spec. Nov., Beih. 1923;10:244–246. [Google Scholar]
- Senghas K. Subtribus Maxillariinae. In: Breiger FG, Maatsch R, Senghas K, Schlechter R, editors. Die Orchideen. Vol. 28. Berlin: Blackwell Wissenschafts-Verlag; 1993. pp. 1727–1745. [Google Scholar]
- Senghas K. Subtribus Maxillariinae. In: Breiger F. G., Maatsch R., Senghas K., Rudolph Schlechter, editors. Die Orchideen. Vol. 28. Berlin: Blackwell Wissenschafts-Verlag; 1994. pp. 1797–1799. [Google Scholar]
- Servettaz O, Bini Maleci L, Grünanger P. Labellum micromorphology in the Ophrys bertolonii agg. and some related taxa (Orchidaceae) Plant Systematics and Evolution. 1994;189:123–131. [Google Scholar]
- Singer RB, Cocucci AA. Pollination mechanisms in four sympatric southern Brazilian Epidendroideae orchids. Lindleyana. 1999;14:47–56. [Google Scholar]
- Singer RB, Koehler S. Pollinarium morphology and floral rewards in Brazilian Maxillariinae (Orchidaceae) Annals of Botany. 2004;93:39–51. doi: 10.1093/aob/mch009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer RB, Sazima M. The pollination mechanism in the ‘Pelexia alliance’ (Orchidaceae: Spiranthinae) Botanical Journal of the Linnean Society. 1999;131:249–262. [Google Scholar]
- Singer RB, Flach A, Koehler S, Marsaioli AJ, Do Carmo E, Amaral M. Sexual mimicry in Mormolyca ringens (Lindl.) Schltr. (Orchidaceae: Maxillariinae) Annals of Botany. 2004;93:755–762. doi: 10.1093/aob/mch091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer RB, Koehler S, Carnevali G. Brasiliorchis: a new genus for the Maxillaria picta alliance (Orchidaceae, Maxillariinae) Novon. 2007;17:91–99. [Google Scholar]
- Stern WL, Judd WS, Carlsward BS. Systematic and comparative anatomy of Maxillarieae (Orchidaceae), sans Oncidiinae. Botanical Journal of the Linnean Society. 2004;144:251–274. [Google Scholar]
- Stpiczyńska M. The structure of nectary of Platanthera bifolia L. Orchidaceae. Acta Societatis Botanicorum Poloniae. 1997;66:5–11. [Google Scholar]
- Stpiczyńska M. Nectar resorption in the spur of Platanthera chlorantha (Custer) Rchb. Orchidaceae – structural and microautoradiographic study. Plant Systematics and Evolution. (a) 2003;238:119–126. [Google Scholar]
- Stpiczyńska M. Kwitnienie i nektarowanie gółki długoostrogowej. Annales Universitatis Mariae Curie-Skłodowska Section EEE Horticultura. (b) 2003;13:109–115. [Google Scholar]
- Stpiczyńska M. Rozprawy Naukowe Akademii Rolniczej. Vol. 286. Lublin: WAW; 2004. Rola nektaru w kwiatach podkolanu zielonawego Platanthera chlorantha (Custer) Rchb. (Orchidaceae) [Google Scholar]
- Stpiczyńska M, Matusiewicz J. Anatomy and ultrastructure of spur nectary of Gymnadenia conopsea L. Orchidaceae. Acta Societatis Botanicorum Poloniae. 2001;70:267–272. [Google Scholar]
- Stpiczyńska M, Davies KL, Gregg A. Nectary structure and nectar secretion in Maxillaria coccinea (Jacq.) L.O. Williams ex Hodge (Orchidaceae) Annals of Botany. 2004;93:87–95. doi: 10.1093/aob/mch008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel S. Remarkable nectaries: structure, ecology organophyletic perspectives. I. Substitutive nectaries. Flora. 1997;192:305–333. [Google Scholar]
- Whitten WM, Williams NH, Chase MW. Subtribal and generic relationships of Maxillarieae (Orchidaceae) with emphasis on Stanhopeinae: combined molecular evidence. American Journal of Botany. 2000;87:1842–1856. [PubMed] [Google Scholar]