Abstract
Background and Aims
Neutral theory predicts that the diversity and relative abundance of species in ecological communities do not depend on their specific traits. This prediction remains controversial, as many studies suggest that variations in the niches of species determine the structure of communities. The aim of this study was to test empirically the relative importance of niche and neutral processes as drivers of species abundance within plant communities along a successional gradient.
Methods
Information on the abundance (density and frequency) and traits (aboveground individual biomass and seed mass) of > 90 species was collected in alpine and sub-alpine meadows of the Tibet Plateau (China). A successional gradient (1, 3, 15 and 30 years after abandonment) was established in a sub-alpine meadow. The relationships between species traits and their abundance were evaluated using regression models.
Key Results
Seed mass was negatively related to both species density (r = –0·6270, P < 0·001) and frequency (r = –0·5335, P = 0·005) in the 1-year meadow. Such relationships disappeared along the successional gradient evaluated (P > 0·07 in the 3-, 15- and 30-year meadows). Data gathered in all sites showed a significant negative relationship between the average individual biomass of a given species and its density within the community (r < –0·30, P < 0·025 in all cases).
Conclusions
The results show that seed mass was a key driver of species abundance in early successional communities, and that niche forces may become more important as succession progresses. They also indicate that predictions from neutral theory, in its current form, do not hold for the meadow communities studied.
Key words: Average individual biomass, niche, neutral theory, seed mass, successional gradient, meadow
INTRODUCTION
The neutral theory of biodiversity proposed by Hubbell (2001) has invigorated research on the mechanisms driving the distribution and abundance of species within communities. This theory assumes that all individuals of all species are functionally equivalent, i.e. individuals do not have species-specific traits influencing their fitness, longevity, movements or likelihood of speciation (Bell, 2001; Hubbell, 2001). Accordingly, it predicts that any given species trait should be uncorrelated with its abundance in a community (Hubbell, 2001; McGill et al., 2006a). Neutral theory strongly emphasizes the role of stochastic events such as dispersal, local extinction and speciation as drivers of community structure and diversity. Predictions from neutral theory are contrary to basic assumptions of niche theory, which asserts that the species must differ in their traits, and often show trade-offs, in order to co-exist within a community for long periods of time (Chesson, 2000; Chase and Leibold, 2003; Condit et al., 2006; McGill et al., 2006b; Westoby and Wright, 2006).
Empirical support for the neutral theory remains equivocal, with studies both supporting (Hubbell, 2001; Condit et al., 2002; Volkov et al., 2003; Hubbell, 2005, 2006) and rejecting (McGill, 2003; Gilbert and Lechowicz, 2004; Volkov et al., 2005; Wootton, 2005; Gilbert et al., 2006; Harpole and Tilman, 2006; Thompson and Townsend, 2006) its predictions. The fact that random forces alone cannot explain many patterns observed in nature suggests that factors such as variations in the niches of species are important determinants of the structure of ecological communities. Indeed, recent studies are incorporating elements of both niche and neutral theory to develop a more general framework for explaining such structure (Uriarte and Reeve, 2003; Tilman, 2004; Gravel et al., 2006; Zhou and Zhang, 2006; Adler et al., 2007). In this regard, the continuum hypothesis proposed by Gravel et al. (2006) assumes that communities are located in a continuum determined by stochastic and deterministic forces depending on three factors: species niche overlap, species richness and dispersal capabilities. According to their model, diversity and abundance patterns along environmental gradients will be the consequence of the balance between stochastic processes and competitive exclusion. Additionally, Gravel et al. (2006) show that there is a positive relationship between neutrality and the dispersal capabilities of species, suggesting that the latter may be a key process to link niche and neutral approaches.
Many studies have shown that the dispersal ability of a species is closely correlated with traits such as its seed mass (Leishman and Murray, 2001; Coomes et al., 2002; Mabry, 2004) which, indeed, is strongly correlated with plant abundance in many communities (Leishman and Murray, 2001; Muller-Landau, 2003; Murray and Leishman, 2003; Murray et al., 2004, 2005). Leishman and Murray (2001) proposed a successional model to explain the relationship between seed mass and the abundance of species within communities. In their model, communities in early successional stages are dominated by small-seeded species with good dispersal capacities (Leishman and Murray, 2001; Schippers et al., 2001; Coomes et al., 2002; Mabry, 2004; Mouquet et al., 2004). As succession progresses, communities become dominated by large-seeded species with competitive superiority (Leishman and Murray, 2001; Murray and Leishman, 2003; Murray et al., 2005). These predictions have been theoretically evaluated in a series of studies (Leishman and Murray, 2001; Guo, 2003; Eriksson, 2005), but very few empirical tests have been conducted so far to test them (but see Leishman and Murray, 2001; Coomes et al., 2002). Leishman and Murray (2001) found similar results in open woodland communities along a successional gradient determined by fire. These authors found a negative and non-significant relationship between seed size and abundance in areas burned < 10 years ago, but this relationship became significant and positive in sites burned > 15 years ago. Coomes et al. (2002) found that at least some large-seeded species are restricted to late-successional, nutrient-rich sites in dune communities.
To our knowledge, no previous study has empirically evaluated the relative importance of niche and neutral processes as determinants of community structure along a successional gradient. The aim of this study was to do this using the relationship between species abundance and both seed mass and average individual biomass (hereafter AIB) in species-rich alpine and sub-alpine meadow communities. The working hypothesis was that both stochastic and niche processes dominate in the early stage of succession, while the niche process dominates in later successional stages (Coomes et al., 2002; Gravel et al., 2006). The main objectives of the study were to: (a) determine empirically the relative importance of niche and neutral processes along a successional gradient; (b) explore whether successional time affects the neutrality of a community; and (c) evaluate whether AIB determines species abundance in sub-alpine and alpine meadows.
MATERIALS AND METHODS
Study sites
A series of experiments were carried out in alpine (Maqu and Azi) and sub-alpine (Hezuo) meadows of the eastern part of the Qing-Hai Tibet Plateau, China (Table 1). The vegetation at the alpine sites is typical of species-rich alpine meadows, and is dominated by species such as Scirpus pumilus Vahl, Kobresia capillifolia (Decne.) C. B. Clarke, Festuca ovina Linn, Poa poophagorum Bor, Roegneria nutans (Keng) Keng, Anemone rivularis Buch.-Ham. and Kobresia macrantha Boeck. In the sub-alpine meadow, the vegetation is dominated by Agrostis hugoniana Rendle, Stipa aliena Keng and Kobresia humilis (C. A. Mey.) Serg.
Table 1.
Characteristics of the study sites evaluated
| Parameters | Sites | ||
|---|---|---|---|
| Hezuo | Maqu | Azi | |
| Latitude | 34°55′N | 35°58′N | 33°58′N |
| Longitude | 102°53′E | 101°53′E | 101°53′E |
| Altitude (m) | 2900 | 3500 | 3500 |
| Annual mean temperature (°C) | 2·4 | 1·2 | 1·2 |
| Annual mean precipitation (mm) | 532 | 620 | 620 |
| Vegetation type | SM | AM | AM |
SM, sub-alpine meadow; AM, alpine meadow.
Field sampling
To evaluate the relationships between seed mass and species abundance along a successional gradient, experimental plots were established in Hezuo in 2003. These were laid out in fields abandoned 1, 3, 15 and 30 years ago. The fields, located within an area of 10 km2, had the same orientation, facing aspect and position within the slope. At each plot, thirty 50 × 50 cm sampling quadrats were randomly arranged in August, during the peak of the growing season, and the number of ramets of every species in each quadrat (i.e. density) was counted. After these measurements, the aboveground biomass of each species was cut, dried at 80°C to constant weight and weighed. A total of 91 species were found in the surveys. Seeds of each species were collected in nearby sites when mature between the years 2000 and 2003. Seeds were air-dried and kept in the laboratory. To estimate seed mass, seeds from the collections, were pooled, 100 seeds for each species were weighed three times, and then the average weight was taken.
To evaluate the relationship between species abundance and AIB in natural communities, additional data were collected at the Hezuo, Maqu and Azi sites during 2001 and 2006, respectively. A total of 30 (Hezuo), 45 (Maqu) and 20 (Azi) 50 × 50 cm sampling quadrats were randomly arranged in August, during the peak of the growing season. The density and aboveground biomass of each species were obtained as described above. A total of 55, 87 and 50 species were found at the Hezuo, Maqu and Azi sites, respectively. The AIB of each species in a given quadrat was calculated as its biomass divided by its density in the quadrat.
Statistical analyses
To avoid confusion, density (number of individuals per sampling quadrat) and frequency (number of quadrats a species appeared in) were used as surrogates of species abundance (Guo, 2003; Murray and Leishman, 2003). The relationship between species abundance and both seed mass and AIB was evaluated using linear regressions. Seed mass, AIB and abundance data were in all cases log10-transformed prior to these analyses.
In the regressions involving AIB, density is both the response variable and the denominator of the predictor variable (AIB, see above). Statistically, this causes non-independence between predictor and response variables, and may lead to spurious analyses because of violations in the assumptions of regression (Berges, 1997; Brett, 2004). To overcome this issue, and following Brett (2004), the relationship between AIB and species abundance was evaluated using bootstrapping (Roff, 2006). Regression and bootstrapping analyses were conducted with the SigmaPlot 2006 (SPSS Inc., Chinago, IL, USA) and LSS (http://biol-chem.uwb.edu.pl/IP/ENG/biologia/statystyka/stats.php) software, respectively. A total of 10 000 bootstrap replications were used in the bootstrapping analyses.
RESULTS
At the Hezuo site, seed mass showed a significant negative relationship with both frequency and density in the field abandoned 1 year ago (Table 2). For the fields abandoned 3, 15 and 30 years ago, the relationships were not significant. However, it is interesting to note a change in the sign of the relationship from negative in the fields abandoned 1, 3 and 15 years ago to positive in the field abandoned 30 years ago (Fig. 1, Table 2). In both alpine and sub-alpine natural meadow communities, species density showed a significant negative relationship with AIB (Table 3). However, species frequency only showed a significant relationship with AIB in one of the study sites (Table 3).
Table 2.
Linear regression models fitted to the relationship between species abundance (Y) and seed mass (X) along the successional gradient evaluated
| Years after abandonment | n | Abundance measure | Regression equation | r | P |
|---|---|---|---|---|---|
| 1 | 26 | D | Y = 0·10 – 0·64X | −0·6270 | <0·001 |
| F | Y = 0·15 – 0·31X | −0·5335 | 0·005 | ||
| 3 | 39 | D | Y = 0·67 – 0·35X | −0·2851 | 0·079 |
| F | Y = 0·28 – 0·12X | −0·2236 | 0·171 | ||
| 15 | 49 | D | Y = 1·40 – 0·04X | −0·0275 | 0·851 |
| F | Y = 0·55 – 0·0004X | −0·0006 | 0·997 | ||
| 30 | 43 | D | Y = 1·54 + 0·14X | 0·1204 | 0·442 |
| F | Y = 0·70 + 0·08X | 0·1264 | 0·419 |
n refers to the total number of species used in the analysis. D, density, F, frequency. P-values < 0·05 are in bold.
Fig. 1.
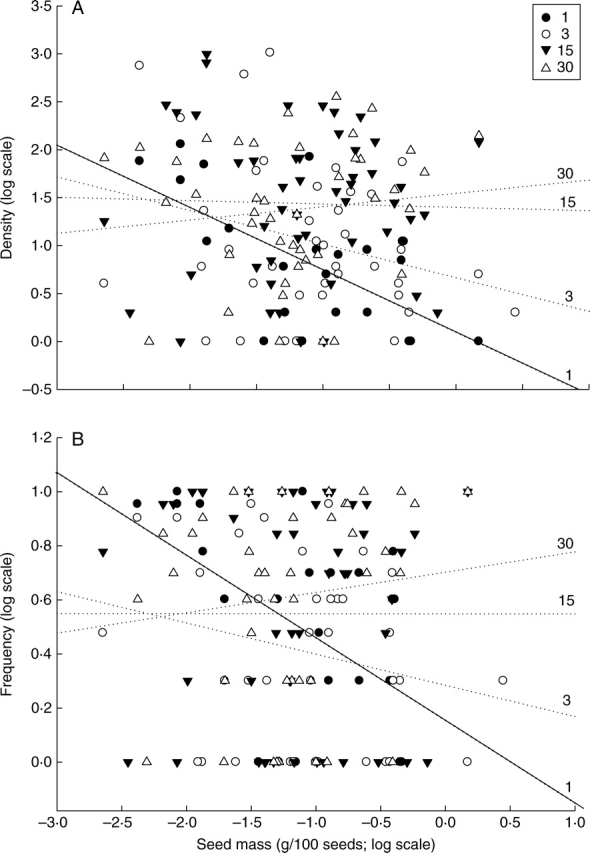
The relationship between abundance, i.e. (A) density and (B) frequency, and seed mass along a successional gradient (1-, 3-, 15- and 30-year meadows). Significant relationships (P < 0·05) are denoted with solid lines.
Table 3.
Linear regression models fitted to the relationship between species abundance (Y) and average individual biomass (X) in the alpine and sub-alpine meadows evaluated
| Sites | n | Abundance measure | Regression equation | r | P |
|---|---|---|---|---|---|
| Azi | 50 | D | Y = −0·20 – 0·76X | −0·3579 | 0·0105 |
| F | Y = 0·71 – 0·19X | −0·1782 | 0·2110 | ||
| Maqu | 87 | D | Y = −0·52 – 1·01X | −0·3767 | 0·0001 |
| F | Y = 0·73 – 0·55X | −0·3243 | 0·0021 | ||
| Hezuo | 55 | D | Y = 1·29 – 0·50X | −0·3003 | 0·0243 |
| F | Y = 0·60 – 0·07X | −0·0930 | 0·4955 |
n refers to the total number of species used in the analyses. P and r values shown for density were obtained after bootstrapping with 10 000 simulations. D, density; F, frequency. P-values < 0·05 are in bold.
DISCUSSION
Is the relationship between species abundance and seed mass consistent along a successional gradient in sub-alpine meadow communities?
The results showed that the magnitude and direction of the relationship between seed mass and species abundance varied along succession, with a negative relationship during early successional stages and a trend towards a positive relationship as succession progresses (Fig. 1, Table 2). This pattern is consistent with the successional model proposed by Leishman and Murray (2001). The model predicts that in the early stages of succession, superior colonists (including those with strong dispersal abilities) are the most abundant species, resulting in a negative correlation between seed mass and abundance. Over time, larger-seeded species arrive, and they progressively outcompete the smaller-seeded species. Thus, in intermediate stages of succession, no clear relationship between seed mass and abundance should occur. As time progresses and with no disturbance, there will be a positive correlation between seed mass and abundance. As illustrated in classical studies of MacArthur (1962) and MacArthur and Wilson (1967), high disturbance levels select for species that produce many and well-dispersed offspring, while low disturbance levels would favour competitive species.
It is important to point out that the model of Leishman and Murray (2001) is based on the trade-off between seed mass and seed number within species throughout succession, and that such trade-offs are always considered as evidence of niche processes (Chase and Leibold, 2003; Kneitel and Chase, 2004; Kearney and Porter, 2006). The strong dispersal ability of small-seeded species in early stages of succession, and the strong competition ability of large-seeded species in later successional stages, are key functional traits implying that niche forces are at work. The present results showed that small-seeded species were much more abundant than large-seeded species in the early phase of succession, indicating that dispersal processes were an important determinant of the structure of the studied meadows (Gravel et al., 2006). Dispersal itself is not only a key trait of species but also an important process shaping the structure of plant communities. Therefore, and according to the initial hypothesis, it could be concluded that both random and niche processes have an impact on the structure of community in early successional stages, while deterministic forces such as competitive ability of large-seeded species are dominant in later successional stages. According to the present results, successional stage may also be an important factor to determine where on a continuum from niche to neutrality a particular community will fall (Gravel et al., 2006).
What is the relationship between abundance and AIB in both alpine and sub-alpine meadow communities?
The clear negative relationship between species density and AIB found in all the sites evaluated was in clear contradiction to the neutral theory prediction that any given species trait should be uncorrelated with their abundance in the local community. The results agree with predictions from the metabolic theory of ecology, which seeks to explain the consistent relationship between a species' body size and metabolic rate (Brown, 2004), and match those of studies conducted with different organisms showing that the individual body mass of a species is correlated significantly and negatively with its abundance (Damuth, 1981, 1991; Brown and Nicoletto, 1991; Enquist et al., 1998; White et al., 2007). Body mass is perhaps the most fundamental property of an organism, and is related to many biological traits, such as abundance, lifespan and metabolism (White et al., 2007). The importance of body mass–abundance relationships has been widely recognized in both terrestrial and aquatic ecology (Brown, 1995; Gaston and Blackburn, 2000; Kerr and Dickie, 2001). Such relationships are also essential to link species- and population-specific traits to the structure and dynamics of ecological communities (Kerr and Dickie, 2001; Woodward et al., 2005). The present results clearly suggest that AIB plays an important role in shaping the abundance of species in the studied meadows. In addition to the merging of neutral and niche theories, recent theoretical developments are also attempting to combine neutral theory with the theory of metabolic scaling (Gewin, 2006). Such a combination will provide better predictions on the relationship between AIB and species abundance.
Concluding remarks
A set of experiments was conducted to test the relative importance of niche and neutral processes as drivers of community structure along succession, and to evaluate if the AIB of a species is related to its abundance within communities. The results indicate that, in the early stages of succession, both stochastic processes, such as dispersal, and deterministic processes, such as the trade-off between seed mass and number, were important determinants of the structure of the studied communities. In the meadow communities studied, AIB was negatively related to species abundance. Advances in the development of a unified theoretical body incorporating elements of neutral theory, niche theory and metabolic scaling theory will give rise to refined theory and models, improving our ability to understand the relative importance of those factors driving the composition and structure of plant communities.
ACKNOWLEDGEMENTS
We thank Luqiang Zhao and Qi Li for help with fieldwork and data collection, and A. Ostling, D. Gravel, B. Murray, Yanxin Liu, D. Causton and two anonymous reviewers for their valuable comments on earlier versions of the manuscript. The study was supported by the National Basic Research Program of China (Grant No. 2002CB111505). F. T. M. was supported by a Ramón y Cajal contract from the Spanish Ministerio de Educación y Ciencia, by an Early Career Project Grant (ECPG 231/607) from the British Ecological Society, and by the Comunidad de Madrid-funded project REMEDINAL (S-0505/AMB/0335).
LITERATURE CITED
- Adler PB, HilleRisLambers J, Levine JM. A niche for neutrality. Ecology Letters. 2007;10:95–104. doi: 10.1111/j.1461-0248.2006.00996.x. [DOI] [PubMed] [Google Scholar]
- Bell G. Neutral macroecology. Science. 2001;293:2413–2418. doi: 10.1126/science.293.5539.2413. [DOI] [PubMed] [Google Scholar]
- Berges JA. Ratios, regression statistics and ‘spurious’ correlations. Limnology and Oceanography. 1997;42:1006–1007. [Google Scholar]
- Brett MT. When is a correlation between non-independent variables ‘spurious’? Oikos. 2004;105:647–656. [Google Scholar]
- Brown JH. Macroecology. Chicago: The University of Chicago Press; 1995. [Google Scholar]
- Brown JH. Toward a metabolic theory of ecology. Ecology. 2004;85:1771–1789. [Google Scholar]
- Brown JH, Nicoletto PF. Spatial scaling of species composition: body masses of North American land mammals. American Naturalist. 1991;138:1478–1512. [Google Scholar]
- Chase JM, Leibold MA. Ecological niche: linking classical and contemporary approaches. Chicago: The University of Chicago Press; 2003. [Google Scholar]
- Chesson P. Mechanisms of maintenance of species diversity. Annual Review of Ecology, Evolution, and Systematics. 2000;31:343–366. [Google Scholar]
- Condit R, Pitman N, Leigh EG, Jr, Chave J, Terborgh J, Foster RB, et al. Beta-diversity in tropical forest trees. Science. 2002;295:666–669. doi: 10.1126/science.1066854. [DOI] [PubMed] [Google Scholar]
- Condit R, Ashton P, Bunyavejchewin S, Dattaraja HS, Davies S, Esufali S, et al. The importance of demographic niches to tree diversity. Science. 2006;313:98–101. doi: 10.1126/science.1124712. [DOI] [PubMed] [Google Scholar]
- Coomes DA, Rees M, Grubb PJ, Turnbull L. Are differences in seed mass among species important in structuring plant communities? Evidence from analyses of spatial and temporal variation in dune-annual populations. Oikos. 2002;96:421–432. [Google Scholar]
- Damuth J. Population density and body size in mammals. Nature. 1981;290:699–700. [Google Scholar]
- Damuth J. Of size and abundance. Nature. 1991;351:268–269. [Google Scholar]
- Enquist BJ, Brown JH, West GB. Allometric scaling of plant energetics and population density. Nature. 1998;395:163–165. [Google Scholar]
- Eriksson O. Game theory provides no explanation for seed size variation in grasslands. Oecologia. 2005;144:98–105. doi: 10.1007/s00442-005-0001-z. [DOI] [PubMed] [Google Scholar]
- Gaston KJ, Blackburn T. Pattern and process in macroecology. Oxford: Blackwell Science; 2000. [Google Scholar]
- Gewin V. Beyond neutrality – ecology finds its niche. Plos Biology. 2006;4:1306–1310. doi: 10.1371/journal.pbio.0040278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert B, Lechowicz MJ. Neutrality, niches, and dispersal in a temperate forest understory. Proceedings of the National Academy of Sciences, USA. 2004;101:7651–7656. doi: 10.1073/pnas.0400814101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert B, Laurance WF, Leigh EG, Jr, Nascimento HEM. Can neutral theory predict the responses of Amazonian tree communities to forest fragmentation? American Naturalist. 2006;168:304–317. doi: 10.1086/506969. [DOI] [PubMed] [Google Scholar]
- Gravel D, Canham CD, Beaudet M, Messier C. Reconciling niche and neutrality: the continuum hypothesis. Ecology Letters. 2006;9:399–409. doi: 10.1111/j.1461-0248.2006.00884.x. [DOI] [PubMed] [Google Scholar]
- Guo QF. Plant abundance: the measurement and relationship with seed size. Oikos. 2003;101:639–642. [Google Scholar]
- Harpole WS, Tilman D. Non-neutral patterns of species abundance in grassland communities. Ecology Letters. 2006;9:15–23. doi: 10.1111/j.1461-0248.2005.00836.x. [DOI] [PubMed] [Google Scholar]
- Hubbell S. The unified neutral theory of biodiversity and biogeography. Princeton, NJ: Princeton University Press; 2001. [Google Scholar]
- Hubbell SP. Neutral theory in community ecology and the hypothesis of functional equivalence. Functional Ecology. 2005;19:166–172. [Google Scholar]
- Hubbell SP. Neutral theory and the evolution of ecological equivalence. Ecology. 2006;87:1387–1398. doi: 10.1890/0012-9658(2006)87[1387:ntateo]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Kearney M, Porter WP. Ecologists have already started rebuilding community ecology from functional traits. Trends in Ecology and Evolution. 2006;21:481–482. doi: 10.1016/j.tree.2006.06.019. [DOI] [PubMed] [Google Scholar]
- Kerr SR, Dickie LM. Biomass spectrum. New York: Columbia University Press; 2001. [Google Scholar]
- Kneitel JM, Chase JM. Trade-offs in community ecology: linking spatial scales and species coexistence. Ecology Letters. 2004;7:69–80. [Google Scholar]
- Leishman MR, Murray BR. The relationship between seed size and abundance in plant communities: model predictions and observed patterns. Oikos. 2001;94:151–161. [Google Scholar]
- Mabry CM. The number and size of seeds in common versus restricted woodland herbaceous species in central Iowa, USA. Oikos. 2004;107:497–504. [Google Scholar]
- MacArthur RH. Some generalized theorems of natural selection. Proceedings of the National Academy of Sciences, USA. 1962;48:1893–1897. doi: 10.1073/pnas.48.11.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacArthur RH, Wilson EO. The theory of island biogeography. Princeton, NJ: Princeton University Press; 1967. [Google Scholar]
- McGill BJ. A test of the unified neutral theory of biodiversity. Nature. 2003;422:881–885. doi: 10.1038/nature01583. [DOI] [PubMed] [Google Scholar]
- McGill BJ, Maurer BA, Weiser MD. Empirical evaluation of neutral theory. Ecology. (a) 2006;87:1411–1423. doi: 10.1890/0012-9658(2006)87[1411:eeont]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- McGill BJ, Enquist BJ, Weiher E, Westoby M. Rebuilding community ecology from functional traits. Trends in Ecology and Evolution. (b) 2006;21:178–185. doi: 10.1016/j.tree.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Mouquet N, Leadley P, Me'riguet J, Loreau M. Immigration and local competition in herbaceous plant communities: a three-year seed-sowing experiment. Oikos. 2004;104:77–90. [Google Scholar]
- Muller-Landau HC. Seeds of understanding of plant diversity. Proceedings of the National Academy of Sciences, USA. 2003;100:1469–1471. doi: 10.1073/pnas.0438004100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray BR, Leishman MR. On the relationship between seed mass and species abundance in plant communities. Oikos. 2003;101:643–645. [Google Scholar]
- Murray BR, Brown AHD, Dickman CR, Crowther MS. Geographical gradients in seed mass in relation to climate. Journal of Biogeography. 2004;31:379–388. [Google Scholar]
- Murray BR, Kelaher BP, Hose GC, Figueira WF, Leishman MR. A meta-analysis of the interspecific relationship between seed size and plant abundance within local communities. Oikos. 2005;110:191–194. [Google Scholar]
- Roff DA. Introduction to computer-intensive methods of data analysis in biology. Cambridge: Cambridge University Press; 2006. [Google Scholar]
- Schippers P, van Groenendael JM, Vleeshouwers LM, Hunt R. Herbaceous plant strategies in disturbed habitats. Oikos. 2001;95:198–210. [Google Scholar]
- Thompson R, Townsend C. A truce with neutral theory: local deterministic factors, species traits and dispersal limitation together determine patterns of diversity in stream invertebrates. Journal of Animal Ecology. 2006;75:476–484. doi: 10.1111/j.1365-2656.2006.01068.x. [DOI] [PubMed] [Google Scholar]
- Tilman D. Niche tradeoffs, neutrality, and community structure: a stochastic theory of resource competition, invasion, and community assembly. Proceedings of the National Academy of Sciences, USA. 2004;101:10854–10861. doi: 10.1073/pnas.0403458101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uriarte M, Reeve HK. Matchmaking and species marriage: a game-theory model of community assembly. Proceedings of the National Academy of Sciences, USA. 2003;100:1787–1792. doi: 10.1073/pnas.0337167100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkov I, Banavar JR, Hubbell SP, Maritan A. Neutral theory and relative species abundance in ecology. Nature. 2003;424:1035–1037. doi: 10.1038/nature01883. [DOI] [PubMed] [Google Scholar]
- Volkov I, Banavar JR, He F, Hubbell SP, Maritan A. Density dependence explains tree species abundance and diversity in tropical forests. Nature. 2005;438:658–661. doi: 10.1038/nature04030. [DOI] [PubMed] [Google Scholar]
- Westoby M, Wright IJ. Land-plant ecology on the basis of functional traits. Trends in Ecology and Evolution. 2006;21:261–268. doi: 10.1016/j.tree.2006.02.004. [DOI] [PubMed] [Google Scholar]
- White EP, Morgan Ernest SK, Kerkhoff AJ, Enquist BJ. Relationships between body size and abundance in ecology. Trends in Ecology and Evolution. 2007 doi: 10.1016/j.tree.2007.03.007. in press. [DOI] [PubMed] [Google Scholar]
- Woodward G, Ebenman B, Emmerson M, Montoya JM, Olesen JM, Valido A, et al. Body size in ecological networks. Trends in Ecology and Evolution. 2005;20:402–409. doi: 10.1016/j.tree.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Wootton JT. Field parameterization and experimental test of the neutral theory of biodiversity. Nature. 2005;433:309–312. doi: 10.1038/nature03211. [DOI] [PubMed] [Google Scholar]
- Zhou SR, Zhang DY. Allele effects and the neutral theory of biodiversity. Functional Ecology. 2006;20::509–513. [Google Scholar]


