Abstract
Background and Aims
The leaf rosettes of the carnivorous Pinguicula moranensis follow a spiral phyllotaxis approaching a Fibonacci pattern while the stalked flowers arise from extra-axillary sites between the leaves. The organization of this rosette has been discussed by various authors, with various results. The aim of the present study was to clarify the development of the flowering rosettes of this species.
Methods
The formation of the rosettes is shown with the aid of scanning electron microscopy.
Key Results and Conclusions
The scanning electron micrographs show that each flower terminates an article (sympodial unit). The leaves of consecutive articles of such sympodially constructed rosettes are arranged along a spiral Fibonacci pattern (with divergence angles around 137°). This results from homodromy of leaf initiation in consecutive articles with the first leaf (prophyll) of a new article inserted in an obliquely transverse position next to the floral scape that terminates the former article. Sympodial construction of flowering shoots and leaf rosettes is also known from Aloe, Gunnera and Philodendron. As a by-product of this study, the unidirectional development of the Pinguicula flower is confirmed and discussed.
Key words: Article, Fibonacci, flower development, homodromy, leaf initiation, Lentibulariaceae, phyllotaxis, Pinguicula moranensis, sympodial unit
INTRODUCTION
Among the carnivorous family Lentibulariaceae, Pinguicula is the most basal genus (Jobson et al., 2003). The family appears in the Lamiales where its final position is yet to be determined (Albert et al., 1992; Bremer et al., 2002; Müller et al., 2004).
The studies of Goebel (1891, 1928–1933) and Lloyd (1942) have already provided interesting insight into the developmental morphology of various Lentibulariaceae. The vegetative bauplans of the genera Pinguicula (86 spp.), Utricularia (including Polypompholyx, 220 spp.) and Genlisea (21 spp.) are still not yet properly understood. Morphological analyses of the Lentibulariaceae are badly needed in order to make molecular phylogenies (e.g. Jobson et al., 2003; Müller et al., 2004) more meaningful.
The hygrophilous genus Pinguicula is characterized by a basal rosette of more or less broadly ovate leaves that, by means of secretory glands, capture and digest insects (Casper, 1966; Legendre, 2000). There are two distinct growth-forms in the genus, a temperate one (over-wintering in a hibernaculum, i.e. a winter bud, from which the new plant will grow in the coming spring) and a tropical one (winter rosette with usually narrower leaf blades) (Casper, 1966). Besides their carnivory, there are other features that make them of particular interest for morphologists. Although they all have roots, Pinguicula roots often lack rootcaps and are unbranched (Rutishauser and Isler, 2001). Furthermore, Pinguicula is one of the few genera in eudicots where monocotyledony has been observed (Troll, 1937; Casper, 1966).
Pinguicula moranensis (syn. P. longicaudata) is a well-known flycatcher plant in greenhouses. This species, originating from Mexican Highlands, Guatemala and El Salvador, belongs to the tropical growth form, flowering nearly year-round (Goebel, 1891; Zamudio and Rzedowsky, 1991; Barthlott et al., 2004).
Branching patterns of flower-producing leaf rosettes in P. moranensis have been discussed by various authors. Casper (1966), for example, considered that the flowers of Pinguicula arise from the axils of rudimentary leaves, while the rosettes themselves show monopodial construction. In opposition to this view, Raju (1969) thought that the flowers in Pinguicula, especially P. vulgaris, occupy leaf sites, similar to what is known from Nymphaea spp. (Cutter, 1961; Grob et al., 2006).
Among vascular plants, the most frequent phyllotaxis is the Fibonacci pattern. There are two ways to detect such a pattern. One is the measurement of the divergence angle between two consecutive leaves, and the second is the observation of the contact parastichies. The divergence angle is the stem sector enclosed by two consecutive leaves or leaf primordia along the ontogenetic spiral (see Fig. 2A). For the Fibonacci-type phyllotaxis this angle is around 137° (120–144°, see Rutishauser, 1998). Along the ontogenetic spiral consecutive primordia are separated by the above-mentioned divergence angle. The time interval between the initiation of two consecutive primordia at the shoot apical meristem (SAM) is termed plastochrone. In a densely packed shoot tip, young leaves are in contact with certain other leaves. A contact parastichy is the curved line by which contacting leaves are linked together (see Fig. 1B). In the Fibonacci-type phyllotaxis, there are always contact parastichies between leaves that are x, y and z plastochrones apart, with x, y and z being consecutive members of the Fibonacci sequence: 3, 5, 8, 13, 21, … (Rutishauser and Peisl, 2001).
Fig. 2.
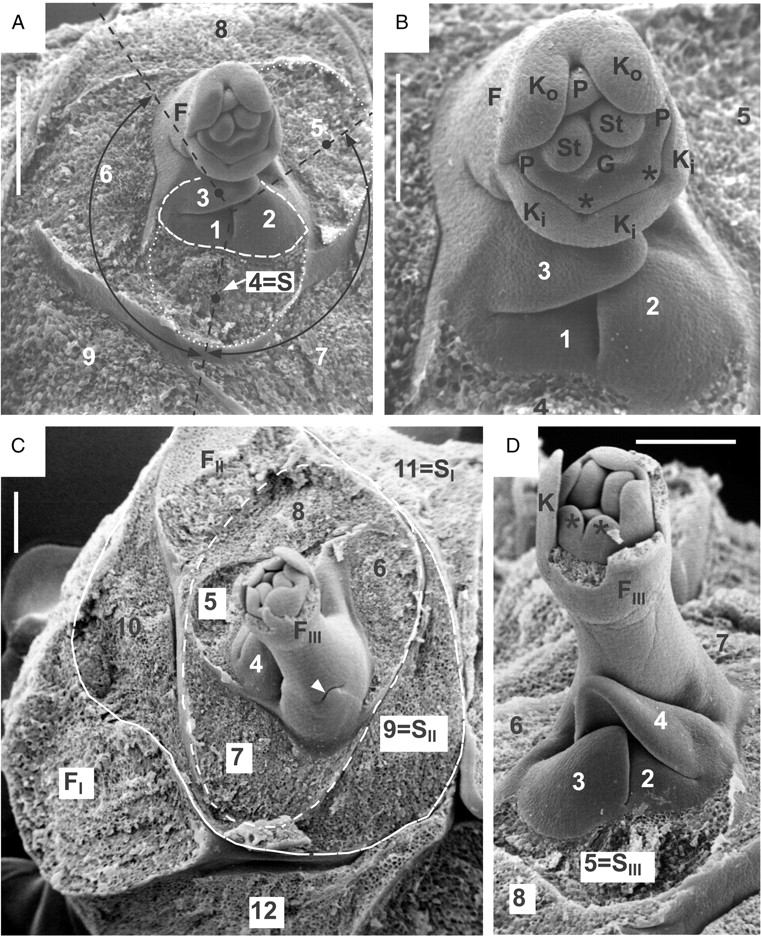
Pinguicula moranensis. (A, B) Centre of another dissected rosette with flower bud; (A) overview and (B) close-up. All leaves removed, except leaf primordia 1–3. Leaves 1–3 (surrounded by dashed line) belong to a sympodial unit (new article), which is subtended by leaf 4 (S = subtending leaf). Former article (with leaves 4–9) is terminated by flower (F). Two divergence angles (both around 137°) are marked for illustration with curved double arrows: between leaves 4 and 5 (within article) and between leaves 3 and 4 (across articles). Note primordial floral organs: Ko, outer sepals; Ki, inner sepals; P, five petals, with two lobes forming upper lip (i.e. flag petals) marked with asterisks; St, two primordial stamens; G, primordial gynoecium. Scale bars: (A) 0·5 mm, (B) 0·25 mm. (C, D) Centre of another rosette with a dissected flower bud; (C) overview and (D) close-up. All leaves removed, except leaf primordia 1–4. Leaves 1–4 belong to a new sympodial unit (i.e. youngest article), which is subtended by leaf 5 (SIII). Next older article (with leaves 5–8, surrounded by dashed line) is terminated by flower (FIII) and subtended itself by leaf 9 (SII) of the previous article. This article is highlighted by a solid line and consists of two leaves (9 and 10) and a terminal flower (FII, removed). Oldest observable article terminates with flower (FI, again removed) after formation of leaves 11 and 12. Leaf 11 acts as subtending leaf (SI) for the next inner article. Arrowhead points to an additional scaly leaf at the base of scape (FIII). Other abbreviations as above. Scale bars = 0·25 mm.
Fig. 1.
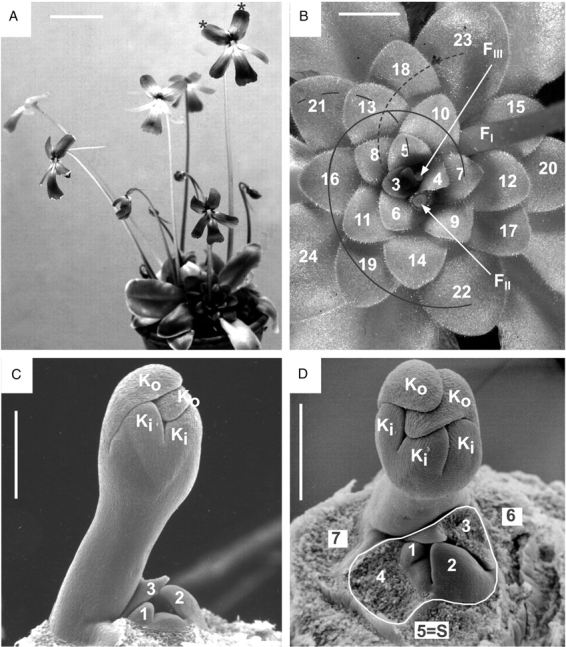
Pinguicula moranensis. (A) Flowering individual, dividing vegetatively into daughter rosettes. Each rosette has one, two or three flowers on long stalks (scapes). In one flower, the flag petals are marked with asterisks. Scale bar = 40 mm. (B) Top view of rosette. The observable leaves are labelled along the ontogenetic spiral (also called genetic spiral, i.e. according to their initiation order) from the centre to the periphery. Note Fibonacci pattern with contact parastichies connecting leaves with age differences of three (solid line), five (short dashed line) and eight (long dashed line) plastochrones. The positions of three flowers FI, FII and FIII are shown by their scape bases (flowers and upper scape portions removed). Scale bar = 15 mm. (C, D) Centre of dissected rosette, lateral and top view. Flower bud on a stalk (young scape). Calyx lobes show descending cochlear aestivation, with two outer lobes (Ko) covering three inner lobes (Ki). (C) All leaves except leaf primordia 1–3 partly or totally removed. (D) Leaves 1–4 (surrounded by dashed line) belong to a sympodial unit (new article), which is subtended by leaf 5 (S = subtending leaf). Former article (with leaves 5–7) is terminated by the flower bud. Scale bars = 0·5 mm.
In a monopodium, one and the same SAM is responsible for the growth of the main axis of the plant. Pluripotent stem cells at the SAM differentiate into all organs necessary for plant development. Often leaves are initiated spirally, with a divergence angle of about 137° (120–144°). In a sympodium one SAM is responsible for the growth of an article (sympodial unit) only. The entire plant may then be made up of several articles. With the termination of an article the SAM ceases to act and a new SAM (born as part of an axillary shoot bud) takes over the growth of the plant.
The aim of the present paper is to show growth and phyllotaxis of Pinguicula rosettes by means of scanning electron micrographs. The presence of the Fibonacci pattern in the rosettes is detected by the observation of contact parastichies. Possible reasons for this organization are discussed, in the hope that molecular studies can clarify these findings. Moreover, this study also confirms the unidirectional floral development in Pinguicula, as already described by Buchenau (1865).
MATERIAL AND METHODS
The data presented in this study are based on fixed material of Pinguicula moranensis H.B.K. (syn. P. caudata Schlechtendal) cultivated in the Botanical Gardens of the University of Zürich. Voucher specimens are housed in Z/ZT. The material used for this study was fixed and preserved in 70 % ethyl alcohol. For scanning electron microscopy (SEM), the dissected material was critical-point dried and sputter-coated (gold). The micrographs were taken with Cambridge S4 and JEOL scanning electron microscopes operating at 20 kV. Thirty fully grown rosettes were checked in order to verify the observations here. The authorities of additional species names mentioned in the text are equivalent to the species and author names given by Barthlott et al. (2004).
RESULTS
Morphology of the adult plant
Flowers of Pinguicula moranensis are bright carmine red. The corolla (length 30–64 mm including spur) consists of a short tube with two lobes forming the upper lip and three lobes forming the lower lip. The prominent narrow spur is up to 44 mm long (Fig. 1A). The remaining floral organs are five sepals, two stamens and a superior ovary consisting of two fused carpels (Fig. 2B). Fully grown summer rosettes consist of up to 30 sticky leaves. They can be taken as parts of one and the same ontogenetic spiral, which always twines in the same direction, e.g. anticlockwise when seen from the rosette centre to its periphery (leaves 3–24 in Fig. 1B). In addition, there are direct contacts between leaves along parastichies 3, 5 and 8. These curved lines connect leaves with age differences of three, five and eight plastochrones, respectively (Fig. 1B).
There are up to three flowers present in a fully grown rosette with the older flowers (e.g. FI in Fig. 1B) towards the periphery and the younger flowers (FII, FIII) towards the rosette centre. Each flower is provided with a leafless stalk (scape) up to 20 cm long (Fig. 1A). Each scape arises from extra-axillary positions between leaves. Subtending leaves associated with the flowers and their scapes are lacking. For example, flower FI shown in Fig. 1B seems to originate in the gap between leaf 15 and leaf 23, whereas flower FII is inserted in the gap between leaf 6 and leaf 9. This position of the flower does not fit with the monopodial growth-form and therefore requires a more thorough investigation of the shoot tip.
Sympodial construction of Fibonacci-type flower-producing rosettes
Dissected rosettes reveal the proper position of the stalked flowers. Each flower, although arising from a seemingly homogeneous rosette, terminates a sympodial unit (article). Leaves 1–4 (in Fig. 1C, D) belong to a new article (i.e. sympodial unit), which is subtended by leaf 5. The former article (with 5–7 as its uppermost leaves) is terminated by a stalked flower bud (with Ki/ Ko in Fig. 1C, D). A similar situation is seen in Fig. 2A and B, showing the centre of another rosette. Leaves 1–3 (surrounded by a dashed line) belong to a new article, which is subtended by leaf 4. The former article (with leaves 4–9) again is terminated by a flower (F). Three consecutive articles (I–III) with terminating flowers (FI, FII and FIII, respectively) are shown in Fig. 2C and D as part of the same rosette. Leaves 2–4 belong to the youngest article in the axil of leaf 5, which acts as subtending leaf (SIII). The next older article (with leaves 5–8) is marked by a dashed line. It is terminated by flower FIII and subtended by leaf 9 (SII) of the next older article II. This article consists of two leaves (9 and 10) prior to the formation of a terminal flower FII (removed) and is highlighted by a solid line. The oldest observable article I terminates with the flower FI (removed) after the formation of at least two leaves (11 and 12 in Fig. 2C). Leaf 11 acts as subtending leaf (SI) for article II.
Surprisingly, the leaves within an article as well as the leaves of consecutive articles of the sympodially constructed rosettes are arranged along a spiral Fibonacci pattern, twining in the same direction throughout. Therefore, leaves 4 and 5 within an article, as well as leaves 3 and 4 from consecutive articles, both have divergence angles of around 137° (Fig. 2A). However, to measure the exact divergence angle, the centre between two consecutive leaves needs to be determined. As the example here shows consecutive leaves (e.g. leaves 3 and 4 in Fig. 2A), belonging to the same ontogenetic spiral but originating from consecutive shoot apical meristems, the definition of a centre can only be approximated (as shown in Fig. 2A). The Fibonacci pattern for the rosette is therefore deduced from the contact parastichies (as explained in the Introduction). Figure 1B shows that leaves with three, five and eight plastochrone age differences contact each other. The only explanation for this set of parastichies is the Fibonacci-type phyllotaxis. This pattern results from homodromy of leaf initiation in consecutive articles. This means that the respective direction of the ontogenetic spiral of each article does not change from one article to the next. When seen from the periphery to the centre, all leaves of consecutive articles are arranged either in a clockwise (Figs 1B, D and 2A, B) or in an anticlockwise ontogenetic spiral (Fig. 2C, D). In order to continue the Fibonacci pattern of the former article, the first leaf (prophyll) of a new article is inserted in an obliquely transverse position next to the floral scape that terminates the former article. For example, leaf 3 in Fig. 2B and leaf 4 in Fig. 2D are prophylls. Occasionally, there is an additional scale at the base of a floral scape (see arrowhead in Fig. 2C). This seems to be the very last leaf of the former article, also fitting into the Fibonacci spiral of the rosette leaves (e.g. 8–7–6–5 in Fig. 2C). Such a scale may rarely subtend another flower bud in P. moranensis.
We may therefore formulate the following rule for flowering rosettes of P. moranensis: looking from the periphery to the centre we can draw an ontogenetic spiral that connects all leaves in a rosette. Within this rosette consisting of two or more articles the prophylls of consecutive articles are always positioned on the same side of the flower that terminates the preceding article: prophyll (e.g. leaf 3) on the ‘right’ side of the flower in rosettes with clockwise ontogenetic spiral (Fig. 2A, B), prophyll (e.g. leaf 4) on the ‘left’ side of the flower in rosettes with anticlockwise ontogenetic spiral (as in Fig. 2C, D).
Flower development
As is typical for Pinguicula, the flower buds arise on stalks (scapes) that usually do not carry additional scales or leaves (Fig. 1C, D). Dorsiventral flower symmetry is already evident in calyx formation. The five calyx lobes are arranged in two lips (Fig. 2B). The two sepal lobes (Ko) pointing towards the rosette periphery show an accelerated growth as compared with the three inner ones (Ki). In a later stage (Fig. 1C, D) the two outer sepal lobes (Ko) are covering the three inner ones (Ki). This pattern is known as descending cochlear aestivation. Corolla formation starts with five lobes, which are soon fused into one ring. The two inner petal lobes (marked by asterisks in Fig. 2B, D) are pointing towards the centre of the rosette. They are slightly delayed as compared with the development of the three outer petal lobes pointing towards the rosette periphery. The three outer lobes will finally turn into the lower corolla lip, whereas the two inner lobes will turn into the upper flag petals (marked with asterisks in Fig. 1A). The two stamens first appear as hemispherical bumps and the gynoecium arises as a lens-shaped bulge (Fig. 2B). The spur, which is prominent in the functional flower, is not yet observable in the micrographs shown.
DISCUSSION
Sympodial construction of Fibonacci-type leaf rosettes in Pinguicula
Observational evidence, presented in this paper, point to the fact that the rosettes of Pinguicula are constructed sympodially with the flowers terminating consecutive articles (sympodial units). This fact has been known for P. vulgaris since Buchenau (1865) and Troll (1937) but has not subsequently been discussed. New publications on reproductive biology have overlooked such data. For example, neither Svensson et al. (1993) nor Worley and Harder (1999) in their papers on preformation of flowers in Pinguicula vulgaris mentioned either Buchenau (1865) or Troll (1937). In addition recent, accurate overviews on the genus Pinguicula such as presented by Legendre (2000) and Barthlott et al. (2004) do not cover the sympodial construction of the leaf rosettes.
Similar to Wydler (1857), Raju (1969 : 511) assumed that consecutive primordia along the same ontogenetic (= genetic) spiral have the ability to develop into either a radial floral stalk or a dorsiventral leaf. Raju (1969: 514) wrote: ‘The presence of two kinds of genetic spirals, clockwise, or counter clockwise, in Pinguicula vulgaris is also an interesting morphological problem. In a long-lived plant one or the other genetic spiral is maintained in spite of the interruption caused by the appearance of flowers in leaf sites. The floral stalks developed, thus, are ebracteate similar to the cases reported for Nuphar and Nymphaea (Cutter 1961)’. Similarity with the situation proposed by Raju (1969) for Pinguicula and Nymphaea consists of more than the ebractate floral stalks. Indeed, the situation of flowers appearing in leaf sites has been confirmed for Nymphaea (Cutter, 1961; Grob et al., 2006). In other nymphaeaceous genera, such as Nuphar and Ondinea, this means of flower inception can also be found (Schneider et al., 2003). The SAM in these basal angiosperms obviously has the plasticity of initiating leaves or flowers along the same ontogenetic spiral. The observations on Pinguicula moranensis as presented herein clearly show that Raju (1969) was wrong in accepting a homeotic replacement of certain leaves (leaf sites) by flowers in Pinguicula. The presence of extra-axillary flowers (not subtended by leaves) in Pinguicula is due to sympodial construction with homodromy of spiral phyllotaxis of consecutive sympodial units. Similarly, sympodial construction of flowering shoots and leaf rosettes (even showing prophylls with expanded blades) is also known from various Araceae (Ray, 1987), Asphodelaceae such as Aloë (Dahlgren et al., 1985), Gunneraceae (Rutishauser et al., 2004) and Solanaceae (Reinhardt and Kuhlemeier, 2002). In Pistia stratiotes (Araceae) the sympodially constructed leaf rosettes again approach Fibonacci phyllotaxis. The sympodial units of Pistia rosettes, however, consist of two different leaves each: a prophyll with reduced blade and a foliage leaf (Lemon and Posluszny, 2000).
Regulation of spiral phyllotaxis
Phyllotaxis is the result of how resources (most importantly space) in the SAM are distributed among the primordia. In a review paper on phyllotaxis, Reinhardt (2005) asked the question ‘Why did regular phyllotaxis evolve?’ He answered this question for monopodial stems with a continuously active SAM as follows (p. 544): ‘Here, regular phyllotaxis may represent a selective advantage over random leaf position, allowing the meristem to optimally allocate founder cells, thus avoiding depletion or over-proliferation of organogenic cells on one side of the apex’. More specifically, Reinhard (2005) as well as Reinhardt et al. (2003) proposed an ‘auxin-sink’ model where auxin transport proteins are responsible for the generation of regular phyllotactic patterns such as the Fibonacci system. Does Reinhardt's explanation of optimal allocation of founder cells and the related ‘auxin-sink’ model also fit to the reproductive shoot systems of, for example, Pinguicula, Pistia and Gunnera, which give rise to regular Fibonacci systems in spite of the lack of monopodiality? In fact, the rosettes of Pinguicula, Pistia and Gunnera are constructed as chains of sympodial units (articles) with the foliage leaves of consecutive articles often being arranged according to a Fibonacci pattern or nearly so (for Pistia see Lemon and Posluszny, 2000; for other araceous members see Ray, 1988; for Gunnera see Rutishauser et al., 2004). In order to get a single ontogenetic spiral over several sympodial units (as a result of several consecutive but independent SAMs), the first leaf (prophyll) of each new axillary bud needs to be added always on the same side with respect to the clockwise or anticlockwise turning ontogenetic spiral. Because the SAM of each sympodial unit (article) terminates into a flower or an inflorescence, any continuity of founder cells (as proposed by Reinhardt, 2005) inside a long-lasting SAM is impossible. Nevertheless, Pinguicula, Pistia and Gunnera are able to produce Fibonacci-type shoot systems (leaf rosettes). Thus, there must also be other mechanisms than those proposed by Reinhardt (2005) and Reinhardt et al. (2003) leading to Fibonacci-type patterns in sympodial shoot systems. In non-flowering (sterile, vegetative) leaf rosettes of Pistia the sympodial chain of articles (with a bract-like prophyll and a foliage leaf each) appears to be ‘monopodized’, i.e. coming close to monopodial shoot growth (Lemon and Posluszny, 2000). Such sterile ‘monopodized’ shoots may have evolved from reproductive sympodial shoots, as already proposed in Salpichroa and other Solanaceae by Huber (1980), and in members of Alismataceae, Limnocharitaceae and Hydrocharitaceae by Charlton and Posluszny (1999).
Unidirectional floral development in Pinguicula as compared with Utricularia
For earlier descriptions of flower development in Pinguicula the reader is referred to Legendre (2000 : 78) and Casper (1966 : 23). Tucker (1999) cites Buchenau (1865), saying that floral development in Pinguicula is unidirectional, i.e. initiation of organs starts on one side (adaxial or abaxial) and continues through to the other side. This pattern is also observable in Leguminosae, Solanaceae, Scrophulariaceae, Plantaginaceae and Utricularia (Tucker, 1999). The present observations confirm a unidirectional mode of floral development in P. moranensis, especially for the calyx (Fig. 1C, D) and the corolla (Fig. 2A–D). The ‘abaxial’ floral organs pointing towards the periphery of the rosette develop earlier than the ‘adaxial’ ones pointing towards the centre of the rosette. On the ‘adaxial’ side, there are the two flag petals, forming the upper corolla lip, while the three ‘abaxial’ petals become the lower corolla lip. This means that the flower in the course of its development is turned outwards with the ‘adaxial’ side of the bud becoming the upper side (flag petals) in the fully-grown flower. A similar unidirectional mode of floral development is also observable in other members of the Lentibulariaceae. In young flower buds of P. vulgaris and Utricularia spp. (such as U. minor or U. vulgaris) the flag petals are smaller than the lobes of the lower corolla lip (Buchenau, 1865).
ACKNOWLEDGEMENTS
The technical assistance (scanning electron microscopy) of U. Jauch (Institut für Pflanzenbiologie der Universität Zürich) is gratefully acknowledged. We also would like to thank two anonymous referees for valuable comments on an earlier version of the manuscript. This paper is part of a research project supported by the Swiss National Science Foundation (grant No.3100AO-105974/1).
LITERATURE CITED
- Albert VA, Williams SE, Chase MW. Carnivorous plants: phylogeny and structural evolution. Science. 1992;257:1491–1495. doi: 10.1126/science.1523408. [DOI] [PubMed] [Google Scholar]
- Barthlott W, Porembski S, Seine R, Theisen I. Karnivoren. Stuttgart: Verlag Eugen Ulmer; 2004. [Google Scholar]
- Bremer B, Bremer K, Heidari N, Erixon P, Olmstead RG, Anderberg AA, et al. Phylogenetics of asterids based on 3 coding and 3 non-coding chloroplast DNA markers and the utility of non-coding DNA at higher taxonomic levels. Molecular Phylogenetics and Evolution. 2002;24:274–301. doi: 10.1016/s1055-7903(02)00240-3. [DOI] [PubMed] [Google Scholar]
- Buchenau F. Morphologische Studien an deutschen Lentibularieen. 2. Der Blüthenstand von. Pinguicula. Botanische Zeitung. 1865;23:69–71. [Google Scholar]
- Casper SJ. Monographie der Gattung. Pinguicula L. Bibliotheca Botanica. 1966;127/128:1–209. [Google Scholar]
- Charlton WA, Posluszny U. Morphological traffic between inflorescence and vegetative shoots in helobial monocotyledons. Botanical Review. 1999;65:370–384. [Google Scholar]
- Cutter EG. The inception and distribution of flowers in the Nymphaeaceae. Journal of the Proceedings of the Linnean Society, Botany. 1961;172:93–100. [Google Scholar]
- Dahlgren RMT, Clifford HAT, Yeo PF. The families of the monocotyledons: structure, evolution and taxonomy. Berlin: Springer-Verlag; 1985. [Google Scholar]
- Goebel K. Pflanzenbiologische Schilderungen 1. + 2. Teil. Marburg: Elwert'sche Verlagsbuchhandlung; 1891. [Google Scholar]
- Goebel K. Organographie der Pflanzen. 3rd edn. Vol. 3. Jena: Fischer; 1928–1933. [Google Scholar]
- Grob V, Moline P, Pfeifer E, Novelo AR, Rutishauser R. Developmental morphology of branching flowers in Nymphaea prolifera. Journal of Plant Research. 2006;119:561–570. doi: 10.1007/s10265-006-0021-8. [DOI] [PubMed] [Google Scholar]
- Huber K. Morphologische und entwicklungsgeschichtliche Untersuchungen an Blüten und Blütenständen von Solanaceen und von Nolana paradoxa Lindl. (Nolanaceae) Dissertationes Botanicae. 1980;55:1–291. [Google Scholar]
- Jobson RW, Playford J, Cameron KM, Albert VA. Molecular phylogenetics of Lentibulariaceae inferred from plastid rps16 intron and trnL-F DNA sequences: implications for character evolution and biogeography. Systematic Botany. 2003;28:157–171. [Google Scholar]
- Legendre L. The genus Pinguicula L. (Lentibulariaceae): an overview. Acta Botanica Gallica. 2000;147:77–95. [Google Scholar]
- Lemon GD, Posluszny U. Shoot development and evolution in Pistia stratiotes (Araceae) International Journal of Plant Sciences. 2000;161:721–732. [Google Scholar]
- Lloyd FE. The carnivorous plants. Waltham: Chronica Botanica; 1942. [Google Scholar]
- Müller K, Borsch T, Legendre L, Porambski S, Theisen I, Barthlott W. Evolution of carnivory in Lentibulariaceae and the Lamiales. Plant Biology. 2004;6:477–490. doi: 10.1055/s-2004-817909. [DOI] [PubMed] [Google Scholar]
- Raju MVS. Development of floral organs in the sites of leaf primordia in Pinguicula vulgaris. American Journal of Botany. 1969;56:507–514. [Google Scholar]
- Ray TS. Diversity of shoot organization in the Araceae. American Journal of Botany. 1987;74:1373–1387. [Google Scholar]
- Ray TS. Survey of shoot organisation in the Araceae. American Journal of Botany. 1988;75:56–84. [Google Scholar]
- Reinhardt D. Regulation of phyllotaxis. International Journal of Developmental Biology. 2005;49:539–546. doi: 10.1387/ijdb.041922dr. [DOI] [PubMed] [Google Scholar]
- Reinhardt D, Kuhlemeier C. Plant architecture. EMBO Reports. 2002;3:846–851. doi: 10.1093/embo-reports/kvf177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt D, Pesce ER, Stieger P, Mandel T, Baltensperger K, Bennett M, et al. Regulation of phyllotaxis by polar auxin transport. Nature. 2003;426:255–260. doi: 10.1038/nature02081. [DOI] [PubMed] [Google Scholar]
- Rutishauser R. Plastochrone ratio and leaf arc as parameters of a quantitative phyllotaxis analysis in vascular plants. In: Jean RV, Barabé D, editors. Symmetry in plants. Singapore: World Scientific; 1998. pp. 171–212. [Google Scholar]
- Rutishauser R, Isler B. Fuzzy Arberian morphology: Utricularia, developmental mosaics, partial shoot hypothesis of the leaf and other FAMous ideas of Agnes Arber (1879–1960) on vascular plant bauplans. Annals of Botany. 2001;88:1173–1202. [Google Scholar]
- Rutishauser R, Peisl P. Phyllotaxy. Encyclopedia of Life Sciences. Macmillan Publishers; 2001. (electronic encyclopedia, see http://www.els.net. ) [Google Scholar]
- Rutishauser R, Wanntorp L, Pfeifer E. Gunnera herteri – developmental morphology of a dwarf from Uruguay (Gunneraceae) Plant Systematics and Evolution. 2004;248:219–241. [Google Scholar]
- Schneider EL, Tucker SC, Williamson PS. Floral development in the Nymphaeales. International Journal of Plant Sciences. 2003;119:561–570. [Google Scholar]
- Svensson BM, Carlsson BA, Karlsson SP, Nordell OK. Comparative long-term demography of three species of Pinguicula. Journal of Ecology. 1993;81:635–645. [Google Scholar]
- Troll W. Vergleichende Morphologie der höheren Blütenpflanzen. Berlin: Gebrüder Borntraeger; 1937. [Google Scholar]
- Tucker SC. Evolutionary lability of symmetry in early floral development. International Journal of Plant Sciences. 1999;116:S25–S39. doi: 10.1086/314212. [DOI] [PubMed] [Google Scholar]
- Worley AC, Harder LD. Consequences of preformation for dynamic resource allocation by a carnivorous herb, Pinguicula vulgaris (Lentibulariaceae) American Journal of Botany. 1999;86:1136–1145. [PubMed] [Google Scholar]
- Wydler H. Pinguicula. Vol. 39. Flora: 1857. Morphologische Mittheilungen; pp. 609–616. [Google Scholar]
- Zamudio S, Rzedowski J. Dos especies nuevas de Pinguicula (Lentibulariaceae) del estado de Oaxaca, Mexico. Acta Botanica Mexicana. 1991;14:23–32. [Google Scholar]


