Abstract
Background and Aims
This study analysed the differences in nitrogen (N), non-structural carbohydrates (NSC) and biomass allocation to the roots and shoots of 18 species of Mediterranean dwarf shrubs with different shoot-rooting and resprouting abilities. Root N and NSC concentrations of strict root-sprouters and species resprouting from the base of the stems were also compared.
Methods
Soluble sugars (SS), starch and N concentrations were assessed in roots and shoots. The root : shoot ratio of each species was obtained by thorough root excavations. Cross-species analyses were complemented by phylogenetically independent contrasts (PICs).
Key Results
Shoot-rooting species showed a preferential allocation of starch to shoots rather than roots as compared with non-shoot-rooting species. Resprouters displayed greater starch concentrations than non-sprouters in both shoots and roots. Trends were maintained after PICs analyses, but differences became weak when root-sprouters versus non-root-sprouters were compared. Within resprouters, strict root-sprouters showed greater root concentrations and a preferential allocation of starch to the roots than stem-sprouters. No differences were found in the root : shoot ratio of species with different rooting and resprouting abilities.
Conclusions
The shoot-rooting ability of Mediterranean dwarf shrubs seems to depend on the preferential allocation of starch and SS to shoots, though alternative C-sources such as current photosynthates may also be involved. In contrast to plants from other mediterranean areas of the world, the resprouting ability of Mediterranean dwarf shrubs is not related to a preferential allocation of N, NSC and biomass to roots.
Key words: Mediterranean dwarf shrubs, nitrogen, non-structural carbohydrates, sprouting, resprouting, disturbance response, root : shoot ratio, shoot-rooting ability, adventitious roots, allocation pattern, starch, chamaephytes
INTRODUCTION
Disturbances are unpredictable events that reduce plant biomass. They are a crucial factor affecting ecological strategies, population biology, physiology and architecture of terrestrial plants worldwide (DeSouza et al., 1986; Pate et al., 1990; Hansen et al., 1991; Midgley, 1996; Bell, 2001). Plant responses to disturbances have typically been classified dichotomously, separating species that survive and persist vegetatively (the resprouters) from those that are killed and regenerate by seeds (the seeders or non-sprouters) (Wells, 1968; Midgley, 1996). This dichotomous classification is adequate for severe disturbances causing the loss of all above-ground biomass such as intense fires (Pate et al., 1990; Verdaguer and Ojeda, 2002; Vesk et al., 2004). However, when a wider range of disturbance intensities and frequencies is considered, plant responses seem to vary continuously along disturbance gradients (Bellingham and Sparrow, 2000).
Disturbances can also affect below-ground biomass, a variable that is frequently ignored in regeneration classifications. For example, plants from eroded lands or mobile substrates are frequently uprooted or buried by geomorphological processes (Sakai et al., 1997; Guerrero-Campo and Montserrat-Martí, 2000). Most species can survive a certain degree of uprooting; however, once a certain threshold is surpassed, the plant canopy dries out and only root-sprouters survive (Guerrero Campo, 1998). Under such circumstances, the ability of plants to root-sprout may be crucial for their persistence (Guerrero-Campo et al., 2006). Similarly, many tropical and temperate trees are able to survive wind throws and hurricanes by forming adventitious roots from the layering branches (Del Tredeci, 1995; Everham and Brokaw, 1996). Classifications including the shoot-rooting and the root-suckering abilities of plants are particularly scarce. Indeed, the ability to form shoot-borne roots is often neglected in disturbance-response analyses, and most studies on resprouting do not discriminate between sprouts produced from roots, stems or stem-derived structures (e.g. stolons or rhizomes) (Klimešová and Klimeš, 2003; but see Koop, 1987; Del Tredici, 2001). Root-sprouters are plants with the ability to form adventitious buds on their roots in response to special stimuli (Klimešová and Martínková, 2004). Many root-sprouters show shallow horizontal roots that sprout spontaneously, performing more or less extensive clonal growth (Klimešová and Martínková, 2004). Nevertheless, apart from this typical form, root-sprouters show a vast diversity of growth forms and not all of them display clonal growth (Sachs, 2002; Klimešová and Martínková, 2004). In this study, ‘root-sprouters’ will be referred to as plants with the ability to form adventitious shoots from their roots, irrespective of their ability to perform clonal growth. Similarly, ‘stem-sprouters’ will be considered as plants with the ability to resprout from stems or stem-derived structures such as rhizomes, lignotubers, burls or stolons, but not roots (Groff and Kaplan, 1988; Del Tredici, 2001). These different modes of vegetative regeneration have important implications for plant persistence (Groff and Kaplan, 1988; Bond and Midgley, 2003).
Groff and Kaplan (1988) proposed four different types of relationships among the root and shoot systems of vascular plants: class 1, ‘bipolar plants’, in which neither shoot-borne roots nor root-borne shoots are formed; class 2, plants that form shoot-borne roots but not root-borne shoots; class 3, plants that root-sprout but do not form shoot-borne roots; and class 4, plants that can form both shoot-borne roots and root-borne shoots. Although Groff and Kaplan did not discuss the effect of disturbances, the classes they proposed may be useful for understanding the response of vascular plants to disturbance events.
Irrespective of the portion of the plant that is killed by disturbance, most species that persist vegetatively maintain protected reservoirs of dormant meristems, non-structural carbohydrates (NSC) and mineral nutrients in safe places to support initial regrowth of the lost biomass (Canadell and López-Soria, 1998; Bell and Ojeda, 1999; Verdaguer and Ojeda, 2002; El Omari et al., 2003; Vesk and Westoby, 2004). The amount of carbon (C) needed may be very small before the plant becomes self-sustained (Richards and Caldwell, 1985). Indeed, it is still not clear that resprouting ability is related to the availability of NSC and nitrogen (N) stores. When disturbances affect only the roots or do not eliminate all above-ground parts, C resources for resprouting may be derived from current photosynthesis (Sakai and Sakai, 1998). However, when disturbances remove most or all above-ground biomass, the ability of plants to resprout depends on the remobilization of resources stored below ground, at least in the initial stages of regrowth (Iwasa and Kubo, 1997). Resprouters generally allocate more biomass and show greater starch concentrations in the roots than non-sprouters (Pate et al., 1990; Bell et al., 1996; Bell and Ojeda, 1999). Nevertheless, a lack of correlation between resprouting vigour and carbohydrate reserves in below-ground organs has also been documented (Cruz et al., 2003). Some investigations have also demonstrated the relevance of below-ground stores of N in supporting regrowth (El Omari et al., 2003). Nutrient availability may exert an important effect on the carbohydrate stores available for resprouting (Knox and Clarke, 2005). However, resprouting ability was unrelated to N content in the storage organs of the Mediterranean shrub Erica australis (Cruz et al., 2003). Indeed, resprouters do not seem to accumulate more macronutrients than non-sprouters in roots (Pate et al., 1990).
Similarly, apart from showing phylogenetical, ecological and developmental constraints (Itoh et al., 2002; Tousignant et al., 2003; Kibbler et al., 2004), the formation of shoot-borne roots seems to be limited by a threshold in the NSC concentrations of stems (Veierskov, 1988). Indeed, it has been suggested that apart from serving as an energy source and yielding the carbon skeletons needed for the construction of new roots (Van't Hoff, 1968), carbohydrates such as phosphorylated sugars may play a key role in regulating root initiation (Haissig, 1982, 1986; Veierskov, 1988). It could therefore be expected that the ability of a plant to root-sprout or to form roots from the shoots might be related to NSC and N levels of roots and shoots, respectively. However, as far as is known no previous studies have analysed such relationships simultaneously and in a broad group of species. In addition, it is currently not known whether different types of resprouters, i.e. strict root-sprouters versus species resprouting from stems or stem-derived structures, show differences in concentrations of N and NSC in roots.
In this study, the aim was to test the general hypothesis that the shoot-rooting and root-sprouting ability of plants are related to N and NSC concentrations of shoots and roots, respectively. As a consequence of this hypothesis, the following were expected: (a) shoot-rooting species would show higher NSC and N concentrations and allocate more biomass to shoots than non-shoot-rooting species, and (b) root-sprouters would have higher concentrations of NSC and N and allocate more biomass to roots than non-root-sprouters. An additional aim was to compare root N and NSC concentrations of strict root-sprouters and species resprouting from the base of the stems. The expectation was that the former would show higher N and NSC concentrations.
The restriction of the present analysis to dwarf shrubs aimed to avoid possible variations of plant response to disturbance associated with life-form (Vesk et al., 2004; Vesk, 2006).
MATERIALS AND METHODS
Species and study area
Eighteen species of Mediterranean dwarf shrubs were selected for analysis (Table 1): five bipolar plants (class 1), seven shoot-rooting species (class 2), three root-sprouting species (class 3) and three species with both the ability to root-sprout and form shoot-borne roots (class 4). The choice and definition of these species was based on a previous study on the shoot-rooting and root-sprouting abilities of the 123 most frequent phanerogams in the same area (Guerrero-Campo et al., 2006).
Table 1.
Main characteristics of study species
| Species | Family | Root : shoot relationship* | Sprouting† | Period of minimum biomass | Study site |
|---|---|---|---|---|---|
| Artemisia herba-alba | Asteraceae | 2 | 0a | Summer | Ayerbe (Hu) |
| Bupleurum fruticescens ssp fruticescens | Apiaceae | 1 | 0h | Winter | Ayerbe (Hu) |
| Dorycnium pentaphyllum ssp pentaphyllum | Fabaceae | 4 | 1b,c,d,h | Winter | Bernués (Hu) |
| Echinospartum horridum | Fabaceae | 2 | 0a,b,e | Winter | Las Peñas de Riglos (Hu) |
| Fumana hispidula | Cistaceae | 2 | 0b,c | Summer | Villamayor 1 (Z) |
| Genista scorpius | Fabaceae | 3 | 1b | Summer | Ayerbe (Hu) |
| Hedysarum boveanum ssp. europaeum | Fabaceae | 3 | 1a,c | Summer | Villamayor 1 (Z) |
| Helianthemum syriacum | Cistaceae | 1 | 0b | Summer | Villamayor 1 (Z) |
| Lepidium subulatum | Brassicaceae | 2 | 0f | Summer | Villamayor 2 (Z) |
| Linum tenuifolium ssp. appresum | Linaceae | 4 | 1a | Winter | Jaca (Hu) |
| Linum suffruticosum | Linaceae | 4 | 1a, b | Summer | Villamayor 2 (Z) |
| Lithodora fruticosa | Boraginaceae | 3 | 1c | Summer | Ayerbe (Hu) |
| Ononis fruticosa | Fabaceae | 2 | 1a | Winter | Bernués (Hu) |
| Ononis tridentata | Fabaceae | 1 | 0c | Summer | Villamayor 2 (Z) |
| Salvia lavandulifolia | Lamiaceae | 2 | 1a | Summer | Villamayor 1 (Z) |
| Satureja montana ssp. innota | Lamiaceae | 2 | 0a | Winter | Lasieso (Hu) |
| Staehelina dubia | Asteraceae | 1 | 1a,b,c | Winter | Villamayor 1 (Z) |
| Thymelaea tinctoria ssp. tinctoria | Thymelaeaceae | 1 | 1b,c | Summer | Villamayor 1 (Z) |
* Root : shoot relationship according to Groff and Kaplan's classification: class 1, bipolar species; class 2, shoot-rooting species; class 3, root-sprouters; class 4, species with both the ability to form roots from shoots and root-sprout.
† Sprouting ability after fire or clipping: 0 = non-sprouters (when 0–20 % of the individuals resprouted, but most resprouts died after 1 year of clipping); and 1 = resprouters (when >20 % of the individuals resprouted and most resprouts survived). Sources of information: a = authors' personal observation through experimental clipping or field prospects, b = Villar Salvador (2000), c = Guerrero Campo (1998), d = Riera (2000), e = Montserrat-Recoder et al. (1984), f = A. Escudero, pers. comm., h = Cucó (1987).
The study area was located between the middle Ebro Valley and the pre-Pyrenees, in north-eastern Spain and the main characteristics of the study sites are summarized in Table 2. Each species was collected from only one natural population within the study area. Due to their different distributions, it was impossible to find a single location where all 18 species co-occurred. Similarly, it was not feasible to find a single location with a sufficient number of dwarf shrubs representative of the four different types of root : shoot relationship analysed. Climate in the higher parts of the study area is sub-Mediterranean, with short summer drought periods and cold winters, and increased risk of winter frosts with altitude (Creus-Novau, 1983). In the lower parts, the risk of winter frosts is lower, but the length of summer drought increases. This type of climate can be considered to be Mediterranean with a ‘continental influence’ (Rivas-Martínez, 1987). For more details about the study area see Guerrero Campo (1998) and Palacio et al. (2006).
Table 2.
Main geological and climatic characteristics of study sites
| Study site | Substrate | pH | SOM (%) | N (%) | CaCO3 (%) | Gypsum (%) | P (mm) | T (ºC) | Altitude (m a.s.l.) | UTM |
|---|---|---|---|---|---|---|---|---|---|---|
| Ayerbe (Hu) | Miocene clays intermingled with marls | 8·2 | 1·2 | 0·11 | 31·1 | – | 630·2 | 13·2 | 580 | XM9280 |
| Huesca (Hu) | Miocene clays intermingled with marls | 8·3 | 0·4 | 0·04 | 37·8 | – | 587 | 13·4 | 488 | YM0560, YM0359 |
| Bernués (Hu) | Dominant sandstones intermingled with marls and conglomerates | 8·5 | 0·4 | 0·04 | 48·8 | – | 693·2 | 12·0 | 810–1020 | XN9704, YN0108 |
| Jaca (Hu) | Badlands on Eocene marls. | 8·2 | 0·6 | 0·05 | 39·0 | – | 824 | 11·4 | 920 | XN9913 |
| Las Peñas de Riglos (Hu) | Massive calcareous conglomerates | 7·9 | 6·7 | 0·36 | 2·4 | – | 1246·6* | 8·0* | 1380 | XN8908 |
| Lasieso (Hu) | Calcareous alluviums | 8·7 | 0·6 | 0·03 | 54·1 | – | 653·2 | 12·1 | 675 | YM1099 |
| Villamayor 1 (Z) | Gypsum sediments intermingled with marls and clays | 7·8 | 4·7 | 0·25 | 44·8 | 2·0 | 402·9 | 14·1 | 340 | XM8920 |
| Villamayor 2 (Z) | Powdery gypsum outcrops | 7·7 | 1·9 | 0·14 | 15·6 | 55·5 | 402·9 | 14·1 | 320 | XM8820 |
P = precipitation (mm) and T = temperature (°C): values were obtained from the closest weather station to the study population of each species (always located less than 10 km away from the study site). No weather information was available for the population of Echinospartum horridum (Las Peñas de Riglos, Huesca). Therefore, mean annual rainfall and temperature values for this site (*) were extrapolated from values recorded at the meteorological station of Jaca located 25 km away (31 years of record, 840 m a.s.l.), following the vertical gradients proposed for the Pyrenees by López-Moreno (2005) and García-Ruíz et al. (1985). Soil organic matter content (SOM, %) was analysed by the method of Walkley and Black (1934). Total N concentrations (N, %) were measured with an elementar analyser (Elementar VarioMAX N/CM, Hanau, Germany). Carbonate content (CaCO3, %) was assessed by Bernard's calcimetry (Porta-Casanellas et al., 1986) and gypsum content (%) was determined with a C/S elementar analyser (ISC144DR, Leco, St Joseph, Michigan).
Plants response to disturbance
Information on the shoot-rooting and root-sprouting ability of the study species was obtained from Guerrero-Campo et al. (2006) and Villar Salvador (2000). Similarly, data on the resprouting ability of most study species after fire or clipping were obtained from the literature (see Table 1). For the species whose resprouting ability was not reported in the literature, clipping experiments (by clipping 20 individuals at the ground level) were performed to determine the percentage of resprouting individuals remaining 6 and 12 months after treatment. Resprouting ability of species was classified in one of two categories (Table 1): ‘0’ non-sprouters (when 0–20 % of the individuals resprouted, but most new sprouts died after 1 year of clipping); and ‘1’ resprouters (when >20 % of the individuals resprouted and most new sprouts survived).
Collection of plant material
Plant material was collected during summer (August 2003) and winter (January 2004), the two main periods of stress in Mediterranean-type climate (Mitrakos, 1980). Most Mediterranean dwarf shrubs show periodic oscillations of their living and photosynthetic biomass following the seasonality of Mediterranean climate (Orshan, 1989). For some species, summer is the main period of stress and hence photosynthetic and living biomass is reduced to a minimum during this time of the year (Orshan, 1954). However, in colder Mediterranean areas winter is the most limiting period of the year, and plants of such environments show minimum living biomass during winter (Palacio et al., 2006). When plants from diverse Mediterranean-type environments are considered, samples taken during summer or winter may represent different phenological stages and data may not be comparable. Indeed, previous studies on the seasonal N and NSC dynamics of six of the 18 study species showed that seasonal trends of the concentrations of NSC and N are strongly affected by leaf phenology, even for plants that co-exist at the same site (Palacio et al., 2007a, b). Following the above reasoning, the data obtained were sorted according to phenological state at the time of sampling. Accordingly, the period of the year when each study species showed minimum living biomass was identified (Table 1) and the data were sorted by periods of high (i.e. when living biomass was minimum) and low stress (otherwise), instead of by summer or winter periods. The analysis focused on data from the periods of high stress, when plants display minimum phenological activity and hence they are more comparable.
On each sampling date (August 2003 and January 2004), five medium-sized adult individuals of each species were excavated down to 30–40 cm from their rooting point for N and NSC concentration analyses. The excavated plants were separated into coarse roots (>3 mm in width) and main and secondary stems (older than 3 years). Therefore, 5 replicates × 18 species × 2 organs × 2 sampling dates, totalling 360 samples, were collected. Samples were processed within <24 h from collection or else they were stored at −20 °C. All plant material was vigorously brushed to remove soil particles, cut in small pieces, and stored at −20 °C until freeze-dried (Cryodos, Telstar Industrial SL, Terrasa, Spain).
In August 2004, three adult individuals similar to those used for chemical analyses were excavated to their full rooting depth for biomass measurements. Most roots, except very fine ones, were followed until their end and collected. The excavated plants were partitioned into their above- and below-ground biomass for root : shoot biomass estimations. Dry leaves and branches were carefully eliminated to ensure the sampling of living materials. Samples were oven-dried to a constant weight at 60 °C and weighed.
Chemical analyses
Dried samples were milled (IKA MF10, IKA-Werke, Staufen, Denmark) to a fine powder. Nitrogen mass-based concentrations were measured with an elementar analyser (Elementar VarioMAX N/CM, Hanau, Germany). Soluble sugars were extracted with 80 % (v/v) ethanol and concentrations were determined colorimetrically using the phenol-sulfuric method of Dubois et al. (1956) as modified by Buysse and Merckx (1993). Starch and complex sugars remaining in the undissolved pellet after ethanol extractions were enzymatically reduced to glucose and analysed as described in Palacio et al. (2007a). Non-structural carbohydrates measured after ethanol extraction are referred to as soluble sugars (SS) and carbohydrates measured after enzymatic digestion in glucose equivalents are referred to as starch.
Statistics
All data were checked for normality and homoscedasticity prior to statistical analyses. When data lacked normality they were log- or inversely transformed (Zar, 1984). To determine whether any of the measured factors were associated with shoot-rooting, all species with the capacity to form roots from shoots (classes 2 and 4) were compared with all species that did not (classes 1 and 3). The parallel organization of the data was used to look at root-sprouting, i.e. root-sprouting species (classes 3 and 4) were compared with non-root-sprouting species (classes 1 and 2). Differences among the four types of root : shoot relationships (Groff and Kaplan's classification) were also assessed by comparing the four classes independently. It is important to note that non-root-sprouters (classes 1 and 2) may include species with the ability to resprout from stem-derived structures, which are widespread among Mediterranean-type species (James, 1984). To assess the relationship between the resprouting ability after clipping (irrespective of the plant part sprouts were formed from) and N and NSC concentrations, species with and without the ability to resprout after fire or clipping (Table 1) were compared.
Differences in SS, starch and N concentrations among disturbance responses (shoot-rooting, root-sprouting, Groff and Kaplan classes and resprouting after clipping) were explored by nested ANOVAs by considering ‘species’ as a nested factor within ‘disturbance response’. Similarly, differences in NSC and N concentrations of roots between strict root-sprouters and stem-sprouters were analysed by using nested ANOVAs, with ‘species’ nested within the factor ‘resprouting type’ (i.e. root-sprouters versus stem-sprouters).
To analyse differences in the internal allocation of NSC and N between roots and shoots of study species, the quotient between root and shoot concentrations was calculated for the concentration of starch, SS and N in all individuals sampled. This provides an estimate of the relative allocation to roots and shoots of each resource within individuals. Differences in the relative allocation of carbohydrates and N among disturbance responses were explored by nested ANOVAs, with ‘species’ as a nested factor within ‘disturbance response’.
Root : shoot ratios of the study species did not follow a normal distribution, not even after being transformed. Therefore, differences in the root : shoot ratio of shoot-rooting versus non-shoot-rooting species, root-sprouters versus non-root-sprouters and species with and without ability to resprout after clipping, were analysed by non-parametric U Mann–Whitney tests. These analyses were performed separately for species showing minimum above-ground biomass in summer and winter. All statistical analyses were conducted with SPSS 13·0 (SPSS Inc., Chicago, IL, USA).
Analyses that treat species as independent data points do not take into account that species may share traits through common phylogenetic origin and may lead to correlations that are artefacts of relatedness (Felsenstein, 1985; Harvey and Pagel, 1991). Therefore, in addition to analyses treating each species as an independent data point, trends in NSC and N concentrations were assessed using phylogenetically independent contrasts (PICs). One way to perform PICs is to compare pairs of taxa below a node in a bifurcating phylogeny. The relationship between variables is then phylogenetically independent because both taxa in a comparison have evolved independently (Felsenstein, 1985). Felsenstein's method requires that the true phylogeny is known. However, Purvis and Rambaut (1995) developed a model [Comparative Analysis by Independent Contrasts (CAIC)] that applies Felsenstein's approach to data sets for which only approximate phylogenies are available. This model obtains a single value (called ‘contrast’) for each variable within the taxa below a node, showing its magnitude and direction of change. Contrasts are then scaled using information of the length of the branches leading from that node (or an assumption on the length of the branches if it is not known; Harvey and Pagel, 1991). When analysing both quantitative and categorical variables, as in this study, differences between categories have to be analysed by the BRUNCH module of CAIC (Purvis and Rambaut, 1995). This algorithm uses the categorical variable as the predictor and sets all contrasts in the predictor variable to +1. If there is no tendency for taxa in any of the categories to have higher or lower values of the quantitative variable (in this study N and NSC concentrations), contrasts in the dependent variable should show no significant tendency to be different from zero. The existence of such a tendency is assessed by using one-sample t-tests against a population of mean zero (Purvis and Rambaut, 1995).
Here, a phylogenetic tree was obtained using the method of Soltis et al. (2000) for the structure down to the family level and that of Wojciechowski et al. (2004) for the Fabaceae, the only family with more than two species, and used to compute independent standardized contrasts for all variables. In the absence of node age, all branch lengths were assumed to be equal (Purvis and Rambaut, 1995). Comparisons between disturbance response types were undertaken using t-tests on the contrasts generated by CAIC using the BRUNCH option (n = 8, 5 and 7 contrasts for shoot-rooting, root-sprouting and resprouting after clipping, respectively).
RESULTS
Comparison between shoot-rooting and non-shoot-rooting species
There were no significant differences among carbohydrate and N concentrations in the shoots and roots of shoot - rooting and non-shoot-rooting species (Fig. 1). However, shoot-rooting species showed marginally higher SS concentrations in shoots than non-shoot-rooting species after the analysis of PICs (P = 0·054). Shoot-rooting species showed significantly lower relative starch concentrations in roots than in shoots (P = 0·012), which indicates preferential allocation of starch to shoots (Table 3). Such a trend was still noticeable after the analysis of PICs, though differences between groups were weak (P = 0·076). No significant differences were found between the root : shoot ratios of both types of species (P = 0·931 and P = 0·190 for species showing minimum above-ground biomass in summer and winter, respectively).
Fig. 1.
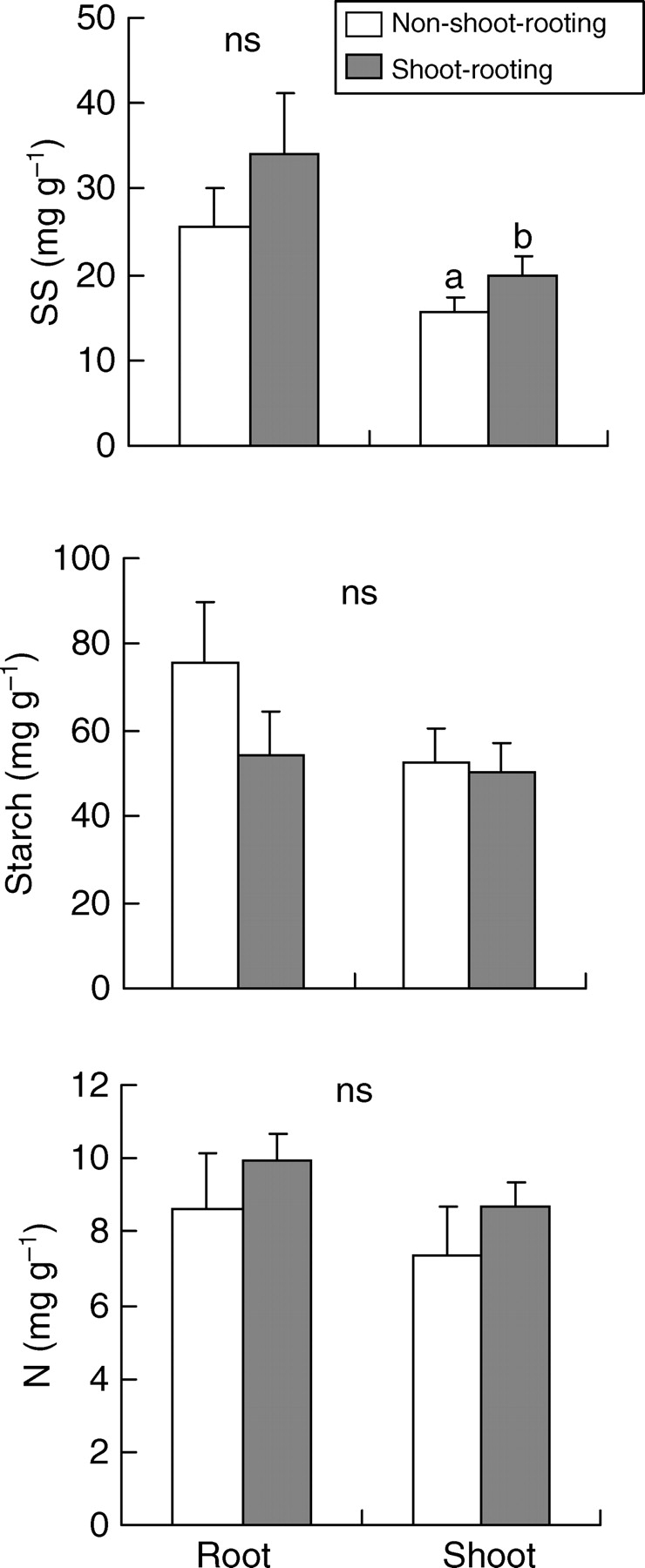
Concentrations of soluble sugars (SS), starch and nitrogen (N) in shoot-rooting and non-shoot-rooting species. Values are means +2 s.e. ns indicates no significant differences according to a nested ANOVA (P > 0·05). n = 8 for non-shoot-rooting species and n = 10 for shoot-rooting species. See text for further details on calculations.
Table 3.
Relative allocation of starch, soluble sugars (SS) and nitrogen (N) to roots and shoots of species with different rooting and sprouting abilities
| Disturbance response | n | Starch | SS | N |
|---|---|---|---|---|
| Shoot rooting ability: | ||||
| Rooting | 10 | 1·1 (0·5) | 1·8 (1·2) | 1·2 (0·3) |
| Non-rooting | 8 | 1·4 (0·5) | 1·8 (1·3) | 1·2 (0·3) |
| Root-sprouting ability | ||||
| Root-sprouters | 6 | 1·3 (0·5) | 1·9 (1·2) | 1·2 (0·3) |
| Non-root-sprouters | 12 | 1·2 (0·4) | 1·7 (1·3) | 1·2 (0·3) |
| Sprouting after clipping: | ||||
| Non-sprouters | 8 | 1·2 (0·5) | 1·8 (1·5) | 1·1 (0·2) |
| Resprouters | 10 | 1·3 (0·4) | 1·8 (1·2) | 1·3 (0·3) |
| Root- versus stem-sprouters | ||||
| Root-sprouters | 6 | 1·3 (0·5) | 1·9 (1·2) | 1·2 (0·3) |
| Stem-sprouters | 4 | 1·1 (0·3) | 1·5 (0·9) | 1·3 (0·3) |
Mean values of the quotient between root and shoot concentrations are shown along with standard deviations (in parentheses). Values in bold indicate significant differences between groups after a nested ANOVA (P < 0·05; see text for further details on calculations).
Comparison between resprouters and non-sprouters
Root-sprouters had significantly higher concentrations of starch than non-root-sprouters in both shoots and roots (Fig. 2). Indeed, starch concentration in roots of root-sprouters was almost double that of non-root-sprouters. However, no significant differences were found between SS concentrations of both organs and the NSC, N or biomass allocation patterns in both groups of plants (Fig. 2; Table 3; P = 0·850 and P = 1·000 for the root : shoot ratio of species showing maximum stress in summer and winter, respectively; see also Supplementary Information available online). Trends were similar after the analysis of PICs, though differences in starch concentrations were not significant (P = 0·077 and P = 0·053 for shoots and roots, respectively). Nitrogen concentrations were significantly higher in the shoots of root-sprouters, though such differences disappeared when PICs were analysed (P = 0·202). Results were very similar when resprouting after clipping instead of strict root-sprouting was considered (Fig. 3 and Table 3). Nevertheless, according to the analysis of PICs, differences in the starch concentrations of shoots (P = 0·051) and roots (P = 0·009) were stronger when species with different ability to sprout after clipping instead of species with different root-sprouting ability were compared. Finally, when the analysis was conducted on the classes of Groff and Kaplan, root-sprouters (classes 3 and 4) showed significantly greater starch concentrations than non-root-sprouters (classes 1 and 2) in both their shoots and roots (Fig. 4).
Fig. 2.
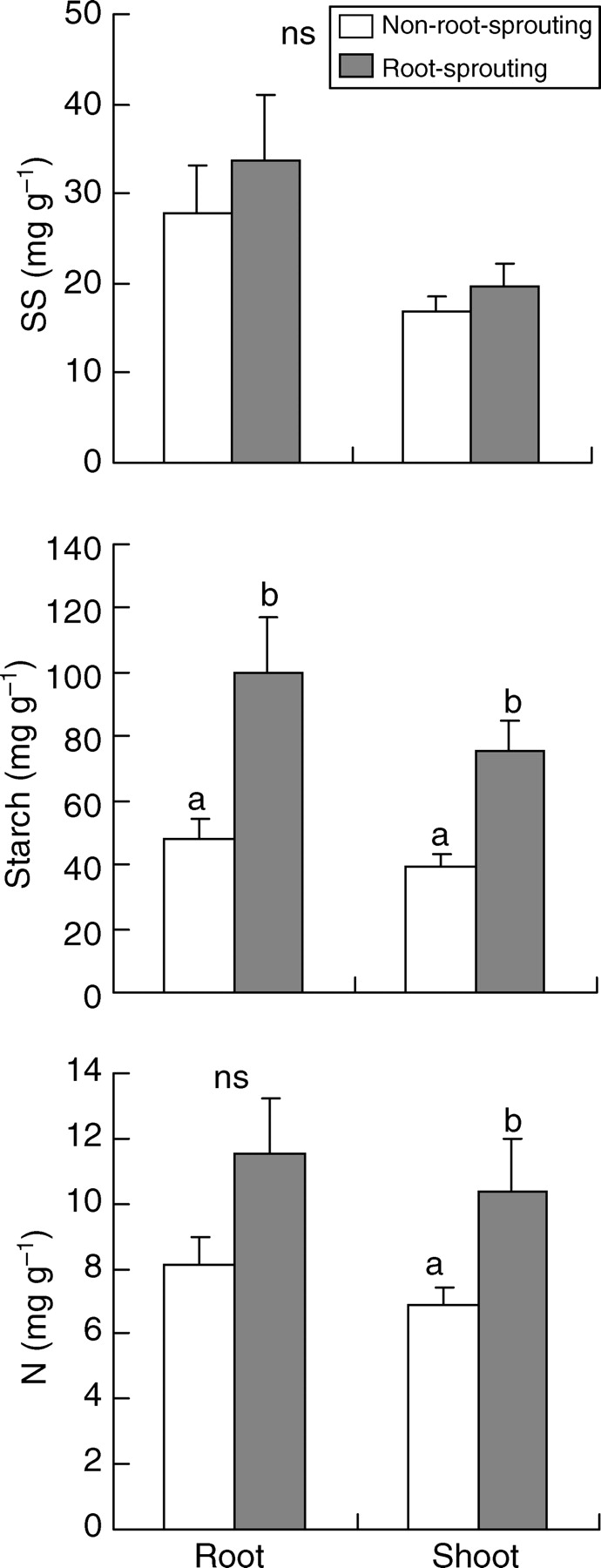
Concentrations of soluble sugars (SS), starch and nitrogen (N) in root-sprouting and non-root-sprouting species. Values are means +2 s.e. Different letters indicate significant differences according to a nested ANOVA (P < 0·05). ns indicates no significant differences. n = 12 for non-root-sprouters and n = 6 for root-sprouters. See text for further details on calculations.
Fig. 3.
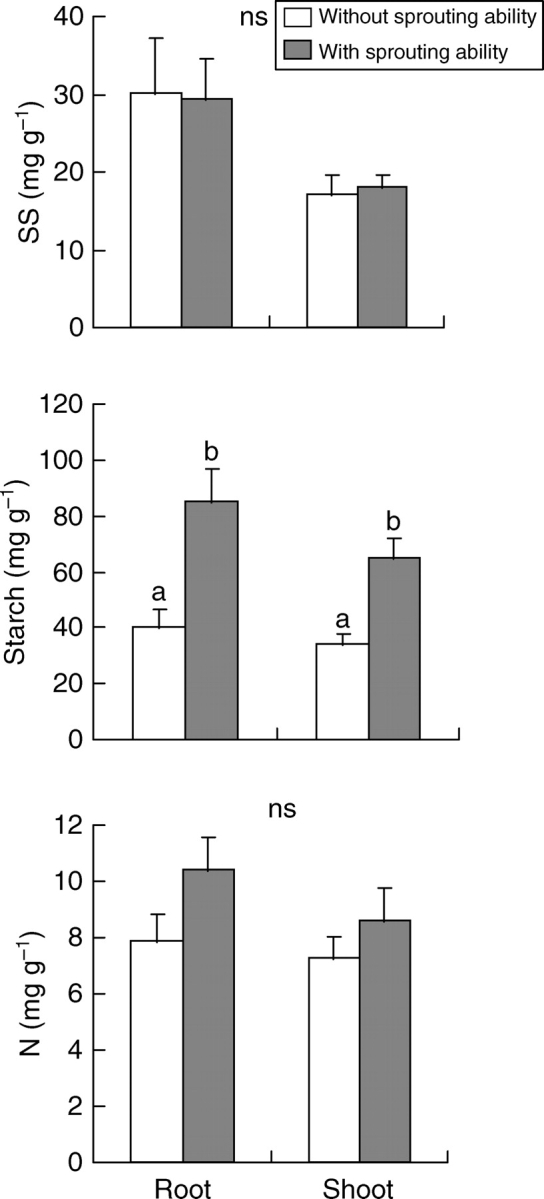
Concentrations of soluble sugars (SS), starch and nitrogen (N) in species with (dark columns) and without the ability to sprout after fire or clipping (open columns). Values are means +2 s.e. Different letters indicate significant differences according to a nested ANOVA (P < 0·05). ns indicates no significant differences. n = 8 for non-sprouters and n = 10 for sprouters. See text for further details on calculations.
Fig. 4.
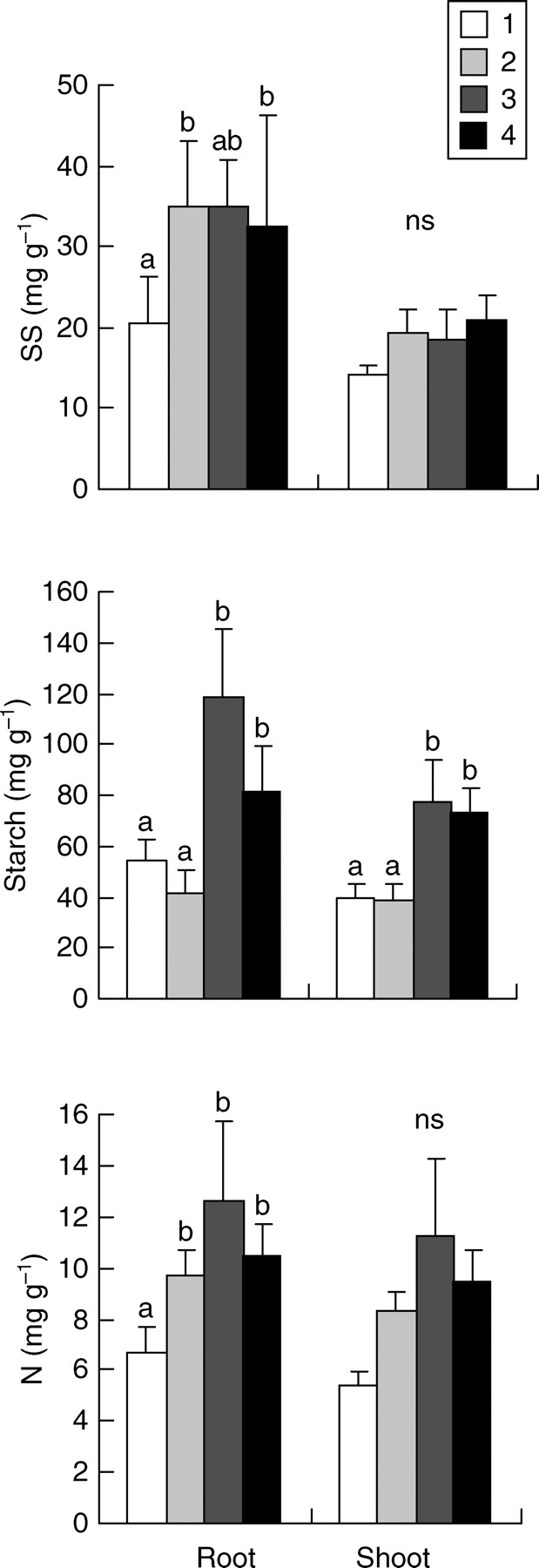
Concentrations of soluble sugars (SS), starch and nitrogen (N) in species with different root : shoot relationship according to Groff and Kaplan's classification: class 1, bipolar species; class 2, shoot-rooting species; class 3, root-sprouters; class 4, species with both the ability to form roots from shoots and root-sprout. Values are means +2 s.e. Different letters indicate significant differences according to a nested ANOVA (P < 0·05). ns indicates no significant differences. n = 5 for class 1 species, n = 7 for class 2 species, n = 3 for class 3 species and n = 3 for class 4 species. See text for further details on calculations.
Comparison between root-sprouters and stem-sprouters
Root-sprouters had higher mean concentrations of starch in roots than stem-sprouters (99·99 ± 8·71 versus 49·99 ± 5·13 of stem-sprouters), but differences were weak (P = 0·051). For SS and N, differences were not significant (P > 0·05). Similarly, strict root-sprouters showed significantly greater allocation of starch in roots than stem-sprouters (P = 0·021; Table 3).
DISCUSSION
The results indicate that the different types of root : shoot relationship analysed (Groff and Kaplan, 1988) do not entail different biomass allocation patterns within Mediterranean dwarf shrubs. Nevertheless, some important differences were found in the concentration and relative allocation to roots and shoots of NSC in species with different shoot-rooting and root-sprouting abilities. Resprouting ability showed a clear relationship with increased starch concentrations both in roots and shoots, while the shoot-rooting ability of Mediterranean dwarf shrubs may depend on a preferential allocation of starch to shoots and increased shoot SS concentrations.
Results obtained with cross-species and PICs led to similar trends, though some differences were noticeable. In some cases, significant differences disappeared after PICs analyses, as for N concentrations in roots of root-sprouters and non-root-sprouters. Yet in other cases, differences were significant, such as differences in shoot SS concentrations of shoot-rooting species. This may indicate phylogenetic biases due to sampling (Felsenstein, 1985). Nevertheless, in most cases the analysis of PICs reduced the strength of the observed differences while maintaining the trends. This was the case, for example, of differences in starch concentrations in roots and shoots of species with different root-sprouting ability. Though such a loss in significance may be indicative of phylogenetic biases, it may also be due to a loss of statistical power linked to the reduced number of contrasts available (n = 5 in this particular case). Further studies dealing with a broader array of species of Mediterranean dwarf shrubs are needed to clarify these preliminary results.
This study included species from a range of sites, with different climates and soils, and it is difficult to evaluate whether this could have influenced the results. Nutrient and water availability may affect NSC accumulation and biomass allocation patterns (Cruz et al., 2002; Cheng et al., 2004). Most study sites showed similar nutrient availability (N and soil organic matter), which was quite low (Table 2). However, water availability was quite different between sites, lower sites showing a marked summer drought period (Table 2). It is impossible to assess how such differences in water availability may have affected the results. Nevertheless, a recent study has shown that, although differences in resource availability may lead to changes in starch and biomass allocation of congeneric sprouter and non-sprouter shrubs, the overall effect of nutrient or water availability is small when compared with differences between resprouters and non-sprouters (Knox and Clarke, 2005).
In accordance with previous results (Veierskov, 1988) the shoot-rooting species analysed showed greater relative allocation of starch to shoots than roots and marginally greater SS concentrations in shoots than non-shoot-rooting species after the analysis of PICs. However, their starch and N concentrations in shoots were similar to those of non-shoot-rooting species. Roots without large parenchyma storage tissue, and hence ‘cheaper’ in terms of energy requirements, may be selected for in these species with a fast root formation and presumably also a high root turnover. This indicates that their reliance on stores is weaker than expected. Other C-sources apart from in situ stores may be involved in the formation of new roots. Carbohydrates needed to complement the construction of new roots may be directly diverted from photosynthesis (Sakai and Sakai, 1998). Similar results were obtained for rooting branches of the tree Backhousia citriodora (Kibbler et al., 2004). In this species, rooting success of branch cuttings depends on the developmental stage of the leaves: branch cuttings with mature leaves form roots faster than cuttings with immature leaves, presumably due to the greater capacity to export resources from mature leaves (Kibbler et al., 2004).
It is well known from previous studies that woody resprouters and non-sprouters show differences in allocation of resources to roots (Pate et al., 1990; Iwasa and Kubo, 1997; Bell and Ojeda, 1999). The results presented here complement the classical resprouter versus non-sprouter view by presenting new data on differences in N and carbohydrate allocation between different types of resprouters (i.e. strict root-sprouters and stem-sprouters). In the current study, root-sprouters had greater concentrations and allocated relatively more NSC to roots than stem-sprouters. Strict root-sprouters may therefore show a stronger tendency for root storage than species resprouting from the base of their stems.
According to the present results, no differences in the concentration of N in shoots and roots were found between resprouting and non-sprouting species when phylogenetic biases were excluded from the analyses. Although the relevance of nutrients such as N to support regrowth of resprouters has been demonstrated experimentally (El Omari et al., 2003), it does not seem to be followed by a differential accumulation of N in below-ground organs (Pate et al., 1990; Cruz et al., 2003). Nutrient availability interacts with resprouting ability and NSC-storing capacity of plants (Knox and Clarke, 2005), and such interaction may be complex. For example, in a study on Erica australis, high nutrient availability favoured the growth of resprouts by increasing their biomass and length, but at the same time, reduced plant investment on the lignotuber, leading to a reduced number of resprouting buds (Cruz et al., 2002). Another important factor that may affect the relationship between nutrient availability, resprouting ability and amount of stored reserves is the nature and ecophysiology of microsymbionts such as mycorrhizae or N2-fixing microbia. Species with N2-fixing symbionts such as the Fabaceae show higher N concentrations in their roots than species from other families (Chapin, 1980). The higher root N concentrations of Fabaceae are followed by increased root starch concentrations both in seeders and resprouters of this family (Pate et al., 1990). Similarly, differences in mycorrhizal fungi between resprouters and non-sprouters could result in differences in the accessibility of plants to soil nutrients and ultimately in their investment to stored reserves. However, as far as is known, no previous studies have assessed the differences in mycorrhizal fungi of both groups of plants. Further studies on the internal cycling of N, and on the activity of microsymbionts of resprouters and non-sprouters are needed to clarify these issues.
One important difference found in this study compared with previous studies is that resprouters show significantly greater concentrations of starch than non-sprouters both in shoots and roots, while in other studies resprouters accumulated more carbohydrates than non-sprouters in roots but not shoots (Pate et al., 1984; Bell and Pate, 1996; Bell and Ojeda, 1999). Furthermore, previous studies reported significant differences in the root : shoot allocation patterns between resprouters and non-sprouters, the former investing more biomass in roots. However, this study found no significant differences in the root : shoot biomass ratios of resprouters and non-sprouters. Comparative studies have focused on species from fire-prone environments of Australia (Pate et al., 1990; Bowen and Pate, 1993; Bell et al., 1996) and South Africa (Bell and Ojeda, 1999; Verdaguer and Ojeda, 2002), where the selective pressure for fire persistence traits is strong (Bond et al., 2005). Contrastingly, this study used species from Mediterranean Basin shrublands, where fire-based selection is thought to have been weaker than in other Mediterranean areas of the world (Pausas et al., 2006). Other disturbances such as herbivory may have exerted a strong influence on the evolution of plant species in Mediterranean shrublands (Naveh, 1975). Indeed, grazing has historically been very important in these areas, and Mediterranean species show various adaptations to avoid being consumed by herbivores (Margaris and Vokou, 1982; Lev-Yadun and Ne'eman, 2004). Disturbances such as herbivory may exert a weaker selection towards allocating below-ground starch and biomass than fire.
In conclusion, this study shows that significant differences in starch allocation exist between rooting and non-rooting species of Mediterranean dwarf shrubs and that, although root-sprouters show slightly greater concentrations of starch in roots than stem-sprouters, both types of resprouters display similarly higher concentrations of carbohydrates when compared with non-sprouters. The classical root : shoot biomass and starch allocation trade-offs observed in species from fire-prone ecosystems of other Mediterranean-type areas of the world do not seem to apply to Mediterranean dwarf shrubs, which might account for different allocation patterns of biomass and starch.
SUPPLEMENTARY INFORMATION
Supplementary information is available online at http://aob.oxfordjournals.org/. Data on mean soluble sugars (SS), starch, total non-structural carbohydrate (NSC) and nitrogen (N) concentrations in the roots and shoots of the study species are provided together with the mean root : shoot ratios (R : S) of study species in August 2004.
ACKNOWLEDGEMENTS
The authors are grateful to Rubén Milla, Tina L. Bell, Adrián Escudero, Txemi Olano, David Causton and two anonymous referees for valuable comments on earlier versions of the manuscript, to Susana Paula and Juli Pausas for a helpful introduction to PICs, to Luis Erneta for assistance with field sampling and to Adela Lamana, M. Luisa Dehesa and Patricia Fustero for their help with chemical analyses. This study was supported by a research grant from the Instituto de Estudios Altoaragoneses and by the research projects RTA 2005-00100 and SUM2006-00025-00-00 from the MEC (INIA) and CGL2006-11619/HID, CGL2004-04919-C02-01/HID, REN2003–08678/HID from the MCT, Spanish Government.
This study is dedicated to the memory of Dr Alberto Cruz.
LITERATURE CITED
- Bell DT. Ecological response syndromes in the flora of southwestern Western Australia: fire resprouters versus reseeders. The Botanical Review. 2001;67:417–440. [Google Scholar]
- Bell TL, Ojeda F. Underground starch storage in Erica species of the Cape Floristic Region – differences between seeders and resprouters. New Phytologist. 1999;144:143–152. [Google Scholar]
- Bell TL, Pate JS. Growth and fire response of selected Epacridaceae of south-western Australia. Australian Journal of Botany. 1996;44:509–526. [Google Scholar]
- Bell TL, Pate JS, Dixon KW. Relationships between fire response, morphology, root anatomy and starch distribution in south-west Australian Epacridaceae. Annals of Botany. 1996;77:357–364. [Google Scholar]
- Bellingham PJ, Sparrow AD. Resprouting as a life history strategy in woody plant communities. Oikos. 2000;89:409–416. [Google Scholar]
- Bond WJ, Midgley JJ. The evolutionary ecology of sprouting in woody plants. International Journal of Plant Sciences. 2003;164:103–114. [Google Scholar]
- Bond WJ, Woodward F, Midgley GF. The global distribution of ecosystems in a world without fire. New Phytologist. 2005;165:525–538. doi: 10.1111/j.1469-8137.2004.01252.x. [DOI] [PubMed] [Google Scholar]
- Bowen BJ, Pate JS. The significance of root starch in post-fire shoot recovery of the resprouter Stirlingia latifolia R. Br. (Proteaceae) Annals of Botany. 1993;72:7–16. [Google Scholar]
- Buysse J, Merckx R. An improved colorimetric method to quantify sugar content of plant tissue. Journal of Experimental Botany. 1993;44:1627–1629. [Google Scholar]
- Canadell J, López-Soria L. Lignotuber reserves support regrowth following clipping of two Mediterranean shrubs. Functional Ecology. 1998;12:31–38. [Google Scholar]
- Chapin FS. The mineral nutrition of wild plants. Annual Review on Ecology and Systematics. 1980;11:233–260. [Google Scholar]
- Cheng L, Ma F, Ranwala D. Nitrogen storage and its interaction with carbohydrates of young apple trees in response to nitrogen supply. Tree Physiology. 2004;24:91–98. doi: 10.1093/treephys/24.1.91. [DOI] [PubMed] [Google Scholar]
- Creus-Novau J. El clima del alto Aragón occidental. Jaca: Instituto de Estudios Pirenaicos. CSIC. Diputación Provincial de Huesca; 1983. [Google Scholar]
- Cruz A, Pérez B, Quintana JR, Moreno JM. Resprouting in the Mediterranean-type shrub Erica australis is affected by soil resource availability. Journal of Vegetation Science. 2002;13:641–650. [Google Scholar]
- Cruz A, Pérez B, Moreno JM. Plant stored reserves do not drive resprouting of the lignotuberous shrub. Erica australis. New Phytologist. 2003;157:251–261. doi: 10.1046/j.1469-8137.2003.00668.x. [DOI] [PubMed] [Google Scholar]
- Cucó ML. Mecanismes de regeneració. In: Terradas J, editor. Quaderns d'ecologia aplicada. Ecosistemes terrestres. La resposta als indendis i a d'altres pertorbacions. Barcelona: Servei del Medi Ambient, Diputació de Barcelona; 1987. pp. 45–62. [Google Scholar]
- Del Tredeci P. Shoots from roots: a horticultural review. Arnoldia Fall. 1995:3–19. [Google Scholar]
- Del Tredici P. Sprouting in temperate trees: a morphological and ecological review. The Botanical Review. 2001;67:121–140. [Google Scholar]
- DeSouza J, Silka PA, Davis SD. Comparative physiology of burned and unburned Rhus laurina after chaparral wildfire. Oecologia. 1986;71:63–68. doi: 10.1007/BF00377322. [DOI] [PubMed] [Google Scholar]
- Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Analytical Chemistry. 1956;28:350–356. [Google Scholar]
- El Omari B, Aranda X, Verdaguer D, Pascual G, Fleck I. Resource remobilization in Quercus ilex L. resprouts. Plant and Soil. 2003;252:349–357. [Google Scholar]
- Everham EM, Brokaw NVL. Forest damage and recovery from catastrophic wind. The Botanical Review. 1996;62:113–185. [Google Scholar]
- Felsenstein J. Phylogenies and the comparative method. American Naturalist. 1985;125:1–15. doi: 10.1086/703055. [DOI] [PubMed] [Google Scholar]
- García-Ruiz JM, Puigdefábregas J, Creus J. Los recursos hídricos superficiales del Alto Aragón. Huesca: Instituto de Estudios Altoaragoneses; 1985. [Google Scholar]
- Groff PA, Kaplan DR. The relation of root systems to shoot systems in vascular plants. The Botanical Review. 1988;54:387–422. [Google Scholar]
- Guerrero Campo J. Respuestas de la vegetación y de la morfología de las plantas a la erosión del suelo. Zaragoza: Consejo de la Protección de la Naturaleza de Aragón; 1998. [Google Scholar]
- Guerrero-Campo J, Montserrat-Martí G. Effects of soil erosion on the floristic composition of plant communities on marl in northeast Spain. Journal of Vegetation Science. 2000;11:329–336. [Google Scholar]
- Guerrero-Campo J, Palacio S, Pérez Rontomé C, Montserrat-Martí G. Effect of root system morphology on root-sprouting and shoot-rooting abilities in 123 plant species from eroded lands in north-east Spain. Annals of Botany. 2006;98:439–447. doi: 10.1093/aob/mcl122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haissig BE. Activity of some glycolytic and pentose phosphate pathways enzymes during the development of adventitious roots. Physiologia Plantarum. 1982;55:261–272. [Google Scholar]
- Haissig BE. Metabolic processes in adventitious rooting of cuttings. In: Jackson MB, editor. New root formation in plants and cuttings. Dordrecht/Boston/Lancaster: Martinus Nijhoff Publisher; 1986. pp. 141–191. [Google Scholar]
- Hansen A, Pate JS, Hansen AP. Growth and reproductive performance of a seeder and a resprouter species of Bossiaea as a function of plant age after fire. Annals of Botany. 1991;67:497–509. [Google Scholar]
- Harvey PH, Pagel MD. The comparative method in evolutionary biology. Oxford: Oxford University Press; 1991. [Google Scholar]
- Itoh A, Yamakura T, Kanzaki M, Ohkubo T, Palmiotto PA, LaFrankie JV, et al. Rooting ability of cuttings relates to phylogeny, habitat preference and growth characteristics of tropical rainforest trees. Forest Ecology and Management. 2002;168:275–287. [Google Scholar]
- Iwasa Y, Kubo T. Optimal size of storage for recovery after unpredictable disturbances. Evolutionary Ecology. 1997;11:41–65. [Google Scholar]
- James S. Lignotubers and burls: their structures, function and ecological significance in Mediterranean ecosystems. The Botanical Review. 1984;50:225–266. [Google Scholar]
- Kibbler H, Johnston ME, Williams RR. Adventitious root formation in cuttings of Backhousia citriodora F. Muell. 1. Plant genotype, juvenility and characteristics of cuttings. Scientia Horticulturae. 2004;102:133–143. [Google Scholar]
- Klimešová J, Klimeš L. Resprouting of herbs in disturbed habitats: is it adequately described by Bellingham-Sparrow's model? Oikos. 2003;103:225–229. [Google Scholar]
- Klimešová J, Martínková J. Intermediate growth forms as a model for the study of plant clonality functioning: an example with root sprouters. Evolutionary Ecology. 2004;18:669–681. [Google Scholar]
- Knox KJE, Clarke PJ. Nutrient availability induces contrasting allocation and starch formation in resprouting and obligate seeding shrubs. Functional Ecology. 2005;19:690–698. [Google Scholar]
- Koop H. Vegetative reproduction of trees in some European natural forest. Vegetatio. 1987;72:103–110. [Google Scholar]
- Lev-Yadun S, Ne'eman G. When may green plants be aposematic? Biological Journal of the Linnean Society. 2004;81:413–416. [Google Scholar]
- López-Moreno I. Disponibilidad de recursos hídricos y gestión de los embalses en el Pirineo central español: interacciones entre variabilidad espacio-temporal de los recursos y el uso del agua bajo condiciones de cambio ambiental. University of Zaragoza; 2005. PhD Thesis. [Google Scholar]
- Margaris NS, Vokou D. Structural and physiological features of woody plants in phryganic ecosystems related to adaptive mechanisms. Ecologia Mediterranea. 1982;8:449–459. [Google Scholar]
- Midgley JJ. Why the world's vegetation is not totally dominated by resprouting plants; because resprouters are shorter than reseeders. Ecography. 1996;19:92–95. [Google Scholar]
- Mitrakos KA. A theory for Mediterranean plant life. Acta Oecologica. 1980;1:245–252. [Google Scholar]
- Montserrat-Recoder P, Montserrat-Martí JM, Montserrat-Martí G. Estudio de las comunidades de Echinospartum horridum en el Pirineo español. Acta Biologica Montana. 1984;4:249–257. [Google Scholar]
- Naveh Z. Evolutionary significance of fire in the Mediterranean region. Vegetatio. 1975;29:199–208. [Google Scholar]
- Orshan G. Surface reduction and its significance as a hydroecological factor. Journal of Ecology. 1954;42:442–444. [Google Scholar]
- Orshan G. Plant pheno-morphological studies in Mediterranean type ecosystems. Dordrecht: Kluwer Academic Publisher; 1989. [Google Scholar]
- Palacio S, Millard P, Montserrat-Martí G. Aboveground biomass allocation patterns within Mediterranean sub-shrubs: a quantitative analysis of seasonal dimorphism. Flora. 2006;201:612–622. [Google Scholar]
- Palacio S, Maestro M, Montserrat-Martí G. Seasonal dynamics of non-structural carbohydrates in two species of Mediterranean sub-shrubs with different leaf phenology. Environmental and Experimental Botany. (a) 2007;59:34–42. [Google Scholar]
- Palacio S, Millard P, Maestro M, Montserrat-Martí G. Non-structural carbohydrates and nitrogen dynamics in Mediterranean sub-shrubs: an analysis of the functional role of overwintering leaves. Plant Biology. (b) 2007;9:49–58. doi: 10.1055/s-2006-924224. [DOI] [PubMed] [Google Scholar]
- Pate JS, Dixon KW, Orshan G. Growth and life form characteristics of Kwongan species. In: Pate JS, Beard JS, editors. Kwongan plant life of the sandplain. Nedlands, Australia: University of Western Australia Press; 1984. pp. 84–100. [Google Scholar]
- Pate JS, Froend RH, Bowen BJ, Hansen A, Kuo J. Seedling growth and storage characteristics of seeder and resprouter species of Mediterranean-type ecosystems of SW Australia. Annals of Botany. 1990;65:585–601. [Google Scholar]
- Pausas JG, Keeley JE, Verdu M. Inferring differential evolutionary processes of plant persistence traits in northern hemisphere Mediterranean fire-prone ecosystems. Journal of Ecology. 2006;94:31–39. [Google Scholar]
- Porta-Casanellas J, Lopez-Acebedo M, Rodriguez Ochoa M. Técnicas y experimentos en edafología. Barcelona: Col.legi Oficial d'Enginyers Agrònoms de Catalunya; 1986. [Google Scholar]
- Purvis A, Rambaut A. Comparative analysis by independent contrasts (CAIC): an Apple Macintosh application for analysing comparative data. CABIOS. 1995;11:247–251. doi: 10.1093/bioinformatics/11.3.247. [DOI] [PubMed] [Google Scholar]
- Richards JH, Caldwell MM. Soluble carbohydrates, concurrent photosyntesis and efficiency in regrowth following defoliation: a field study with Agropyron species. Journal of Applied Ecology. 1985;22:907–920. [Google Scholar]
- Riera J. Efectes de l'incendi de 1994 sobre la vegetació del Garraf: estat actual de la regeneració. Barcelona: Diputació de Barcelona; 2000. [Google Scholar]
- Rivas-Martínez S. Memoria del mapa de series de vegetación de España. Madrid: Ministerio de Agricultura, Pesca y Alimentación. ICONA; 1987. [Google Scholar]
- Sachs T. Developmental processes and the evolution of plant clonality. Evolutionary Ecology. 2002;15:485–500. [Google Scholar]
- Sakai A, Sakai S. A test for the resource remobilization hypothesis: tree sprouting using carbohydrates from above-ground parts. Annals of Botany. 1998;82:213–216. [Google Scholar]
- Sakai A, Sakai S, Akiyama F. Do sprouting tree species on erosion-prone sites carry large reserves of resources? Annals of Botany. 1997;79:625–630. [Google Scholar]
- Soltis DE, Soltis PS, Chase MW, Mort ME, Albach DC, Zanis M, et al. Angiosperm phylogeny inferred from 18S rDNA, rbcL, and atpB sequences. Botanical Journal of the Linnean Society. 2000;133:330–336. [Google Scholar]
- Tousignant D, Richer C, Rioux JA, Brassard N, Mottard JP. Vegetative propagation of sugar maple: relating stem water content and terminal bud developmental stage to adventitious rooting of stem cuttings. Canadian Journal of Plant Science. 2003;83:859–867. [Google Scholar]
- Van't Hoff J. Control of cell progression through the mitotic cycle by carbohydrate provision. Journal of Cell Biology. 1968;37:773–778. doi: 10.1083/jcb.37.3.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veierskov B. Relations between carbohydrates and adventitious root formation. In: Davis TD, Haissig BE, Sankhla N, editors. Adventitious root formation in cuttings. Portland, OR: Dioscorides Press; 1988. pp. 70–78. [Google Scholar]
- Verdaguer D, Ojeda F. Root starch storage and allocation patterns in seeder and resprouter seedlings of two Cape Erica (Ericaceae) species. American Journal of Botany. 2002;89:1189–1196. doi: 10.3732/ajb.89.8.1189. [DOI] [PubMed] [Google Scholar]
- Vesk PA. Plant size and resprouting ability: trading tolerance and avoidance of damage? Journal of Ecology. 2006;94:1027–1034. [Google Scholar]
- Vesk PA, Westoby M. Funding the bud bank: a review of the costs of buds. Oikos. 2004;106:200–208. [Google Scholar]
- Vesk PA, Warton DI, Westoby M. Sprouting by semi-arid plants: testing a dichotomy and predictive traits. Oikos. 2004;107:72–89. [Google Scholar]
- Villar Salvador P. Estrategias ecológicas y funcionales del xilema en plantas leñosas mediterráneas. University of Valencia, Spain; 2000. PhD Thesis. [Google Scholar]
- Walkley A, Black IA. An examination of the Degtjareff method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Science. 1934;37:29–38. [Google Scholar]
- Wells PV. The relation between mode of reproduction and extent of speciation in woody genera of the California chaparral. Evolution. 1968;23:264–267. doi: 10.1111/j.1558-5646.1969.tb03510.x. [DOI] [PubMed] [Google Scholar]
- Wojciechowski MF, Lavin M, Sanderson MJ. A phylogeny of legumes (Leguminosae) based on analyses of the plastic matKgene resolves many well-supported subclades within the family. American Journal of Botany. 2004;91:1846–1862. doi: 10.3732/ajb.91.11.1846. [DOI] [PubMed] [Google Scholar]
- Zar JH. Biostatistical analysis. Upper Saddle River, NJ: Prentice-Hall International; 1984. [Google Scholar]


