Abstract
Background
There is widespread recognition of the potential promise of vaginal microbicides as a tool to combat global HIV/AIDS and STI epidemics, and candidate product development has maintained a rapid pace in recent years; however, rectal microbicide development has received less attention. As it is likely that commercial products developed for vaginal use will also be used rectally, there is a clear need to assess the safety and efficacy of candidate microbicide products specifically in the rectal compartment.
Methods
We have developed a standardized protocol for preclinical rectal safety and (chlamydial) efficacy assessment of topical microbicide candidates in a non-human primate model. We evaluated a total of twelve test compounds for rectal safety (via rectal pH, microflora, and rectal lavage) and one compound for efficacy against rectal chlamydial infection.
Results
In this paper, we describe our methods in detail and summarize our results, particularly noting the ability of our model to distinguish products with deleterious effects on the rectal environment. We also outline the specific criteria used to recommend products move into preclinical rectal efficacy trials or be recommended for reformulation to the product developer. In sum, we observed significant adverse effects in two products. The single product that underwent efficacy evaluation was not observed to be protective against rectal chlamydial infection.
Conclusions
A preclinical safety and efficacy model is critical to promoting rectal microbicide development, which will ultimately offer a significant opportunity for intervention in the global HIV/AIDS epidemic.
Keywords: topical microbicides, rectal compartment, Chlamydia trachomatis
Introduction
The development of topical microbicides represents an important opportunity and a major source of hope in the global fight against HIV/AIDS and other sexually transmitted infections (STIs). A microbicide product that is safe, effective, and inexpensive has the potential to significantly reduce global transmission of STIs. While most global microbicide development has focused on vaginal use, development of products for rectal use has lagged behind for complex reasons, and it is not immediately obvious that products developed for vaginal use will necessarily be safe and effective when used rectally. In light of the facts that the epidemic of HIV/AIDS continues to disproportionately affect men who have sex with men (MSM) in both developed, and increasingly in many developing countries[1–3] and that women of many cultures report practicing receptive anal intercourse (RAI)[4], the advancement of microbicides assessed specifically for rectal use is critically needed.
The U.S. Food and Drug Administration (FDA) requires that safety, and whenever possible, in vivo activity, of candidate topical microbicide products be evaluated in animals prior to their use in humans. Preclinical animal studies are particularly useful in that both acute and cumulative effects of product exposures can be assessed, and to a certain extent the behavior of the test subjects can be controlled (e.g. timed product administration, abstinence from intercourse). The standardized nature of our particular animal studies allows for findings from any single test product to be compared to those of another. The main purpose of these studies is to provide evidence which helps to move promising products forward in development, and perhaps more importantly, to recommend reformulation for products which are observed to cause significant, deleterious changes or toxicity to product-exposed tissues.
Pigtailed Macaque Model Background
Our laboratory was founded on the establishment of a macaque model for Chlamydia trachomatis (Ct) infection, which has been used to further our understanding of the pathogenesis of experimentally induced Chlamydia trachomatis cervicitis and salpingitis since the mid-1980s.[5–9] This model was developed in the pigtailed macaque (Macaca nemestrina) for several reasons, including the facts that this macaque is subject to year round hormonal changes (unlike the rhesus macaque, M. mulatta, which is a seasonal breeder), they experience a 28-day menstrual cycle very similar to that of women; they have pronounced tumescence of perineal tissues which allow for visual estimation of the female’s current stage of menstrual cycle; the female reproductive tract tissues are physically similar to humans; pigtailed macaques are ideally sized animals, i.e. smaller than baboons for example (making them easier to work with on a daily basis for research exams), yet larger than M.fascicularis (whose vaginal anatomy is small enough to make vaginal and cervical exams rather difficult)[10]; this macaque is naturally susceptible to infection with human isolates of Ct, with no need to pretreat the animals or the pathogen; and the Washington National Primate Research Center (WaNPRC) primarily houses Macaca nemestrina.
With the advent of topical microbicide development efforts, we expanded the Ct model to further describe the normal vaginal and eventually rectal environments of the pigtailed macaque, as compared to those of humans. [11],[12] As the anatomy, microbiologic constituents and, to a lesser degree pH, have been shown to model those of humans, we have continued to use the M. nemestrina model to study safety issues of repeated daily use of topical microbicide test agents. Because chlamydial infections are very common and are associated with increased risk of acquiring HIV, we have also assessed the efficacy of certain test products to prevent both cervically and rectally acquired chlamydial infection.
This paper summarizes the preclinical rectal microbicide candidate safety and efficacy studies we have completed in the pigtailed macaque model.
Materials and Methods
Test Products
Candidate topical microbicide formulations were provided by product developers; in some cases, developers also provided matched placebo gels for comparison (i.e., formulations identical to the product except for the active compound). Products with acceptable vaginal safety profiles as previously evaluated by our laboratory were eligible for rectal safety evaluations at the discretion of the developer.
A total twelve candidate products were evaluated for rectal safety and one for rectal chlamydial efficacy.
Animals
Sexually mature Macaca nemestrina were obtained from a colony of animals at the Washington National Primate Research Center (WaNPRC); both male and female animals were eligible for rectal studies. Prior approval for use of monkeys in this protocol was obtained from the Institutional Animal Care and Use Committee at the University of Washington. Animals were handled humanely, and experiments were performed within the National Institutes of Health’s animal use guidelines. In the event that the results of a safety study indicated significant adverse findings related to a test product, repeat experiments could be conducted with additional macaques in order to increase the overall study size and clarify the findings. Every animal was allowed at least two weeks of recovery time between studies. All animal exams and research procedures were conducted under ketamine sedation.
Rectal Safety Study Design
An initial rectal safety study was performed with Conceptrol, a 4% nonoxynol-9 formulation available over the counter for vaginal use as a spermicide; results of this study have been published.[13] The experimental protocol for rectal lavage collection has since been modified to minimize the potential for mechanically induced tissue disruption. All of the studies discussed in this paper followed the modified protocol, which includes animal positioning and standardized insertion and retraction of the lavage collection tubing.
Because each product developer and funding source had slightly different requirements, the study designs and protocols vary somewhat for each product. In general, a pool of macaques was kept available for random enrollment into specific studies. For each safety evaluation, six to eight animals were randomly selected for each study arm; the total number of study arms was dependent on the number of products being tested, but most often involved two to three arms: a test product being compared to a placebo formulation and/or to no product application at all. Placebo formulations, when assessed, were either product-specific or hydroxyethylcellulose (HEC) universal placebo.[14]
All but one study followed a crossover design, wherein each animal controlled for itself by completing all arms of the safety study in random order. In a simple two-arm study with six animals for example, three animals undergo the first experiment in the test product arm, while the other three make up the negative control (placebo or no product) arm. After two to three weeks of recovery time, the same animals complete the experiment once again, but cross over to the other study arm. Given the small number of animals available and the expectation that individual animals would show some degree of correlation in their results over time, a crossover design is not only practical but also statistically efficient. The combination gel studies were halted prematurely, due to the degree of tissue irritation we observed. Therefore, a simple independent study design resulted, with different animals used in product and placebo groups with no crossover.
According to study protocol, 2.5 ml of product or placebo gel was applied intrarectally on days 1–3, with day 4 being follow-up [Table 1]. For three product studies (0.1% UC781, 1.0% UC781, and ComboGel), product application was extended through day 4, and day 5 was considered follow-up.
Table 1.
Rectal safety study design.
| Day 1 | Day 2 | Day 3 | Follow-Up | ||||
|---|---|---|---|---|---|---|---|
| T-0* | T-30 | T-0 | T-30 | T-0 | T-30 | Day 4** | |
| Microbiology | X | X | X | X | |||
| Rectal pH | X | X | X | X | X | X | X |
| Rectal Lavage | X | X | X | X | X | X | X |
After each T-0 sampling, 2.5 ml product or placebo gel was applied intrarectally.
For later studies, product application continued through day 4, and follow-up samples were collected on day 5.
Safety measures
All rectal safety studies assessed rectal pH, microbiologic constituents and components of rectal lavage samples before and after daily exposures to test gels. We consider these measures to be indicators of general health of the rectal environment which can be transiently affected by topical product exposure. Variations in these measures can be easily captured from samples collected shortly after (15 or 30 minutes) product exposure. Importantly, each of these measures provide information which may have significant impact on pathogen transmission: pH may affect the pathogen’s ability to infect tissues; an imbalance in normal rectal flora may affect innate protection against introduced pathogens; and evidence of fresh epithelium and/or blood in rectal lavages indicate a potential break in the epithelial integrity which would provide easy access for invading pathogens.
Rectal pH was ascertained daily at baseline (time 0) and 15 or 30 minutes post-gel application and at follow-up by collection of a rectal swab, and measured in increments of 0.5 pH units. Samples were collected by rolling a Dacron-tipped swab against the wall of the rectum, approximately 3cm beyond the anal sphincter, then dabbing the end of the swab onto a pH (ColorpHast ®) indicator strip. After color change has ceased with the strip still moist, the pH is determined by matching the color block on the strip to the color provided standard. The normal range of rectal pH values in the Macaca nemestrina model has been previously established as 6.0–7.5 (95% confidence interval).[12]
To evaluate rectal microbiology, a swab was collected each day at baseline (T0) and placed in Port-a-Cul tube, which is specifically designed for transport of anaerobic, facultative, and aerobic organisms on swabs; this device has been shown to preserve viability of bacteria in clinical specimens.[15] All samples were shipped on ice overnight to the Reproductive Infectious Disease Laboratory at Magee-Womens Research Institute (Pittsburgh, PA) for analysis. The presence and quantity of each microbe was reported on a semi-quantitative scale (0/1+/2+/3+/4+), where numbers represent the quadrants of organism growth noted on appropriate agar plate.
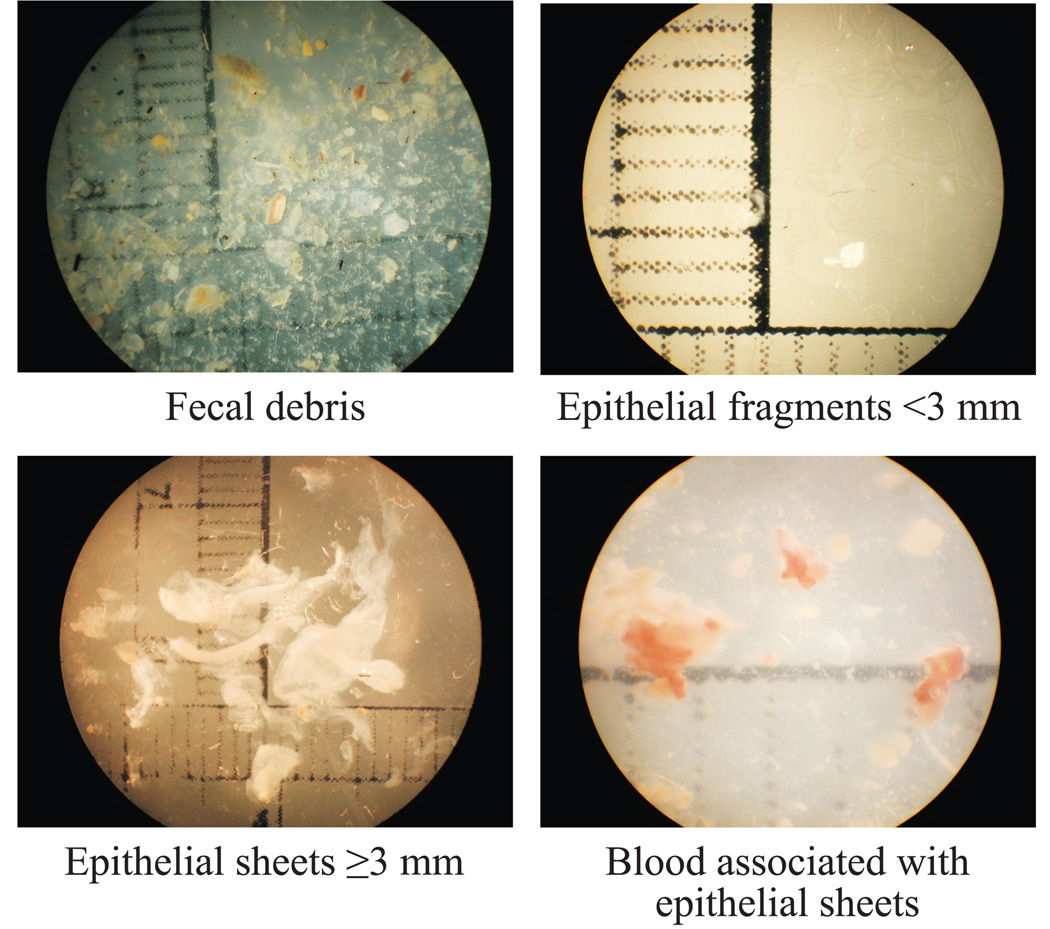
Rectal lavage was performed daily at baseline (T0) and 15 or 30 minutes post-gel application (depending on project-specific protocol) and at follow-up (24 hours after the final product exposure) in order to assess gross tissue disruption. The lavage sample was obtained by inserting an infant feeding tube attached to a 10 ml syringe, approximately 3–5 cm beyond the anal sphincter into the rectum of an animal positioned on a 30° angled ramp and expelling 8 ml of saline wash, which was then gently recovered after twenty seconds and stored for analysis. Within a few hours of collection, lavage samples were examined under a dissecting microscope at 7X magnification for evidence of fecal matter, cellular debris, epithelial desquamation (epithelial sheets ≥3mm in any dimension) and blood associated with the epithelial sheets. Epithelial sheets were measured, and the lavage samples were photographed for documentation. Examples of rectal lavage findings, including fecal debris, epithelial sheets and blood are displayed in Figure 1.
Figure 1.
Cytokine and blood (pharmacokinetic) analyses were conducted in two product safety evaluations (0.1% and 1.0% UC781); these methods and results have been described in detail elsewhere.[16]
Product development criteria
Product development guidelines for rectal safety studies were established to allow for forward movement or recommended reformulation of a topical microbicide product [Table 2]. Product development was recommended to continue if the standardized macaque safety study indicated (1) rectal microflora remained stable, (2) pH remained stable or changed only transiently, and (3) there was no evidence of gross tissue damage such as significant epithelial desquamation or the presence of blood in rectal lavage samples. Reformulation was recommended if any of the following were observed: (1) presence of grossly identifiable blood/red blood cells, (2) epithelial sheets ≥3mm in any dimension were noted in quantities greatly exceeding that of the control arm, (3) persistent shift in pH with no documentation of rectal pH returning to baseline levels, (4) profound decrease (transient or permanent suppression to zero-level) in H202 producing microorganisms, (5) increase (by two steps or more on the semi-quantitavie scale) in pathogenic organisms, or (6) suppression of microflora.
Table 2.
Product development criteria.
| Safety Profile | ACCEPTABLE | UNACCEPTABLE |
|---|---|---|
| Recommendation | Efficacy studies | Reformulation |
| Rectal Lavage | •Epithelial sheets <3mm in all dimensions | •Increased presence of epithelial sheets ≥3mm in any dimension, in majority of test animals |
| •Presence of blood | ||
| Microbiology | •Preserved H202 producing flora •Transient shifts in other flora |
•Increase in pathogenic organisms |
| •Decreased H202 production | ||
| •All flora suppressed | ||
| Rectal pH | •Unchanged or transient shift | •Persistent shift |
Experimental Efficacy Protocol: Rectal chlamydia challenge
After completion of a rectal safety study with acceptable results, product developers were offered the option of having their products continue on to efficacy testing against rectal chlamydial infection. However, as many of the products have been designed to have antiviral and specifically anti-HIV activity, chlamydial efficacy testing was not always relevant, and most developers chose not to submit their products for further testing. To date, one product (BufferGel) has completed rectal chlamydial efficacy testing.
Eight animals were randomly assigned to test product (n=5) or placebo (n=3) groups. Baseline culture and nucleic acid amplification test (NAAT) samples were collected from all animals. Test product animals then received 2.5ml of BufferGel intrarectally. Fifteen minutes later, all animals received a single rectal inoculation of C. trachomatis.
The University of Washington Chlamydia Reference Laboratory provided the clinical rectal isolate of C. trachomatis strain D for these studies. The chlamydial isolate was prepared in McCoy cell culture and purified by renografin methylglucamine diatrizonate linear gradient column. The stock inocula were titered (1.0–1.3 × 107 IFU/ml), in sucrose-phosphate-glutamate buffer (SPG) and frozen (− 70°) until dilutions were prepared for experimental inoculations. For inoculation, a frozen aliquot of chlamydia was thawed and diluted to 1–5 × 105 inclusion forming units (IFU) per milliliter just prior to use, and administered rectally with a 1-cc tuberculin syringe inserted just past the anal sphincter.
Chlamydia trachomatis Detection Assays
Rectal specimens were collected with Dacron-tipped swabs 3–4 cm beyond the anal sphincter on days 2, 7, 14, and 21 post-inoculation. All samples were transported to the University of Washington Chlamydia Reference Laboratory for assessment.
Cell culture
Rectal swab specimens were cultured on cycloheximide-treated McCoy cells in 96-well microtiter plates[17] and stained with a monoclonal antibody specific for C. trachomatis detection.
GenProbe APTIMA Combo 2
Rectal swab specimens were collected, stored, and transported to the laboratory and assessed according to the manufacturer’s instructions (Gen-Probe, Inc., San Diego, CA).[18] The APTIMA Combo 2 assay employs the amplification technology of transcription-mediated amplification (TMA), in which the RNA target molecule from C. trachomatis is isolated and specific regions are amplified by using a separate capture oligomer and a unique set of primers for the target. This test uses TMA to detect a specific C. trachomatis 23S ribosomal RNA target.
Serology
Serum IgG and IgM chlamydial antibody titers were measured using the micro-immunofluorescence technique.[19]
Results
Safety Evaluations
Rectal pH and microbiologic constituents underwent transient fluctuations in each study, but values generally rebounded by 24 hours after each gel administration. No product application was associated with a persistent shift in pH or a change in level of any microflora species.
Rectal lavage assessments provided the most provocative information regarding tissue responses to daily perturbations with candidate products. In the macaque model, it is not rare for rectal lavage samples to contain evidence of shed epithelium. Approximately 90% of baseline rectal lavage samples included small clusters (<3mm) of epithelial cells, and 23% of all baseline lavage samples contained epithelial sheets measuring at least 3mm in one dimension. At later timepoints, shedding of epithelial sheets was frequently noted in rectal lavage samples, whether animals had been exposed to test product or not. In fact, every test arm of every study included at least one animal with epithelial sheets noted in rectal lavage samples. The presence of blood associated with these epithelial sheets is believed to be indicative of an adverse tissue response.
In a preliminary rectal safety study using a different protocol, we previously reported adverse effects of rectal nonoxynol-9 exposure including significant epithelial desquamation with blood and stroma observed in rectal lavage samples.[13] However, when nonoxynol-9 was re-tested under the current protocol (with efforts to reduce trauma due to mechanical abrasion during the collection of lavage sample), blood and stroma were no longer common components of the lavage samples, while epithelial sloughing was still apparent. Using the revised protocol, nonoxynol-9 resulted in a less caustic safety profile after three daily exposures.
To date, one test product has been shown to cause unacceptable irritation with daily rectal usage. This study assessed two similar formulations of a combination product, but was halted prior to completion due to the detrimental findings. Both formulations resulted in epithelial desquamation, with evidence of stroma and blood present in rectal lavage samples, as described below.
Combination gel studies
We investigated the safety profile of two SPL7013 dendrimer combination formulations (Combo+ and Combo−, provided by Starpharma) after repeated rectal application. The individual components of these gels (formulated 7013 dendrimer and BufferGel) had each been determined to have acceptable safety profiles as stand-alone products in rectal studies conducted in this model. Additionally, freshly prepared lots of these combination formulations had previously completed vaginal safety studies in the macaque model, with favorable results. The combination test formulations were compared to the HEC gel (as placebo) and to no product use in the rectal safety model. Significant adverse effects including presence of blood in rectal lavage samples collected 30-minutes after exposure, were noted after rectal use of each of the combination formulations. No such findings were noted in the HEC gel or no product arms of these studies. These adverse effects, noted in 2 of 2 animals that had received each formulation (four animals total), led us to stop the rectal safety study before completing test arms of n=6 per product. In an attempt to determine potential causes of the rectal toxicity, the original test formulation was reassayed and found to contain a dendrimer degradant. Therefore, safety studies were conducted using a freshly prepared combination formulation, and formulations with two concentrations of the dendrimer degradant in acid buffering gel without dendrimer. However, none of these gels resulted in toxicity similar to that noted in the original rectal safety assessment. The combination microbicide gels showed a differential toxicity profile compared with either standalone product. This study highlights the importance of testing the safety of combination products rather than inferring safety by individual assessments of active components. Importantly, this study has confirmed that rectal lavage assessments are sufficiently sensitive to identify product toxicity in our macaque model.
Efficacy Study
BufferGel application prior to C. trachomatis inoculation was not observed to be protective against rectal chlamydial infection, as discussed in detail elsewhere.[20] All five animals in the test product (BufferGel) group tested positive by NAAT on days 2–21 and by culture on day 7 (3 of 5 animals) and day 14 (5 of 5 animals), and all remained positive thereafter. All three placebo animals also established rectal chlamydial infection, testing positive by NAAT only on day 2 and by NAAT and culture on day 7 and thereafter. Serologic assays documented humoral chlamydial antibody in 2 of 5 test animals and in 1 of 3 control animals.
Discussion
We developed a pigtailed macaque model in order to be able to comprehensively evaluate the safety and efficacy of topical microbicide candidate products in the rectal compartment. Our initial efforts toward developing this model were instigated by David Phillips’ early work in a small number of human subjects, which indicated that rectal use of a nonoxynol-9 lubricating product caused epithelial cells to be sloughed in rectal lavage samples.[21] In this study, as well as in a larger follow up study, no epithelium was noted in baseline lavage samples, nor in rectal lavage samples collected after exposure to products that do not contain nonoxynol-9.[22] Dr. Phillips’ studies used naked eye and electron microscopy assessments of lavage samples. While our initial macaque studies conducted with nonoxynol-9 product did result in severe adverse findings, specifically bloody lavage samples with numerous sheets of exfoliated epithelium in 5 of 8 macaques, we now recognize that some of the damage noted may have been caused by mechanical trauma associated with our lavage collection technique. In this original study, sheets of epithelium and bloody lavage samples were also noted, though to a lesser extent, in the placebo gel and no product arms (2 of 8 macaques and 3 of 8 macaques, respectively). We have since refined our lavage collection procedure to revise the placement of the catheter through which the lavage fluid is expelled and captured. As a result, we have documented fewer findings of bloody lavage samples, though we still document the presence of exfoliated epithelium in samples from each arm of every study. Using this new technique, we reassessed the nonoxynol-9 product with far more subtle results. In an attempt to distinguish “normal” epithelial shedding (that noted at baseline and in no product arms), from “notable” epithelial shedding, we have devised several approaches to describing the content of rectal lavage samples. For each of the studies described in this paper, lavage samples were assessed in the manner described in the Methods section. Study results were summarized by comparing lavage contents (most notably shed epithelial sheets measuring at least 3mm on one dimension) for each study arm. By this method, most products did not generate adverse safety profiles.
In total, twelve products underwent rectal safety evaluations and one was additionally tested for efficacy against rectal chlamydial infection. These safety investigations identified two formulations of one product that showed severe deleterious effects on the rectal environment (combination formulations of SPL7013 and BufferGel).
Because the rectal compartment is both anatomically and histologically distinct from the vaginal compartment, it follows that safety (and/or efficacy) in one compartment does not imply similar results in the other. Specifically, it may be that the rectal compartment is more sensitive to deleterious effects of candidate products due to differences in the mucosal layer thickness. The rectal lining is a single cell layer of columnar epithelium covering the luminal surface and lining the crypt-like folds; by contrast, the mucosal lining of the vaginal and ectocervix consists of approximately 35 – 40 layers of stratified squamous epithelium.[23] Due to the fragile nature of the rectal compartment, it is likely more vulnerable to irritation, sloughing, and infections.
In light of recent concerns regarding the safety of topical microbicides in international clinical trials, the importance of comprehensive preclinical safety investigations is especially clear. Prevention methods like microbicide products offer a critical opportunity in the fight against HIV and other STIs globally, but such products must first undergo rigorous safety and efficacy testing. Preclinical evaluations in an animal model such as ours can provide significantly more detailed information obtained under standardized laboratory conditions, offering early warning of products that may have harmful effects and helping to usher promising products into clinical trials more quickly.
However, it is also important to bear in mind that preclinical testing necessarily is limited by the small numbers of animals involved, and we cannot always detect rare adverse events or safety problems which may arise when larger clinical trials are conducted. Thus, our overall goal is not to definitively establish whether a product is safe or not, but rather to detect any major toxicities and recommend reformulation before the product enters clinical testing. When toxicity problems are observed, we may be able to conduct further testing and isolate the specific component of the product which is responsible for the deleterious effects, information which can be very useful for the developer in terms of reformulation. This learning and refinement process is critical to future product development. Extensive preclinical testing is an efficient way to promote microbicide research while also ensuring that obviously harmful products do not inadvertently move forward into clinical trials.
Table 3.
Rectal microbicide candidate safety evaluations.
| Safety Profile | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Test compound |
Supplier | N | Days of Product Application |
Formulation | Overall | Microflora | pH | Lavage | |
| BufferGel®15 | ReProtect, Inc. | 6 | 3 | gel | Acceptable | Within normal ranges/no significant shifts. | Transient decreased pH. | Slightly increased incidence of epithelial desquamation. | |
| Nonoxynol-9/Conceptrol7 | Advanced Care Products | 8 | 3 | 4.0% gel | Acceptable | Within normal ranges/no significant shifts | Transient increased pH, within normal range. | No differences in epithelial sheets or blood | |
| C31G18 | CONRAD | 6 | 3 | 1.0% gel | Acceptable | Transient decrease in anaerobic GNRs, rebounded by follow-up. | Increased pH, but within normal range. | No significant differences in prevalence of epithelial sheets by group at any time point. | |
| Liposome-encapsulated octylglycerol (OG)20 | Magee-Womens Research Institute (MWRI) | 6 | 3 | 1% gel | Acceptable | Within normal ranges. | Transient fluctuations, but within normal range. | No differences in epithelial sheets or blood | |
| 3-Octylglycerol (3-OG) | MWRI | 8 | 3 | 0.5% | Acceptable | Within normal ranges. | Within normal range. | No differences in epithelial sheets or blood | |
| ZA-SEO-1065A (Polystyrene sulfate) | CONRAD | 6 | 3 | 10.0% | Acceptable | Within normal ranges | Transient increase in pH in both groups. | No differences in epithelial sheets or blood | |
| ZA-SEO-1066A (Cellulose sulfate) | CONRAD | 6 | 3 | 6.0% | Acceptable | Within normal ranges | Transient increase in pH in both groups. | Some minor evidence of increased epithelial shedding, only observed in two animals. | |
| SPL7013 Dendrimer17 | Starpharma Ltd. | 8 | 3 | 3.0% gel | Acceptable | Within normal ranges/no significant shifts | Within normal range/not different than placebo | No significant differences in presence of epithelial sheets or blood | |
| Carraguard19 | Population Council | 6 | 3 | gel | Acceptable | Within normal ranges/no significant differences. | Transient increase in pH in both groups. | No differences in epithelial sheets or blood | |
| UC-78111 | CONRAD | 6 | 4 | 0.1% gel | Acceptable | Within normal ranges/no significant differences by group. | Transient increased pH. | No differences in epithelial sheets or blood | |
| CONRAD | 6 | 4 | 1.0% gel | Increased expression of numerous cytokines. | Within normal ranges/no significant differences by group. | Transient increased pH. | No differences in epithelial sheets or blood.. | ||
| Combo (SPL 7013 + BufferGel®+ LA + AA) | Starpharma Ltd. | 2* | 4 | 3.0% | Unacceptable | Transient suppression of black GNRs. | Transient decrease in pH, rebounded by 24 hours. | Presence of blood and epithelial sheets. Degradation of epithelial sheets noted. | |
Study halted early after only two animals had completed follow-up due to adverse findings (see results and discussion)
Acknowledgements
This work was supported by grants P01-AI-39061, U19 AI 051661, U19 AI 060598, and WaNPRC RR00166. The contents of this work are solely the responsibility of the authors and do not necessarily represent the official views of the Division of AIDS.
REFERENCES
- 1.Jaffe HW, VR, De Cock KM. The reemerging HIV/AIDS epidemic in men who have sex with men. JAMA. 2007;298:2412–2412. doi: 10.1001/jama.298.20.2412. [DOI] [PubMed] [Google Scholar]
- 2.CDC. Subpopulation Estimates from the HIV Incidence Surveillance System—United States, 2006. MMWR. 2008;57(36):985–989. [PubMed] [Google Scholar]
- 3.Baral S, Sifakis F, Cleghorn F, et al. Elevated risk for HIV infection among men who have sex with men in low- and middle-income countries 2000–2006: A systematic review. PLoS Med. 2007;4(12):e339. doi: 10.1371/journal.pmed.0040339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koblin BA, Hoover DR, Guozhen Xu, et al. Correlates of Anal Intercourse Vary by Partner Type Among Substance-Using Women: Baseline Data from the UNITY Study. AIDS and Behavior. 2008 doi: 10.1007/s10461-008-9440-y. (epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 5.Patton DL, Halbert SA, Kuo CC, et al. Host response to primary Chlamydia trachomatis infection of the fallopian tube in pig-tailed monkeys. Fertil Steril. 1983;40(6):829–840. [PubMed] [Google Scholar]
- 6.Patton DL, Kuo CC, Wang SP, et al. Chlamydial infection of subcutaneous fimbrial transplants in cynomolgus and rhesus monkeys. J Infect Dis. 1987;155(2):229–235. doi: 10.1093/infdis/155.2.229. [DOI] [PubMed] [Google Scholar]
- 7.Patton DL, Kuo CC, Wang SP, et al. Distal tubal obstruction induced by repeated Chlamydia trachomatis salpingeal infections in pig-tailed macaques. J Infect Dis. 1987;155(6):1292–1299. doi: 10.1093/infdis/155.6.1292. [DOI] [PubMed] [Google Scholar]
- 8.Patton DL. Immunopathology and histopathology of experimental chlamydial salpingitis. Rev Infect Dis. 1985;7(6):746–753. doi: 10.1093/clinids/7.6.746. [DOI] [PubMed] [Google Scholar]
- 9.Patton DL, Kuo CC. Histopathology of Chlamydia trachomatis salpingitis after primary and repeated reinfections in the monkey subcutaneous pocket model. J Reprod Fertil. 1989;85(2):647–656. doi: 10.1530/jrf.0.0850647. [DOI] [PubMed] [Google Scholar]
- 10.Patton DL, Sweeney YC, Tsai CC, et al. Macaca fascicularis vs. Macaca nemestrina as a model for topical microbicide safety studies. J Med Primatol. 2004;33(2):105–108. doi: 10.1111/j.1600-0684.2004.00059.x. [DOI] [PubMed] [Google Scholar]
- 11.Patton DL, Sweeney Y, Rabe LK, et al. The vaginal microflora of pig-tailed macaques and the effects of chlorhexidine and benzalkonium on this ecosystem. Sex Transm Dis. 1996;23:489–493. doi: 10.1097/00007435-199611000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Patton DL, Cosgrove Sweeney YT, Rabe LK, et al. The pig-tailed macaque rectal model: microflora and chlamydial infection. Sex Transm Dis. 2001;28(7):363–366. doi: 10.1097/00007435-200107000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Patton DL, Cosgrove Sweeney YT, Rabe LK, et al. Rectal applications of nonoxynol-9 cause tissue disruption in a monkey model. Sex Transm Dis. 2002;29(10):581–587. doi: 10.1097/00007435-200210000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Tien D, Schnaare RL, Kang F, et al. In vitro and in vivo characterization of a potential universal placebo designed for use in vaginal microbicide clinical trials. AIDS Res Hum Retroviruses. 2005;21(10):845–853. doi: 10.1089/aid.2005.21.845. [DOI] [PubMed] [Google Scholar]
- 15.Baron EJ, Strong CA, McTeague M, et al. Survival of anaerobes in original specimens transported by overnight mail services. Clin Infect Dis. 1995;20 Suppl 2:S174–S177. doi: 10.1093/clinids/20.supplement_2.s174. [DOI] [PubMed] [Google Scholar]
- 16.Patton DL, Cosgrove Sweeney YT, Balkus JE, et al. Preclinical Safety Assessments of UC781 Anti-Human Immunodeficiency Virus Topical Microbicide Formulations. Antimicrob Agents Chemother. 2007;51(5):1608–1615. doi: 10.1128/AAC.00984-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stamm WE, Tam M, Koester M, et al. Detection of Chlamydia trachomatis inclusions in McCoy cell cultures with fluorescein-conjugated monoclonal antibodies. J Clin Microbiol. 1983;17(4):666–668. doi: 10.1128/jcm.17.4.666-668.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gaydos CA, Quinn TC, Willis D, et al. Performance of the APTIMA Combo 2 assay for detection of Chlamydia trachomatis and Neisseria gonorrhoeae in female urine and endocervical swab specimens. J Clin Microbiol. 2003;41(1):304–309. doi: 10.1128/JCM.41.1.304-309.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang SP, Grayston JT, Alexander ER, et al. Simplified micro-immunofluorescence test with trachoma lymphogranuloma venereum (Chlamydia trachomatis) antigens for use as a screening test for antibody. J Clin Microbiol. 1975;1:250. doi: 10.1128/jcm.1.3.250-255.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patton DL, Sweeney YC, Cummings PK, et al. Safety and efficacy evaluations for vaginal and rectal use of BufferGel in the macaque model. Sex Transm Dis. 2004;31(5):290–296. doi: 10.1097/01.olq.0000124614.91448.d4. [DOI] [PubMed] [Google Scholar]
- 21.Phillips DM, Taylor CL, Zacharopoulos VR, et al. Nonoxynol-9 causes rapid exfoliation of sheets of rectal epithelium. Contraception. 2000;62(3):149–154. doi: 10.1016/s0010-7824(00)00156-6. [DOI] [PubMed] [Google Scholar]
- 22.Phillips DM, Sudol KM, Taylor CL, et al. Lubricants containing N-9 may enhance rectal transmission of HIV and other STIs. Contraception. 2004;70(2):107–110. doi: 10.1016/j.contraception.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 23.Sternberg SS. Histology for Pathologists. New York: Raven Press; 1992. [Google Scholar]
- 24.Patton DL, Sweeney Y, Balkus JE, et al. Vaginal and rectal topical microbicide development: safety and efficacy of 1.0% Savvy (C31G) in the pigtailed macaque. Sex Transm Dis. 2006;33(11):691–695. doi: 10.1097/01.olq.0000216022.18321.d3. [DOI] [PubMed] [Google Scholar]
- 25.Patton DL, Rohan L, Issacs C, et al. 15th International Society for Sexually Transmitted Diseases Research Congress. Vol. 277. Ottawa, Canada: 2003. Octylglycerol as a topical microbicide: Preclinical safety evaluations for rectal use of a liposome formulation; p. 98. Abstract. [Google Scholar]
- 26.Patton DL, Cosgrove Sweeney YT, McCarthy TD, et al. Preclinical safety and efficacy assessments of dendrimer-based (SPL7013) microbicide gel formulations in a nonhuman primate model. Antimicrob Agents Chemother. 2006;50(5):1696–1700. doi: 10.1128/AAC.50.5.1696-1700.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patton D, Ballweber L, Cosgrove Sweeney Y, et al. Safety evaluation for rectal use of buffergel and carraguard in the macaque model; Microbicides 2002 Conference; Antwerp, Belgium: 2002. p. 101. Abstract A-157. [Google Scholar]