Abstract
The serotonin system is a collection of neural pathways whose overall level of functioning (from low to high) relates to diverse kinds of psychological and behavioral variability. Individual differences in serotonergic function are important both in personality and in vulnerability to psychological disorders. These disorders range widely—from impulsive aggression to depression. One way to understand such diverse reflections of differences in serotonergic function is by viewing serotonergic function through the lens of two-mode (or dual-process) models of self-regulation. Such theories posit a lower-order system that responds quickly to associative cues of the moment and a higher-order system that responds reflectively and planfully. Low serotonergic function appears to enhance influence of the lower-order system. This often yields impulsive reactivity. Why, then, does low serotonergic function also relate to depression, which is characterized by lethargy and unresponsiveness? The answer must be that ascendance of the lower system interacts with other factors. One hypothesis is that low serotonergic function plus high sensitivity to incentives yields vulnerability to impulsive approach, whereas low serotonergic function plus low incentive sensitivity yields vulnerability to depression. Conceptualizing serotonergic function this way helps integrate information pertaining to very different disorders into a coherent picture.
Keywords: serotonin, depression, aggression, impulsiveness, dual-process theories
Controlling impulsive reactivity is crucial to successful self-management (Baumeister & Vohs, 2004). Control helps people pursue long-term goals; it may also protect against certain kinds of psychopathology. Increasing evidence implicates responsiveness of the neural pathways that form the serotonergic system in the ability to exert this control. We propose that this effect of the serotonergic system maps easily to two-mode models of thought and action (also termed dual-process models) that are in widespread use in psychology. A puzzling fact is that low serotonergic function is linked to disorders with very different surface characteristics. This fact can be interpreted by positing interactions between level of serotonergic function and other factors.
The article begins with a brief overview of two-mode models of self-regulation. Then we turn to some of the effects of variation in serotonergic function, which ultimately we interpret in terms of the two-mode viewpoint. We then consider how this viewpoint conceptualizes effects of low serotonergic function that are associated with depression.
TWO-MODE MODELS
Numerous two-mode models of cognition and behavior exist in psychology (Barrett, Tugade, & Engle, 2004; MacDonald, 2008; Strack & Deutsch, 2004). The core idea is that the nervous system is organized such that people simultaneously process experiences in two ways, one more basic (evolutionarily primitive) than the other (Epstein, 1994). The two processing modes appear to use different aspects of available information, they seem to learn in different ways, and they create potentially competing paths to action. The more primitive mode operates largely outside consciousness. The other is the familiar symbolic processor of the rational mind.
Characterizations of the more primitive mode typically use such terms as impulsive, reflexive, reactive, implicit, heuristic, and associative. This mode is responsive to situational cues of the moment, schematic associations, and strong emotions. Its strengths are its quickness and its low demand on processing resources. It spontaneously creates action when its schemas or production systems are sufficiently activated. It thus can act even with little available information and high time urgency.
Characterizations of the other mode typically use such terms as reflective, explicit, strategic, deliberative, and logical. Its strength is its ability to take into account circumstances that go beyond the immediate present. This mode requires substantial processing resources and thus loses efficiency when cognitive capacity is limited.
The premise of two processing modes also connects to increasingly influential literatures on implicit versus explicit attitudes, self-concepts, and so on. Theories on such topics hold that people build up networks of associations that are not easily accessed verbally, and also form (separately) verbally mediated logical structures. The associations and the logical structures both influence behavior. Of most interest are cases in which the influences conflict with each other. The connection here is that the associative network represents the reactive system, and the logical structures represents the deliberative system.
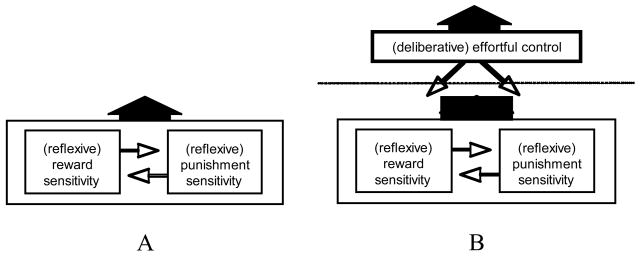
There are also influential developmental versions of the two-mode viewpoint (e.g., Rothbart, Ellis, & Posner, 2004). These are perhaps clearest in indicating that each mode can promote either action or inaction (Fig. 1). That is, in the reactive mode, strong reward focus can yield impulsive action; strong punishment focus can yield immobility or withdrawal. Immobility might not seem impulsive (i.e., no action), but the automatic nature of the response suggests an impulsive quality (i.e., impulsive as automatic). Either of these reactive tendencies can be overridden, once the capacity for what is called effortful control develops. Effortful control (which closely resembles what Depue & Lenzenweger, 2005, called nonaffective constraint) can counter the more basic reactions, overcoming either an impulse to act or an impulse to become immobile.
Fig. 1.
Three temperamental influences on behavior. Panel A shows a reactive temperament for approaching rewards and a reactive temperament for avoiding punishment compete for influence; in the absence of effortful control (whether due to early stage of development, individual differences, or situational pressures), the resultant from that competition is expressed behaviorally. Panel B shows how the engagement of an emergent effortful control system permits the resultant from the competition of the reactive systems to be overridden, thus dampening the role of the reactive systems in behavior. This figure reflects various statements by Rothbart, Eisenberg, and others; it is reproduced from Carver, Johnson, & Joormann (2008).
The properties that differentiate the reflective from the reflexive mode resemble what are generally termed executive processes. An example is working-memory capacity (WMC), the ability to maintain information in working memory and shield it from interference or distraction from competing stimuli, including strong emotions. Another example is the ability to remove information from working memory when it is not relevant to the task at hand. Both WMC and the ability to disengage from irrelevant information help the deliberative system operate.
Variables such as these are subject to both individual differences and situational influences, yielding variations in the ability to exert effortful control. For example, when WMC is high, people act according to their explicit attitudes (i.e., WMC helps them use the deliberative, planful system); when WMC is low, people act according to implicit attitudes (i.e., in this circumstance the reactive system guides behavior; Hofmann, Gschwender, Friese, Wiers, & Schmidt, 2008).
Effortful Control: Serotonergic Function
There is a substantial basis for arguing that the relative influence of reactive and effortful systems is affected by the serotonergic system. The processes by which this system operates are complex and not fully understood (Hensler, 2006), but clearly more is involved than simply the level of serotonin. Other factors include sensitivity and density of several kinds of serotonin receptors, efficiency of reuptake of serotonin from the synaptic cleft, dietary intake of certain amino acids, and recent history of the cell’s firing. Each of these factors can influence the key issue, the overall “functioning” of the system.
There are several ways to study serotonergic function, which vary in how direct they are. One way is to challenge the system; systems with lower functioning show greater perturbation. Another is to experimentally increase or decrease tryptophan (a precursor to serotonin) or administer drugs that affect serotonergic function. There also are genetic markers of serotonergic function. In general, results converge impressively across methods.
Experimentally increasing serotonergic function reduces responsiveness to negative emotional stimuli, decreases aggression, and increases cooperativeness and social potency. Experimentally lowering serotonergic function increases behavioral impulsivity. Experimentally lowering serotonergic function also increases aggression, though the effect appears to depend at least partly on preexisting tendencies: Persons low in aggressiveness sometimes are unaffected and sometimes even decrease aggression when serotonin function is lowered.
Naturally existing low serotonergic function has also been linked to behavioral impulsivity, particularly impulsive responses to anger (Manuck, Kaplan, & Lotrich, 2006). In genetic and drug-challenge studies, low serotonergic function related to self-reported hostility, sensation seeking, and impulsiveness. Large-scale studies link low serotonergic function to low conscientiousness, low agreeableness, and elevations in the anger-hostility and depression facets of neuroticism.
These various associations seem readily interpretable via the two-mode models described earlier. Associations of low serotonergic function with hostile and depressive tendencies reflect intrusion of strong emotions, which characterizes reflexive-mode processing. Associations with impulsive aggression (and other behavioral impulsiveness) may reflect limited effortful control over spontaneous action. Associations of higher serotonergic function with higher agreeableness and conscientiousness suggest that higher serotonergic function promotes a general broadening of perspective: Conscientiousness implies taking future contingencies into account; agreeableness implies taking the needs of others into account.
Low serotonergic function appears to tune the person to be more responsive to cues of the moment, particularly emotional cues, fitting the reflexive system in two-mode models. People with low serotonergic function tend to be highly reactive. They grab what they want when they want it rather than delaying gratification. They react to associative cues of the moment rather than thinking matters through. They react impulsively to salient or intense emotions (Spoont, 1992).
Disorders of Impulse
Fitting the idea that low serotonergic function yields impulsive, reactive behavior, evidence links low serotonergic function to childhood conduct disorders and attention-deficit hyperactivity disorder (ADHD), and to emotionally explosive disorders of adulthood, including violent aggression, borderline personality disorder, and violent suicide. Indeed, impulsive externalizing problems are probably the most widely known manifestations of low serotonergic functioning in disorders (cf. Raine, 2008).
SEROTONERGIC FUNCTION AND DEPRESSION
Serotonergic function has also been linked to vulnerability to depression. The surface appearance of depression is quite different from that of impulsive disorders. If low serotonergic function promotes impulsiveness, why is impulsiveness not a salient characteristic of depression? What sense can be made of this pattern?
An important point here is that the term impulsiveness applies to many qualities. Among them are lack of planfulness, lack of follow-through, sensation seeking, jumping to conclusions, taking risks, being swept up in emotions, reacting quickly to emotions, taking actions without consideration of longer-term consequences, being easily distracted, and more. These qualities are not uniform. Some of them in fact pertain directly to depression. The ones that appear to be least descriptive of depression involve impulsive overt action. We focus first on that property.
Interactions Between Serotonergic Function and Another System
It is important to recall the conditions under which the dual-process models predict that lack of effortful control promotes impulsive action. Specifically, this prediction follows only when there are action impulses to express. What happens if those impulses are lacking? For example, what if the person’s reactive approach subsystem is insensitive? In this case, effortful control would be required in order to act, rather than to refrain from acting. Deficient control here would lead to inaction, lethargy, and lack of effort toward rewards—qualities that sound more like depression.
This reasoning suggests that depression vulnerability may involve, in addition to low serotonergic function, blunted sensitivity of the approach system. Indeed, there is evidence of such blunted sensitivity in depression from studies of asymmetry in cortical activation patterns, studies of behavioral responses to rewards, and studies of self-reported sensitivity to rewards.
Blunted approach motivation associated with depression may reflect low dopaminergic function (Dunlop & Nemeroff, 2007). Dopaminergic pathways are believed to be critical in the engagement of goal-directed effort (Salamone, Correa, Farrar, & Mingote, 2007)—“wanting” for appetitive outcomes (Berridge, 2007) and effort in pursuit of them. With lower dopaminergic function, people would have lower appetitive interests and greater difficulty sustaining goal-directed efforts.
The evidence thus suggests an interaction involving serotonergic function and dopaminergic function. With low incentive sensitivity, the person is unmotivated to approach reward; with lack of effortful control, it is hard to override this lack of motivation. The result is apathy, passivity, and fatigue, which characterize many cases of depression.
Two-Mode Models and Depression
Other manifestations of depression more easily fit the picture of low serotonergic function described earlier. Once again the picture requires a combination of low serotonergic function and other variables, but in this case the variables are psychological. Specifically, low serotonergic function allows emotion-related brain areas to be more active, yielding greater salience of emotion and emotional associations (Spoont, 1992). What follows, however, depends on the nature of the emotional associations.
An array of evidence supports the view that persons vulnerable to depression have associative networks incorporating links from negative emotions to the self, the world, and the future, making negatively toned self-oriented rumination likely (Beevers, 2005). Once these emotions are evoked, it is difficult to restrain them, in the relative absence of effortful control. This aspect of the phenomenology of depression would closely resemble that of impulsive externalizing problems—high emotional salience—but with different emotions being salient. The salient emotions here are sadness and hopelessness.
Neurobiological evidence also fits this view of uncontrolled emotions. Imaging studies of depressed persons show hyperactivity in areas that are related to the reflexive mode, such as the amygdala (Mayberg, 2004), while areas associated with the reflective mode are hypoactive. Indeed, amygdala activation correlates positively with depression severity and normalizes with antidepressant treatment (see Carver, Johnson, & Joormann, 2008, for more on brain function and depression).
FUTURE DIRECTIONS
Vulnerability to depression and vulnerability to disorders of behavioral impulsivity both relate to low serotonergic function, which we suggest reduces the influence of the deliberative mode compared to the reactive mode. To account for divergent effects of low serotonergic function, we proposed an interaction: that both types of disorder reflect low serotonergic function but differ regarding engagement of the system responsible for eager, effortful approach.
Further Interactions?
A key question for the future is whether there are other interactions involving the serotonin system and other systems (for wide-ranging discussion of this idea see Depue & Lenzenweger, 2005). As an example, vulnerability to anxiety disorders may also depend partly on low serotonergic function (Leonardo & Hen, 2006). Anxiety disorders thus may reflect the interactive effect of a highly sensitive threat system and low serotonergic functioning.
Indeed, this view also suggests an angle on the high rates of comorbidity between depression and anxiety disorders. Other things being equal, a blunted approach system implies a threat system that is therefore relatively more sensitive (i.e., relative to the approach system). Even without a highly engaged threat system, a person with a blunted approach system may be more responsive to threat than an otherwise similar person with a more sensitive approach system may be.
Low Serotonergic Function as Amplifier
Many observers have noted that the attempt to link any given neurotransmitter to the operation of a single behavioral system is likely to be a great oversimplification. Many have also noted that effects of one neurotransmitter system likely depend on the functioning of another neurotransmitter system. The picture we believe is emerging fits these cautionary statements quite well, yet the picture also has its own coherence.
Specifically, it does not seem too far an extrapolation to suggest that low serotonergic function promotes a stronger manifestation of whatever tendencies the person has at the reactive level (Depue, 1995; Nigg, 2006; Spoont, 1992). In an incentive-sensitive person, low serotonergic function exaggerates the pursuit of incentives. In an incentive-insensitive person, low serotonergic function exaggerates the lack of effortful engagement. In a threat-sensitive person, low serotonergic function exaggerates vigilance to threat.
The specific cases of depression, impulsive disorders, and anxiety disorders are only three possibilities, reflecting interactions of a serotonergic system with two other systems. Other possibilities may exist. Diversity among disorders may reflect dysregulation of systems unique to those disorders, but low serotonergic function may be a common factor across them, diminishing constraint over the other, more reactive systems (Depue & Lenzenweger, 2005).
Interestingly, this view of low serotonergic function—as a risk factor for multiple disorders rather than just one type of disorder—resembles arguments about other risk factors that seem to operate across multiple disorders, such as sleep disruption (Harvey, 2008). It is also interesting, in that regard, that both low serotonergic function and sleep deficits are implicated as contributors to poor effortful control. Further exploration of such transdiagnostic risk factors seems an important avenue for future work.
Finally, the interactive principle does not apply only to disorder. Recall that low serotonergic function has been linked to lower conscientiousness and lower agreeableness. One might rephrase our characterization of low serotonergic function as follows: Among people at relative extremes of extraversion, neuroticism, and openness, overt display of those traits should be exaggerated by lower conscientiousness and agreeableness (or lower constraint). Thus the interactive view has testable implications for the normal ranges of personality as well.
Acknowledgments
Preparation of this manuscript was facilitated by support from the National Cancer Institute (CA64710), the National Science Foundation (BCS0544617), the National Institute of Mental Health (MH076021), and a NARSAD Young Investigator Award.
Recommended Reading
- Carver CS, Johnson SL, Joormann J. A more detailed review of the literatures that are reviewed in this article, as well as more specific aspects of brain function 2008 (See References) [Google Scholar]
- Depue RA, Lenzenweger MF. A review that addresses potential interactions among diverse biobehavioral systems in normal and pathological personality 2005 (See References) [Google Scholar]
- Manuck SB, Kaplan JR, Lotrich FE. A comprehensive review of evidence linking serotonergic function to aggression. 2006. (See References) [Google Scholar]
- Rothbart MK, Ellis LK, Posner MI. A review of a two-mode theory of self-regulation in developmental psychology 2004 (See References) [Google Scholar]
- Strack F, Deutsch R. Theoretical statement of one particular two-mode theory in social psychology 2004 (See References) [Google Scholar]
References
- Barrett LF, Tugade MM, Engle RW. Individual differences in working memory capacity and dual-process theories of the mind. Psychological Bulletin. 2004;130:553–573. doi: 10.1037/0033-2909.130.4.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumeister RF, Vohs KD, editors. Handbook of self-regulation: Research, theory, and applications. New York: Guilford; 2004. [Google Scholar]
- Beevers CG. Cognitive vulnerability to depression: A dual process model. Clinical Psychology Review. 2005;25:975–1002. doi: 10.1016/j.cpr.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Berridge KC. The debate over dopamine’s role in reward: The case for incentive salience. Psychopharmacology. 2007;191:391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- Carver CS, Johnson SL, Joormann J. Serotonergic function, two-mode models of self-regulation, and vulnerability to depression: What depression has in common with impulsive aggression. Psychological Bulletin. 2008;134:912–943. doi: 10.1037/a0013740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depue RA. Neurobiological factors in personality and depression. European Journal of Personality. 1995;9:413–439. [Google Scholar]
- Depue RA, Lenzenweger MF. A neurobiological dimensional model of personality disturbance. In: Lenzenweger MF, Clarkin JF, editors. Major theories of personality disorder. 2. New York: Guilford; 2005. [Google Scholar]
- Dunlop BW, Nemeroff CB. The role of dopamine in the pathophysiology of depression. Archives of General Psychiatry. 2007;64:327–337. doi: 10.1001/archpsyc.64.3.327. [DOI] [PubMed] [Google Scholar]
- Epstein S. Integration of the cognitive and the psychodynamic unconscious. American Psychologist. 1994;49:709–724. doi: 10.1037//0003-066x.49.8.709. [DOI] [PubMed] [Google Scholar]
- Harvey AG. Insomnia, psychiatric disorders, and the transdiagnostic perspective. Current Directions in Psychological Science. 2008;17:299–303. [Google Scholar]
- Hensler JG. Serotonergic modulation of the limbic system. Neuroscience Biobehavioral Review. 2006;30:203–214. doi: 10.1016/j.neubiorev.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Hofmann W, Gschwender T, Friese M, Wiers RW, Schmitt M. Working memory capacity and self-regulatory behavior: Toward an individual differences perspective on behavior determination by automatic versus controlled processes. Journal of Personality and Social Psychology. 2008;95:962–977. doi: 10.1037/a0012705. [DOI] [PubMed] [Google Scholar]
- Leonardo ED, Hen R. Genetics of affective and anxiety disorders. Annual Review of Psychology. 2006;57:117–137. doi: 10.1146/annurev.psych.57.102904.190118. [DOI] [PubMed] [Google Scholar]
- MacDonald KB. Effortful control, explicit processing, and the regulation of human evolved predispositions. Psychological Review. 2008;115:1012–1031. doi: 10.1037/a0013327. [DOI] [PubMed] [Google Scholar]
- Manuck SB, Kaplan JR, Lotrich FE. Brain serotonin and aggressive disposition in humans and nonhuman primates. In: Nelson RJ, editor. Biology of Aggression. New York: Oxford University Press; 2006. pp. 65–102. [Google Scholar]
- Mayberg HS. Depression: A neuropsychiatric perspective. In: Panksepp J, editor. Textbook of biological psychiatry. New York: Wiley; 2004. pp. 197–229. [Google Scholar]
- Nigg JT. Temperament and developmental psychopathology. Journal of Child Psychology and Psychiatry. 2006;47:395–422. doi: 10.1111/j.1469-7610.2006.01612.x. [DOI] [PubMed] [Google Scholar]
- Raine A. From genes to brain to antisocial behavior. Current Directions in Psychological Science. 2008;17:323–328. [Google Scholar]
- Rothbart MK, Ellis LK, Posner MI. Temperament and self-regulation. In: Baumeister RF, Vohs KD, editors. Handbook of self-regulation: Research, theory, and applications. New York: Guilford; 2004. pp. 357–370. [Google Scholar]
- Salamone JD, Correa M, Farrar A, Mingote SM. Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology. 2007;191:461–482. doi: 10.1007/s00213-006-0668-9. [DOI] [PubMed] [Google Scholar]
- Spoont MR. Modulatory role of serotonin in neural information processing: Implications for human psychopathology. Psychological Bulletin. 1992;112:330–350. doi: 10.1037/0033-2909.112.2.330. [DOI] [PubMed] [Google Scholar]
- Strack F, Deutsch R. Reflective and impulsive determinants of social behavior. Personality and Social Psychology Review. 2004;8:220–247. doi: 10.1207/s15327957pspr0803_1. [DOI] [PubMed] [Google Scholar]