Abstract
Acute administration of neuropeptide Y (NPY) modulates alcohol intake in genetic and chemical models of high intake, while leaving intake unaffected during ‘normal’ or baseline conditions. In non-selected, normal rat lines, alcohol consumption can be increased by prolonged exposure to alcohol, and it is unclear what effect a constitutive increase in NPY function will have on alcohol intake. The purpose of the present study was to examine the effects on alcohol intake of an inducible, constitutive overexpression of NPY, one of the most abundant neuropeptides in the central nervous system. A liquid diet was used in combination with repeated alcohol deprivation sessions to increase alcohol intake in normal Wistar rats. We then examined the effect of NPY overexpression in the amygdala on excessive alcohol intake produced by prolonged exposure to alcohol and alcohol deprivation. Repeated withdrawal increased alcohol consumption in a 24-h continuous access two-bottle choice model. Both the number of withdrawals as well as the length of the withdrawal periods affected alcohol consumption with an increased intake resulting from multiple withdrawals and the alcohol deprivation effect being enhanced by longer periods of abstinence. The increase in intake following repeated abstinence was blunted by intra-amygdala administration of a Sindbis viral vector containing NPY cDNA. Amygdala NPY overexpression also was demonstrated to be anxiolytic in the open field test. Repeated withdrawal in combination with a history of alcohol consumption significantly elevated alcohol intake, and the amygdala may mediate the transition to high-drinking states in this model.
Keywords: alcoholism, animal model, neuropeptide Y, viral vector, amygdala
Introduction
Neuropeptide Y (NPY), a 36 amino acid peptide expressed at high levels within the mammalian nervous system, has been shown to have effects on emotional behaviour and alcohol intake in rodent models. Nanomolar doses of NPY injected centrally are anxiolytic in a number of animal models of anxiety-related behaviour, including the elevated plus-maze (Broqua et al., 1995), social interaction task (Sajdyk et al., 1999), fear potentiated startle and conditioned fear responses (Heilig et al., 1989, 1992; Broqua et al., 1995; Tovote et al., 2004). In addition, intracerebroventricular (i.c.v.) administration of NPY prevents gastric ulceration induced by water restraint, a strong stressor (Heilig and Murison, 1987). Transgenic rats overexpressing NPY in the hippocampus were shown to be resistant to stress-induced increases in anxiety-like behaviour (Thorsell et al., 2000; Carvajal et al., 2004). These studies indicate that activation of NPY signalling attenuates stress-related effects.
NPY also has been demonstrated to have a role in the regulation of alcohol intake, dependence and withdrawal. The effect profile of NPY shows numerous similarities with that of established anti-anxiety compounds and also that of alcohol. A direct link between NPY signalling and regulation of alcohol consumption was first shown in a study where mice with a null-mutation in the NPY gene demonstrated increased alcohol consumption, while mice with a transgenic overexpression of NPY consumed less alcohol (Thiele et al., 1998). The effect of NPY on alcohol consumption appears to be in part dependent on the individual's history and state of alcohol consumption. In animal studies, central administration of NPY into the lateral ventricles, central nucleus of the amygdala or the third ventricle leaves levels of alcohol intake unaffected in normal, outbred rat strains (Badia-Elder et al., 2001; Katner et al., 2002a, b). However, a significant suppression of alcohol intake can be seen in the high-drinking alcohol-preferring P line of rats compared to non-preferring NP and normal Wistar rats, and in the high alcohol drinking (HAD) rat line (Badia-Elder et al., 2001, 2003). Further evidence for NPY's involvement in the regulation of alcohol intake comes from a study using animals in which dependence and high alcohol preference were induced using 8-week exposure to intermittent alcohol vapour (14 h on/10 h off per day; target blood alcohol level 200 mg%). Intermittent alcohol exposure models chronic alcohol consumption and produces behavioural changes similar to clinical manifestations as well as long-term changes in neurochemistry and increases in alcohol intake (Rimondini et al., 2002). In this model, NPY was shown to significantly suppress alcohol intake in exposed animals compared to saline treatment. Notably, consumption was reduced back to, but not below, pre-vapour exposure levels (Thorsell et al., 2005).
The efficacy of NPY to suppress alcohol intake in the preferring lines which consume alcohol for its pharmacological properties, and the non-efficacy of NPY in states of low intake, are important in understanding a basic distinction in the mechanisms of alcohol intake. A basal component of alcohol consumption is unrelated to the pharmacological/rewarding properties of alcohol, but is related to its properties as a caloric nutrient and can be regulated by factors modulating appetite. This component is not suppressed by NPY—it is stimulated by hypothalamic NPY injections, as would be expected from the well-established property of NPY to stimulate appetite (Kelley et al., 2001). A further illustration of the changes in neurobiology induced by a history of alcohol exposure/intake is the observation that suppression of alcohol consumption by i.c.v. administration of an NPY Y2 receptor antagonist, while present in the non-exposed animal, is strongly facilitated in animals with a history of alcohol exposure, indicating sensitization to the effect of the antagonist in these animals (Thorsell et al., 2002; Rimondini et al., 2005).
The different facets of alcohol-related behaviour can be examined using different animal models. The alcohol deprivation effect (ADE), defined as a temporary increase in absolute alcohol intake and in the ratio of alcohol intake/total fluid intake seen when access to alcohol is reinstated after a period of abstinence, has been suggested to model alcohol craving (Sinclair and Li, 1989; Heyser et al., 1997). The ADE has been used to test pharmacological strategies to prevent relapse to alcohol intake (Spanagel and Zieglgansberger, 1997; Heyser et al., 1998) and has been demonstrated in a number of species, including rats (Rodd-Henricks et al., 2000a, b), mice (Salimov and Salimova, 1993), monkeys (Kornet et al., 1990a) and humans (Burish et al., 1981).
The amygdala has been the most prominent region of interest with regard to emotionality. The central nucleus of the amygdala was initially suggested to be the mediating site of anxiolytic NPY actions (Heilig et al., 1993), as well as the lateral/basolateral complex (Sajdyk et al., 1999). Furthermore, in clinical studies of alcohol dependence, a correlation between initial anxiety and subsequent alcohol abuse, possibly due to the anxiolytic action of alcohol, has been demonstrated (Pandey, 2003; Pandey et al., 2003). While this may only be true for a subgroup of alcoholics, it may partially explain some of the changes and effects seen for NPY in alcoholism.
Previous work has involved only the acute administration of NPY, and it is unknown whether a constitutive change in NPY in the amygdala will convey similar effects. Overexpression in the central nervous system can be accomplished using viral vectors. Viral vectors derived from Sindbis and the related Semliki Forest virus—members of the family of alpha-viruses—have been extensively used to transduce post-mitotic neurons (Xiong et al., 1989; Ehrengruber et al., 1999). Sindbis selectively infects neurons and confers a rapid onset and high level of expression of foreign genes. For the present study, we used a vector derived from a Sindbis virus mutant in which a single change in the viral nsP2 protein from proline to serine inhibits virus-induced suppression of host RNA synthesis that otherwise occurs with Sindbis virus. The decreased suppression of host RNA synthesis is interestingly accompanied by higher levels of expression of a reporter gene (Dryga et al., 1997).
The hypothesis that constitutive changes in NPY activity may mediate the increased alcohol consumption associated with forced exposure and repeated alcohol deprivations was tested. A 24 h two-bottle free choice paradigm was used with administration of a Sindbis viral vector to induce localized overexpression of NPY within the central nucleus of the amygdala. To examine anxiety-related behaviour in these animals, behaviour in the open field also was tested.
Material and methods
Animals
Male Wistar rats (220–240 g at start of experiment; n = 16) were used. Animals were kept single-caged throughout the study with food and water available ad libitum except during the liquid diet. Animals were housed in a 12 : 12 h light/dark cycle. All procedures were conducted in strict adherence to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of The Scripps Research Institute.
Viral vector procedures
Host shut-off deficient Sindbis virus vector was obtained from Dr Andreas Jeromin (Jeromin et al., 2003). With this system, separate transcripts are co-transfected by electroporation into Baby Hamster Kidney (BHK) cells—one containing the genomic RNA coding for the replicase and gene(s) of interests, and the other containing structural proteins. The NPY propeptide and green fluorescent protein (GFP) were co-expressed through double subgenomic promoters (Okada et al., 2001). Virus expressing GFP only from an identical vector was used for control. Sindbis structural proteins expressed on a separate vector and the vector expressing non-structural proteins and the proteins of interest (both NPY and GFP, or GFP alone) were transcribed using in vitro transcription with SP6 RNA polymerase and co-transfected by electroporation into BHK-21 cells for virion production. Virions were harvested 4 days later and concentrated by ultracentrifugation at 120 000 × g at 4°C for 3 h to obtain a titre of 1010 transducing virions per millilitre.
Alcohol two-bottle choice
Alcohol two-bottle choice was performed as previously described (Moller et al., 1997). Briefly, single-housed animals were given a choice between a bottle containing water with decreasing concentrations of saccharin and a bottle with decreasing concentrations of saccharin and increasing concentrations of alcohol. The end concentrations of each were 0% saccharin and 10% (w/v) ethanol. The bottles were shifted side to side during the experiment to avoid biased results due to side preference. The bottles were weighed daily, and the amount of consumed alcohol in g/kg body weight was determined. Preference for the alcohol solution also was calculated.
Alcohol deprivation schedule
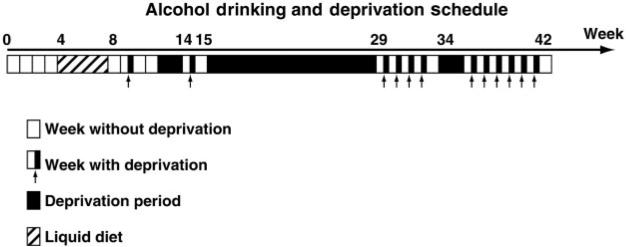
Animals were repeatedly deprived of alcohol in this study (Fig. 1). The first deprivation was performed at around 10 weeks of ‘alcohol history’. The 12 weeks (the first deprivation occurred in week 10, but the drinking continued for another 2 weeks) consisted of an initial 4-week baseline of two-bottle free choice drinking followed by 4 weeks of liquid diet, and then another 4-week session of two-bottle free choice. The deprivations were for 72 h (Friday morning to Monday morning) except when longer time-intervals are indicated (Table 1). The initial deprivation was for 72 h followed by 2 weeks of alcohol access and then a 2-week deprivation period. Following this 2-week deprivation, drinking was reestablished, and another 72 h deprivation was performed. A long (13 weeks) deprivation followed. Following the extended deprivation, seven short (72 h) deprivations were done prior to the next 2-week deprivation. After the 2-week deprivation, drinking baseline was reestablished for 3 weeks, and animals were selected for surgery. Following surgery and recovery, 5 weeks of drinking studies were done that included four deprivation sessions. After the last drinking measure, animals were taken off of the alcohol and allowed a 1-week period of abstinence prior to examination of anxiety-related behaviour in the open field.
Fig. 1.
Outline of the deprivation schedule prior to viral vector treatment. All 72 h deprivations took place Friday—Monday, and the longer deprivation periods were initiated Friday of the preceding week.
Table 1.
Alcohol intake and preference during repeated deprivations prior to viral vector treatment
| Week of study | Alcohol deprivation effect (g/kg/24 h) | Alcohol deprivation effect (% preference) | Intake during days 2–4 (g/kg/24 h) | Deprivation period |
|---|---|---|---|---|
| 10 | 1.4 ± 0.1 | 58 ± 4 | 1.13 ± 0.2 | 72 h |
| 14 | 2.1 ±0.2 | 61 ±5.5 | 1.43 ± 0.12 | 2 weeks |
| 15 | 1.6 ± 0.3 | 55 ± 3.3 | 1.3 ± 0.15 | 72 h |
| 29 | 5.2 ± 0.6 | 74 ± 6 | 2.5 ± 0.2 | 13 weeks |
| 30 | 3.2 ± 0.4 | 54 ± 5 | 2.1 ±0.34 | 72 h |
| 31 | 3.3 ± 0.3 | 57 ±6 | 2.4 ± 0.3 | 72 h |
| 32 | 3.8 ± 0.5 | 52 ± 6 | 2.8 ± 0.21 | 72 h |
| 33 | 3.4 ± 0.5 | 54 ± 5 | 2.7 ± 0.16 | 72 h |
| 35 | 3.6 ± 0.4 | 75 ± 4 | 2.7 ± 0.17 | 2 weeks |
| 36 | 5.4 ± 0.6 | 62 ± 6 | 3.1 ±0.16 | 72 h |
| 37 | 3.9 ± 0.4 | 67 ± 5 | 2.8 ± 0.23 | 72 h |
| 38 | 4.1 ±0.4 | 66 ± 4 | 2.9 ± 0.17 | 72 h |
| 39 | 3.8 ± 0.4 | 69 ± 3 | 2.8 ± 0.13 | 72 h |
| 40 | 3.8 ± 0.3 | 64 ± 5 | 3.2 ± 0.13 | 72 h |
| 41 | 4.5 ± 0.4 | 71 ±4 | 3.5 ± 0.2 | 72 h |
The deprivation schedule was as pictured in Fig. 1.
Alcohol liquid diet
To induce a history of dependence in the animals, an alcohol liquid-diet was used. The diet was maintained for 4 weeks, and during this time no additional food or water was available to the animals. The diet was prepared by mixing ‘Boost’ with vitamins and minerals and ethanol to yield a 10% v/v solution. The liquid was checked and changed on a daily basis, and the animals were weighed to detect any weight-loss due to the diet.
The open field test
Anxiety/activity testing was done using an open field. The animal was allowed to explore an open surface (1 × 1 m2; divided into 25 squares) freely for 10 min. The amount of time spent in the nine central squares, the four corner squares and the 16 peripheral squares was measured as was the number of line-crossings made within the entire field and within each subset of squares. The open field was performed under red light conditions during the animals’ active phase. Behaviour was observed by an individual blind to treatment condition.
Surgery
Animals were anaesthetized using isoflurane, and Sindbis viral vector (control GFP or NPY) was injected using a microsyringe into the central nucleus of the amygdala (AP: −2.1 mm, L: ±4.6 mm, VD: −8.7 mm) (Paxinos and Watson, 1997). GFP expression was used to validate vector expression of both NPY and control vector-injected animals; the NPY-expressing vector also expressed GFP. Injection accuracy was verified by GFP expression in a separate set of control animals. Cannula placement could not be determined in the experimental animals because of the disappearance of injection tracts due to the long time which had elapsed since the injection, which extended past the duration of GFP expression. In the present study, the behavioural response of the animals in the open field was used as an index of a successful injection. Great care was taken not to induce any lesions.
Following surgery, animals were allowed a 1-week recovery prior to restarting the two-bottle free choice and anxiety/activity testing.
Statistics
Statistical analyses were performed using STATISTICA 7.0 software. A repeated-measures ANOVA was used for the drinking data followed by Tukey's HSD post hoc test when appropriate. The open field data were analysed using the t-test for independent samples.
Results
Viral vector construct
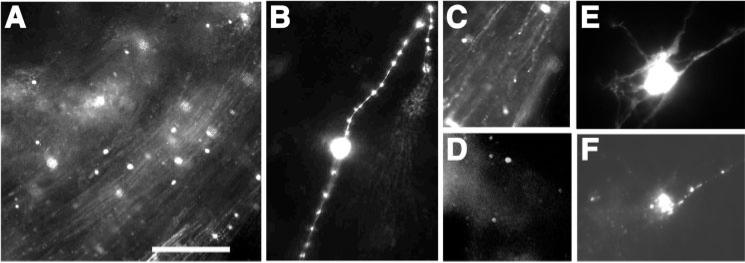
Transduction of NPY- and GFP-transducing Sindbis viral vector deficiency in the host shut-off function is shown in Fig. 2. Expression was robust in central amygdala neurons and quite protracted as expected from previous applications of this vector (Jeromin et al., 2003). The injection was highly localized. High magnification pictures are shown because of the small size of the neurons and to demonstrate immunoreactive fibres. Tissue was not fixed to limit quenching of GFP by fixative; therefore, additional pictures could not be taken. As mentioned earlier, the injection site could not be verified in the experimental rats because of the long duration of the experiment and the disappearance of the injection tracts. One animal was excluded from the data analysis because of a failure to observe an anxiolytic-like effect in the open field.
Fig. 2.
(A) Detection of green fluorescent protein (GFP)/NPY-expressing neurons in the central nucleus of the amygdala 1 week after injection with a high titre Sindbis viral vector by GFP fluorescence (neurons and fibres are seen at 10× magnification). The vector used is deficient in viral-induced shut-off of protein synthesis of the infected cell resulting in protracted expression and apparent lack of toxicity without compromising expression levels of the transduced genes (Jeromin et al., 2003). (B) Higher magnification of GFP/NPY-expressing neurons and fibres (at 40× magnification). (C) Expression remained robust 2 weeks after infection and (D) was still detectable 4 weeks after infection. (E) Co-detection of GFP and (F) NPY in primary neurons infected with the GFP/NPY-expressing Sindbis vector. Scale bar = 100 μm in A, 25 μm in B, E, F, and 200 μm in C, D.
Baseline alcohol intake: effect of liquid diet on intake
Baseline intake of alcohol and preference for the alcohol solution are shown in Table 1. The baseline intake prior to animal exposure to the liquid diet was 1.1 ± 0.2 g/kg/24 h. Following the liquid diet during which animals consumed on average 11.4 ± 0.7 g/kg/24 h of pure ethanol, the consumption was slightly, but not significantly, elevated to 1.53 g/kg/24 h.
Alcohol deprivation effect and alcohol intake
The ADE was defined as consumption during the first 24 h following a deprivation period. Data are shown in Table 1. The initial 72 h deprivation did not significantly elevate ADE-induced intake. A longer deprivation time, 2 weeks, significantly elevated intake on day 1 compared to baseline while leaving drinking on day 2–4 unaffected. The 13-week deprivation led to an increase from 1.5 ± 0.2 to 5.2 ± 0.6 g/kg/24 h. The day 2–4 intake following 72 h deprivation slowly increased and had, after the last deprivation, increased by 132 ± 8.5% (P < 0.001) and 310 ± 14% (P < 0.0001) compared to post-13-week deprivation and initial intake baseline, respectively.
Effect of amygdala NPY overexpression on alcohol intake, water intake and body weight
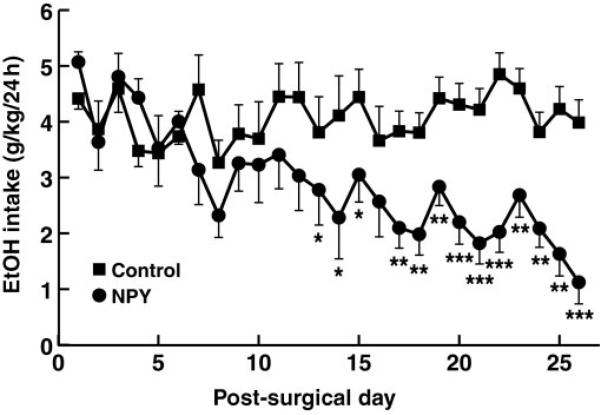
Overexpression of NPY in the amygdala significantly decreased alcohol intake starting at day 13 post-surgery [Fig. 3; overall treatment × day interaction: F(25,225) = 3.03, P < 0.00001] while leaving water intake unaffected [Table 2; F(25,225) = 0.02, P = 1.0]. Overexpression of NPY in the amygdala did not significantly affect body weight development in treated animals compared to controls [Table 2; F(25,225) = 0.13, P = 0.98].
Fig. 3.
Effect of Sindbis viral vector-mediated overexpression of NPY in the amygdala on alcohol consumption during baseline and alcohol deprivation effect. Animals were injected on day 0, and two-bottle free choice drinking was initiated directly after surgery. Treatment significantly suppressed drinking from day 13 onward. *P < 0.05, **P < 0.01 and ***P < 0.001.
Table 2.
Water intake and body-weight development following intra-amygdala administration of viral vector [control (GFP) or NPY]
| Time | Controls | NPY | |
|---|---|---|---|
| Water intake (ml) | Pre-surgery | 13.6 ± 1.2 | 14.2 ± 1.1 |
| Post-surgery measure 1 | 14.9 ± 1.1 | 13.2 ± 1.1 | |
| Post-surgery measure 10 | 13.2 ± 2.0 | 14.7 ± 2.1 | |
| Post-surgery measure 20 | 13.6 ± 1.7 | 12.9 ± 1.3 | |
| Post-surgery last measure | 14.3 ± 1.2 | 14.1 ± 1.2 | |
| Body weight (g) | Pre-surgery | 761 ± 4.5 | 758 ± 4.4 |
| Post-surgery measure 1 | 771 ±4.5 | 773 ± 3.5 | |
| Post-surgery last measure | 768 ± 3.0 | 765 ± 3.5 |
Viral vector treatment did not significantly affect either water intake or body weight.
Effect of amygdala NPY overexpression on open field behaviour
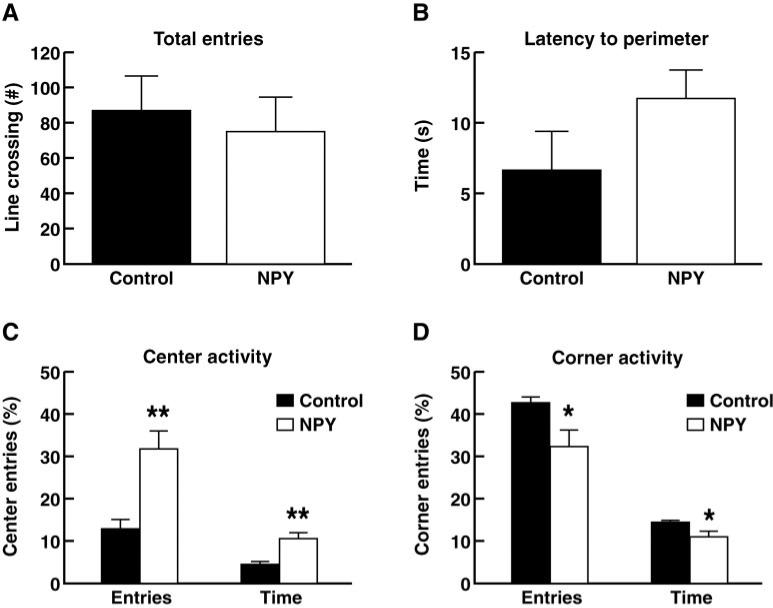
Amygdala NPY overexpression did not significantly affect latency to reach the perimeter of the open field, though there was a trend toward increased latency in the NPY-treated animals (Fig. 4). The amount of time spent in the centre of the open field was significantly increased, and the time spent in the corners of the maze was significantly decreased (P < 0.001 and P < 0.01, respectively; t-test for independent samples), indicating an anxiolytic-like effect of the viral vector overexpression.
Fig. 4.
Effect of Sindbis viral vector-mediated overexpression of NPY in the amygdala on anxiety-related behaviour in the open field. (A) Total activity measured as number of lines crossed in the open field. (B) Latency for animals to reach the perimeter of the open field upon introduction into the apparatus. (C) Presence of animals in centre squares of the open field. (D) Presence of animals in the corners of the open field. *P < 0.01 and **P < 0.001.
Effect of amygdala NPY overexpression on general behaviour
The novel Sindbis viral vector did not induce any visible aversive side-effects as determined by a general assessment of the animals’ health and physical appearance. Subjects treated with control vector and animals treated with the NPY-containing construct recovered well from surgery and had normal appearance (fur, eye colour, body posture, etc.), weight gain and locomotor skills.
Discussion
We have demonstrated that repeated alcohol deprivation periods increased subsequent alcohol intake measured both as ADE and post-ADE alcohol intake when alcohol was presented after a history of dependence induction. Furthermore, we showed that overexpression of NPY locally in the central nucleus of the amygdala significantly reduced the elevated alcohol consumption induced by a prolonged history of alcohol consumption and deprivation cycles.
The NPY system may be a particularly interesting target system involved in the development of alcohol dependence as well as mood disorders such as depression and anxiety syndromes. NPY is an endogenous anxiolytic compound. It functions as an antidepressant and is effective in modifying alcohol intake in high-drinking states. Evidence indicating an involvement of NPY in the modulation of neurobiological responses to alcohol and in regulation of alcohol intake stems from the observation that NPY expression levels are inversely related to alcohol intake in mice (Thiele et al., 1998). In addition, a number of genetic mapping studies in animals bred for high and low alcohol preference/consumption reveal significant differences in NPY and NPY receptor expression patterns (Ehlers et al., 1998; Hwang et al., 1999; Caberlotto et al., 2001; Murphy et al., 2002). Low NPY signalling in animal models correlates with high alcohol drinking in alcohol-preferring P rats and high-alcohol-drinking HAD rats, which have low levels of NPY in the amygdala compared to controls (Ehlers et al., 1998; Hwang et al., 1999). Furthermore, administration of alcohol and alcohol withdrawal alter central NPY expression and levels (Clark et al., 1998; Ehlers et al., 1998; Roy and Pandey, 2002). In addition, i.c.v. administration of NPY reduces alcohol drinking in alcohol-preferring P rats (Badia-Elder et al., 2001) as well as in rats with a history of alcohol vapour exposure (Thorsell et al., 2005).
In the present study, we induced a state of high intake by repeated alcohol deprivations and reexposures to alcohol. The fact that the intake levels and/or the resulting blood-alcohol levels were not as high as those seen during alcohol vapour exposure does not detract from the importance of the demonstrated dramatic increase in alcohol intake and the effects produced by a constitutive increase in NPY expression on both ADE amplitude and intake during post-ADE days 2–4. These results demonstrate that a long period of moderate-to-high intake in combination with repeated deprivations may induce behavioural changes similar to those seen after exposure to models inducing significantly higher blood alcohol levels. Furthermore, a suppression of intake in this prolonged intake model by constitutive NPY overexpression demonstrates that there may be underlying changes in neuronal processes (as well as at the molecular level) that are similar to more ‘extreme’ methods of inducing a high intake state.
The anxiolytic effects of NPY are in part mediated through the amygdala. Thus, it has been hypothesized that increased levels of NPY in this region lead to a reduction in anxiety-related behaviour and may lead to a reduction in alcohol intake in high-drinking states. Previous studies in animals without a history of alcohol exposure/high intake showed no effect of NPY on alcohol intake (Katner et al., 2002a). However, the amygdala has been demonstrated numerous times to be a site of action for the anxiolytic actions of NPY (Heilig, 1995). The anxiety induced by repeated withdrawal cycles may indeed be directly attenuated by NPY and/or NPY may substitute for ethanol's acute anti-anxiety effect and by this lead to decreased intake. Other non-benzodiazepine drugs have been shown to indeed also affect alcohol withdrawal and relapse to alcohol intake, for example gabapentin, vigabatrin and topiramate (Book and Myrick, 2005).
In the present study, the hypothesis that a constitutive increase in amygdala NPY would alter increased drinking in response to alcohol deprivation was tested. Alcohol intake was the primary measure, and anxiety-related behaviour in the open field also was tested.
The ADE has been suggested to be an animal model of human relapse. Relapse behaviour for individuals with a history of alcohol abuse or alcoholism is a substantial problem. Approximately 60–80% of abstinent alcoholics will relapse during their lifetime. A number of criteria for relapse exist, but the primary criterion suggests that an increase in intake, or at least consumption at pre-abstinence levels, constitutes a relapse. The ADE is defined as a temporary increase in alcohol intake and in the preference for the alcohol solution over other solution(s) present upon reinstatement of alcohol following a period of deprivation. The ADE is present after both short (12 h or less) (Sinclair and Li, 1989) and long (up to 75 days) (Sinclair et al., 1973) periods of abstinence and thus is not a result of acute physiological withdrawal. In addition, while the ADE does occur in animals with a short history of alcohol consumption not sufficient to result in physical dependence, the amplitude of the ADE may be increased in animals with a history of physical dependence (Rodd-Henricks et al., 2000a, b). The magnitude of the ADE also may be correlated with the length of the abstinence period (Kornet et al., 1990b; Heyser et al., 1997). In outbred rats, the ADE has been examined using both 24 h free choice drinking and operant self-administration (Wolffgramm and Heyne, 1995; Roberts et al., 2000). The ADE may be used as a measure of increases in alcohol intake and/or preference in rodent models of alcohol consumption.
The ADE has been demonstrated in numerous studies using both outbred animals as well as animals bred for high alcohol consumption. Using outbred rats, an increase in alcohol intake from a ‘controlled’ level to a ‘high’ level (2–3.5 g/kg/day; thought to reflect ‘loss of control’ or an ‘addicted state’) has been demonstrated (Wolffgramm and Heyne, 1995). However, it is a concern that these ‘high’ levels of intake may not be indeed ‘high’ but rather should be defined as ‘moderate’. We demonstrated that it is possible to increase the ADE to levels almost twice that are defined here as ‘high’. In the present paradigm, where the animals had a history of dependence, a longer deprivation session elevated ADE-induced drinking to over 5 g/kg/day, a level of intake similar to that seen in some of the selectively bred high consumption lines as well as the intake level seen following cyclic exposure to alcohol vapour. While the level of intake seen during the ADE did not persist during the following days of alcohol intake (days 2–4 in this study), the level of intake during these days slowly elevated with the number of deprivation cycles and reached a stable level of around 3–3.5 g/kg/day, a level significantly higher than the original baseline intake of 1.13 g/kg/day.
In summary, prolonged moderate alcohol intake developed into high intake when combined with repeated deprivation periods and a history of dependence. Local constitutive NPY overexpression in the amygdala blunted this increase. These results provide further support for NPY dysregulation as a mediator of excessive drinking in alcohol abuse and alcoholism.
Acknowledgements
This is publication number 18342 from The Scripps Research Institute. Research was supported by grants AA06420 and AA08459 from the National Institute on Alcohol Abuse and Alcoholism. The authors would like to thank Mike Arends and Mellany Santos for editorial assistance.
Abbreviations
- ADE
alcohol deprivation effect
- BHK
Baby Hamster Kidney
- GFP
green fluorescent protein
- HAD
high alcohol drinking
- NPY
neuropeptide Y
References
- Badia-Elder NE, Stewart RB, Powrozek TA, Murphy JM, Li TK. Effects of neuropeptide Y on sucrose and ethanol intake and on anxiety-like behavior in high alcohol drinking (HAD) and low alcohol drinking (LAD) rats. Alcohol Clin Exp Res. 2003;27:894–99. doi: 10.1097/01.ALC.0000071929.17974.DA. [DOI] [PubMed] [Google Scholar]
- Badia-Elder NE, Stewart RB, Powrozek TA, Roy KF, Murphy JM, Li TK. Effect of neuropeptide Y (NPY) on oral ethanol intake in Wistar, alcohol-preferring (P), and -nonpreferring (NP) rats. Alcohol Clin Exp Res. 2001;25:386–90. [PubMed] [Google Scholar]
- Book SW, Myrick H. Novel anticonvulsants in the treatment of alcoholism.. Expert Opin Investig Drugs. 2005;14:371–6. doi: 10.1517/13543784.14.4.371. [DOI] [PubMed] [Google Scholar]
- Broqua P, Wettstein JG, Rocher MN, Gauthier-Martin B, Junien JL. Behavioral effects of neuropeptide Y receptor agonists in the elevated plus-maze and fear-potentiated startle procedures. Behav Pharmacol. 1995;6:215–22. [PubMed] [Google Scholar]
- Burish TG, Maisto SA, Cooper AM, Sobell MB. Effects of voluntary short-term abstinence from alcohol on subsequent drinking patterns of college students. J Stud Alcohol. 1981;42:1013–20. doi: 10.15288/jsa.1981.42.1013. [DOI] [PubMed] [Google Scholar]
- Caberlotto L, Thorsell A, Rimondini R, Sommer W, Hyytia P, Heilig M. Diferential expression of NPY and its receptors in alcohol-preferring AA and alcohol-avoiding ANA rats. Alcohol Clin Exp Res. 2001;25:1564–9. [PubMed] [Google Scholar]
- Carvajal CC, Vercauteren F, Dumont Y, Michalkiewicz M, Quirion R. Aged neuropeptide Y transgenic rats are resistant to acute stress but maintain spatial and non-spatial learning. Behav Brain Res. 2004;153:471–80. doi: 10.1016/j.bbr.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Clark JT, Keaton AK, Sahu A, Kalra SP, Mahajan SC, Gudger JN. Neuropeptide Y (NPY) levels in alcoholic and food restricted male rats: implications for site selective function. Regul Pept. 1998;75–76:335–45. doi: 10.1016/s0167-0115(98)00086-x. [DOI] [PubMed] [Google Scholar]
- Dryga SA, Dryga OA, Schlesinger S. Identification of mutations in a Sindbis virus variant able to establish persistent infection in BHK cells: the importance of a mutation in the nsP2 gene. Virology. 1997;228:74–83. doi: 10.1006/viro.1996.8364. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Li TK, Lumeng L, et al. Neuropeptide Y levels in ethanol-naïve alcohol-preferring and nonpreferring rats and in Wistar rats after ethanol exposure. Alcohol Clin Exp Res. 1998;22:1778–82. [PubMed] [Google Scholar]
- Ehrengruber MU, Lundstrom K, Schweitzer C, Heuss C, Schlesinger S, Gahwiler BH. Recombinant Semliki Forest virus and Sindbis virus efficiently infect neurons in hippocampal slice cultures. Proc Natl Acad Sci USA. 1999;96:7041–6. doi: 10.1073/pnas.96.12.7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Stewart RB, Murphy JM, Badia-Elder NE. Neuropeptide Y in the paraventricular nucleus of the hypothalamus increases ethanol intake in high- and low-alcohol-drinking rats. Alcohol Clin Exp Res. 2004;28:1492–8. doi: 10.1097/01.alc.0000141813.27875.d5. [DOI] [PubMed] [Google Scholar]
- Heilig M. Antisense inhibition of neuropeptide Y (NPY)-Y1 receptor expression blocks the anxiolytic like action of NPY in amygdala and paradoxically increases feeding. Regul Pept. 1995;59:201–5. doi: 10.1016/0167-0115(95)00103-i. [DOI] [PubMed] [Google Scholar]
- Heilig M, Murison R. Intracerebroventricular neuropeptide Y protects against stress-induced gastric erosion in the rat. Eur J Pharmacol. 1987;137:127–9. doi: 10.1016/0014-2999(87)90191-9. [DOI] [PubMed] [Google Scholar]
- Heilig M, Soderpalm B, Engel JA, Widerlov E. Centrally administered neuropeptide Y (NPY) produces anxiolytic-like effects in animal anxiety models. Psychopharmacology (Berl) 1989;98:524–9. doi: 10.1007/BF00441953. [DOI] [PubMed] [Google Scholar]
- Heilig M, McLeod S, Brot M, et al. Anxiolytic-like action of neuropeptide Y: mediation by Y1 receptors in amygdala, and dissociation from food intake effects. Neuropsychopharmacology. 1993;8:357–63. doi: 10.1038/npp.1993.35. [DOI] [PubMed] [Google Scholar]
- Heilig M, McLeod S, Koob GK, Britton KT. Anxiolytic-like effect of neuropeptide Y (NPY), but not other peptides in an operant conflict test. Regul Pept. 1992;41:61–9. doi: 10.1016/0167-0115(92)90514-u. [DOI] [PubMed] [Google Scholar]
- Heyser CJ, Schulteis G, Koob GF. Increased ethanol self-administration after a period of imposed ethanol deprivation in rats trained in a limited access paradigm. Alcohol Clin Exp Res. 1997;21:784–91. [PubMed] [Google Scholar]
- Heyser CJ, Schulteis G, Durbin P, Koob GF. Chronic acamprosate eliminates the alcohol deprivation effect while having limited effects on baseline responding for ethanol in rats. Neuropsychopharmacology. 1998;18:125–33. doi: 10.1016/S0893-133X(97)00130-9. [DOI] [PubMed] [Google Scholar]
- Hwang BH, Zhang JK, Ehlers CL, Lumeng L, Li TK. Innate differences of neuropeptide Y (NPY) in hypothalamic nuclei and central nucleus of the amygdala between selectively bred rats with high and low alcohol preference. Alcohol Clin Exp Res. 1999;23:1023–30. [PubMed] [Google Scholar]
- Jeromin A, Yuan LL, Frick A, Pfaffinger P, Johnston D. A modified Sindbis vector for prolonged gene expression in neurons. J Neurophysiol. 2003;90:2741–5. doi: 10.1152/jn.00464.2003. [DOI] [PubMed] [Google Scholar]
- Katner SN, Slawecki CJ, Ehlers CL. Neuropeptide Y administration into the amygdala does not affect ethanol consumption. Alcohol. 2002a;28:29–38. doi: 10.1016/s0741-8329(02)00235-5. [DOI] [PubMed] [Google Scholar]
- Katner SN, Slawecki CJ, Ehlers CL. Neuropeptide Y administration into the third ventricle does not increase sucrose or ethanol self-administration but does affect the cortical EEG and increases food intake. Psychopharmacology (Berl) 2002b;160:146–54. doi: 10.1007/s00213-001-0950-9. [DOI] [PubMed] [Google Scholar]
- Kelley SP, Nannini MA, Bratt AM, Hodge CW. Neuropeptide-Y in the paraventricular nucleus increases ethanol self-administration. Peptides. 2001;22:515–22. doi: 10.1016/s0196-9781(01)00361-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornet M, Goosen C, Ribbens LG, van Ree JM. Analysis of spontaneous alcohol drinking in rhesus monkeys. Physiol Behav. 1990a;47:679–84. doi: 10.1016/0031-9384(90)90077-h. [DOI] [PubMed] [Google Scholar]
- Kornet M, Goosen C, van Ree JM. The effect of interrupted alcohol supply on spontaneous alcohol consumption by rhesus monkeys. Alcohol Alcohol. 1990b;25:407–12. [PubMed] [Google Scholar]
- Moller C, Wiklund L, Sommer W, Thorsell A, Heilig M. Decreased experimental anxiety and voluntary ethanol consumption in rats following central but not basolateral amygdala lesions. Brain Res. 1997;760:94–101. doi: 10.1016/s0006-8993(97)00308-9. [DOI] [PubMed] [Google Scholar]
- Murphy JM, Stewart RB, Bell RL, et al. Phenotypic and genotypic characterization of the Indiana University rat lines selectively bred for high and low alcohol preference. Behav Genet. 2002;32:363–88. doi: 10.1023/a:1020266306135. [DOI] [PubMed] [Google Scholar]
- Okada T, Yamada N, Kakegawa W, et al. Sindbis viral-mediated expression of Ca2+-permeable AMPA receptors at hippocampal CA1 synapses and induction of NMDA receptor-independent long-term potentiation. Eur J Neurosci. 2001;13:1635–43. doi: 10.1046/j.0953-816x.2001.01523.x. [DOI] [PubMed] [Google Scholar]
- Pandey SC. Anxiety and alcohol abuse disorders: a common role for CREB and its target, the neuropeptide Y gene. Trends Pharmacol Sci. 2003;24:456–60. doi: 10.1016/S0165-6147(03)00226-8. [DOI] [PubMed] [Google Scholar]
- Pandey SC, Carr LG, Heilig M, Ilveskoski E, Thiele TE. Neuropeptide y and alcoholism: genetic, molecular, and pharmacological evidence. Alcohol Clin Exp Res. 2003;27:149–54. doi: 10.1097/01.ALC.0000052706.21367.0E. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 3rd edn Academic Press; San Diego: 1997. [Google Scholar]
- Rimondini R, Arlinde C, Sommer W, Heilig M. Long-lasting increase in voluntary ethanol consumption and transcriptional regulation in the rat brain after intermittent exposure to alcohol. FASEB J. 2002;16:27–35. doi: 10.1096/fj.01-0593com. [DOI] [PubMed] [Google Scholar]
- Rimondini R, Thorsell A, Heilig M. Suppression of ethanol self-administration by the neuropeptide Y (NPY) Y2 receptor antagonist BIIE0246: evidence for sensitization in rats with a history of dependence. Neurosci Lett. 2005;375:129–33. doi: 10.1016/j.neulet.2004.10.084. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Heyser CJ, Cole M, Griffin P, Koob GF. Excessive ethanol drinking following a history of dependence: animal model of allostasis. Neuropsychopharmacology. 2000;22:581–94. doi: 10.1016/S0893-133X(99)00167-0. [DOI] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, Mckinzie DL, Murphy JM, McBride WJ, Lumeng L, Li TK. The expression of an alcohol deprivation effect in the high-alcohol-drinking replicate rat lines is dependent on repeated deprivations. Alcohol Clin Exp Res. 2000a;24:747–53. [PubMed] [Google Scholar]
- Rodd-Henricks ZA, Mckinzie DL, Shaikh SR, et al. Alcohol deprivation effect is prolonged in the alcohol preferring (P) rat after repeated deprivations. Alcohol Clin Exp Res. 2000b;24:8–16. [PubMed] [Google Scholar]
- Roy A, Pandey SC. The decreased cellular expression of neuropeptide Y protein in rat brain structures during ethanol withdrawal after chronic ethanol exposure. Alcohol Clin Exp Res. 2002;26:796–803. [PubMed] [Google Scholar]
- Sajdyk TJ, Vandergriff MG, Gehlert DR. Amygdalar neuropeptide Y Y1 receptors mediate the anxiolytic-like actions of neuropeptide Y in the social interaction test. Eur J Pharmacol. 1999;368:143–7. doi: 10.1016/s0014-2999(99)00018-7. [DOI] [PubMed] [Google Scholar]
- Salimov RM, Salimova NB. The alcohol-deprivation effect in hybrid mice. Drug Alcohol Depend. 1993;32:187–91. doi: 10.1016/0376-8716(93)80012-4. [DOI] [PubMed] [Google Scholar]
- Sinclair JD, Li TK. Long and short alcohol deprivation: effects on AA and P alcohol-preferring rats. Alcohol. 1989;6:505–9. doi: 10.1016/0741-8329(89)90059-1. [DOI] [PubMed] [Google Scholar]
- Sinclair JD, Walker S, Jordan W. Behavioral and physiological changes associated with various durations of alcohol deprivation in rats. Q J Stud Alcohol. 1973;34:744–57. [PubMed] [Google Scholar]
- Spanagel R, Zieglgansberger W. Anti-craving compounds for ethanol: new pharmacological tools to study addictive processes. Trends Pharmacol Sci. 1997;18:54–9. [PubMed] [Google Scholar]
- Thiele TE, Marsh DJ, Ste ML, Bernstein IL, Palmiter RD. Ethanol consumption and resistance are inversely related to neuropeptide Y levels. Nature. 1998;396:366–9. doi: 10.1038/24614. [DOI] [PubMed] [Google Scholar]
- Thorsell A, Michalkiewicz M, Dumont Y, et al. Behavioral insensitivity to restraint stress, absent fear suppression of behavior and impaired spatial learning in transgenic rats with hippocampal neuropeptide Y overexpression. Proc Natl Acad Sci USA. 2000;97:12852–7. doi: 10.1073/pnas.220232997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorsell A, Rimondini R, Heilig M. Blockade of central neuropeptide Y (NPY) Y2 receptors reduces ethanol self-administration in rats. Neurosci Lett. 2002;332:1–4. doi: 10.1016/s0304-3940(02)00904-7. [DOI] [PubMed] [Google Scholar]
- Thorsell A, Slawecki CJ, Ehlers CL. Effects of neuropeptide Y and corticotropin-releasing factor on ethanol intake in Wistar rats: interaction with chronic ethanol exposure. Behav Brain Res. 2005;161:133–40. doi: 10.1016/j.bbr.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Tovote P, Meyer M, Beck-Sickinger AG, et al. Central NPY receptor-mediated alteration of heart rate dynamics in mice during expression of fear conditioned to an auditory cue. Regul Pept. 2004;120:205–14. doi: 10.1016/j.regpep.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Wolffgramm J, Heyne A. From controlled drug intake to loss of control: the irreversible development of drug addiction in the rat. Behav Brain Res. 1995;70:77–94. doi: 10.1016/0166-4328(95)00131-c. [DOI] [PubMed] [Google Scholar]
- Xiong C, Levis R, Shen P, Schlesinger S, Rice CM, Huang HV. Sindbis virus: an efficient, broad host range vector for gene expression in animal cells. Science. 1989;243:1188–91. doi: 10.1126/science.2922607. [DOI] [PubMed] [Google Scholar]