Abstract
Summary
Successful mitosis requires that anaphase chromosomes sustain a commitment to move to their assigned spindle poles. This requires stable spindle attachment of anaphase kinetochores. Prior to anaphase, stable spindle attachment depends on tension created by opposing forces on sister kinetochores [1]. Because tension is lost when kinetochores disjoin, stable attachment in anaphase must have a different basis. After expression of nondegradable cyclin B (CYC-BS) in Drosophila embryos, sister chromosomes disjoined normally but their anaphase behavior was abnormal [2]. Chromosomes exhibited cycles of reorientation from one pole to the other. Additionally, the unpaired kinetochores accumulated attachments to both poles (merotelic attachments), congressed (again) to a pseudometaphase plate, and reacquired associations with checkpoint proteins more characteristic of prometaphase kinetochores. Unpaired prometaphase kinetochores, which occurred in a mutant entering mitosis with unreplicated (unpaired) chromosomes, behaved just like the anaphase kinetochores at the CYC-BS arrest. Finally, the normal anaphase release of AuroraB/INCENP from kinetochores was blocked by CYC-BS expression and, reciprocally, was advanced in a CycB mutant. Given its established role in destabilizing kinetochore-microtubule interactions [3], Aurora B dissociation is likely to be key to the change in kinetochore behavior. These findings show that, in addition to loss of sister chromosome cohesion, successful anaphase requires a kinetochore behavioral transition triggered by CYC-B destruction.
Results and Discussion
CYC-B Maintains AuroraB/INCENP Localization at the Kinetochore
Stable cyclins have been shown to block mitotic exit in numerous systems [4-8], and detailed analyses of the cytological consequence of stabilization of each of the cognate mitotic cyclins of Drosophila have begun to reveal regulatory features that were not evident in other experimental systems [2, 9].
A group of chromosomal “passenger proteins” that are localized between paired kinetochores at metaphase usually relocalizes to the central spindle upon onset of anaphase [10]. Previous work showed that this relocalization is blocked upon expression of stable sea urchin cyclin B in mammalian cells [11, 12]. In agreement with this, expression of Drosophila CYC-BS in Drosophila embryos blocked relocalization of two interacting passenger proteins, INCENP (Figures 1A and 1B; Figure S1A in the Supplemental Data) and Aurora B (Figure S1E). Normal metaphase foci of INCENP split in two at anaphase, half segregating with each sister kinetochore without relocalization to the spindle (Figure 1B). Failure to release kinetochore-localized AuroraB/INCENP and a slowing of anaphase A chromosome movements [2] are the earliest perturbations of mitotic progression observed upon CYC-BS expression. The onset of these defects immediately follows or overlaps the time of destruction of normal CYC-B.
Figure 1.
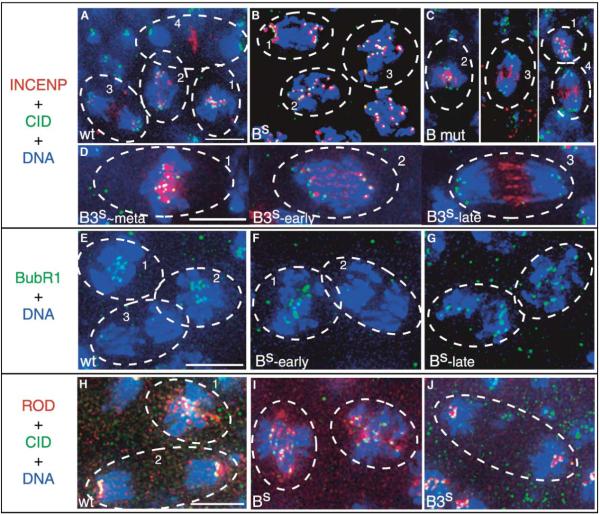
CYC-B Destruction Is Required for Normal Anaphase Distributions of INCENP, BubR1 and ROD
(A-D) INCENP localization in wild-type (A), CYC-BS-expressing (B), CycB mutant (C), and CYC-B3S-expressing (D) cells. INCENP staining is in red, Centromere Identifier (CID) staining in green, and Hoechst staining of DNA in blue (see Figures S1A and S1B for separated channels and tubulin staining). (A) INCENP, which is localized between paired CID staining foci in metaphase (cell #1), disengages from kinetochores during anaphase (cells 2 and 3) to become strongly localized at the spindle midzone by telophase (cell 4) [29]. (B) INCENP foci remain associated with kinetochores in cells expressing CYC-BS. Cell 1 is late anaphase A (i.e., prior to final arrest). Cells 2 and 3 are at a later stage of arrest, with kinetochores largely congregated at a pseudometaphase plate (note the absence of the pairing of CID staining foci as in the orderly arrangement seen in cell 1 of panel A). (C) INCENP, whereas kinetochore-localized during metaphase (cell 1) in CycB null mutant cells, relocalizes prematurely during anaphase (cells 1-3). (D) Cells expressing another stable cyclin, CYC-B3S, show normal anaphase movements and INCENP relocalization as they progress (cells 2 and 3) from metaphase cell 1 to an arrest with chromosomes at separated poles [2]. In all cases examined, Aurora B staining shows the same localization as that for INCENP (Figure S1E and our unpublished data).
(E-G) BubR1 is lost from kinetochores during early anaphase in wild-type (E) and CYC-BS-expressing (F) cells, but kinetochore localization is later restored in CYC-BS-arrested cells (G). BubR1 staining is in green, and Hoechst staining of the DNA is in blue (see Figure S1C for separated channels and tubulin staining). (E) In the wild-type, BubR1 localizes to the kinetochores in metaphase (cells 1 and 2), but is absent from the kinetochores during anaphase (cell 3). (F) In cells expressing CYC-BS, BubR1 localizes normally in metaphase (cell 1) and is absent from the kinetochores during the early anaphase A movements (cell 2). Note that we documented many CYC-BS-expressing cells in early anaphase, and reduction in kinetochore staining by Bub-R1 antibody at this stage is a general finding. (G) BubR1 again localizes to the kinetochores in cells arrested with CYC-BS.
(H-J) ROD localization in wild-type (H), CYC-BS-expressing (I), and CYC-B3S-expressing (J) cells. ROD staining is in red, Hoechst staining of the DNA in blue, and CID staining in green (see Figure S1D for separated channels and tubulin staining). (H) ROD staining is on the kinetochores and along the spindle during metaphase (cell 1), but it is only on the kinetochores during anaphase (cell 2) in wild-type cells. (I) ROD staining in cells arrested with CYC-BS; the staining is localized to some of the kinetochores with some dispersion along the spindle. (J) ROD staining can be seen only at the kinetochores in cells arrested with CYC-B3S. In all cases examined, ZW10 staining shows the same localization as that for ROD (our unpublished data). Heat shock and antibody-staining protocols have been previously described [2]. Antibodies were generously provided by Gary Karpen (chicken α-CID), Bill Earnshaw (rabbit α-INCENP), Claudio Sunkel (rabbit α-BubR1), Roger Karess (rabbit α-ROD), and David Glover (rabbit α-Aurora B). Homozygous cyclin B null mutants were identified by the absence of a balancer chromosome expressing lacZ under the control of the ftz promoter, detected by β-galactosidase staining in a striped pattern. The scale bars indicate 5 μm.
Embryos expressing a different stabilized mitotic cyclin, CYC-B3S, arrested with chromosomes at the spindle poles after normal anaphase movements [2] and normal redistribution of AuroraB/INCENP from the kinetochore to the spindle midzone (Figures 1D and S1B). Thus, CYC-BS and not CYC-B3S maintains kinetochore localization of AuroraB/INCENP.
As a result of partial redundancy among Drosophila cyclins, CycB null mutants undergo mitosis [13]. As in wild-type, AuroraB/INCENP is associated with kinetochores in metaphase cells lacking CYC-B; however, its anaphase relocalization occurs prematurely (compare Figures 1A and 1C). Thus, the endogenous CYC-B in the wild-type inhibits AuroraB/INCENP relocalization, and relocalization appears to await its destruction. Together, precocious relocalization in the CycB mutant, coincidence in the onset of relocalization and the time of CYC-B destruction, and the block to relocalization by persistent CYC-B lead us to conclude that CYC-B destruction times AuroraB/INCENP relocalization.
CYC-B Degradation Switches Chromosomes from Prometaphase to Anaphase Behavior
The dramatic transition in kinetochore-protein interactions upon destruction of CYC-B might serve only to release the sequestered passenger proteins to play their important function at the spindle midzone in cytokinesis [14, 15]. However, elegant studies of Ipl1, the Aurora B kinase homolog of yeast, suggest that Ipl1 can destabilize kinetochore interactions with the spindle [3, 16, 17]. These studies, as well as supporting work in vertebrate cells [18, 19], suggest that loss of Aurora B function upon CYC-B destruction might alter kinetochore behavior. Indeed, our results suggest that CYC-B destruction does have an important influence on anaphase chromosome behavior.
As we previously described, when Drosophila cells enter anaphase in the presence of CYC-BS, poleward movement of unpaired chromosomes is abortive and chromosome behavior is unusual [2]. We suggested that this chromosome behavior might represent an extension of prometaphase/metaphase behavior, differing only in so far as the loss of kinetochore pairing at metaphase/anaphase alters the behavior. The behavior of unpaired prometaphase kinetochores was previously examined in a mutant in maize exhibiting premature loss of chromosome pairing and after microsurgical production of single kinetochore chromosomes in mammalian cells [20, 21]. In these experiments, single-kinetochore chromosomes behaved much as the chromosomes of Drosophila cells that progress to anaphase (to produce unpaired kinetochores) in the presence of CYC-BS [2]. To further test this parallel, we examined the Drosophila mutant, double parked, in which unpaired chromosomes exist in prometaphase. Double Parked is an essential replication protein that is also required for a checkpoint function that ordinarily prevents cells from entering mitosis with unreplicated DNA, and like analogous mutants in S. cerevisiae (e.g., cdc6), Drosophila cells lacking Double Parked enter mitosis with unreplicated DNA [22]. When a maternal supply of Double Parked is depleted, replication fails in double parked embryos and cells accumulate in mitosis (Figure 2A). The mitotic arrest occurs because unpaired chromosomes are incapable of normal bipolar alignment and consequently induce the spindle checkpoint [22, 23].
Figure 2.
Unreplicated Chromosomes in double parked Mitosis Behave Like Disjoined Chromosomes at CYC-BS Arrest
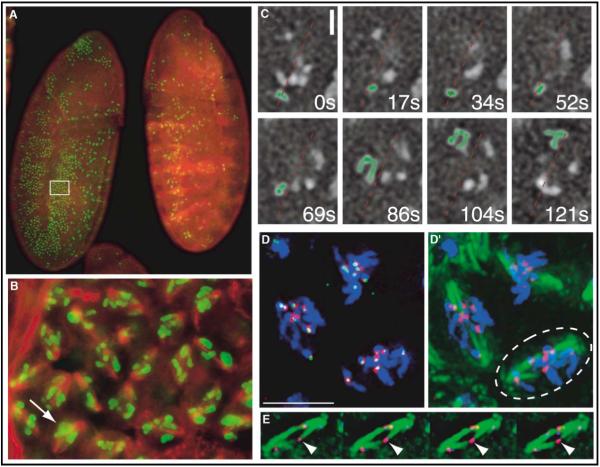
The double parked mutant embryo fails to replicate DNA in S phase of cycle 16 and arrests in the subsequent mitosis [22]. (A) A double parked mutant embryo (left) and a control embryo (right) are stained for phosphohistone H3, which specifically labels mitotic chromosomes, in green and for α-tubulin in red. The mutant embryos, which lack a balancer chromosome, were identified by the absence of stripes of β-galactosidase staining (also red; see control embryo) expressed from a transgene (ftz-lacZ) on the balancer chromosome. (B) A region (boxed) of the double parked mutant embryo from (A) is enlarged to show deranged distribution of chromosomes along the spindle. The arrow indicates clustering of chromosomes at a pseudometaphase plate in some cells. (C) Frames from a real-time movie (Movie 1 in the Supplemental Data) showing dynamic chromosome movements in the double parked mutant mitotic arrest. The approximate spindle orientation is given with the dotted red line. The highlighted chromosome (artificially green), initially oriented toward the lower pole in the first four frames, is seen to undergo spindle reorientation and rapid movement toward the upper pole in the final four frames. The time indicated is given in seconds elapsed from the start of the series. Standard crossing schemes were used to create a line of flies carrying the dupa1 mutation and the histone-GFP transgene for live analysis. The scale bar indicates 2 μm. (D) INCENP staining (red) in a double parked mutant cell showing association of INCENP foci with CID staining (green) of kinetochores (DNA is stained in blue). The image is a projection of multiple focal planes. The scale bar indicates 5 μm. (D’) The same image as (D), but with tubulin staining in green showing merotelic attachments of kinetochores to the spindle. (E) Four focal planes of a cell from (D’) (circled) showing that the kinetochore marked by the arrowhead is associated with kinetochore fibers from both poles. See Figure S2 for the separated channels.
In fixed images of the double parked arrest, most chromosomes were scattered along the spindle, with some clustered in a central pseudometaphase plate (Figures 2B and 2D), just as in CYC-BS-arrested cells [2]. Real-time analysis showed that this is a dynamic situation, with chromosomes making oscillatory movements between the poles (Figure 2C, supplementary data Movie 1). For example, during the double parked arrest, a chromosome highlighted in green in Figure 2C lingered near the lower pole during four frames (52 s), then moved abruptly toward the opposite pole over the next few frames (most movement was within 35 s, between 69 and 104 s). This chromosome movement between the poles resembles that observed during the CYC-BS block and is consistent with reorientation of the kinetochore from one pole to the other, as has been described for prometaphase chromosomes [24].
Despite the absence of prior replication, INCENP and Aurora B localized to the unpaired kinetochores in the double parked arrest (Figures 2D, 2D’, and S2 and our unpublished data), as in the CYC-BS arrest. Furthermore, despite the presence of only a single kinetochore, many of the chromosomes congress to a pseudometaphase plate in double parked and CYC-BS arrests (Figures 2 and 3). We conclude that, when CYC-B persists, unpaired chromosomes behave similarly before and after the metaphase/anaphase transition.
Figure 3.
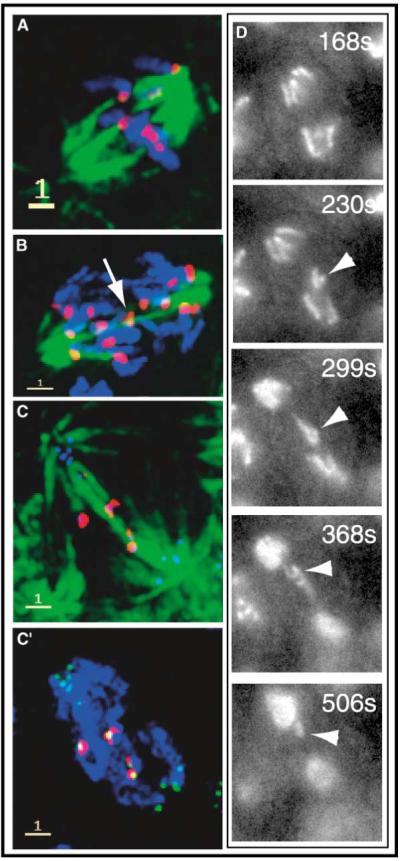
CYC-BS Promotes Attachment of Unpaired Anaphase Kinetochores to Both Spindle Poles and Increases Anomalous Segregation.
In the CYC-BS arrest, kinetochores frequently make merotelic attachments to spindle microtubules, and these kinetochores stain strongly for INCENP. Spindle staining is shown in green (A-C), INCENP staining in red (A-D), DNA staining in blue (A, B, and C’), and CID kinetochore staining in blue (C) or green (C’). (A) A CYC-BS-arrested cell with disjoined chromosomes in a pseudometaphase plate and a spindle exhibiting robust kinetochore fibers impinging on the unpaired kinetochores. (B) A CYC-BS-expressing cell early in the progress toward arrest. One kinetochore with merotelic attachments (arrow) lies at the spindle midzone. Presumably, this is one of the first of the disjoined chromosomes to return to the midzone after earlier anaphase movements [2]. (C and C’) This cell appears to be disrupted (and probably retarded) but not arrested by CYC-BS expression, as suggested by the presence of some decondensing chromosomes at the poles. A few kinetochores remain localized in the middle of the spindle, and only these stain for INCENP. (D) Frames from a movie of a cell proceeding through mitosis despite expression of CYC-BS. The times are approximate times after metaphase and indicate greatly retarded anaphase (about three times normal). Near the end of anaphase movements, a chromosome (arrowheads) can be seen to make a movement from the lower pole toward the upper pole. We believe this phenomenon to be analogous to the fixed image shown in (C) and to represent CYC-BS-stimulated reorientation of a single chromosome instead of the entire complement. Scale bars on the fixed images indicate 1 μm. See Figure S3 for separated channels and an additional example.
Although it was somewhat puzzling that some chromosomes congressed to a pseudometaphase plate in double parked embryos, a similar observation was made when single kinetochore chromosomes were present in prometaphase in mammals [20]. These congressed single kinetochore chromosomes had attachments to both poles (merotelic attachment). We observed robust kinetochore fibers in double parked spindles, and in cases that were not confounded by the clustering of chromosomes in the middle, it was apparent that kinetochore fibers from both poles impinged on single kinetochores (Figures 2E and S2). We interpret these observations as an indication of frequent merotelic attachment in the double parked arrest and have made similar findings in the CYC-BS-arrested cells (see below).
The finding that merotelic attachments accumulate in the double parked arrest suggests that kinetochore pairing normally helps to prevent merotelic attachments under prometaphase conditions. We suggest that such an effect could be explained by an extension of the idea that trial and error processes contribute to bipolar attachment of paired kinetochores in prometaphase [1]. Because kinetochore-spindle interactions are unstable in prometaphase, all modes of attachment can be sampled, at least transiently, but the most stable mode ultimately predominates. Consequently, the most stable (correct bipolar attachment) precludes less stable and incorrect attachments (Figure 4A). Spindle tension stabilizes attachment, and it has been suggested [3] that, upon bipolar arrangement, tension deforms the paired kinetochore, effectively “pulling” the attachment sites away from a centrally localized destabilizing activity (Figure 4). Although tension also deforms a merotelically attached kinetochore [20, 21, 25], we suggest that the distortion is not as orderly as in bipolar attachment and that the separation from the destabilizing activity is less effective. Consequently, when kinetochores are paired, bipolar attachments will accumulate as the most stable outcome and hence exclude merotelic attachments. When kinetochores are unpaired, the dynamics of formation and decay of merotelic attachments appears to favor their accumulation.
Figure 4.
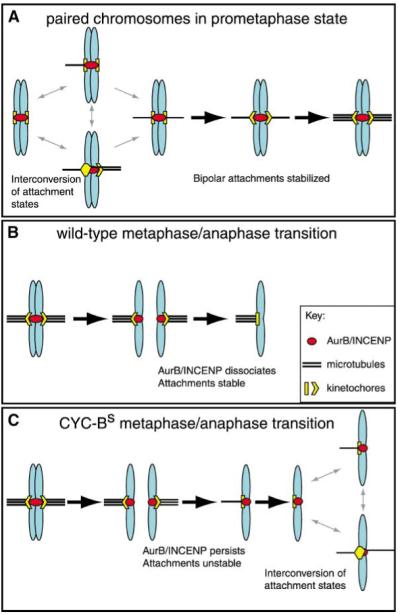
Model of Kinetochore-Spindle Interactions
A model illustrating the different states of kinetochore-spindle interactions in prometaphase and anaphase. Chromosomes are shown in blue, microtubules in black, kinetochores in yellow, and the AuroraB/INCENP complex in red. (A) In prometaphase, destabilization of inappropriate attachments by the AuroraB/INCENP complex allows interconversion of the different attachment states until bipolar attachment is achieved. At that point tension pulls the sister kinetochores away from the AuroraB/INCENP complex, more microtubules are recruited, and the bipolar state is stabilized (as previously proposed [3]). (B) After the metaphase/anaphase transition in the wild-type, the AuroraB/INCENP complex dissociates from the kinetochores, ensuring stable attachment during anaphase. (C) In the presence of CYC-BS, the AuroraB/INCENP complex does not dissociate from the kinetochores, and the instability that characterizes prometaphase kinetochore-spindle interaction now persists. Because the disjoined kinetochores are unpaired, attachments interconvert without the option of normal bipolar attachment. As is the case with unreplicated prometaphase chromosomes in the double parked mutant, this interconversion (gray double-headed arrows at the right) leads to disorderly chromosome movements and merotelic attachments.
Checkpoint Reactivation in CYC-BS-Arrested Cells
Prior to the time at which CYC-B is usually degraded, we see no defects in mitotic progression in cells expressing CYC-BS. Sister chromatids separate from one another, and other substrates of the APC/C are degraded [2, 8]. The dissociation of BubR1 from kinetochores marks the release of checkpoint control. CYC-BS-expressing cells having an anaphase configuration (prior to final arrest) had a greatly decreased level of kinetochore staining (Figures 1F and S1C and our unpublished data). However, at the final arrest point, BubR1 again localized to the kinetochores (Figures 1G and S1C and our unpublished data). BubR1 staining did not completely disappear during anaphase, and levels at final arrest did not match the highest levels at prometaphase (our unpublished data). Nevertheless, since a return of BubR1 to the kinetochore after sister chromatid separation was never observed in wild-type cells, there appears to be some reactivation of the checkpoint at the CYC-BS arrest.
Looking for additional reporters of checkpoint activity, we probed for ROD (Figures 1H-1J and S1D) and ZW10 (our unpublished data), components of a mitotic checkpoint that relocalize in a manner suggesting a role in sensing tension [26, 27]. In prometaphase cells, a ROD/ZW10 complex localizes tightly to kinetochores. As chromosomes develop bipolar attachment, the kinetochore staining for ROD/ZW10 is reduced, and staining appears on kinetochore fibers. Upon disjunction of sister chromosomes, the staining returns to the kinetochores. All of these events appeared to occur normally in CYC-BS- and CYC-B3S-expressing cells as they progressed toward an arrest (our unpublished data). However, during the course of arrest with CYC-BS, but not with CYC-B3S, spindle microtubules once again stained for ROD/ZW10 (Figure 1I versus 1J). The spindle localization of ROD/ZW10 in the CYC-BS arrest is another example of checkpoint components that have reverted to their characteristic preanaphase localization and further suggests that some aspects of the checkpoint have been reactivated, perhaps in response to defective chromosome-spindle interactions.
CYC-BS-Arrested Cells Display Merotelic Spindle Attachments
Spindle staining of ROD/ZW10 during metaphase has been shown to require bipolar attachment and perhaps tension across the kinetochores [26, 27]. The spindle staining in cells at the CYC-BS arrest could imply some level of bipolar attachment. Initially, this seemed unlikely because the anaphase chromosomes are unpaired and contain only one kinetochore when at the arrest point. However, staining for kinetochores and microtubules showed robust kinetochore fibers extending from both poles to the pseudometaphase plate, suggesting merotelic attachment (Figures 3A and S3).
Merotelic attachments were obvious when only one or a few chromosomes remained near the middle of the spindle (Figures 3B, 3C, and S3), as occurred frequently early after the transition to anaphase in the presence of CYC-BS (Figure 3B) and when the arrest was less complete (Figure 3C). At the level of CYC-BS expression in our experiments, some cells are not fully arrested [2]. Live observations revealed occasional cells with slow mitotic progress but without the full complement of arrest behaviors. These prolonged mitoses show a high frequency of chromosome segregation anomalies. For example, Figure 3D shows frames from a movie in which chromosomes separated after a prolonged anaphase A, as seen in cells destined for CYC-BS arrest [2]; however, unlike the complete arrest in which all the chromosomes lose their poleward orientation, most of the chromosomes remained at the poles and decondensed while a single chromosome moved from one pole to the other. Kinetochores successfully retained at the pole after CYC-BS expression lacked INCENP and BubR1, whereas chromosomes localized to the middle of the spindle displayed merotelic attachments and stained strongly for INCENP and BubR1 (Figures 3C, 3C’, and S3). The presence of two categories of kinetochore, one having and one lacking INCENP, suggests that there is a switch-like event at individual kinetochores and that the cells with an incomplete arrest are near the threshold of the switch.
Our findings show that CYC-BS promotes merotelic attachments, which accumulate after the initially successful chromosome disjunction at the transition to anaphase. Furthermore, our results are consistent with proposals that merotelic attachments underlie congression of chromosomes with a single kinetochore [20] and disrupt chromosome segregation [25]. We suggest that the accumulation of merotelic attachments at the CYC-BS arrest is the consequence of persistence of the dynamic phase of kinetochore spindle attachment beyond the time of sister kinetochore disjunction. Rather than preserving the established monopolar orientation of the anaphase kinetochores, persistence of dynamic exchange favors change toward the arrangements that are most stable for unpaired kinetochores, and one such arrangement is merotelic attachment (Figure 4).
Conclusions
Our results show that a change in kinetochore composition and behavior accompanies the metaphase/anaphase transition and that a change in kinetochore behavior is essential for the unerring commitment of chromosomes to their assigned poles. Because the success of mitosis depends on this change, we think of the transition as an integral part of the metaphase/anaphase transition. We show that destruction of CYC-B triggers and times the kinetochore transition at the onset of anaphase and that a second mitotic cyclin, CYC-B3, does not govern this kinetochore transition. The kinetochore transition is coordinated with the disjunction of sister chromosomes as a result of their common regulation by APC/C, which promotes the destruction of CYC-B as well as the sister cohesion regulators, securin and cyclin A [2, 28]. The change in kinetochore behavior can be understood as a change from dynamically exchanging tension-stabilized attachment to fixed stable attachment. The striking coupling of this change with the release of Aurora B/INCENP from the kinetochore, and the identified role of Aurora B kinase in destabilizing kinetochore spindle attachments [3], suggests a plausible mechanism in which the dissociation of Aurora B stabilizes spindle attachments. However, a stable derivative of the sea urchin cyclin B did not produce similar modifications of chromosome behavior in mammalian cells despite blocking the release of GFP-Aurora B from the kinetochores [12]. Clearly, further work is required to elucidate the regulatory paths connecting kinetochore behavior with CYC-B destruction.
We found that unpaired chromosomes developed merotelic attachments whenever AuroraB/INCENP is associated with unpaired kinetochores, whether this occurs in anaphase as a result of CYC-BS expression or in prophase as a result of a failure in DNA replication (in the double parked arrest). We suggest that kinetochore pairing influences the outcome of dynamic reassortment of kinetochore attachments (Figure 4). Evidently, it is important to stabilize kinetochore-spindle attachments upon disjunction of sisters; otherwise attachments reequilibrate to the most stable states available to unpaired kinetochores, including merotelic attachments.
Supplementary Material
Acknowledgments
We thank numerous colleagues (as stated) for reagents, and Dave Morgan, Renny Feldman, and Arnaud Echard for helpful comments. D.H.P. was supported by the Dr. and Mrs. Bernard Kramer Scholarship from the Achievement Rewards for College Scientists Foundation and by National Institutes for Health T32HD07470, and the work was supported by National Institutes of Health grant GM37193 to P.H.O.
References
- 1.Nicklas RB. How cells get the right chromosomes. Science. 1997;275:632–637. doi: 10.1126/science.275.5300.632. [DOI] [PubMed] [Google Scholar]
- 2.Parry DH, O’Farrell PH. The schedule of destruction of three mitotic cyclins can dictate the timing of events during exit from mitosis. Curr. Biol. 2001;11:671–683. doi: 10.1016/s0960-9822(01)00204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tanaka TU, Rachidi N, Janke C, Pereira G, Galova M, Schiebel E, Stark MJ, Nasmyth K. Evidence that the Ipl1-Sli15 (Aurora kinase-INCENP) complex promotes chromosome bi-orientation by altering kinetochore-spindle pole connections. Cell. 2002;108:317–329. doi: 10.1016/s0092-8674(02)00633-5. [DOI] [PubMed] [Google Scholar]
- 4.Murray AW, Solomon MJ, Kirschner MW. The role of cyclin synthesis and degradation in the control of maturation promoting factor activity. Nature. 1989;339:280–286. doi: 10.1038/339280a0. [DOI] [PubMed] [Google Scholar]
- 5.Ghiara JB, Richardson HE, Sugimoto K, Henze M, Lew DJ, Wittenberg C, Reed SI. A cyclin B homolog in S. cerevisiae: chronic activation of the Cdc28 protein kinase by cyclin prevents exit from mitosis. Cell. 1991;65:163–174. doi: 10.1016/0092-8674(91)90417-w. [DOI] [PubMed] [Google Scholar]
- 6.Gallant P, Nigg EA. Cyclin B2 undergoes cell cycle-dependent nuclear translocation and, when expressed as a non-destructible mutant, causes mitotic arrest in HeLa cells. J. Cell Biol. 1992;117:213–224. doi: 10.1083/jcb.117.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hinchcliffe EH, Cassels GO, Rieder CL, Sluder G. The coordination of centrosome reproduction with nuclear events of the cell cycle in the sea urchin zygote. J. Cell Biol. 1998;140:1417–1426. doi: 10.1083/jcb.140.6.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sigrist S, Jacobs H, Stratmann R, Lehner CF. Exit from mitosis is regulated by Drosophila fizzy and the sequential destruction of cyclins A, B and B3. EMBO J. 1995;14:4827–4838. doi: 10.1002/j.1460-2075.1995.tb00164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Echard A, O’Farrell PH. The degradation of two mitotic cyclins contributes to the timing of cytokinesis. Curr. Biol. 2003 doi: 10.1016/s0960-9822(03)00127-1. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adams RR, Carmena M, Earnshaw WC. Chromosomal passengers and the (aurora) ABCs of mitosis. Trends Cell Biol. 2001;11:49–54. doi: 10.1016/s0962-8924(00)01880-8. [DOI] [PubMed] [Google Scholar]
- 11.Wheatley SP, Hinchcliffe EH, Glotzer M, Hyman AA, Sluder G, Wang Y. CDK1 inactivation regulates anaphase spindle dynamics and cytokinesis in vivo. J. Cell Biol. 1997;138:385–393. doi: 10.1083/jcb.138.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murata-Hori M, Tatsuka M, Wang YL. Probing the dynamics and functions of aurora B kinase in living cells during mitosis and cytokinesis. Mol. Biol. Cell. 2002;13:1099–1108. doi: 10.1091/mbc.01-09-0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacobs HW, Knoblich JA, Lehner CF. Drosophila Cyclin B3 is required for female fertility and is dispensable for mitosis like Cyclin B. Genes Dev. 1998;12:3741–3751. doi: 10.1101/gad.12.23.3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaitna S, Mendoza M, Jantsch-Plunger V, Glotzer M. Incenp and an aurora-like kinase form a complex essential for chromosome segregation and efficient completion of cytokinesis. Curr. Biol. 2000;10:1172–1181. doi: 10.1016/s0960-9822(00)00721-1. [DOI] [PubMed] [Google Scholar]
- 15.Severson AF, Hamill DR, Carter JC, Schumacher J, Bowerman B. The aurora-related kinase AIR-2 recruits ZEN-4/CeMKLP1 to the mitotic spindle at metaphase and is required for cytokinesis. Curr. Biol. 2000;10:1162–1171. doi: 10.1016/s0960-9822(00)00715-6. [DOI] [PubMed] [Google Scholar]
- 16.Biggins S, Severin FF, Bhalla N, Sassoon I, Hyman AA, Murray AW. The conserved protein kinase Ipl1 regulates microtubule binding to kinetochores in budding yeast. Genes Dev. 1999;13:532–544. doi: 10.1101/gad.13.5.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kang J, Cheeseman IM, Kallstrom G, Velmurugan S, Barnes G, Chan CS. Functional cooperation of Dam1, Ipl1, and the inner centromere protein (INCENP)-related protein Sli15 during chromosome segregation. J. Cell Biol. 2001;155:763–774. doi: 10.1083/jcb.200105029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murata-Hori M, Wang YL. The kinase activity of aurora B is required for kinetochore-microtubule interactions during mitosis. Curr. Biol. 2002;12:894–899. doi: 10.1016/s0960-9822(02)00848-5. [DOI] [PubMed] [Google Scholar]
- 19.Kallio MJ, McCleland ML, Stukenberg PT, Gorbsky GJ. Inhibition of aurora B kinase blocks chromosome segregation, overrides the spindle checkpoint, and perturbs microtubule dynamics in mitosis. Curr. Biol. 2002;12:900–905. doi: 10.1016/s0960-9822(02)00887-4. [DOI] [PubMed] [Google Scholar]
- 20.Khodjakov A, Cole RW, McEwen BF, Buttle KF, Rieder CL. Chromosome fragments possessing only one kinetochore can congress to the spindle equator. J. Cell Biol. 1997;136:229–240. doi: 10.1083/jcb.136.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu HG, Dawe RK. Functional redundancy in the maize meiotic kinetochore. J. Cell Biol. 2000;151:131–142. doi: 10.1083/jcb.151.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Whittaker AJ, Royzman I, Orr-Weaver TL. Drosophila double parked: a conserved, essential replication protein that colocalizes with the origin recognition complex and links DNA replication with mitosis and the down-regulation of S phase transcripts. Genes Dev. 2000;14:1765–1776. [PMC free article] [PubMed] [Google Scholar]
- 23.Garner M, van Kreeveld S, Su TT. mei-41 and bub1 block mitosis at two distinct steps in response to incomplete DNA replication in Drosophila embryos. Curr. Biol. 2001;11:1595–1599. doi: 10.1016/s0960-9822(01)00483-3. [DOI] [PubMed] [Google Scholar]
- 24.Nicklas RB. Recurrent pole-to-pole movements of the sex chromosome during prometaphase I in Melanoplus Differentialis spermatocytes. Chromosoma. 1961;12:97–115. doi: 10.1007/BF00328917. [DOI] [PubMed] [Google Scholar]
- 25.Cimini D, Howell B, Maddox P, Khodjakov A, Degrassi F, Salmon ED. Merotelic kinetochore orientation is a major mechanism of aneuploidy in mitotic mammalian tissue cells. J. Cell Biol. 2001;153:517–527. doi: 10.1083/jcb.153.3.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williams BC, Gatti M, Goldberg ML. Bipolar spindle attachments affect redistributions of ZW10, a Drosophila centromere/kinetochore component required for accurate chromosome segregation. J. Cell Biol. 1996;134:1127–1140. doi: 10.1083/jcb.134.5.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scaerou F, Aguilera I, Saunders R, Kane N, Blottiere L, Karess R. The rough deal protein is a new kinetochore component required for accurate chromosome segregation in Drosophila. J. Cell Sci. 1999;112:3757–3768. doi: 10.1242/jcs.112.21.3757. [DOI] [PubMed] [Google Scholar]
- 28.Nasmyth K, Peters JM, Uhlmann F. Splitting the chromosome: cutting the ties that bind sister chromatids. Science. 2000;288:1379–1385. doi: 10.1126/science.288.5470.1379. [DOI] [PubMed] [Google Scholar]
- 29.Cooke CA, Heck MM, Earnshaw WC. The inner centromere protein (INCENP) antigens: movement from inner centromere to midbody during mitosis. J. Cell Biol. 1987;105:2053–2067. doi: 10.1083/jcb.105.5.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.