Abstract
Limb development is a complex process involving precise control of both patterning and growth. Great strides have been made in understanding limb morphogenesis and identifying essential patterning genes in Drosophila. Differential expression of these genes divides the future limb into territories, which will give rise to different regions of the adult appendage. Recent analyses have defined the role of territorial boundaries as organizers of both patterning and growth, highlighting the connection between these two processes. The organizing activity of territorial boundaries seems to be mediated through the activity of two locally produced morphogens: Wingless and Decapentaplegic. We propose a model in which these two molecules, distributed in a graded fashion, act in synergy to promote growth of the entire appendage. We also suggest the existence of growth inhibitors that counteract the action of Wingless and Decapentaplegic; by opposing the gradient of these growth factors, the inhibitors guide the near-uniform proliferation that shapes the imaginal discs from which the adult appendages are formed in Drosophila.
Introduction
Morphogenesis — the formation of biological shape and structure — involves the control of processes such as epithelial folding, cell movement, cell death and growth. The size and shape of an appendage, for example, will be determined by where, when and to what degree these processes occur. The program for each of these processes is guided by signaling molecules that act as morphogens to direct the development of particular structures. The actions of morphogenetic molecules have been investigated in the development of appendages in both vertebrates and the fruitfly Drosophila. As large increases in cell number and size accompany the development of appendages, patterning of these structures occurs in the context of extensive growth. In recent years, this field has witnessed a number of spectacular advances, which have identified the key patterning molecules that direct morphogenesis of wings and legs in Drosophila, revealed the interactions that direct their localized expression, and demonstrated that their graded concentrations govern pattern. These advances allow us to consider the basis of the coordination of growth with patterning.
Here we review observations demonstrating that growth and patterning are tightly coupled during appendage formation. We focus on indications that two cell signaling molecules, Decapentaplegic (Dpp) and Wingless (Wg), act as key regulators of both pattern and growth, and we delve into the enigmatic relationship between these localized regulators and the near-uniform growth that they promote. We shall advocate three principles. First, that interactions between different territories create developmental organizers that direct the fates of surrounding cells. Second, that the organizers also direct and coordinate growth to produce structures of an appropriate size and shape. Third, that the regulation of growth and the regulation of morphological pattern are interwoven.
Embryonic organizers
Subdivisions of an epithelium produce organizers
Embryonic organizers have classically been defined as groups of cells that, after transplantation to an ectopic location, can reorganize the surrounding tissue to form structures reminiscent of those found at or around their original location. Spemann and Mangold [1] provided one of the first examples of an organizer by showing that, in a frog embryo, the dorsal lip of the blastopore can induce the formation of a new body axis if transplanted to a new position. Similarly, cells from the posterior margin of a chick limb bud have the capacity to reorganize anterior–posterior pattern after transplantation to the anterior margin [2]. A different approach to studying organizers has been undertaken in Drosophila, where genetic rather than transplantation experiments have proven to be most useful. Analysis of the patterning of adult structures has revealed the existence of organizers in Drosophila and has provided insights into how an organizer can be produced and can function.
Although the morphogenesis and differentiation of structures such as wings and legs occur during pupal stages, we focus on the earlier growth and assignment of cell fate that take place during the larval stages. Discrete groups of precursor cells, the imaginal discs primordia, are set apart during embryogenesis [3]. During the subsequent larval stages, the discs grow about a thousand fold as a convoluted epithelial monolayer. Importantly, although the cells lack marked distinctions, genetic analysis and gene expression studies have revealed that the disc epithelium is divided into territories, each expressing different developmental genes. We briefly summarize the process by which these territories form and focus on the interactions at the points where different territories meet; it is at these interfaces between adjacent territories that organizers are formed.
Territories of cells
The division of discs into territories follows cues laid down when embryonic patterning partitions the embryo into striped zones of localized gene expression [4]. For example, the patch of cells (about 20) destined to form the leg disc occurs at a point uniquely defined by stripes of expression of three important patterning molecules: Engrailed (En), Wg and Dpp. The stripe of En-expressing cells runs through the posterior part of the patch. The Dpp stripe forms at a right angle to this and runs through the dorsal cells of the patch. Finally, a stripe of Wg runs parallel to the En stripe through the anterior part of the future disc, but an interruption in this stripe limits it to ventral cells of the patch (Fig. 1).
Figure 1.
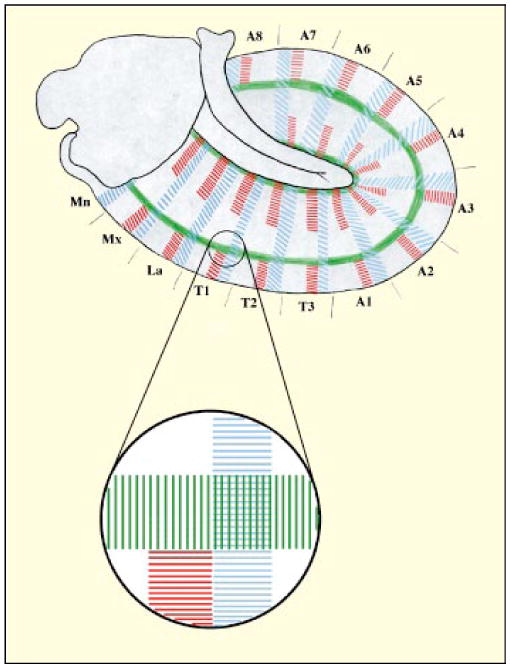
The intersection of En, Dpp and Wg expression stripes defines the position of imaginal disc primordia in Drosophila. The main diagram is a lateral view of a stage 11 Drosophila embryo. Engrailed (En) expression in the posterior compartment cells is shown in blue, Wingless (Wg) expression in adjacent anterior compartment cells in red and Decapentaplegic (Dpp) in green. The circle represents the region where the first leg disc primordium forms, and an enlarged view of this is also shown. Segment abbreviations: Mn, mandibulary; Mx, maxillary; La, labial; T1–3, thoracic segments; A1–8, abdominal segments.
These cues can provide an anterior–posterior and dorsal–ventral coordinate system for patterning the disc only if the cells sustain their distinctions during proliferation. Two types of mechanism stabilize these distinctions between cells of different territories. The first type of mechanism is distinguished by the stable transmission of the distinctions to daughter cells. Even in the absence of overt differences in the cells, heritable — that is, imprinted — differences in expression of specific regulatory genes, known as selector genes, distinguishes their behavior during morphogenesis [5,6]. Territories marked in this way, known as compartments, were initially detected by lineage analysis, which showed that the assignment to a territory is stable over many cell generations [7]. A second type of territory, which is not associated with lineage restriction, has been revealed by the analysis of patterns of gene expression. These territories, which might be more common, appear to be maintained by regulatory loops that involve cell signaling, rather than a heritable imprinting mechanism.
Paracrine regulation plays a transient role in maintaining the subdivision between anterior and posterior territories of segments, after which stable heritable control takes over. After the embryonic regulatory cascade turns on En and Wg in juxtaposed stripes, the Wg-expressing cells supply the En-expressing cells with a signal (Wg itself) that maintains En expression, and the En-expressing cells supply the Wg-expressing cells with a signal, Hedgehog (Hh), which maintains Wg expression [8]. This mutual reinforcement contributes to the maintenance of the subdivision for at least a short period of time. When subdivision maintenance becomes heritable [9], the en gene is stably repressed in anterior cells by Polycomb group regulators, which are thought to function by an imprinting mechanism; in posterior cells en remains active [10,11]. The simultaneous elimination from posterior cells of the functions of both en and the en homolog invected (inv) leads to the creation of a new anterior compartment [12] (Fig. 2; see below). This strongly supports the earlier conclusion that en acts as a selector gene, the expression of which specifies posterior cell fate.
Figure 2.
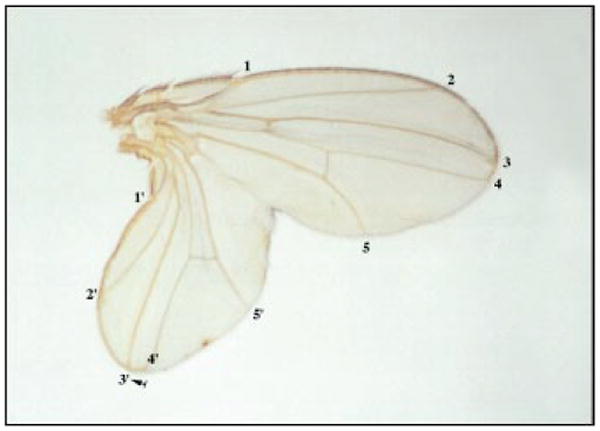
A duplicated Drosophila wing generated by a posterior clone of en/inv mutant cells [12]. The veins in the original wing are indicated by numbers, and the veins in the duplicated wing by primed numbers. The arrow indicates the position of the clone.
In the leg discs, although no lineage restriction is observed between the dorsal and ventral regions, distinctions between them are nonetheless maintained. For instance, these two regions respond differently to Hh. Ventral cells exposed to Hh express high levels of Wg, whereas dorsal cells exposed to Hh express high levels of Dpp (reviewed in [13]). This distinction appears to be maintained by the persistence of two mutually exclusive paracrine signaling loops. Although both ventral and dorsal cells can express either Wg or Dpp, mutual antagonism between these two expression states prevents them from expressing both [14,15]. It appears that the early embryonic cues create the original dorsal–ventral asymmetry between Wg and Dpp expression [4] and that mutual antagonism thereafter prevents the other regulator from gaining the upper hand.
In contrast to the leg disc, the dorsal–ventral distinction in the wing disc is associated with a lineage restriction [16,7], but the late appearance of this restriction suggests that other mechanisms might stabilize the distinction earlier. In any case, by the middle of the first of three larval stages, stable expression of the gene apterous (ap) in dorsal cells specifies the dorsal–ventral distinction [17,18].
Boundaries as organizers: an overview
Although graded morphogens play important roles in patterning both the early embryo and the discs, the physical disposition of these morphogens is dramatically different in these two cases. In the egg, the poles act as the sources of anterior and posterior patterning gradients [19]. In contrast, morphogens originate in the center of discs, near the boundaries between the anterior and posterior territories, and the dorsal and ventral territories. While all the boundaries seem to have organizing activity, full activity occurs at the centrally located intersection of the anterior–posterior and dorsal–ventral boundaries. The potency of these foci to organize pattern was deduced from the effects of experimentally produced ectopic boundaries (see below), and can be rationalized in terms of the activities and distributions of the two signaling molecules Wg and Dpp. Relatively local interactions between the different territories lead to the induction of either Dpp or Wg in cells near the inter-territory boundary (reviewed in [20]). Only at the intersection of boundaries are both morphogens produced at substantial levels (Fig. 3). Their synergistic action confers special properties to this region, which behaves as the distal organizer of appendages [21,22].
Figure 3.
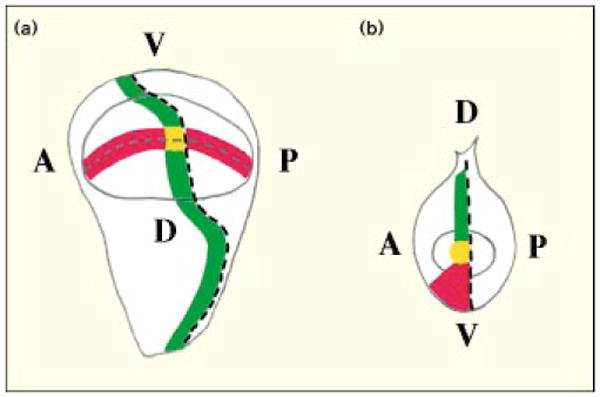
A diagram showing how expression stripes of development genes cross in the Drosophila wing disc (a) but meet in the leg disc (b). The dashed lines represent the boundaries between the anterior (A) and posterior (P), and the dorsal (D) and ventral (V), compartments. Red represents Wg expression, and green Dpp expression; yellow dots represent the regions where Wg and Dpp expression domains intersect, or meet, at the center of the imaginal discs. This central region will give rise to the distal-most structures of the appendages.
The local production of morphogens at a central position within a uniform field of cells ought to direct the formation of symmetric patterns. Indeed, experimental manipulations that produce a focus of morphogen within a single disc territory lead to the outgrowth of symmetric appendages (see below). The normal appendage is asymmetric, because the cells on either side of the source of morphogen belong to different territories and consequently respond differently. Thus, two key features contribute to patterning of the disc: the distinct behaviors of cells in different developmental compartments and the local production of morphogens.
New foci expressing morphogens are more than organizers of pattern: not only do they reorganize existing cells, but they also induce new proliferation, and the resulting cell population produced is recruited to form an ectopic appendage. Dpp and Wg appear to provoke such proliferation [23–25]: their action as both morphogens and growth factors appears to coordinate the growth and patterning of appendages.
A new compartment induces a new appendage
Removing en and inv gene function by mitotic recombination produces clones of cells that are defective only when located in the posterior compartment, where these genes are normally expressed [12]. The cells of such posterior clones behave as anterior cells, both in terms of gene expression and the structures they ultimately produce. Because they are ectopically located in the midst of posterior compartment cells, these clones of anteriorly behaving cells are surrounded by an ectopic anterior–posterior boundary that organizes new patterns.
The new patterns are particularly spectacular when the anterior–posterior boundary surrounding the en mutant clone intersects the normal dorsal–ventral boundary to create an ectopic intersection; in these cases, an entire duplicated wing can be produced (Fig. 2) [12]. This duplicated wing has a reversed polarity: its anterior compartment, comprising en mutant cells, is toward the posterior end, and its posterior compartment, formed from normal posterior compartment cells reprogrammed by the ectopic organizer, emerges from the posterior compartment of the original wing as a mirror-image duplication. It is notable that the size of both the normal and duplicate wing can approach normal.
Patterning molecules
Wg and Dpp are required for proliferation
Drosophila Wg and Dpp are founding members of highly conserved families of signaling molecules: the Wingless/Int (Wnt) and transforming growth factor β (TGFβ) families, respectively [26,27]. Members of these families have been associated with embryonic patterning in diverse organisms. Genetic and biochemical analyses have greatly advanced our understanding of signal transduction in responding cells. While the details of this signal transduction are important to patterning, here we focus on the evidence implicating Wg and Dpp in the control of proliferation.
Partial or complete loss of function of either wg or dpp can compromise the proliferation of cells in a disc. The gene name wingless stems from the phenotype of a particular allele, wg1, that is specifically defective in function in the wing disc [28]. Most other wg alleles are lethal, but the use of mitotic recombination to remove wg function in clones of cells in the wing shows that the wg gene is required in at least some areas of the wing [29], and specifically in those areas in which it is normally expressed (such as the wing margin, which coincides with the boundary between dorsal and ventral territories). Local loss of wg function leads to loss of tissue. Similar analyses of Dpp suggest that it, too, is required for proliferation [30]. Of particular note, a series of partial loss-of-function dpp alleles reveals that, with increased reduction of function, there is progressive loss of structures within appendages, with the distal parts lost first and the proximal parts last. This suggests that the highest levels of Dpp are required to make the most distal structures.
Wg and Dpp are expressed in cells adjacent to boundaries between territories
The mature wing disc shows one type of pattern of Wg and Dpp distribution, and the mature leg disc another (Fig. 3) [31,32]; both are dramatic. In the region of the wing disc that produces the wing blade, Dpp is expressed in a stripe of cells just anterior to the border between anterior and posterior cells. A stripe of Wg-staining cells brackets the dorsal–ventral boundary and crosses the Dpp-expressing stripe (Fig. 3). This intersection becomes the distal part of the wing [21].
In the leg disc, there is no obvious marking along the dorsal–ventral boundary itself; however, the anterior–posterior border is differently marked in the dorsal and ventral territories. The cells immediately anterior to the anterior–posterior border express Dpp in dorsal territories, while the cells anterior to the border express Wg in the ventral territory. It is noteworthy that Dpp is also expressed at low levels in ventral cells, but, because the expression level is lower and loss of dpp function in ventral cells has no consequence, we assume that this ventral Dpp expression is of no functional significance. In the leg disc, juxtaposition of the ends of the two stripes produces a unique region at the intersection of the anterior–posterior and dorsal–ventral boundaries: as in the wing, only this region has significant levels of both Wg and Dpp, and it produces the distal tip of the appendage (Fig. 3) [21,22].
Ectopic Wg or Dpp expression within a territory can induce formation of an ectopic symmetric appendage
Constitutive expression of a transgene can be induced in clones of cells by using site-specific recombination to excise a DNA sequence that interrupts the gene. When such clones produce Dpp, surrounding cells adopt new fates [25]. This is particularly dramatic when the clone of Dpp-expressing cells in the wing crosses the dorsal–ventral border to produce an ectopic intersection of Dpp-expressing and Wg-expressing cells. Such clones induce an ectopic, mirror-symmetric wing blade. If, for example, the clone occurs in the anterior compartment, the ectopic wing blade will consist of two symmetrically arranged anterior compartments projecting out of the original anterior compartment (Fig. 4) [25].
Figure 4.
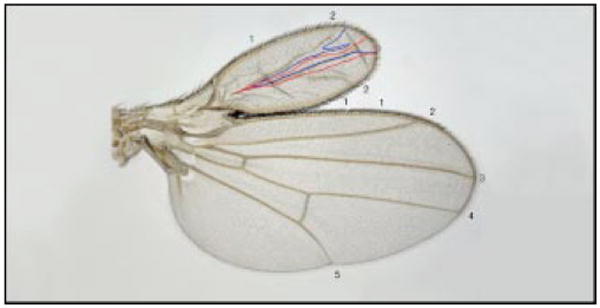
The Drosophila wing pattern is reorganized as a result of ectopic Dpp expression [25]. The generation of a Dpp-expressing clone in the anterior compartment of the wing disc, which contributes to both the dorsal and the ventral surfaces of the wing, induces formation of an ectopic, double-anterior wing. The clone borders are outlined in blue ventrally and in red dorsally; the numbers indicate the veins.
The cells of the clone lie at the center of this duplicated wing, and are presumed to be the source of a symmetric signal that reorganizes the cell fates within the surrounding anterior compartment. When the clone occurs in the posterior compartment it can induce the formation of a double posterior wing blade that once again has the Dpp-expressing cells along its center of symmetry [25]. These results indicate that a focus of Dpp expression can reorganize pattern in both anterior and posterior compartments and that the response is dictated by the location of the clone. Similar results with ectopic Wg expression suggest that this morphogen can similarly reorganize pattern in the leg and wing discs [23,33,34].
A number of patterning genes act upstream of Dpp and Wg to localize their expression
Elegant experiments have defined several important events in the induction of Dpp and Wg expression along compartment borders. For example, the compartment-specific transcription factors En and Cubitus interruptus (Ci) direct posterior cells to express Hh and anterior cells to respond to this signaling molecule [35–38]. Only the anterior cells close to the anterior–posterior border receive a Hh signal adequate to induce Dpp [39,40]. A number of genes involved in the Hh signaling pathway, such as patched, are required for this induction of Dpp [41]. Not surprisingly, mutations in these genes interfere with signal transduction, expression of Dpp and patterning of the disc.
Clones of cells that constitutively express Dpp-inducing functions, or that are deficient in functions required to inhibit Dpp expression, can produce ectopic foci of organizing activity (reviewed in [42]). This organizing activity is associated with ectopic Dpp expression, and the ability of ectopic Dpp on its own to reorganize pattern and to restore pattern to a dpp mutant argues that Dpp is the downstream mediator of these patterning effects. Except for the important roles of the selector-type genes, en and ci, in defining the type of response (anterior or posterior), the upstream genes in the pathway regulating localized expression of Dpp have no demonstrated involvement in subsequent patterning. Indeed, ectopic Dpp can re-pattern without recapitulation of the upstream events that turn on its expression at the correct position during normal development.
Similarly, mutations that perturb Wg expression also affect patterning, and again their effects on patterning can be largely attributed to their perturbation of Wg expression. The effects of ectopic Wg expression demonstrate that, like Dpp, Wg has autonomous patterning capabilities [23,34,43]. Although this suggests that Wg mediates the patterning activities of a set of upstream genes, it should be noted that some of these latter genes might have additional roles downstream of Wg. This is particularly likely to be the case for genes involved in Notch signaling, which is involved in localizing Wg expression [33] and has numerous patterning roles, some of which might be downstream of Wg [44].
Wg and Dpp are directly responsible for long-range organizing activities
The experiments described above do not exclude the possibility that the effects of locally expressed Wg and Dpp on pattern and proliferation could be indirect and due to the activation of a secondary diffusible factor. Direct signaling by Dpp and Wg throughout the presumptive wing blade has, however, been demonstrated by inducing clones of cells defective in genes required to transduce these signals. Regardless of the position of these clones, the affected cells show defects in proliferation.
Several components of the Dpp signaling cascade have been identified, including the receptors Thickveins (Tkv) and Punt and the transcription factor Schnurri. Each of these molecules has been shown by clonal analysis to be required for proliferation in cells throughout the wing disc, indicating that Dpp, or a related ligand that uses the same receptor, must be sensed throughout the wing blade [45]. Furthermore, clonal expression of a constitutively active form of Tkv induces expression of the Dpp-responsive genes optomotor blind (omb) and spalt in a cell autonomous manner, only in the cells expressing the ligand independent receptor [46]. Thus, the assessed responses are not communicated to other cells via a secondary intermediate.
Clonal analysis has similarly demonstrated that armadillo (arm), a mediator of Wg signal transduction, is required in cells throughout the wing blade for their proliferation [47]. In addition, clonal expression of a constitutively activate form of Arm activates Wg-responding genes in a cell-autonomous manner, in contrast to clonal expression of Wg which exerts a long-range effect on target genes [43]. These results support the idea that Wg has a direct function in promoting growth in Drosophila discs.
Wg and Dpp act synergistically to specify distal structures
The central regions of the discs, where the anterior–posterior and dorsal–ventral boundaries intersect, have unique capacities to organize proximal–distal pattern. From the ability of clones ectopically expressing one of the morphogens, either Dpp or Wg, to mimic this organizing activity only when they intersect a stripe of the other morphogen (see above), it appears that the unique organizing activity of the central region of a disc is due to the synergistic action of Dpp and Wg (Fig. 5a).
Figure 5.
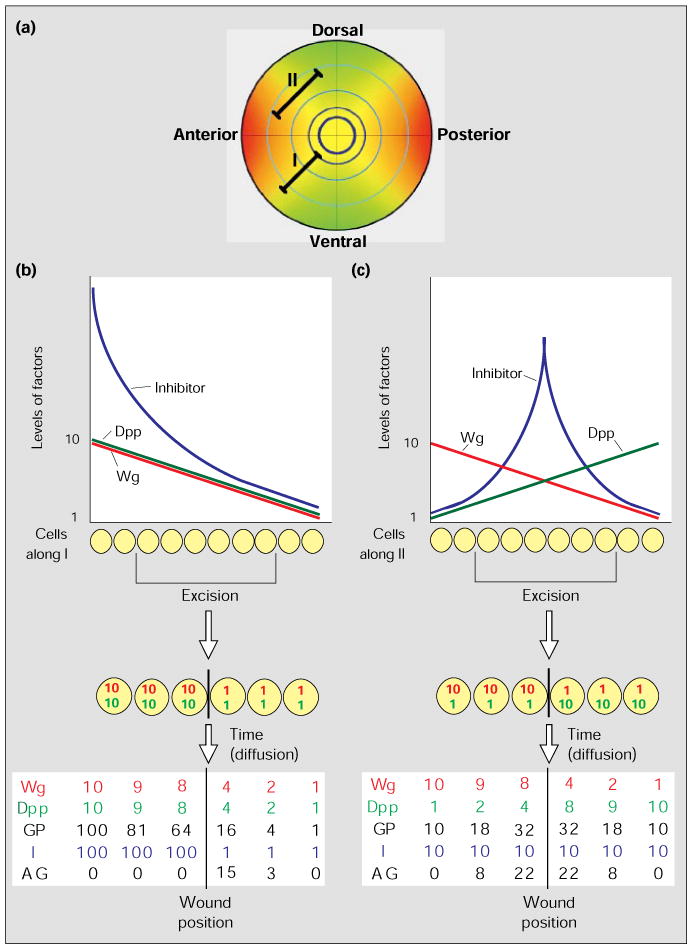
A model for intercalary growth in Drosophila imaginal discs. (a) An idealized diagram of the graded distribution of Wg and Dpp within the wing disc. The distributions of Wg (red) and Dpp (green) expression within the disc are shown by the gradients of color intensity; yellow represents regions where Wg and Dpp expression overlap. The intersecting lines represent the anterior–posterior (vertical line) and dorsal–ventral (horizontal line) boundaries. The pale blue concentric circles are meant to represent proximal–distal distinctions that might result from the joint action of high levels of Dpp and Wg at successive times during the growth of the disc. Lines I and II represent rows of cells: the cells along I maintain the same ratio of Dpp and Wg levels – see (b) – but the absolute levels drop with distance from the sources of morphogen; cells along II differ dramatically in the ratio of Dpp and Wg levels – see (c) – depending on the relative distance from the Wg and Dpp sources. (b,c) The graphs show how the concentration levels, in arbitrary numbers, of Wg (red), Dpp (green) and a hypothetical growth inhibitor (blue) vary along lines I (b) and II (c); we have used linear gradients of morphogen concentration for simplicity, but they could well be exponential. Below the graphs we illustrate the consequence of an excision of some of the cells, which juxtaposes cells from distant positions giving discontinuities in the gradients of Wg (red) and Dpp (green). The table of numbers at the bottom gives the expected levels of the growth factors Wg and Dpp after some diffusion across the wound. Using the model, the table also indicates the growth potential (GP) based on synergy between Dpp and Wg (GP = W × D), the presumed level of inhibitor (I) and the net or actual growth (AG). All numbers are arbitrary and are assigned only to illustrate the principle.
Genes such as aristaless (al) and Distal-less (Dll) are specifically expressed near the intersection point, in response to Wg and Dpp [21,22]. The al and Dll gene products are required for proximal–distal patterning and growth [21,22]. Additionally, ectopic al expression is sufficient to induce formation of supernumerary appendages, at least in some regions of the disc [21]. These results support the idea that the unique abilities of the central regions of the discs to organize proximal–distal structures is the consequence of the simultaneous requirement for Dpp and Wg to express distalizing functions. A detailed dissection of the suggested synergy between Dpp and Wg would benefit from a system in which one could induce clones of cells ectopically expressing both Wg and Dpp.
Growth regulation
The enigma: localized Wg and Dpp promote nearly uniform proliferation
During the thousand-fold increase in wing-disc cell number that occurs during larval growth, the daughter cell progeny of each division seldom move away from one another. Consequently, a mitotic clone usually represents a contiguous group of cells in which the number of marked cells indicates the degree of proliferation since the induction of the clone. The analysis of such clones reveals that cells in all positions within the disc proliferate, and that rates of proliferation do not vary greatly with position [48]. The direct visualization of mitotic cells and detection of S phase cells by labeling with DNA precursors also show widespread proliferation with little variation from one location to another within a disc [49].
There are some exceptions to this generalization. For example, cells near the wing margin withdraw from proliferation earlier than the cells in most parts of the disc [50]. Nonetheless, it is clear that, despite the role of Dpp and Wg in promoting proliferation, proliferation within the wing blade is not confined to, or even concentrated in, the areas that express high levels of Wg and Dpp. It is a challenge to understand how factors that stimulate growth can drive near-uniform proliferation despite their localized expression.
Growth regulation by Wg and Dpp is concentration dependent
One of the simplest models to explain the lack of correspondence between sites of Dpp and Wg expression and sites of proliferation is that the concentration of these morphogens required to stimulate growth is so low that even the lowest concentrations found in the disc exceeds the threshold for growth. If this were the case, growth would not be further stimulated by increasing the levels of these regulators above their normal levels. In contrast to this expectation, rather spectacular expansions in the size of a wing disc result from increased levels of expression of Dpp, or Wg [46,51], and as discussed above, new foci of expression of these regulators act as new organizers that induce extensive proliferation. These results argue that the growth-promoting signal provided by these regulators is not normally saturated in the discs.
Induction of proliferation by Wg and Dpp does not require a concentration gradient
It is not clear how disc size is regulated. One simple model suggests that a locally produced morphogen diffuses to produce a concentration gradient, the slope of which diminishes as a field of cells grows; growth might be appropriately regulated if cells are stimulated by the gradient and grow until the slope becomes too shallow [19]. In addition to providing an attractive model for size control, the proposal that growth responds to the slope of the morphogen gradient, rather than its absolute concentration, provides a possible explanation of why proliferation does not correlate well with the concentration of Wg and Dpp. However, uniform ectopic expression of Dpp [46] or of both morphogens (L. Maves and G. Schubiger, personal communication) induces proliferation, arguing that a gradient of these morphogens is not required to induce proliferation.
A gradient of responsiveness opposite to the morphogen concentration gradient
While the effects of ubiquitous ectopic expression argue that a gradient of Wg or Dpp is not required to induce proliferation, it is possible that cells nonetheless respond to the gradient. For example, a gradient of an inhibitor of proliferation could establish a gradient of responsiveness. In this situation, a cell might sense the Dpp and/or Wg concentration directly, but respond only when the signal strength exceeds a threshold level established by the graded concentration of the inhibitor; growth would stop when the gradient of growth-factor concentrations balanced the gradient of inhibition.
As the growth factors are sensed directly, any increase in their concentration, whether graded or uniform, would disturb the balance of activities and would stimulate growth. We suggest that a gradient of responsiveness opposes the gradient of morphogen concentration so that the proliferation response is relatively uniform. Such a gradient opposing the proliferative activity of Dpp and Wg must selectively flatten out the proliferation response without destroying the concentration-dependent gene induction by these morphogens. This would be achieved if it inhibits proliferation but not the signaling associated with patterning.
Modeling growth regulation
We have suggested that the available data argue that growth is regulated by a balance between the non-uniform growth factors, Wg and Dpp, and a non-uniform inhibitor of proliferation. We now consider this conclusion in more detail to assess its ability to accommodate known properties of Drosophila discs.
A simple model
Although the balancing act between growth factors and inhibitors might be complex, it seems best initially to consider a relatively simple model. It is known that Wg and Dpp are independently required for growth, and that they act synergistically. In a simple synergistic relationship, the growth potential (GP) is a function of the product of the concentrations of Wg (W) and Dpp (D): GP = W × D. Note that a variety of quantitative relationships can underlie synergy, and a mathematical relationship is presented as a conceptual aid rather than a proposal of the precise nature of the synergy. We are suggesting that growth potential is counterbalanced by an inhibitor which sets a threshold that must be exceeded for actual growth (AG), that is: AG = [W × D] − I. Accordingly, the enigmatic relationship between the distributions of Wg and Dpp and the uniform growth would be explained if the strength of inhibition (I) varied as some function of the product of the concentrations of Wg and Dpp (Fig. 5).
There are numerous possible relationships between the negative and positive growth factors, and little information to direct a more detailed model. Nonetheless, to avoid being vague, we have developed our model on the premise that the inhibitor is not diffusible and that, once established, its concentration is independent of subsequent changes in Wg and/or Dpp concentrations. Such a gradient of a non-diffusible inhibitor could be set up, for instance, by localized induction of the inhibitor near the boundaries between territories, in response to high levels of Wg and/or Dpp, and deposition of the inhibitor in the basement membrane: expansion of the basement membrane associated with growth would successively isolate regions of the basement from the local source of the inhibitor, and would then dilute the inhibitor within the basement membrane as growth progressed.
While we assume that normal disc growth requires at least a slight imbalance between the growth factors and the growth inhibitor, our model has not advanced a mechanism to explain the regulation of this imbalance. Nonetheless, the skeleton model we do present can explain a number of observations. According to the above model, if a high Dpp concentration is induced that saturates the Dpp signaling pathway, then the resulting growth should be determined by Wg concentration. This is consistent with the observed expansion of the wing disc along the dorsal–ventral boundary upon expression of high levels of Dpp. Similarly, other results summarized above are compatible with this model, but this is not surprising as it was these results that suggested the model in the first place. It is more relevant to ask if the model offers explanations for other phenomena.
A possible explanation for intercalary growth
Regeneration in numerous systems, including Drosophila discs, is associated with stimulation of proliferation, which, together with subsequent differentiation, erases discontinuities in the pattern by intercalating the missing elements. This has led to the proposal that juxtaposing cells from incongruous positions stimulates growth and differentiation. In other words, cells somehow sense which cells are their correct neighbors and respond to discordant neighbors by proliferating until they restore normal neighbors.
According to this view of intercalary growth, the induction of extensive proliferation around a clone of cells that expresses ectopic Dpp might be a secondary response — first Dpp would transform cell fates, and the presence of incongruous neighbors would then induce proliferation. However, clones expressing a constitutively active form of a Dpp receptor demonstrate that the growth response is an autonomous feature of Dpp signaling [46]. Although cells within these clones over-proliferate in response to the activation of the Dpp signaling pathway, no proliferation — that is, no intercalary growth — is induced in the surrounding cells, despite the incongruous juxtapositioning of cell fates at the border of the clone. The unique feature of the transformed cells within these clones is that they have a fate normally associated with a high concentration of Dpp, but lack high Dpp levels. The failure to activate intercalary growth in this circumstance suggests that it is not the juxtapositioning of incongruous cell fates, but rather the juxtapositioning of cells with discordant levels of Dpp that induces this proliferation. Similar data argue that Wg also has a fairly direct role in stimulating intercalary growth [43]. Thus, the discordance that induces intercalary growth appears to be a discontinuity in Dpp and/or Wg morphogen concentrations.
Our model provides a rationale for the stimulation of proliferation by a discontinuity in the concentration of Dpp and/or Wg. In the undisturbed disc, neighboring cells have a similar balance of growth factors and inhibitor (or near balance, if growth is ongoing). Cells at substantially different distances from the compartment boundaries have a different balance (Fig. 5a). As the growth factors are diffusible, juxtaposing cells from different positions will change the balance of growth factors and inhibitor in cells bordering the junction (Fig. 5b,c). When cells with high levels of diffusible growth factor and high levels of fixed inhibitor abut cells with low levels of growth factor and inhibitor, the growth factors will diffuse across the junction to stimulate the growth of the cells with low inhibitor levels (Fig. 5b). A region that has high Dpp and low Wg levels might have the same growth potential as a region that has low Dpp and high Wg levels; nonetheless, when these two regions are juxtaposed, growth should be induced — as a result of diffusion of the two ligands, the juxtaposed cells will have intermediate levels of both regulators, which synergize to increase the growth potential (Fig. 5c).
According to the above model, intercalary growth ought to result whenever cells with different levels of Wg and/or Dpp are juxtaposed. Within each quadrant of the wing disc, bordered by two compartment boundaries, we expect each cell to experience a unique combination of Wg and Dpp concentrations (Fig. 5a), governed by its distances from the morphogen sources at the compartment boundaries. The excision of cells from within a quadrant should therefore always induce intercalation. While excisions between quadrants might juxtapose cells with similar combinations of Wg and Dpp levels, growth is still expected because the cells will belong to different compartments: interactions between the different compartments should establish a new compartment border and thus a new source of growth factor, Wg or Dpp. Thus, together with other aspects of morphogen regulation, the proposed balance of growth factors and inhibitor can explain some important features of proliferation during regeneration. Although these proposals fail to address several mysteries, such as the mechanisms that lead to de novo regeneration of a compartment after its complete excision, it seems that the roles of Wg and Dpp in intercalary growth are worth exploring.
Inhibitors of proliferation exist
The cutting of imaginal discs without excision leads to transient proliferation, suggesting that normal contacts inhibit proliferation. So while positive signals from incongruous neighbors are generally invoked to explain the proliferation seen during intercalary regeneration, it appears that general negative signals arise simply from contact. If the strength of contact inhibition of growth varies across the disc, contact inhibition could represent the negative signal for growth required by the above model. Recently, it has been found that nitric oxide acts as an inhibitor of proliferation in Drosophila discs [52], and it is a candidate for being an inhibitor that functions in conjunction with the growth factors Wg and Dpp. Perhaps the details of the regulation of this newly defined inhibitor will give us insights into the basis for program of growth control in discs.
Mysteries exposed
One of the most mysterious features of morphogenesis is that structures form themselves as they grow. In considering pattern formation the dominant thoughts are of gradients of morphogens across a preexisting field. But patterns are generally formed in conjunction with the growth that produces the field of cells that is patterned. How are the actions of morphogens coordinated with the process of growth? Studies of Drosophila development have demonstrated tight connections between the regulators of pattern and growth itself. While exposing the mysteries to molecular analyses, the results also issue a challenge: the connections between the concentrations of the growth promoting factors Wg and Dpp, and the growth they promote, is not direct, but it appears that if we could understand it we would have gained real insight into a mechanism that gives shape to biological structures.
Acknowledgments
The authors would like to thank Charles Girdham for his generous help in preparing Figure 5A. We are particularly grateful to Didier Stainier and Peter Follette for useful comments and discussions on the manuscript. We also apologize to many because the format of this review did not permit thorough review and citation of all the work that contributed to the ideas contained herein.
References
- 1.Spemann H, Mangold H. Uber induktion von embryonenanlagen durch implantation artfremder organisatoren. Arch Mikrosk Anat Entw Mech. 1924;100:599–638. [Google Scholar]
- 2.Tickle C, Summerbell D, Wolpert L. Positional signalling and specification of digits in chick limb morphogenesis. Nature. 1975;254:199–202. doi: 10.1038/254199a0. [DOI] [PubMed] [Google Scholar]
- 3.Cohen SM. Imaginal disc development. In: Bate M, Martinez-Arias A, editors. The Development of Drosophila melanogaster. Plainview, New York: Cold Spring Harbor Laboratory Press; 1993. pp. 747–841. [Google Scholar]
- 4.Cohen B, Simcox AA, Cohen SM. Allocation of the thoracic imaginal primordia in the Drosophila embryo. Development. 1993;117:597–608. doi: 10.1242/dev.117.2.597. [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Bellido A. Cell Patterning CIBA symposium. Boston: Little Brown; 1975. Genetic control of wing disc development in Drosophila; pp. 161–183. [DOI] [PubMed] [Google Scholar]
- 6.Lawrence PA, Morata G. The compartment hypothesis. In: Lawrence PA, editor. Insect Development. Oxford: Blackwell Scientific Publishing; 1976. pp. 132–147. [Google Scholar]
- 7.Bryant PJ. Cell lineage relationships in the imaginal wing disc of Drosophila melanogaster. Dev Biol. 1970;22:389–411. doi: 10.1016/0012-1606(70)90160-0. [DOI] [PubMed] [Google Scholar]
- 8.Vincent JP, O'Farrell PH. The state of engrailed expression is not clonally transmitted during early Drosophila development. Cell. 1992;68:923–931. doi: 10.1016/0092-8674(92)90035-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heemskerk J, DiNardo S, Kostriken R, O'Farrell PH. Multiple modes of engrailed regulation in the progression towards cell fate determination. Nature. 1991;352:404–410. doi: 10.1038/352404a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dura JM, Ingham P. Tissue- and stage-specific control of homeotic and segmentation gene expression in Drosophila embryos by the polyhomeotic gene. Development. 1988;103:733–741. doi: 10.1242/dev.103.4.733. [DOI] [PubMed] [Google Scholar]
- 11.Moazed D, O'Farrell PH. Maintenance of the engrailed expression pattern by Polycomb group genes in Drosophila. Development. 1992;116:805–810. doi: 10.1242/dev.116.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tabata T, Schwartz C, Gustavson E, Ali Z, Kornberg TB. Creating a Drosophila wing de novo, the role of engrailed, and the compartment border hypothesis. Development. 1995;121:3359–3369. doi: 10.1242/dev.121.10.3359. [DOI] [PubMed] [Google Scholar]
- 13.Held LIJ. Axes, boundaries and coordinates: the ABCs of fly leg development. BioEssays. 1995;17:721–732. doi: 10.1002/bies.950170809. [DOI] [PubMed] [Google Scholar]
- 14.Jiang J, Struhl G. Complementary and mutually exclusive activities of Decapentaplegic and Wingless organize axial patterning during Drosophila leg development. Cell. 1996;86:401–409. doi: 10.1016/s0092-8674(00)80113-0. [DOI] [PubMed] [Google Scholar]
- 15.Penton A, Hoffman FM. Decapentaplegic restricts the domain of wingless during Drosophila limb patterning. Nature. 1996;382:162–165. doi: 10.1038/382162a0. [DOI] [PubMed] [Google Scholar]
- 16.Garcia-Bellido A, Ripoll P, Morata G. Developmental compartmentalization in the dorsal mesothoracic disc of Drosophila. Dev Biol. 1976;48:132–147. doi: 10.1016/0012-1606(76)90052-x. [DOI] [PubMed] [Google Scholar]
- 17.Cohen B, McGuffin ME, Pfeifle C, Segal D, Cohen SM. Apterous, a gene required for imaginal disc development in Drosophila encodes a member of the LIM family of developmental regulatory proteins. Genes Dev. 1992;6:715–729. doi: 10.1101/gad.6.5.715. [DOI] [PubMed] [Google Scholar]
- 18.Diaz-Benjumea FJ, Cohen SM. Interaction between dorsal and ventral cells in the imaginal disc directs wing development. Cell. 1993;75:741–752. doi: 10.1016/0092-8674(93)90494-b. [DOI] [PubMed] [Google Scholar]
- 19.Lawrence PA. The Making of a Fly: the Genetics of Animal Design. Oxford: Blackwell Scientific Publications; 1992. [Google Scholar]
- 20.Lawrence PA, Struhl G. Morphogens, compartments, and pattern: lessons from Drosophila? Cell. 1996;85:951–961. doi: 10.1016/s0092-8674(00)81297-0. [DOI] [PubMed] [Google Scholar]
- 21.Campbell G, Weaver T, Tomlinson A. Axis specification in the developing Drosophila appendage: the role of wingless, decapentaplegic, and the homeobox aristaless. Cell. 1993;74:1113–1123. doi: 10.1016/0092-8674(93)90732-6. [DOI] [PubMed] [Google Scholar]
- 22.Diaz-Benjumea FJ, Cohen B, Cohen SM. Cell interaction between compartments establishes the proximo-distal axis of Drosophila legs. Nature. 1994;372:175–179. doi: 10.1038/372175a0. [DOI] [PubMed] [Google Scholar]
- 23.Struhl G, Basler K. Organizing activity of Wingless protein in Drosophila. Cell. 1993;72:527–540. doi: 10.1016/0092-8674(93)90072-x. [DOI] [PubMed] [Google Scholar]
- 24.Ingham PW, Fietz MJ. Quantitative effects of hedgehog and decapentaplegic activity on the patterning of the Drosophila wing. Curr Biol. 1995;5:432–440. doi: 10.1016/s0960-9822(95)00084-4. [DOI] [PubMed] [Google Scholar]
- 25.Zecca M, Basler K, Struhl G. Sequential organizing activities of engrailed, hedgehog and decapentaplegic in the Drosophila wing. Development. 1995;121:2265–2278. doi: 10.1242/dev.121.8.2265. [DOI] [PubMed] [Google Scholar]
- 26.Rijsewijk F, Schuermann M, Waganaar E, Parren P, Weigel D, Nusse R. The Drosophila homolog of the mouse mammary oncogene int-1 is identical to the segment polarity gene wingless. Cell. 1987;50:649–657. doi: 10.1016/0092-8674(87)90038-9. [DOI] [PubMed] [Google Scholar]
- 27.Panganiban GEF, Rashka KE, Neitzel MD, Hoffmann FM. Biochemical characterization of the Drosophila dpp protein, a member of the transforming growth factor-β family of growth factors. Mol Cell Biol. 1990;10:2669–2677. doi: 10.1128/mcb.10.6.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sharma RP, Chopra VL. Effect of the wingless (wg1) mutation on wing and haltere development in Drosophila melanogaster. Dev Biol. 1976;48:461–465. doi: 10.1016/0012-1606(76)90108-1. [DOI] [PubMed] [Google Scholar]
- 29.Baker NE. Embryonic and imaginal requirements for wingless, a segment-polarity gene in Drosophila. Dev Biol. 1988;125:96–108. doi: 10.1016/0012-1606(88)90062-0. [DOI] [PubMed] [Google Scholar]
- 30.Posakony LG, Raftery LA, Gelbart WM. Wing formation in Drosophila melanogaster requires decapentaplegic gene function along the anterior–posterior compartment boundary. Mech Dev. 1991;33:69–82. doi: 10.1016/0925-4773(90)90136-a. [DOI] [PubMed] [Google Scholar]
- 31.Masucci JD, Miltenberger RJ, Hoffmann FM. Pattern-specific expression of the Drosophila decapentaplegic gene in imaginal disks is regulated by 3 cis-regulatory elements. Genes Dev. 1990;4:2011–2023. doi: 10.1101/gad.4.11.2011. [DOI] [PubMed] [Google Scholar]
- 32.Baker NE. Transcription of the segment polarity gene wingless in the imaginal discs of Drosophila, and the phenotype of a pupal-lethal wg mutation. Development. 1988;102:489–497. doi: 10.1242/dev.102.3.489. [DOI] [PubMed] [Google Scholar]
- 33.Diaz-Benjumea FJ, Cohen SM. Serrate signals through Notch to establish a Wingless-dependent organizer at the dorsal/ventral compartment boundary of the Drosophila wing. Development. 1995;121:4215–4225. doi: 10.1242/dev.121.12.4215. [DOI] [PubMed] [Google Scholar]
- 34.Ng M, Diaz-Benjumea FJ, Vincent JP, Wu J, Cohen SM. Specification of the wing by localized expression of wingless protein. Nature. 1996;381:316–318. doi: 10.1038/381316a0. [DOI] [PubMed] [Google Scholar]
- 35.Tabata T, Eaton S, Kornberg TB. The Drosophila hedgehog gene is expressed specifically in posterior compartment cells and is a target of engrailed regulation. Genes Dev. 1992;6:2635–2645. doi: 10.1101/gad.6.12b.2635. [DOI] [PubMed] [Google Scholar]
- 36.Lee JJ, von Kessler DP, Parks S, Beachy PA. Secretion and localized transcription suggest a role in positional signalling for products of the segmentation gene hedgehog. Cell. 1992;71:33–50. doi: 10.1016/0092-8674(92)90264-d. [DOI] [PubMed] [Google Scholar]
- 37.Alexandre C, Jacinto A, Ingham PW. Transcriptional activation of hedgehog target genes in Drosophila is mediated directly by the Cubitus interruptus protein, a member of the GLI family of zinc finger DNA-binding proteins. Genes Dev. 1996;10:2003–2013. doi: 10.1101/gad.10.16.2003. [DOI] [PubMed] [Google Scholar]
- 38.Dominguez M, Brunner M, Hafen E, Basler K. Sending and receiving the Hedgehog signal: control by the Drosophila Gli protein cubitus interruptus. Science. 1996;272:1621–1625. doi: 10.1126/science.272.5268.1621. [DOI] [PubMed] [Google Scholar]
- 39.Basler K, Struhl G. Compartment boundaries and the control of Drosophila limb pattern by Hedgehog protein. Nature. 1994;368:208–214. doi: 10.1038/368208a0. [DOI] [PubMed] [Google Scholar]
- 40.Tabata T, Kornberg TB. Hedgehog is a signaling protein with a key role in patterning Drosophila imaginal discs. Cell. 1994;76:89–102. doi: 10.1016/0092-8674(94)90175-9. [DOI] [PubMed] [Google Scholar]
- 41.Johnson RL, Grenier JK, Scott MP. Patched overexpression alters wing disc size and pattern: transcriptional and post-transcriptional effects on hedgehog targets. Development. 1995;121:4161–4170. doi: 10.1242/dev.121.12.4161. [DOI] [PubMed] [Google Scholar]
- 42.Perrimon N. Hedgehog and beyond. Cell. 1995;80:517–520. doi: 10.1016/0092-8674(95)90503-0. [DOI] [PubMed] [Google Scholar]
- 43.Zecca M, Basler K, Struhl G. Direct and long-range action of a Wingless morphogen gradient. Cell. 1996;87:833–844. doi: 10.1016/s0092-8674(00)81991-1. [DOI] [PubMed] [Google Scholar]
- 44.Couso JP, Knust E, Martinez-Arias A. Serrate and wingless cooperate to induce vestigial gene expression and wing formation in Drosophila. Curr Biol. 1995;5:1437–1448. doi: 10.1016/s0960-9822(95)00281-8. [DOI] [PubMed] [Google Scholar]
- 45.Burke R, Basler K. Dpp receptors are autonomously required for cell proliferation in the entire developing Drosophila wing. Development. 1996;122:2261–2269. doi: 10.1242/dev.122.7.2261. [DOI] [PubMed] [Google Scholar]
- 46.Nellen D, Burke R, Struhl G, Basler K. Direct and long-range action of a Dpp morphogen gradient. Cell. 1996;85:357–368. doi: 10.1016/s0092-8674(00)81114-9. [DOI] [PubMed] [Google Scholar]
- 47.Peifer M, Rauskolb C, Williams M, Riggleman B, Wieschaus E. The segment polarity gene armadillo interacts with the wingless signaling pathway in both embryonic and adult pattern formation. Development. 1991;111:1029–1043. doi: 10.1242/dev.111.4.1029. [DOI] [PubMed] [Google Scholar]
- 48.Gonzalez-Gaitan M, Capdevila MP, Garcia-Bellido A. Cell proliferation patterns in the wing imaginal disc of Drosophila. Mech Dev. 1994;40:183–200. doi: 10.1016/0925-4773(94)90070-1. [DOI] [PubMed] [Google Scholar]
- 49.Milan M, Campuzano S, Garcia-Bellido A. Cell cycling and patterned cell proliferation in the wing primordium of Drosophila. Proc Natl Acad Sci USA. 1996;93:640–645. doi: 10.1073/pnas.93.2.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.O'Brochta DA, Bryant PJ. A zone of non-proliferating cells at a lineage restriction boundary in Drosophila. Nature. 1985;313:138–141. doi: 10.1038/313138a0. [DOI] [PubMed] [Google Scholar]
- 51.Neumann CJ, Cohen SM. Distinct mitogenic and cell fate specification functions of wingless in different regions of the wing. Development. 1996;122:1781–1789. doi: 10.1242/dev.122.6.1781. [DOI] [PubMed] [Google Scholar]
- 52.Kuzin B, Roberts I, Peunova N, Enikolopov G. Nitric oxide regulates cell proliferation during Drosophila development. Cell. 1996;87:639–649. doi: 10.1016/s0092-8674(00)81384-7. [DOI] [PubMed] [Google Scholar]


