Abstract
Objectives
To assess whether serial measurements of childhood body mass index (BMI) give clinically useful predictions of the risk of developing adult metabolic syndrome and impaired glucose tolerance or type 2 diabetes.
Design/setting
Follow-up of a community-based birth cohort in Delhi, India.
Participants
1,492 men and women aged 26-32 years whose BMI was recorded 6-monthly throughout childhood.
Main outcome measures
The predictive value of childhood BMI for adult metabolic syndrome (MS) defined using waist circumference, blood pressure and fasting glucose, triglyceride and HDL-cholesterol concentrations, and impaired glucose tolerance (IGT) and diabetes (DM) diagnosed by oral glucose tolerance tests.
Results
Twenty-five percent of subjects had MS and 15% had IGT/DM. Both outcomes were associated with greater childhood BMI gain (MS: OR 1.63 [95% CI 1.44 to 1.85]; IGT/DM: 1.39 [1.20 to 1.60] per unit increase in within-cohort BMI SD-score between 5-14 years). Best predictions of adult disease were obtained using a combined test comprising i) any increase in BMI SD-score between 5-14 years and ii) a BMI SD-score >0 at 14 years (MS: sensitivity 45%, specificity 78%; IGT/DM: 37%, 73%). Likelihood ratios were low (MS: 1.4-2.0; IGT/DM: 1.2-1.4). A single high BMI measurement at 14 years (overweight or obese, International Obesity Task Force criteria) was highly specific but insensitive (MS: sensitivity 7%, specificity 97%; IGT/DM: 8%, 97%). Charts for plotting BMI SD-scores through childhood were produced.
Conclusions
Serial measurements of childhood BMI give useful predictions of adult risk and could guide advice to children and parents on preventing later disease.
Keywords: Childhood body mass index, type 2 diabetes, metabolic syndrome, predictions
INTRODUCTION
The incidence of type 2 diabetes and metabolic syndrome is increasing worldwide, most rapidly in developing countries like India1,2. This has been attributed to greater availability of food, urbanisation and industrialisation, and reduced physical activity, resulting in increased adiposity. It is predicted that by 2030, 75% of the world’s adult diabetic patients will be in developing countries, and that India will have 80 million1. Action to prevent disease is urgently needed.
Childhood obesity is a risk factor for adult diabetes, hypertension and dysplidemia3-5 and treating it may prevent later disease. However, identifying and treating only those children who are already obese is inappropriate for developing countries. Among adults born in Delhi, India, whose body mass index (BMI) was measured serially throughout childhood, those who developed impaired glucose tolerance (IGT)/diabetes or metabolic syndrome were well below internationally-recognised criteria for obesity in childhood, but had accelerated childhood BMI gain relative to the rest of the cohort6-8. Such children would not easily be identified using standard ‘distance’ BMI charts.
We have now used the Delhi data to assess how well childhood BMI predicts adult IGT/diabetes and metabolic syndrome and whether serial BMI measurements give better predictions than single measurements, and to devise charts that could be used by clinicians to monitor BMI changes and thus estimate adult risk in individual children.
METHODS
The cohort was established in 1969-19726. Married women living in a 12 km2 area of Delhi (N=20,755) were followed up. There were 9,169 pregnancies, resulting in 8,181 live births. Trained personnel recorded the weight and length of the babies within 72 hours of birth and 6 monthly (±15 days) until 14-21 years. Gaps in funding interrupted measurements for one year in 1972-1973, and 2.5 years in 1980-1982. At recruitment, 60% of families had an income >50 rupees per month (national average 28 rupees) and 15% of parents were illiterate (national average 66%). Nevertheless, 43% of families lived in one room. Hindus were the majority religious group (84%), followed by Sikhs (12%), Christians (2%), Muslims (1%) and Jains (1%).
Current Study
In 1998-2002 we retraced 2,584 (32%) of the cohort. The study was approved by the All India Institute of Medical Sciences research ethics committee, and informed verbal consent was obtained from each subject. Weight, height and waist circumference were measured using standardized techniques. Blood pressure was recorded using an automated device (Omron 711) with the subjects seated, after five minutes rest (mean of two readings). Plasma triglyceride (fasting) and glucose (fasting and 120-minutes after a 75g glucose load) concentrations were analyzed by standard enzymatic methods using Randox kits on a Beckman autoanalyser. Fasting HDL-cholesterol was estimated using the same method after phosphotungstate precipitation. IGT and diabetes (DM) were defined using WHO criteria9. Metabolic syndrome (MS) was defined using International Diabetes Federation criteria10 (waist circumference ≥90 cm (men) or ≥80 cm (women) plus two or more of the following: a) fasting serum triglyceride ≥1.7 mmol/l; b) HDL-cholesterol <1.0 mmol/l (men) or <1.3 mmol/l (women); c) hypertension defined as on treatment for hypertension or systolic blood pressure ≥139 mmHg or diastolic pressure ≥85 mmHg and d) known diabetes or fasting plasma glucose ≥5.6 mmol/l.
Statistical Analyses
We used all recorded data (not just for subjects recruited for this study) to derive sex-specific within-cohort SD-scores for each subject at birth, age six months and at birthdays from 1-21 years6,11. Interpolated values were used if a measurement was made within 6 months (up to 1 year), 1 year (age of 2 years), 1.5 years (age of 3 years), and 2 years (all older ages). We also derived SD-scores for cohort children relative to an international reference (United States Center for Disease Control, CDC12). Because earlier analyses showed that BMI gain only after age 2 years predicted adult IGT/diabetes6, and because the number of measurements dropped after age 14 years, we limited our analysis to 2-14 years. Associations of childhood BMI SD-scores, and changes in BMI SD-scores, with adult outcomes were analysed using multiple logistic regression. Sex differences were tested using interaction terms. Risks for adult MS and IGT/diabetes were modelled as a function of BMI SD-scores at pairs of ages in childhood, including the linear and quadratic terms for both BMI SD-scores, and their interaction (the product of both SD-scores), regardless of statistical significance, to fit the full quadratic surface. We display the results using contour lines derived from this model (Figure 1). We assessed the ability of combinations of childhood BMI SD-scores and changes in childhood BMI SD-scores to predict adult outcomes, expressed as their sensitivity, specificity, attributable fraction, positive and negative predictive values, and likelihood ratio13. Data analysis was carried out using SPSS version 12.0; graphs were plotted using FigSys (Biosoft, Cambridge, UK).
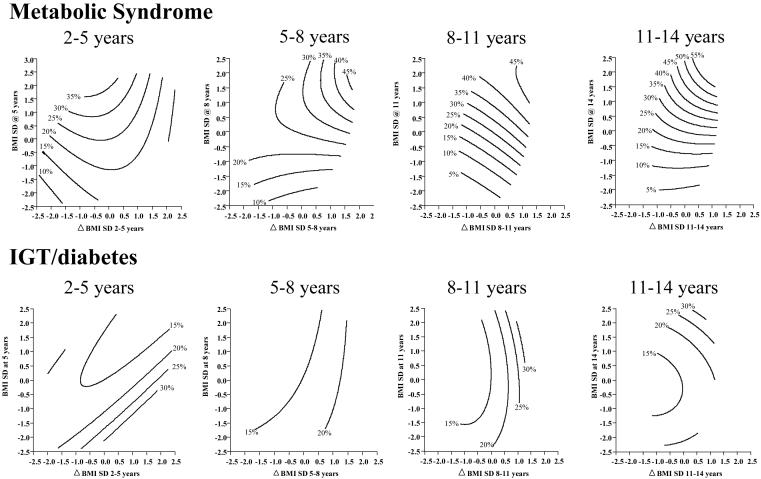
Figure 1. Prevalence (%) of (a) metabolic syndrome and (b) IGT/diabetes according to absolute BMI SD-score and changes in BMI SD-score during childhood.
The contour lines show the prevalence (%) of metabolic syndrome (upper panel) or IGT/diabetes (lower panel) for different combinations of change in BMI SD-score between two ages (x-axis) and BMI SD-score at the later age (y axis). They are drawn so that they cover the most ‘central’ 95% of the observed data points, in order to exclude areas where there were few observations. Perfectly vertical contour lines would indicate that the prevalence varies mainly with change in BMI SD-score, rather than with absolute attained BMI SD-score; horizontal contours indicate the opposite, and diagonal contours indicate that prevalence varies with both these parameters.
RESULTS
Of 2,584 subjects traced, 1,492 had fasting blood samples and 1,442 had complete glucose tolerance tests. Compared with the original cohort, the mean childhood BMI of participants was approximately 0.1 SD lower. At all ages from 2-14 years they had a low mean BMI by international criteria (Table 1). The men and women studied had a mean age of 29 years; 25% had MS, 4% had DM and 11% had IGT (Table 1).
Table 1. Childhood body mass index (BMI), adult anthropometry and risk factors, and prevalence of components of the metabolic syndrome and impaired glucose tolerance and diabetes.
| MALES | FEMALES | |||||
|---|---|---|---|---|---|---|
|
Childhood BMI (kg/m2) |
N | Mean (SD) BMI | CDC†SD-score | N | Mean (SD) BMI | CDC SD-score |
| 2 years 5 years 8 years 11 years 14 years |
831 861 857 828 874 |
15.8 (1.2) 14.9 (1.0) 14.5 (1.3) 15.3 (1.7) 16.7 (2.4) |
-0.8
-0.6 -1.2 -1.2 -1.5 |
600 622 619 600 628 |
15.4 (1.2) 14.7 (1.1) 14.2 (1.2) 15.2 (1.8) 18.2 (2.7) |
-0.9
-0.5 -1.2 -1.3 -0.6 |
| Adult measurements | N | Mean (SD) | N | Mean (SD) | ||
| Age (years) | 884 | 29.2 | (1.3) | 634 | 29.2 | (1.4) |
| Height (cm) | 884 | 169.7 | (6.4) | 632 | 154.9 | (5.7) |
| Weight (kg) | 884 | 71.8 | (14.0) | 634 | 59.3 | (13.4) |
| BMI (kg/m2) | 884 | 24.9 | (4.3) | 632 | 24.7 | (5.1) |
| Waist circumference (cm) |
884 | 90.2 | (12.1) | 634 | 79.6 | (12.4) |
| Triglyceride concentration (mmol/l)* |
868 | 1.57 | (1.7) | 623 | 1.05 | (1.5) |
| HDL cholesterol concentration (mmol/l)* | 869 | 1.12 | (1.3) | 621 | 1.24 | (1.3) |
| Systolic blood pressure (mm Hg) | 878 | 118 | (11) | 625 | 107 | (11) |
| Diastolic blood pressure (mm Hg) | 878 | 78 | (10) | 625 | 73 | (9) |
| Fasting glucose concentration (mmol/l)* | 869 | 5.4 | (1.2) | 623 | 5.3 | (1.2) |
| Prevalence of adult metabolic syndrome components§ and IGT/diabetes | N | No. Positive | % | N | No. Positive | % |
| High waist circumference |
884 | 454 | 51 | 634 | 289 | 46 |
| Hypertriglyceridaemia | 868 | 358 | 41 | 623 | 66 | 11 |
| Low HDL cholesterol | 869 | 297 | 34 | 621 | 345 | 56 |
| High blood pressure or on treatment for hypertension | 878 | 242 | 28 | 626 | 76 | 12 |
| Glucose intolerance, fasting hyperglycaemia or known diabetes | 869 | 359 | 41 | 623 | 228 | 37 |
| Metabolic syndrome | 869 | 265 | 30 | 623 | 114 | 18 |
| Diabetes | 849 | 41 | 5 | 593 | 22 | 4 |
| IGT | 849 | 95 | 11 | 593 | 61 | 10 |
| IGT or diabetes | 849 | 136 | 16 | 593 | 83 | 14 |
Geometric means and standard deviations
SD-scores for childhood BMI according to CDC reference
According to International Diabetes federation criteria10 (see Methods).
The prevalence of MS was higher in men and women whose childhood BMI was higher, and this association strengthened with increasing age of the childhood BMI measurement (Table 2a, Figure 1). The prevalence of IGT/DM was higher in subjects who had a lower BMI up to the age of 8 years, but from 11 years onwards, the pattern was similar to that for MS. The prevalence of both outcomes was higher in men and women whose BMI SD-score increased with time, especially at older ages (Table 2b, Figure 1). All associations were similar in both sexes. When adult BMI was included in the regression models almost all the associations became non-significant. Odds ratios at all ages were similar if CDC rather than Delhi SD-scores were used (Table 2).
Table 2. Percentage of subjects with metabolic syndrome and IGT or diabetes in young adult life according to (a) BMI SD-score and (b) change in BMI SD-score at different ages during childhood.
| (a) BMI SD-score | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) |
Within-cohort (Delhi) SD-Score for body mass index | Odds ratio per unit SD-score* (95% CI) | p value* | CDC, OR per unit SD-score* | ||||||||
| Below -1 | -1 to 0 | 0 to +1 | Above +1 | |||||||||
| Metabolic syndrome | ||||||||||||
| 2 | 23.8 | (181) | 21.7 | (530) | 27.5 | (516) | 30.4 | (181) | 1.20 | (1.04 to 1.37) | 0.01 | 1.18 |
| 5 | 19.1 | (188) | 24.3 | (506) | 26.0 | (534) | 31.4 | (229) | 1.19 | (1.05 to 1.35) | 0.006 | 1.20 |
| 8 | 17.7 | (209) | 22.4 | (558) | 27.6 | (486) | 37.6 | (197) | 1.36 | (1.21 to 1.53) | <0.001 | 1.41 |
| 11 | 11.1 | (190) | 21.4 | (585) | 31.2 | (448) | 39.7 | (179) | 1.63 | (1.43 to 1.85) | <0.001 | 1.69 |
| 14 | 10.8 | (231) | 19.4 | (588) | 33.5 | (463) | 42.3 | (194) | 1.87 | (1.65 to 2.12) | <0.001 | 1.78 |
| IGT/Diabetes | ||||||||||||
| 2 | 21.6 | (176) | 16.1 | (510) | 12.1 | (496) | 13.6 | (177) | 0.84 | (0.71 to 1.00) | 0.04 | 0.85 |
| 5 | 17.3 | (185) | 16.8 | (487) | 13.9 | (518) | 12.4 | (218) | 0.89 | (0.76 to 1.04) | 0.14 | 0.90 |
| 8 | 17.4 | (207) | 15.4 | (538) | 12.6 | (468) | 18.1 | (188) | 1.00 | (0.86 to 1.16) | 1.00 | 0.97 |
| 11 | 16.8 | (184) | 14.2 | (564) | 12.9 | (433) | 22.0 | (173) | 1.13 | (0.97 to 1.31) | 0.11 | 1.09 |
| 14 | 12.9 | (225) | 14.0 | (563) | 14.2 | (450) | 23.2 | (190) | 1.22 | (1.06 to 1.41) | 0.005 | 1.14 |
| (b) Change in BMI SD-score | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) |
Change in within-cohort (Delhi) SD-score for body mass index | Odds ratio per unit change in SD-score* (95% CI) | p value* | CDC, OR per unit change in SD-score* | ||||||||
| Drop >1 | Drop 0 to 1 | Rise 0 to 1 | Rise >1 | |||||||||
| Metabolic syndrome | ||||||||||||
| 2-5 | 20.0 | (170) | 26.0 | (497) | 27.4 | (507) | 21.4 | (206) | 1.03 | (0.90 to 1.18) | 0.065 | 1.01 |
| 5-8 | 21.2 | (113) | 23.5 | (681) | 26.1 | (537) | 40.0 | (100) | 1.35 | (1.15 to 1.59) | <0.001 | 1.31 |
| 8-11 | 13.8 | (29) | 23.1 | (714) | 28.1 | (587) | 42.2 | (45) | 1.69 | (1.33 to 2.15) | <0.001 | 1.84 |
| 11-14 | 25.9 | (27) | 21.5 | (656) | 28.8 | (681) | 36.4 | (33) | 2.04 | (1.57 to 2.66) | <0.001 | 1.97 |
| IGT/Diabetes | ||||||||||||
| 2-5 | 11.0 | (164) | 16.7 | (484) | 13.7 | (489) | 17.4 | (195) | 1.02 | (0.86 to 1.20) | 0.83 | 1.04 |
| 5-8 | 13.6 | (110) | 14.2 | (657) | 15.3 | (518) | 23.7 | (97) | 1.25 | (1.02 to 1.52) | 0.03 | 1.21 |
| 8-11 | 10.3 | (29) | 12.8 | (680) | 16.6 | (574) | 31.8 | (44) | 1.74 | (1.30 to 2.32) | <0.001 | 1.60 |
| 11-14 | 30.4 | (23) | 12.9 | (635) | 16.8 | (659) | 15.2 | (33) | 1.33 | (0.97 to 1.82) | 0.08 | 1.21 |
adjusted for sex and adult age by logistic regression; figures in parentheses are the number of subjects (not cases) in each cell.
Predictive tests
We investigated combinations of within-cohort BMI SD-scores as predictors of adult risk. The most practical test, and the one that gave optimal predictions, defined ‘test-positive’ children as those with a combination of any increase in BMI SD-score between two ages and a BMI SD-score ≥0 at the later age. For example (Table 3), for MS, and BMI SD-scores from 5-14 years, this test identified 28% of children as positive, and had a sensitivity (test-positive given that disease is positive) of 45%, specificity (test-negative given that disease is negative) of 78%, attributable fraction of 24% (the percentage of cases of MS that could potentially be avoided if all children were test-negative, assuming causality), positive predictive value (disease positive given that the test is positive) of 41%, negative predictive value (disease negative given that the test is negative) of 81% and likelihood ratio (the multiplicative factor by which test-positivity increases the odds of disease) of 2.0. Predictions were better at older ages, and with longer time intervals between BMI measurements. For IGT/DM the corresponding figures were: ‘test-positive’ 29%, sensitivity 37%, specificity 73%, attributable fraction 12%, positive predictive value 20%, negative predictive value 87% and likelihood ratio 1.4, similar at all ages and time intervals of BMI measurements (Table 3). There were no significant differences between the sexes.
Table 3. Prediction of adult metabolic syndrome and IGT/diabetes.
| Starting age (years) |
Finishing Age (years) |
Metabolic Syndrome | IGT/Diabetes | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ‘test positive’ (%) |
Sensitivity (%) |
Specificity (%) |
Attributable fraction (%) |
Positive predictive value (%) |
Negative predictive value (%) |
Likelihood ratio |
‘test positive’ (%) |
Sensitivity (%) |
Specificity (%) |
Attributable fraction (%) |
Positive predictive value (%) |
Negative predictive value (%) |
Likelihood ratio |
||
| Delhi reference | |||||||||||||||
| 5 | 8 | 26 | 32 | 76 | 9 | 32 | 77 | 1.4 | 26 | 30 | 75 | 6 | 18 | 86 | 1.2 |
| 11 | 25 | 38 | 79 | 18 | 39 | 79 | 1.9 | 25 | 32 | 76 | 9 | 19 | 86 | 1.3 | |
| 14 | 28 | 45 | 78 | 24 | 41 | 81 | 2.0 | 29 | 37 | 73 | 12 | 20 | 87 | 1.4 | |
| 8 | 11 | 23 | 30 | 80 | 9 | 34 | 77 | 1.5 | 23 | 29 | 78 | 7 | 19 | 86 | 1.3 |
| 14 | 28 | 41 | 77 | 18 | 38 | 79 | 1.8 | 28 | 37 | 74 | 12 | 20 | 87 | 1.4 | |
| 11 | 14 | 28 | 44 | 77 | 21 | 39 | 80 | 1.9 | 29 | 37 | 72 | 11 | 19 | 87 | 1.3 |
| CDC reference | |||||||||||||||
| 5 | 8 | 11 | 17 | 91 | 7 | 40 | 76 | 2.0 | 11 | 14 | 90 | 3 | 19 | 85 | 1.3 |
| 11 | 14 | 22 | 89 | 9 | 41 | 77 | 2.0 | 14 | 21 | 87 | 8 | 22 | 86 | 1.6 | |
| 14 | 25 | 39 | 79 | 18 | 39 | 79 | 1.9 | 25 | 34 | 76 | 11 | 20 | 87 | 1.4 | |
| 8 | 11 | 21 | 30 | 82 | 11 | 36 | 77 | 1.6 | 22 | 27 | 79 | 7 | 19 | 86 | 1.3 |
| 14 | 39 | 51 | 65 | 19 | 33 | 79 | 1.5 | 39 | 44 | 62 | 9 | 17 | 86 | 1.2 | |
| 11 | 14 | 41 | 53 | 63 | 20 | 33 | 80 | 1.4 | 41 | 44 | 60 | 5 | 16 | 86 | 1.1 |
| Overweight or obese (IOTF) | |||||||||||||||
| 5 | 1 | 1 | 98 | -1 | 11 | 74 | 0.3 | 1 | 1 | 99 | 0 | 13 | 85 | 0.8 | |
| 8 | 1 | 1 | 99 | 0 | 30 | 75 | 1.3 | 1 | 1 | 99 | 0 | 22 | 85 | 1.6 | |
| 11 | 1 | 2 | 99 | 1 | 37 | 75 | 1.7 | 1 | 2 | 99 | 1 | 22 | 85 | 1.6 | |
| 14 | 4 | 7 | 97 | 3 | 47 | 75 | 2.6 | 4 | 8 | 97 | 4 | 33 | 86 | 2.8 | |
| >0.67 SD above the within-cohort mean (>75th percentile) | |||||||||||||||
| 5 | 26 | 31 | 76 | 7 | 31 | 76 | 1.3 | 25 | 23 | 74 | -4 | 13 | 84 | 0.9 | |
| 8 | 22 | 32 | 81 | 12 | 36 | 78 | 1.6 | 22 | 25 | 78 | 3 | 17 | 85 | 1.1 | |
| 11 | 19 | 29 | 85 | 13 | 40 | 78 | 2.0 | 19 | 23 | 82 | 5 | 19 | 86 | 1.3 | |
| 14 | 21 | 35 | 84 | 18 | 43 | 79 | 2.2 | 21 | 26 | 80 | 7 | 19 | 86 | 1.3 | |
Definition of ‘test-positive’: An increase in BMI SD-scores between the starting and finishing ages AND a BMI SD-score at the finishing age of above 0 (Delhi reference) or above minus 1 (CDC reference). ‘Overweight or obese’ defined according to International Obesity Task Force (IOTF) criteria14.
When using the CDC reference, we defined ‘test-positive’ as an increase in BMI SD-score between two ages, plus a BMI SD-score at the later age of ≥-1.0. Percentages of ‘test-positive’ children and predictive parameters varied widely with age (Table 3).
Predictions based on single BMI measurements
We defined children who were overweight or obese at 5 (n=19), 8 (n=10), 11 (n=19) and 14 (n=53) years according to the International Obesity Task Force BMI criteria14. Forty-seven percent of the overweight/obese children at 14 years developed MS and 33% developed IGT/DM. Being overweight/obese was highly specific for identifying children at risk of MS or IGT/DM (Table 3), but because few of the children who developed adult pathology were overweight/obese, the sensitivity and attributable fraction were very low. If a lower BMI cut-off was selected (>75th within-cohort percentile), obtaining similar percentages of ‘test-positive’ children to the combined test, the sensitivity improved but remained inferior to that of the combined test (Table 3). Likelihood ratios were similar (regardless of the cut-off) to those obtained using the combined test.
Charts
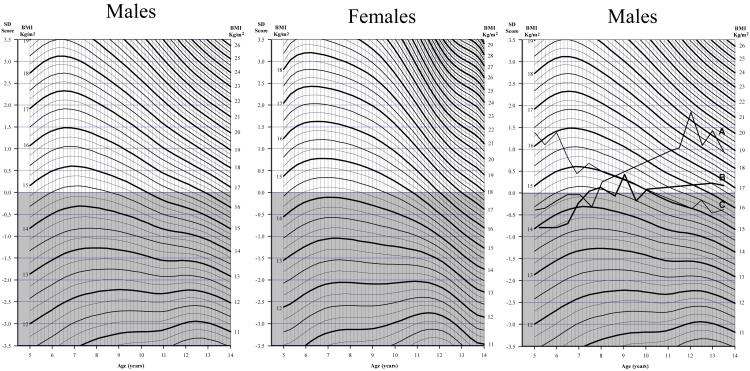
Figure 2 shows charts based on the Delhi data and designed to estimate BMI SD-scores at all ages. The continuous wavy lines are used to plot actual BMI, and the equivalent SD-score is indicated on the left-hand scale. The upper unshaded zone of the graph indicates SD-scores >0. A child whose BMI is in this zone at the end of any age interval and who moves horizontally upwards on the chart is ‘test-positive’. A similar chart based on CDC reference data is available on request.
Figure 2. Chart for plotting BMI at different ages during childhood and converting BMI values into SD-scores (based on within-cohort data).
Measured BMI is plotted on the appropriate wavy line, at the child’s age. The SD-score can be read off the y-axis at the same horizontal level. This procedure is repeated at later ages. ‘Test-positive’ is defined as any increase in SD-score between measurements and an SD-score above the median at the later measurement. The right-hand chart shows the BMI trajectory of 3 cohort children; children A is ‘test positive’ at ages 5-8 and 8-11; child B is ‘test positive’ at ages 5-8 and 11-14 and child C is ‘test-negative’ at all ages.
DISCUSSION
We studied a large sample of young Indian adults who had serial measurements of BMI in childhood. As children, they were thin by international standards, but as adults had a high prevalence of overweight/obesity, diabetes and metabolic syndrome, an increasingly common scenario in urban developing country populations. Accelerated BMI gain during childhood was associated with an increased risk of adult IGT/DM and MS, probably mediated by increased adult adiposity7. Our main objective was to determine whether screening tests could be derived from serial childhood BMI measurements to detect high-risk children. Data from the US Bogalusa Heart Study was used to predict risk of MS from single childhood BMI values15 but as far as we know, this is the first such analysis using longitudinal BMI data.
Strengths of the study were that it was population-based, and anthropometric data were collected by trained personnel at unusually frequent intervals. Only 19% of the original cohort participated, and they are likely to be unrepresentative. However, the differences in their mean childhood BMI from the rest of the cohort, though statistically significant, were small.
Predictions for MS were superior to those for IGT/DM. Sensitivity was 30-45% for MS and 29-37% for IGT/DM. Positive predictive values ranged from 32-41% for MS and 18-20% for IGT/DM. Thus a substantial proportion, though a minority, of ‘test-positive’ children developed disease, and a positive test could thus cause unwarranted anxiety for families. Specificity and negative predictive values were high (76-81% for MS and 72-87% for IGT/DM) indicating that a negative test is reassuring. The attributable fraction (9-24% for MS, 6-12% for IGT/DM) and likelihood ratio (1.4-2.0 for MS and 1.1-1.6 for IGT/DM) were low.
The longitudinal test gave superior predictions to those based on single overweight/obese BMI values, currently often used to identify children needing intervention. The latter, though highly specific (>97%), picked up few of the children at risk and thus had a low sensitivity (≤7%). This differs from findings in the Bogalusa study, in which a single overweight/obese BMI value recorded between the ages of 4 and 15 years had a sensitivity of 38% and specificity of 87% for predicting adult MS15. If a lower BMI cut-off was used in Delhi, predictions were closer to those obtained using the combined test, but sensitivity remained lower.
Opinions will vary on the usefulness of these predictions. Likelihood ratios of >5.0 or <0.2 are generally considered important clinically16 and those for our combined test ranged from 1.1 to 2.0. No single predictive test can be taken in isolation, and likelihood ratios were similar for overweight/obesity, which most clinicians would consider worthy of intervention. Likelihood ratios do not change according to disease prevalence, and a 10 to 100% increase in the odds of developing disease is important if that disease is common and serious. Considering that these are predictions across an intervening period of several decades, we think they are impressive, although better predictive indices, possibly metabolic profiles in adolescence, should be sought.
Given the very high prevalence of disease in young adulthood in this population, it could be argued that screening tests are redundant; there should clearly be population-wide attempts to reduce adiposity. Such interventions, however, have not been adopted even in rich countries, and are unlikely to happen quickly in developing countries. Parents in India who can afford to, do take their children to paediatricians regularly, and individual-level risk-assessment could be useful. Since observational studies cannot prove causation, intervening to limit BMI gain will not necessarily prevent adult disease. Furthermore, no consistently effective interventions have been identified17-20. However, the seriousness of the diabetes epidemic in India demands the formulation of policy and clinical guidelines based on best evidence. Although randomised trials extending from childhood into adulthood have not been performed, lifestyle intervention trials have documented short-term improvements in adiposity, metabolic parameters and endothelial function17-22. A commonsense approach would be to suggest that parents of test-positive children should be advised to consider lifestyle changes, to continue monitoring the child’s BMI, and to obtain relevant investigations (blood pressure, lipid profile and glucose concentrations).
Our analysis raises several practical issues. It would be possible to identify ‘test-positive’ children using a conventional BMI chart (crossing centiles upwards and above the 50th percentile at the later measurement). These changes could, however, appear subtle on a standard chart, and furthermore, clinicians may not be concerned about a child of average BMI, even if climbing centiles. Our new charts show upward and downward movement more clearly. They are more complex than standard charts, however, and clinicians would need additional explanation/guidance to use them. Since detailed growth data is not available for all populations, an external reference would be advantageous and also facilitate global comparisons. We adapted our test to overcome the large BMI differences between the Delhi and CDC populations. The CDC reference, however, produced widely varying predictions because these differences varied with age. Our results suggest that ‘local’ reference data, based on children as similar as possible to those in the population under consideration, are best for identifying high-risk children. We cannot, therefore, recommend the clinical use of our chart in non-Indian populations, or in children that differ greatly in BMI from the Delhi cohort. Comparable analyses need to be carried out, and charts derived, in different populations. Because our measurements were obtained as part of a research study, they are likely to have been more precise, resulting in better predictions, than could be achieved in clinical practice. However, in a simulation exercise, introducing random BMI measurement error, and misclassification of cases and controls, predictive parameters were little changed (details available on request).
Some of the predictive indices (positive and negative predictive values and attributable fraction) are influenced by the prevalence of the outcome. Although the prevalence of metabolic syndrome and IGT/DM was already high in the Delhi cohort, it will increase further with time. We are currently re-studying the cohort after an interval of 5 years, and will re-assess the predictions.
Acknowledgements
We thank the participants, and the field and laboratory staff. The original cohort study was funded by the National Centre for Health Statistics, USA and the Indian Council of Medical Research and this study was funded by the British Heart Foundation. Jane Pearce typed the manuscript and prepared the figures.
Source of funding: British Heart Foundation
Funding
The original cohort study was funded by the National Centre for Health Statistics, USA and the Indian Council of Medical Research and this study was funded by the British Heart Foundation. All the researchers are independent of the funder, and the funder played no part in the study design, collection, analysis and interpretation of data, the writing of the report, or the decision to submit the article for publication.
Footnotes
Competing interests
All authors declare that the answer to the questions on the BMJPG’s competing interest form are all NO and therefore have nothing to declare.
Copyright
The corresponding author has the right to grant on behalf of all authors, and does grant on behalf of all authors, an exclusive license on a worldwide basis to the BMJ Publishing Group Ltd, and its Licensees to permit this article (if accepted) to be published in BMJ editions and any other BMJPGL products and to exploit all subsidiary rights as set out in the license (bmj.com/advice/copyright.shtml).
What is already known on this topic
- Developing countries making rapid economic progress are experiencing ‘epidemic’ rates of type 2 diabetes and metabolic syndrome in young adults.
- Obesity in childhood is a risk factor for these adult disorders, but is an unsatisfactory marker for identifying at-risk children in developing countries, where childhood obesity is still rare.
What this study adds
- In a developing country setting, we have shown that children gaining body mass index faster than their peers, even though they are well below international definitions of obesity, are at increased risk of developing diabetes or metabolic syndrome in adult life.
- We have devised a screening test and suitable body mass index charts that could be used by clinicians to identify at-risk children, based on serial changes in their body mass index, who could be offered advice about healthy diet and lifestyle as one approach to disease prevention.
REFERENCES
- 1.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes. Estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 2.Ghaffar A, Reddy KS, Singhi M. Burden of non-communicable diseases in South Asia. BMJ. 2004;328:807–10. doi: 10.1136/bmj.328.7443.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vanhala M, Vanhala P, Kumpusalo E, Halonen P, Takala J. Relation between obesity from childhood to adulthood and the metabolic syndrome: population based study. BMJ. 1998;317:319. doi: 10.1136/bmj.317.7154.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Srinivasan SR, Myers L, Berenson GS. Predictability of childhood adiposity and insulin for developing insulin resistance syndrome (Syndrome X) in young adulthood; the Bogalusa Heart Study. Diabetes. 2002;51:204–9. doi: 10.2337/diabetes.51.1.204. [DOI] [PubMed] [Google Scholar]
- 5.Sinaiko AR, Donahue RP, Jacobs DR, Prineas RJ. Relation of weight and rate of increase in weight during childhood and adolescence to body size, blood pressure, fasting insulin and lipids in young adults; the Minneapolis children’s blood pressure study. Circulation. 1999;99:1471–6. doi: 10.1161/01.cir.99.11.1471. [DOI] [PubMed] [Google Scholar]
- 6.Bhargava SK, Sachdev HPS, Fall CHD, Osmond C, Lakshmy R, Barker DJP, et al. Relation of serial changes in childhood body mass index to impaired glucose tolerance in young adulthood. New England J Med. 2004;350:865–75. doi: 10.1056/NEJMoa035698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sachdev HPS, Fall CHD, Osmond C, Lakshmy R, Dey Biswas SK, Leary SD, et al. Anthropometric indicators of body composition in young adults: relation to size at birth and serial measurements of body mass index in childhood; the New Delhi birth cohort. Am J Clin Nutr. 2005;82:456–66. doi: 10.1093/ajcn.82.2.456. [DOI] [PubMed] [Google Scholar]
- 8.Fall CHD, Sachdev HPS, Osmond C, Lakshmy R, Dey Biswas SK, Prabhakaran D, Tandon N, Ramji S, Reddy KS, Barker DJP, Bhargava SK. Body mass index from birth to adulthood and metabolic risk factors for cardiovascular disease; data from the New Delhi birth cohort. Early Human Dev. 2007;83(Suppl 1) S80-6E-4 (conference abstract) [Google Scholar]
- 9.World Health Organization . Definition, Diagnosis and Classification of Diabetes Mellitus and its Complications. Part 1: Diagnosis and Classification of Diabetes Mellitus. Report of a WHO Consultation. World Health Organization, WHO/NCD/NCS/99.2; Geneva: 1999. [Google Scholar]
- 10.International Diabetes Federation The IDF consensus worldwide definition of the metabolic syndrome. [accessed Jan 15th 2008]. http://www.idf.org/webdata/docs/Metac_syndrome_def.pdf.
- 11.Royston P. Constructing time-specific reference ranges. Stat Med. 1991;10:675–90. doi: 10.1002/sim.4780100502. [DOI] [PubMed] [Google Scholar]
- 12.National Centre for Health Statistics [Accessed on 5th March 2007]. http://www.cdc.gov/nchs/about/major/nhanes/growthcharts/datafiles.
- 13.Lewis RJ. The analysis of 2X2 tables: odds, likelihood and other useful and confusing terms. [Accessed January 15th 2008]. http://www.saem.org/download/01lewis1.pdf.
- 14.Cole TJ, Bellizzi MC, Flegel KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ. 2000;320:1240–3. doi: 10.1136/bmj.320.7244.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Janssen I, Katzmarzyk PT, Srinivasan SR, Chen W, Malina RM, Bouchard C, Berenson GS. Utility of childhood BMI in the prediction of adulthood disease: comparisons of National and international references. Obesity Research. 2005;13:1106–1115. doi: 10.1038/oby.2005.129. [DOI] [PubMed] [Google Scholar]
- 16.Jaeschke R, Guyatt G, Lijmer J, The Evidence-Based Medicine Working Group . Diagnostic tests. In: Guyatt G, Rennie D, editors. Users’ Guides to the Medical Literature. Essentials of Evidence Based Clinical Practice. American Medical Association Press; 2004. pp. 187–217. JAMA and Archives Journals. [Google Scholar]
- 17.Summerbell CD, Waters E, Edmunds LD, Kelly S, Brown T, Campbell KJ. Interventions for preventing obesity in children. The Cochrane Database of Systematic Reviews. 2005 doi: 10.1002/14651858.CD001871.pub2. CD001871. [DOI] [PubMed] [Google Scholar]
- 18.Cruz ML, Shaibi GQ, Weigensberg MJ, Spruijt-Metz D, Ball GDC, Goran MI. Pediatric obesity and insulin resistance : chronic disease risk and implications for treatment and prevention beyond body weight modification. Annu Rev Nutr. 2005;25:435–468. doi: 10.1146/annurev.nutr.25.050304.092625. [DOI] [PubMed] [Google Scholar]
- 19.Bhave S, Bavdekar A, Otiv M. Indian Academy of Paediatrics National Task Force for Childhood Prevention of Adult Diseases: Childhood Obesity. Indian Pediatr. 2004;41:559–75. [PubMed] [Google Scholar]
- 20.NHS Centre for Reviews and Dissemination The prevention and treatment of childhood obesity. Effective Health Care. 2002;7:1–12. [Google Scholar]
- 21.Monzavi R, Dreimane D, Geffner ME, Braun S, Conrad B, Klier M, Kaufman FR. Improvement in risk factors for metabolic syndrome and insulin resistance in overweight youth who are treated with lifestyle intervention. Pediatrics. 2006;117:1111–1118. doi: 10.1542/peds.2005-1532. [DOI] [PubMed] [Google Scholar]
- 22.Raitakari OT, Ronnemaa T, Jarvisalo MJ, Volanen I, Kallio K, Lagstrom H, Jokinen E, Niinikosk H, Viikari JS, Simell O. Endothelial function in healthy 11-year-old children after dietary intervention with onset in infancy: the Special Turku Coronary Risk Factor Intervention Project for children (STRIP) Circulation. 2005;112:3786–3794. doi: 10.1161/CIRCULATIONAHA.105.583195. [DOI] [PubMed] [Google Scholar]