Abstract
Objective
The purpose of this study was to evaluate the effect of 12/15-lipoxygenase (12/15LO) in macrophage ABCG1 expression and function associated with cholesterol efflux.
Methods and Results
12/15LO was stably overexpressed in J774 macrophages. 12/15LO-overexpressing macrophages had a 30% reduction in HDL-mediated cholesterol efflux, corresponding with significantly reduced ABCG1 protein expression. Treatment of 12/15LO-overexpressing macrophages with a 12/15LO ribozyme to reduce 12/15LO restored HDL-mediated efflux and ABCG1 protein expression. Treating macrophages with 12/15LO unsaturated fatty acid substrates or eicosanoid products also reduced HDL-mediated cholesterol efflux. Additionally, both 12/15LO overexpression in macrophages and incubation of macrophages with eicosanoids reduced ABCG1 protein, but not mRNA, expression. However, incubation of macrophages with linoleic or arachidonic acids significantly reduced both ABCG1 mRNA and protein expression, suggesting that 12/15LO substrates and eicosanoid products differentially regulate ABCG1 expression. 12/15LO fatty acids did not decrease ABCG1 translation; however, 12/15LO fatty acids increased ABCG1 degradation when blocked by cyclohexidmide. ABCG1 degradation may be regulated through post-translational modifications. Treatment with the 12/15LO eicosanoid product 12SHETE increased serine phosphorylation of ABCG1.
Conclusions
We conclude that serine phosphorylation may increase the degradation rate of ABCG1, and as a result cause macrophage cholesterol accumulation. These findings provide evidence that 12/15LO activity in the vessel wall contributes to atherogenesis by impairing the macrophage ABCG1 cholesterol efflux pathway.
Keywords: Lipoxygenase, ABCG1, macrophage, fatty acid, eicosanoid
Atherosclerosis is a chronic inflammatory disease characterized by monocyte recruitment to the arterial wall and their subsequent accumulation as lipid-laden macrophages, or ‘foam’ cells. Oxidation of low-density lipoprotein (LDL) plays an essential role in the early events leading to foam cell formation, and the enzyme 12/15-lipoxygenase (12/15LO) has been implicated in LDL oxidation1,2.
12/15LO is a non-heme iron containing dioxygenase which incorporates molecular oxygen stereospecifically to arachidonic and linoleic acid to produce the hydroperoxy fatty acids 12S- and 15S-hydroxyeicosatetranoic acids (12SHETE/15SHETE) and 13S-hydroxyoctadecadienoic acid (13SHODE), respectively3,4. The 12/15-lipoxygenase family consists of “platelet-type” 12-lipoxygenase and “leukocyte-type” 12/15-lipoxygenase. Based on the ratios of 12S- and 15SHETE produced from arachidonic acid, the enzyme is designated 15-lipoxygenase (15LO) in humans and leukocyte 12-lipoxygenase in mice and pigs. Murine leukocyte 12/15LO and human 15LO proteins are highly homologous5.
12/15LO activation has been implicated in the pathogenesis of atherosclerosis and diabetes. We and others have shown that mice deficient in 12/15LO are protected from the development of atherosclerosis6,7 while mice that overexpress the enzyme are more prone to lesion development8. The mechanisms by which 12/15LO regulates atherogenesis remain unknown, but may be due to inflammatory actions of 12/15LO eicosanoids on the endothelium and macrophages in the vessel wall and/or due to its ability to oxidize LDL1,8,9. 12/15LO protein has been localized within atherosclerotic lesions in both rabbits and humans10,11. In vitro data from Kuhn et al. suggested a role for 12/15LO in the oxidation of LDL12, while Cathcart and colleagues demonstrated that human monocytes generate superoxide that mediates the oxidation of LDL in an enzyme-dependent manner2.
Initially in lesion development monocytes are recruited to the vessel wall, where they differentiate into macrophages and upregulate scavenger receptors such as CD36 and SR-A to facilitate the uptake of oxidized LDL13. Conversely, differentiated macrophages also upregulate members of the ATP binding cassette transporter family (ABC transporters), namely ABCA1 and ABCG1, to mediate the transfer or efflux of modified cholesterol moieties to high density lipoproteins (HDL)14. HDL transports this cholesterol back to the liver for subsequent conversion to bile and removal from the body during the process of reverse cholesterol transport (RCT). ABCA1-mediated removal of cholesterol to lipid poor HDL particles has been extensively studied15. Fatty acids can regulate ABCA1 expression and function. Oram and colleagues have demonstrated that unsaturated fatty acids, such as arachidonic and linoleic acid, inhibit cholesterol efflux from macrophages by enhancing the protein degradation and serine phosphorylation of ABCA116,17. Moreover, apolipoprotein A-I (apoA-I) binds and cross-links ABCA1 to stabilize and protect the transporter from degradation18.
Much less is known about the regulation of ABCG1, and the role of this transporter in the development of atherosclerosis remains controversial19. ABCG1 mediates the removal of cholesterol to mature HDL particles and facilitates the clearance of esterified cholesterol from macrophages20. Uehara et al. have demonstrated that unsaturated fatty acids suppress ABCG1 gene transcription21. In the current study, we show that fatty acids of the 12/15LO pathway decrease HDL-mediated cholesterol efflux from macrophages. This reduction in cholesterol efflux is associated with enhanced degradation and serine phosphorylation of ABCG1. These novel results support the concept that 12/15LO activity in the vessel wall impairs the clearance of excess cholesterol from macrophages through regulation of ABCG1, thereby contributing to the development of macrophage foam cell formation.
Materials and Methods
For detailed methodology, please see the data supplement, available online at http://atvb.ahajournals.org. J774a.1 (J774) cells were cultured in DMEM containing 10% fetal bovine serum (FBS). J774 cells stably overexpressing porcine leukocyte 12/15LO (Plox) and mock-transfected (Mock) J774 cells were kind gifts of Dr. Tanihiro Yoshimoto (Kanazawa University School of Medicine, Kanazawa, Japan)22.
Results
12/15LO activity reduces HDL-mediated cholesterol efflux
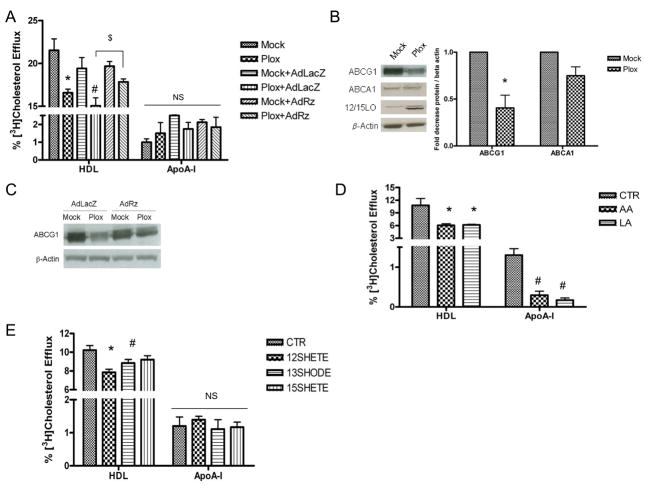
Macrophages in atherosclerotic lesions have been shown to express high levels of 12/15LO23. Thus, we assessed the role of 12/15LO and its eicosanoid products in regulating macrophage function associated with RCT. We used mouse J774 macrophages overexpressing porcine leukocyte 12/15LO (Plox) or mock-transfected (Mock) control cells. We have previously shown that Plox macrophages have increased production of fatty acid hydroxy derivatives and a 3-fold increase in 12SHETE production compared to Mock controls9. In the current study, Mock and Plox macrophages were labeled with [3H]cholesterol, and the effects of 12/15LO overexpression on HDL- or apoA-I-mediated [3H]cholesterol efflux were measured. Tall and colleagues have reported that HDL is the preferred cholesterol acceptor from ABCG1 while lipid free apoA-I is the preferred cholesterol acceptor from ABCA124. 12/15LO-overexpressing Plox macrophages had a significant 30% reduction in HDL-mediated cholesterol efflux compared to Mock controls (Figure 1A). No significant change in apoA-I-mediated cholesterol efflux between the two groups was observed, suggesting that 12/15LO activity preferentially modulates ABCG1-mediated cholesterol efflux.
FIGURE 1. 12/15LO activity reduces cholesterol efflux to HDL.
Panel A. Mock-transfected (Mock) or 12/15LO-overexpressing (Plox) J774 macrophages were incubated with recombinant adenovirus expressing either the 12/15LO ribozyme (+AdRz) or control LacZ vector (+AdLacZ). Cholesterol efflux to either HDL or to lipid free apoA-I (apoA-I) was measured. Data represent the mean ± SE of 3 experiments (*significantly lower than Mock p<0.006; #significantly lower than Mock+AdLacZ p<0.007; $significantly higher than Plox+AdLacZ p<0.05 by ANOVA). Panel B. Left, Total cell lysates were analyzed by immunoblotting for ABCG1, ABCA1, or 12/15LO. Right, Densitometry of immunoblots normalized to beta actin. Data represent the mean ± SE of 4 experiments (*significantly lower than Mock p<0.02 by ANOVA). Panel C. Mock or Plox macrophages were incubated with recombinant adenovirus expressing either the 12/15LO ribozyme (AdRz) or control LacZ vector (AdLacZ). Total cell lysates were analyzed by immunoblotting for ABCG1. Results are representative of 2 similar experiments. Panel D. HDL- or apoA-I mediated cholesterol efflux was measured in J774 macrophages treated with vehicle control (CTR) or 125 μM arachidonic (AA) or linoleic acid (LA). Data represent the mean ± SE of 6 experiments (*significantly lower than CTR p<0.005; #significantly lower than CTR p<0.0001 by ANOVA). Panel E. HDL- or apoA-I mediated cholesterol efflux was measured in J774 macrophages treated with vehicle control (CTR) or 500nM 12SHETE, 13SHODE, or 15SHETE. Data represent the mean ± SE of 6 experiments (*significantly lower than CTR p<0.001; #significantly lower than CTR p<0.04 by ANOVA).
To further determine that 12/15LO activity is directly responsible for the reduction in cholesterol efflux, we inhibited expression of 12/15LO in Mock and Plox macrophages using an adenovirus expressing a ribozyme to 12/15LO (AdRz). We have previously used this ribozyme to reduce 12/15LO expression by 60%25,26. The 12/15LO ribozyme restored HDL-mediated cholesterol efflux in Plox macrophages to levels similar to control (Figure 1A), indicating that 12/15LO is able to regulate HDL-mediated cholesterol efflux.
12/15LO activity reduces ABCG1 cellular expression
Because ABCG1 is known to regulate HDL-mediated cholesterol efflux, we assayed the effects of 12/15LO activity on ABCG1 expression. We confirmed that 12/15LO protein was overexpressed in Plox macrophages (Figure 1B). Plox macrophages had a significant 60% reduction in ABCG1 protein expression (Figure 1B). No significant change in ABCA1 protein expression was observed (Figure 1B). Inhibition of 12/15LO in Plox macrophages with the 12/15LO ribozyme prevented the downregulation of ABCG1 protein expression (Figure 1C). These data suggest that the reduction in ABCG1 expression and cholesterol efflux to HDL is regulated by 12/15LO activity.
12/15LO eicosanoids reduce cholesterol efflux to HDL
When macrophages are presented with an amount of cholesterol that exceeds that which can be incorporated into cellular membranes, the excess cholesterol is stored in macrophages as cholesteryl esters. The enzyme acyl-coenzyme A:cholesterol acyltransferase (ACAT) esterifies excess cholesterol with long chain fatty acids and the resulting cholesteryl esters are stored in the cytoplasm as neutral lipid droplets. To examine whether fatty acid treatment enhanced cholesteryl ester formation, we treated J774 macrophages with arachidonic or linoleic acid for 16 hours and measured esterified cholesterol accumulation (Wako Chemicals USA, Inc). Treatment with arachidonic or linoleic acids did not significantly alter macrophage cholesteryl ester content (data not shown). We next incubated J774 macrophages with fatty acid precursors and eicosanoid products of the 12/15LO pathway and measured cholesterol efflux. J774 macrophages were labeled with [3H]cholesterol and incubated with arachidonic and linoleic acids. Subsequently, HDL- and apoA-I-mediated cholesterol efflux was measured. Incubation of J774 macrophages with arachidonic or linoleic acids significantly reduced HDL-mediated cholesterol efflux by 43% (Figure 1D), compared to control. Arachidonic and linoleic acids also significantly reduced apoA-I-mediated cholesterol efflux by 77% and 87% respectively. These data are consistent with the findings of Oram and colleagues indicating that unsaturated fatty acids downregulate ABCA1 expression and function16.
To assess the role of 12/15LO eicosanoids on cholesterol efflux, J774 macrophages were labeled with [3H]cholesterol and treated with 12SHETE, 13SHODE, or 15SHETE. Subsequently, HDL- and apoA-I-mediated cholesterol efflux was measured. 12SHETE and 13SHODE significantly reduced cholesterol efflux to HDL by 23% and 13% respectively (Figure 1E). Treatment with 15SHETE produced a 10% reduction in HDL-mediated cholesterol efflux, although this reduction was not statistically significant (Figure 1E). No significant change in apoA-I-mediated cholesterol efflux was observed, indicating that products of the 12/15LO pathway preferentially regulate ABCG1-mediated cholesterol efflux.
Eicosanoid products regulate ABCG1 expression in macrophages
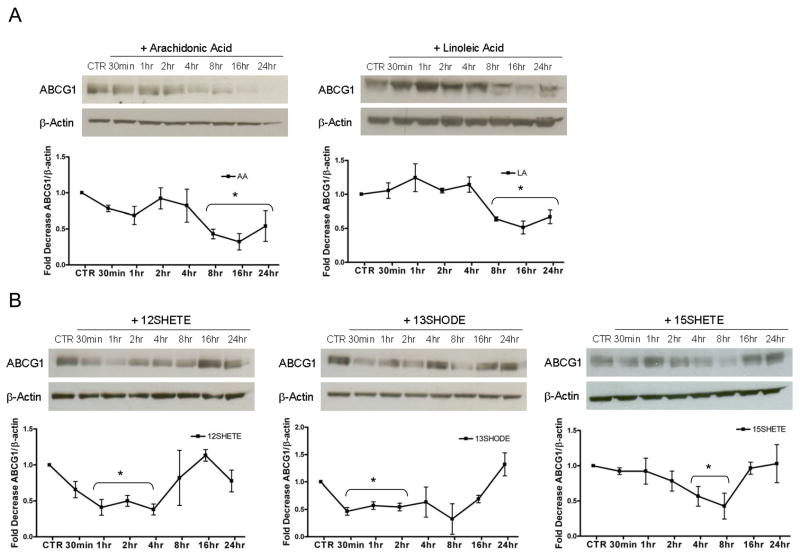
We next examined whether 12/15LO fatty acids regulate ABCG1 protein expression. Oram and colleagues have previously described the role of unsaturated fatty acids in reducing ABCA1 membrane content16. In a similar manner, we found that arachidonic and linoleic acid significantly reduced ABCG1 protein expression within 8 hours and continuing through 24 hours compared to control (e.g., time 0) (Figure 2A).
FIGURE 2. 12/15LO activity reduces cellular ABCG1.
Cell lysates were analyzed by immunoblotting for ABCG1 at each time point. Each time point is compared to control (CTR, e.g. time 0) for statistical evaluation. Panel A. Top, J774 macrophages were incubated with vehicle control (CTR) or with arachidonic (AA) or linoleic (LA) acid (125 μM) for up to 24 hours. Bottom, Densitometry of immunoblot normalized to beta actin. Data represent the mean ± SE of 3 experiments (*significantly lower than CTR p<0.04 by ANOVA). Panel B. Top, J774 macrophages were incubated with vehicle control (CTR) or with the indicated eicosanoids (500nM) for up to 24 hours. Bottom, Densitometry of immunoblot normalized to beta actin. Data represent the mean ± SE of 3 experiments (*significantly lower than CTR p<0.05 by ANOVA).
To determine if specific 12/15LO products regulated ABCG1 expression, we incubated J774 macrophages with 12/15LO eicosanoids for up to 24 hours. Treatment with the 12/15LO eicosanoids also reduced ABCG1 protein expression; however, these eicosanoids reduced ABCG1 expression at much earlier times than did arachidonic and linoleic acids (Figure 2B). Compared to control (e.g., time 0), 12SHETE significantly reduced ABCG1 protein expression from 1 to 4 hours, 13SHODE significantly reduced ABCG1 protein expression from 30 minutes to 2 hours, and 15SHETE significantly reduced ABCG1 expression from 4 to 8 hours (Figure 2B). Thus, 12/15LO eicosanoids seem to produce more rapid downregulation of ABCG1 than did arachidonic and linoleic acids.
12/15LO activity does not affect ABCG1 mRNA expression
Based on the findings of Uehara and colleagues indicating that unsaturated fatty acids suppress ABCA1 and ABCG1 gene transcription21, we examined the effects of 12/15LO on ABCG1 mRNA expression. Plox macrophages had no change in relative ABCG1 mRNA levels compared to Mock controls (supplemental Figure IA, see www.ahajournals.org). Interestingly, ABCA1 mRNA was significantly reduced in 12/15LO-overexpressing macrophages by 40% (Figure IA). However, the reduction in ABCA1 mRNA does not appear to translate to decreased ABCA1 protein expression or decreased apoA-I-mediated cholesterol efflux (Figures 1A and B).
Treatment of J774 macrophages with arachidonic or linoleic acid significantly reduced ABCG1 and ABCA1 mRNA expression (supplemental Figure IB), consistent with the findings of Uehara and colleagues21. However, treatment of J774 macrophages with 12/15LO eicosanoids resulted in no changes in ABCG1 or ABCA1 mRNA levels (supplemental Figure IC). These results suggest that the inhibitory effects of 12/15LO activity on HDL-mediated cholesterol efflux and ABCG1 protein expression are not due to inhibition of ABCG1 transcription or message stability. These data also indicate that unsaturated fatty acids and 12/15LO eicosanoids regulate ABCG1 expression differently. Thus, unsaturated fatty acids transcriptionally regulate ABCG1 expression while 12/15LO eicosanoids regulate ABCG1 expression post-transcriptionally.
The 12/15LO product, 13SHODE, is a ligand for peroxisome proliferator-activated receptor gamma (PPARγ)27. PPARγ has been shown to induce the expression of liver X receptor α (LXRα) thereby stimulating the expression of ABCA1 and ABCG1, leading to enhanced cholesterol efflux28. Addition of 13SHODE to J774 macrophages resulted in a significant increase in LXRα expression (supplemental Figure IC); however, 13SHODE treatment did not significantly induce ABC transporter expression, indicating that 13SHODE may regulate ABCA1 and ABCG1 post-translationally.
12/15LO products enhance ABCG1 degradation
We hypothesized that 12/15LO activity may reduce ABCG1 protein expression through decreased translation or increased protein degradation. To measure the effect of 12/15LO on translation, J774 macrophages were treated with 12SHETE, arachidonic, or linoleic acids for 2 or 16 hours. Cells were labeled with [35S]methionine for 20 minutes, and [35S]methionine incorporation into immunoprecipitated ABCG1 was measured. Treatment with 12/15LO fatty acids did not change methionine incorporation into ABCG1 (supplemental Figure II), indicating that reduced translation efficiency cannot account for the 12/15LO-mediated decrease in ABCG1 expression.
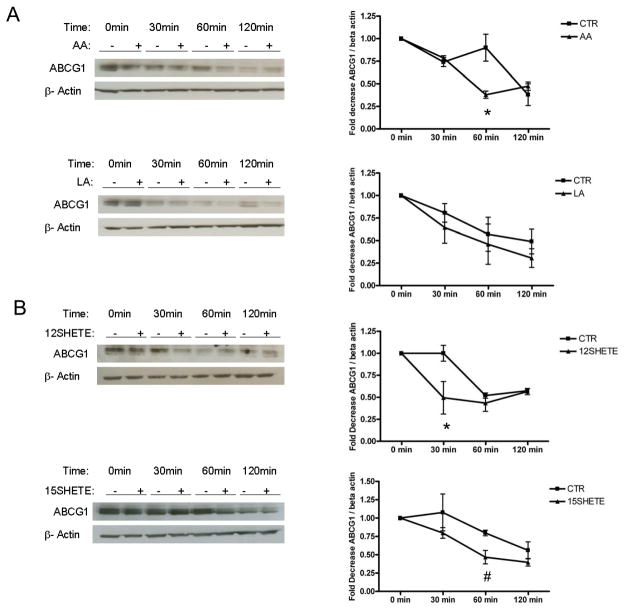
To address the possibility that the 12/15LO pathway regulates ABCG1 degradation, we treated J774 macrophages with 12/15LO fatty acids in the presence of cycloheximide to arrest protein synthesis. We pretreated J774 macrophages with arachidonic or linoleic acids for 2 hours prior to the addition of cycloheximide. Arachidonic but not linoleic acid significantly reduced ABCG1 protein expression after treatment with cycloheximide for 60 minutes (Figure 3A). To assess the role of the 12/15LO eicosanoids on ABCG1 degradation, we treated J774 macrophages with 12SHETE or 15SHETE immediately prior to the addition of cycloheximide. 12SHETE or 15SHETE also reduced ABCG1 protein expression, but seemed to have a more rapid effect than arachidonic acid. 12SHETE and 15SHETE significantly reduced ABCG1 expression after treatment with cycloheximide for 30 or 60 minutes respectively (Figure 3B). These data are consistent with those of Figure 2, which shows rapid degradation of ABCG1 with 12/15LO eicosanoids compared to unsaturated fatty acids.
FIGURE 3. 12/15LO products enhance ABCG1 degradation.
Panel A. Left, J774 macrophages were treated with either vehicle control (CTR), arachidonic (AA), or linoleic (LA) acids for 2 hours prior to the addition of cycloheximide for the indicated time points. Cell lysates were analyzed by immunoblotting for ABCG1. Right, Densitometry of immunoblot normalized to beta actin. Data represent the mean ± SE of 3 experiments (*significantly lower than CTR 60min p<0.002 by ANOVA). Panel B. Left, J774 macrophages were treated with cycloheximide and either vehicle control (CTR), 12SHETE, or 15SHETE for the indicated time points. Cell lysates were analyzed by immunoblotting for ABCG1. Right, Densitometry of immunoblots normalized to beta actin. Data represent the mean ± SE of 3 experiments (*significantly lower than CTR 30min p<0.003; #significantly lower than CTR 60min p<0.05 by ANOVA).
12/15LO products enhance ABCG1 serine phosphorylation
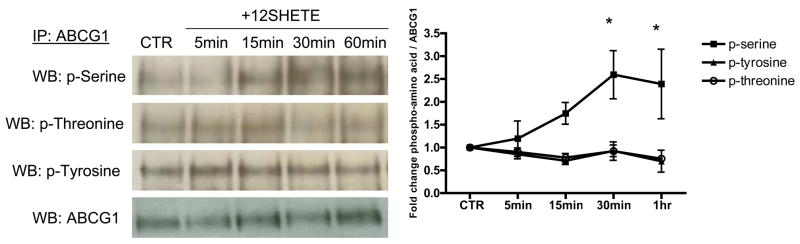
We hypothesized that enhanced degradation of ABCG1 may be associated with post-translational modifications. Oram and colleagues determined that enhanced degradation of ABCA1 is associated with serine phosphorylation17. To measure phosphorylation changes in ABCG1, we used phosphoamino acid-specific antibodies to determine if 12/15LO eicosanoids regulate serine, threonine, or tyrosine phosphorylation. Immunoblots of immunoprecipitated ABCG1 revealed that serine, threonine, and tyrosine phosphorylation occurs at a basal state (Figure 4). 12SHETE treatment significantly enhanced serine, but not threonine or tyrosine, phosphorylation (Figure 4). ABCG1 serine phosphorylation was induced within 15 minutes after 12SHETE treatment and continued through one hour of exposure. Thus, serine phosphorylation occurs concurrently with 12/15LO eicosanoid-regulated degradation of ABCG1 (Figure 3B) and may account for the enhanced degradation rate.
FIGURE 4. 12SHETE enhances ABCG1 serine phosphorylation.
Left, J774 macrophages were treated with either vehicle control (CTR) or 500nM 12SHETE for the indicated time points. ABCG1 was isolated by immunoprecipitation, and blots were probed with phospho-serine, phospho-threonine, phospho-tyrosine, or ABCG1 antibodies. Right, Densitometry of immunoblots normalized to ABCG1. Data represent the mean ± SE of 3 experiments (*significantly higher than CTR p<0.05 by ANOVA).
Discussion
12/15-lipoxygenase (12/15LO) is expressed in high levels in macrophages found in early atherosclerotic lesions10,11. 12/15LO has been implicated in the pathogenesis of diabetes and atherosclerosis. Studies in mice have clearly shown that 12/15LO activity contributes to the atherosclerotic burden 6,8. Moreover, studies have reported increased 12/15LO activity in patients with Type 2 diabetes, including increased renal excretion 12/15LO eicosanoid metabolites 29. The 12/15LO pathway is upregulated by glucose, growth factors, and cytokines, so it is quite likely that this enzyme plays a significant role in the cardiovascular complications associated with diabetes. We have previously shown that 12/15LO activity is upregulated in diabetic db/db mice25, and that these diabetic mice have reduced macrophage ABCG1 expression and function30. In the current study, we demonstrate for the first time that 12/15LO activity in macrophages increases the degradation of ABCG1. ABCG1 is a key regulator of cholesterol efflux from peripheral cells to HDL in RCT31. Thus, factors that reduce ABCG1 expression and function will impact macrophage foam cell formation and cholesterol homeostasis in the arterial wall, and may contribute to the accelerated cardiovascular disease observed in patients with Type 2 diabetes.
12/15LO overexpression in macrophages significantly reduces HDL-mediated but not apoA-I-mediated cholesterol efflux, and this reduction in efflux corresponds with a significant reduction in ABCG1, but not ABCA1, protein expression (Figure 1). Moreover, arachidonic and linoleic acids, and 12/15LO eicosanoid products also reduced HDL-mediated cholesterol efflux (Figure 1). Treatment of macrophages with 12/15LO eicosanoids did not reduce cholesterol efflux to apoA-I, indicating a preferential role in targeting ABCG1 (Figure 1).
Although the reduction in cholesterol efflux may appear modest, we have previously found that a 20% reduction in cholesterol efflux can lead to a 4-fold increase in cholesteryl ester accumulation30. Rader and colleagues have demonstrated that knock-down of ABCG1 levels in J774 macrophages by approximately 50% results in a significant reduction in RCT31. Thus, the changes in macrophage cholesterol efflux in response to fatty acids or 12/15LO products would have a significant impact on RCT.
12/15LO activity in macrophages had no effect on ABCG1 mRNA expression (supplemental Figure I). Uehara and colleagues reported that unsaturated fatty acids can suppress ABCG1 and ABCA1 gene expression21. Consistent with their findings, we observed downregulation of ABCG1 and ABCA1 mRNA expression by linoleic and arachidonic acids (supplemental Figure I). Interestingly, neither 12/15LO overexpression in macrophages nor incubation of macrophages with 12/15LO eicosanoids produced significant changes in ABCG1 mRNA expression (supplemental Figure I). Both ABCA1 and ABCG1 gene expression are regulated by the nuclear hormone receptor, liver X receptor (LXR). It has been previously shown that unsaturated fatty acids antagonize the activation of LXRα by oxysterols32. Thus arachidonic and linoleic acids might regulate ABCG1 and ABCA1 gene transcription through inhibition of LXRα activation. Regulation of LXRα is incompletely understood, and studies have demonstrated important roles for phosphorylation and acetylation of LXRα in regulating the activity of this transcription factor33,34. Post-translational modifications of LXRα may account for upregulation of LXRα expression by 13SHODE with no corresponding upregulation of ABC transporter mRNA expression (supplemental Figure I). Studies to examine the effects of 12/15LO eicosanoids on LXR activation are underway in the laboratory.
12/15LO could reduce ABCG1 protein expression through decreased message translation or enhanced protein degradation. 12/15LO fatty acids or eicosanoids did not alter translation efficiency as determined by methionine incorporation into de novo ABCG1 protein synthesis (supplemental Figure II). Treatment of J774 macrophages with 12SHETE in the presence of cycloheximide indicated that protein degradation appears to be the likely mechanism by which 12/15LO products regulate ABCG1 levels (Figure 3). Surprisingly, unsaturated fatty acids and 12/15LO eicosanoids had differential effects on ABCG1 protein expression. Incubation of macrophages with 12/15LO eicosanoids produced a rapid reduction in ABCG1 protein expression within 30 minutes to 1 hour that continued through 4 to 8 hours, while incubation of macrophages with either arachidonic or linoleic acids reduced ABCG1 expression only after approximately 8 hours of incubation (Figure 2). The time needed to convert fatty acid substrate into eicosanoid product might account for the disparity in ABCG1 expression. Arachidonic and linoleic acid substrates also produced a more lasting effect in reducing ABCG1 expression that continued 16 to 24 hours after treatment, while levels of ABCG1 returned to control (e.g., time 0) levels by 16 to 24 hours of treatment with 12/15LO eicosanoids. Since cells rapidly metabolize eicosanoids, the return of ABCG1 levels to control levels is most likely due to the metabolism of the eicosanoid and subsequent loss of regulation. Alternatively, the return of ABCG1 levels to control levels may be due to attempts of the macrophage to restore ABCG1 expression and cholesterol homeostasis in a compensatory manner.
We anticipate that 12/15LO mediates post-translational modifications of ABCG1, resulting in enhanced degradation. Indeed, we observed the presence of phosphorylated serine, threonine, and tyrosine residues in ABCG1 in the basal state (Figure 4). Treatment with 12SHETE enhanced ABCG1 serine, but not threonine or tyrosine phosphorylation (Figure 4). These data suggest that phosphorylation may be one potential mechanism of regulation of ABCG1 protein expression. Oram and colleagues have elegantly shown that unsaturated fatty acids reduce ABCA1 expression, at least in part through phosphorylation that enhances degradation17. These investigators did not examine the effects of 12/15LO eicosanoids on ABCA1 in their study; however, our data in Figure 1 indicate that 12/15LO overexpression does not significantly impact ABCA1 protein expression. We are currently working to identify the role that post-translational modifications to ABCG1 play in regulating ABCG1 degradation by unsaturated fatty acids and eicosanoids. A direct role for serine phosphorylation in regulating ABCG1 degradation will need to be verified by mutational analysis.
Interestingly, Belkner et al. demonstrated that 12/15LO-overexpressing macrophages have reduced intracellular lipid accumulation and foam cell formation due to reductions in SR-A expression and enhanced cholesteryl ester degradation35. However, these authors found that the 12/15LO products 12SHETE and 13SHODE failed to reduce intracellular cholesterol deposition and tended to enhance lipid accumulation35. Moreover, cholesterol efflux to fetal calf serum was not different between control and 12/15LO-overexpressing macrophages over 18 hours of incubation35. Our study used specific acceptors (lipid free apoA-I or HDL) to delineate the contribution of ABC transporters and shorter incubation time (4 hours), which may account for the differences between the studies. Moreover, we demonstrated that 12/15LO eicosanoids can directly regulate cholesterol efflux and ABCG1 expression. Thus, 12/15LO activity may regulate both macrophage lipid uptake and efflux in a compensatory manner, depending on the surrounding lipid environment in the arterial wall. Additional mechanistic studies are needed to delineate the “pro-atherogenic” versus “anti-atherogenic” properties of this enzyme.
The findings of our current study have important implications. Our data indicate that 12/15LO plays a role in regulating cholesterol efflux from macrophages, thus providing a novel, important link between 12/15LO activity in the vessel wall and macrophage foam cell formation. Elevated levels of plasma free fatty acids are prevalent among subjects with metabolic syndrome and Type 2 diabetes and these free fatty acids can accumulate in atherosclerotic lesions. The action of these fatty acids is two-fold in the vessel wall. First, as shown by us and by Oram’s group, fatty acids have the ability to directly regulate ABCG1 and ABCA1 expression and function by enhancing ABC transporter degradation and inhibiting ABC transporter transcription. Second, these fatty acids act as substrates for the 12/15LO enzyme to convert them into eicosanoids, which cause ABCG1 degradation and reduced cholesterol efflux. We have previously reported regulation of ABCG1 transcription by chronic elevated glucose in Type 2 diabetes30. Taken together, our data indicate that ABCG1 is regulated by both glucose and free fatty acids, which has important clinical implications for diabetic atherosclerosis. Understanding these novel mechanisms by which unsaturated fatty acids and products of the 12/15LO pathway enhance ABCG1 degradation will be beneficial for developing new therapies to regulate ABCG1 and ABCA1 expression in atherogenesis, especially in the setting of Type 2 diabetes.
Supplementary Material
Acknowledgments
The authors thank Dr. John S. Parks (Wake Forest University) for recombinant apoA-I and helpful advice regarding cholesterol efflux, and Dr. David L. Brautigan (University of Virginia) for providing invaluable advice regarding immunoprecipitation studies.
Sources of Funding- This work was supported by NIH P01 HL55798 (to JLN and CCH) and NIH R01 HL085790 (to CCH).
Footnotes
Disclosures- None
References
- 1.Sigari F, Lee C, Witztum JL, Reaven PD. Fibroblasts that overexpress 15-lipoxygenase generate bioactive and minimally modified LDL. Arterioscler Thromb Vasc Biol. 1997;17:3639–3645. doi: 10.1161/01.atv.17.12.3639. [DOI] [PubMed] [Google Scholar]
- 2.McNally AK, Chisolm GM, III, Morel DW, Cathcart MK. Activated human monocytes oxidize low-density lipoprotein by a lipoxygenase-dependent pathway. J Immunol. 1990;145:254–259. [PubMed] [Google Scholar]
- 3.Yamamoto S, Suzuki H, Ueda N. Arachidonate 12-lipoxygenases. Prog Lipid Res. 1997;36:23–41. doi: 10.1016/s0163-7827(97)00002-7. [DOI] [PubMed] [Google Scholar]
- 4.Kuhn H, Borngraber S. Mammalian 15-lipoxygenases. Enzymatic properties and biological implications. Adv Exp Med Biol. 1999;447:5–28. [PubMed] [Google Scholar]
- 5.Yoshimoto T, Takahashi Y. Arachidonate 12-lipoxygenases. Prostaglandins Other Lipid Mediat. 2002;68–69:245–262. doi: 10.1016/s0090-6980(02)00034-5. [DOI] [PubMed] [Google Scholar]
- 6.Cyrus T, Pratico D, Zhao L, Witztum JL, Rader DJ, Rokach J, Fitzgerald GA, Funk CD. Absence of 12/15-lipoxygenase expression decreases lipid peroxidation and atherogenesis in apolipoprotein e-deficient mice. Circulation. 2001;103:2277–2282. doi: 10.1161/01.cir.103.18.2277. [DOI] [PubMed] [Google Scholar]
- 7.Bolick DT, Srinivasan S, Whetzel A, Fuller LC, Hedrick CC. 12/15 lipoxygenase mediates monocyte adhesion to aortic endothelium in apolipoprotein E-deficient mice through activation of RhoA and NF-kappa B. Arteriosclerosis Thrombosis and Vascular Biology. 2006;26:1260–1266. doi: 10.1161/01.ATV.0000217909.09198.d6. [DOI] [PubMed] [Google Scholar]
- 8.Reilly KB, Srinivasan S, Hatley ME, Patricia MK, Lannigan J, Bolick DT, Vandenhoff G, Pei H, Natarajan R, Nadler JL, Hedrick CC. 12/15-lipoxygenase activity mediates inflammatory monocyte/endothelial interactions and atherosclerosis in vivo. J Biol Chem. 2004;279:9440–9450. doi: 10.1074/jbc.M303857200. [DOI] [PubMed] [Google Scholar]
- 9.Wen Y, Gu J, Chakrabarti SK, Aylor K, Marshall J, Takahashi Y, Yoshimoto T, Nadler JL. The role of 12/15-lipoxygenase in the expression of interleukin-6 and tumor necrosis factor-alpha in macrophages. Endocrinology. 2007;148:1313–1322. doi: 10.1210/en.2006-0665. [DOI] [PubMed] [Google Scholar]
- 10.Yla-Herttuala S, Rosenfeld ME, Parthasarathy S, Sigal E, Sarkioja T, Witztum JL, Steinberg D. Gene expression in macrophage-rich human atherosclerotic lesions. 15-lipoxygenase and acetyl low density lipoprotein receptor messenger RNA colocalize with oxidation specific lipid-protein adducts. J Clin Invest. 1991;87:1146–1152. doi: 10.1172/JCI115111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simon TC, Makheja AN, Bailey JM. Relationship of vascular 15-lipoxygenase induction to atherosclerotic plaque formation in rabbits. Prostaglandins Leukot Essent Fatty Acids. 1990;41:273–278. doi: 10.1016/0952-3278(90)90142-8. [DOI] [PubMed] [Google Scholar]
- 12.Kuhn H, Belkner J, Suzuki H, Yamamoto S. Oxidative modification of human lipoproteins by lipoxygenases of different positional specificities. J Lipid Res. 1994;35:1749–1759. [PubMed] [Google Scholar]
- 13.Kunjathoor VV, Febbraio M, Podrez EA, Moore KJ, Andersson L, Koehn S, Rhee JS, Silverstein R, Hoff HF, Freeman MW. Scavenger receptors class A-I/II and CD36 are the principal receptors responsible for the uptake of modified low density lipoprotein leading to lipid loading in macrophages. J Biol Chem. 2002;277:49982–49988. doi: 10.1074/jbc.M209649200. [DOI] [PubMed] [Google Scholar]
- 14.Vaughan AM, Oram JF. ABCA1 and ABCG1 or ABCG4 act sequentially to remove cellular cholesterol and generate cholesterol-rich HDL. Journal of Lipid Research. 2006;47:2433–2443. doi: 10.1194/jlr.M600218-JLR200. [DOI] [PubMed] [Google Scholar]
- 15.Wang N, Silver DL, Costet P, Tall AR. Specific binding of ApoA-I, enhanced cholesterol efflux, and altered plasma membrane morphology in cells expressing ABC1. J Biol Chem. 2000;275:33053–33058. doi: 10.1074/jbc.M005438200. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y, Oram JF. Unsaturated fatty acids inhibit cholesterol efflux from macrophages by increasing degradation of ATP-binding cassette transporter A1. J Biol Chem. 2002;277:5692–5697. doi: 10.1074/jbc.M109977200. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, Oram JF. Unsaturated fatty acids phosphorylate and destabilize ABCA1 through a phospholipase D2 pathway. J Biol Chem. 2005;280:35896–35903. doi: 10.1074/jbc.M506210200. [DOI] [PubMed] [Google Scholar]
- 18.Arakawa R, Yokoyama S. Helical apolipoproteins stabilize ATP-binding cassette transporter A1 by protecting it from thiol protease-mediated degradation. Journal of Biological Chemistry. 2002;277:22426–22429. doi: 10.1074/jbc.M202996200. [DOI] [PubMed] [Google Scholar]
- 19.Curtiss LK. Is two out of three enough for ABCG1? Arterioscler Thromb Vasc Biol. 2006;26:2175–2177. doi: 10.1161/01.ATV.0000243741.89303.27. [DOI] [PubMed] [Google Scholar]
- 20.Schmitz G, Langmann T, Heimerl S. Role of ABCG1 and other ABCG family members in lipid metabolism. J Lipid Res. 2001;42:1513–1520. [PubMed] [Google Scholar]
- 21.Uehara Y, Miura SI, von Eckardstein A, Abe S, Fujii A, Matsuo Y, Rust S, Lorkowski S, Assmann G, Yamada T, Saku K. Unsaturated fatty acids suppress the expression of the ATP-binding cassette transporter G1 (ABCG1) and ABCA1 genes via an LXR/RXR responsive element. Atherosclerosis. 2006 doi: 10.1016/j.atherosclerosis.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 22.Sakashita T, Takahashi Y, Kinoshita T, Yoshimoto T. Essential involvement of 12-lipoxygenase in regiospecific and stereospecific oxidation of low density lipoprotein by macrophages. Eur J Biochem. 1999;265:825–831. doi: 10.1046/j.1432-1327.1999.00803.x. [DOI] [PubMed] [Google Scholar]
- 23.Folcik VA, Nivar-Aristy RA, Krajewski LP, Cathcart MK. Lipoxygenase contributes to the oxidation of lipids in human atherosclerotic plaques. J Clin Invest. 1995;96:504–510. doi: 10.1172/JCI118062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang N, Lan D, Chen W, Matsuura F, Tall AR. ATP-binding cassette transporters G1 and G4 mediate cellular cholesterol efflux to high-density lipoproteins. Proc Natl Acad Sci U S A. 2004;101:9774–9779. doi: 10.1073/pnas.0403506101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hatley ME, Srinivasan S, Reilly KB, Bolick DT, Hedrick CC. Increased Production of 12/15 Lipoxygenase Eicosanoids Accelerates Monocyte/Endothelial Interactions in Diabetic db/db Mice. J Biol Chem. 2003;278:25369–25375. doi: 10.1074/jbc.M301175200. [DOI] [PubMed] [Google Scholar]
- 26.Patricia MK, Natarajan R, Dooley AN, Hernandez F, Gu JL, Berliner JA, Rossi JJ, Nadler JL, Meidell RS, Hedrick CC. Adenoviral delivery of a leukocyte-type 12 lipoxygenase ribozyme inhibits effects of glucose and platelet-derived growth factor in vascular endothelial and smooth muscle cells. Circ Res. 2001;88:659–665. doi: 10.1161/hh0701.088838. [DOI] [PubMed] [Google Scholar]
- 27.Nagy L, Tontonoz P, Alvarez JG, Chen H, Evans RM. Oxidized LDL regulates macrophage gene expression through ligand activation of PPARgamma. Cell. 1998;93:229–240. doi: 10.1016/s0092-8674(00)81574-3. [DOI] [PubMed] [Google Scholar]
- 28.Chawla A, Boisvert WA, Lee CH, Laffitte BA, Barak Y, Joseph SB, Liao D, Nagy L, Edwards PA, Curtiss LK, Evans RM, Tontonoz P. A PPAR gamma-LXR-ABCA1 pathway in macrophages is involved in cholesterol efflux and atherogenesis. Mol Cell. 2001;7:161–171. doi: 10.1016/s1097-2765(01)00164-2. [DOI] [PubMed] [Google Scholar]
- 29.Antonipillai I, Nadler J, Vu EJ, Bughi S, Natarajan R, Horton R. A 12-lipoxygenase product, 12-hydroxyeicosatetraenoic acid, is increased in diabetics with incipient and early renal disease. J Clin Endocrinol Metab. 1996;81:1940–1945. doi: 10.1210/jcem.81.5.8626861. [DOI] [PubMed] [Google Scholar]
- 30.Mauldin JP, Srinivasan S, Mulya A, Gebre A, Parks JS, Daugherty A, Hedrick CC. Reduction in ABCG1 in type 2 diabetic mice increases macrophage foam cell formation. J Biol Chem. 2006 doi: 10.1074/jbc.M510952200. [DOI] [PubMed] [Google Scholar]
- 31.Wang X, Collins HL, Ranalletta M, Fuki IV, Billheimer JT, Rothblat GH, Tall AR, Rader DJ. Macrophage ABCA1 and ABCG1, but not SR-BI, promote macrophage reverse cholesterol transport in vivo. J Clin Invest. 2007;117:2216–2224. doi: 10.1172/JCI32057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoshikawa T, Shimano H, Yahagi N, Ide T, Amemiya-Kudo M, Matsuzaka T, Nakakuki M, Tomita S, Okazaki H, Tamura Y, Iizuka Y, Ohashi K, Takahashi A, Sone H, Osuga J, Gotoda T, Ishibashi S, Yamada N. Polyunsaturated fatty acids suppress sterol regulatory element-binding protein 1c promoter activity by inhibition of liver X receptor (LXR) binding to LXR response elements. Journal of Biological Chemistry. 2002;277:1705–1711. doi: 10.1074/jbc.M105711200. [DOI] [PubMed] [Google Scholar]
- 33.Torra IP, Ismaili N, Feig JE, Xu CF, Cavasotto C, Pancratov R, Rogatsky I, Neubert TA, Fisher EA, Garabedian MJ. Phosphorylation of liver X receptor alpha selectively regulates target gene expression in macrophages. Mol Cell Biol. 2008;28:2626–2636. doi: 10.1128/MCB.01575-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li X, Zhang S, Blander G, Tse JG, Krieger M, Guarente L. SIRT1 deacetylates and positively regulates the nuclear receptor LXR. Mol Cell. 2007;28:91–106. doi: 10.1016/j.molcel.2007.07.032. [DOI] [PubMed] [Google Scholar]
- 35.Belkner J, Chaitidis P, Stender H, Gerth C, Kuban RJ, Yoshimoto T, Kuhn H. Expression of 12/15-lipoxygenase attenuates intracellular lipid deposition during in vitro foam cell formation. Arterioscler Thromb Vasc Biol. 2005;25:797–802. doi: 10.1161/01.ATV.0000157580.26858.2d. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.