Abstract
This review deals with the pharmacological properties of an alkylated monosaccharide mimetic, N-butyldeoxynojirimycin (NB-DNJ). This compound is of pharmacogenetic interest because one of its biological effects in mice – impairment of spermatogenesis, leading to male infertility – depends greatly on the genetic background of the animal. In susceptible mice, administration of NB-DNJ perturbs the formation of an organelle, the acrosome, in early post-meiotic male germ cells. In all recipient mice, irrespective of reproductive phenotype, NB-DNJ has a similar biochemical effect: inhibition of the glucosylceramidase β-glucosidase 2 and subsequent elevation of glucosylceramide, a glycosphingolipid. The questions that we now need to address are: how can glucosylceramide specifically affect early acrosome formation, and why is this contingent on genetic factors? Here we discuss relevant aspects of reproductive biology, the metabolism and cell biology of sphingolipids, and complex trait analysis; we also present a speculative model that takes our observations into account.
Keywords: acrosome, glucosylceramide, glycosphingolipid, imino sugar, semen parameters, sperm morphology, spermatid, spermatogenesis
Drug pharmacokinetics, distribution & systemic effects
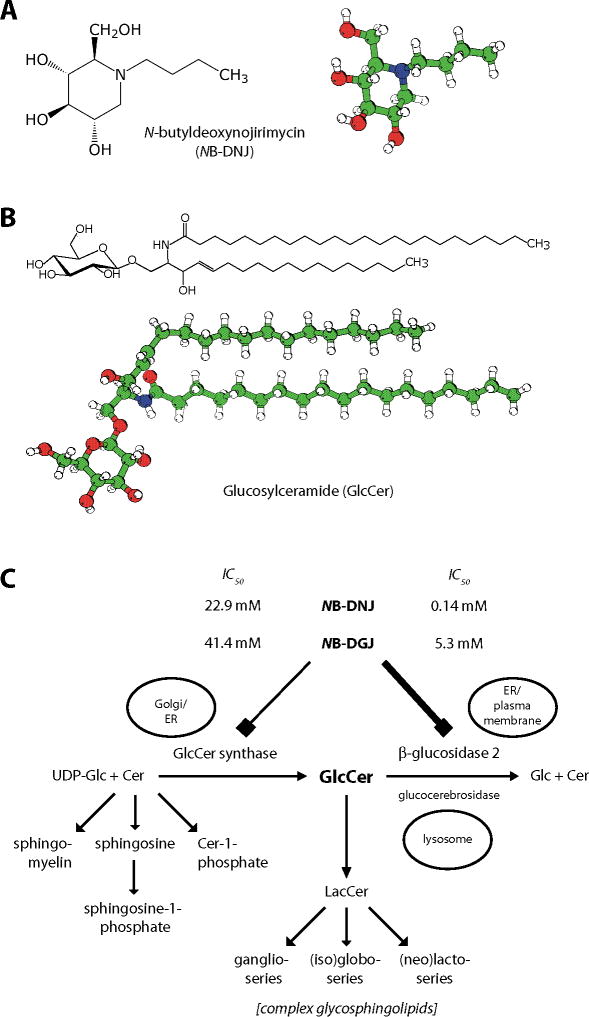
Chemically, N-butyldeoxynojirimycin (NB-DNJ) is a monosaccharide mimetic that is an N-alkylated imino sugar ([2R,3R,4R,5S]-1-butyl-2-[hydroxymethyl]piperidine-3,4,5-triol), resembling glucose (Figure 1A). The parental nonalkylated compound (deoxy)nojirimycin can be found in several plant and microbial systems [1–3]. In the literature, NB-DNJ is also referred to as OGT918, miglustat and Zavesca®; its perbutyrated prodrug derivative is named SC-48334.
Figure 1. Structural formulas and metabolic pathways.
(A) Structural formula and model of NB-DNJ (miglustat, Zavesca®). The molecule differs from glucose in the presence of a nitrogen in the ring (where glucose has an oxygen), the butyl moiety linked to the ring nitrogen, and in the absence of a hydroxyl group from position 1. (B) Structural formula and model of glucosylceramide with an acyl chain of 24 carbons, without unsaturated bonds. (C) Metabolism of sphingolipids, focusing on glucosylceramide. Both the biosynthetic enzyme GCS as well as the degradative enzyme GBA2 can be inhibited by NB-DNJ and NB-DGJ, but GBA2 is much more sensitive to these compounds. This is evident from the differences between the IC50 values of the two drugs towards GCS and GBA2.
Colour code for molecular models: White: Hydrogen; Green: Carbon; Red: Oxygen; Blue: Nitrogen.
Cer: Ceramide; GBA2: β-glucosidase 2; GCS: GlcCer synthase; Glc: Glucose; GlcCer: Glucosylceramide; LacCer: Lactosylceramide; NB-DGJ: N-butyldeoxygalactonojirimycin; NB-DNJ: N-butyldeoxynojirimycin.
Panel (C) adapted from [6].
NB-DNJ can be efficiently orally administered to mice by mixing the compound as a dry crystalline powder with powdered mouse chow. After oral administration or injection in mammals, NB-DNJ does not bind to proteins, is not metabolically converted and is efficiently excreted in the urine [4,5]. In C57BL/6 mice, the serum level of NB-DNJ varies from 0.5 ± 0.3 μM to 21.5 ± 7.8 μM (measured at 15 and 2400 mg/kg/day, respectively) [6]. It is unlikely that genetic background affects the serum level of NB-DNJ, as FVB/N mice have a similar serum concentration to C57BL/6 mice [7]. The compound is not preferentially distributed to the testes or any other tissue [5].
NB-DNJ is nontoxic to cultured cells [5,8]. The systemic effects of long-term low-dose NB-DNJ administration have been evaluated in mice and other mammals [9,201]. Body weight, testicular and epididymal weights remain unchanged over 12 months of drug administration; the same applies to reproductive endocrinology, germ cell apoptosis, and general indicators of liver and kidney functions and animal behavior (co-ordination, muscle strength and spontaneous exploratory behavior [9]).
Drug-induced phenotype – reproductive biology
Compared with nucleated somatic cells, normal mammalian spermatozoa are morphologically exceptional, as there is very little shape variation between individual cells. The murine sperm head has a characteristic curved (falciform) shape, and it is laterally flattened; this shape is closely followed by the sperm nucleus (Figure 2A & G). The sperm head contains a large, specialized secretory vesicle, the acrosome, which overlies the sperm nucleus on the dorsal and adjacent lateral sides. Upon contact of a spermatozoon with the outer shell (zona pellucida) of an oocyte, the acrosomal contents are released via a massive exocytotic event, whereby the outer acrosomal membrane fuses at many points with the closely overlying sperm plasma membrane [10]. This is an essential process, without which the spermatozoon cannot penetrate the zona pellucida, and thus will not fertilize the oocyte [11]. A very small fraction of spermatozoa from C57BL/6 mice develops in an aberrant fashion [12]. This is more pronounced in a few other mouse strains, including BALB/c mice [13,14].
Figure 2. Effect of NB-DNJ on morphology of spermatozoa from C57BL/6 mice.
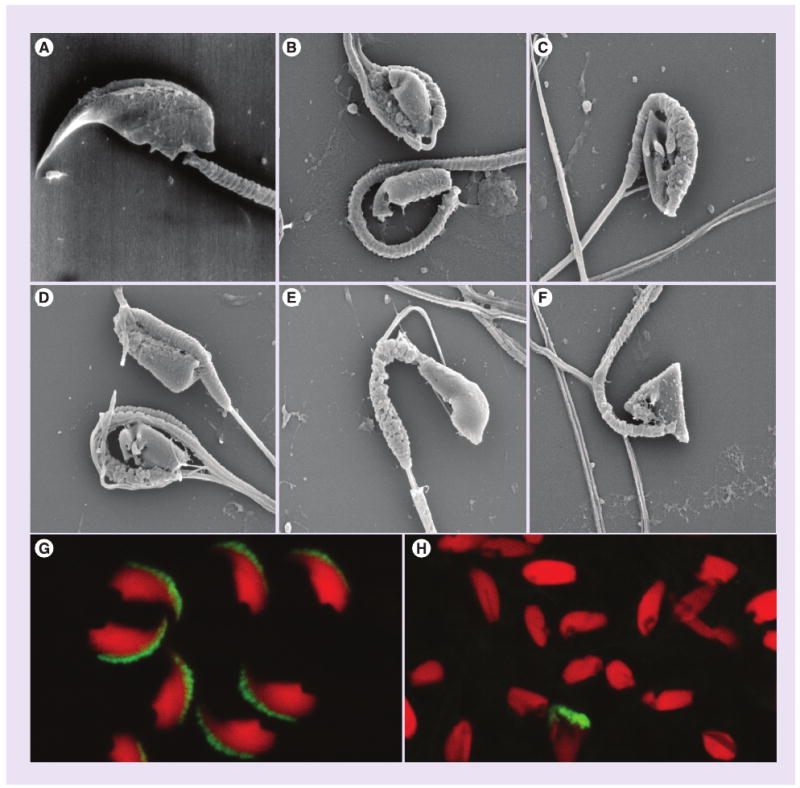
Cells were from control mice (A, G) and from NB-DNJ-treated animals (15–150 mg/kg/day) (B–F, H). Images were obtained by scanning electron microscopy (A–F) and fluorescence microscopy (G, H). In the latter panels, acrosomal structures were detected with an antiacrosomal monoclonal antibody (green) and nuclei were stained with propidium iodide (red). Evident is the lack of acrosomes from the majority of spermatozoa from drug-treated mice, as well as the morphological heterogeneity of sperm heads and nuclei, which is in great contrast with the uniformity of spermatozoa from control mice.
NB-DNJ: N-butyldeoxynojirimycin
After administration of low-dose NB-DNJ or N-butylgalacto(deoxy)nojirimycin (NB-DGJ), male C57BL/6 mice become infertile [15,16]. The drug-treated animals are infertile because they produce spermatozoa that have a grossly abnormal morphology, especially in the sperm head and nucleus [15,16]. The sperm heads and nuclei of imino sugar-treated C57BL/6 mice are not curved or flattened, but exhibit a variety of aberrant shapes, being either triangular, oblate or elongate, the majority being spatulate (Figure 2B–F & H), in morphological terms defined by Hollander et al. [17]. At 15 mg/kg/day of NB-DNJ, which is the minimal dose that causes C57BL/6 mice to become infertile, around 95 ± 2% of epididymal spermatozoa have a grossly aberrant nuclear shape (compared with 1 ± 1% in untreated mice). In addition, 97 ± 2% of sperm cells from the drug-treated mice are without acrosomal features, whereas 5 ± 3% of sperm cells from normal mice are negative for acrosomal antigens (Figure 2G & H) [7,15,16]. Also, the percentage of spermatozoa (from drug-treated C57BL/6 mice) that displays forward motility is much lower than that of control mice, and the swimming speeds of the few sperm cells that do move are considerably reduced [15]. These structural and functional sperm deficiencies explain the infertility seen in imino sugar-administered C57BL/6 mice. After withdrawal of NB-DNJ from C57BL/6 mice, the mice resume the production of morphologically normal spermatozoa, and concomitantly regain their fertility. This is seen after both short- and long-term drug administration (5 vs 52 weeks) [9,15]. The capacity of imino sugars to induce infertility in male mice is not limited to butylated derivative imino sugars, as N-(5-adamantane-1-yl-methoxy)pentyl-deoxynojirimycin also has this effect [18]; nonalkylated deoxynojirimycin, however, does not [15,16].
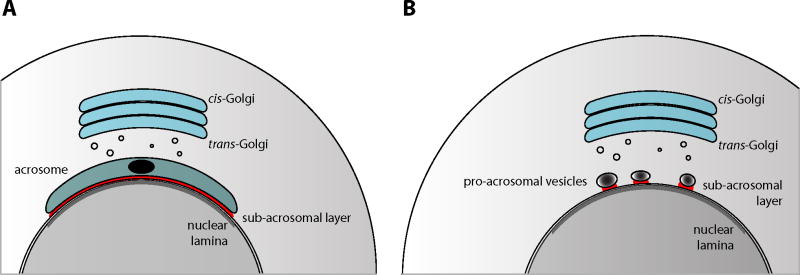
The lack of acrosomes from spermatozoa from NB-DNJ- and NB-DGJ-treated C57BL/6 mice stems from a perturbation at the start of acrosome formation. This process normally commences not long after completion of the second meiotic division, when the germ cells are in the round spermatid phase, and do not yet possess any of the characteristic features of spermatozoa [19]. However, in contrast to many somatic cells, in round spermatids the Golgi is consistently oriented with its trans-cisternae facing the nucleus. What marks the departure from the somatic cell organization is the appearance, at first only observable at the ultrastructural level, of a small number of dense-core vesicles in the space between the trans-Golgi and the spermatid nucleus. These vesicles coalesce to form one single pro-acrosomal vesicle (having a dense core and an electron-lucent halo), which becomes much larger than surrounding Golgiderived vesicles. At this step, several proteins are allied with the pro-acrosomal vesicle, at its cytoplasmic side. These proteins become components of the subacrosomal cytoskeleton (perinuclear theca) in the mature spermatozoon [20–23]. During subsequent steps in round spermatid development, the pro-acrosomal vesicle docks on the nuclear envelope, and grows in size while expanding laterally to cover a large proportion of the nuclear surface, forming a round cap that subtends an angle of approximately 150° (Figure 3A) [19]. Here, the part of the nuclear envelope that is covered by the acrosome has also a distinguishing feature at the opposite (internal) face of the nuclear envelope, an electron-dense lamina (Figure 3A). Next, the murine spermatid begins its process of morphological transformation, with nuclear elongation, loss of symmetry, lateral flattening and extensive chromatin condensation, while forming a flagellum, and seceding of most of its cytoplasm and cytoplasmic organelles [19].
Figure 3. Acrosome development.
(A) Depicts a murine step 5 spermatid, with its acrosome covering a significant portion of the nuclear envelope. Indicated are the subacrosomal layer (red) in between the acrosome and the nuclear envelope, and the nuclear lamina (gray), on the inner aspect of the nuclear envelope. Also important to note that in these cells the Golgi is oriented with its trans-cisternae facing the nucleus.
(B) Depicts a round spermatid from a C57BL/6 mouse that has been treated with a low-dose of NB-DNJ or NB-DGJ. Associated with the nuclear envelope are multiple proacrosomal vesicles, each accompanied by a small amount of subacrosomal material. The nuclear lamina is present as in control spermatids.
NB-DGJ: N-butyldeoxygalactonojirimycin; NB-DNJ: N-butyldeoxynojirimycin.
In spermatids of NB-DNJ-treated C57BL/6 mice, the nuclear lamina is present, underlying the area of the nuclear envelope that normally is covered by the developing acrosome, encompassing the anterior nucleus up to the anterior boundary of the manchette (nuclear ring) in elongating spermatids (Figure 3B) [24]. However, in the ‘drug-treated’ spermatids, only a fraction of this part of the nuclear envelope is occupied by acrosomal structures, if at all, even when the spermatids start elongating. Not one, but multiple dense-core pro-acrosomal vesicles can be observed in association with the nuclear envelope (Figure 3B) [24]. Alternatively, a larger developing acrosome can be found harboring two dense cores [Walden CM and van der Spoel AC, Unpublished Data]. By light microscopy, most of the ‘drug-treated’ round spermatids are lacking their distinctive acrosomal structures [9]. The further development of these spermatids is highly aberrant, but also irregular, so that the spermatozoa from the imino sugar-treated mice are heterogeneous in shape (Figure 2B–F & H) [7,15,16].
The imino sugar-induced phenotype of C57BL/6 mice resembles, at least morphologically, the phenotypes of a number of spontaneous mouse mutants and knock-out mouse models. Early acrosome formation and production of acrosome-less spermatozoa is also seen in male mice that are deficient in Hrb or GOPC [25–27], and those with mutations in HERC2 or Vps54, which are found in the rjs and Wobbler (wr) mutants, respectively [28–30]. Interesting from a genetic perspective is the Wobbler mutation, as the accompanying sperm phenotype differs between C57BL/6 mice and C57BL/6 × CAST/Ei animals [31]. In addition, multiple dense-core proacrosomal vesicles are seen on the nuclear envelope in the early spermatids from blind-sterile (bs) mice [32]. Also, similar to imino sugar-treated C57BL/6 mice is the normal development of the manchette, and the presence of the electron-dense lamina on the inner aspect of the nuclear envelope of round spermatids from blind-sterile mice, in the absence of an acrosome on the opposite side of the envelope [32]. Finally, in humans, a mutation in the SPATA16 gene has been found to be responsible for the round-headed sperm phenotype (globozoospermia) [33]. Future developments may reveal any involvement of the products of these genes in the imino sugar/glucosylceramide-sensitive pathway.
Pharmacogenetics & pharmacogenomics
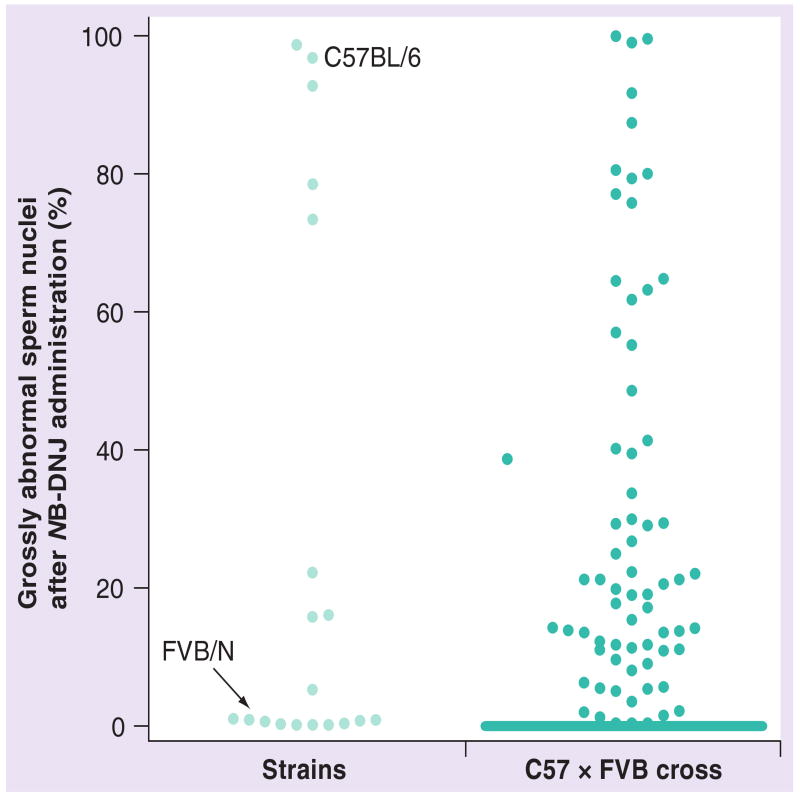
In inbred mice, the consequences of the administration of low doses of NB-DNJ or NB-DGJ on spermatogenesis are highly dependent on the genetic background of the animals. Most strains from the C57 lineage are sensitive to NB-DNJ and produce high percentages (± 92%) of grossly abnormal spermatozoa lacking an acrosome following administration of the drug [7]. By contrast, a few mouse strains (AKR, BALB/c and MA/My) display an intermediate level (± 18% grossly abnormal spermatozoa) of susceptibility, whereas most strains from the Swiss and Castle lineages (e.g., FVB and 129S1/SvImJ) were not affected in this way by the imino sugar (± 0.5% grossly abnormal spermatozoa) (Figure 4) [7]. Males with intermediate proportions of acrosome-deficient spermatozoa with grossly abnormal nuclei (e.g., from the Ma/My and AKR strains) were able to produce offspring when mated with C57BL/6 females [7]. The reproductive effect of a low-dose NB-DNJ regimen thus differs widely between different mouse strains. Furthermore, NB-DNJ does not cause infertility in rabbits or in men [7,34].
Figure 4. Imino sugar pharmacogenetics.
(Strains) Graphical representation of the differences in the reproductive effect of NB-DNJ between 19 inbred mouse strains. The mice were from the Swiss (FVB/N) and Castle (129S1/SvImJ, A/J, AKR/J, BALB/c, C3H/HeN, CBA/Ca, DBA/2, NZB, NZW and SM/J) families of strains, from the C57 lineage (C57BL/10, C57BR, C57L, C58, MA/My and YBR), and from a mixed background (MRL/Mp). Average standard deviation in percentage abnormal sperm nuclei was 2% (range 0.2–9%, n = 2–3 mice per strain). Indicated are the positions of C57BL/6 and FVB/N mice, which differ widely in their responses to NB-DNJ. (C57 × FVB cross) Indicated are the results of drug administration in individual C57BL/6 × FVB/N intercross mice. As most – but not all – hybrid mice had a similar response as FVB/N mice, the dots around the baseline are overlapping. The effect of NB-DNJ in the remainder of the intercross mice varied widely.
NB-DNJ: N-butyldeoxynojirimycin.
In mouse strains of which the males do not become infertile after NB-DNJ treatment, the drug has a milder effect. A high percentage of their spermatozoa have an abnormal acrosomal morphology, and mildly dysmorphic nuclei [7]. These spermatozoa carry acrosomes that are aberrantly structured, while their nuclei can be classified as falciform, being laterally flattened, and to some extent curved at the anterior end. Clearly, what is critical for the fertility of the drug-treated mice is the percentage of nonfalciform acrosome-less spermatozoa (inversely correlated) [7]. Thus from a reproductive perspective, it is relevant and sufficient to discriminate between two categories of spermatozoa from imino sugar-treated mice, either falciform-with-acrosome or nonfalciform/lacking an acrosome.
To understand how the sensitivity to NB-DNJ is inherited, intercross hybrid mice have been generated from two inbred strains that differ widely in their biological response to imino sugar administration, C57BL/6 and FVB (having 95 ± 2% and 0.2 ± 0.3% nonfalciform spermatozoa, respectively). Over 200 fourth-generation hybrid males have been evaluated for their response to the drug, and scored for their percentage of grossly abnormal spermatozoa. The majority of these hybrid mice responded to imino sugar administration in a similar fashion to FVB mice, while a minority showed a wide range of effects, with very few animals having a similar proportion of grossly aberrant sperm nuclei as C57BL/6 mice (Figure 4) [7]. The drug responses of approximately a third of the interstrain hybrid mice were exceptional. Each of the animals from this group produced spermatozoa from both of the categories described above, for example, spermatozoa with an acrosome and a falciform nuclear shape, as well as acrosome-deficient spermatozoa with grossly aberrant nuclei. What was remarkable about this group of C57BL/6 × FVB/N hybrid mice was that they produced the two types of spermatozoa in proportions that were not seen in either of their progenitor strains. Their percentages of nonfalciform spermatozoa ranged from 2 to 90%, spanning the extremes of the scale set by the progenitor strains.
Clearly, the sensitivity of murine spermatogenesis for NB-DNJ is not inherited in a Mendelian fashion. As a genetic trait, the reproductive outcome of imino sugar administration may be regarded in different ways. At the cellular level, distinguishing only two categories of spermatozoa (falciform-with-acrosome versus nonfalciform/acrosome-less), the reproductive trait of imino sugar-treated mice is binary. In turn, as very large numbers of spermatozoa are produced, the trait can be expressed as the percentage of total spermatozoa that are nonfalciform. In this sense, the trait of imino sugar-treated mice can be presented as quantitative. Even so, in this way it is not comparable with other quantitative traits, like blood pressure, tumor size or tibia length. As one arrives at the percentage of abnormal spermatozoa by counting cells, instead of taking one measurement, the trait may be viewed as meristic. The number of grossly aberrant spermatozoa only differs from, for example, the number of abdominal bristles on Drosophila [35], by being very large – too large to count. It remains to be seen what the most fitting model is to describe the reproductive phenotype of imino sugar-treated mice.
Biochemistry
As a glucose- and short-chain ceramide-mimetic, NB-DNJ can inhibit a number of carbohydrate or glycoconjugate-modifying enzymes [36–38]. The biochemical property of NB-DNJ that is responsible for its reproductive effect in male mice can be delineated by comparing its characteristics with those of its galactose analogue, NB-DGJ. Administration of NB-DGJ results in a similar reproductive phenotype to NB-DNJ [16], but the galactose analogue affects a different set of enzymes [36]. The two alkylated imino sugars share the capacity to inhibit two enzymes that are involved in the metabolism of glucosylceramide, a ubiquitous eukaryotic cellular glycosphingolipid (Figure 1B, molecular model after [39])
Glycosphingolipids
Glycosphingolipids are produced by the transfer of one or more monosaccharide residue to the sphingolipid ceramide, and are thus amphipathic constituents of cellular membranes [40,41]. A large number (>400) of structurally distinct glycosphingolipids has been described, with variations in the carbohydrate domain, as well as in the ceramide moiety [202]. Glycosphingolipid levels are regulated in space and time, as animal tissues differ considerably in the occurrence and quantity of individual sphingolipid species, and tissue glycosphingolipid profiles (including those of murine testes) change during postnatal development (e.g., [42–44]). At present, the regulatory processes that underlie these differences are being investigated [45].
Glycosphingolipids are involved in many essential biological processes, including cell motility and adhesion [46], embryonic development [47], establishment of skin impermeability [48,49], postnatal development of the CNS [50,51], insulin signaling [52,53], invariant natural killer T cell maturation [54,55] and spermatogenesis [56,57]. In addition, in a number of inherited diseases, lysosomal accumulation of glycosphingolipids causes severe pathology in several organ systems (reviewed in [58–61]). Examples of these conditions include Fabry, Gaucher, Niemann–Pick type C, Sandhoff and Tay–Sachs diseases. These disorders are caused by defects in the transport of glycosphingolipids to lysosomes, or by deficiencies in the lysosomal catabolism of one or more glycosphingolipids [58–61]. High doses of NB-DNJ are effective in reducing or delaying the accumulation of glycosphingolipids in mouse models of Sandhoff and Tay–Sachs disease, with improvement in survival and well-being of the Sandhoff mice [62,63]. Also, in type 1 Gaucher disease patients, NB-DNJ has shown efficacy [64,65] (reviewed in [66–70]). NB-DNJ has been licensed for clinical use in Type 1 Gaucher patients (tradename Zavesca®), and is currently in clinical trials for other glycosphingolipid storage disorders [71,72].
Glucosylceramide
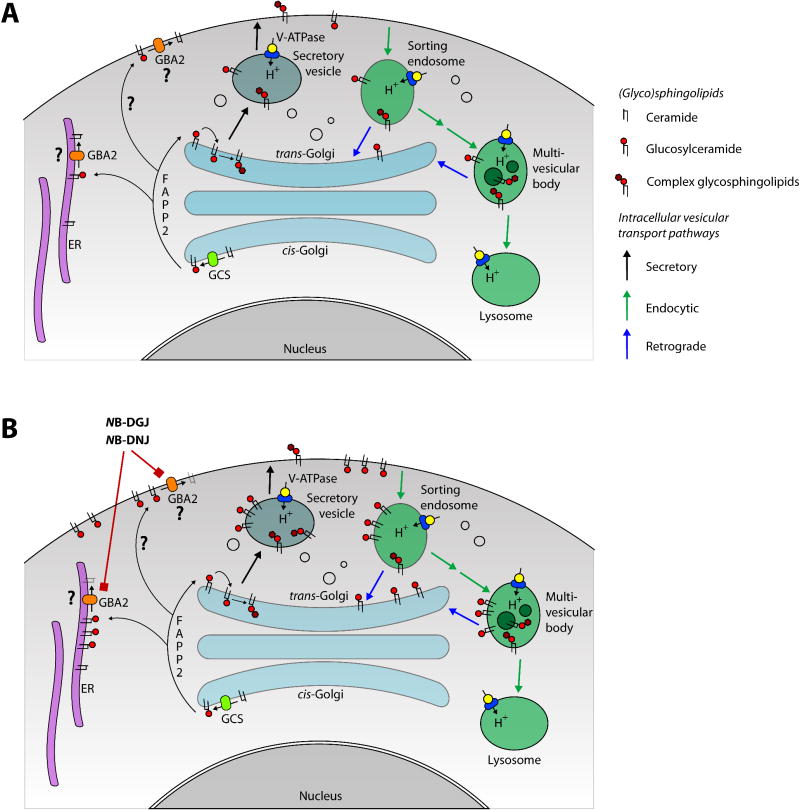
The first step in the biosynthetic pathway of most complex glycosphingolipids is the production of glucosylceramide. This step is catalyzed by glucosylceramide synthase (GCS, UDP-glucose: N-acylsphingosine D-glucosyltransferase), which transfers the monosaccharide from UDP-glucose to ceramide (Figure 1C). Among sphingolipids, glucosylceramide occupies a unique position, both from a biochemical and a cell biological perspective. Firstly, in biosynthetic pathways it is situated between ‘simple’ sphingolipids (including the signaling molecules sphingosine-1-phosphate and ceramide-1-phosphate [73,74]) and sphingolipids with complex glycosylation (see previous paragraph). Glucosylceramide is the single entity that connects these two families of sphingolipids, which each have branching metabolic pathways (Figure 1C). Secondly, cell biologically, glucosylceramide is the only glycosylated sphingolipid that is located in the cytoplasmic leaflet of cellular membranes, so that it is oriented with its hydrophilic headgroup extending in the cytoplasmic compartment [75,76]. The cytoplasmic orientation of glucosylceramide is the consequence of the topology of glucosylceramide synthase, which has its active site facing the cytoplasmic side of the Golgi (Figure 5A) [77–79]. In stably transfected HeLa cells, glucosylceramide synthase is distributed over all Golgi stacks, but is more concentrated in the medial/trans-cisternae [80]. All other glycosyltransferases involved in glycosphingolipid biosynthesis, including ceramide galactosyltransferase are active on the lumenal side of the Golgi. After translocating to the lumenal side of the Golgi, glucosylceramide is further metabolized by these enzymes, to produce lactosylceramide and more complex glycosphingolipids (Figure 1C & 5A). Glucosylceramide can be degraded to glucose and ceramide in lysosomes, by glucocerebrosidase (GBA, β-glucosidase 1), and in extralysosomal locations by β-glucosidase 2 (GBA2, nonlysosomal glucosylceramidase/bile acid β-glucosidase) (Figure 1C) [18,81].
Figure 5. Glycosphingolipid cell biology.
Depicts part of the metabolism and intracellular movements of glucosylceramide and complex glycosphingolipids in somatic cells, based on the available experimental data (see text for references). (A) Control cell. Glucosylceramide is produced by GCS, situated at the cytoplasmic face of the cis-Golgi. Glucosylceramide is transported through the Golgi stack by vesicular transport, but it can also be transferred to the trans-Golgi and other locations in the cell, by the protein FAPP2. At the trans-Golgi, glucosylceramide is translocated from the cytoplasmic to the lumenal side, and there it is converted to complex glycosphingolipids. Alternatively, glucosylceramide is transported to the cytoplasmic leaflet of plasma membrane via vesicular or nonvesicular means. It is here (or at the ER) that this glycolipid is cleaved by GBA2, to yield ceramide and glucose. (B) Cell cultured in the presence of a low concentration of NB-DNJ or NB-DGJ. These drugs reduce the activity of GBA2. Consequently, glucosylceramide accumulates at sites where GBA2 is present (either the ER or the plasma membrane – see text). From the plasma membrane, supraphysiological levels of glucosylceramide may enter the endocytic pathway. Most of the internalized glucosylceramide is transported to lysosomes, where it is degraded, while a small fraction of this pool is recycled to the trans-Golgi.
ER: Endoplasmic reticulum; GBA2: β-glucosidase 2; GCS: Glucosylceramide synthase; NB-DGJ: N-butyldeoxygalactonojirimycin; NB-DNJ: N-butyldeoxynojirimycin.
Recent studies have suggested that glucosylceramide can be transferred from its site of synthesis at the Golgi to other organelles through the cytoplasmic compartment, via a specific transfer protein, FAPP2 (Figure 5A) [80,82]. In vitro, FAPP2 can mediate the transfer of glucosylceramide between phospholipid vesicles, and, in FAPP2-deficient HeLa cells, glucosylceramide remains mostly at the cis-side of the Golgi stack, instead of being in the trans-cisternae and the trans-Golgi network [82]. Other data indicate that, in MEB4 cells, FAPP2 is instrumental in the transport of glucosylceramide from its site of synthesis to the endoplasmic reticulum (ER), where it translocates to the lumenal leaflet of the limiting membrane (Figure 5A) [80]. It can be envisaged that the internalized glucosylceramide then becomes available for the biosynthesis of complex glycosphingolipids via vesicular transport to the Golgi. This explains the observation that in various cultured cells that have been depleted for FAPP2, newly synthesized glucosylceramide accumulates, while the rate of biosynthesis of complex glycosphingolipids diminishes [80,82].
Alkylated imino sugars & glucosylceramide
As mentioned above, NB-DNJ and NB-DGJ are inhibitors of enzymes involved in carbohydrate, glycoprotein and glucosylceramide metabolism. Each of these imino sugars affects a distinct set of enzymes, but it has become apparent that these two compounds share two common enzyme targets. Thus, two enzymes have been identified that are sensitive to both NB-DNJ and NB-DGJ. These enzymes are both involved in the metabolism of glucosylceramide. They are GCS [83,84] and GBA2 (Figure 1C) [85,86]. It is important to note that these enzymes differ in their sensitivities to inhibition by NB-DNJ and NB-DGJ – GBA2 being the more susceptible enzyme (Figure 1C) [6]. Therefore, in vivo, drug dosage determines whether one or both of these enzymes is/are inhibited. Low doses of NB-DNJ and NB-DGJ (15–150 mg/kg/day, respectively) will only affect GBA2, whereas at higher doses (600–2400 mg/kg/day) the activity of both enzymes will be reduced. At low imino sugar doses the substrate of this enzyme, glucosylceramide, accumulates in tissues of drug-treated mice, reaching (in testes) 6–8-times the levels found in control animals (Figure 5B) [6]. In turn, at higher drug doses the biosynthesis of glucosylceramide is increasingly attenuated, so that whole-testis glucosylceramide levels are not as high as at 15–150 mg/kg/day; at the highest dose used (2400 mg/kg/day NB-DNJ) the biosynthetic enzyme is inhibited to such an extent that whole-testis glucosylceramide levels are similar to those of untreated mice [6]. When administration of low-dose NB-DNJ is extended from a few weeks to several months, testicular glucosylceramide levels do not rise any further, but remain at 6–8 times control levels [6]. The drug-induced elevation of glucosylceramide is reversible: its level returns to normal within a few weeks of drug withdrawal, even after 12 months of administration [6].
The data on the relationships between doses of NB-DNJ and NB-DGJ and testicular glucosylceramide levels all come from studies in C57BL/6 mice. However, more recently glucosylceramide levels have also been measured in ten other inbred mouse strains (129S1/SvImJ, AKR, BALB/c, C3H, C57BL/10, C57BR, DBA2, FVB, MA/My and NZW). After treating males of these strains with low-dose NB-DNJ, testicular glucosylceramide levels were found to be raised in all strains, comparable to drug-treated C57BL/6 mice [6]. Thus the genetic background of the mice does not determine the primary biochemical effect of the imino sugar when it is administered at 15–150 mg/kg/day.
In this context it is important to consider the cell biology of glucosylceramide. A major uncertaintly here is the subcellular location of GBA2, as current reports on the subcellular localization of this glucosylceramidase are not in agreement. By density gradient electrophoresis of organelles from Mel JuSo human melanoma cells, the β-glucosidase activity was found in fractions containing light endosomes [86]. Similarly, in transfected COS-7 cells, human GBA2-GFP fusion proteins were preferentially localized at the plasma membrane (Figure 5) [18]. However, in COS cells overexpressing GBA2 by itself, the protein displayed a distribution indicative of an association with the ER (as detected with an anti-GBA2 antiserum) (Figure 5) [81].
Assuming that GBA2 is present at the plasma membrane, glucosylceramide will initially accumulate at this location upon low-dose NB-DNJ administration. Subsequently, this sphingolipid will be subject to the constitutive endocytosis of cell-surface glycosphingolipids (reviewed in [87,88]). Accordingly, most of the internalized glucosylceramide will be transported to lysosomes, where it is degraded, while a small proportion may traffic to the trans-Golgi (Figure 5B). The latter pool of glucosylceramide may translocate to the Golgi lumen and be converted to complex glycosphingolipids. Two experimental findings support this scenario. Firstly, in tissues from drug-treated animals, the level of glucosylceramide is several-fold higher than in controls, but it is stationary – there is no continuous increase in glucosylceramide in these tissues [6], in spite of the fact that the biosynthesis of glucosylceramide is not held back. Secondly, in the testes from low-dose NB-DNJ-treated animals, there is a modest increase in the levels of complex glycosphingolipids [6]. This may be the result of a fraction of the internalized glucosylceramide being recycled to the trans-Golgi. Therefore, current evidence suggests that, when GBA2 is inhibited, a localized pool of supraphysiological glucosylceramide is generated, the size of which is primarily limited by lysosomal degradation, and, to a much smaller extent, by recycling via the Golgi. Nevertheless, it is important to note that most studies on (glyco)sphingolipid biochemistry and cell biology have been carried out with somatic cells, and that at present our knowledge on these processes in haploid male germ cells is limited.
Glucosylceramide metabolism & acrosome formation
Considering that, in C57BL/6 mice, NB-DNJ and NB-DGJ give rise to a reproductive phenotype at low doses (≥15–150 mg/kg/day, respectively), and that, at such drug doses, the serum level of NB-DNJ exceeds its in vitro IC50 towards GBA2 (but remains well below the corresponding value for ceramide glucosyltransferase), it is most likely that the reproductive phenotype is owing to inhibition of GBA2 [6]. This is in agreement with the finding that GBA2-deficient mice, which have a reproductive phenotype that is comparable to that of imino sugar-treated mice, also have increased levels of glucosylceramide in their testes [81]. The available data thus support the idea that, in C57BL/6 mice, the imino sugar-induced disturbance of acrosome formation is mediated by inhibition of GBA2 and subsequent increase in glucosylceramide [6].
The causative cascade, through which the higher level of glucosylceramide has an impact on acrosome development, remains to be delineated. To this end it is important to identify those lipids and proteins of which the functioning is altered by an abundance of glucosylceramide. One such protein has been put forward by van Meer et al. They suggest that glucosylceramide positively regulates the activity of the vacuolar proton-ATPase (V-ATPase) [80], which controls the luminal pH of intracellular compartments, including endosomes, lysosomes and the Golgi (Figure 5) (for reviews, see [89,90]). However, the impact of glucosylceramide on the vacuolar ATPase in germ cells is not clear at present.
Genetics, beyond genetics (& mice)
Ultimately, it is our aim to build a model that takes into account that after NB-DNJ treatment the highly aberrant sperm phenotype is only seen in a subset of spermatozoa from each mouse (regardless of its genotype), and that the size of this subset depends on the genetic constitution of the mouse.
It is intriguing that after NB-DNJ treatment only a proportion (small, large or anywhere inbetween) of the spermatozoa from a mouse develops abnormally. Similarly, it is fascinating that in man different spermatids develop in different ways, as within one man only a proportion of spermatozoa has a ‘normal’ morphology and functionality, while the remainder develop in various abnormal ways, resulting in a heterogeneous collection of spermatozoa (for example [91–93]). The fact that spermatids of one individual mouse/man, experiencing the same environmental influences (e.g., small molecules), can develop in different ways, points to a heterogeneity among the germ cells that determines the course of their development. The relevant heterogeneity between individual spermatids may be present at the genetic level, a consequence of recombinations in the progenitor spermatocyte. Alternatively, the heterogeneity between spermatids may be present at the transcriptional, translational or metabolic level. There is indeed increasing appreciation for the significance of differences between morphologically similar cells of one individual, in levels of gene expression, translational efficiency or metabolic processes. These differences may be stochastic in nature [94–97]. Thus, cellular individuality may underlie the variation in the course of spermatid maturation observed in individual NB-DNJ-treated mice; this may also apply to germ cell development in man. It is important to determine at what level the crucial differences between spermatids lie, and characterize the relevant parameters, especially considering the current concern regarding spermatogenesis in man (for example [98–100]). This will require analysis of mRNA, protein and metabolite levels in individual germ cells, and may benefit from new analytical methodologies for single cells [101,102].
We have established that one of the factors that can affect spermatid development in mice is the level of glucosylceramide. It can be envisaged that the outcome of NB-DNJ administration for an individual spermatid depends on the following: the level of glucosylceramide in that particular cell (dependent on the local imino sugar concentration and the flux through GBA2 in this cell); the genetic constitution of the spermatid, specifying which allelic variants of proteins forming the glucosylceramide-sensitive pathway are expressed, and at what level; and stochastic variation between cells, in glucosylceramide level, and in levels of proteins that make up the glucosylceramide-sensitive pathway. In this model, C57BL/6 mice can be assumed to carry exclusively those alleles of proteins that are highly sensitive to glucosylceramide, so that the level of glucosylceramide in almost all spermatids is sufficient to impair acrosome formation. Thus, in this case the stochastic variation between cells in glucosylceramide level is of very little consequence. By contrast, FVB/N mice will possess those allelic variants that are insensitive to glucosylceramide. Thus in the large majority of FVB/N spermatids, the glucosylceramide concentration is not sufficient to have an effect. Finally, in mice with a mixed genetic constitution, the glucosylceramide-sensitive pathway may have an intermediate affinity for glucosylceramide. In these mice, the glucosylceramide level in individual spermatids (having a stochastic distribution over all spermatids) is the crucial parameter determining the course of germ cell development.
Within the framework of this model, one can speculate on the reasons for the lack of a reproductive effect of NB-DNJ in male rabbits and in man. Possibly, the flux of glucosylceramide through GBA2 may be much lower than in mice, so that, after drug administration, the level of glucosylceramide is increased, but remains well below the level reached in murine testes, so that the glucosylceramide-sensitive proteins are not affected and acrosome formation proceeds unperturbed. Alternatively, these species have in common with the drug-insensitive mouse strains that they express an allelic variant of a protein that is less sensitive to glucosylceramide.
Future perspective
Clearly, the reproductive phenotype induced by NB-DNJ in male mice is highly variable, and is dependent on genetic background. We have also seen that the susceptibility of post-meiotic male germ cells to the consequences of imino sugar administration can be regarded as a complex pharmacogenetic trait, involving the pharmacodynamics of the drug. Thus it is to be expected that the imino sugar susceptibility, like many other complex traits, is determined by multiple genetic features. The anticipation is therefore that multiple quantitative trait loci (QTLs) will be found that together are responsible for the observed variation in imino sugar sensitivity.
The challenge is now to move from imino sugar pharmacogenetics to pharmacogenomics, to be able to explain the variation in response to the drug on the basis of nucleotide differences between mouse strains. This is indeed a challenge as of the many complex quantitative traits, for which QTLs have been mapped, only very few have been resolved at the nucleotide level (reviewed in [103]). Current mapping strategies to identify the genetic variation underlying a QTL are limited by the effect size of the QTL, the percentage of the total phenotypic variation that is explained by one QTL (or a combination of QTLs) [103]. Individual QTLs with a substantial contribution to the total phenotypic variation are more likely to be reduced to quantitative trait nucleotides than those with small effect sizes. The QTLs that have at present been resolved at the nucleotide level, have in common that on average they control approximately a quarter of the total phenotypic variation [103]. Therefore, in order to estimate the likelihood of identifying quantitative trait nucleotides linked to the pharmacogenetics of NB-DNJ, it will be informative to determine how many high-resolution QTLs underlie this complex trait. Currently, efforts are underway to identify and fine-map QTLs that are associated with reproductive effects of the imino sugar. This endeavor, as well as other murine pharmacogenetic studies, are likely to benefit from the increasing array of resources and approaches that are available for complex trait analysis in mice (see [103–107] for overviews). Recently, Su et al. successfully followed a mouse intercross strategy to identify QTLs and candidate genes that control aspects of lipid metabolism and distribution [108].
Our knowledge regarding the biochemical pathways that are affected by NB-DNJ and of the cell biology that is impacted by the drug may facilitate the design of functional studies needed to substantiate the involvement of candidate proteins. Most of the enzymes involved in the metabolism of simple and complex sphingolipids have been identified, and improved methods for quantitation of sphingolipids in biological samples are being developed [109–111]. As both sphingolipid biochemistry and male germ cell development are of significant biological importance and require further understanding, it is of considerable interest to take this project to the next step.
Executive summary.
The alkylated imino sugar N-butyldeoxynojirimycin can cause male mice to become infertile
The affected mice produce over 90% grossly abnormal spermatozoa that do not have an acrosome and are poorly motile.
Administration of the drug to C57BL/6 mice first impacts the early steps of acrosome formation: it leads to aberrant behavior of pro-acrosomal dense-core vesicles.
Imino sugar pharmacogenetics
The reproductive effect of N-butyldeoxynojirimycin (NB-DNJ) is limited to a number of inbred strains from the C57 lineage.
Intercross mice generated from a high- and a low-responding strain display a continuous range of effects of the drug.
The susceptibility for the spermatogenic impact of NB-DNJ is not inherited in a Mendelian fashion, and can be considered a quantitative trait.
NB-DNJ affects glycosphingolipid metabolism
NB-DNJ acts in vitro and in vivo as inhibitor of β-glucosidase 2, which is an extralysosomal membrane-bound glucosylceramidase, it cleaves glucosylceramide in glucose and ceramide.
Administration of NB-DNJ at low doses significantly elevates testicular glucosylceramide in inbred mice, irrespective of strain background.
Impact of glucosylceramide on acrosome formation
In C57BL/6 mice and in related strains, some of the enzymes and other proteins that are involved in early acrosome formation may be affected by higher glucosylceramide levels.
Other inbred strains may express allelic variants of such enzymes that differ in their susceptibility to glucosylceramide.
Model for sperm heterogeneity within individual mice (or men)
A model is presented that takes into account that after NB-DNJ treatment the highly aberrant sperm phenotype is only seen in a subset of spermatozoa from each mouse (regardless of its genotype), and that the size of this subset depends on the genetic constitution of the mouse.
This model can also be applied to human spermatogenesis, and predicts that the morphological and functional heterogeneity between human spermatozoa may be owing to either genetic or stochastic nongenomic differences between individual germ cells.
Imino sugar pharmacogenomics
Efforts are underway to identify quantitative trait loci and the relevant genes that control the reproductive outcome of NB-DNJ administration in male mice.
This endeavor may provide novel insights in the biochemical role of glucosylceramide, a ubiquitous eukaryotic sphingolipid, and in the early steps of acrosome formation, an essential event in mammalian spermatogenesis.
Footnotes
Financial & competing interests disclosure: ACS and FMP are in receipt of a grant from Bayer Schering Pharma AG, Berlin, Germany. The authors would like to acknowledge the support of Oxford GlycoSystems, The Oxford Glycobiology Institute, the NIH/NICHD (ACS and FMP, grant U01 HD045861), Bayer Schering Pharma, and the Wellcome Trust (RM). We also want to thank Wilhelm Bone (Bayer Schering Pharma), Terry Butters, Raymond Dwek and Mark Wormald (Department of Biochemistry, University of Oxford, Oxford, UK), Chia-Chen Chuang (Department of Pharmacology, University of Oxford, Oxford, UK), Harry Moore (University of Sheffield, Sheffield, UK), Richard Oko (Queen's University, Kingston, ON, Canada), Ioannis Ragoussis (Wellcome Trust Centre for Human Genetics, Oxford, UK), Roger Sandhoff (German Cancer Research Centre, Heidelberg, Germany), David Smith (Department of Pharmacology), Charlotte M Walden, the MRC Drosophila Cooperative Group (Department of Zoology, University of Oxford, Oxford, UK), and the Electron Microscopy and Microanalysis Group (Department of Materials, University of Oxford, Oxford, UK) for their help with this project. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Compain P, Martin OR, editors. Iminosugars: From Synthesis to Therapeutic Applications. John Wiley and Sons; Chichester, UK: 2007. [Google Scholar]
- 2.Stutz AE, editor. Iminosugars as Glycosidase Inhibitors: Nojirimycin and Beyond. Wiley VCH; Weinheim, Germany: 1998. [Google Scholar]
- 3.Asano N. Naturally occurring iminosugars and related compounds: structure, distribution, and biological activity. Curr Top Med Chem. 2003;3:471–484. doi: 10.2174/1568026033452438. [DOI] [PubMed] [Google Scholar]
- 4.Treiber A, Morand O, Clozel M. The pharmacokinetics and tissue distribution of the glucosylceramide synthase inhibitor miglustat in the rat. Xenobiotica. 2007;37:298–314. doi: 10.1080/00498250601094543. [DOI] [PubMed] [Google Scholar]
- 5.Mellor HR, Nolan J, Pickering L, et al. Preparation, biochemical characterization and biological properties of radiolabelled N-alkylated deoxynojirimycins. Biochem J. 2002;366:225–233. doi: 10.1042/BJ20020466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walden CM, Sandhoff R, Chuang CC, et al. Accumulation of glucosylceramide in murine testis, caused by inhibition of β-glucosidase 2: implications for spermatogenesis. J Biol Chem. 2007;282:32655–32664. doi: 10.1074/jbc.M702387200. [DOI] [PubMed] [Google Scholar]; • Here the consequences of N-butyldeoxynojirimycin (NB-DNJ) administration on in vivo glycosphingolipid biochemistry are analyzed.
- 7.Bone W, Walden CM, Fritsch M, et al. The sensitivity of murine spermiogenesis to miglustat is a quantitative trait: a pharmacogenetic study. Reprod Biol Endocrinol. 2007;5:1. doi: 10.1186/1477-7827-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Describes the extent of the variation in sperm phenotype between mice of different inbred strains, following NB-DNJ administration.
- 8.Durantel D, Branza-Nichita N, Carrouee-Durantel S, Butters TD, Dwek RA, Zitzmann N. Study of the mechanism of antiviral action of iminosugar derivatives against bovine viral diarrhea virus. J Virol. 2001;75:8987–8998. doi: 10.1128/JVI.75.19.8987-8998.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walden CM, Butters TD, Dwek RA, Platt FM, van der Spoel AC. Long-term non-hormonal male contraception in mice using N-butyldeoxynojirimycin. Hum Reprod. 2006;21:1309–1315. doi: 10.1093/humrep/dei463. [DOI] [PubMed] [Google Scholar]
- 10.Mayorga LS, Tomes CN, Belmonte SA. Acrosomal exocytosis, a special type of regulated secretion. IUBMB Life. 2007;59:286–292. doi: 10.1080/15216540701222872. [DOI] [PubMed] [Google Scholar]
- 11.Yanagimachi R. Mammalian fertilization. In: Knobil E, Neill JD, editors. The physiology of reproduction. Raven Press; New York, USA: 1994. [Google Scholar]
- 12.Hillman N, Nadijcka M. A study of spermatozoan defects in wild-type and T:t-bearing mice. J Embryol Exp Morphol. 1978;44:263–280. [PubMed] [Google Scholar]
- 13.Kishikawa H, Tateno H, Yanagimachi R. Chromosome analysis of BALB/c mouse spermatozoa with normal and abnormal head morphology. Biol Reprod. 1999;61:809–812. doi: 10.1095/biolreprod61.3.809. [DOI] [PubMed] [Google Scholar]
- 14.Pogany GC, Balhorn R. Quantitative fluorometry of abnormal mouse sperm nuclei. J Reprod Fertil. 1992;96:25–34. doi: 10.1530/jrf.0.0960025. [DOI] [PubMed] [Google Scholar]
- 15.van der Spoel AC, Jeyakumar M, Butters TD, et al. Reversible infertility in male mice following oral administration of alkylated imino sugars: a non-hormonal approach to male contraception. PNAS. 2002;99:17173–17178. doi: 10.1073/pnas.262586099. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Discovery of the contraceptive effect of NB-DNJ in C57BL/6 mice.
- 16.Suganuma R, Walden CM, Butters TD, et al. Alkylated imino sugars, reversible male infertility-inducing agents, do not affect the genetic integrity of male mouse germ cells during short-term treatment despite induction of sperm deformities. Biol Reprod. 2005;72:805–813. doi: 10.1095/biolreprod.104.036053. [DOI] [PubMed] [Google Scholar]
- 17.Hollander WF, Bryan JHD, Gowen JW. A male sterile pink-eyed mutant type in the mouse. Fertil Steril. 1960;11:316–324. doi: 10.1016/s0015-0282(16)33789-x. [DOI] [PubMed] [Google Scholar]
- 18.Boot RG, Verhoek M, Donker-Koopman W, et al. Identification of the non-lysosomal glucosylceramidase as β-glucosidase 2. J Biol Chem. 2007;282:1305–1312. doi: 10.1074/jbc.M610544200. [DOI] [PubMed] [Google Scholar]
- 19.Russell LD, Ettlin RA, Sinha Hikim AP, Clegg ED. Histological and histopathological evaluation of the testis. Cache River Press; Clearwater, FL, USA: 1990. [Google Scholar]
- 20.Oko R. Occurrence and formation of cytoskeletal proteins in mammalian spermatozoa. Andrologia. 1998;30:193–206. doi: 10.1111/j.1439-0272.1998.tb01161.x. [DOI] [PubMed] [Google Scholar]; •• Discovery of the close association between the pro-acrosomal vesicle and components of the perinuclear theca, at the early spermatid stage.
- 21.Oko RJ. Developmental expression and possible role of perinuclear theca proteins in mammalian spermatozoa. Reprod Fertil Dev. 1995;7:777–797. doi: 10.1071/rd9950777. [DOI] [PubMed] [Google Scholar]
- 22.Korley R, Pouresmaeili F, Oko R. Analysis of the protein composition of the mouse sperm perinuclear theca and characterization of its major protein constituent. Biol Reprod. 1997;57:1426–1432. doi: 10.1095/biolreprod57.6.1426. [DOI] [PubMed] [Google Scholar]
- 23.Aul RB, Oko RJ. The major subacrosomal occupant of bull spermatozoa is a novel histone H2B. Dev Biol. 2002;242:376–387. [PubMed] [Google Scholar]
- 24.Wu AT, Sutovsky P, Xu W, van der Spoel AC, Platt FM, Oko R. The postacrosomal assembly of sperm head protein, PAWP, is independent of acrosome formation and dependent on microtubular manchette transport. Dev Biol. 2007;312:471–483. doi: 10.1016/j.ydbio.2007.08.051. [DOI] [PubMed] [Google Scholar]
- 25.Kang-Decker N, Mantchev GT, Juneja SC, McNiven MA, van Deursen JM. Lack of acrosome formation in Hrb-deficient mice. Science. 2001;294:1531–1533. doi: 10.1126/science.1063665. [DOI] [PubMed] [Google Scholar]
- 26.Yao R, Ito C, Natsume Y, et al. Lack of acrosome formation in mice lacking a Golgi protein, GOPC. Proc Natl Acad Sci USA. 2002;99:11211–11216. doi: 10.1073/pnas.162027899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Juneja SC, van Deursen JM. A mouse model of familial oligoasthenoteratozoospermia. Hum Reprod. 2005;20:881–893. doi: 10.1093/humrep/deh719. [DOI] [PubMed] [Google Scholar]
- 28.Ji Y, Walkowicz MJ, Buiting K, et al. The ancestral gene for transcribed, low-copy repeats in the Prader- Willi/Angelman region encodes a large protein implicated in protein trafficking, which is deficient in mice with neuromuscular and spermiogenic abnormalities. Hum Mol Genet. 1999;8:533–542. doi: 10.1093/hmg/8.3.533. [DOI] [PubMed] [Google Scholar]
- 29.Lehman AL, Nakatsu Y, Ching A, et al. A very large protein with diverse functional motifs is deficient in rjs (runty, jerky, sterile) mice. Proc Natl Acad Sci USA. 1998;95:9436–9441. doi: 10.1073/pnas.95.16.9436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmitt-John T, Drepper C, Mussmann A, et al. Mutation of Vps54 causes motor neuron disease and defective spermiogenesis in the wobbler mouse. Nat Genet. 2005;37:1213–1215. doi: 10.1038/ng1661. [DOI] [PubMed] [Google Scholar]
- 31.Mussmann A, Drepper C, Heimann P, et al. Expresion and metabolite profiling of the murine Wobbler globozoospermia and mapping of a fertility-restoring allele. Presented at: 10th International Symposium on Spermatology; El Escorial, Spain. 17–22 September (2006). [Google Scholar]
- 32.Sotomayor RE, Handel MA. Failure of acrosome assembly in a male sterile mouse mutant. Biol Reprod. 1986;34:171–182. doi: 10.1095/biolreprod34.1.171. [DOI] [PubMed] [Google Scholar]
- 33.Dam AH, Koscinski I, Kremer JA, et al. Homozygous mutation in SPATA16 is associated with male infertility in human globozoospermia. Am J Hum Genet. 2007;81:813–820. doi: 10.1086/521314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amory JK, Muller CH, Page ST, et al. Miglustat has no apparent effect on spermatogenesis in normal men. Hum Reprod. 2007;22:702–707. doi: 10.1093/humrep/del414. [DOI] [PubMed] [Google Scholar]
- 35.Long AD, Mullaney SL, Reid LA, Fry JD, Langley CH, Mackay TF. High resolution mapping of genetic factors affecting abdominal bristle number in Drosophila melanogaster. Genetics. 1995;139:1273–1291. doi: 10.1093/genetics/139.3.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Andersson U, Butters TD, Dwek RA, Platt FM. N-butyldeoxygalactonojirimycin: a more selective inhibitor of glycosphingolipid biosynthesis than N-butyldeoxynojirimycin, in vitro and in vivo. Biochem Pharmacol. 2000;59:821–829. doi: 10.1016/s0006-2952(99)00384-6. [DOI] [PubMed] [Google Scholar]
- 37.Elbein AD. Inhibitors of the biosynthesis and processing of N-linked oligosaccharide chains. Annu Rev Biochem. 1987;56:497–534. doi: 10.1146/annurev.bi.56.070187.002433. [DOI] [PubMed] [Google Scholar]
- 38.Butters TD, van den Broek LAGM, Fleet GWJ, et al. Molecular requirements of imino sugars for the selective control of N-linked glycosylation and glycosphingolipid biosynthesis. Tetrahedron Asymmetry. 2000;11:113–124. [Google Scholar]
- 39.Nyholm PG, Pascher I, Sundell S. The effect of hydrogen bonds on the conformation of glycosphingolipids. Methylated and unmethylated cerebroside studied by x-ray single crystal analysis and model calculations. Chem Phys Lipids. 1990;52:1–10. doi: 10.1016/0009-3084(90)90002-9. [DOI] [PubMed] [Google Scholar]
- 40.Hakomori S, Ineo I. Encyclopedia of Life Sciences. John Wiley and Sons; Chichester, UK: 1996. Glycolipids: Animal. [Google Scholar]
- 41.Kolter T, Proia RL, Sandhoff K. Combinatorial ganglioside biosynthesis. J Biol Chem. 2002;277:25859–25862. doi: 10.1074/jbc.R200001200. [DOI] [PubMed] [Google Scholar]
- 42.Futerman AH, Boldin SA, Brann AB, Pelled D, Meivar-Levy I, Zisling R. Regulation of sphingolipid and glycosphingolipid metabolism during neuronal growth and development. Biochem Soc Trans. 1999;27:432–437. doi: 10.1042/bst0270432. [DOI] [PubMed] [Google Scholar]
- 43.Rabionet M, van der Spoel AC, Chuang CC, et al. Male germ cells require polyenoic sphingolipids with complex glycosylation for completion of meiosis: a link to ceramide synthase-3. J Biol Chem. 2008;283(19):13357–13369. doi: 10.1074/jbc.M800870200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu RK, Bieberich E, Xia T, Zeng G. Regulation of ganglioside biosynthesis in the nervous system. J Lipid Res. 2004;45:783–793. doi: 10.1194/jlr.R300020-JLR200. [DOI] [PubMed] [Google Scholar]
- 45.Ngamukote S, Yanagisawa M, Ariga T, Ando S, Yu RK. Developmental changes of glycosphingolipids and expression of glycogenes in mouse brains. J Neurochem. 2007;103:2327–2341. doi: 10.1111/j.1471-4159.2007.04910.x. [DOI] [PubMed] [Google Scholar]
- 46.Todeschini RA, Hakomori SI. Functional role of glycosphingolipids and gangliosides in control of cell adhesion, motility, and growth, through glycosynaptic microdomains. Biochim Biophys Acta. 2008;1780:421–433. doi: 10.1016/j.bbagen.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yamashita T, Wada R, Sasaki T, et al. A vital role for glycosphingolipid synthesis during development and differentiation. Proc Natl Acad Sci USA. 1999;96:9142–9147. doi: 10.1073/pnas.96.16.9142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Choi MJ, Maibach HI. Role of ceramides in barrier function of healthy and diseased skin. Am J Clin Dermatol. 2005;6:215–223. doi: 10.2165/00128071-200506040-00002. [DOI] [PubMed] [Google Scholar]
- 49.Holleran WM, Takagi Y, Uchida Y. Epidermal sphingolipids: metabolism, function, and roles in skin disorders. FEBS Lett. 2006;580:5456–5466. doi: 10.1016/j.febslet.2006.08.039. [DOI] [PubMed] [Google Scholar]
- 50.Jennemann R, Sandhoff R, Wang S, et al. Cell-specific deletion of glucosylceramide synthase in brain leads to severe neural defects after birth. Proc Natl Acad Sci USA. 2005;102:12459–12464. doi: 10.1073/pnas.0500893102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yamashita T, Wu YP, Sandhoff R, et al. Interruption of ganglioside synthesis produces central nervous system degeneration and altered axon-glial interactions. Proc Natl Acad Sci USA. 2005;102:2725–2730. doi: 10.1073/pnas.0407785102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kabayama K, Sato T, Saito K, et al. Dissociation of the insulin receptor and caveolin-1 complex by ganglioside GM3 in the state of insulin resistance. Proc Natl Acad Sci USA. 2007;104:13678–13683. doi: 10.1073/pnas.0703650104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yamashita T, Hashiramoto A, Haluzik M, et al. Enhanced insulin sensitivity in mice lacking ganglioside GM3. Proc Natl Acad Sci USA. 2003;100:3445–3449. doi: 10.1073/pnas.0635898100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Paget C, Mallevaey T, Speak AO, et al. Activation of invariant NKT cells by toll-like receptor 9-stimulated dendritic cells requires type I interferon and charged glycosphingolipids. Immunity. 2007;27:597–609. doi: 10.1016/j.immuni.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 55.Speak AO, Salio M, Neville DC, et al. Implications for invariant natural killer T cell ligands due to the restricted presence of isoglobotrihexosylceramide in mammals. Proc Natl Acad Sci USA. 2007;104:5971–5976. doi: 10.1073/pnas.0607285104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Takamiya K, Yamamoto A, Furukawa K, et al. Complex gangliosides are essential in spermatogenesis of mice: possible roles in the transport of testosterone. Proc Natl Acad Sci USA. 1998;95:12147–12152. doi: 10.1073/pnas.95.21.12147. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Discovery of the involvement of complex glycosphingolipids in spermatogenesis in the mouse.
- 57.Sandhoff R, Geyer R, Jennemann R, et al. Novel class of glycosphingolipids involved in male fertility. J Biol Chem. 2005;280:27310–27318. doi: 10.1074/jbc.M502775200. [DOI] [PubMed] [Google Scholar]; • Characterization of the unusual glycosphingolipids present in murine testes.
- 58.Jeyakumar M, Butters TD, Dwek RA, Platt FM. Glycosphingolipid lysosomal storage diseases: therapy and pathogenesis. Neuropathol Appl Neurobiol. 2002;28:343–357. doi: 10.1046/j.1365-2990.2002.00422.x. [DOI] [PubMed] [Google Scholar]
- 59.Winchester B, Vellodi A, Young E. The molecular basis of lysosomal storage diseases and their treatment. Biochem Soc Trans. 2000;28:150–154. doi: 10.1042/bst0280150. [DOI] [PubMed] [Google Scholar]
- 60.Kacher Y, Futerman AH. Genetic diseases of sphingolipid metabolism: pathological mechanisms and therapeutic options. FEBS Lett. 2006;580:5510–5517. doi: 10.1016/j.febslet.2006.08.041. [DOI] [PubMed] [Google Scholar]
- 61.Kolter T, Sandhoff K. Sphingolipid metabolism diseases. Biochim Biophys Acta. 2006;1758:2057–2079. doi: 10.1016/j.bbamem.2006.05.027. [DOI] [PubMed] [Google Scholar]
- 62.Jeyakumar M, Butters TD, Cortina-Borja M, et al. Delayed symptom onset and increased life expectancy in sandhoff disease mice treated with N-butyldeoxynojirimycin. Proc Natl Acad Sci USA. 1999;96:6388–6393. doi: 10.1073/pnas.96.11.6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Platt FM, Neises GR, Reinkensmeier G, et al. Prevention of lysosomal storage in Tay-Sachs mice treated with N-butyldeoxynojirimycin. Science. 1997;276:428–431. doi: 10.1126/science.276.5311.428. [DOI] [PubMed] [Google Scholar]
- 64.Cox T, Lachmann R, Hollak C, et al. Novel oral treatment of Gaucher's disease with N-butyldeoxynojirimycin (OGT 918) to decrease substrate biosynthesis. Lancet. 2000;355:1481–1485. doi: 10.1016/S0140-6736(00)02161-9. [DOI] [PubMed] [Google Scholar]
- 65.Elstein D, Hollak C, Aerts JM, et al. Sustained therapeutic effects of oral miglustat (Zavesca, N-butyldeoxynojirimycin, OGT 918) in Type I Gaucher disease. J Inherit Metab Dis. 2004;27:757–766. doi: 10.1023/B:BOLI.0000045756.54006.17. [DOI] [PubMed] [Google Scholar]
- 66.Jeyakumar M, Dwek RA, Butters TD, Platt FM. Storage solutions: treating lysosomal disorders of the brain. Nat Rev Neurosci. 2005;6:713–725. doi: 10.1038/nrn1725. [DOI] [PubMed] [Google Scholar]
- 67.Butters TD, Dwek RA, Platt FM. Imino sugar inhibitors for treating the lysosomal glycosphingolipidoses. Glycobiology. 2005;15:43R–52R. doi: 10.1093/glycob/cwi076. [DOI] [PubMed] [Google Scholar]
- 68.Cox TM. Substrate reduction therapy for lysosomal storage diseases. Acta Paediatr. 2005;(Suppl 94):69–75. doi: 10.1111/j.1651-2227.2005.tb02116.x. discussion 57. [DOI] [PubMed] [Google Scholar]
- 69.Lachmann RH. Miglustat: substrate reduction therapy for glycosphingolipid lysosomal storage disorders. Drugs Today (Barc) 2006;42:29–38. doi: 10.1358/dot.2006.42.1.937457. [DOI] [PubMed] [Google Scholar]
- 70.Aerts JM, Hollak CE, Boot RG, Groener JE, Maas M. Substrate reduction therapy of glycosphingolipid storage disorders. J Inherit Metab Dis. 2006;29:449–456. doi: 10.1007/s10545-006-0272-5. [DOI] [PubMed] [Google Scholar]
- 71.Chien YH, Lee NC, Tsai LK, et al. Treatment of Niemann-Pick disease type C in two children with miglustat: initial responses and maintenance of effects over 1 year. J Inherit Metab Dis. 2007;30:826. doi: 10.1007/s10545-007-0630-y. [DOI] [PubMed] [Google Scholar]
- 72.Patterson MC, Vecchio D, Prady H, Abel L, Wraith JE. Miglustat for treatment of Niemann-Pick C disease: a randomised controlled study. Lancet Neurol. 2007;6:765–772. doi: 10.1016/S1474-4422(07)70194-1. [DOI] [PubMed] [Google Scholar]
- 73.Futerman AH, Hannun YA. The complex life of simple sphingolipids. EMBO Rep. 2004;5:777–782. doi: 10.1038/sj.embor.7400208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lahiri S, Futerman AH. The metabolism and function of sphingolipids and glycosphingolipids. Cell Mol Life Sci. 2007;64:2270–2284. doi: 10.1007/s00018-007-7076-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Degroote S, Wolthoorn J, van Meer G. The cell biology of glycosphingolipids. Semin Cell Dev Biol. 2004;15:375–387. doi: 10.1016/j.semcdb.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 76.van Meer G, Wolthoorn J, Degroote S. The fate and function of glycosphingolipid glucosylceramide. Philos Trans R Soc Lond B Biol Sci. 2003;358:869–873. doi: 10.1098/rstb.2003.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Coste H, Martel MB, Got R. Topology of glucosylceramide synthesis in Golgi membranes from porcine submaxillary glands. Biochim Biophys Acta. 1986;858:6–12. doi: 10.1016/0005-2736(86)90285-3. [DOI] [PubMed] [Google Scholar]
- 78.Futerman AH, Pagano RE. Determination of the intracellular sites and topology of glucosylceramide synthesis in rat liver. Biochem J. 1991;280(Pt 2):295–302. doi: 10.1042/bj2800295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jeckel D, Karrenbauer A, Burger KN, van Meer G, Wieland F. Glucosylceramide is synthesized at the cytosolic surface of various Golgi subfractions. J Cell Biol. 1992;117:259–267. doi: 10.1083/jcb.117.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Halter D, Neumann S, van Dijk SM, et al. Pre- and post-Golgi translocation of glucosylceramide in glycosphingolipid synthesis. J Cell Biol. 2007;179:101–115. doi: 10.1083/jcb.200704091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yildiz Y, Matern H, Thompson B, et al. Mutation of β-glucosidase 2 causes glycolipid storage disease and impaired male fertility. J Clin Invest. 2006;116:2985–2994. doi: 10.1172/JCI29224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.D'Angelo G, Polishchuk E, Di Tullio G, et al. Glycosphingolipid synthesis requires FAPP2 transfer of glucosylceramide. Nature. 2007;449:62–67. doi: 10.1038/nature06097. [DOI] [PubMed] [Google Scholar]
- 83.Platt FM, Neises GR, Dwek RA, Butters TD. N-butyldeoxynojirimycin is a novel inhibitor of glycolipid biosynthesis. J Biol Chem. 1994;269:8362–8365. [PubMed] [Google Scholar]
- 84.Platt FM, Neises GR, Karlsson GB, Dwek RA, Butters TD. N-butyldeoxygalactonojirimycin inhibits glycolipid biosynthesis but does not affect N-linked oligosaccharide processing. J Biol Chem. 1994;269:27108–27114. [PubMed] [Google Scholar]
- 85.Matern H, Heinemann H, Legler G, Matern S. Purification and characterization of a microsomal bile acid β-glucosidase from human liver. J Biol Chem. 1997;272:11261–11267. doi: 10.1074/jbc.272.17.11261. [DOI] [PubMed] [Google Scholar]; •• Astounding report on the purification of β-glucosidase 2 (GBA2), describing several idiosyncrasies that needed to be overcome in order to obtain highly purified and active GBA2 – a biochemical spine-tingler.
- 86.Overkleeft HS, Renkema GH, Neele J, et al. Generation of specific deoxynojirimycintype inhibitors of the non-lysosomal glucosylceramidase. J Biol Chem. 1998;273:26522–26527. doi: 10.1074/jbc.273.41.26522. [DOI] [PubMed] [Google Scholar]
- 87.Tettamanti G. Ganglioside/glycosphingolipid turnover: new concepts. Glycoconj J. 2004;20:301–317. doi: 10.1023/B:GLYC.0000033627.02765.cc. [DOI] [PubMed] [Google Scholar]; •• Excellent review on the intracellular traffic of glycosphingolipids that have been endocytosed.
- 88.Maxfield FR, McGraw TE. Endocytic recycling. Nat Rev Mol Cell Biol. 2004;5:121–132. doi: 10.1038/nrm1315. [DOI] [PubMed] [Google Scholar]
- 89.Forgac M. Vacuolar ATPases: rotary proton pumps in physiology and pathophysiology. Nat Rev Mol Cell Biol. 2007;8:917–929. doi: 10.1038/nrm2272. [DOI] [PubMed] [Google Scholar]
- 90.Sun-Wada GH, Wada Y, Futai M. Diverse and essential roles of mammalian vacuolar-type proton pump ATPase: toward the physiological understanding of inside acidic compartments. Biochim Biophys Acta. 2004;1658:106–114. doi: 10.1016/j.bbabio.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 91.Chia SE, Tay SK, Lim ST. What constitutes a normal seminal analysis? Semen parameters of 243 fertile men. Hum Reprod. 1998;13:3394–3398. doi: 10.1093/humrep/13.12.3394. [DOI] [PubMed] [Google Scholar]
- 92.Guzick DS, Overstreet JW, Factor-Litvak P, et al. Sperm morphology, motility, and concentration in fertile and infertile men. N Engl J Med. 2001;345:1388–1393. doi: 10.1056/NEJMoa003005. [DOI] [PubMed] [Google Scholar]
- 93.van der Merwe FH, Kruger TF, Oehninger SC, Lombard CJ. The use of semen parameters to identify the subfertile male in the general population. Gynecol Obstet Invest. 2005;59:86–91. doi: 10.1159/000082368. [DOI] [PubMed] [Google Scholar]
- 94.Spudich JL, Koshland DE., Jr Non-genetic individuality: chance in the single cell. Nature. 1976;262:467–471. doi: 10.1038/262467a0. [DOI] [PubMed] [Google Scholar]
- 95.Kaern M, Elston TC, Blake WJ, Collins JJ. Stochasticity in gene expression: from theories to phenotypes. Nat Rev Genet. 2005;6:451–464. doi: 10.1038/nrg1615. [DOI] [PubMed] [Google Scholar]
- 96.Kaufmann BB, van Oudenaarden A. Stochastic gene expression: from single molecules to the proteome. Curr Opin Genet Dev. 2007;17:107–112. doi: 10.1016/j.gde.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 97.Rao CV, Wolf DM, Arkin AP. Control, exploitation and tolerance of intracellular noise. Nature. 2002;420:231–237. doi: 10.1038/nature01258. [DOI] [PubMed] [Google Scholar]
- 98.Aitken RJ, Sawyer D. The human spermatozoon – not waving but drowning. Adv Exp Med Biol. 2003;518:85–98. doi: 10.1007/978-1-4419-9190-4_8. [DOI] [PubMed] [Google Scholar]
- 99.Swan SH. Does our environment affect our fertility? Some examples to help reframe the question. Semin Reprod Med. 2006;24:142–146. doi: 10.1055/s-2006-944420. [DOI] [PubMed] [Google Scholar]
- 100.Jorgensen N, Asklund C, Carlsen E, Skakkebaek NE. Coordinated European investigations of semen quality: results from studies of Scandinavian young men is a matter of concern. Int J Androl. 2006;29:54–61. doi: 10.1111/j.1365-2605.2005.00635.x. discussion 105–108. [DOI] [PubMed] [Google Scholar]
- 101.Sweedler J, Arriaga E. Single cell analysis. Anal Bioanal Chem. 2007;387:1–2. [Google Scholar]
- 102.Turner EH, Cohen D, Pugsley HR, et al. Chemical cytometry: the chemical analysis of single cells. Anal Bioanal Chem. 2008;390:223–226. doi: 10.1007/s00216-007-1665-5. [DOI] [PubMed] [Google Scholar]
- 103.Flint J, Valdar W, Shifman S, Mott R. Strategies for mapping and cloning quantitative trait genes in rodents. Nat Rev Genet. 2005;6:271–286. doi: 10.1038/nrg1576. [DOI] [PubMed] [Google Scholar]
- 104.Peters LL, Robledo RF, Bult CJ, Churchill GA, Paigen BJ, Svenson KL. The mouse as a model for human biology: a resource guide for complex trait analysis. Nat Rev Genet. 2007;8:58–69. doi: 10.1038/nrg2025. [DOI] [PubMed] [Google Scholar]
- 105.Arbilly M, Pisante A, Devor M, Darvasi A. An integrative approach for the identification of quantitative trait loci. Anim Genet. 2006;37(Suppl 1):7–9. doi: 10.1111/j.1365-2052.2006.01472.x. [DOI] [PubMed] [Google Scholar]
- 106.Flaherty L, Herron B, Symula D. Genomics of the future: identification of quantitative trait loci in the mouse. Genome Res. 2005;15:1741–1745. doi: 10.1101/gr.3841405. [DOI] [PubMed] [Google Scholar]
- 107.Mott R. Finding the molecular basis of complex genetic variation in humans and mice. Philos Trans R Soc Lond B Biol Sci. 2006;361:393–401. doi: 10.1098/rstb.2005.1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Su Z, Tsaih SW, Szatkiewicz J, Shen Y, Paigen B. Candidate genes for plasma triglyceride, free fatty acid, and glucose revealed from an intercross between inbred mouse strains NZB/B1NJ × NZW/LacJ. J Lipid Res. 2008 doi: 10.1194/jlr.M800053-JLR200. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Sophisticated approach is followed to identify quantitative trait loci and reduce their genomic dimensions so that candidate genes can be identified.
- 109.Neville DC, Coquard V, Priestman DA, et al. Analysis of fluorescently labeled glycosphingolipid-derived oligosaccharides following ceramide glycanase digestion and anthranilic acid labeling. Anal Biochem. 2004;331:275–282. doi: 10.1016/j.ab.2004.03.051. [DOI] [PubMed] [Google Scholar]
- 110.Whitmore CD, Hindsgaul O, Palcic MM, Schnaar RL, Dovichi NJ. Metabolic cytometry. Glycosphingolipid metabolism in single cells. Anal Chem. 2007;79:5139–5142. doi: 10.1021/ac070716d. [DOI] [PubMed] [Google Scholar]
- 111.Lee YM, Venkataraman K, Hwang SI, Han DK, Hla T. A novel method to quantify sphingosine 1-phosphate by immobilized metal affinity chromatography (IMAC) Prostaglandins Other Lipid Mediat. 2007;84:154–162. doi: 10.1016/j.prostaglandins.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Websites
- 201.European public assessment reports for authorised medicinal products for human use www.emea.europa.eu/humandocs/Humans/EPAR/zavesca/zavesca.htm
- 202.SphinGOMAP http://sphingolab.biology.gatech.edu/sources.html