Abstract
In this paper we present data indicating that mammalian stem cell pluripotency-inducing factors are expressed during lens and limb regeneration in newts. The apparent expression even in intact tissues and the ensued regulation during regeneration raises the possibility that these factors might regulate tissue-specific reprogramming and regeneration. Furthermore, these factors should enable us to understand the similarities and differences between animal regeneration in the newt and stem cell strategies in mammals.
Keywords: newt regeneration, transdifferentiation, stem cell, pluripotency factors
Introduction
Some amphibians and especially adult newts can regenerate several tissues, organs or even body parts. Newts achieve this remarkable process by transdifferentiation of the terminally differentiated tissues at the site of tissue removal (Sánchez Alvarado and Tsonis, 2006). Obviously, the newt cells have to be reprogrammed and then differentiate to cells that constitute the lost tissue. One of the most clear-cut cases is that of lens regeneration where after lentectomy the pigment epithelial cells (PECs) of the tip of the dorsal iris dedifferentiate and then form the lost lens. Importantly, the ventral iris PECs are not able to contribute to regeneration (Del Rio-Tsonis and Tsonis, 2003). Recently, it has been shown that differentiated mammalian cells, including human, can be also reprogrammed to become induced pluripotent stem cells (iPSCs) that can subsequently differentiate to different tissues. This reprogramming is mediated by inducing the expression of four factors, Oct4, Sox-2, c-myc and Klf4. Another factor, nanog, seems to be also important (Takahashi et al., 2006; Takahashi et al., 2007; Yu et al., 2007; Okita et al., 2007; Wernig et al., 2007). All these are transcriptional factors expressed in embryonic stem cells. The critical question that arises from these studies is: Does reprogramming that mediates the formation iPSCs share any similarities to reprogramming during regeneration in newts? There is no evidence indicating that newt cells reprogram to pluripotency, but ample evidence exists indicating that differentiated newt cells do change their genetic activity in order to transdifferentiate to another cell type. To further investigate this process, we cloned and examined the expression of the above-mentioned factors during lens and limb regeneration in newts.
Experimental Procedures
Animals
Adult newts (Notophthalmus viridescens) were purchased from Charles D. Sullivan Co., Inc.
cDNA cloning
Partial cDNAs for stem cell factors from Notophthalmus viridescens were cloned using degenerate PCR and rapid amplification of cDNA ends. Accession numbers of the cloned genes were AB476740, FJ617548, AB476741, FJ617547 and FJ622934 for Oct4, Sox2, c-myc, Klf4, and Nanog, respectively.
Phylogenetic analysis
Phylogenetic analysis of cloned cDNAs was carried out using Genetyx-mac ver. 13. 1. 4 (Genetyx corp.). Sequences of cloned cDNA, homologues and outgroups were aligned together and the end regions which did not corresponding to cloned cDNA sequence were trimmed off. Using only the corresponding regions, phylogenetic trees were made with UPGMA method.
Quantitative PCR
Apical halves of dorsal and ventral 90° sector-iris (20 each from 10 animals for each time point) were collected at each time point of lens regeneration. For analysis during limb regeneration, hind limbs were amputated at the middle stylopodium level and stumps, about 2 mm in thickness from the edge, were collected (6 samples per time point). For day 0, limb stumps were collected immediately after the amputation. Early and late blastema was observed on day 7 and 15 day, respectively. Total RNA was extracted from pooled samples and reverse transcription (RT) for Sox2, c-Myc, Klf4, Nanog, and ribosomal protein L27 (RPL27) was carried out with a first-strand cDNA synthesis kit (Amersham Bioscience) using an oligo(dT) primer and for Oct4 using the iScript cDNA Synthesis Kit (Bio-Rad), in which RT reaction was primed by both of an oligo(dT) and random primers. qPCR was performed using iQ SYBR Green super mix (Bio-Rad) and the following gene specific primers, Oct4-F, 5′-GAGCAAGAGACCTGCCTCAC-3; Oct4-R, 5′-TCCTTGGAGAGGAGAACTGC-3′; Sox2-F, 5′-ATGCACCGCTACGACGTCA-3′; Sox2-R, 5′-CGGAGGGATTCATGGAGTTGT-3′; c-Myc-F, 5′-ACTCACAATGTTCTGGAGCGC-3′; c-Myc-R, 5′-GGTGCTTTTTCATTGTCCGC-3′; Klf4-F, 5′-AGATACACTGCCATCCCCACAT-3′; Klf4-R, 5′-CATGCTGAACTGTCCGTGAAAC-3′; Nanog-F, 5′-TATCTGAGTCCCCTGCAGATCC-3′; Nanog-R, TGGCCCAACAGCACTTTTTT-3′; RPL27-F, 5′-ATTTATGAAACCCGGGAAGG-3′; RPL27-R, 5′-CCAGGGCATGACTGTAAGGT-3′. In order to quantitate the expression of each gene, Ct values were compared to a standard curve generated using a series of dilutions of cloned cDNAs. The amount of mRNA was normalized to that of RPL27, a gene that shows no variation between dorsal and ventral iris (Makarev et al., 2007). The specificity of qPCR was checked by melting curve analysis.
Results and Discussion
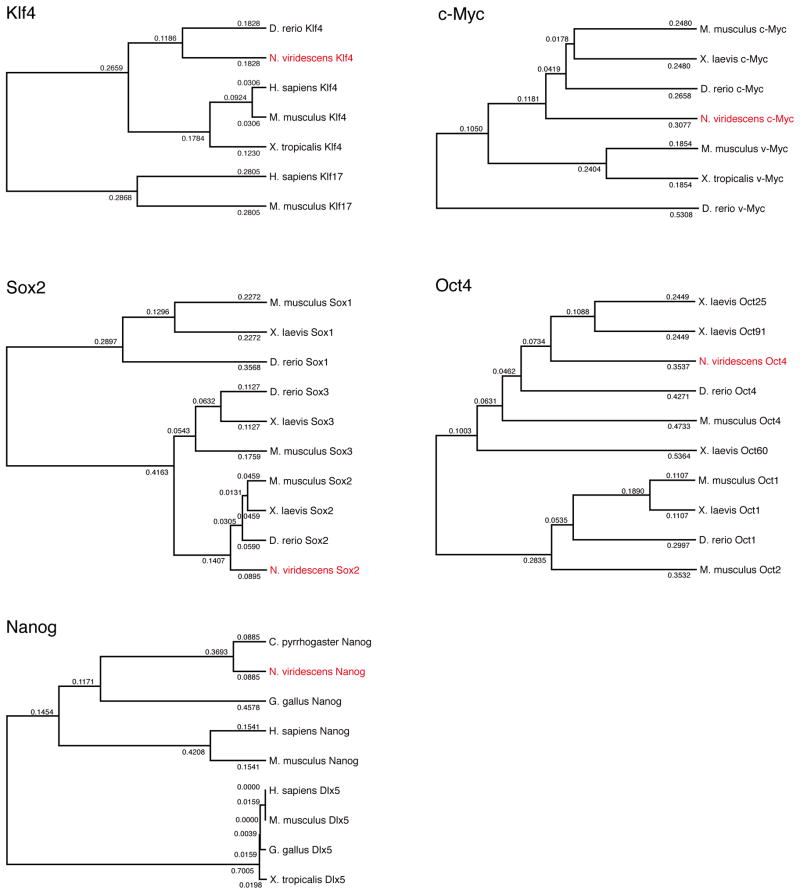
Phylogenetic tree analysis confirmed the identity of all 5 genes (Fig. 1). Based on this and the extensive sequence similarities (Supplementary Figure 1) we are quite confident that these are true orthologs. However, lack of whole newt genome sequence cannot rule out the existence of other possible paralogs. For expression studies we removed lenses or amputated hind limbs and collected tissues at different times after tissue removal. For lens regeneration, dorsal and ventral irises were collected at days 0 (intact tissue), 2, 4, 6, 8 and 10 post-lentectomy. By day 10 an undifferentiated vesicle forms devoid of lens-specific markers. After day 10, the cells in the vesicle begin to differentiate into a lens. For limb regeneration, we collected tissues at day 0 (immediately after amputation; intact tissue) and 7 and 15 after amputation. Because we wished to examine stages before differentiation occurred, we chose day 7 and 15 post-amputation as these mark the appearance of the early and late blastema, respectively. Interestingly, there was significant regulation of three of the factors that we examined, Sox-2, Klf4 and c-myc. Oct4 and nanog were not detected in these tissues beyond the levels of negative control (−RT), however, they were expressed in ovary (Fig. 2).
Fig. 1.
Phylogenetic tree analysis of all cloned cDNAs, indicating their identity with orthologs from other species.
Fig. 2.
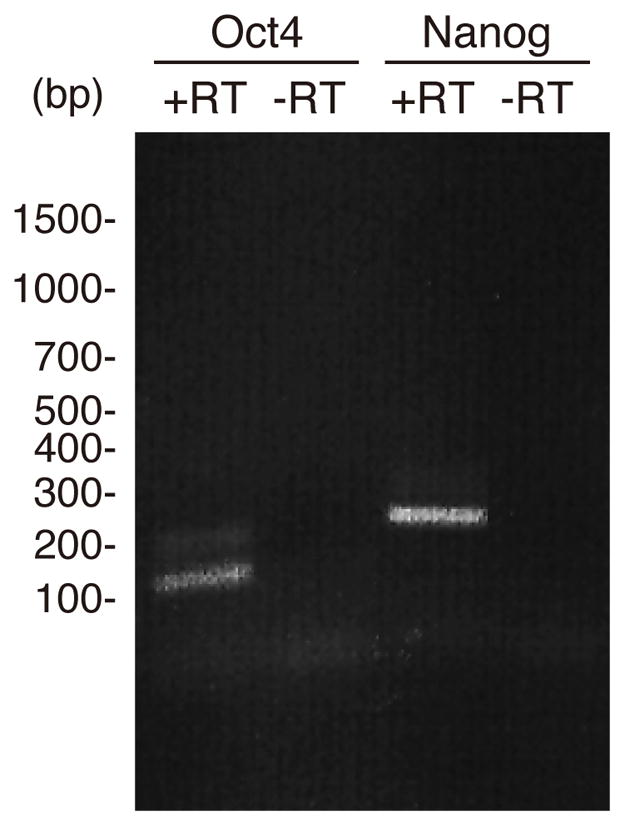
Expression of Oct-4 and nanog detected via PCR in newt ovaries. The expected size for Oct4 was 139bp and for nanog 237bp
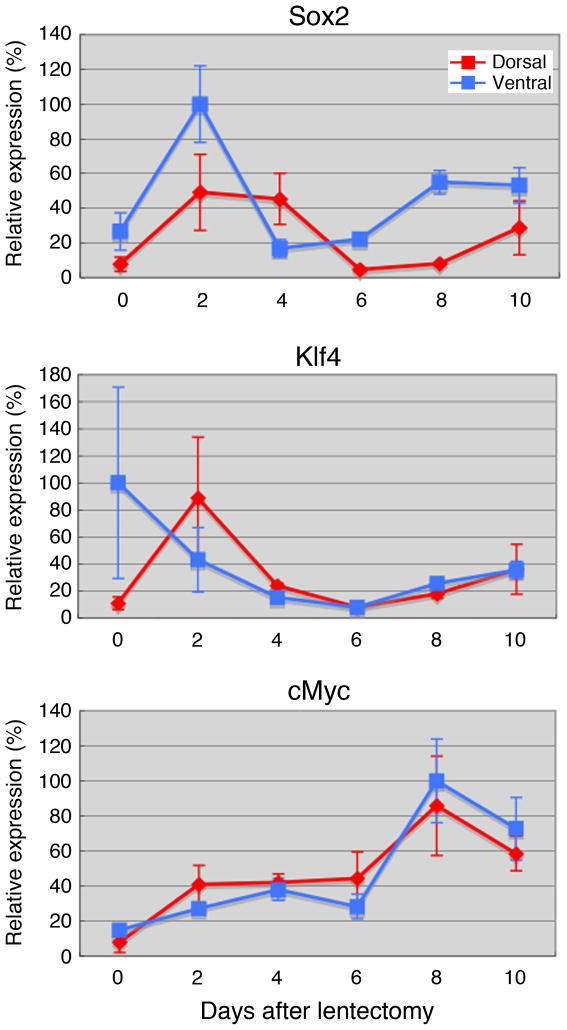
During lens regeneration, Sox2 and Klf4 were upregulated during the very early stages of regeneration (Fig. 3, day 2), while c-myc showed a peak of expression at day 8. Day 2 marks an early response to lens removal and is expected to be characterized by events that may prepare pre-existing tissues for reprogramming and cell cycle re-entry. In fact, cell proliferation is not detected until day 4. Those rapid responses to lens removal prior to cell cycle re-entry are similar to those observed for nucleostemin, a stem cell and cancer cell marker (Maki et al., 2007). c-myc showed quite opposite patterns to Sox2 and Klf4. It was highly expressed at day 8, which correlated with the establishment of the vesicle, but without major differences between dorsal and ventral iris. It should be noted here that the regeneration-incompetent ventral iris does show activity such as cell cycle re-entry and gene expression, which must be suppressed later (Grogg et al., 2005; Madhavan et al., 2006). Therefore, expression differences between dorsal and ventral iris might be correlated with these events. Statistics between the values of dorsal and ventral iris or between different times in dorsal or ventral iris are presented in Supplementary Table 1.
Fig. 3.
qPCR analysis of expression of Sox2, Klf4 and c-myc during lens regeneration. For each time point we collected 20 irises, that were pooled for the analysis.
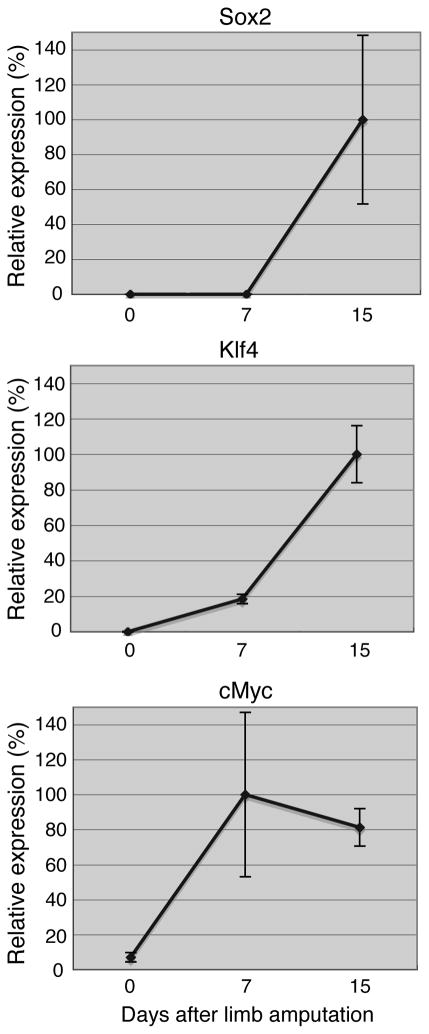
After limb amputation, blastema cells are produced by all tissues of the stump, such as muscle, cartilage, bone, dermis, etc (Tsonis, 1996). All three factors were strongly up-regulated with the emergence of the undifferentiated blastema cells. Sox2 and Klf 4 showed similar patterns with high expression at day 15, while c-myc was higher at day 7 (Fig. 4). Statistics are presented in Supplementary Table 1.
Fig. 4.
qPCR analysis of expression of Sox2, Klf4 and c-myc during regenerating limb blastema formation. For each time point we selected 6 limb stumps or blastemas that were pooled for the analysis.
Even though beyond the scope of the present paper, it will be of interest to extend these studies to single cells and find out whether all these three genes are present in the same cell. Such studies, however, will be possible only when appropriate markers (providing that they are not regulated during dedifferentiation) for contributing cells can be used to sort them out and study their properties in detail.
These expression patterns may bear significance to the field of reprogramming, stem cells and regeneration in general. While the newt does not reprogram its adult differentiated cells to form pluripotent progenitors, it does reprogram them to specific progenitor stem cell state according to their origin and to their contribution, as evidenced by their ability to regenerate with precision only those parts lost to injury. Indeed, aggregates from dorsal iris PECs will transdifferentiate only to lens even if transplanted to regenerating limb (Ito et al, 1999; Tsonis, 2000). Our data might suggest a possible mechanism for this property in the newt. The specific stage-related regulation of Sox2, Klf4 and c-myc, and the absence of Oct4 and nanog expression might indicate why the newt cells do not become pluripotent. Manipulating these factors in the newt will open new avenues in the field and should be of enormous importance to compare or contrast the biology of regeneration in classical models and in stem cells.
Supplementary Material
Acknowledgments
NIH; Grant number: EY10540
Supported by NIH grant EY10540 to PAT
References
- Ito M, Hayashi T, Kuroiwa A, Okamoto M. Lens formation by pigmented epithelial cell reaggregate from dorsal iris implanted into limb blastema in the adult newt. Dev Growth Diff. 1999;41:429–440. doi: 10.1046/j.1440-169x.1999.00447.x. [DOI] [PubMed] [Google Scholar]
- Del Rio-Tsonis K, Tsonis PA. Eye regeneration at the molecular age. Dev Dyn. 2003;26:211–224. doi: 10.1002/dvdy.10224. [DOI] [PubMed] [Google Scholar]
- Grogg MW, Call MK, Okamoto M, Vergara MN, Del Rio-Tsonis K, Tsonis PA. BMP inhibition-driven regulation of six-3 underlies induction of newt lens regeneration. Nature. 2005;438:858–862. doi: 10.1038/nature04175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhavan M, Haynes TL, Frisch NC, Call MK, Minich CM, Tsonis PA, Del Rio-Tsonis K. The role of Pax-6 in lens regeneration. Proc Natl Acad Sci USA. 2006;103:14848–14853. doi: 10.1073/pnas.0601949103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarev E, Call MK, Grogg MW, Atkinson DL, Milash B, Odelberg SJ, Tsonis PA. Gene expression signatures in the newt irises during lens regeneration. FEBS Lett. 2007;581(9):1865–1870. doi: 10.1016/j.febslet.2007.03.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki N, Takechi K, Sano S, Tarui H, Sasai Y, Agata K. Rapid accumulation of nucleostemin in nucleolus during newt regeneration. Dev Dyn. 2007;236:941–950. doi: 10.1002/dvdy.21027. [DOI] [PubMed] [Google Scholar]
- Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–317. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- Sánchez Alvarado A, Tsonis PA. Bridging the regeneration gap: Genetic insights from diverse animal models. Nat Rev Genet. 2006;7:873–884. doi: 10.1038/nrg1923. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichikawa T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Tsonis PA. Limb Regeneration. Cambridge University Press; 1996. p. 241. [Google Scholar]
- Tsonis PA. Regeneration in vertebrates. Dev Biol. 2000;221:273–284. doi: 10.1006/dbio.2000.9667. [DOI] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science. 318(5858):1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- Wernig M, Meissner A, Foreman R, Brambrink T, Ku M, Hochedlinger K, Bernstein BE, Jaenisch R. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature. 2007;448:318–324. doi: 10.1038/nature05944. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.