Summary
Flavin-dependent thymidylate synthases (FDTSs) catalyze the production of 2′-deoxythymidine-5′-monophosphate (dTMP) from 2′-deoxyuridine-5′-monophosphate (dUMP) and N5, N10-methylene-5,6,7,8-tetrahydrofolate (CH2H4folate). In contrast to human and other classical thymidylate synthases, the activity of FDTS depends on a flavin adenine dinucleotide (FAD) coenzyme, and its catalytic mechanism is very different. Several human pathogens rely on this recently discovered enzyme, making it an attractive target for novel antibiotics. Like many other flavoenzymes, FDTS can function as an oxidase, which catalyzes the reduction of oxygen (O2) to hydrogen peroxide (H2O2) using reduced nicotinamide adenine dinucleotide 2′-phosphate (NADPH) or other reducing agents. In the present study we exploit the oxidase activity of FDTS from Thermatoga maritima to probe the binding and release features of the substrates and products during its synthase activity. The results from both steady state and single turnover experiments suggest a sequential kinetic mechanism of substrate binding during FDTS oxidase activity. CH2H4folate competitively inhibits the oxidase activity, which indicates that CH2H4folate and O2 compete for the same reduced and dUMP-activated enzymatic complex (FDTS-FADH2-NADP+-dUMP). These studies imply that the binding of CH2H4folate precedes NADP+ release during FDTS synthase activity. The inhibition constant of CH2H4folate towards the oxidase activity was determined to be rather small (2 μM), which indicates a tight binding of CH2H4folate to the FDTS-FADH2-NADP+-dUMP complex.
Keywords: enzyme kinetics, flavin, thymidylate synthase, oxidase, competitive substrates
Introduction
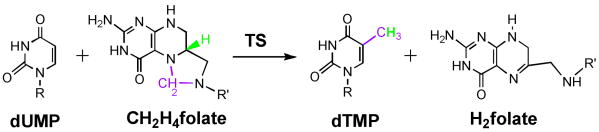
Thymidylate synthases (TSs, encoded by the thyA and tymS genea) catalyze the reductive methylation of 2′-deoxyuridine-5′-monophosphate (dUMP) to form 2′-deoxythymidine-5′-monophosphate (dTMP) in nearly all eukaryotes including humans. This reaction employs N5, N10-methylene-5,6,7,8-tetrahydrofolate (CH2H4folate) as both the methylene and the hydride donor [1], producing 7,8-dihydrofolate (H2folate), as illustrated in Scheme 1. The product, H2folate, is reduced to 5,6,7,8-tetrahydrofolate (H4folate) by dihydrofolate reductase (DHFR, encoded by the folA gene), and then methylenated back to CH2H4folate. Thus, the genomes of thyA-dependent organisms have always been found to contain folA as well, forming a TS-DHFR coupled catalytic cycle that is essential for thymidine biosynthesis.
Scheme 1.
The reaction catalyzed by classical TS. R is 2′-deoxyribose-5′-phosphate and R′ is p-aminobenzoyl-glutamate.
Since 2002, thyX, a new gene that encodes for flavin-dependent thymidylate synthases (FDTSs), has been identified in a number of microorganisms, including some severe human pathogens [2-5]. FDTS is a homotetramer with four identical active sites, each of which is formed at an interface of three of the four subunits [6]. This is quite different from the structure of classical TS, which is a homodimer with one active site per subunit [1]. Recent studies have suggested that the catalytic mechanisms of TS and FDTS also differ substantially [7-10]. Since dTMP is a vital metabolite for DNA biosynthesis, this newly discovered enzyme is a promising target for novel antibiotics that could be designed to selectively inhibit FDTS activity with potentially low toxicity for humans.
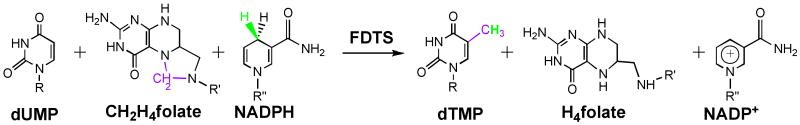
In order to direct future drug design, the molecular mechanism by which FDTS catalyzes thymidylate synthesis must be clarified to reveal the enzyme-substrate complexes and intermediates present along the reaction pathway. In contrast to classical TS, FDTS takes the hydride from reduced nicotinamides or other reductants, while CH2H4folate serves as the methylene donor only and produces H4folate instead of H2folate [2, 4, 5], as illustrated in Scheme 2. This explains the absence of both thyA and folA in the genomes of some thyX-dependent organisms [11]. Preliminary mechanistic studies have demonstrated that the FDTS mechanism is substantially different from the common bi-functional enzymes with both TS and DHFR activities [7-9]. During the reductive half reaction, reduced nicotinamide adenine dinucleotide 2′-phosphate (NADPH) reduces the non-covalently bound flavin adenine dinucleotide (FAD) cofactor to its reduced form (FADH2); during the oxidative half reaction, the enzyme catalyzes the transfer of the methylene group from CH2H4folate to dUMP, while FADH2 serves as the reducing agent to produce dTMP. Several proposed kinetic mechanisms suggested that the product of the reductive half reaction (nicotinamide adenine dinucleotide 2′-phosphate, NADP+) leaves before CH2H4folate binds to the enzyme [7-9]. This putative kinetic mechanism, however, remains to be experimentally tested.
Scheme 2.
The reaction catalyzed by FDTS. R is 2′-deoxyribose-5′-phosphate, R′ is p-aminobenzoyl-glutamate, and R″ is adenine-2′-phosphate-ribose-5′-pyrophosphate-ribose.
Like many other flavoenzymes, FDTS can function as an NADPH oxidase, consuming molecular oxygen (O2) and producing NADP+ and hydrogen peroxide (H2O2). Our recent studies revealed a close connection between the synthase activity (dUMP → dTMP) and oxidase activity (O2 → H2O2) of FDTS [12, 13]. Several aspects of the proposed mechanism, however, have not been experimentally confirmed thus far. In the present paper, we report pre-steady state and steady state studies on the oxidase activity of FDTS from Thermotoga maritima, and elucidated the binding and release features of its synthase substrates NADPH, CH2H4folate, and dUMP.
Results and Discussion
Initial Velocity Studies of FDTS Oxidase Activity
Previous studies suggested that NADPH binds to the FDTS-FAD complex, and that after the flavin is reduced the product of the reductive half reaction, NADP+, dissociates before the initiation of the oxidative half reaction [7-9]. This proposed mechanism was examined by measuring the steady state initial velocities of FDTS oxidase activity while varying NADPH concentrations at several O2 concentrations (8 μM, 20 μM, 50 μM, 210 μM, 1 mM). These experiments were conducted in the presence of saturating concentrations of dUMP, to ensure examination of the dUMP-activated form of the enzyme [12, 13]. The results revealed that in the absence of CH2H4folate, FDTS oxidase activity exhibits Michaelis-Menten kinetics for O2 with an unusually small Km. The apparent Km values of O2 with 100 μM NADPH were determined to be 7 ± 1 μM at 37 °C, and 29 ± 2 μM at 65 °C. This may imply that either the enzyme has a binding site for O2, or, more likely, that an O2 independent step becomes rate limiting as the concentration of O2 increases [14].
The double reciprocal Lineweaver-Burk plot (1/rate vs. 1/[substrate]) of FDTS oxidase activity shows an intersecting pattern (Figure 1), which suggests a sequential kinetic mechanism. If the product NADP+ left the enzymatic complex before O2 binds, these lines would have been parallel (i.e., Ping Pong mechanism [15, 16]). The data were globally fit to a bi-substrate sequential mechanism (eq 1, from Ref. [16]) to estimate the kinetic parameters:
Figure 1. Steady state sequential mechanism of FDTS oxidase activity.
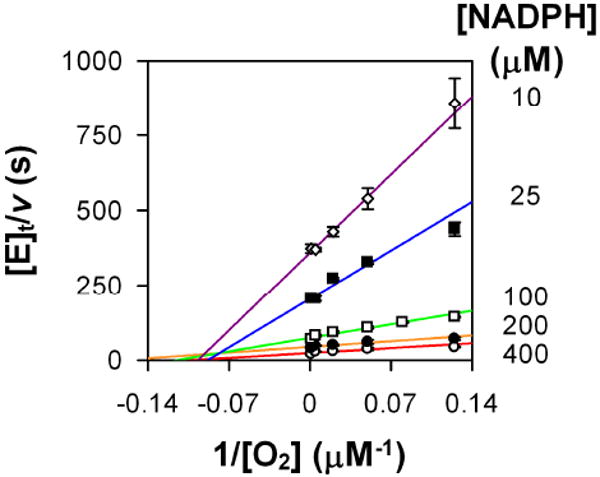
Data are presented as a Lineweaver-Burk double reciprocal plot. Experiments were performed at 37 °C. NADPH concentrations used were (○, red line) 400 μM, (λ, orange line) 200 μM, (□, green line) 100 μM, (ν, blue line) 25 μM, and (◊, purple line) 10 μM.
| (1) |
where [A], [B], and [E]t are the concentrations of NADPH and O2, and total concentration of enzyme active sites, respectively; Ka is the Michaelis constant of NADPH, Kb is the Michaelis constant of O2; and Kia is the dissociation constant of the substrate from the enzymatic complex. The kinetic parameters determined from this global fitting are: kcat = 0.0830 ± 0.0002 s-1, Ka = 522 ± 2 μM, Kia = 3.61 ± 0.06 mM, Kb = 1.12 ± 0.02 μM.
Assessment of the rate of product NADP+ release by examination of the FADH2-NADP+ charge-transfer complex
The progress of the reductive half reaction was monitored by recording UV-Vis spectra continuously in anaerobic single turnover experiments. The decrease of absorbance at 450 nm follows the reduction of enzyme-bound FAD. When NADPH is used as the reducing agent, the spectra also show an increase in the absorbance of a wide absorbance band (550-900 nm) with an isosbestic point at 510 nm (Figure 2, a). This wide band is not observed during FAD reduction by dithionite, and is identified as a charge-transfer complex between the reduced flavin and the oxidized nicotinamide [17-19]. The first-order rate constant of the formation of the charge-transfer complex was determined to be identical to that of FAD reduction (Figure 2, a, Inset), which, together with the isosbestic point, indicates that the two changes occur simultaneously and represent the same process. This observation demonstrates the stability of the enzyme bound FADH2-NADP+ complex that is formed during the reductive half reaction, and suggests a close proximity of the oxidized nicotinamide ring to the reduced isoalloxazine ring. This observation is significant since no other structural information is currently available regarding the binding site of the nicotinamide cofactor.
Figure 2. The kinetics of FADH2-NADP+ charge-transfer complex during the reductive half reaction of FDTS.
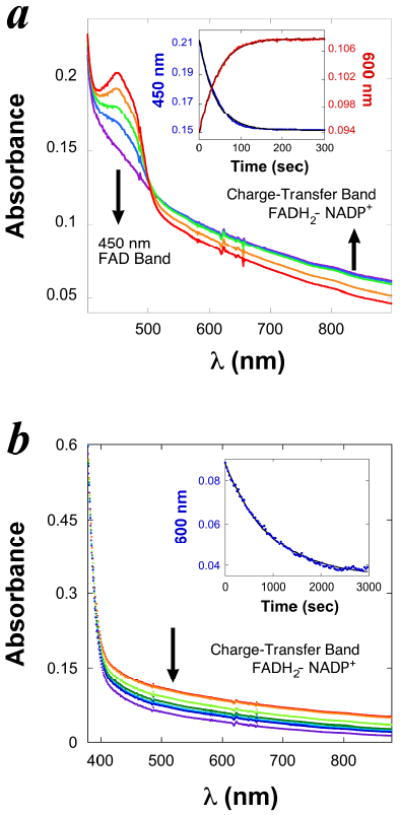
a. Spectra of 10 μM (active site concentration) tmFDTS-FAD being reduced anaerobically by 200 μM NADPH in 200 mM Tris/HCl buffer (pH 7.9) at 37 °C. Each spectrum was sampled at a different time during one single-turnover experiment. The absorbance of FAD (450 nm) decreases as the charge-transfer band of the FADH2-NADP+ complex (550 - 900 nm) increases. Inset: A typical time course of the enzyme-bound FAD reduction by NADPH. The decrease of absorbance at 450 nm is presented as red traces, and the increase of the charge-transfer band from 550 nm to 900 nm is presented as blue traces. Fitting each time course (black curves in the inset) to an exponential equation yields a first-order rate constant, both equal 0.025 ± 0.002 s-1. b. Continuation of the experiments described in panel a., but the first spectrum was recorded after the last one in panel a., and the spectra were recorded in intervals of 30 min. The wide charge-transfer band disappears slowly while the enzyme remains reduced and with no change in total enzyme concentration (as judged from absorbance at 230-300 nm range). The decrease in charge transfer bend is interpreted as NADP+ release. The inset presents the exponential fitting (black curve) of the time course of absorbance change at 600 nm (blue traces). The first-order rate constant of disappearance of the charge-transfer band was determined to be 0.00135 ± 0.00005 s-1.
After the completion of the reductive single turnover experiment, the rate of NADP+ release from the FDTS-FADH2-NADP+-dUMP complex was measured by following the disappearance of the charge-transfer band while no change was observed at 450 nm (Figure 2, b). The rate constant of NADP+ release was determined to be 0.00135 ± 0.00005 s-1 at 37 °C (Figure 2, b, Inset). In comparison, the first order rate constant of FADH2 oxidation by O2 was determined to be 0.131± 0.010 s-1 at 0 °C in the oxidative single turnover experiment. Thus, NADP+ release from the enzyme is at least 2 orders of magnitude slower than FADH2 oxidation by O2, indicating that NADP+ has a very high affinity for the reduced enzyme. This suggests that NADP+ does not leave the FDTS-FADH2-NADP+-dUMP complex at the end of the reductive half reaction, but rather remains bound to the enzymatic complex during the oxidative half reaction. This observation supports the sequential mechanism suggested above from steady state kinetic measurements. Neither the rate of FAD reduction nor that of FADH2-NADP+ formation is dependent on dUMP concentrations, which confirms our previous suggestion that dUMP does not influence the reductive half reaction [13].
Assessment of NADP+ binding to the oxidized enzyme from product inhibition studies
Since NADP+ appears to bind tightly to the reduced enzyme, it is of interest to assess its binding to the oxidized enzyme. Additionally, product inhibition studies can discriminate between the steady state ordered and random mechanisms, which is not easy to do via initial velocity measurements in the absence of products [15, 16]. Therefore, the effect of NADP+ on initial velocities was examined by measuring the steady state initial velocities of FDTS oxidase activity with 100 μM NADPH at both saturating (210 μM) and sub-saturating (10 μM) concentrations of O2. These measurements show no observable inhibition up to the solubility limit of ∼550 mM NADP+ under the experiment conditions. Regardless of the enzymatic complex from which NADP+ dissociates, the lack of any inhibitory effect corroborates the low affinity of NADP+ for the oxidized enzyme, despite its high affinity for the reduced enzyme.
Inhibition of FDTS oxidase activity by CH2H4folate
CH2H4folate appears to inhibit FDTS oxidase activity, and addition of 400 μM CH2H4folate completely suppresses this activity under atmospheric concentration of O2 (210 μM). In order to investigate the nature of inhibition of FDTS oxidase activity by CH2H4folate, steady state initial velocities were measured while varying CH2H4folate concentrations at several O2 concentrations (8 μM, 12.5 μM, 20 μM, 210 μM, 1 mM), in the presence of a saturating concentration of dUMP. The initial velocities under atmospheric concentration of O2 were also studied in the absence of dUMP, and although the rates are slower (in accordance with ref [13]), dUMP does not seem to affect the nature of CH2H4folate inhibition of the oxidase activity. To examine the relation between CH2H4folate and O2, and to ascertain the binding constant of CH2H4folate to the reduced and dUMP-activated enzyme, we used a simplified model in which CH2H4folate is treated as a dead-end inhibitor [15, 16] of the oxidase activity. The Appendix examines and verifies the validity of this simplification.
To determine the inhibition pattern of CH2H4folate toward O2, initial velocities were analyzed by the secondary slope and intercept replots of the Lineweaver-Burk double reciprocal plot (Figure 3, a) [16]. The slope of the double reciprocal plot increases linearly with the concentration of CH2H4folate (Figure 3, b), while the intercept is independent of the concentration of CH2H4folate (Figure 3, c). According to this analysis, CH2H4folate appears to be a competitive inhibitor of O2 in FDTS oxidase activity. The initial velocities were thus fit to the competitive inhibition model to estimate the kinetic parameters [15, 16]:
Figure 3. Competitive inhibition of FDTS oxidase activity by CH2H4folate.
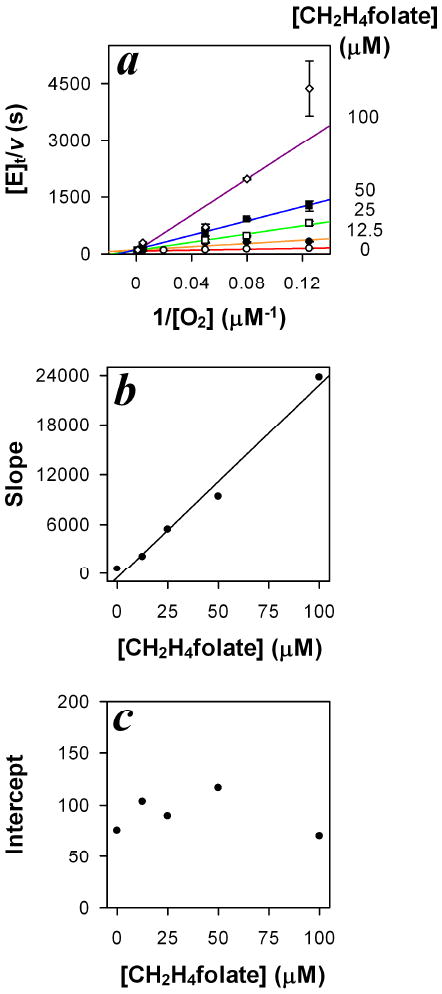
a. The Lineweaver-Burk double reciprocal plot (1/rate vs. 1/[O2]). CH2H4folate concentrations used were (○, red line) 0 μM, (λ, orange line) 12.5 μM, (□, green line) 25 μM, (ν, blue line) 50 μM, and (◊, purple line) 100 μM. b. The secondary slope-replot of the Lineweaver-Burk plot (a), which increases linearly with [CH2H4folate]. c. The secondary intercept-replot of the Lineweaver-Burk plot (a), which is independent of [CH2H4folate]. Experiments were performed at 37 °C.
| (2) |
where kcat is the first-order rate constant, describing the maximal reaction rate per enzyme active site; [S], [I], and [E]t are the concentrations of O2, CH2H4folate, and total concentration of enzyme active sites, respectively; Km is the Michaelis constant of O2, and KI is the inhibition constant of CH2H4folate. The kinetic parameters determined from this fitting were: kcat = 0.0127 ± 0.0004 s-1, Km = 7 ± 1 μM, KI = 1.9 ± 0.3 μM. The inhibition pattern was also analyzed by globally fitting the initial velocities to the mixed-type inhibition model [15], which is a general equation for competitive, non-competitive, or un-competitive inhibition. The results also suggest that the inhibition is best described by the competitive pattern. A detailed analysis is presented in the Appendix. In summary, the observed competitive inhibition of CH2H4folate towards O2 indicates that CH2H4folate and O2 indeed compete for the same enzymatic complex (FDTS-FADH2-NADP+-dUMP).
The sequential binding order of NADPH and O2 in FDTS oxidase activity, together with the competitive inhibition pattern between O2 and CH2H4folate, suggests that the binding order of NADPH and CH2H4folate in FDTS synthase activity is also sequential. This conclusion disagrees with the kinetic schemes proposed in previous studies, in which NADP+ leaves before the oxidation of FADH2 [7-9]. A recent kinetic study on the synthase activity of FDTS from Mycobacterium tuberculosis corroborates our data [20]. The presence of NADP+ in complexes during the oxidative half reaction is important in various attempts to mimic these complexes, which may assist in the design of inhibitors and drugs as well as in the crystallization of the long sought enzymatic complexes with nicotinamide cofactors and/or folate derivatives.
The inhibition constant (KI = 1.9 ± 0.3 μM) obtained from this experiment is a direct measure of the dissociation constant of CH2H4folate from the FDTS-FADH2-NADP+-dUMP-CH2H4folate complex. This measurement affords a good estimate of the binding constant of CH2H4folate to the FDTS-FADH2-NADP+-dUMP complex (1/ KI ≈ 0.5 μM-1), which reflects the high affinity of CH2H4folate for the reduced and dUMP-activated enzyme. This complex seems to be unique to FDTS, therefore such information may assist in rational design of inhibitors and drugs. This is significant because hitherto no specific inhibitors or drugs targeting FDTS have been identified. The current finding may also direct efforts towards crystallization of complexes of FDTS with FADH2, dUMP, NADP+, and folate derivatives under anaerobic conditions. Solving structures with nicotinamide and folate entities would help identify the binding sites of both NADPH and CH2H4folate, and provide important structural information for FDTS studies.
Kinetic scheme
Based on the results presented in this report and in previous studies [7-9, 12, 13], thymidylate synthesis catalyzed by FDTS follows a sequential kinetic mechanism with respect to all its substrates, as illustrated in Scheme 3. The reaction is composed of a reductive half reaction and an oxidative half reaction, and NADP+ only leaves the enzymatic complex after the oxidation of flavin. Since dUMP acts as an activator for the oxidative half reaction, but not for the reductive half reaction [12, 13], we propose that it binds at the beginning of the oxidative half reaction. After dUMP binds to and activates the enzyme, CH2H4folate and O2 compete for the reduced and dUMP-activated enzymatic complex. No direct evidence has been shown so far to support the exact order of product release after the oxidation of FADH2, so Scheme 3 follows a “first come, last leave” principle.
Scheme 3.
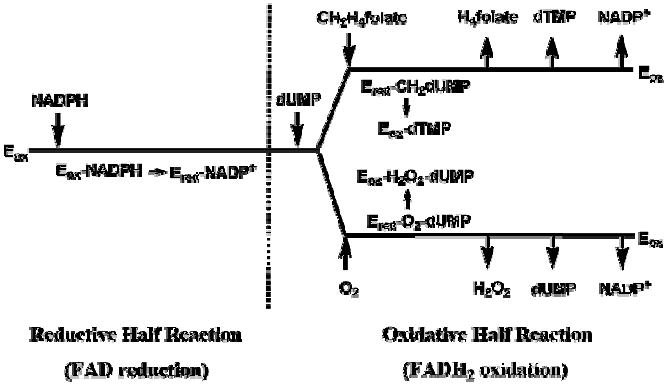
The proposed binding and release kinetic mechanism of FDTS (see text for details). Ered and Eox represent the reduced and the oxidized enzymatic complexes, respectively. Adopted from ref [13]. All the arrows represent reversible process but the formation of dTMP that appears to be irreversible.
Conclusions
The oxidase activity of tmFDTS was exploited to probe several aspects of the kinetic mechanism of FDTS-catalyzed thymidylate synthesis. CH2H4folate and O2 appear to be competitive substrates of FDTS, supporting the notion that both compete for the same reduced form of the enzyme (i.e., the FDTS-FADH2-NADP+-dUMP complex). The binding constant of CH2H4folate to the reduced form of the enzyme is determined to be rather large (1/KI = 0.5 μM), suggesting a tightly bound reactive FDTS-FADH2-NADP+-dUMP-CH2H4folate complex. Binding constants of a substrate to a pre-activated enzyme are usually difficult to measure. We developed a method to assess such a binding constant, by studying an alternative activity of the enzyme where the substrate of interest acts as an inhibitor (or competitive substrate, see Appendix). The high binding affinity of CH2H4folate to the reactive enzymatic complex, and the observation that the oxidase activity of FDTS is faster than the synthase activity, implies that steps following CH2H4folate binding are rate-limiting for the oxidative half reaction of FDTS synthase activity. These results agree with previous observations that the presence of CH2H4folate slows the consumption of NADPH under aerobic conditions [8]. Additionally, the oxidase activity of FDTS calls for caution when studying the synthase activity under aerobic conditions, which has been the case in many previous studies [2, 5, 8, 9, 20, 21]. Aerobic experiments where non-saturating CH2H4folate concentrations were used may need to be revisited, while the results of kinetic measurements with saturating concentrations of CH2H4folae should be valid, as the oxidase activity of the FDTS would be completely suppressed.
In contrast to the suggestions from previous studies [7-9], our data indicate that the product of the reductive half reaction, NADP+, does not leave the enzymatic complex after the reductive half reaction (in accordance with ref [20]). The findings identify a potentially stable complex of reduced FDTS with dUMP, NADP+, and folate derivatives (Scheme 3). The existence of such complexes may lead to new directions in inhibitor and drug design as well as direct attempts to gain structural information of FDTS complexes with folates and nicotinamides. The lack of such information is currently a major obstacle to understanding FDTS in general.
Materials and Methods
Materials
All chemicals were purchased from Sigma-Aldrich, USA, unless otherwise specified. Formaldehyde solution (37.3% by weight) was purchased from Fisher Scientific, USA. CH2H4folate was a generous gift from Eprova Inc., Switzerland. All chemicals were used as purchased without further purification. Thermotoga maritima FDTS (tmFDTS) enzyme was expressed and purified as previously described [6].
Methods
Analytical Methods
A Varian Cary 300 Bio UV-Vis spectrophotometer was used for concentration determinations and steady state kinetic measurements. A Hewlett-Packard 8453 series diode-array UV-Vis spectrophotometer was used in single-turnover experiments. All the measured velocities were normalized by the concentration of enzyme active sites. All the reported concentrations refer to the final reaction mixture. FDTS concentration refers to its active site concentration as determined from 450 nm absorbance of bound FAD (ε450 nm = 11,300 M-1cm-1, [12]). To analyze the data from steady state initial velocity measurements, kinetic parameters were assessed from least-square non-linear regression of the data to the appropriate rate equation with Grafit 5.0. For graphical presentation and further analysis, we used the Lineweaver-Burk double reciprocal plot and secondary replots to further discriminate the kinetic patterns [16].
Steady state Kinetic Measurements
Initial velocities of FDTS oxidase activity were measured with the coupled horseradish peroxidase (HRP, type VIA)/Amplex Red assay, by following the oxidation of Amplex Red by H2O2 as indicated by the increase of absorbance at 575 nm (ε575 nm = 67,000 M-1cm-1) [22]. Experiments were performed at 37 °C in 200 mM tris(hydroxymethyl)aminomethane (Tris)/HCl buffer (pH=7.9), with 100 μM dUMP (to ensure examination of the dUMP-activated enzyme [13]), 50 μM Amplex Red, 1 unit/ml HRP, and 2 μM FDTS. Reactions were initiated by addition of FDTS. The final volume of the reaction mixture was 210 μl. Three different Tris/HCl buffers were prepared: (i) buffer under atmospheric concentration of O2 (210 μM), (ii) buffer under 1 atm of purified argon, ([O2] = 0), and (iii) buffer saturated with O2 (1 atm of pure oxygen, [O2] = 1050 μM). In order to obtain various O2 concentrations, different combinations of these buffers were mixed in preparation of each experiment. Air-tight syringes were used to transfer the solutions under anaerobic conditions controlled by a dual manifold Schlenk line. Control experiments were performed under Ar atmosphere with the same experiment techniques, where no oxidase activity was observed.
The apparent Michaelis constant of O2 at 100 μM NADPH was determined with O2 concentrations ranging from 2 to 990 μM. The binding order of NADPH and O2 was studied by varying the NADPH concentration from 10 to 400 μM over an O2 concentration range of 8 μM - 1 mM. The product inhibition by NADP+ was examined with 100 μM NADPH at both saturating (210 μM) and sub-saturating (10 μM) O2 concentrations. NADP+ concentrations ranged from 0 to its solubility limit (∼550 mM) in 200 mM Tris/HCl buffer (pH=7.9) at 37 °C. The inhibition of FDTS oxidase activity by CH2H4folate was studied by varying the CH2H4folate concentration from 0 to 100 μM over an O2 concentration range of 8 μM - 1 mM. This inhibition study was conducted in the presence of fixed concentrations of NADPH (100 μM) and formaldehyde (10 mM, to stabilize CH2H4folate).
FADH2 oxidation by O2
Single-turnover experiments of the oxidative half reaction were conducted to examine the oxidation of the enzyme bound FADH2 by O2. Experiments were performed at 0 °C in 200 mM Tris/HCl buffer (pH=7.9) with a dUMP concentration range of 0-1 mM. 10 μM FDTS-bound FAD was first reduced to FADH2 by titrating with one equivalent of sodium dithionite [23] under anaerobic conditions. The anaerobic conditions were controlled by the Schlenk line. Reactions were then initiated by addition of O2-containing buffer ([O2]=14 μM in the final reaction mixture). The final volume of the reaction mixture was 300 μl. FADH2 oxidation was followed by increase of absorbance at 450 nm (ε450 nm=11,300 M-1cm-1, [12]). Data from each time course were fit to an exponential equation to obtain the rate constant for this reaction.
Formation of the FADH2-NADP+ charge-transfer complex
In order to examine the formation of the FADH2-NADP+ charge-transfer complex, single-turnover experiments were conducted on the reductive half reaction under anaerobic conditions (Ar) maintained by a glucose/glucose oxidase (type X) O2-consuming system [13]. Experiments were performed at 37 °C in 200 mM Tris/HCl buffer (pH=7.9) with 10 mM glucose, 100 units/ml glucose oxidase, 200 μM NADPH, and 10 μM FDTS, at various concentrations of dUMP (0-200 μM). Reactions were initiated by addition of NADPH stock solution. The final volume of the reaction mixture was 300 μl. The reduction of FAD and the formation of the FADH2-NADP+ complex were followed by changes in the absorbance at 450 nm [12] and in the charge-transfer band from 550 to 900 nm [17-19], respectively. Data from each time course were fit to an exponential equation to obtain the rate constant for this process.
Acknowledgments
This work was supported by NIH R01 GM065368 and NSF CHE- 0715448 to AK, and the Iowa Center for Biocatalysis and Bioprocessing Predoctoral Fellowships to ZW and EMK. The authors are grateful to Bryce Plapp, Daniel Quinn, and Judith Klinman for insightful discussions regarding this work.
Abbreviations
- FDTS
flavin-dependent thymidylate synthase
- TS
Thymidylate synthase
- DHFR
dihydrofolate reductase
- FAD
flavin adenine dinucleotide (oxidized form)
- FADH2
flavin adenine dinucleotide (reduced form)
- dUMP
2′-deoxyuridine-5′-monophosphate
- dTMP
2′-deoxythymidine-5′-monophosphate
- NADPH
nicotinamide adenine dinucleotide 2′-phosphate (reduced form)
- NADP+
nicotinamide adenine dinucleotide 2′-phosphate (oxidized form)
- CH2H4folate
N5, N10-methylene-5,6,7,8-tetrahydrofolate
- H2folate
7,8-dihydrofolate
- H4folate
5,6,7,8-tetrahydrofolate
- HRP
horseradish peroxidase
- Tris
tris(hydroxymethyl)aminomethane
- H2O2
hydrogen peroxide
Appendix
This appendix presents the details of two analytical procedures we employed: (i) Determination of the inhibition pattern of CH2H4folate, which is treated as a dead-end inhibitor for FDTS oxidase activity; and (ii) Examination and validation of the assumption that using the inhibition constant from item i leads to direct assessment of the binding constant of CH2H4folate to the reduced and dUMP-activated enzymatic complex.
(i) Analysis of the inhibition pattern of FDTS oxidase activity by CH2H4folate
The traditional analysis to determine the inhibition pattern [16] has been shown in the Results and Discussion section. Here we present an alternative way to analyze the same data. The inhibition pattern of CH2H4folate is examined by fitting the steady state initial velocities to the mixed-type inhibition model (eq 3). As presented below, this general model can distinguish between various patterns of dead-end inhibition with a single substrate and a single inhibitor:
| (3) |
where kcat is the first-order rate constant of the reaction when [S] approaches infinity and [I] approaches zero; [S], [I], and [E]t are the concentrations of O2, CH2H4folate, and total concentration of enzyme active sites, respectively; Km is the Michaelis constant of O2; and KI is the inhibition constant of CH2H4folate. The coefficient α is the ratio between the dissociation constants of the inhibitor from the enzyme (EI) and from the enzyme-substrate complex (ESI), which reflects the difference in the inhibitor's affinities for these two different enzymatic complexes. The magnitude of α discriminates between various types of inhibition [15]: when α ≪ 1, the inhibition is un-competitive; when α ∼ 1, it is non-competitive; and when α ≫ 1, it is competitive. Fitting our data to eq 3 yields a value for α that is much larger than unity α = 101 ± 46, Table 1), thus the second term of the denominator approaches [S], and the mixed inhibition model (eq 3) is reduced to the competitive inhibition model (eq 2).
Table 1. The kinetic parameters determined from the fittings of data for the inhibition of FDTS oxidase activity by CH2H4folate to competitive and mixed-type inhibition (Eq. 2 and Eq. 3, respectively).
The F-test (a statistical test of validity of going from a complicated model to a simpler one, [24]) suggests that the mixed-type inhibition (eq 3) does not provide a statistically better fitting than the competitive inhibition (eq 2). Furthermore, kinetic parameters for both fittings were determined to be identical within experimental error (Table 1). In accordance with the linearized analysis presented in the main text, the current analysis indicates that the inhibition of FDTS oxidase activity by CH2H4folate is best described by a competitive pattern.
(ii) Estimating the binding constant of CH2H4folate to the reduced and dUMP-activated enzymatic complex from its apparent inhibition constant
The analysis of data from the inhibition study of FDTS oxidase activity with CH2H4folate, presented above, treated CH2H4folate as a dead-end inhibitor. Yet, when the reduced complex is activated by dUMP [13], CH2H4folate is actually an alternative substrate competing with O2. This part of the appendix examines the validity of treating CH2H4folate as a dead-end inhibitor to assess its binding constant to the reactive enzymatic complex.
The initial velocity of the oxidase activity, in the presence of CH2H4folate, can be best described by the equation for a bi-substrate system with an alternative second substrate [15]:
| (4) |
where [A], [B], [I] and [E]t are the concentrations of NADPH, O2, and CH2H4folate, and total concentration of enzyme active sites, respectively; KmA, KmB, and KmI are the Michaelis constants of NADPH, O2, and CH2H4folate, respectively; Kia is the dissociation constant of NADPH from the FDTS-FAD-NADPH-dUMP complex, and Kii is the dissociation constant of CH2H4folate from the FDTS-FADH2-NADP+-dUMP-CH2H4folate complex (i.e., the reciprocal of its binding constant to the reactive FDTS-FADH2-NADP+-dUMP complex). Eq 5 can be derived from eq 4:
| (5) |
Eq 5 has the same form as eq 2, where
| (6) |
| (7) |
| (8) |
Therefore, with a fixed concentration of substrate A (NADPH), fitting our data to eq 2 provides the estimated values for the parameters , , and . To test whether , which is KI in eq 2, can represent Kii, which is the dissociation constant of CH2H4folate from the reduced enzymatic complex, eq 8 is transformed to eq 9:
| (9) |
Under the conditions of our experiments ([NADPH] = 100 μM), , and , so . Eq 9 is therefore reduced to eq 10:
| (10) |
Thus, the apparent value for CH2H4folate determined in the inhibition study is a reasonable estimate of the dissociation constant (Kii) of CH2H4folate from the FDTS-FADH2-NADP+-dUMP-CH2H4folate complex.
Footnotes
Enzymes: FDTS, flavin-dependent thymidylate synthase, EC 2.1.1.148; TS, Thymidylate synthase, EC 2.1.1.45; DHFR, dihydrofolate reductase, EC 1.5.1.3; HRP, horseradish peroxidase, EC 1.11.1.7.
Subdivision: Enzymology
The gene that encodes TS (EC 2.1.1.45) in mouse, rat, and human is currently named tymS rather than thyA.
References
- 1.Carreras CW, Santi DV. The catalytic mechanism and structure of thymidylate synthase. Annu Rev Biochem. 1995;64:721–762. doi: 10.1146/annurev.bi.64.070195.003445. [DOI] [PubMed] [Google Scholar]
- 2.Myllykallio H, Lipowski G, Leduc D, Filee J, Forterre P, Liebl U. An alternative flavin-dependent mechanism for thymidylate synthesis. Science. 2002;297:105–107. doi: 10.1126/science.1072113. [DOI] [PubMed] [Google Scholar]
- 3.Murzin AG. Biochemistry: DNA building block reinvented. Science. 2002;297:61–62. doi: 10.1126/science.1073910. [DOI] [PubMed] [Google Scholar]
- 4.Mathews II, Deacon AM, Canaves JM, McMullan D, Lesley SA, Agarwalla S, Kuhn P. Functional analysis of substrate and cofactor complex structures of a thymidylate synthase-complementing protein. Structure. 2003;11:677–690. doi: 10.1016/s0969-2126(03)00097-2. [DOI] [PubMed] [Google Scholar]
- 5.Leduc D, Graziani S, Meslet-Cladiere L, Sodolescu A, Liebl U, Myllykallio H. Two distinct pathways for thymidylate (dTMP) synthesis in (hyper)thermophilic Bacteria and Archaea. Biochem Soc Trans. 2004;32:231–235. doi: 10.1042/bst0320231. [DOI] [PubMed] [Google Scholar]
- 6.Kuhn P, Lesley SA, Mathews II, Canaves JM, Brinen LS, Dai X, Deacon AM, Elsliger MA, Eshaghi S, Floyd R, et al. Crystal structure of thy1, a thymidylate synthase complementing protein from Thermotoga maritima at 2.25 A resolution. Proteins: Struct, Funct, Genet. 2002;49:142–145. doi: 10.1002/prot.10202. [DOI] [PubMed] [Google Scholar]
- 7.Agrawal N, Lesley SA, Kuhn P, Kohen A. Mechanistic studies of a flavin-dependent thymidylate synthase. Biochemistry. 2004;43:10295–10301. doi: 10.1021/bi0490439. [DOI] [PubMed] [Google Scholar]
- 8.Graziani S, Bernauer J, Skouloubris S, Graille M, Zhou CZ, Marchand C, Decottignies P, van Tilbeurgh H, Myllykallio H, Liebl U. Catalytic mechanism and structure of viral flavin-dependent thymidylate synthase ThyX. J Biol Chem. 2006;281:24048–24057. doi: 10.1074/jbc.M600745200. [DOI] [PubMed] [Google Scholar]
- 9.Griffin J, Roshick C, Iliffe-Lee E, McClarty G. Catalytic mechanism of Chlamydia trachomatis flavin-dependent thymidylate synthase. J Biol Chem. 2005;280:5456–5467. doi: 10.1074/jbc.M412415200. [DOI] [PubMed] [Google Scholar]
- 10.Koehn EM, Fleischmann T, Conrad JA, Palfey BA, Lesley SA, Mathews II, Kohen A. A novel mechanism of thymidylate biosynthesis in organisms containing the thyX gene. Nature. 2009 doi: 10.1038/nature07973. Accepted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Myllykallio H, Leduc D, Filee J, Liebl U. Life without dihydrofolate reductase FolA. Trends Microbiol. 2003;11:220–223. doi: 10.1016/s0966-842x(03)00101-x. [DOI] [PubMed] [Google Scholar]
- 12.Mason A, Agrawal N, Washington MT, Lesley SA, Kohen A. A lag-phase in the reduction of flavin dependent thymidylate synthase (FDTS) revealed a mechanistic missing link. Chem Commun. 2006:1781–1783. doi: 10.1039/b517881a. [DOI] [PubMed] [Google Scholar]
- 13.Chernyshev A, Fleischmann T, Koehn EM, Lesley SA, Kohen A. The relationships between oxidase and synthase activities of flavin dependent thymidylate synthase (FDTS) Chem Commun. 2007:2861–2863. doi: 10.1039/b700977a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mattevi A. To be or not to be an oxidase: challenging the oxygen reactivity of flavoenzymes. Trends Biochem Sci. 2006;31:276–283. doi: 10.1016/j.tibs.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 15.Segel IH. Enzyme kinetics: behavior and analysis of rapid equilibrium and steady state enzyme systems. Wiley; New York: 1975. [Google Scholar]
- 16.Cook PF, Cleland WW. Enzyme kinetics and mechanism. Taylor & Francis Group; New York: 2007. [Google Scholar]
- 17.Blankenhorn G. Flavin-nicotinamide biscoenzymes: models for the interaction between NADH (NADPH) and flavin in flavoenzymes. Reaction rates and physicochemical properties of intermediate species. Eur J Biochem. 1975;50:351–356. doi: 10.1111/j.1432-1033.1975.tb09810.x. [DOI] [PubMed] [Google Scholar]
- 18.Massey V, Ghisla S. Role of Charge-Transfer Interactions in Flavoprotein Catalysis. Ann N Y Acad Sci. 1974;227:446–465. [Google Scholar]
- 19.Filisetti L, Valton J, Fontecave M, Niviere V. The flavin reductase ActVB from Streptomyces coelicolor: characterization of the electron transferase activity of the flavoprotein form. FEBS Lett. 2005;579:2817–2820. doi: 10.1016/j.febslet.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 20.Hunter JH, Gujjar R, Pang CK, Rathod PK. Kinetics and ligand-binding preferences of Mycobacterium tuberculosis thymidylate synthases, ThyA and ThyX. PLoS ONE. 2008;3:e2237. doi: 10.1371/journal.pone.0002237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ulmer JE, Boum Y, Thouvenel CD, Myllykallio H, Sibley CH. Functional analysis of the Mycobacterium tuberculosis FAD-dependent thymidylate synthase, ThyX, reveals new amino acid residues contributing to an extended ThyX motif. J Bacteriol. 2008;190:2056–2064. doi: 10.1128/JB.01094-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou M, Diwu Z, Panchuk-Voloshina N, Haugland RP. A stable nonfluorescent derivative of resorufin for the fluorometric determination of trace hydrogen peroxide: applications in detecting the activity of phagocyte NADPH oxidase and other oxidases. Anal Biochem. 1997;253:162–168. doi: 10.1006/abio.1997.2391. [DOI] [PubMed] [Google Scholar]
- 23.Gattis SG, Palfey BA. Direct observation of the participation of flavin in product formation by thyX-encoded thymidylate synthase. J Am Chem Soc. 2005;127:832–833. doi: 10.1021/ja0432214. [DOI] [PubMed] [Google Scholar]
- 24.Motulsky H, Christopoulos A. Fitting models to biological data using linear and nonlinear regression: a practical guide to curve fitting. Oxford University Press, Oxford; New York: 2004. [Google Scholar]