Abstract
FM1-43, a fluorescent styryl dye that penetrates into and stains membranes, was used to investigate kinetics of constitutive endocytosis and to visualize the fate of endocytic organelles in resting and activated human T lymphocytes. The rate of dye accumulation was strongly temperature dependent and ~10-fold higher in activated than in resting T cells. Elevation of cytosolic free Ca2+ concentration with thapsigargin or ionomycin further accelerated the rate of FM1-43 accumulation associated with cytosolic actin polymerization. Direct modulation of actin polymerization affected membrane trafficking. Actin condensation beneath the plasma membrane with calyculin A abolished FM1-43 internalization, whereas actin depolymerization with cytochalasin D had no effect. Photoconversion of DAB by FM1-43 revealed altered endocytic compartment targeting associated with T cell activation. Internalized cargo was carried to lysosome-like compartments in resting T cells and to multivesicular bodies (MVB) in activated T cells. Externalization of exosomes from MVB occurred commonly in activated but not in resting T cells. T cell exosomes contained raft-associated CD3 proteins, GM1 glycosphingolipids, and phosphatidylserine at the outer membrane leaflet. The present study demonstrates the utility of FM1-43 as a marker of membrane trafficking in T cells and reveals possible mechanisms of its modulation during T cell activation.
Keywords: Endocytosis, Endosomes, Exosomes, Calcium, Photoconversion, Cytoskeleton
Introduction
Membrane trafficking plays an important role in regulating the surface expression of membrane proteins in T lymphocytes and provides an important mechanism to modulate immune responses. For example, T cell receptors (TCR) undergo continuous recycling [1–3], resulting in a steady-state level of receptor expression at the plasma membrane (PM). Receptor ligation by antigen/MHC complexes reduces TCR expression on the T cell surface [4–7], either by preventing recycling [2] or by shifting the equilibrium between recycling/degradation pathways in favor of lysosomal degradation [7,8]. Long-lasting down-regulation of TCR expression is thought to be critical for termination of the immune response [9,10]. Some viruses, including HIV, alter receptor internalization/recycling mechanisms and shift the equilibrium in favor of receptor degradation to evade immune surveillance [11].
Despite the importance of constitutive membrane trafficking, the origin and fate of endosomal compartments have not been studied extensively in T lymphocytes, and kinetics of endocytosis and mechanisms of its regulation in T cells remain undefined. Lymphocyte activation by antigen/MHC induces T cell proliferation, lymphokine secretion, and changes in expression of membrane proteins. However, effects of lymphocyte activation on membrane trafficking remain unclear. Several biochemically distinct compartments for membrane uptake and turnover have been identified in other cell types, including primary endocytic vesicles, early endosomes (EE), late endosomes (LE), and lysosomes [12]. Hematopoietic cells possess LE that may serve as a secretory compartment [13]. Vesiculated LE, also known as multivesicular bodies (MVB), can fuse with the plasma membrane to release vesicles, termed exosomes [14–16]. Selected membrane and cytosolic proteins are released with exosomes into the extracellular space. Exosomes produced by dendritic cells enhance antigen presentation and have been shown to induce potent antitumor immune responses in mice [17]. Human PHA/TCR-activated T cells, T cell clones, and Jurkat T cells have been found to release microvesicles that contain MHC II molecules and several other PM and endosomal proteins into the culture medium [14,18,19], suggesting that externalization of exosomes may also occur in T lymphocytes. Difficulties in elucidating the mechanisms of forming and releasing vesicle-associated proteins have been compounded in T cells because the fate of endocytic vesicles and the dynamics of transport intermediates remain uncertain. Structural relationships among endocytic vesicles, intracellular compartments, and externalized material have not been established.
To address the issue of endocytic compartment biogenesis in T cells it is necessary to elucidate the dynamic interactions between membrane compartments. Substantial insight into the dynamics of synaptic vesicle cycling has been made using the styryl dye FM1-43 [20,21]. FM1-43 is an amphipathic molecule that intercalates spontaneously into the outer leaflet of cell membranes without diffusing across the membrane. Within the lipid environment, FM1-43 exhibits 50- to 100-fold increased fluorescence intensity than the dye in aqueous solution. FM1-43 can be internalized by vesicular endocytosis, and an increase in intracellular fluorescence can therefore be used as an index of endocytosis. Moreover, intense illumination of FM1-43 in fixed specimens in the presence of oxygen and diaminobenzidine (DAB) catalyzes the polymerization of DAB into an insoluble osmiophilic reaction product that can be visualized by electron microscopy [22]. The photoconversion procedure allows for identification of endocytic compartments in living cells by real-time fluorescence monitoring, followed by a snapshot with EM resolution, thereby permitting correlation of fluorescence measurements with the ultrastructure [23]. Here, we introduce the use of the FM1-43 to investigate real-time membrane trafficking dynamics and mechanisms of its regulation in human T lymphocytes. Photoconversion of FM1-43 allows definition of structural intermediates of endocytic compartments, tracked from the PM to early and late endosomes and back to the PM with EM resolution.
Materials and methods
Cell culture and chemicals
Unless otherwise indicated, all chemicals were from Sigma (St. Louis, MO). Normal human peripheral blood mononuclear cells were isolated using Accuspin System-Histoplaque-1077 density gradient separation tubes. CD3+ cells were purified using human T cell enrichment columns (R&D Systems, Minneapolis, MN) and cultured in media containing 88% RPMI (Fisher Scientific, Pittsburgh, PA), 1% MEM, 1% Na-pyruvate, 1% 1-glutamine, and 0.035 μL/L β-mercaptoethanol supplemented with 10% FCS (Omega Scientific, Tarzana, CA). Unless otherwise indicated, T cells were stimulated in vitro with phytohemagglutinin P (PHA, 40 μg/ml, Difco, Detroit, MI) for 48–96 h to activate T cell proliferation and lymphokine secretion. In some experiments, T cells were activated with ionomycin (200 nM) + PMA (10 nM). Cells were placed on a poly-L-lysine-coated coverslip 10 min before the experiment. Apoptosis/necrosis of adherent cells was assessed by annexin V and propidium iodide staining (Vibrant Apoptosis Assay kit #2, Molecular Probes, Eugene, OR). Ionomycin, thapsigargin Calyculin A, and Cytochalasin D were from Calbiochem (La Jolla, CA). FM1-43 and FM4-64 (Molecular Probes, Eugene, OR) were used at 5 μM final concentration in Tyrode solution.
Fluorescence measurements
A coverslip with adherent cells was mounted onto the recording chamber on the stage of a Zeiss Axiovert 10 microscope. Cells were continuously superfused with external solutions of different composition. Most experiments were done in modified Tyrode solution containing (in mM) 160 NaCl, 5.6 KCl, 1 MgCl2, 2 CaCl2, 5 Hepes, 10 glucose, pH = 7.3 adjusted with NaOH. For some experiments, Ca2+ and Mg2+ were omitted from the solution and 5 mM EGTA was added (Ca2+-free Tyrode solution). Fluorescence images were acquired with a Biorad MRC 600 laser scanning confocal imaging system using a 63x/1.25 n.a. oil immersion Zeiss objective. For FM1-43 and Alexa Fluor 488-conjugated annexin V and choleratoxin B (Molecular Probes), 488 nm excitation and 515 nm emission filters were used. For FM4-64 fluorescence, 514 nm excitation and 600 nm emission filters were used. The pinhole was opened to 20% of its maximal diameter. For kinetic analysis all images were taken at identical acquisition settings. Temperature control was achieved by heated/cooled water circulation around the objective, and by continuous superfusion of the recording chamber with solution of the desired temperature. Perfusion solutions were delivered through a temperature-controlled multitube pre-heater (Cell Micro Controls, Virginia Beach, VA) placed ~100 μm from the field of view.
Kinetic analysis of FM1-43 accumulation
Off-line analysis was performed with Scion Image software (Scion Corporation, Frederick, MD). The total fluorescence intensity was measured in unprocessed images from the manually defined area within each individual cell (shown in Fig. 1). Plasma membrane and bright peripheral fluorescent patches stained immediately after dye application were excluded from consideration. Total fluorescence intensity was normalized to the area and plotted against time. The value obtained from the first image acquired 30 s after application of FM1-43 was taken as a background fluorescence and was subtracted from all measurements. The rate constants (r) were estimated by fitting the experimentally derived time courses of FM1-43 accumulation with the first-order exponential function: [FM1-43]in(t) = [FM1-43]max × (1−e−rt), where [FM1-43]in(t) is a mean fluorescence intensity at a given time; [FM1-43]max is a mean fluorescence intensity of maximally loaded dye; r is the rate constant; and t is the time. Given the assumptions that dye did not change fluorescence intensity within the endocytic compartment and that dye did not recycle back to the extracellular space to a significant degree during the short incubation period, the r value approximates the rate of constitutive endocytosis. Because the cytosolic region is enlarged in activated T cells it is reasonable to expect that a larger area within the plane of focus could account for some of the increase in fluorescence intensity. From thin-section electron microscope images, the ratio of nucleus to total cell diameter was 0.7 ± 0.02 and 0.63 ± 0.02 (n = 16) for resting and activated cells, respectively. From this, we estimate that the increase in cytosolic area (outside of the nucleus) in activated compared to resting T cells would account for a 20%–25% increase in mean fluorescence intensity measured from confocal planes, but cannot account for the observed 10-fold increase. The relative change in the rate constants for a 10 °C change in temperature, the Q10, was calculated by: Q10 = r2/r1)10/(T2−T1), where, r1 is the rate constant of FM1-43 accumulation at lower temperature T1 and r2 is the rate constant of FM1-43 accumulation at higher temperature T2. Arrhenius activation energies were determined from: Ea = (RT1T2/(T2−T1))×ln(r2/r1), where R is the gas constant and T1and T2 are temperatures in degrees Kelvin. In practice, experimental data were transferred into plots and Q10 and Ea determined from the slopes of linear regression fits of experimental data (Microcal Origin 6.0, Microcal Software, Inc., Northampton, MA). All plots represent mean value ± s.e.m.
Fig. 1.
FM1-43 accumulation in resting and activated T cells is temperature dependent. T cells were exposed to 5 μM FM1-43 in Tyrode solution for 21 min while confocal images were acquired every 7 min. Resting (A–F) and activated (G–O) T cells exposed to FM1-43 for 30 s (top panels), 21 min (middle panels), and following 1 min wash (lower panels) at temperatures indicated above each column. Note the lack of nuclear staining and dye accumulation within the cytosol after washout. Scale bars are 10 μm. (P) Mean fluorescence intensity in resting and activated T cells plotted against time (left and middle panels). Intracellular fluorescence for each cell was measured within a circled area to exclude membrane fluorescence and then divided by the total measurement area to yield the mean fluorescence intensity. Bright peripheral fluorescence patches were excluded from analysis. Each time course is an average of four experiments from different cultures. Smooth lines are exponential fits of experimental data. At 34 °C, the rates of endocytosis were 0.07 min−1 in activated T cells, compared to 0.008 min−1 in resting T cells. Arrhenius plot (right panel) of rate constants (r) of FM1-43 accumulation in activated T cells. T is the absolute temperature. The activation energies (Ea) were calculated from the slopes of linear regression fits within the temperature ranges of 14–24 °C and 24–34 °C. If not shown, the error bars are smaller than the symbol size.
Photoconversion of DAB and EM imaging
Photoconversion experiments were performed as described [24]. Briefly, living cells were loaded with FM1-43 for different times and conditions as specified in Results. Cells were washed and then fixed with 2% glutaraldehyde (Electron Microscopy Sciences, Ft. Washington, PA) in sodium cacodylate buffer (0.1 M, pH 7.4, Ted Pella Inc., Redding, CA) for 20 min, rinsed in buffer, and treated for 5 min in KCn (20 mM), aminotriazole (5 mM), glycine in buffer (50 mM) to reduce nonspecific background. For photoconversion, diaminobenzidine (DAB, 1 mg/ml) in oxygenated sodium cacodylate (0.1 M) was added to the chamber. DAB solution was refreshed every 3 min and cells were illuminated by a 75 W xenon lamp through a Zeiss 63x/1.25 n.a. objective using a 488 nm filter for 10 to 15 min until a brownish reaction product appeared in place of the green fluorescence. Cells were then washed in buffer and postfixed in 1% osmium tetroxide (Electron Microscopy Sciences) for 30 min. Cells were rinsed in distilled water, dehydrated in ethanol, embedded in Durcupan resin, and polymerized at 60 °C for 48 h. To distinguish any nonspecific reaction between intracellular components and DAB, we performed photoconversion of DAB in cells that were never exposed to FM1-43. Except for mitochondria, no nonspecific staining of internal organelles was induced by illumination in the absence of FM1-43 even after 1.5 h exposure to light. The nonspecific staining of the mitochondria was easily recognizable and was excluded from consideration. Photoconversion of cells bathed with FM1-43-containing solution for ~30 s before fixation revealed a thin ring of electron-dense reaction product at the PM due to dye partitioning into the lipid bilayer. All other images presented in the results were taken from cells that were fixed after FM1-43 was briefly washed from the extracellular solution and therefore contained only remaining traces of the photoconversion reaction product at the PM. Morphometric measurement of intracellular organelles was performed in thin section images using Scion Image software.
Immunohistochemistry
Adherent cells were fixed with 4% paraformaldelhyde in 2XPBS (Fisher Scientific, Pittsburgh, PA) for 30 min, then washed three times with 2X PBS for 15 min. Cells were permeablilized in a saponin solution (0.075% w/v saponin, 1% bovine serum albumin, 1% goat serum in 2X PBS) for 1 h. For F-actin staining, permeablilized cells were incubated with 2 U/ml Oregon Green-conjugated phalloidin (Molecular Probes) in 2X PBS for 20 min at room temperature, washed three times with 2X PBS for 15 min, and then mounted on slides with Slow Fade glycerol buffer (Molecular Probes). For TCR staining, permeablilized cells were incubated with primary mouse anti-human CD3ε antibodies (UCHT1, BD Biosciences Pharmingen, San Diego, CA) diluted 1:500 for 45 min at room temperature, washed three times with 2X PBS containing 2% of goat serum, and then incubated with rabbit anti-mouse Alexa Fluor 488 secondary antibodies (Molecular Probes) for 30 min at room temperature. Cells were washed three times with 2X PBS for 15 min and then mounted on slides. For IL2 staining, primary rat anti-human IL2 antibodies (BD Biosciences Pharmingen, San Diego, CA) were used as primary, and chicken anti-rat Alexa Fluor 488 antibodies (Molecular Probes) were used as secondary. Only background fluorescence was detected when permeabilized cells were stained with secondary antibodies only. For the IL2 assay, cells were incubated with Golgi Stop solution (BD Biosciences, Franklin Lakes, NJ) for 6 h before fixation.
Assessment of mitochondrial transmembrane potential (Δψm)
Δψm was measured using 3,3′-dihexyloxacarbocynine iodide (DiOC6(3), Molecular Probes) at a final concentration of 40 nM in PBS (stock 1 mM in methanol). Adherent cells were incubated with DiOC6(3) for 15 min at 37 °C, followed by confocal imaging analysis (excitation 488 nm; emission 515 nm). As a positive control, the mitochondrial uncoupling agent carbonyl cyanide m-chlorophenyl-hydrazone (CCCP) was applied for 10 min to the same cells at room temperature, or apoptosis was induced by incubation of cells with 1 μM staurosporine (Calbiochem, San Diego, CA) for 6 h at 37 °C.
Results
Accelerated endocytosis in activated T cells
To visualize endocytosis in resting and activated human T lymphocytes, we exposed cells to FM1-43 for varying times and monitored fluorescence by confocal microscopy. FM1-43 rapidly stained the plasma membrane (PM) of both resting and PHA-activated T cells as a result of dye partitioning into the lipid bilayer (Fig. 1 top panels). In resting T cells, the ring of staining indicated a uniform distribution of dye within the PM. In activated T cells, brightly fluorescent patches averaging 2.4 × 1.4 μm in size (n = 271) appeared at the cell periphery immediately after addition of FM1-43 to the extracellular solution, simultaneously with PM staining (Fig. 1G, J, and M). Surface membrane staining and peripheral patches of FM1-43 fluorescence did not change after several minutes of exposure to FM1-43 (Fig. 1, middle panels). Upon washout of FM1-43, PM staining and brightness of the peripheral patches was substantially reduced, but peripheral patches remained visible for several minutes (Fig. 1, lower panels). The bright peripheral patches, found only in activated T cells, consisted of clusters of external vesicles adherent to the PM, as shown below by electron microscopy (EM). After several minutes of exposure, in addition to staining the cell surface, FM1-43 accumulated inside the cell but was excluded from the nucleus. After washout, dye was retained within the cells (Fig. 1, lower panels). Intracellular accumulation was especially apparent in activated T cells at or above room temperature. Similar results were obtained with T cells activated by ionomycin + phorbol myristate acetate (PMA, data not shown).
The accumulation of dye within T cells may reflect constitutive endocytosis. Consistent with this mechanism, we found that rates of dye uptake were strongly temperature dependent. In resting T cells, intracellular FM1-43 accumulation was not observed after 20 min of incubation at 24 °C (Fig. 1A–C), but patches of FM1-43 fluorescence appeared within the cytosol at 34 °C (Fig. 1D–F). Activated T cells accumulated substantially more dye than resting T cells at 24 °C (Fig. 1J–L), and dye accumulation was strongly enhanced at 34 °C (Fig. 1M–O) but completely blocked at 14 °C (Fig. 1G–I). To assess endocytosis quantitatively, we fitted the time course of intracellular FM1-43 fluorescence accumulation in resting and activated T cells at different temperatures with first-order exponential functions (Fig. 1P). The rate constant (r) of dye uptake was strongly dependent upon temperature and was approximately one order of magnitude larger at any given temperature in activated T cells, consistent with a strong enhancement of the rate of endocytosis, compared to resting T cells (Fig. 1P, left and middle panels). An Arrhenius plot of the temperature dependence of r in activated T cells (Fig. 1P, right panel) deviates from a straight line with an inflection point in the vicinity of 24 °C, indicating that the temperature dependence is stronger at lower temperatures. The energies of activation (Ea) calculated from the slopes of linear regression fits are 153 kJ/mol (Q10 = 7.8) and 76 kJ/mol (Q10 = 3.9) in the lower (14–24 °C) and high (24–34 °C) temperature ranges, respectively. The nonlinear Arrhenius plot may indicate that lipids undergo a phase transition [25] and that membrane fluidity is a rate-limiting factor for constitutive endocytosis in T cells.
Rate of endocytosis increased by elevated [Ca2+]i
Ca2+ plays an important role in shaping T cell responses [26]. In addition, Ca2+ regulates endocytosis in neuronal cells and hematopoeitic cells [27,28]. Therefore, we explored the effects of Ca2+ on rates of FM 1-43 dye accumulation, using either ionomycin, a Ca2+ ionophore, or thapsigargin, an inhibitor of the SERCA pump Ca2+-ATPase, to elevate cytosolic free Ca2+ concentration ([Ca2+]i). Both ionomycin (Fig. 2A–C) and thapsigargin (Fig. 2D–F) significantly accelerated the rate of FM1-43 accumulation in activated T cells, in comparison to control cells (Fig. 1J–L and Fig. 2J). Ionomycin also increased the rate of FM1-43 accumulation in resting T cells but to a lesser extent than in activated T cells (n = 4, data not shown). Removal of external Ca2+ did not affect the basal rate of FM1-43 internalization (Fig. 2G–I, J), indicating that extracellular Ca2+ influx is not required to maintain the basal rate of membrane internalization. Consistent with the previous finding by Zweifach [29], ~10% of cells displayed annexin V staining around the entire plasma membrane (not shown) and increased FM1-43 surface fluorescence, presumably due to phosphatidylserine (PS) scrambling. These cells were excluded from analysis. Our data demonstrate that the rate of endocytosis in human T cells is accelerated by elevated [Ca2+]i although basal endocytosis does not require Ca2+ influx.
Fig. 2.
Elevation of [Ca2+]i accelerates the rate of FM1-43 accumulation in activated T cells. Activated T cells exposed to FM1-43 for 30 s (A, D, G), and 21 min (B, E, H) in the presence of 0.5 μM ionomycin (B, C); 1 μM thapsigargin (E, F); and in Ca2+ -free Tyrode solution (G–I). All experiments were done at 24 °C. Scale bars are 10 μm. (J) Time courses of FM1-43 accumulation in normal Tyrode solution (closed circles); in Ca2+ -free Tyrode solution (open circles); in the presence of 0.1 μM (closed squares) and 0.5 μM (closed triangles) ionomycin; and in the presence of 1 μM thapsigargin (open squares). Each time course is an average of six experiments from different cultures.
Effects of cytoskeletal modulators
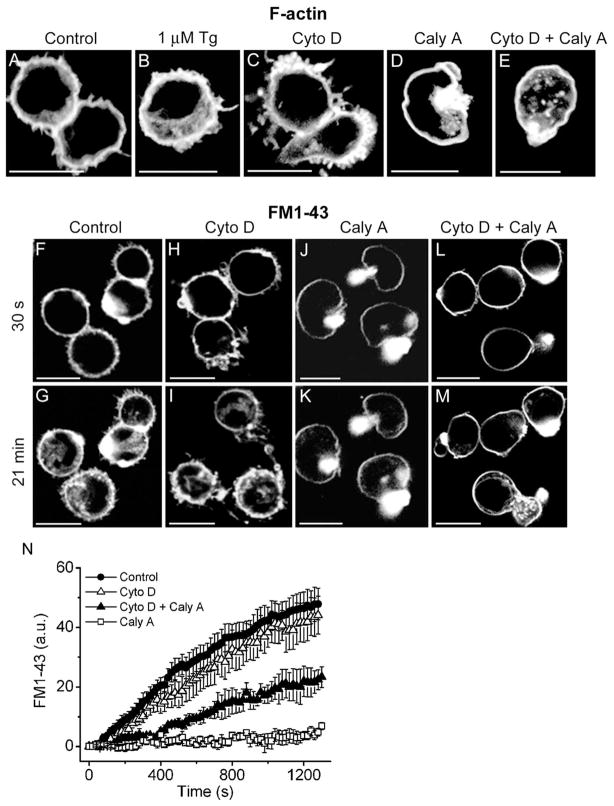
Engagement of T cell receptors during antigen presentation results in dramatic changes in cytoskeleton organization [30]. We tested whether changes in actin polymerization affect the rate of membrane trafficking in T cells. In normal Tyrode solution, F-actin was distributed uniformly near the membrane as revealed with phalloidin staining (Fig. 3A). Elevation of [Ca2+]i with thapsigargin produced actin polymerization in the cytosol (Fig. 3B) and, as shown above (Fig. 2), was accompanied by an increased rate of membrane internalization. Incubation of T cells with cytochalasin D (Cyto D), a membrane permeable inhibitor of actin polymerization [31], induced formation of numerous processes and PM shedding. In these cells, tight aggregates of polymerized actin were located in cell extremities (Fig. 3C), and some patches were found within the cytosol. The polymerized actin layer that appeared to be resistant to Cyto D was observed in cell processes and beneath the PM. These findings are compatible with observations of cytoskeletal rearrangement in other cell types [31,32]. Monitoring of FM1-43 fluorescence within the cytosol revealed that treatment with Cyto D did not significantly affect the rate of FM1-43 accumulation (Fig. 3F–I, N) in activated T cells. In contrast, treatment with calyculin A (Caly A), a serine/threonine phosphatase (PP1 and PP2A) inhibitor, abolished uptake of FM1-43 (Fig. 3J, K, N). Caly A induced the formation of a dense actin ring beneath the plasma membrane (Fig. 3D), consistent with effects described in other cell types [32–36]. Caly A-treated cells acquired a typical kidney-like shape and appeared to be curved around a single tightly condensed cytosolic actin bundle. To explore whether the inhibition of vesicular trafficking by Caly A was due to modification of the cytoskeleton, Caly A was applied together with Cyto D. Coapplication of Caly A and Cyto D produced an intermediate effect on polymerized actin distribution; the dense near-membrane actin ring was present, but actin patches within the cytosol were also observed (Fig. 3E). FM1-43 accumulation was partially rescued in these cells (Fig. 3L–N). We conclude that actin polymerization can affect the vesicular trafficking in T cells in different ways. Polymerized actin in the cytosol could support vesicle transport and facilitate membrane internalization upon elevation of [Ca2+]i. In contrast, tight condensation of actin beneath the PM creates a barrier that inhibits membrane trafficking. The limited amount of Cyto D-resistant actin is probably sufficient to maintain constitutive vesicular trafficking in Cyto D-treated cells.
Fig. 3.
Cytoskeleton polymerization may affect the rate of FM1-43 accumulation in activated T cells. Activated T cells were stained with Oregon Green phalloidin after fixation. Before fixation, cells were incubated in normal Tyrode solution (A), in the presence of 1 μM thapsigargin (5 min, B), 10 μM cytochalasin D (20 min, C), 200 nM calyculin A (20 min, D), or both 10 μM cytochalasin D and calyculin A (20 min, E). Pretreatments with cytochalasin D and calyculin A were done at 37 °C for 20 min whereas thapsigargin was applied at 24 °C. In parallel experiments, activated T cells were exposed to FM1-43 for 30 s (F, H, J, L), or 21 min (G, I, K, M) in normal Tyrode solution (F, G); after pretreatment with 10 μM cytochalasin D (H, I); or 200 nM calyculin A (J, K); or both 10 μM cytochalasin D and 200 nM calyculin A (L, M). Note that in panels J and K a bright FM1-43 fluorescence spot that appears to be located in the cytosol (cell on the left) is in the extracellular space, because staining emerged immediately after dye application. This area was excluded from the analysis. The time courses of FM1-43 accumulation were acquired at 24 °C. Scale bars are 10 μm. (N) Time courses of FM1-43 accumulation in normal Tyrode solution (closed circles); in the presence of 10 μM cytochalasin D (open triangles); in the presence of 200 nM calyculin A (open squares); and in the presence of both 10 μM cytochalasin D and 200 nM calyculin A (closed triangles). Each time course is an average of six experiments from different cultures.
Changes in trafficking compartments revealed by EM
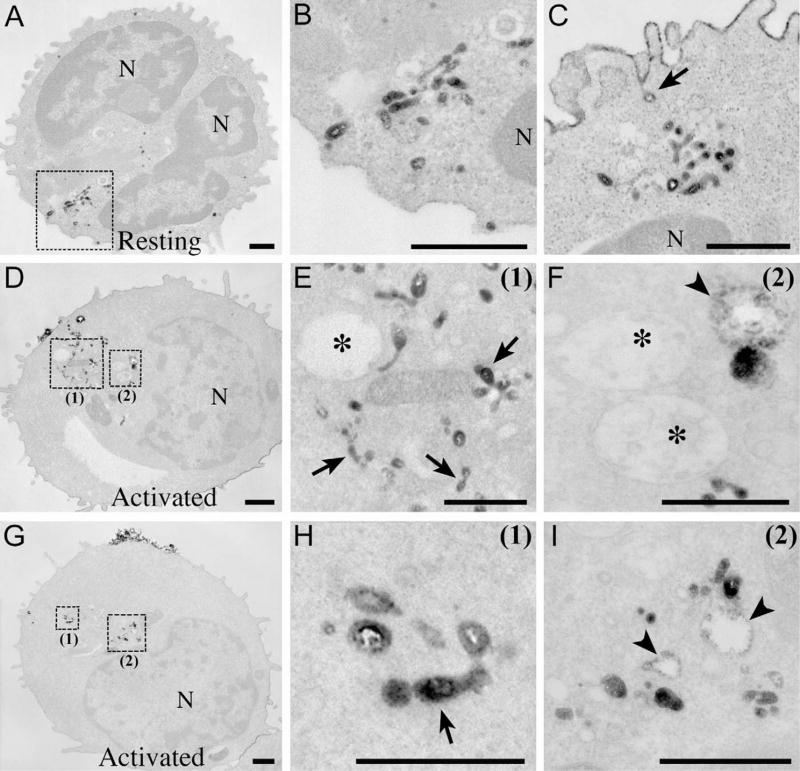
Photoconversion of DAB by FM1-43 allows the subcellular origin of FM1-43 fluorescence to be visualized with EM resolution [22]. When cells were incubated with FM1-43 for 20 min at room temperature, followed by washing to remove dye from the PM, the electron-dense reaction product was found within a pool of vesiculo-tubular compartments located within the organelle-enriched pole of both resting (Fig. 4A–C) and activated (Fig. 4D–I) T cells. Some vesicles with reaction product appeared to pinch inward from the PM (Fig. 4C) and probably represent primary endocytic vesicles. The average size of FM1-43-positive vesicular compartments within the cytosol was 72 ± 2 nm (n = 312); long tubular structures with similar diameter (68 ± 4 nm) were 234 ± 20 nm in length (n = 73). Some of the tubular structures appeared to be formed by clusters of small vesicles (Fig. 4B, E, H). The appearance of FM1-43-positive compartments in resting and activated T cells was similar to early endosomes (EE) described in other cell types [12,37].
Fig. 4.
Photoconversion of DAB by FM1-43 reveals similar early endocytic compartments in resting and activated T cells. Electron micrographs showing representative images obtained from resting (A–C) and activated (D–I) T cells exposed to FM1-43 for 20 min at 24 °C. Note that (A) shows two parts of a single nucleus (N) separated by a cleft. The photoconversion reaction product (dark staining) appeared within the cytosol of resting (A–C) and activated (D–I) T cells. After 5 min of incubation with FM1-43 at room temperature, an electron-dense reaction product was found only around the PM (not shown). (B) Enlargement of the boxed area in (A), and (C) showing examples of EE compartments in resting T cells. The arrow indicates a vesicle that appears to pinch off from the PM and may represent a primary endocytic vesicle. Staining around the PM is due to residual FM1-43 in the PM after washout. (D and G) A pool of EE (dark staining) stretching from the PM toward the nucleus. (E and F) and (H and I) Enlargements of the boxed areas (1) and (2) in (D) and (G). Note tubular structures that appear to be formed by several small vesicles (arrows in (E) and (H)). Note fusion of FM1-43-positive vesicles with small perinuclear MVB (arrowheads in (F) and I), whereas large MVB (*in E and F) remain FM1-43−. N, nucleus. Scale bars are 0.5 μm.
Many FM1-43-positive vesiculo-tubular structures were observed in continuity with intermediate-sized (153 ± 28 nm, n = 14) vacuoles located in the perinuclear region of activated T cells (Fig. 4F and I). These vacuoles often contained a small amount of reaction product and displayed early signs of inward vesiculation suggesting that they are late endosomes (LE) targeted by EE. Within single cells, FM1-43-positive intermediate-sized LE coexisted with large vesiculated vacuoles (388 ± 30 nm, n = 18) that contained no dye (FM1-43−), even though FM1-43-positive EE were located in close proximity to the FM1-43− vacuoles (Fig. 4E and F). These results suggested that EE target a specific pool of perinuclear LE.
In order to visualize late endocytic compartments, resting and activated T cells were incubated with FM1-43 for 1.5 h at 37 °C. In resting T cells the photoconversion reaction product was found in the EE adjacent to perinuclear vacuoles (Fig. 5A and B) and inside peripheral vacuoles that displayed no internal vesiculation but appeared as homogeneously filled compartments (Fig. 5A and C) typical of lysosomes [38,39]. Strikingly different FM1-43-positive LE containing numerous vesicles (mulivesicular bodies, MVB) were observed in activated T cells after 1.5 h of incubation with FM1-43 (Fig. 5D–G). These FM1-43-positive MVB were found anywhere in the cytosol, and were larger (367 ± 24 nm, n = 18) than perinuclear FM1-43-positive LE. In addition, large MVB containing a small amount of photoconversion product were also observed within a single cell (Fig. 5F). Interestingly, small vesicles/tubules were found in continuity with a large FM1-43-positive MVB (Fig. 5G), suggesting that directed targeting continues until the MVB reaches a critical size or state. Alternatively, vesicles may bud off from the mature MVB, as was suggested for dendritic cells [40]. Thus, our data indicate that resting and activated T cells employ similar structural intermediates that operate from the PM to perinuclear LE. In activated T cells the perinuclear LE enlarge, vesiculate, and drift away from the nucleus within 1.5 h. In contrast, LE in resting T cells appear to mature to lysosomes within the same period of time.
Fig. 5.
Photoconversion of DAB by FM1-43 reveals different late endocytic compartments in resting and activated T cells. Representative images obtained from resting (A–C) and activated (D–G) T cells exposed to FM1-43 for 1.5 h at 37 °C. (B) Enlargement of the boxed area (1) in (A). Note small FM1-43-positive vesicles docked to the perinuclear vacuole. (C) Enlargement of the boxed area (2) in (A). Note that FM1-43-positive endosomes in resting T cells display no internal vesiculation. (E and F) Enlargements of boxed areas (1) and (2) in (D). Note that FM1-43-positive endosomes in activated T cells in (E) have a multivesicular structure. (F) A large MVB weakly stained with FM1-43. (G) EE continue to target large FM1-43-positive MVB. N, nucleus. Scale bars are 0.5 μm.
In many cell types, MVB undergo maturation into lysosomes as a standard pathway for degradation of internalized membrane proteins [12]. However, in activated T cells we observed fusion of the limiting membrane of FM1-43-positive MVB with the PM (Fig. 6A and B), resulting in release of vesicles enclosed in the MVB, a secretory process known as exosome externalization [16]. Externalized vesicles were 120 ± 10 nm (n = 121) in diameter, a size typical for exosomes. Fusion of FM1-43-positive MVB with the PM and externalization of exosomes was observed as early as 1.5 h after dye was allowed to internalize. Extracellular vesiculated membrane aggregates composed of ~110–160 nm vesicles adherent to the plasma membrane were present in the majority of activated T cells (Fig. 6C and D). The external vesicles were often found in areas of cell-to cell contact, sometimes within the extensive net of lamellopodia stretching from one cell to another (Fig. 6E and F). Interestingly, exosomes retained FM1-43 even after PM staining was significantly reduced by extensive washing. Because clusters of externalized exosomes tend to retain dye, we conclude that they correspond to large peripheral patches of FM1-43 fluorescence in activated T cells (Figs. 1 and 2).
Fig. 6.
Photoconversion reveals exosome externalization by activated T cells. (A) Fusion of a MVB with the PM and exosome externalization in activated T cells. Cells were loaded with FM1-43 for 1 h and 20 min at 37 °C, washed for 5 min in Tyrode solution, fixed, and subjected to photoconversion. (B) Enlargement of the boxed area in (A). (C and D) Clusters of external membranes adherent to the PM of activated T cell. Cells were exposed to FM1-43 for 20 min, and dye was then washed out. Note that many vesicles display cup-shaped morphology typical of exosomes. (E) Photoconversion reaction product is found trapped within external vesicles in regions of cell– cell contact. (F) Enlargement of the boxed area in (E) Note that EE (dark staining) and MVB (*) are located in the vicinity of cell– cell contact. N, nucleus; m, mitochondria. Scale bars are 0.5 μm.
Taken together, our data demonstrate that human T cells constantly internalize their PM by endocytosis into EE that then carry their cargo into lysosome-like LE in resting T cells and into MVB in activated T cells. In activated T cells, but not resting T cells, the MVB can externalize exosomes into the extracellular space by means of fusion of the limiting membrane of MVB with the PM.
Colocalization of exosomes with CD3ε, cholera toxin B, and annexin V staining
In order to assess whether FM1-43 follows the same pathway of internalization as surface receptors, we performed double staining of endocytic compartments with FM4-64 (a red-shifted variant of FM1-43) and Alexa Fluor 488-conjugated anti-CD3ε. Fig. 7A–C demonstrates in activated T cells that internalized anti-CD3ε, visible as punctate staining in the cytosol, colocalizes with FM4-64 fluorescence. Although colocalization is restricted to a few intracellular compartments, this observation strongly suggests that CD3ε is internalized to the same endosomes as FM4-64. On the cell periphery, bright spots of FM4-64 fluorescence originated from the clusters of exosomes also colocalized with anti-CD3ε immunofluorescence. Colocalization of exosome clusters with anti-CD3ε immunofluorescence suggests that CD3ε downregulation may follow the pathway of exosome externalization (Figs. 5 and 6). To further characterize the molecular composition of exosomes, we stained surface membranes with Alexa Fluorconjugated cholera toxin B (CT-B), a marker of lipid rafts [41, 42], or annexin V, a marker of phosphatidylserine (PS). Fig. 7D–F, demonstrates that CT-B staining was confined to small areas that colocalized with bright fluorescence patches of FM4-64, indicating that exosomes bear raft-associated GM1 glycosphingolipid. In addition, annexin V staining was restricted to the same areas (Fig. 7G–I), indicating exposure of PS on the outer surface of exosomes.
Fig. 7.
FM4-64 fluorescence colocalizes with CD3ε, cholera toxin-B staining and annexin V staining in activated T cells. (A–C) Anti-CD3ε and FM4-64 in fixed cells. Activated T cells were first incubated with FM4-64 for 30 min at 37 °C. Ionomycin (0.1 μ M) and PMA (50 nM) were added to promote internalization of TCR/CD3 complexes [3]. After FM4-64 was thoroughly washed out, cells were fixed and costained with primary anti-CD3ε antibody and Alexa Fluor 488-conjugated secondary antibody. (A, green) Distribution of anti-CD3ε -Alexa Fluor 488 fluorescence, acquired with 515 nm band pass emission filter. Note PM distribution of CD3ε in two cells and complete internalization of CD3ε in one cell. (B, red) FM4– 64 fluorescence acquired with 600 nm long pass emission filter. (C) Superimposed images from (A) and (B); yellow indicates colocalization of FM4– 64 fluorescence with anti-CD3ε fluorescence. Note colocalization within endosomes (arrows) and within extracellular exosome clusters (arrowheads) (D–I) Living T cells were stained for 15 min at room temperature with CT-B-Alexa Fluor 488 (D, green) or annexin V-Alexa Fluor 488 (G, green) and then images were acquired with 515 nm band pass emission filter. Cells were then briefly (30 s) exposed to FM4-64 and fluorescence acquired with 600 nm long pass emission filter (E and H, red). (F and I) Superimposed images from (D) and (E), and (G) and (H), correspondingly. Note that surface CT-B and annexin V staining colocalize with exosome clusters revealed with FM4– 64. Scale bars are 10 μ m.
Because transverse redistribution of plasma membrane PS is an early indicator of apoptosis [43], we investigated whether exosome externalization is a characteristic of viable, nonapoptotic cells. We found that 81% of adherent activated T cells (363 out of 445 cells from four donors) expressed exosomes positively stained by annexin V; none of these cells were stained with PI, indicating that the cells were not necrotic. Surface PS exposure by apoptotic cells is associated with loss of mitochondrial transmembrane potentials (Δψm) [44]. However, we found that cells bearing exosomes at their surface maintained a high Δψm that could be dissipated by CCCP, the mitochondrial uncoupler, or by treatment with staurosporine, an agent that induces apoptosis (Fig. 8). In addition, the majority (67%) of activated T cells were producing IL2 (102 out of 162 cells tested), as assessed by immunohistochemical staining (not shown). Only 27 out of 445 cells (4.5%) were uniformly stained with annexin V, of which seven displayed nuclear PI staining. Thus, the majority of adherent activated T cells displayed high mitochondrial potentials and were competent to produce cytokines. These data demonstrate that viable, activated T cells release exosomes that bear properties of raft membranes and expose PS locally at their outer membrane leaflet.
Fig. 8.
Exosomes at the surface of viable activated T cells. (A) FM4– 64 surface fluorescence (red) obtained from living cells after brief (30 s) exposure to FM4– 64. (B) Same cells stained with DiOC6 (3) (green). (C) Same cells after incubation with 10 μM CCCP for 15 min at room temperature. (D) Histograms of DiOC6 (3) intensity measured from untreated activated T cells (control, top panel); from activated T cells treated with 10 μM CCCP for 15 min at room temperature (middle panel); and from activated T cells preincubated with 1 μM staurosporine for 6 h at 37 °C to induce apoptosis (lower panel). Fluorescence intensities were measured from randomly selected individual adherent T cells. Scale bars are 10 μm.
Discussion
Dynamic monitoring of membrane trafficking
In this study, we show that FM1-43 is rapidly and constitutively internalized by vesicular endocytosis in human T cells and, therefore, can be utilized for unraveling fundamental mechanisms that regulate membrane trafficking. Real-time monitoring of FM1-43 fluorescence indicated that the rate of constitutive membrane trafficking was ~10-fold higher in activated T cells compared to resting. Fast and dynamic regulation of membrane protein expression may help to shape specific responses in activated T cells. Furthermore, elevation of [Ca2+]i significantly accelerated membrane internalization. Ca2+ signaling was shown previously to be enhanced in activated T cells, compared to resting T cells [45,46]. Therefore, augmented Ca2+ signaling may enhance membrane traffic when activated T cells are restimulated by antigen. Extracellular Ca2+ influx was not required for basal endocytosis in T cells, in good agreement with previous reports in neuronal cells [47,48], and in rat peritoneal mast cells in which high-affinity Ca2+ sites were implicated in membrane uptake [27].
Cytoskeletal elements are involved in different steps of the endocytic process, including membrane invagination and fission, as well as vesicle movement in the cytoplasm [49,50]. We found that [Ca2+]i elevation in human T cells produced actin polymerization and accelerated FM1-43 accumulation, thus implying the involvement of the actin cytoskeleton in regulation of endocytosis. However, actin disruption with Cyto D had no effect on the rate of FM1-43 accumulation, consistent with results in several other mammalian cells [51–53]. Increased mobility of endocytic vesicles after treatment with agents that sequester monomeric actin could be due to removal of a diffusional barrier [54]. It is also possible that a cortical layer of actin underlying the PM in Cyto D-treated cells (Fig. 3C) could still support vesicle formation and movement away from the PM. On the other hand, strong condensation of cortical actin beneath the PM with Caly A abolished FM1-43 accumulation, consistent with the diffusional barrier hypothesis. Thus, our data confirm that vesicular trafficking in T cells can be fine-tuned by spatially restricted cytoskeleton modification.
Rates of endocytosis in T cells were strongly temperature dependent, consistent with studies of receptor-mediated and fluid-phase endocytosis in other cell types [55–58]. The Arrhenius plot of endocytic rates in Fig. 1 deviates from a straight line at ~24 °C and reveals values of activation energy similar to those measured for cleavage of the GPI-anchored protein, 5′-nucleotidase, from the surface of porcine lymphocyte PM [59]. A biphasic Arrhenius plot is common for many membrane-bound enzymes and may result from a lipid phase transition from a gel to a fluid liquid crystalline phase, from lipid phase separation within the membrane [60,25], or from other factors such as a change in activation entropy or changes in membrane components other than lipids [59].
Endocytic compartments and exosome externalization
The ability of FM1-43 to convert DAB into an electron-dense product provides a unique opportunity to relate real-time fluorescence monitoring of endocytosis in living cells to the ultrastructural compartments within the cell. T cells employ structurally different primary endocytic vesicles to mediate membrane receptor trafficking. TCR and transferrin receptor are internalized via clathrin-coated pits [61,62], whereas IL2 R, CD4, CD8, CD19, and CD20 are internalized primarily via uncoated vesicles [63,64]. The size of FM1-43-positive intracellular vesicles (50 –100 nm) is consistent with either coated or uncoated endocytic vesicles found in T lymphocytes [64]. Colocalization with anti-CD3ε within the cell indicates that the dye follows the clathrin-coated vesicle pathway, among other endocytic pathways. Therefore, FM1-43 appears to be a powerful tool for investigation of T cell membrane dynamics and mechanisms that regulate membrane receptor internalization.
Tracking the fate of membrane-associated FM1-43 within the cell revealed a significant change in the organization of endocytic compartments as T cells become activated. Early endosomes (EE) in resting and activated T cells displayed similar morphology to those in other cell types [12], but late endosomes appear to take different paths when T cells are activated. In resting T cells, FM1-43 was found in lysosome-like vacuoles that lacked internal membranes, whereas activated T cells readily accumulated the dye within MVB. Our results indicate that fusion of MVB with the PM of activated (but not resting) human T cells results in externalization of exosomes. This pathway may allow certain proteins to avoid degradation and initiate signal transduction in neighboring cells. The mechanisms that regulate the fate of MVB within the activated T cell remain to be determined.
Clusters of exosomes at the surface of activated T cells were revealed as bright fluorescence patches of FM1-43/FM4-64 that appeared simultaneously with PM staining immediately after dye application. Exosome clusters were usually located in cell– cell contacts and differed in membrane composition from the PM by enrichment of raft-associated GM1 and by exposure of PS on the outer membrane leaflet (Fig. 7). The presence of PS at the outer surface of T cell exosomes is consistent with platelet-derived exosomes [65]. FM1-43 can change its spectral properties depending on the lipid environment [29,66], a property that may account for the bright fluorescence from exosomes in the presence of dye and the prolonged fluorescence from exosome clusters after dye washout (Fig. 1). Exosome clusters observed by electron microscopy were composed of single vesicles 100 –200 nm in diameter. Previous studies showed that similar-sized microvesicles at the surface of activated T cells, T cell clones, and Jurkat T cells bear biologically active proteins including TCR, adhesion molecules CD2 and LFA-1, MHC class I and class II, and the chemokine receptor CXCR4 [18]. Our colocalization study revealed the presence of raft-associated CD3ε (a TCR component) in exosomes, in addition to GM1 glycosphingolipid, indicating that exosomes are formed by raft membranes. In lymphocytes that lack caveolae [41], raft domains were implicated in GPI-anchored protein endocytosis [67]. We hypothesize that the composition of internalized raft domains may be preserved when processed via LE compartments and then externalized by exosomes. It is possible that MVB also serve as vehicles for externalization of newly synthesized and recycled raft components. Externalization of exosomes formed by raft membranes from endocytic compartments may also participate in the formation of larger, micron-sized raft aggregates at the surface of T cells after CT-B or TCR cross-linking [68].
The functional role of exosomes in the immune response remains controversial [16]. Recently, costimulatory rafts that appear to originate from cytoplasmic vesicles were implicated as important amplifiers of the later phases of TCR-mediated stimulation [69, 70]. We suggest that specific types of raft aggregates can be delivered by exosome externalization and may play a role in providing costimulatory signals for activated T cells. Moreover, it is established that exposure of PS on apoptotic cells triggers phagocytosis by macrophages and DCs [71,72] in turn resulting in presentation of antigens that were expressed by the phagocytosed cells [73]. We speculate that exosomes released by nonapoptotic T cells can also be phagocytosed, resulting in enhanced antigen presentation by professional APC. In addition, Fas and APO2/TRAIL death ligands have been found in microvesicles released by Jurkat and PHA-activated human T cells [14,19] and may suppress the immune response. Finally, exosome externalization can result in permanent TCR down-regulation, similar to down-regulation of the transferrin receptor during reticulocyte maturation [74,75]. T cell exosomes carrying membrane receptors and exposed PS may underlie long-range cell-to-cell interactions by phagocytosis or transendocytosis.
Acknowledgments
This work was supported by NIH grants NS14609 and GM41514 (M.D.C.), NIH/NCRR grant P41RR04050 (M.H.E.), a Scientist Development Grant 0030275N from the American Heart Association (A.F.F.), and by Public Health Service Research grant M01 RR00827 from the National Center for Research Resources (UCI General Clinical Research Center). The authors thank Craig Walsh and Nicolas Blanchard for insightful comments. Tatiana Krasieva, Luette Forrest, and Olga Safrina provided valuable experimental assistance.
References
- 1.Krangel MS. Endocytosis and recycling of the T3-T cell receptor complex, The role of T3 phosphorylation. J Exp Med. 1987;165:1141–1159. doi: 10.1084/jem.165.4.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu H, Rhodes M, Wiest DL, Vignali DA. On the dynamics of TCR:CD3 complex cell surface expression and downmodulation. Immunity. 2000;13:665–675. doi: 10.1016/s1074-7613(00)00066-2. [DOI] [PubMed] [Google Scholar]
- 3.Minami Y, Samelson LE, Klausner RD. Internalization and cycling of the T cell antigen receptor, Role of protein kinase C. J Biol Chem. 1987;262:13342–13347. [PubMed] [Google Scholar]
- 4.Hemmer B, Stefanova I, Vergelli M, Germain RN, Martin R. Relationships among TCR ligand potency, thresholds for effector function elicitation, and the quality of early signaling events in human T cells. J Immunol. 1998;160:5807–5814. [PubMed] [Google Scholar]
- 5.Itoh Y, Hemmer B, Martin R, Germain RN. Serial TCR engagement and down-modulation by peptide: MHC molecule ligands: relationship to the quality of individual TCR signaling events. J Immunol. 1999;162:2073–2080. [PubMed] [Google Scholar]
- 6.Valitutti S, Muller S, Cella M, Padovan E, Lanzavecchia A. Serial triggering of many T-cell receptors by a few peptide-MHC complexes. Nature. 1995;375:148–151. doi: 10.1038/375148a0. [DOI] [PubMed] [Google Scholar]
- 7.Valitutti S, Muller S, Salio M, Lanzavecchia A. Degradation of T cell receptor (TCR)-CD3-zeta complexes after antigenic stimulation. J Exp Med. 1997;185:1859–1864. doi: 10.1084/jem.185.10.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D’Oro U, Vacchio MS, Weissman AM, Ashwell JD. Activation of the Lck tyrosine kinase targets cell surface T cell antigen receptors for lysosomal degradation. Immunity. 1997;7:619–628. doi: 10.1016/s1074-7613(00)80383-0. [DOI] [PubMed] [Google Scholar]
- 9.Cai Z, Kishimoto H, Brunmark A, Jackson MR, Peterson PA, Sprent J. Requirements for peptide-induced T cell receptor downregulation on naive CD8+ T cells. J Exp Med. 1997;185:641–651. doi: 10.1084/jem.185.4.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.La Face DM, Couture C, Anderson K, Shih G, Alexander J, Sette A, Mustelin T, Altman A, Grey HM. Differential T cell signaling induced by antagonist peptide-MHC complexes and the associated phenotypic responses. J Immunol. 1997;158:2057–2064. [PubMed] [Google Scholar]
- 11.Roche PA. Intracellular protein traffic in lymphocytes: “How do I get THERE from HERE”? Immunity. 1999;11:391–398. doi: 10.1016/s1074-7613(00)80114-4. [DOI] [PubMed] [Google Scholar]
- 12.Mellman I. Endocytosis and molecular sorting. Annu Rev Cell Dev Biol. 1996;12:575–625. doi: 10.1146/annurev.cellbio.12.1.575. [DOI] [PubMed] [Google Scholar]
- 13.Blott EJ, Griffiths GM. Secretory lysosomes. Nat Rev Mol Cell Biol. 2002;3:122–131. doi: 10.1038/nrm732. [DOI] [PubMed] [Google Scholar]
- 14.Denzer K, Kleijmeer MJ, Heijnen HF, Stoorvogel W, Geuze HJ. Exosome: from internal vesicle of the multivesicular body to intercellular signaling device. J Cell Sci. 2000;113:3365–3374. doi: 10.1242/jcs.113.19.3365. [DOI] [PubMed] [Google Scholar]
- 15.Stoorvogel W, Kleijmeer MJ, Geuze HJ, Raposo G. The biogenesis and functions of exosomes. Traffic. 2002;3:321–330. doi: 10.1034/j.1600-0854.2002.30502.x. [DOI] [PubMed] [Google Scholar]
- 16.Thery C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2:569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 17.Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D, Ricciardi-Castagnoli P, Raposo G, Amigorena S. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat Med. 1998;4:594–600. doi: 10.1038/nm0598-594. [DOI] [PubMed] [Google Scholar]
- 18.Blanchard N, Lankar D, Faure F, Regnault A, Dumont C, Raposo G, Hivroz C. TCR activation of human T cells induces the production of exosomes bearing the TCR/CD3/zeta complex. J Immunol. 2002;168:3235–3241. doi: 10.4049/jimmunol.168.7.3235. [DOI] [PubMed] [Google Scholar]
- 19.Monleon I, Martinez-Lorenzo MJ, Monteagudo L, Lasierra P, Taules M, Iturralde M, Pineiro A, Larrad L, Alava MA, Naval J, Anel A. Differential secretion of Fas ligand- or APO2 ligand/TNF-related apoptosis-inducing ligand-carrying microvesicles during activation-induced death of human T cells. J Immunol. 2001;167:6736–6744. doi: 10.4049/jimmunol.167.12.6736. [DOI] [PubMed] [Google Scholar]
- 20.Betz WJ, Mao F, Smith CB. Imaging exocytosis and endocytosis. Curr Opin Neurobiol. 1996;6:365–371. doi: 10.1016/s0959-4388(96)80121-8. [DOI] [PubMed] [Google Scholar]
- 21.Cochilla AJ, Angleson JK, Betz WJ. Monitoring secretory membrane with FM1-43 fluorescence. Annu Rev Neurosci. 1999;22:1–10. doi: 10.1146/annurev.neuro.22.1.1. [DOI] [PubMed] [Google Scholar]
- 22.Henkel AW, Simpson LL, Ridge RM, Betz WJ. Synaptic vesicle movements monitored by fluorescence recovery after photobleaching in nerve terminals stained with FM1-43. J Neurosci. 1996;16:3960–3967. doi: 10.1523/JNEUROSCI.16-12-03960.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harata N, Pyle JL, Aravanis AM, Mozhayeva M, Kavalali ET, Tsien RW. Limited numbers of recycling vesicles in small CNS nerve terminals: implications for neural signaling and vesicular cycling. Trends Neurosci. 2001;24:637–643. doi: 10.1016/s0166-2236(00)02030-0. [DOI] [PubMed] [Google Scholar]
- 24.Gaietta G, Deerinck TJ, Adams SR, Bouwer J, Tour O, Laird DW, Sosinsky GE, Tsien RY, Ellisman MH. Multicolor and electron microscopic imaging of connexin trafficking. Science. 2002;296:503–507. doi: 10.1126/science.1068793. [DOI] [PubMed] [Google Scholar]
- 25.Sandermann H. Regulation of membrane enzymes by lipids. Biochim Biophys Acta. 1978;515:209–237. doi: 10.1016/0304-4157(78)90015-1. [DOI] [PubMed] [Google Scholar]
- 26.Crabtree GR. Generic signals and specific outcomes: signaling through Ca2+, calcineurin, and NF-AT. Cell. 1999;96:611–614. doi: 10.1016/s0092-8674(00)80571-1. [DOI] [PubMed] [Google Scholar]
- 27.Almers W, Neher E. Gradual and stepwise changes in the membrane capacitance of rat peritoneal mast cells. J Physiol (London) 1987;386:205–217. doi: 10.1113/jphysiol.1987.sp016530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Camilli P, Takei K. Molecular mechanisms in synaptic vesicle endocytosis and recycling. Neuron. 1996;16:481–486. doi: 10.1016/s0896-6273(00)80068-9. [DOI] [PubMed] [Google Scholar]
- 29.Zweifach A. FM1-43 reports plasma membrane phospholipid scrambling in T-lymphocytes. Biochem J. 2000;349:255–260. doi: 10.1042/0264-6021:3490255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Acuto O, Cantrell D. T cell activation and the cytoskeleton. Annu Rev Immunol. 2000;18:165–184. doi: 10.1146/annurev.immunol.18.1.165. [DOI] [PubMed] [Google Scholar]
- 31.Cooper JA. Effects of cytochalasin and phalloidin on actin. J Cell Biol. 1987;105:1473–1478. doi: 10.1083/jcb.105.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patterson RL, van Rossum DB, Gill DL. Store-operated Ca2+ entry: evidence for a secretion-like coupling model. Cell. 1999;98:487–499. doi: 10.1016/s0092-8674(00)81977-7. [DOI] [PubMed] [Google Scholar]
- 33.Kreienbuhl P, Keller H, Niggli V. Protein phosphatase inhibitors okadaic acid and calyculin A alter cell shape and F-actin distribution and inhibit stimulus-dependent increases in cytoskeletal actin of human neutrophils. Blood. 1992;80:2911–2919. [PubMed] [Google Scholar]
- 34.Downey GP, Takai A, Zamel R, Grinstein S, Chan CK. Okadaic acid-induced actin assembly in neutrophils: role of protein phosphatases. J Cell Physiol. 1993;155:505–519. doi: 10.1002/jcp.1041550309. [DOI] [PubMed] [Google Scholar]
- 35.Hosoya N, Mitsui M, Yazama F, Ishihara H, Ozaki H, Karaki H, Hartshorne DJ, Mohri H. Changes in the cytoskeletal structure of cultured smooth muscle cells induced by calyculin-A. J Cell Sci. 1993;105:883–890. doi: 10.1242/jcs.105.4.883. [DOI] [PubMed] [Google Scholar]
- 36.Shinoki N, Sakon M, Kambayashi J, Ikeda M, Oiki E, Okuyama M, Fujitani K, Yano Y, Kawasaki T, Monden M. Involvement of protein phosphatase-1 in cytoskeletal organization of cultured endothelial cells. J Cell Biochem. 1995;59:368–375. doi: 10.1002/jcb.240590308. [DOI] [PubMed] [Google Scholar]
- 37.Robinson MS, Watts C, Zerial M. Membrane dynamics in endocytosis. Cell. 1996;84:13–21. doi: 10.1016/s0092-8674(00)80988-5. [DOI] [PubMed] [Google Scholar]
- 38.Luzio JP, Mullock BM, Pryor PR, Lindsay MR, James DE, Piper RC. Relationship between endosomes and lysosomes. Biochem Soc Trans. 2001;29:476–480. doi: 10.1042/bst0290476. [DOI] [PubMed] [Google Scholar]
- 39.Luzio JP, Rous BA, Bright NA, Pryor PR, Mullock BM, Piper RC. Lysosome-endosome fusion and lysosome biogenesis. J Cell Sci. 2000;113:1515–1524. doi: 10.1242/jcs.113.9.1515. [DOI] [PubMed] [Google Scholar]
- 40.Murk J, Stoorvogel W, Kleijmeer M, Geuze H. The plasticity of multivesicular bodies and the regulation of antigen presentation. Semin Cell Dev Biol. 2002;13:303–311. doi: 10.1016/s1084952102000605. [DOI] [PubMed] [Google Scholar]
- 41.Fra AM, Williamson E, Simons K, Parton RG. Detergent-insoluble glycolipid microdomains in lymphocytes in the absence of caveolae. J Biol Chem. 1994;269:30745–30748. [PubMed] [Google Scholar]
- 42.Harder T, Scheiffele P, Verkade P, Simons K. Lipid domain structure of the plasma membrane revealed by patching of membrane components. J Cell Biol. 1998;141:929–942. doi: 10.1083/jcb.141.4.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stuart MC, Damoiseaux JG, Frederik PM, Arends JW, Reutelingsperger CP. Surface exposure of phosphatidylserine during apoptosis of rat thymocytes precedes nuclear changes. Eur J Cell Biol. 1998;76:77–83. doi: 10.1016/S0171-9335(98)80019-8. [DOI] [PubMed] [Google Scholar]
- 44.Kroemer G, Zamzami N, Susin SA. Mitochondrial control of apoptosis. Immunol Today. 1997;18:44–51. doi: 10.1016/s0167-5699(97)80014-x. [DOI] [PubMed] [Google Scholar]
- 45.Hess SD, Oortgiesen M, Cahalan MD. Calcium oscillations in human T and natural killer cells depend upon membrane potential and calcium influx. J Immunol. 1993;150:2620–2633. [PubMed] [Google Scholar]
- 46.Fomina AF, Fanger CM, Kozak JA, Cahalan MD. Single channel properties and regulated expression of Ca2+ release-activated Ca2+ (CRAC) channels in human T cells. J Cell Biol. 2000;150:1435–1444. doi: 10.1083/jcb.150.6.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ryan TA, Reuter H, Wendland B, Schweizer FE, Tsien RW, Smith SJ. The kinetics of synaptic vesicle recycling measured at single presynaptic boutons. Neuron. 1993;11:713–724. doi: 10.1016/0896-6273(93)90081-2. [DOI] [PubMed] [Google Scholar]
- 48.Ryan TA, Smith SJ, Reuter H. The timing of synaptic vesicle endocytosis. Proc Natl Acad Sci USA. 1996;93:5567–5571. doi: 10.1073/pnas.93.11.5567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Qualmann B, Kessels MM, Kelly RB. Molecular links between endocytosis and the actin cytoskeleton. J Cell Biol. 2000;150:F111–F116. doi: 10.1083/jcb.150.5.f111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lanzetti L, Di Fiore PP, Scita G. Pathways linking endocytosis and actin cytoskeleton in mammalian cells. Exp Cell Res. 2001;271:45–56. doi: 10.1006/excr.2001.5369. [DOI] [PubMed] [Google Scholar]
- 51.Gottlieb TA, Ivanov IE, Adesnik M, Sabatini DD. Actin micro-filaments play a critical role in endocytosis at the apical but not the basolateral surface of polarized epithelial cells. J Cell Biol. 1993;120:695–710. doi: 10.1083/jcb.120.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fujimoto LM, Roth R, Heuser JE, Schmid SL. Actin assembly plays a variable, but not obligatory role in receptor-mediated endocytosis in mammalian cells. Traffic. 2000;1:161–171. doi: 10.1034/j.1600-0854.2000.010208.x. [DOI] [PubMed] [Google Scholar]
- 53.Jackman MR, Shurety W, Ellis JA, Luzio JP. Inhibition of apical but not basolateral endocytosis of ricin and folate in Caco-2 cells by cytochalasin D. J Cell Sci. 1994;107:2547–2556. doi: 10.1242/jcs.107.9.2547. [DOI] [PubMed] [Google Scholar]
- 54.Gaidarov I, Santini F, Warren RA, Keen JH. Spatial control of coated-pit dynamics in living cells. Nat Cell Biol. 1999;1:1–7. doi: 10.1038/8971. [DOI] [PubMed] [Google Scholar]
- 55.Illinger D, Poindron P, Kuhry JG. Fluid phase endocytosis investigated by fluorescence with trimethylamino-diphenylhexatriene in L929 cells; the influence of temperature and of cytoskeleton depolymerizing drugs. Biol Cell. 1991;73:131–138. doi: 10.1016/0248-4900(91)90095-5. [DOI] [PubMed] [Google Scholar]
- 56.Mamdouh Z, Giocondi MC, Laprade R, Le Grimellec C. Temperature dependence of endocytosis in renal epithelial cells in culture. Biochim Biophys Acta. 1996;1282:171–173. doi: 10.1016/0005-2736(96)00077-6. [DOI] [PubMed] [Google Scholar]
- 57.Tomoda H, Kishimoto Y, Lee YC. Temperature effect on endocytosis and exocytosis by rabbit alveolar macrophages. J Biol Chem. 1989;264:15445–15450. [PubMed] [Google Scholar]
- 58.Weigel PH, Oka JA. Temperature dependence of endocytosis mediated by the asialoglycoprotein receptor in isolated rat hepatocytes. Evidence for two potentially rate-limiting steps. J Biol Chem. 1981;256:2615–2617. [PubMed] [Google Scholar]
- 59.Lehto MT, Sharom FJ. PI-specific phospholipase C cleavage of a reconstituted GPI-anchored protein: modulation by the lipid bilayer. Biochemistry. 2002;41:1398–1408. doi: 10.1021/bi011579w. [DOI] [PubMed] [Google Scholar]
- 60.Kumamoto J, Raison JK, Lyons JM. Temperature “breaks” in Arrhenius plots: a thermodynamic consequence of a phase change. J Theor Biol. 1971;31:47–51. doi: 10.1016/0022-5193(71)90120-2. [DOI] [PubMed] [Google Scholar]
- 61.Dietrich J, Hou X, Wegener AM, Geisler C. CD3 gamma contains a phosphoserine-dependent di-leucine motif involved in down-regulation of the T cell receptor. EMBO J. 1994;13:2156–2166. doi: 10.1002/j.1460-2075.1994.tb06492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Luton F, Legendre V, Gorvel JP, Schmitt-Verhulst AM, Boyer C. Tyrosine and serine protein kinase activities associated with ligand-induced internalized TCR/CD3 complexes. J Immunol. 1997;158:3140–3147. [PubMed] [Google Scholar]
- 63.Lamaze C, Dujeancourt A, Baba T, Lo CG, Benmerah A, Dautry-Varsat A. Interleukin 2 receptors and detergent-resistant membrane domains define a clathrin-independent endocytic pathway. Mol Cell. 2001;7:661–671. doi: 10.1016/s1097-2765(01)00212-x. [DOI] [PubMed] [Google Scholar]
- 64.Burkhardt O, Merker HJ. Immunoelectron microscopic investigations of patching, capping, endocytotic and shedding processes in T and B lymphocytes. Ann Anat. 2002;184:45–53. doi: 10.1016/S0940-9602(02)80034-6. [DOI] [PubMed] [Google Scholar]
- 65.Heijnen HF, Schiel AE, Fijnheer R, Geuze HJ, Sixma JJ. Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. Blood. 1999;94:3791–3799. [PubMed] [Google Scholar]
- 66.Schote U, Seelig J. Interaction of the neuronal marker dye FM1-43 with lipid membranes. Thermodynamics and lipid ordering. Biochim Biophys Acta. 1998;1415:135–146. doi: 10.1016/s0005-2736(98)00188-6. [DOI] [PubMed] [Google Scholar]
- 67.Deckert M, Ticchioni M, Bernard A. Endocytosis of GPI-anchored proteins in human lymphocytes: role of glycolipid-based domains, actin cytoskeleton, and protein kinases. J Cell Biol. 1996;133:791–799. doi: 10.1083/jcb.133.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Janes PW, Ley SC, Magee AI. Aggregation of lipid rafts accompanies signaling via the T cell antigen receptor. J Cell Biol. 1999;147:447–461. doi: 10.1083/jcb.147.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tuosto L, Parolini I, Schroder S, Sargiacomo M, Lanzavecchia A, Viola A. Organization of plasma membrane functional rafts upon T cell activation. Eur J Immunol. 2001;31:345–349. doi: 10.1002/1521-4141(200102)31:2<345::aid-immu345>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 70.Horejsi V. Membrane rafts in immunoreceptor signaling: new doubts, new proofs? Trends Immunol. 2002;23:562–564. doi: 10.1016/s1471-4906(02)02330-x. [DOI] [PubMed] [Google Scholar]
- 71.Henson PM, Bratton DL, Fadok VA. The phosphatidylserine receptor: a crucial molecular switch? Nat Rev Mol Cell Biol. 2001;2:627–633. doi: 10.1038/35085094. [DOI] [PubMed] [Google Scholar]
- 72.Schlegel RA, Callahan MK, Williamson P. The central role of phosphatidylserine in the phagocytosis of apoptotic thymocytes. Ann NY Acad Sci. 2000;926:217–225. doi: 10.1111/j.1749-6632.2000.tb05614.x. [DOI] [PubMed] [Google Scholar]
- 73.Albert ML, Pearce SF, Francisco LM, Sauter B, Roy P, Silverstein RL, Bhardwaj N. Immature dendritic cells phagocytose apoptotic cells via alphavbeta5 and CD36, and cross-present antigens to cytotoxic T lymphocytes. J Exp Med. 1998;188:1359–1368. doi: 10.1084/jem.188.7.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes) J Biol Chem. 1987;262:9412–9420. [PubMed] [Google Scholar]
- 75.Johnstone RM, Mathew A, Mason AB, Teng K. Exosome formation during maturation of mammalian and avian reticulocytes: evidence that exosome release is a major route for externalization of obsolete membrane proteins. J Cell Physiol. 1991;147:27–36. doi: 10.1002/jcp.1041470105. [DOI] [PubMed] [Google Scholar]