Abstract
Background
Nuclear receptors are a superfamily of metazoan transcription factors that regulate diverse developmental and physiological processes. Sequenced genomes from an increasing number of bilaterians have provided a more complete picture of duplication and loss of nuclear receptors in protostomes and deuterostomes but have left open the question of which nuclear receptors were present in the cnidarian-bilaterian ancestor. In addition, nuclear receptor expression and function are largely uncharacterized within cnidarians, preventing determination of conserved and novel nuclear receptor functions in the context of animal evolution.
Results
Here we report the first complete set of nuclear receptors from a cnidarian, the starlet sea anemone Nematostella vectensis. Genomic searches using conserved DNA- and ligand-binding domains revealed seventeen nuclear receptors in N. vectensis. Phylogenetic analyses support N. vectensis orthologs of bilaterian nuclear receptors in four nuclear receptor subfamilies within nuclear receptor family 2 (COUP-TF, TLL, HNF4, TR2/4) and one putative ortholog of GCNF (nuclear receptor family 6). Other N. vectensis genes grouped well with nuclear receptor family 2 but represented lineage-specific duplications somewhere within the cnidarian lineage and were not clear orthologs of bilaterian genes. Three nuclear receptors were not well-supported within any particular nuclear receptor family. The seventeen nuclear receptors exhibited distinct developmental expression patterns, with expression of several nuclear receptors limited to a subset of developmental stages.
Conclusion
N. vectensis contains a diverse complement of nuclear receptors including orthologs of several bilaterian nuclear receptors. Novel nuclear receptors in N. vectensis may be ancient genes lost from triploblastic lineages or may represent cnidarian-specific radiations. Nuclear receptors exhibited distinct developmental expression patterns, which are consistent with diverse regulatory roles for these genes. Understanding the evolutionary relationships and developmental expression of the N. vectensis nuclear receptor complement provides insight into the evolution of the nuclear receptor superfamily and a foundation for mechanistic characterization of cnidarian nuclear receptor function.
Background
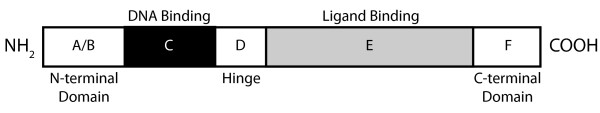
Nuclear receptors (NRs) are one of the most abundant classes of transcription factors in metazoans and coordinate diverse processes ranging from developmental patterning to physiological regulation. NRs appear to be restricted to animals because extensive work has shown nuclear receptors are present in all metazoan phyla (e.g., sponges [1,2], cnidarians [3,4], and bilaterians [5]) but not in plants, fungi or choanoflagellates [6]. The signature motifs of NRs are the DNA-binding domain (DBD), which includes a Cys-Cys zinc coordinating region, and the ligand-binding domain (LBD), a carboxy-terminal domain that binds ligands and facilitates receptor dimerization and coactivator recruitment (Figure 1). High sequence conservation within these domains, particularly the DBD, has permitted identification of NRs from a number of animals and phylogenetic analysis of the NR superfamily [5].
Figure 1.
Nuclear receptor gene structure. Nuclear receptors are composed of two well-conserved regions, the DNA binding domain (DBD) and the ligand binding domain (LBD).
Genes in the NR superfamily have been classified into six major NR families, many of which are further divided into subfamilies of orthologs and paralogs. All six families are represented in both the protostome and deuterostome lineages, supporting the hypothesis that NRs had undergone extensive radiation prior to the divergence of triploblastic animals. An analysis by Bertrand et al. [5] of NRs from nine bilaterian genomes did much to resolve the evolutionary history of this superfamily. They inferred 25 NRs that likely existed in the Urbilaterian with at least one gene from each of the six families. After this divergence, many NRs underwent extensive duplication in the vertebrates, particularly fishes, and one gene underwent extensive duplication in nematodes [7]. With the increasing availability of sequenced genomes and PCR-based surveys of NR diversity, particularly from non-ecdysozoan protostomes [8-10], it has become apparent that a number of NRs have also been lost since the Urbilaterian ancestor, particularly in insects and nematodes.
Because the NR superfamily had already diversified prior the divergence of the protostomes and deuterostomes, it is essential to characterize NRs in "basal" metazoans to understand not only when the NR families evolved but also the evolutionary relationships among these families. NRs have been reported from a handful of cnidarians from three of the four classes (Anthozoa [4], Cubozoa [11], Hydrozoa [3]) and two sponges [1,2]. Sequence and phylogenetic analyses of identified NRs have led these authors to suggest that many or all of these genes belong to NR family 2 with homologs of TLL (tailless, NR2E) in corals [4], HNF4 (hepatocyte nuclear factor 4, NR2A) in coral [4] and sponge [1], RXR (retinoic × receptor, NR2B) in the box jelly [11], and COUP-TF (chicken ovalbumin upstream promoter transcription factor, NR2F) in coral and hydra ([3,4]).
Despite the importance of understanding how NR regulation of development and physiology has evolved, little has been reported regarding the expression and function of NRs in non-bilaterian animals. Some cnidarian NRs have been shown to share conserved features with their vertebrate orthologs. For example, the RXR homolog in the box jelly Tripedalia cystophora specifically binds 9-cis retinoic acid with an affinity similar to that of vertebrate RXRs, supporting a conserved function of RXR in these evolutionary distant taxa [11]. The COUP-TF homolog from hydra is expressed in neuronal cells, suggesting an evolutionary origin in nerve cell specification [3]. Many of the identified NRs in cnidarians are orphan receptors (lacking known ligands). Cnidarian tissues contain diverse lipids including prostaglandins, fatty acids, sterols and steroids, and it is plausible that some of these compounds act as ligands for nuclear receptors [12-18]. In addition, the developmental expression of most cnidarian NRs remains uncharacterized. Temporal patterns of expression can provide significant insight into the potential role for NRs during development and metamorphosis as well as the opportunity for co-expressed NRs to interact with one another [19].
Here we present the first genomic analysis of nuclear receptors in the starlet sea anemone, Nematostella vectensis, and quantify expression of all identified NRs throughout the life cycle. We identified seventeen nuclear receptors in the genome of N. vectensis. Many of the NRs did not show clear orthology to bilaterian NRs, and some N. vectensis NRs appear to have resulted from lineage specific gene duplications. Expression of most NRs varied significantly during development, with clusters of NRs exhibiting similar expression patterns.
Results
Phylogenetic relationships of N. vectensis nuclear receptors
We identified 17 NRs from N. vectensis with a combination of bioinformatic searches of the assembled N. vectensis genome [20]. We have designated these as NvNR1-17 (Accession Numbers Table 1, numbering is arbitrary and does not indicate phylogenetic position within the NR superfamily; for amino acid sequences see Additional file 1). All of these genes were amplified from cDNA preparations and confirmed as expressed transcripts.
Table 1.
Accession numbers of N. vectensis nuclear receptors from NCBI GenBank, JGI Nematostella Database, and ESTs (where available).
| Gene Name | GenBank Acc. | JGI Gene Model | Matching ESTs |
| NvNR1 | XP_001634258 | 101676 | NA |
| NvNR2 | XP_001636937 | 99425 | NA |
| NvNR3 | XP_001632045 | 108851 | JGI_CAAB2572.fwd |
| NvNR4 | XP_001638550 | 89471 | JGI_CAAD1662 |
| NvNR5 | XP_001630386 | 114090 | JGI_CAGN9558.fwd |
| NvNR6 | XP_001635112 | 183874 | JGI_CAGN4016 |
| NvNR7 | XP_001630385 | 169225 | JGI_CAGN3217.fwd |
| NvNR8 | XP_001634999 | 99425 | NA |
| NvNR9 | XP_001624815 | 247458 | JGI_CAGG3386.fwd |
| NvNR10 | XP_001629708 | 189134 | JGI_CAAB5823 |
| NvNR11 | XP_001634340 | 242271 | JGI_CAGN16932 |
| NvNR12 | XP_001634378 | 165424 | JGI_CAGH2972 |
| NvNR13 | XP_001636010 | 203423 | NA |
| NvNR14 | XP_001636637 | 202735 | NA |
| NvNR15 | XP_001631902 | 167880 | JGI_CAGH3462.fwd |
| NvNR16 | XP_001631058 | 244121 | JGI_CAGN9351 |
| NvNR17 | XP_001624292 | 218255 | JGI_CAGG4622.fwd |
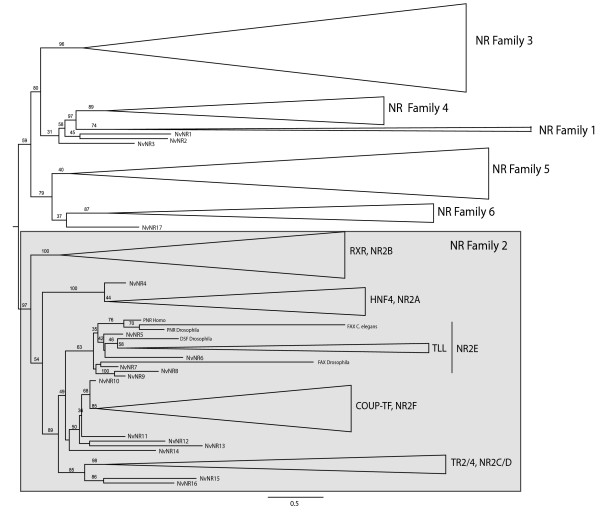
When DBD plus LBD were used for phylogenetic analyses of N. vectensis and bilaterian NRs, the topology of the tree with the highest likelihood was consistent with monophyletic relationships of the recognized NR families. Nodes for NR families 1-4 were each supported by high bootstrap scores (Figure 2, for a more detailed tree with nodes expanded see Additional file 2). Although NR families 5 and 6 were each weakly supported (BS = 40 and 37, respectively), a clade grouping these two families was fairly well supported (BS = 79) as previously shown by Thornton [21]. Phylogenetic analyses using just the DBD or portion of the LBD resulted in more poorly supported nodes throughout the tree and in some cases failed to recover monophyletic NR families (see Additional files 3 and 4). In addition, a complementary analysis using neighbor joining with the DBD and LBD also resulted in weakly supported nodes for the NR families (see Additional file 5). Therefore, we inferred the phylogenetic relationships of the N. vectensis NRs from the likelihood analysis based on the DBD plus LBD.
Figure 2.
Phylogenetic relationships of N. vectensis NRs (NvNRs) depicted by a maximum-likelihood tree with percent bootstrap support of 1000 bootstraps for nodes of evolutionary significance. The alignment was constructed using the DBD and a portion of the LBD (additional details in text). Monophyletic relationships for bilaterian sequences in particular families or subfamilies were recovered in all cases but one (NR2E) and are depicted as horizontal triangle. A full tree with all taxa depicted as individual branches is presented in Additional File 2. The tree was rooted with NR family 2 because this family is monophyletic, represents a defined nuclear receptor family, and includes what is likely the original nuclear receptor, represented by an HNF4-like homolog from the sponge Amphimedon queenslandica [1]. Most N. vectensis NRs group within NR family 2 and many (e.g., NvNR11- NvNR14) are supported as independent radiations of subfamilies within this family. N. vectensis also has a NR related to GCNF (NR family 6), but with low support. Three NRs did not group with any previously described family (NvNR1-3). These genes may represent ancestral genes that later diversified into one or more of the NR 1, 3, and 4 families.
NvNR1, 2, and 3 cannot be unambiguously assigned to a family and nest as equally related to NR families 1 and 4. A majority of N. vectensis NRs (NvNR4-16) are strongly supported as homologs of genes in NR family 2. These N. vectensis NRs are associated with four subfamilies within family 2. NvNR4 is an ortholog of HNF4 (subfamily 2A), which has also been identified in vertebrates, insects, and nematodes [22,23]. NvNR5-9 cluster with subfamily 2E (TLL, FAX, PNR). NvNR5 and 6 are most closely related to TLL with moderate bootstrap support. NvNR7-9 do not have a clear orthologs with the bilaterian genes from this subfamily and thus may represent a cnidarian-specific radiation. NvNR10-14 group with subfamily 2F (COUP-TFs), and among these, NvNR10 is most closely related to the bilaterian COUP-TFs. NvNR15-16 are supported as homologs of the testicular orphan receptors (TR2/TR4, subfamily 2C/D). An RXR homolog (jRXR, subfamily 2B) has been identified in the box jelly Tripedalia cystophora [11], but an RXR homolog is not apparent in the N. vectensis genome. NvNR17 groups with NR family 6 (GCNF, germ cell nuclear factor) but with relatively weak support. In the neighbor joining analysis, NvNR17 was positioned as an outgroup to NR families 5 and 6 but with low bootstrap support. A GCNF-like gene has not been previously reported from a cnidarian.
Gene structure
N. vectensis NRs are composed of between 3 and 7 exons and span 2059 to 11741 bp of genomic sequence (see Additional file 6). Because we currently have complete transcript sequence with 5- and 3- prime untranslated region (UTR) for fewer than half of the NRs (6 of 17), additional exons may be identified when complete transcripts are confirmed. In some cases (e.g., NvNR10), exons were composed completely of UTR sequence; thus it is likely that additional exons will be identified for some of the NRs with uncharacterized UTR sequence.
Phylogenetic analyses indicated that several NRs in N. vectensis are most closely related to one another suggesting potential lineage-specific duplication. With the exception of two genes (NvNR11 and NvNR12), the NRs in N. vectensis are located on different scaffolds. Thus, the NR diversity in N. vectensis does not appear to have resulted from recent tandem duplications, but instead may represent more ancient duplications within the cnidarian lineage. Some closely related genes also shared conserved intron-exon structure (see Additional file 7). For example, NvNR8 and NvNR9 group together with 100% bootstrap support and share two of three splice sites.
Developmental expression of nuclear receptors
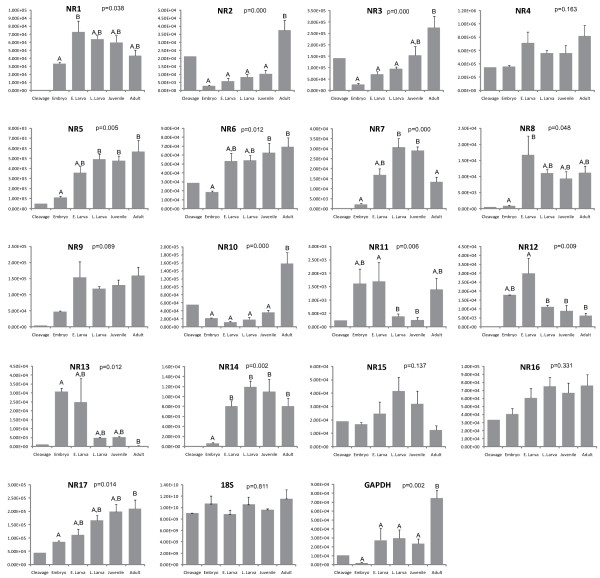
One-way ANOVA indicated that 13 of the 17 NRs varied significantly in their level of expression during development (Figure 3). Even with relatively coarse developmental staging, we observed clear temporal patterns of gene expression (Figure 4a). NvNR8, 11, 12 and 13 were primarily expressed during embryonic and/or early larval stages with little expression in the juvenile and adult stages. NvNR1, 7, 14 and 15 were most highly expressed during larval and juvenile stages. NvNR4, 5, 6, 9, 16, and 17 showed increased expression during larval and juvenile stages and continued high expression in adults. Finally, NvNR2, 3, and 10 were primarily expressed in adults, and NvNR10 was only minimally expressed in earlier stages. 18S expression did not vary significantly across the stages and is apparently a good housekeeping gene with respect to N. vectensis development. We also tested GAPDH as a potential control gene to standardize gene expression. GAPDH expression was highly variable across these developmental stages and was not used for comparisons of NR expression among stages.
Figure 3.
Developmental expression of Nematostella vectensis nuclear receptors (NR1-NR17) and putative housekeeping genes (18S and GAPDH). Expression measured using qPCR, as described in text. Expression in molecules per μl cDNA indicated on the y-axis. Bar indicate the mean ± standard error of 3-5 biological replicates. The first bars ("cleavage") represents a single sample, which was not included in statistical analysis. Expression patterns were analyzed with a one-way ANOVA followed by pairwise comparisons with Tukey's Honestly Significant Difference Test. Letters indicate groups that were different at a level of p = 0.05. GAPDH showed variable expression over the sampled stages and was not used as a standard for comparing gene expression of NRs among developmental stages.
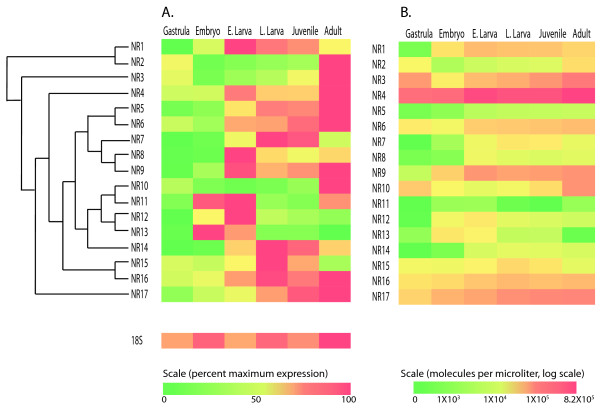
Figure 4.
Heat maps indicating developmental expression of Nematostella vectensis nuclear receptors. Cladogram indicates the topology from Figure 2 with other taxa removed. (A) For each gene, expression is shown on a linear scale as a percentage of the stage with the highest expression. (B) Expression is shown on a log scale as molecules per microliter, calculated in relation to standard curves constructed from serially diluted plasmids.
Absolute levels of gene expression, normalized to plasmid standards, indicated large differences in number of expressed transcripts among genes (Figure 4b). Although its expression was developmentally variable (Figure 3, 4a), NvNR4 was the most highly expressed NR over all stages (Figure 4b). NvNR3, 9 and 17 also showed high levels of overall expression.
In several cases, phylogenetically closely related genes showed divergent temporal expression patterns during development. For example, NR11 was expressed at very low levels (Figure 4b), primarily during embryonic and early larval stages (Figure 4a). In contrast, NR10 was expressed at moderate levels, mostly in adults.
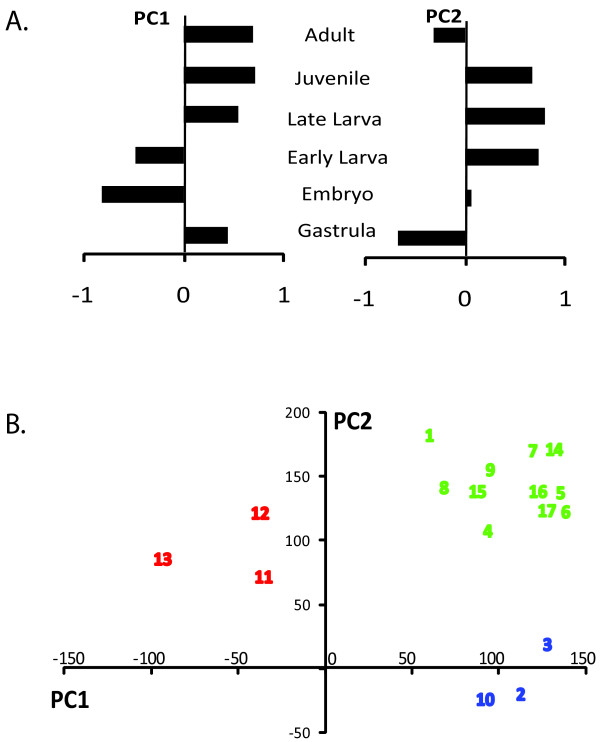
Principal components analysis (PCA) is a statistical technique for reducing the dimensionality of a dataset, in this case facilitating identification of NRs with similar expression patterns. PCA was conducted for all 17 genes from all developmental stages (Figure 5). The first principal component (PC1) accounted for 39% of the overall variance in gene expression and second principal component (PC2) accounted for 36% of the variance. When the values for PC1 and PC2 are shown on a scatter plot (Figure 5B), three clusters emerged, NvNR11, 12 and 13 clustered to the exclusion of other genes along PC1 and were each highly expressed in early developmental stages (embryo, early larva). A second cluster contained NvNR2, 3, and 10, which are most strongly expressed in adults. The remaining genes constituted a third cluster and were not strongly distinguished from one another by this analysis.
Figure 5.
Principal components analysis of nuclear receptor relative expression data. Analysis was conducted on gene expression normalized to the maximum expression for each gene, as in Figure 4A. (A) Eigen vectors showing relative contribution of each life stage to each principal component. Longer bars indicate stronger relative contribution. The first and second principal components explained 39% and 36% of the variance, respectively. (B) Scatter plot of first and second principal component scores for all seventeen nuclear receptors. Colors indicate three distinct clusters of NRs showing similar expression patterns. Numbers 1-17 indicate N. vectensis nuclear receptors 1-17, respectively.
Discussion
Cnidarians both occupy a key evolutionary position and are ecologically important as predators, prey and structure-builders in marine and freshwater environments. As "basal" metazoans, cnidarians form an outgroup to the bilaterian animals and are intermediate in complexity between sponges and bilaterians. In spite of the acknowledged importance of understanding cnidarians from both an ecological and an evolutionary perspective, cnidarian physiology is poorly understood, particularly at the molecular level. Thus, characterizing cnidarian NRs provides insight both into evolution of NR signaling and bioregulatory processes in a major group of aquatic animals.
Homology and expression of NvNRs
Analysis of the phylogenetic relationships among NRs from the starlet sea anemone, Nematostella vectensis, provides strong support for the diversification of NR family 2 prior to the divergence of the cnidarian and bilaterian lineages. Phylogenetic analyses of N. vectensis and bilaterian NRs revealed cnidarian orthologs of HNF4, COUP, TLL, and TR2/4. Grasso et al. [4] identified 10 NRs in the coral Acropora millepora, including several members of NR family 2. Our results support their conclusion that family 2 was well diversified prior to the split between the cnidarian and bilaterian lineages.
NvNR4 is an apparent homolog of hepatocyte nuclear factor 4 (HNF4, subfamily 2A), which has also been identified in vertebrates, insects, nematodes, and corals [4,22,23]. In mammals, HNF4α binds endogenous fatty acid ligands [24,25] and regulates hepatocyte differentiation, energetic metabolism and xenobiotic detoxification. HNF4 also regulates insect gut development [26,27]. HNF4 underwent extensive duplications in C. elegans [28] such that inferring homologous functions for HNF4 from the nematode is difficult. One C. elegans paralog, nhr-49, is involved in energetic metabolism as a regulator of fat storage [29]. In addition, an HNF4 homolog has been cloned from sponges and may be similar to the ancestral NR [1]. Sponge HNF4 is expressed throughout development in ciliated column cells of the outer epithelium [1]. In N. vectensis, NvNR4 was expressed at all developmental time points with higher mean expression from larval to adult stages. We predict that NvNR4 will be expressed in endodermal tissue, which would be consistent with conserved roles in development of the digestive epithelium, energetic metabolism, and/or detoxification.
NvNR5- NvNR9 cluster with subfamily 2E (TLL/TLX, FAX, PNR); members of this subfamily have previously been reported in corals [4,30]. In our phylogenetic analyses, we were unable to fully resolve the evolutionary relationships of N. vectensis and bilaterian NRs within subfamily 2E (Figure 2). In other animals, members of subfamily 2E are involved in neural differentiation. Tailless homologs in mammals (TLX) and insects (TLL) are particularly important for eye and forebrain development [31-34] and embryonic anterior-posterior patterning [35]. FAX-1 regulates neural patterning in C. elegans [36,37], and PNR plays a more specialized role by regulating retinal development in vertebrates [38]. Several N. vectensis members of subfamily 2E (i.e., NvNR5, 7, 8) were highly expressed during early developmental stages coinciding with neurogenesis and embryonic patterning [39]. The stage of maximal expression varied among members of this subfamily, indicating potential diversification of function within the cnidarian lineage.
NvNR10-NvNR14 group with subfamily 2F (COUP-TFs); COUP-TFs have also been identified in corals, hydra, flatworms, sea urchin, and lancelets [3,4,40-42]. NvNR10 was most closely related to bilaterian COUP-TFs, while NvNR11- NvNR14 are supported as an independent radiation with this subfamily. COUP-TFs generally act as transcriptional repressors and regulate development of muscles, the heart and the nervous system, particularly differentiation of the hindbrain and photoreceptors [43-46]. In hydra, COUP-TF is expressed in nematoblasts (nematocyte precursors) and in a subset of neuronal cells, consistent with a conserved role of COUP-TF in regulating neural differentiation [3]. COUP-TFs can also affect reproductive processes through cross-talk with estrogen receptors and ecdysteroid receptors in vertebrates and insects, respectively [47-50]. COUP-TF homologs from N. vectensis showed diverse expression patterns during development. For example, NvNR12 and13 were primarily expressed during embryonic and/or early larval stages with little expression in the juvenile and adult stages. The expression of these COUP-TF-like genes coincides with neurogenesis and embryo patterning [39]. In contrast, we observed relatively high expression of NvNR10 in adults but lower expression during embryogenesis and larval development.
We identified two members of the subfamily 2C/D (TR2/4, NvNR15-16). TR2/4 homologs have been identified in a range of animals including vertebrates, sea urchins, ascidians, nematodes, insects, flatworms, and corals [51-54]. Protostomes and most deuterostomes have a single TR2/4 homolog. Interestingly, both N. vectensis and the coral Acropora millepora [4] contain two TR2/4 homologs, suggesting a cnidarian-specific duplication. In general, TR2/4 homologs act as transcriptional repressors through several mechanisms including competition with other nuclear receptors for binding sites and co-factors [55,56]. TR2/4 homologs are broadly expressed in vertebrate tissues [57] and throughout development in mammals [57], ascidians [51], and flatworms [58]. We observed similar ubiquitous expression in N. vectensis where both NvNR15 and 16 were expressed throughout all developmental stages.
NvNR17 forms a monophyletic grouping with NR family 6 (GCNFs, germ cell nuclear factors). Although bootstrap support for this assignment is relatively weak, likelihood analyses of the DBD plus LBD and each of these domains individually group NvNR17 as a homolog to the GCNFs. While neighbor joining analysis placed NvNR17 as an outgroup to NR families 5 and 6, bootstrap support for deep nodes of this tree were low relative to the maximum likelihood analyses, potentially obscuring evolutionary relationships. GCNF has been reported from a number of bilaterian phyla. This is the first report of a putative cnidarian homolog from this NR family. Expression of GCNF homologs varies greatly among taxa. In D. melanogaster, DmHR4 is expressed following pulses of 20-hydroxyecdysone during discrete stages of embryogenesis, the second larval instar and prepupal development [59,60]. In C. elegans, CeNHR-91 is expressed in response to ATP-binding cassette protein E in embryos, larvae and several adult tissues [61]. In vertebrates, GCNF is expressed primarily expressed in gonadal tissues and during embryogenesis [62,63]. In vertebrates, GCNF is involved in neuronal differentiation, germ cell development, and axial patterning, and gametogenesis [63-65]. In our study, NvNR17 expression increased throughout the sampled developmental stages. Highest expression was in adult tissues, which may reflect a role in gametogenesis.
NvNR1-3 did not group well with any previously defined NR family. These genes are most closely related to NR Family 1 and 4. With the available data, we cannot conclude whether these represent the descendents of ancient NRs that later diversified into recognized NR families or if these genes represent an independent radiation somewhere within the cnidarian lineage. Further sampling within the Cnidaria will be necessary to help resolve the evolutionary relationship of these NRs.
We did not identify a member of NR family 3 in N. vectensis. NR family 3 is represented in protostomes and deuterostomes [5,21,66,67], and has been more recently identified in the placozoan Trichoplax adhaerens [[68], Reitzel and Tarrant, unpublished data] but has not yet been reported from any cnidarian. Thus, NR 3 homologs have apparently been lost early in the cnidarian lineage. NR family 3 contains the vertebrate-type steroid (i.e., non-ecdysteroid) receptors. While NR3 family members were not identified in N. vectensis, estrogens and other steroids have been detected in cnidarian tissues [12,15,69], are apparently released during spawning events [12,69], and can experimentally affect coral growth and reproduction [70]. The mechanism for steroid action in cnidarians is currently unknown and may be mediated through nuclear receptors that are not orthologs of the vertebrate steroid receptors or through alternative mechanisms.
RXR loss in N. vectensis
N. vectensis appears to have lost the ortholog of RXR because this gene is present in another cnidarian, the cubozoan Tripedalia cystophora [11]. The RXR homolog (jRXR) from T. cystophora bound 9-cis retinoic acid, and heterodimerized with vertebrate thyroid hormone receptor [11]. This evidence suggests that this cnidarian RXR functions in a similar way as vertebrate RXRs. In contrast to the box jelly, an RXR ortholog was not identified in a PCR-based survey of NRs from the coral Acropora millepora [4]. However, RXR immunoreactivity has been reported in epithelial nerve tissue of the coral A. millepora and the sea pansy Renilla koellikeri, but the genes were not identified [71]. Indeed the authors of the immunological study indicated that the epitope for the antibody has highest sequence similarity to A. millepora nuclear receptor 8, but phylogenetic analyses indicate that AmNR8 is most similar to TR2/4 [4]. The absence of an RXR ortholog in N. vectensis and in corals is particularly interesting from a functional perspective because vertebrate and insect RXRs form heterodimers with many other nuclear receptors to regulate gene expression [48,72-74]. In a parallel example, C. elegans independently lost an RXR ortholog, but another NR (nhr-49, HNF4-like) can dimerize with several other NRs [75]. Thus, it has been suggested that nhr-49 may have assumed an RXR-like function as a common heterodimerization partner [76]. Similarly, N. vectensis and other cnidarians may have one or more receptors that "promiscuously" form heterodimers with other NRs in a manner similar to bilaterian RXRs. Alternatively, it is possible that RXR has been lost and not replaced with a functional equivalent.
Gene duplication and divergence
Within NR family 2, our data from N. vectensis support independent radiations of several subfamilies. Phylogenetic analyses support two TR2/4 genes and multiple COUP-TF-like NRs in N. vectensis. Similarly, we observed 5 N. vectensis NRs that group with bilaterian NRs in the subfamily NR 2E. These genes are likely cnidarian-specific radiations within these NR 2 subfamilies and may represent interesting cases for better understanding the diversification of NR function within an early diverging metazoan.
After gene duplications, paralogs may be selected for divergent functions that may be reflected by different expression patterns [77,78]. Previous reports of N. vectensis paralogs have suggested that duplicated genes have varying degrees of distinct spatial and temporal gene expression patterns (e.g., snail [79], Otx [80]). In some cases, NvNRs supported as likely lineage specific duplications showed divergent temporal gene expression patterns consistent with subfunctionalization. For example, NvNR1 was highly expressed in the larval and juvenile stages, and the closely related NvNR2 was most highly expressed during cleavage and in adults. In situ hybridization studies of N. vectensis will reveal spatial expression patterns (e.g., anterior-posterior gradients, neural expression), providing further insight into the potential subfuctionalization of duplicated NRs.
Conclusion
In this study, we have identified 17 nuclear receptors in the sea anemone Nematostella vectensis. Phylogenetic analyses indicate that a majority of these NRs are members of NR family 2 and are consistent with previous studies indicating that this family had diversified prior to the cnidarian-bilaterian split. We also identified a putative homolog to GCNF in NR family 6 as well as three NRs that do not group with any previously identified NR family. Expression of N. vectensis NRs varied during development, consistent with stage-specific functions related to development, metamorphosis and adult physiology. Future studies of NR function in N. vectensis will provide a critical understanding of the evolution the nuclear receptor superfamily in animals.
Methods
Animals
The starlet sea anemone, Nematostella vectensis, is a small burrowing anemone found in estuaries along the Atlantic Coast of the United States, as well as in populations in England and along the Pacific Coast of the United States. We collected adults from Great Sippewissett Marsh, MA USA and cultured them under standard conditions (0.45 μm filtered seawater diluted to 13 ppt, room temperature, fed twice weekly brine shrimp and once mussel ovary; similar to previously described conditions [81,82]). Under these conditions, N. vectensis undergoes a predictable reproductive cycle that includes weekly spawning. Within 2-3 hours after spawning, embryos were gathered from dishes, rinsed with diluted seawater, and placed in sterile dishes. At specific stages (i.e., cleavage, early planula, late planula [pyramid shape], and juvenile, [81]) we collected individuals with a pipette, lightly centrifuged, decanted excess seawater, and snap froze with liquid nitrogen for later analysis. Adults were left unfed for at least one week prior to freezing to minimize contamination from food sources.
RNA extraction and cDNA synthesis
For cloning of nuclear receptors, total RNA was extracted from N. vectensis of varying developmental stages, ranging from embryos to adult. RNA was extracted by homogenization of whole individuals in STAT-60 (Tel-Test, Inc). RNA for cloning was then pooled from developmental stages. RNA purity and yield were quantified using a ND-1000 spectrophotometer (Nanodrop). RNA quality was visualized for representative samples on a denaturing agarose gel. Polyadenylated-enriched RNA (polyA-RNA) was prepared from total RNA using the MicroPoly(A)Purist Kit (Ambion). Complementary DNA (cDNA) was synthesized from polyA-RNA using the Iscript cDNA Synthesis Kit (Bio-Rad) using 1 μg of polyA-RNA per 20 μl reaction.
For characterization of developmental expression patterns, RNA was extracted using STAT-60 and the Aurum Total RNA Mini Kit with on-column DNAse digestion, as described previously [83]. Each extraction included multiple individuals from a single spawning event or in the case of adults, multiple individuals from the breeding stock. From the total RNA, cDNA was synthesized with the Iscript cDNA Synthesis Kit using 2 μg of RNA per 30 μl reaction. cDNA was prepared from the following developmental stages: cleavage (0.5 days post fertilization [dpf], n = 1), embryos (1 dpf, n = 3), early larvae (3-4 dpf, n = 4), late larvae (7-11 dpf, not metamorphosed, n = 4), juvenile (8-23 dpf, metamorphosed, n = 4), and adult (>100 dpf, n = 4).
Database searching and cloning
N. vectensis nuclear receptors were identified through a combination of BLASTp and domain searches through the Joint Genome Institute N. vectensis assembly. Genes were additionally queried against the EST database at JGI in order to annotate more complete transcripts. To confirm the predicted sequences we amplified, cloned, and sequenced large portions of each predicted NR transcript (primers designed with Primer3, primer sequences listed in Table S1). The PCR mixture consisted of 11.8 μl of molecular biology grade distilled water, 0.1 μl of AmpliTaq Gold (Applied Biosystems), 2 μl of the accompanying 10× Buffer, 3.75 mM MgCl2, 0.8 mM dNTPs, 0.5 μl of 10 μM gene-specific primers, and 0.5 μl of 1:5 diluted polyA cDNA. PCR conditions were as follows: 95°C for 5 min; 40 cycles of 95°C for 15 s, 60°C for 30 s, and 72°C for 60 s, followed by a 10 min extension at 72°C. Products were gel purified, ligated into pGEM-T Easy vector (Promega), and sequenced to confirm the targeted amplicon.
Phylogenetic analysis
We used a likelihood based approach to determine evolutionary relationships of the N. vectensis NRs with bilaterian NRs. NRs from Homo sapiens, Danio rerio, Xenopus laevis, Drosophila melanogaster, and Caenorhabditis elegans from Bertrand et al. [5] were used as representative bilaterians. We included sequences for these species from each of the defined families and subfamilies in order to fully represent the broad-scale diversity of NRs. We also included NR sequences from Ciona intestinalis reported in [51] and an estrogen receptor homolog from the mollusc Aplysia californica [84]. Additional reports of NR diversity from other animals [9,67,85,86] have shown that these taxa fully represent the diversity of NRs to the sub-family level, despite lineage-specific losses in some ecdysozoans and gene-specific duplications in a variety of taxa. Full length sequences for all taxa were aligned with Muscle 3.6 [87] and edited manually in the case of clear errors. Maximum likelihood analyses were run using RAxML (version 7.0.4, [88]) with a JTT+G matrix (model determined by AIC criteria with ProtTest v1.4, [89]). Separate analyses were conducted using the DBD only, a portion of the LDB, and the DBD plus a portion of LBD. We also performed a complementary neighbor joining analysis of the DBD and LBD alignment (PHYLIP v3.6). Trees were visualized and illustrated with FigTree v1.1.2 http://tree.bio.ed.ac.uk/software/figtree/.
Gene structure
We assembled the most complete transcripts for each of N. vectensis' NRs with a combination of cloned sequence, ESTs, and, where necessary, gene prediction models from the Joint Genome Institute (JGI). Intron-exon structure was determined by aligning the assembled transcripts to the most recent genome scaffolds in the JGI N. vectensis database. Gene structure was illustrated with GenePalette v1.21 [90].
Quantitative real-time RT-PCR (qPCR)
Oligonucleotide primers (see Additional file 8) were designed to amplify each N. vectensis NR, as well as glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and 18S ribosomal RNA (18S). Primers were 20-21 nt, with a GC content of 40-60%, either overlapped predicted exon-exon boundaries by 3-4 bp or spanned a large intron, and produced predicted amplicons of 55-146 bp with minimal predicted secondary structure (m-fold, [91]). A standard curve was constructed from serially-diluted plasmids containing the amplicon of interest. The standard curve was used in qPCR reactions to quantify amplification efficiency and to calculate the number of molecules per reaction (as in [92]). qPCR was performed using iQ SYBR Green Supermix (Bio-Rad), and reactions were run a MyCycler Real-Time PCR detection system (Bio-Rad). For each gene, standards were run in triplicate wells and experimental samples were run in duplicate wells (technical replicates) on a single plate. The PCR mixture consisted of 11.5 μl of molecular biology grade distilled water, 12.5 μl of IQ SYBR Green Supermix, 0.5 μl of 10 μM gene-specific primers, and 0.5 μl of cDNA. PCR conditions were as follows: 95°C for 3 min; 40 cycles of 95°C for 15 s and 64°C for 45 s. After 40 cycles, the PCR products from each reaction were subjected to melt curve analysis to ensure that only a single product was amplified. Selected reactions for each gene were visualized on 15% TBE gels (Bio-Rad) and consistently yielded single bands of the predicted size. The number of molecules per μl for each gene was calculated by comparing the threshold cycle (Ct) from the sample with the standard curve. Expression was compared among developmental stages using one-way analysis of variance (ANOVA) with Tukey's Honestly Significant Difference Test as a posthoc test (SYSTAT 12). Relative gene expression patterns were compared with principal component analysis (PCA, calculated with SYSTAT 12). This statistical approach is similar to that previously described by Tarrant and colleagues [83].
Authors' contributions
AMR conducted bioinformatic, gene structure, and phylogenetic analysis, designed primers, cloned nuclear receptor fragments, and drafted the manuscript. AMT conceived of the study, conducted qPCR assays and statistical analysis, and contributed to the draft. The authors together interpreted the data and revised the manuscript. Both authors read and approved of the final manuscript.
Supplementary Material
N. vectensis deduced amino acid sequences used for phylogenetic analyses. List of amino acid sequences.
Maximum likelihood tree using the DBD and portion of the LBD. NRs were taken from N. vectensis, Homo sapiens, Danio rerio, Xenopus laevis, Drosophila melanogaster, Caenorhabditis elegans, Aplysia californica, and Ciona intestinalis. Tree was rooted with the NR 2 family. Values above branches indicate percent of 1000 bootstraps.
Maximum likelihood tree using only the DBD portion of the NR alignment. Tree was rooted with the NR 2 family. Values above branches indicate percent of 1000 bootstraps.
Maximum likelihood tree using only the alignable portions of the LBD of the NR alignment. Tree was rooted with the NR 2 family. Values above branches indicate percent of 1000 bootstraps.
Neighbor joining tree constructed using the DBD and portion of LDB of representative NRs. NRs from N. vectensis, Homo sapiens, Danio rerio, Xenopus laevis, Drosophila melanogaster, Caenorhabditis elegans, Aplysia californica, and Ciona intestinalis. Tree was rooted with the NR 2 family. Values above branches indicate percent of 1000 bootstraps.
Intron-exon structure for seventeen N. vectensis NRs. White boxes indicate exons with coding sequence, gray boxes are exons or portions of exons containing the untranslated regions, and lines between boxes indicate introns.
Exon boundaries (vertical bars) for two sets of inferred cnidarian-specific NR radiations. (A) NvNR8 and 9, TLL-like genes, share 2 of the 3 exon positions. We could not compare the first boundary in NvNR9 with NvNR8 because we do not have corresponding sequence data for this portion of the protein. (B) Four NvNRs inferred to be duplications related to COUP-TF. NvNR12, 13 and 14 each has 3 exons with two conserved splice sites in all three genes. These two positions are also conserved in NvNR11, but this gene has two additional exon boundaries without conservation in the other three NRs from this subfamily.
Primer sequences for amplifying pieces of NvNRs for cloning and for qPCR. Table of primer sequences.
Acknowledgments
Acknowledgements
We are grateful for financial support from the Woods Hole Oceanographic Institution (WHOI) through the Tropical Research Initiative, the Ocean Life Institute (AMT), the Academic Programs Office, and to the Beacon Institute for Rivers and Estuaries (AMR). We thank Natasha Rabinowitz for assistance with animal care. We would also like to thank Mark Hahn, Jed Goldstone, John McLachlan, and Tung-chin Chiang for assistance in formulating ideas and approaches during this study.
Contributor Information
Adam M Reitzel, Email: areitzel@whoi.edu.
Ann M Tarrant, Email: atarrant@whoi.edu.
References
- Larroux C, Fahey B, Liubicich D, Hinman V, Gauthier M, Gongora M, Green K, Wörheide G, Leys S, Degnan B. Developmental expression of transcription factor genes in a demosponge: insights into the origin of metazoan multicellularity. Evolution and Development. 2006;8:150–173. doi: 10.1111/j.1525-142X.2006.00086.x. [DOI] [PubMed] [Google Scholar]
- Wiens M, Batel R, Korzhev M, Müller W. Retinoid × receptor and retinoic acid response in the marine sponge Suberites domuncula. Journal of Experimental Biology. 2003;206:3261–71. doi: 10.1242/jeb.00541. [DOI] [PubMed] [Google Scholar]
- Gauchat D, Escriva H, Miljkovic-Licina M, Chera S, Langlois M-C, Begue A, Laudet V, Galliot B. The orphan COUP-TF nuclear receptors are markers for neurogenesis from cnidarians to vertebrates. Developmental Biology. 2004;275:104–23. doi: 10.1016/j.ydbio.2004.07.037. [DOI] [PubMed] [Google Scholar]
- Grasso L, Hayward D, Trueman J, Hardie K, Janssens P, Ball E. The evolution of nuclear receptors: evidence from the coral Acropora. Molecular Phylogenetics and Evolution. 2001;21:93–102. doi: 10.1006/mpev.2001.0994. [DOI] [PubMed] [Google Scholar]
- Bertrand S, Brunet F, Escriva H, Parmentier G, Laudet V, Robinson-Rechavi M. Evolutionary genomics of nuclear receptors: from twenty-five ancestral genes to derived endocrine systems. Molecular Biology and Evolution. 2004;21:1923–37. doi: 10.1093/molbev/msh200. [DOI] [PubMed] [Google Scholar]
- King N, Westbrook MJ, Young SL, Kuo A, Abedin M, Chapman J, Fairclough S, Hellsten U, Isogai Y, Letunic I, et al. The genome of the choanoflagellate Monosiga brevicollis and the origin of metazoans. Nature. 2008;451:783–788. doi: 10.1038/nature06617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluder A, Matthews S, Hough D, Yin V, Maina C. The nuclear receptor superfamily has undergone extensive proliferation and diversification in nematodes. Genome Research. 1999;9:103–20. [PubMed] [Google Scholar]
- Keay J, Bridgham J, Thornton J. The Octopus vulgaris estrogen receptor is a constitutive transcriptional activator: evolutionary and functional implications. Endocrinology. 2006;147:3861–9. doi: 10.1210/en.2006-0363. [DOI] [PubMed] [Google Scholar]
- Wu W, Niles EG, El-Sayed N, Berriman M, LoVerde PT. Schistosoma mansoni (Platyhelminthes, Trematoda) nuclear receptors: Sixteen new members and a novel subfamily. Gene. 2006;366:303–315. doi: 10.1016/j.gene.2005.09.013. [DOI] [PubMed] [Google Scholar]
- Wu W, Niles EG, Hirai H, LoVerde PT. Evolution of a novel subfamily of nuclear receptors with members that each contain two DNA binding domains. BMC Evolutionary Biology. 2007;7:27. doi: 10.1186/1471-2148-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostrouch Z, Kostrouchova M, Love W, Jannini E, Piatigorsky J, Rall J. Retinoic acid × receptor in the diploblast, Tripedalia cystophora. Proceedings of the National Academy of Science, USA. 1998;95:13442–7. doi: 10.1073/pnas.95.23.13442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarrant A, Atkinson S, Atkinson M. Estrone and estradiol-17β concentration in tissue of the scleractinian coral, Montipora verrucosa. Comparative Biochemistry and Physiology A. 1999;122 doi: 10.1016/s1095-6433(98)10155-1. [DOI] [PubMed] [Google Scholar]
- Twan W-H, Hwang J-S, Chang C-F. Sex steroids in scleractinian coral, Euphyllia ancora: implication in mass spawning. Biol Reprod. 2003;68:2255–60. doi: 10.1095/biolreprod.102.012450. [DOI] [PubMed] [Google Scholar]
- Slattery M, Hines G, Starmer J, Paul V. Chemical signals in gametogenesis, spawning, and larval settlement and defense of the soft coral Sinularia polydactyla. Coral Reefs. 1999;18:75–84. doi: 10.1007/s003380050158. [DOI] [Google Scholar]
- Pernet V, Anctil M. Annual variations and sex-related differences of estradiol-17beta levels in the anthozoan Renilla koellikeri. General and Comparative Endocrinology. 2002;129:63–8. doi: 10.1016/S0016-6480(02)00513-0. [DOI] [PubMed] [Google Scholar]
- Eterovic V, Hann R, Ferchmin P, Rodriques A, Li L, Lee Y, McNamee M. Diterpenoids from Caribbean gorgonians act as noncompetitive inhibitors of the nicotinic acetylcholine receptor. Cell Mol Neurobiol. 1993;13:99–110. doi: 10.1007/BF00735367. [DOI] [PubMed] [Google Scholar]
- Fleury B, Lages B, Barbosa J, Kaiser C, Pinto A. New hemiketal steroid from the introduced soft coral Chromonephthea braziliensis is a chemical defense against predatory fishes. Journal of Chemical Ecology. 2008;34:987–93. doi: 10.1007/s10886-008-9499-y. [DOI] [PubMed] [Google Scholar]
- Koljak R, Järving I, Kurg R, Boeglin W, Varvas K, Valmsen K, Ustav M, Brash A, Samel N. The basis of prostaglandin synthesis in coral. Journal of Biological Chemistry. 2001;276:7033–40. doi: 10.1074/jbc.M009803200. [DOI] [PubMed] [Google Scholar]
- Sullivan AA, Thummel CS. Temporal profiles of nuclear receptor gene expression reveal coordinate transcriptional responses during Drosophila development. Mol Endocrinol. 2003;17:2125–2137. doi: 10.1210/me.2002-0430. [DOI] [PubMed] [Google Scholar]
- Putnam N, Srivastava M, Hellsten U, Dirks B, Chapman J, Salamov A, Terry A, Shapiro H, Lindquist E, Kapitonov V, et al. Sea anemone genome reveals ancestral eumetazoan gene repertoire and genomic organization. Science. 2007;317:86–94. doi: 10.1126/science.1139158. [DOI] [PubMed] [Google Scholar]
- Thornton JW. Nuclear receptor diversity: phylogeny, evolution and endocrine disruption. Pure and Applied Chemistry. 2003;75:1827–1839. doi: 10.1351/pac200375111827. [DOI] [Google Scholar]
- Maglich J, Sluder A, Guan X, Shi Y, McKee D, Carrick K, Kamdar K, Willson T, Moore J. Comparison of the complete nuclear receptor sets from the human, Caenorhabditis elegans, and Drosophila genomes. Genome Biology. 2001;2:1–7. doi: 10.1186/gb-2001-2-8-research0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sladek F, Zhong W, Lai E, Darnell J. Liver-enriched transcription factors HNF-4 is a novel member of the steroid hormone receptor superfamily. Genes Dev. 1990;4:2353–65. doi: 10.1101/gad.4.12b.2353. [DOI] [PubMed] [Google Scholar]
- Dhe-Paganon S, Duda K, Iwamoto M, Chi Y, Shoelson S. Crystal structure of the HNF4 alpha ligand binding domain in complex with endogenous fatty acid ligand. Journal of Biological Chemistry. 2002;277:37973–6. doi: 10.1074/jbc.C200420200. [DOI] [PubMed] [Google Scholar]
- Wisely G, Miller A, Davis R, Thornquest AJ, Johnson R, Spitzer T, Sefler A, Shearer B, Moore J, Miller A, et al. Hepatocyte nuclear factor 4 is a transcription factor that constitutively binds fatty acids. Structure. 2002;10:1225–34. doi: 10.1016/S0969-2126(02)00829-8. [DOI] [PubMed] [Google Scholar]
- Watt A, Garrison W, Duncan S. HNF4: a central regulator of hepatocyte differentiation and function. Hepatology. 2003;37:1249–53. doi: 10.1053/jhep.2003.50273. [DOI] [PubMed] [Google Scholar]
- Zhong W, Sladek F, Darnell J. The expression patttern of a Drosophila homolog to the mouse transcription factor HNF-4 suggests a determinative role in gut formation. The EMBO Journal. 1993;12:537–44. doi: 10.1002/j.1460-2075.1993.tb05685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson-Rechavi M, Maina C, Gissendanne C, Laudet V, Sluder A. Explosive lineage-specific expansion of the orphan nuclear receptor HNF4 in nematodes. Journal of Molecular Evolution. 2004;60:577–86. doi: 10.1007/s00239-004-0175-8. [DOI] [PubMed] [Google Scholar]
- Van Gilst MR, Hadjivassiliou H, Jolly A, Yamamoto KR. Nuclear hormone receptor NHR-49 controls fat consumption, storage, and composition in C. elegans. PLoS Biol. 2004;3:e53. doi: 10.1371/journal.pbio.0030053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarrant A, Cortés J, Atkinson M, Atkinson S, Johanning K, Chiang T-C, Vargas J, McLachlan J. Three orphan nuclear receptors in the scleractinian coral Pocillopora damicornis. Revista de Biologia Tropical. pp. 39–48.
- Stenman J, Yu R, Evans R, Campbell K. Tlx and Pax6 co-operate genetically to establish the pallio-subpallial boundary in the embryonic mouse telencephalon. Development. 2003;130:1113–22. doi: 10.1242/dev.00328. [DOI] [PubMed] [Google Scholar]
- Yu R, Chiang M-Y, Tanabe T, Kobayashi M, Yasuda K, Evans R, Umesono K. The orphan nuclear receptors Tlx regulates Pax2 and is essential for vision. Proceedings of the National Academy of Science, USA. 2000;97:2621–5. doi: 10.1073/pnas.050566897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M, Takezawa S, Hara K, Yu R, Umesono K, Agata K, Taniwaki M, Yasuda K, Umesono K. Identification of a photoreceptor cell-specific nuclear receptor. Proceedings of the National Academy of Science, USA. 1999;96:4814–9. doi: 10.1073/pnas.96.9.4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy K, Thiels E, Monaghan A. Loss of the tailless gene affects forebrain development and emotional behavior. Physiol Behav. 2002;77:595–600. doi: 10.1016/S0031-9384(02)00902-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steingrimsson E, Pignoni F, Liaw G, Lengyel J. Dual role of the Drosophila pattern gene tailless in embryonic termini. Science. 1991;254:418–21. doi: 10.1126/science.1925599. [DOI] [PubMed] [Google Scholar]
- Much JW, Slade DJ, Klampert K, Garriga G, Wightman B. The fax-1 nuclear hormone receptor regulates axon pathfinding and neurotransmitter expression. Development. 2000;127:703–712. doi: 10.1242/dev.127.4.703. [DOI] [PubMed] [Google Scholar]
- Wightman B, Ebert B, Carmean N, Weber K, Clever S. The C. elegans nuclear receptor gene fax-1 and homeobox gene unc-42 coordinate interneuron identity by regulating the expression of glutamate receptor subunits and other neuron-specific genes. Developmental Biology. 2005;287:74–85. doi: 10.1016/j.ydbio.2005.08.032. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Hara K, Yu RT, Yasuda K. Expression and functional analysis of Nr2e3, a photoreceptor-specific nuclear receptor, suggest common mechanisms in retinal development between avians and mammals. Development Genes and Evolution. 2008;218 doi: 10.1007/s00427-008-0232-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matus D, Thomsen G, Martindale M. FGF signaling in gastrulation and neural development in Nematostella vectensis, an anthozoan cnidarian. Development Genes and Evolution. 2007;217:137–148. doi: 10.1007/s00427-006-0122-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escriva H, Safi R, Hanni C, Langlois M-C, Saumitou-Laprade P, Stehelin D, Capron A, Pierce R, Laudet V. Ligand binding was acquired during the evolution of nuclear receptors. Proceedings of the National Academy of Science, USA. 1997;94:6803–8. doi: 10.1073/pnas.94.13.6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlois M-C, Vanacker J-M, Holland N, Escriva H, Queva C, Laudet V, Holland L. Amphicoup-TF, a nuclear orphan receptor of the lancelet Branchiostoma floridae, is implicated in retinoic acid signalling pathways. Development, Genes and Evolution. 2000;210:471–82. doi: 10.1007/s004270000087. [DOI] [PubMed] [Google Scholar]
- Chan S-M, Xu N, Niemeyer C, Bone J, Flytzanis C. SpCOUP-TF: a sea urchin member of the steroid/thyroid hormone receptor family. Proceedings of the National Academy of Science, USA. 1992;89:10568–72. doi: 10.1073/pnas.89.22.10568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson GW, Larson RJ, Oas DR, Sandhofer CR, Schwartz HL, Mariash CN, Oppenheimer JH. Chicken ovalbumin upstream promoter-transcription factor (COUP-TF) modulates expression of the Purkinje cell protein-2 gene. A potential role for COUP-TF in repressing premature thyroid hormone action in the developing brain. J Biol Chem. 1998;273:16391–16399. doi: 10.1074/jbc.273.26.16391. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Fujinuma M, Tanaka M, Dräger UC, McCaffery P. Sagittal band expression of COUP-TF2 gene in the developing cerebellum. Mechanisms of Development. 1999;84:143–146. doi: 10.1016/S0925-4773(99)00054-4. [DOI] [PubMed] [Google Scholar]
- Kerber B, Fellert S, Hoch M. Seven-up, the Drosophila homolog of the COUP-TF orphan receptors, controls cell proliferation in the insect kidney. Genes Dev. 1998;12:1781–1786. doi: 10.1101/gad.12.12.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjose A, Nornes S, Weber U, Mlodzik M. Functional conservation of vertebrate seven-up related genes in neurogenesis and eye development. The EMBO Journal. 1993;12:1403–14. doi: 10.1002/j.1460-2075.1993.tb05784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Yang N, Teng CT. COUP-TF acts as a competitive repressor for estrogen receptor-mediated activation of the mouse lactoferrin gene. Mol Cell Biol. 1993;13:1836–1846. doi: 10.1128/mcb.13.3.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Miuma K, Chen L, Raikhel A. Cyclicity of mosquito vitellogenic ecdysteroid-mediated signaling is modulated by alternative dimerization of the RXR homolog Ultraspiraclei. Proceedings of the National Academy of Science, USA. 2003;100:544–9. doi: 10.1073/pnas.0235695100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura K, Zhu J, Dittmer N, Chen L, Raikhel A. A COUP-TF/Svp homolog is highly expressed during vitellogenesis in the mosquito Aedes aegypti. Journal of Molecular Endocrinology. 2002;29:223–38. doi: 10.1677/jme.0.0290223. [DOI] [PubMed] [Google Scholar]
- Klinge CM. Role of estrogen receptor ligand and estrogen response element sequence on interaction with chicken ovalbumin upstream promoter transcription factor (COUP-TF) The Journal of Steroid Biochemistry and Molecular Biology. 1999;71:1–19. doi: 10.1016/S0960-0760(99)00124-7. [DOI] [PubMed] [Google Scholar]
- Devine C, Hinman VF, Degnan BM. Evolution and developmental expression of nuclear receptor genes in the ascidian Herdmania. International Journal of Developmental Biology. 2002;46:687–692. [PubMed] [Google Scholar]
- Lee Y-F, Lee H-J, Chang C. Recent advances in the TR2 and TR4 orphan receptors of the nuclear receptor superfamily. The Journal of Steroid Biochemistry and Molecular Biology. 2002;81:291–308. doi: 10.1016/S0960-0760(02)00118-8. [DOI] [PubMed] [Google Scholar]
- Hu Y, Shyr C, Che W, Mu X, Kim E, Chang C. Suppression of estrogen receptor-mediated transcription and cell growth by interaction with TR2 orphan receptor. Journal of Biological Chemistry. 2002;277:33571–9. doi: 10.1074/jbc.M203531200. [DOI] [PubMed] [Google Scholar]
- Yagi K, Satou Y, Mazet F, Shimeld S, Degnan B, Rokhsar D, Levine M, Kohara Y, Satoh N. A genome-wide survey of developmental genes in Ciona intestinalis III. Genes for Fox, ETS, nuclear receptors and NF-kB. Development, Genes and Evolution. 2003;213:235–44. doi: 10.1007/s00427-003-0322-z. [DOI] [PubMed] [Google Scholar]
- Tanabe O, Shen Y, Liu Q, Campbell AD, Kuroha T, Yamamoto M, Engel JD. The TR2 and TR4 orphan nuclear receptors repress Gata1 transcription. Genes Dev. 2007;21:2832–2844. doi: 10.1101/gad.1593307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Dufau ML, Gerald L. Vitamins & Hormones. Vol. 68. Academic Press; 2004. Gene Silencing by Nuclear Orphan Receptors; pp. 1–48. [DOI] [PubMed] [Google Scholar]
- Lee Y-F, Lee H-J, Chang C. Recent advances in the TR2 and TR4 orphan receptors of the nuclear receptor superfamily. Journal of Steroid Biochemistry and Molecular Biology. 2002;81:291–308. doi: 10.1016/S0960-0760(02)00118-8. [DOI] [PubMed] [Google Scholar]
- Hu R, Wu W, Niles EG, LoVerde PT. SmTR2/4, a Schistosoma mansoni homologue of TR2/TR4 orphan nuclear receptor. International Journal for Parasitology. 2006;36:1113–1122. doi: 10.1016/j.ijpara.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Sullivan A, Thummel C. Temporal profiles of nuclear receptor gene expression reveal coordinate transcriptional responses during Drosophila development. Molecular Endocrinology. 2003;17:2125–37. doi: 10.1210/me.2002-0430. [DOI] [PubMed] [Google Scholar]
- King-Jones K, Charles J-P, Lam G, Thummel C. The ecdysone-induced DHR4 orphan nuclear receptor coordinates growth and maturation in Drosophila. Cell. 2005;121:773–84. doi: 10.1016/j.cell.2005.03.030. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Fang L, Johnsen R, Baillie D. ATP-binding cassette protein E is involved in gene transcription and translation in Caenorhabditis elegans. Biochemical and Biophysical Research Communications. 2004;323:104–111. doi: 10.1016/j.bbrc.2004.08.068. [DOI] [PubMed] [Google Scholar]
- Lan Z-J, Gu P, Xu X, Cooney A. Expression of the orphan nuclear receptor, germ cell nuclear factor in mouse gonads and preimplantation embryos. Biology of Reproduction. 2003;68:282–9. doi: 10.1095/biolreprod.102.008151. [DOI] [PubMed] [Google Scholar]
- Chung ACK, Katz D, Pereira FA, Jackson KJ, DeMayo FJ, Cooney AJ, O'Malley BW. Loss of orphan receptor Germ Cell Nuclear Factor function results in ectopic development of the tail bud and a novel posterior truncation. Mol Cell Biol. 2001;21:663–677. doi: 10.1128/MCB.21.2.663-677.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgmeyer U. Dimeric binding of the mouse germ cell nuclear factor. European Journal of Biochemistry. 1997;244:120–7. doi: 10.1111/j.1432-1033.1997.00120.x. [DOI] [PubMed] [Google Scholar]
- Cooney A, Hummelke G, Herman T, Chen F, Jackson K. Germ cell nuclear factor is a response element-specfic repressor of transcription. Biochemical and Biophysical Research Communications. 1998;245:94–100. doi: 10.1006/bbrc.1998.8391. [DOI] [PubMed] [Google Scholar]
- Keay J, Bridgham JT, Thornton JW. The Octopus vulgaris estrogen receptor is a constitutive transcriptional activator: evolutionary and functional implications. Endocrinology. 2006;147:3861–3869. doi: 10.1210/en.2006-0363. [DOI] [PubMed] [Google Scholar]
- Keay J, Thornton JW. Hormone-Activated Estrogen Receptors in Annelid Invertebrates: Implications for Evolution and Endocrine Disruption. Endocrinology. 2009;150:1731–1738. doi: 10.1210/en.2008-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker ME. Trichoplax, the simplest known animal, contains an estrogen-related receptor but no estrogen receptor: Implications for estrogen receptor evolution. Biochemical and Biophysical Research Communications. 2008;375:623–627. doi: 10.1016/j.bbrc.2008.08.047. [DOI] [PubMed] [Google Scholar]
- Atkinson S, Atkinson M. Detection of estradiol-17β during a mass coral spawn. Coral Reefs. 1992;11:33–5. doi: 10.1007/BF00291932. [DOI] [Google Scholar]
- Tarrant A, Atkinson M, Atkinson S. Effects of steroidal estrogens on coral growth and reproduction. Marine Ecology Progress Series. 2004;269:121–9. doi: 10.3354/meps269121. [DOI] [Google Scholar]
- Bouzaiene M, Angers A, Anctil M. Immunohistochemical localization of a retinoic acid-like receptor in nerve cells of two colonial anthozoans (Cnidaria) Tissue and Cell. 2007;39:123–130. doi: 10.1016/j.tice.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Chawla A, Repa J, Evans R, Mangelsdorf D. Nuclear receptors and lipid physiology: opening the X-files. Science. 2001;294:1866–70. doi: 10.1126/science.294.5548.1866. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf D, Evans R. The RXR heterodimers and orphan receptors. Cell. 1995;83:841–50. doi: 10.1016/0092-8674(95)90200-7. [DOI] [PubMed] [Google Scholar]
- Amoutzias G, Pichler E, Mian N, De Graaf D, Imsiridou A, Robinson-Rechavi M, Bornberg-Bauer E, Robertson D, Oliver S. A protein interaction atlas for the nuclear receptors: properties and quality of a hub-based dimerisation network. BMC Systems Biology. 2007;1:34. doi: 10.1186/1752-0509-1-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Armstrong CM, Bertin N, Ge H, Milstein S, Boxem M, Vidalain P-O, Han J-DJ, Chesneau A, Hao T, et al. A map of the interactome network of the metazoan C. elegans. Science. 2004;303:540–543. doi: 10.1126/science.1091403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antebi A. Nuclear hormone receptors in C. elegans. Wormbook, The C. elegans Research Community; 2006. http://www.wormbook.org doi/10.1895/wormbook.1.64.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno S. Evolution by Gene Duplication. Heidelberg Springer-Verlag; 1970. [Google Scholar]
- Zhang J. Evolution by gene duplication: an update. Trends in Ecology & Evolution. 2003;18:292–298. [Google Scholar]
- Martindale MQ, Pang K, Finnerty JR. Investigating the origins of triploblasty: 'mesodermal' gene expression in a diploblastic animal, the sea anemone Nematostella vectensis (phylum, Cnidaria; class, Anthozoa) Development. 2004;131:2463–2474. doi: 10.1242/dev.01119. [DOI] [PubMed] [Google Scholar]
- Mazza ME, Pang K, Martindale MQ, Finnerty JR. Genomic organization, gene structure, and developmental expression of three Clustered otx genes in the sea anemone Nematostella vectensis. Journal of Experimental Zoology Part B: Molecular and Developmental Evolution. 2007;308B:494–506. doi: 10.1002/jez.b.21158. [DOI] [PubMed] [Google Scholar]
- Reitzel AM, Burton PM, Krone C, Finnerty JR. Comparison of developmental trajectories in the starlet sea anemone Nematostella vectensis: embryogenesis, regeneration, and two forms of asexual fission. Invertebrate Biology. 2007;126:99–112. [Google Scholar]
- Fritzenwanker J, Technau U. Induction of gametogenesis in the basal cnidarian Nematostella vectensis (Anthozoa) Development, Genes and Evolution. 2002;212:99–103. doi: 10.1007/s00427-002-0214-7. [DOI] [PubMed] [Google Scholar]
- Tarrant A, Baumgartner M, Verslycke T, Johnson C. Differential gene expression in diapausing and active Calanus finmarchicus (Copepoda) Marine Ecology Progress Series. 2008;355:193–207. [Google Scholar]
- Thornton JW, Need E, Crews D. Resurrecting the ancestral steroid receptor: ancient origin of estrogen signaling. Science. 2003;301:1714–1717. doi: 10.1126/science.1086185. [DOI] [PubMed] [Google Scholar]
- Maglich JM, Caravella JA, Lambert MH, Willson TM, Moore JT, Ramamurthy L. The first completed genome sequence from a teleost fish (Fugu rubripes) adds significant diversity to the nuclear receptor superfamily. Nucl Acids Res. 2003;31:4051–4058. doi: 10.1093/nar/gkg444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert M, Brunet F, Paris M, Bertrand S, Benoit G, Laudet V. Nuclear hormone receptor signaling in amphioxus. Development Genes and Evolution. 2008;218:651–665. doi: 10.1007/s00427-008-0251-y. [DOI] [PubMed] [Google Scholar]
- Edgar R. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research. 2004;32:1792–7. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis A. RAxML-VI-HPC: Maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 2006;22:2688–90. doi: 10.1093/bioinformatics/btl446. [DOI] [PubMed] [Google Scholar]
- Abascal F, Zardoya R, Posada D. ProtTest: selection of best-fit models of protein evolution. Bioinformatics. 2005;21:2104–5. doi: 10.1093/bioinformatics/bti263. [DOI] [PubMed] [Google Scholar]
- Rebeiz M, Posakony J. GenePalette: a universal software tool for genome sequence visualization and analysis. Developmental Biology. 2004;271:431–8. doi: 10.1016/j.ydbio.2004.04.011. [DOI] [PubMed] [Google Scholar]
- Zuker M. Mfold web server for nucleic acid folding and hybridization prediction. Nucl Acids Res. 2003;31:3406–3415. doi: 10.1093/nar/gkg595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarrant A, Greytak S, Callard G, Hahn M. Estrogen receptor-related receptors in the killifish Fundulus heteroclitus: diversity, expression, and estrogen responsiveness. Journal of Molecular Endocrinology. 2006;37:105–20. doi: 10.1677/jme.1.01976. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
N. vectensis deduced amino acid sequences used for phylogenetic analyses. List of amino acid sequences.
Maximum likelihood tree using the DBD and portion of the LBD. NRs were taken from N. vectensis, Homo sapiens, Danio rerio, Xenopus laevis, Drosophila melanogaster, Caenorhabditis elegans, Aplysia californica, and Ciona intestinalis. Tree was rooted with the NR 2 family. Values above branches indicate percent of 1000 bootstraps.
Maximum likelihood tree using only the DBD portion of the NR alignment. Tree was rooted with the NR 2 family. Values above branches indicate percent of 1000 bootstraps.
Maximum likelihood tree using only the alignable portions of the LBD of the NR alignment. Tree was rooted with the NR 2 family. Values above branches indicate percent of 1000 bootstraps.
Neighbor joining tree constructed using the DBD and portion of LDB of representative NRs. NRs from N. vectensis, Homo sapiens, Danio rerio, Xenopus laevis, Drosophila melanogaster, Caenorhabditis elegans, Aplysia californica, and Ciona intestinalis. Tree was rooted with the NR 2 family. Values above branches indicate percent of 1000 bootstraps.
Intron-exon structure for seventeen N. vectensis NRs. White boxes indicate exons with coding sequence, gray boxes are exons or portions of exons containing the untranslated regions, and lines between boxes indicate introns.
Exon boundaries (vertical bars) for two sets of inferred cnidarian-specific NR radiations. (A) NvNR8 and 9, TLL-like genes, share 2 of the 3 exon positions. We could not compare the first boundary in NvNR9 with NvNR8 because we do not have corresponding sequence data for this portion of the protein. (B) Four NvNRs inferred to be duplications related to COUP-TF. NvNR12, 13 and 14 each has 3 exons with two conserved splice sites in all three genes. These two positions are also conserved in NvNR11, but this gene has two additional exon boundaries without conservation in the other three NRs from this subfamily.
Primer sequences for amplifying pieces of NvNRs for cloning and for qPCR. Table of primer sequences.