Abstract
Heparin is a widely used anticoagulant and antithrombotic agent. Recently, a contaminant, oversulfated chondroitin sulfate (OSCS), was discovered within heparin preparations. The presence of OSCS within heparin likely led to clinical manifestations, most prevalently, hypotension and abdominal pain leading to the deaths of several dozens of patients. Given the biological effects of OSCS, one continuing item of concern is the ability for existing methods to identify other persulfonated polysaccharide compounds that would also have anticoagulant activity and would likely elicit a similar activation of the contact system. To complete a more extensive analysis of the ability for NMR and capillary electrophoresis (CE) to capture a broader array of potential contaminants within heparin, we completed a systematic study of NMR, both mono- and bidimensional, and CE to detect both various components of sidestream heparin and their persulfonated derivatives. We show that given the complexity of heparin samples, and the requirement to ensure their purity and safety, use of orthogonal analytical techniques is effective at detecting an array of potential contaminants that could be present.
Keywords: anticoagulant, capillary electrophoresis, contamination, NMR, oversulfated chondroitin
Heparin, a complex pharmaceutical agent, has been used clinically since the early part of the 20th century in the prevention and initial treatment of thrombosis. Heparin is a sulfated glucosaminoglycan comprised of polysaccharide chains of different molecular weights, with a well-defined statistical composition in terms of disaccharide units and pattern of sulfation. Although the chains within a heparin preparation can vary based on length and sulfation pattern, each is comprised of a disaccharide repeat unit of N-sulfo/N-acetylglucosamine linked 1 → 4 to a uronic acid, either α-l-iduronic acid or β-d-glucuronic. Differential O-sulfonation of each disaccharide, leading to structural heterogeneity, is possible at the 2-O- of the uronic acid as well as the 6-O- and 3-O-positions of N-sulfo/N-acetyl-d-glucosamine (1, 2).
Pharmaceutical grade heparin is commonly derived from whole porcine intestine or porcine intestinal mucosa (3, 4). In the preparation of heparin, the first step in the production scheme is the fractionation of crude (or raw) heparin from tissue (4) (Fig. S1). The constituents of crude heparin include heparin itself, other related glycosaminoglycans (GAGs), including heparan sulfate (HS), dermatan sulfate (DS), chondroitin sulfate (CS), and hyaluronic acid (HA) (Fig. S2), and some percentage of nonpolysaccharidic components, such as nucleic acid and proteins. Subsequent purification leads to the conversion of crude heparin into pharmaceutical heparin [active pharmaceutical ingredient (API)] through a series of isolation steps as well as specific steps to inactivate adventitious agents, including viruses. These isolation steps take advantage of the physicochemical attributes of heparin such as its high anionic charge density, aqueous solubility, and relatively high stability to heat, acid, base, and oxidants (3, 4).
One consequence of this purification strategy is differential isolation of various polysaccharide chains that together constitute “heparin”. At one end of the continuum, and largely eliminated during the purification, are heparan sulfate-like sequences (HS), having a lower anionic charge density and characterized by a ratio of N-acetylglucosamine to N-sulfoglucosamine of >3.0, a sulfo-to-carboxyl ratio of <1.6 and a D-glucuronic to L-iduronic acid ratio of >2 (5). On the other end of the continuum, pharmaceutical heparin has a sulfo-to-carboxyl group ratio of 2.4–2.8 and contains primarily N-sulfoglucosamine and L-iduronic acid, with >50% of its disaccharide units comprised of the trisulfated disaccharide, I2SHNS,6S (6). Between these two ends of the continuum is a diversity of chains with differential N- and O-sulfonation. For example, various heparin/HS fractions can be isolated from crude heparin with distinct structural and functional attributes (5). Several studies have identified methods to separate and characterize various subfractions of heparin, including identification and quantification of slow- and fast-moving components, based on electrophoretic mobility (7–9). These designations, and others, are useful in that they enable the comparison of heparin across different manufacturers and species. For example, fast moving heparin is enriched in the anticoagulant function of heparin and can be used as a screen to assess the anticoagulant activity of heparins isolated from different tissues or organisms (7, 8).
Pharmaceutical grade heparin has historically been defined based on units of activity using plasma clotting assays (10), rather than on the basis of molecular properties. This is changing because of the heparin contamination crisis and the advent of sophisticated methods for polysaccharide analysis. Various compendia standard-setting organizations across the globe, including the United States Pharmacopeia and the European Pharmacopeia, have proposed implementation of tests, based on defined biochemical assays, NMR spectroscopy and capillary electrophoresis (CE)/high performance liquid chromatography (HPLC), to measure specific structural and charge properties of heparin (10). Reexamination of historical lots of pharmaceutical heparin, prepared since 1941, using these types of methods, suggest a surprising level of purity and structural consistency (11).
Changes in testing are warranted because an oversulfated (OS) chondroitin sulfate (CS) contaminant, associated with anaphylactoid reactions, entered the heparin supply chain in a variety of countries, including the United States (12–14). In late 2007 and early 2008, clusters of serious adverse events, including acute hypotension, were reported in patients undergoing hemodialysis and receiving heparin. The observed patient reactions were linked to suspect heparin lots containing a then unidentified contaminant that was subsequently determined to be OSCS (structure represented in Fig. S2). The predominant structure of OSCS was found to have 4 sulfates per disaccharide, a disaccharide unit that, to date, has never been found in nature and is structurally distinct from other GAG impurities, such as DS, which may arise from an incomplete purification process. Additionally, the structure of OSCS suggests that it was persulfonated (complete or nearly complete sulfonation of all hydroxyl groups) before its introduction into heparin. Accordingly, the presence of OSCS in heparin raises the concern that other components of the heparin purification process, including DS, HA, or the so-called side-stream products in the manufacture of heparin, could also be present as persulfonated derivatives. In this study, we present a detailed analysis of side-stream products in the manufacture of heparin and identify characteristic signatures that can be used to identify persulfonated derivatives of these components, either alone or as a mixture, when present within pharmaceutical grade heparin. Thus, this study seeks to define the makeup of various components of side-stream heparin, and identify the distinctive features in the NMR/CE analysis of persulfonated derivatives of each polysaccharide component of side-stream heparin.
Results
Composition of Side-Stream Heparin.
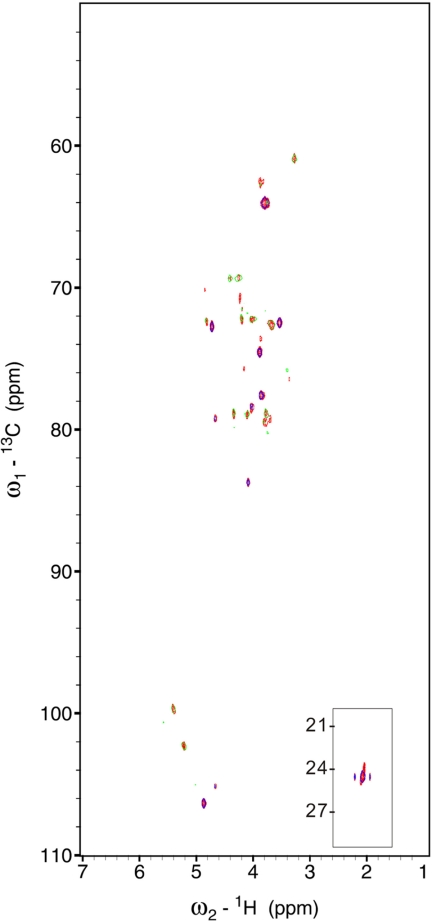
Starting from crude heparin, a number of chemical and enzymatic treatment steps, precipitations, and other purification/recovery processes are performed to derive purified, pharmaceutical grade heparin. Previous studies have determined that the heparin purification process largely eliminates a number of related GAGs and other impurities, present within crude heparin (3, 4). Examination of the 1H NMR of crude heparin (Fig. S3) indicates that it is a mixture of polysaccharide components, many of which are substantially removed after purification. In addition, the spectrum of crude heparin exhibits broader peaks when compared with that of pharmaceutical heparin; this can be attributed at least in part to the heterogeneity of the sample. To further define molecular changes that occur during the purification of heparin, we used two-dimensional heteronuclear single quantum coherence (HSQC) spectroscopy to examine the compositional makeup of crude heparin compared with purified heparin (Fig. 1). Inspection of the NMR spectrum focusing on the H-1/C-1 (anomeric) region for crude heparin indicates that it contains a mixture of GAG components, including hyaluronic acid (HA), CS, and DS, and undersulfated heparan sulfate (HS)-like sequences, all of which are substantially removed after purification, as well as heparin. For example, in the HSQC spectrum, the H-1/C-1 signals for the glucuronate are clearly observed for HA (4.47/106.0 ppm), CS A (4.46/106.6), and CS C (4.50/107.1). In addition, the iduronic acid of DS is present at 4.87/106.1 ppm and N-acetylgalactosamine signal (4.67/104.9) is also clearly distinguishable. These signals are largely absent from purified heparin, indicative of the fact that these species are removed as part of the purification procedure.
Fig. 1.
HSQC analysis of crude heparin (black) compared with purified heparin (gold). Whereas crude heparin contains a number of components, purified heparin contains largely (or only) heparin. Signals associated with CS A and C are identified in red; those arising from HA are shown in light blue. Signals from DS are shown in green, whereas those that arise primarily or exclusively from heparin are identified in black. ANS, N-sulfoglucosamine; ANAc, N-acetylglucosamine; I, iduronic acid; G, glucuronic acid; LR, linkage region.
For the heparin component of the mixture, we find that, consistent with previous findings, crude heparin is less sulfated than purified heparin (Fig. 1) (2, 5). In addition to an overall increase in N-sulfo substituted disaccharides as well as 2-O-sulfoiduronic acid and 3-O-sulfoglucosamine (accounting for the overall increase in specific activity), purified heparin has lower levels of undersulfated domains (15), including the linkage region (16), unsulfated glucuronic acid, and N-acetylglucosamine residues (17). For example, the unsulfated glucuronic acid content in crude heparin (considering only the heparin/heparan sulfate component) is 40–50%, whereas in purified heparin the amount is closer to 18–22% (2, 5). Moreover, the N-acetyl glucosamine content in crude heparin is ≈30% whereas that of purified heparin is closer to 12–18% (17). Because of the intrinsic structural heterogeneity of HS and heparin, most of the NMR signals of HS overlap with those of heparin. However, HS content in heparin preparations can be estimated based on the relative intensity of signals associated with N-acetylglucosamine and d-glucuronic acid-containing sequences and the corresponding decrease in the relative intensity of signals associated with N-sulfoglucosamine and 2-O-sulfo-l-iduronic acid-rich sequences.
During the purification of crude heparin, heparin is differentially precipitated and/or isolated over multiple steps (3, 4). We examined the side-stream products from one such purification process. First, we analyzed material referred to as tank bottom, waste product produced early in the conversion of crude heparin to purified heparin. Next we analyzed material referred to as GAG waste, which is produced later in the purification process. In this manner, we were not only able to identify the composition of the various side-stream products, we were also able to identify changes that occur within heparin as a function of its purification.
Analysis of the tank bottom and the GAG waste material by PAGE and comparison to purified heparin indicates that the tank bottom material largely contains CS A, CS C, and DS, with very little heparin present. Conversely, the GAG waste contains heparin and DS. PAGE has been shown to be able to resolve individual components of GAG mixtures (11). From this initial analysis, it is clear that undersulfated polysaccharide components are largely removed early in the heparin purification process, with DS being removed throughout the purification process. As such, the composition of side-stream byproducts changes as the purification process proceeds. We confirmed and extended PAGE analysis using 1H NMR (Fig. S4). In the case of tank bottom material, the proton NMR spectrum largely matches that of pure DS. In addition, the lack of appreciable signals at 5.2 and 5.5 ppm, assigned to the H-1 of 2-O sulfo-l-iduronic acid and H-1 of N-sulfoglucosamine, respectively, indicates that tank bottom material likely contains mostly DS with very little heparin-like material. We confirmed this analysis through heteronuclear multiple quantum coherence (HMQC) analysis and comparison of the profile to a heparin reference standard (Fig. 2).
Fig. 2.
HMQC of pure heparin (green), tank bottom (red), and DS (blue).
In contrast, analysis of the proton NMR spectrum for the GAG waste fraction indicates that it contains heparin and DS. Quantification of the signals for DS, including the N-acetylgalactosamine signal, indicates that GAG waste contains at least 10% DS. Other minor alterations in the proton NMR profile for the GAG waste compared with purified heparin are likely due to subtle alterations in the sequence composition of this material compared with purified heparin.
We also compared the tank bottom material to GAG waste material and to purified GAGs using capillary electrophoresis (Table 1, Fig. S5). Through this analysis, we find that the tank bottom material can tentatively be assigned as mostly containing DS. As expected from the above NMR-based analysis, GAG waste material appears to contain heparin, with detectable levels of DS. In addition, consistent with the presence of undersulfated sequences in the GAG waste, this material exhibited a wider range of sequence heterogeneity in CE analysis (exhibited as a higher polydispersity) compared with purified heparin, with a slightly changed migration time.
Table 1.
CE analysis of tank bottom and GAG waste material and comparison to reference standards
| Material | Migration time, min | |
|---|---|---|
| Standards | Heparan Sulfate | 9.8 |
| Chondroitin Sulfate A | 7.5 | |
| Chondroitin Sulfate C | 7.0 | |
| Dermatan Sulfate | 8.3 | |
| Heparin | 5.0–5.1 | |
| Samples | Tank Bottom | 8.3 |
| GAG Waste | 5.8, 8.3 |
Therefore, taken together, these results indicate that CE and NMR can be important tools to assess the overall purity of heparin preparations. In addition, two-dimensional NMR analysis resolves individual components from one another, enabling increased confidence in both structural assignment, as well as ensuring the detection of different GAG components.
Analysis of Oversulfated Side-Stream Byproducts of the Heparin Purification Process.
Having defined the composition of side-stream byproducts of the heparin purification process and their relationship to both crude and purified heparin, we next examined the ability of CE and NMR (both proton and two-dimensional NMR) to identify the presence of various persulfonated derivatives, both individually and in the context of a side-stream byproduct within heparin. Given our analysis of crude heparin as well as tank bottom and GAG waste side-stream material, we persulfonated CS, DS, and heparin individually as well as tank bottom and GAG waste material directly (Fig. S2). This analysis is of importance for several reasons: (1) based on findings in the literature, persulfonated polysaccharides, and not just OSCS, likely activate the contact system and thus, if present within a heparin preparation, might be capable of eliciting an adverse response; (2) persulfonated GAGs have been reported to have anticoagulant activity, primarily mediated through heparin co-factor II (18) and thus their presence might not be detected by global anticoagulant assays; and (3) the presence of OSCS in heparin suggests that contamination with other persulfonated polysaccharides might be possible.
First, the tank bottom and GAG waste material were persulfonated (19), and analyzed by 1H (Fig. S6) and HMQC (Fig. 3) NMR to determine the chemical signatures associated with each. The spectra for these materials were also compared with OSCS-contaminated heparin and to isolated contaminant. Neither of the waste streams or their persulfonated products had spectral properties similar to the OSCS contaminant. Consistent with the analysis from above, persulfonated tank bottom material mostly resembled oversulfated DS. Oversulfated GAG waste was clearly distinguished from both OSCS-contaminated heparin and OSCS. Based on chemical shifts at ≈4.55 and 4.60 ppm (assigned to H-2 and H-3 of 3-O-sulfoiduronic acid) and 4.40 ppm (assigned to H-3 of 3-O-sulfoglycosamine) (18), it appears that persulfonated GAG waste largely contains persulfonated heparin components. This assignment was confirmed through the use of CE. Analysis of a solution of heparin (+OSCS) spiked with either 10% wt/wt of persulfonated tank bottom or GAG waste material indicated that these materials are clearly distinguishable from both heparin and OSCS (Fig. 4). Finally, analysis of persulfonated crude heparin yields a complicated spectrum, as expected because it contains CS, DS, and HA as well as HS/heparin, which nonetheless yields signals, which can clearly be distinguished from either heparin, OSCS, or OSCS-contaminated heparin (Fig. S7).
Fig. 3.
Comparison of the HMQC spectra of pure heparin, OSCS-contaminated heparin, OSCS, persulfonated GAG waste, and persulfonated tank bottom. (A) HMQC of pure heparin(red), OSCS-contaminated heparin (blue), isolated OSCS contaminant (green). (B) HMQC of pure heparin (red), OSCS-contaminated heparin (blue), and persulfonated GAG waste (cyan). (C) HMQC of pure heparin (red), OSCS-contaminated heparin (blue), and persulfonated tank bottom (yellow).
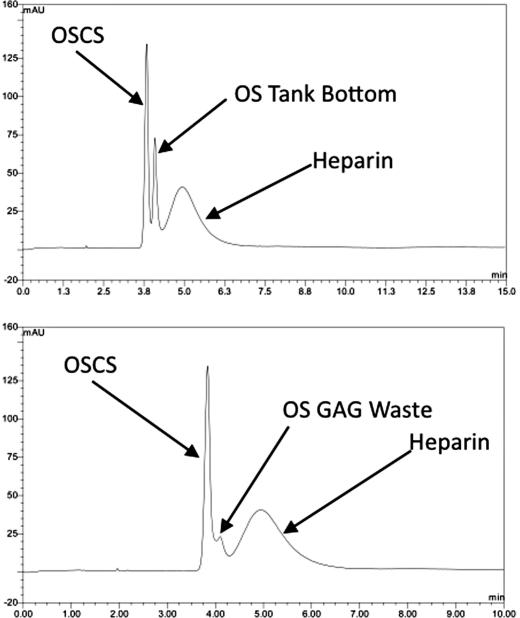
Fig. 4.
CE analysis of heparin mixed with OSCS and OS tank bottom or OS GAG waste. (Top) CE analysis of a 10% wt/wt spike of oversulfated (OS) tank bottom material in 50 mg/mL USP reference standard (heparin +OSCS). Consistent with the NMR analysis, OS tank bottom is clearly distinguishable from either OSCS or heparin. (Bottom) CE analysis of OS GAG waste spiked into the same standard.
To further this analysis, we persulfonated individual components of side-stream heparin, that is, CS, DS, and heparin, and determined whether these could be separated/identified as distinct from heparin. After persulfonation of CS, DS, and heparin, to create OSCS, OSDS, and OS heparin, respectively, we examined the proton NMR spectra of the purified compounds (Fig. S8). Analysis of the region at ≈2 ppm, representing the N-acetyl portion of the spectra, indicates that, under conditions of identical sample concentration, pH, and temperature, the chemical shift for each persulfonated species, with the exception of oversulfated heparin (OS-heparin), was distinct from heparin's N-acetyl signal. Analysis of the entire proton NMR spectra for these persulfonated GAGs indicate distinct differences for each compared with heparin. Consistent with persulfonation, OSCS, and OSDS have relatively simple proton spectra which can be readily defined and which are distinguished from that of heparin or OS-heparin. The spectrum for OS heparin is somewhat more complicated; however, there are differences in the one-dimensional (1D) 1H NMR spectrum for OS-heparin compared with that of heparin, including a pronounced signal from the H-2 and H-3 of 3-O-sulfo-N-sulfo glucosamine as well as the H-2 of 2-O-sulfo-3-O-sulfoiduronic acid (assignments are presented in Table S1). Taken together these results indicate that 1H NMR can be used as a tool to identify potential contaminants within heparin.
Given the fact that oversulfated heparin contains an N-acetyl region within the proton NMR spectrum that is similar to heparin's, we also completed HSQC analysis of heparin mixed with 30% of OS heparin (Fig. 5). We find that oversulfated heparin can be detected in the HSQC spectrum. For example, consistent with the literature (18, 20, 21), we could readily detect signatures associated with 2-O-sulfo, 3-O-sulfo iduronic acid as well as signals associated with 3-O-sulfo-6-O-sulfo-glucosamine (Table S1). Based on the studies presented here, and others (17, 22), we estimate that a wide variety of contaminants, if present at a level >3–5%, will be detected in the HSQC under the experimental conditions used in this study; use of a higher field magnet with a cryoprobe can lower the limit of detection to <1%.
Fig. 5.
Analysis of a 30% wt/wt mix of oversulfated heparin in heparin. HSQC signals associated with the H-2/C-2, H-3/C-3, and H-4/C-4 of 2-O sulfo, 3-O sulfo iduronic acid (labeled 2 I2S,3S, 3 I2S,3S and 4 I2S,3S, respectively) as well as the H-2/C-2 and H-3/C-3 of 3-O-sulfo, N-sulfoglucosamine (labeled 2 GlcNNS,3S and 3 GlcNNS,3S, respectively).
In parallel we also completed capillary electrophoretic analysis of OSDS and OS heparin alone and with OSCS-contaminated heparin using the United States Pharmacopeia system suitability reference standard (Fig. S9). As expected based on their charge density, these persulfonated species migrate differently from heparin and OSCS and would be detected, if present.
Discussion
Since the initial report discovery and structural elucidation of OSCS in heparin (13), a number of analytical tools and methods have been used in the overall analysis and are described in a series of recent publications, including NMR (23–25), MS (26), IR-Raman (27, 28), CE (29–31), HPLC (32), biosensor (33), and bioassays (34–36). Also, the structural assignment of OSCS has been independently confirmed by other independent investigations (41), providing additional support to the fact that OSCS is the major contaminant within suspect heparin lots. In subsequent analysis of multiple lots of heparin, no other major contaminants have been detected; notably, DS impurities are seen in many preparations of pharmaceutical heparins. Thus, it appears that OSCS was added either inadvertently or purposely by itself, rather than in the context of a side-stream product. Nevertheless, we sought to define other potential contaminants and determine whether these contaminants, resulting from persulfonation of either a component or the entirety of side-stream heparin, could be detected and identified using a combination of NMR and CE.
One of the key definitional requirements for this study is to define what is meant by side-stream heparin or heparin byproducts. To this end, we analyzed crude heparin and compared the composition of it to the corresponding purified material. We find that crude heparin contains heparin and undersulfated polysaccharide components, including CS, DS, and HA. As such, crude heparin has a continuum of polysaccharide chains, both in terms of the type of polysaccharide, as well as their overall sulfation levels. Because of the intrinsic structural heterogeneity of heparin, most of the NMR signals of the lower sulfated heparin-like chains (referred to as HS) overlap with those of heparin, and HS impurities in heparin preparations can usually be detected through quantitative analysis, such as the relative increase of the intensity of signals associated with N-acetylglucosamine- and glucuronic acid-containing sequences and the corresponding decrease of the relative intensity of signals associated with N-sulfoglucosamine and 2-O-sulfoiduronic acid-rich sequences.
In the case of oversulfated heparin side-streams, we find that there are characteristic signatures which can be used to differentiate the presence of an oversulfated heparin side-stream with heparin. Consistent with the studies of Perlin and Yates, the presence of the unnatural 3-O-sulfoiduronic acid (either with or without 2-O-sulfo group) is a key signature as is the presence of enriched levels of 3-O-sulfo, 6-O-sulfo, N-sulfoglucosamine residues (21, 37, 38). Moreover, if the iduronic-to-glucuronic ratio is maintained it can be determined whether persulfonation was completed on a heparin or on a HS- mixture. Finally, it is clear that persulfonation of heparin, and to a lesser extent HS, is complicated by the lability of N-sulfo groups. Thus, unless the product is subsequently N-sulfonated, another signature of OSHS/OS heparin is the presence of unsubstituted amino groups, which can be detected by NMR (typical signals at 3.36/57.1 ppm) (39) or by labeling of these moieties by an amine specific reagent (40). The need for a second N-sulfonation step to escape such detection would likely make contamination of heparin with oversulfated heparin (or oversulfated HS) uneconomical. Nevertheless, as mentioned above, persulfonated polysaccharides, including OS heparin/HS are expected to activate the complement system and have global anticoagulant activity, including activity mediated through heparin co-factor II activity.
From this study, we find that both NMR and CE are useful to distinguish purified heparin from heparin containing OSCS or other potential persulfonated derivatives of side-stream heparin or components of it. If completed in a carefully controlled manner with the appropriate reference material, the resolving power of these tests is significant and can, for example, distinguish OSCS, OSDS, and OS heparin from one another. One potential caveat is that analysis here involved persulfonated derivatives. Partially sulfonated derivatives have different chemical shifts in the NMR spectrum, different migration times in CE, and a more heterogeneous composition, complicating detection.
Finally, multidimensional NMR methods, such as HMQC and HSQC, as demonstrated here and elsewhere (13), are powerful techniques to resolve and identify potential contaminants in heparins. For example, in the case of OS-heparin spiking of heparin (Fig. 5), HSQC analysis clearly identifies signals associated with OS-heparin that are well resolved from those of heparin. Conversely, OS-heparin is much less distinguishable from heparin in the one-dimensional NMR experiments (Fig. S10). In conclusion, given the complexity of heparin samples, and the requirement to ensure their purity, it is clear that orthogonal analysis of heparin samples is critical. Of course, although CE and NMR are valuable analytical tools in this regard, other enzymatic approaches, including digestion, separation, and quantification of the resulting fragments, also provide important information. We demonstrate here how such orthogonal analytical strategies can detect a wide array of potential contaminants in heparin, including both OSCS, the major contaminant within suspect heparin lots, and other potential persulfonated polysaccharide components.
Materials and Methods
Pure heparin sodium salt (from porcine intestinal mucosa), heparan sulfate [(HS), from porcine intestinal mucosa], chondroitin sulfate A [(CS A), bovine trachea], and DS (from porcine intestinal mucosa) were purchased from Celsus. Contaminated heparin, isolated contaminant (OSCS), GAG waste, and tank bottom were provided by Baxter.
Persulfonation of Various GAGs, GAG Waste, and Tank Bottom.
Fully sulfated GAGs were prepared according to a modification of a literature procedure (19) using sulfur trioxide-pyridine complex (Py·SO3) as the sulfonating agent. Briefly, the sodium salt of a GAG or GAG mixture (0.5 g) was converted to its tributylammonium (TrBA) salt. After freeze-drying, the GAG TrBA salt (0.7 g) was dissolved in anhydrous dimethylformamide (8 mL). Py·SO3 (12 g) was then added and the reaction mixture was stirred overnight at 40 °C under an argon atmosphere. The reaction mixture was cooled to 0 °C and 16 mL of water was added to stop the reaction. Precipitation of the fully sulfated GAG waste was accomplished by the addition of 50 mL of absolute ethanol. Isolation afforded the GAG PyH+ salt (1.2 g) as a white powder. This material was dissolved in 10 mL water and a saturated solution of sodium acetate in ethanol (30 mL) was added. The resulting precipitate was recovered by centrifugation, washed with ethanol, and dried under vacuum, affording persulfonated GAG as the sodium salt (0.5–0.6 g).
NMR.
Samples (10–20 mg) of pure heparin and mixtures of heparin containing GAG and/or contaminant were dissolved in 0.5 mL D2O (99.996%, Sigma or Euriso-Top) and freeze-dried 3 times to remove the exchangeable protons. The samples were redissolved in 0.5–0.6 mL D2O. Spectra were recorded at 300 K on Bruker Avance II 600 MHz or 800 MHz spectrometers equipped with a cryogenically cooled probe (HCN or TCI). The spectrum for Fig. 5 was recorded at 308 K on Bruker Avance 500 MHz spectrometer equipped with a 5-mm TXI probe.
Monodimensional 1H spectra were obtained with presaturation of residual HDO signal, 32 scans, and a recycle delay of 12 s. Two-dimensional gradient enhanced HSQC spectra were recorded with carbon decoupling during acquisition with 320 increments of 16 scans for each. In all HSQC experiments the polarization transfer delay (D = 1/[2 · 1JC–H]) was set with a 1JC−H coupling values of 150 Hz. The matrix size 1K · 512 was 0 filled to 4K · 2K by application of a squared cosine function before Fourier transformation. Chemical shift values were measured downfield from trimethylsilyl propionate sodium salt (TSP) in all spectra.
CE.
Capillary electrophoresis was completed on an Agilent Technologies CE System using a modification of a published procedure (29). The buffer was 1 M Li Phosphate, pH 2.5. A 25-μm capillary was used with a 33.0-cm total length and a 24.5-cm effective length. The capillary temperature was kept constant at 20 °C. The voltage for the run was maintained at −14 kV in reverse polarity. Injection of samples was completed by pressure injection at 500 mbar*s. All samples were detected at 195 nm. Individual components, either unmodified or persulfonated, were injected at a concentration of 5 mg/mL. In addition, for selected samples a 10% wt/wt solution of the persulfonated material was spiked into the USP System Suitability Reference Standard at 50 mg/mL.
Supplementary Material
Acknowledgments.
We thank Edward Chess and Edwin Moore (Baxter International, Round Lake, IL) for supply of materials and helpful discussions and Todd Wielgos, Mark Nordhaus, and Baxter International for technical assistance and expertise. This work was supported by National Institutes of Health Grants GM 57073 and HL59966 (to R.S.).
Footnotes
Conflict of interest statement: R.L. (until June 2009) and R.S. have been directors of Momenta Pharmaceuticals since 2001. R.J.L. has served as a scientific advisor to Baxter International since mid-February 2008.
This article contains supporting information online at www.pnas.org/cgi/content/full/0906861106/DCSupplemental.
References
- 1.Linhardt RJ. 2003 Claude S. Hudson Award address in carbohydrate chemistry. Heparin: Structure and activity. J Med Chem. 2003;46:2551–2564. doi: 10.1021/jm030176m. [DOI] [PubMed] [Google Scholar]
- 2.Casu B, Lindahl U. Structure and biological interactions of heparin and heparan sulfate. Adv Carbohydr Chem Biochem. 2001;57:159–206. doi: 10.1016/s0065-2318(01)57017-1. [DOI] [PubMed] [Google Scholar]
- 3.Linhardt RJ, Gunay NS. Production and chemical processing of low molecular weight heparins. Semin Thromb Hemost. 1999;25:5–16. [PubMed] [Google Scholar]
- 4.Liu H, Zhang Z, Linhardt RJ. Lessons learned from the contamination of heparin. Nat Prod Rep. 2009;26:313–321. doi: 10.1039/b819896a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Griffin CC, et al. Isolation and characterization of heparan sulfate from crude porcine intestinal mucosal peptidoglycan heparin. Carbohydr Res. 1995;276:183–197. doi: 10.1016/0008-6215(95)00166-q. [DOI] [PubMed] [Google Scholar]
- 6.Conrad HE. Heparin-Binding Proteins. San Diego: Academic; 1998. [Google Scholar]
- 7.Volpi N. “Fast moving” and “slow moving” heparins, dermatan sulfate, and chondroitin sulfate: Qualitative and quantitative analysis by agarose-gel electrophoresis. Carbohydr Res. 1993;247:263–278. doi: 10.1016/0008-6215(93)84259-9. [DOI] [PubMed] [Google Scholar]
- 8.Bianchini P, et al. Fractionation and structural features of two heparin families with high antithrombotic, antilipemic and anticoagulant activities. Arzneimittelforschung. 1985;35:1215–1219. [PubMed] [Google Scholar]
- 9.Casu B. Methods is Structural Analysis. In: Lane DA, Lindahl U, editors. Heparin: Chemical and Biological Properties and Clinical Applications. New York: CRC Press; 1989. [Google Scholar]
- 10.Anonymous. (Proposed USP Monograph Revision.)
- 11.Zhang Z, et al. Analysis of pharmaceutical heparins and potential contaminants using 1H-NMR and PAGE. J Pharm Science. 2009 doi: 10.1002/jsp.21729. [DOI] [PubMed] [Google Scholar]
- 12.Kishimoto TK, et al. Contaminated heparin associated with adverse clinical events and activation of the contact system. N Engl J Med. 2008;358:2457–2467. doi: 10.1056/NEJMoa0803200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guerrini M, et al. Oversulfated chondroitin sulfate is a contaminant in heparin associated with adverse clinical events. Nat Biotechnol. 2008;26:669–675. doi: 10.1038/nbt1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blossom DB, et al. Outbreak of adverse reactions associated with contaminated heparin. N Engl J Med. 2008;359:2674–2684. doi: 10.1056/NEJMoa0806450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gallagher JT, Turnbull JE, Lyon M. Patterns of sulphation in heparan sulphate: Polymorphism based on a common structural theme. Int J Biochem. 1992;24:553–560. doi: 10.1016/0020-711x(92)90326-v. [DOI] [PubMed] [Google Scholar]
- 16.Liu J, Desai UR, Han XJ, Toida T, Linhardt RJ. Strategy for the sequence analysis of heparin. Glycobiology. 1995;5:765–774. doi: 10.1093/glycob/5.8.765. [DOI] [PubMed] [Google Scholar]
- 17.Guerrini M, Naggi A, Guglieri S, Santarsiero R, Torri G. Complex glycosaminoglycans: Profiling substitution patterns by two-dimensional nuclear magnetic resonance spectroscopy. Anal Biochem. 2005;337:35–47. doi: 10.1016/j.ab.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 18.Toida T, et al. Preparation and anticoagulant activity of fully O-sulphonated glycosaminoglycans. Int J Biol Macromol. 1999;26:233–241. doi: 10.1016/s0141-8130(99)00088-4. [DOI] [PubMed] [Google Scholar]
- 19.Maruyama T, Toida T, Imanari T, Yu G, Linhardt RJ. Conformational changes and anticoagulant activity of chondroitin sulfate following its O-sulfonation. Carbohydr Res. 1998;306:35–43. doi: 10.1016/s0008-6215(97)10060-x. [DOI] [PubMed] [Google Scholar]
- 20.Casu B, et al. Heparin-like compounds prepared by chemical modification of capsular polysaccharide from E. coli K5. Carbohydr Res. 1994;263:271–284. doi: 10.1016/0008-6215(94)00172-3. [DOI] [PubMed] [Google Scholar]
- 21.Yates EA, et al. Effect of substitution pattern on 1H, 13C NMR chemical shifts and 1J(CH) coupling constants in heparin derivatives. Carbohydr Res. 2000;329:239–247. doi: 10.1016/s0008-6215(00)00144-0. [DOI] [PubMed] [Google Scholar]
- 22.Guerrini M, Guglieri S, Naggi A, Sasisekharan R, Torri G. Low molecular weight heparins: Structural differentiation by bidimensional nuclear magnetic resonance spectroscopy. Semin Thromb Hemost. 2007;33:478–487. doi: 10.1055/s-2007-982078. [DOI] [PubMed] [Google Scholar]
- 23.Yamaguchi H, Shinagawa M, Shimba N, Miyano H, Suzuki E. NMR analysis of a contaminant in heparin. Yakugaku Zasshi. 2008;128:1513–1515. doi: 10.1248/yakushi.128.1513. [DOI] [PubMed] [Google Scholar]
- 24.Bigler P, Brenneisen R. Improved impurity fingerprinting of heparin by high resolution (1)H NMR spectroscopy. J Pharm Biomed Anal. 2009;49:1060–1064. doi: 10.1016/j.jpba.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 25.Beyer T, et al. Quality assessment of unfractionated heparin using 1H nuclear magnetic resonance spectroscopy. J Pharm Biomed Anal. 2008;48:13–19. doi: 10.1016/j.jpba.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 26.Hu P, Fang L, Chess EK. Source-induced fragmentation of heparin, heparan, and galactosaminoglycans and application. Anal Chem. 2009;81:2332–2343. doi: 10.1021/ac802626e. [DOI] [PubMed] [Google Scholar]
- 27.Spencer JA, et al. Screening of heparin API by near infrared reflectance and Raman spectroscopy. J Pharm Science. 2009 doi: 10.1002/jps.21660. [DOI] [PubMed] [Google Scholar]
- 28.Norwig J, et al. Prediction of the oversulphated chondroitin sulphate contamination of unfractionated heparin by ATR-IR spectrophotometry. Pharmeur Sci Notes. 2009;2009:17–24. [PubMed] [Google Scholar]
- 29.Wielgos T, Havel K, Ivanova N, Weinberger R. Determination of impurities in heparin by capillary electrophoresis using high molarity phosphate buffers. J Pharm Biomed Anal. 2009;49:319–326. doi: 10.1016/j.jpba.2008.11.034. [DOI] [PubMed] [Google Scholar]
- 30.Volpi N, Maccari F, Linhardt RJ. Quantitative capillary electrophoresis determination of oversulfated chondroitin sulfate as a contaminant in heparin preparations. Anal Biochem. 2009;388:140–145. doi: 10.1016/j.ab.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Somsen GW, Tak YH, Torano JS, Jongen PM, de Jong GJ. Determination of oversulfated chondroitin sulfate and dermatan sulfate impurities in heparin by capillary electrophoresis. J Chromatogr A. 2009;1216:4107–4112. doi: 10.1016/j.chroma.2009.02.063. [DOI] [PubMed] [Google Scholar]
- 32.Trehy ML, Reepmeyer JC, Kolinski RE, Westenberger BJ, Buhse LF. Analysis of heparin sodium by SAX/HPLC for contaminants and impurities. J Pharm Biomed Anal. 2009;49:670–673. doi: 10.1016/j.jpba.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 33.Wang L, Buchanan S, Meyerhoff ME. Detection of high-charge density polyanion contaminants in biomedical heparin preparations using potentiometric polyanion sensors. Anal Chem. 2008;80:9845–9847. doi: 10.1021/ac801879t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tami C, et al. Inhibition of Taq polymerase as a method for screening heparin for oversulfated contaminants. Biomaterials. 2008;29:4808–4814. doi: 10.1016/j.biomaterials.2008.08.024. [DOI] [PubMed] [Google Scholar]
- 35.Bairstow S, McKee J, Nordhaus M, Johnson R. Identification of a simple and sensitive microplate method for the detection of oversulfated chondroitin sulfate in heparin products. Anal Biochem. 2009;388:317–321. doi: 10.1016/j.ab.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 36.Li B, et al. Oversulfated chondroitin sulfate interaction with heparin-binding proteins: New insights into adverse reactions from contaminated heparins. Biochem Pharmacol. 2009;78:292–300. doi: 10.1016/j.bcp.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Naggi A, et al. Toward a biotechnological heparin through combined chemical and enzymatic modification of the Escherichia coli K5 polysaccharide. Semin Thromb Hemost. 2001;27:437–443. doi: 10.1055/s-2001-17954. [DOI] [PubMed] [Google Scholar]
- 38.Rej RN, Ludwig-Baxter KG, Perlin AS. Sulfation of some chemically-modified heparins. Formation of a 3-sulfate analog of heparin. Carbohydr Res. 1991;210:299–310. doi: 10.1016/0008-6215(91)80130-f. [DOI] [PubMed] [Google Scholar]
- 39.Yates EA, et al. 1H and 13C NMR spectral assignments of the major sequences of twelve systematically modified heparin derivatives. Carbohydr Res. 1996;294:15–27. doi: 10.1016/s0008-6215(96)90611-4. [DOI] [PubMed] [Google Scholar]
- 40.Toida T, et al. Structural differences and the presence of unsubstituted amino groups in heparan sulphates from different tissues and species. Biochem J. 1997;322:499–506. doi: 10.1042/bj3220499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Viskov C, et al. Isolation and characterization of contaminants in recalled unfractionated heparin and low-molecular-weight heparin. Clin Appl Thromb Hemost. 2009 doi: 10.1177/1076029609338710. in press. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.