Abstract
To better understand androgen function in the mammalian nose, we have determined the levels of testosterone (T) in the olfactory mucosa (OM, which harbors the olfactory receptor neurons) and the lateral nasal gland (LNG, which is the largest anterior nasal gland) of C57BL/6 mice. The results indicated that, in adult male mice, T levels in the LNG were substantially higher than those in the OM and other non-reproductive or non-endocrine tissues examined, including liver, kidney, and brain. Furthermore, in the LNG, the high T levels were accompanied by high levels of salivary androgen-binding protein (sABP) and low microsomal T-hydroxylase activities. The high abundance of T and sABP in the LNG suggests not only that the LNG is a storage site for androgen, but also the possibility that unusually high T levels may occur in other organs that have abundant expression of sABP but low expression of steroid-metabolizing enzymes. Our findings suggest a critical need to determine androgen levels in various organs, as well as to establish the functional significance of a usually high T level in the LNG, a gland known for its secretion of biologically active molecules, such as odorant binding proteins and immunoglobulin A, to the nasal cavity.
Keywords: Salivary androgen-binding protein, P450, olfactory mucosa, lateral nasal gland, testosterone, metabolism
Sex steroid hormones, including testosterone (T), estradiol (E2), and progesterone (P), are believed to play important physiological roles in the mammalian olfactory chemosensory system [see Ref. 1 for a review]. Numerous studies in rodents have shown that the chemosensory and reproductive behaviors are influenced by status of sex steroid hormones (e.g., [2,3]). The olfactory mucosa (OM), located in the dorsal, posterior part of the nasal cavity, where the olfactory receptor neurons reside, is a known target tissue for sex steroid hormone action [4,5]. In male rats, OM morphology is altered by castration, and T replacement counteracts these alterations [6]. Castration also leads to decreases in neurosensory epithelial volume in the vomeronasal organ (VNO) during development [7], while circulating sex steroids can modulate VNO pheromone receptor expression [8].
Current understanding of the effects of sex steroids on olfactory chemoreception is based on studies in which circulating steroid levels were modulated and/or measured. Detailed mechanisms of hormone action, including mechanisms of regulation of hormone bioavailability in various nasal organs or tissue compartments, are still poorly understood. Little is known about the homeostasis of sex steroids in the nasal organs, other than reports from in vitro studies on nasal steroid hydroxylase enzymes and activities. In that regard, mammalian OM has very high activities in the metabolism of various sex steroids, including T, E2, and P [1]. The VNO is also capable of metabolizing these sex steroids [9]. The steroid hydroxylases are believed to have critical roles in keeping levels of steroid hormones in the OM low, although in vivo evidence in support of this hypothesis has not been reported. Accumulation of sex steroids, which are normally removed through metabolism by nasal steroid hydroxylases, could affect olfactory signal transduction, by competing for odorant/pheromone receptors, or it could influence regeneration of olfactory receptor neurons following tissue injury [1]. However, the levels of sex steroid hormones in the OM or other nasal organs have not been determined.
In the present study, we have examined the metabolism and homeostasis of T in the OM and the lateral nasal gland (LNG), of mice. The LNG (also known as Steno's gland) is a secretory gland, which drains its contents through ducts that open by the nostril [10]. It is the largest anterior nasal gland in mice and rats, and it is also found in other mammalian species, including man [10-12]. The LNG is a major site for the synthesis and secretion of odorant-binding proteins (OBPs), which function mainly as carriers for odorants in the nasal mucus [13]. The LNG also synthesizes vomeromodulin, a putative pheromone and odorant transporter [14], and it secretes large amounts of immunoglobulin A [15], which may be important for the immune barrier function of the OM [16].
We first compared T levels in various tissues, as well as in serum, of adult, male mice. We found that T levels in the LNG were substantially higher than those in the OM or in other non-reproductive or non-endocrine tissues examined. We also determined T levels in OM, LNG, and serum, in male mice of various ages, in order to determine whether developmental changes in LNG T levels paralleled changes in circulating T level. The finding of unusually high T levels in the LNG prompted us to examine the expression of genes that are potentially responsible in the LNG and OM; this effort led to the additional discovery of high levels of salivary androgen-binding protein (sABP), very low microsomal T-hydroxylase activities, and near-absence of expression of genes for other T-metabolizing enzymes, in the LNG. To establish the functional significance of the high T levels in the LNG, we also determined whether androgen receptor is expressed. Our findings demonstrate, for the first time, a high abundance of T and sABP in the LNG. We propose that although the LNG is probably a target organ for androgens, it is as well a storage site for androgen, and that the stored androgen can be secreted to the nasal mucus, through which T can reach other parts of the nasal cavity. Our findings also suggest a critical need to determine androgen levels in various other organs.
Materials and methods
Determination of serum and tissue T levels
Male and female C57BL/6J (B6) mice were obtained from a breeding stock maintained at the Wadsworth Center. Animal-use protocols were approved by the Institutional Animal Care and Use Committee of the Wadsworth Center. Serum (obtained through cardiac puncture) and tissues from individual mice were used for T determination. For the nasal tissues, LNG was dissected first, according to a procedure described previously [17], and then the nasal cavity was split open by cutting the dorsal part along either side of the nasal septum, for dissection of the OM.
For liquid chromatography-mass spectrometry (LC-MS) analysis of T, OM or LNG from one mouse was homogenized in 1 ml saline, whereas, other tissues were homogenized in saline at 100 mg/ml. The internal standard, 1,2-D2-T (98%, Cambridge Isotope Laboratories, Andover, MA), was added to tissue homogenate or serum samples at 0.1 ng/ml. Samples were extracted twice, each time with 5 volumes of hexane/ethyl acetate (6:4); the extracts were dried with nitrogen, and then reconstituted in 20% (v/v) methanol in water. The resultant mixture was further extracted with an Isolute Extraction Cartridge (C18, 1 ml/100 mg) (Biotage, Charlottesville, VA). Samples were eluted from the cartridge with 1 ml methanol, dried with nitrogen, and reconstituted in 50% (v/v) methanol in water for LC-MS analysis. The recovery of the added T was >90%, at all concentrations tested. Authentic compounds were added to charcoal-stripped bovine serum (Hyclone, Logan, UT) for construction of calibration curves.
An ABI 4000 Q-Trap LC/MS/MS system (Applied Biosystem, Foster City, CA), fitted with a 1.8-μm XDB-C18 column (4.6 × 50 mm, Agilent Technologies, Santa Clara, CA), was used. The samples were eluted at a flow rate of 0.5 ml/min, with a mobile phase consisted of solvent A (0.1% formic acid in acetonitrile/water (95:5)) and solvent B (0.1% formic acid in acetonitrile/water (5:95)). The column was equilibrated with 60%A:40%B; the solvent gradient consisted linear increases from 40%B to 90%B, between 4 and 5 min, and then to 100%B, between 5 and 6 min. The retention time for T and the internal standard was 4.5 min. The MS was operated in the positive ion mode, using atmospheric pressure chemical ionization. The parent/product ion pairs of m/z 289.2/97.1 and 289.2/109.1 (for T), and m/z 291.2/99.1 and 291.2/111.1 (for 1,2-D2-T), were measured in the Multiple Reaction Monitoring (MRM) scan mode. The parameters for the chamber were as follows: curtain gas 35 psig, needle current 3.5 μA, heated nebulizer temperature 350°C, nebulizing gas 40 psig, declustering potential 80 V, and entrance potential 8.0 V.
Assay for T metabolism
OM from four, or LNG from five, 8- to 12-week-old male or female mice were pooled for preparation of microsomes, as described previously [9]. Reaction mixtures contained 50 mM potassium phosphate buffer, pH 7.4, 10 μM T, 1 mM ascorbic acid, 0.1 mg/ml (for OM) or 0.5 mg/ml (for LNG) microsomal protein, and 1 mM NADPH, in a final volume of 0.5 ml. Reactions were carried out at 37°C for 10 min (for OM) or 30 min (for LNG). Control reactions were carried out without the addition of NADPH. The sources of T metabolites and the internal standard have been described previously [18,19].
For LC-MS analysis of T metabolites, reactions were terminated by the addition of cold methanol, to a final concentration of 67%. An internal standard, 16α-hydroxyprogesterone, was added at 200 pg to each sample. Metabolites were enriched through solid-phase extraction (SPE), using Isolute C18 columns (Biotage). The extracted samples were reconstituted in 50% methanol in water for LC-MS analysis. Recovery of the metabolites was ∼90%. Identity of T metabolites was confirmed by matching retention time and MS spectrum with those of the authentic compound. Authentic compounds were used to construct calibration curves. T metabolites were determined using the same LC-MS/MS system, LC column, mobile phase, and flow rate as described above for T detection. The column was equilibrated with 85%A:15%B; the solvent gradient consisted linear increases from 15%B to 30%B (between 3 and 25 min), and then from 30%B to 40%B (between 25 and 26 min), followed by a 4-min wash with 100%B.
The MS was operated in the positive ion mode, using atmospheric pressure chemical ionization. Metabolites were identified by matching retention times and MS spectra (obtained in the Enhanced Product Ion scan mode) with those of the authentic standards. The parent/product ion pairs of m/z 305.2/97.1 (15β-OH-T), 305.2/109.2 (15α-OH-T), 305.2/269.1 (2β-OH-T), and 331.2/97.2 (for 16α-hydroxyprogesterone) were measured in the MRM scan mode. The parameters for the chamber were as follows: curtain gas 30 psig, needle current 3.0 μA, heated nebulizer temperature 350°C, nebulizing gas 50 psig, declustering potential 68 V, and entrance potential 7.0 V.
RNA PCR
Tissues (LNG or OM) from three mice (8- to 12-week-old, male or female) were pooled for total RNA preparation. Total RNA was isolated with use of the RNeasy Mini kit (QIAGEN, Valencia, CA). All RNA samples were treated with DNase I (Life Technologies, Carlsbad, CA) before reverse transcription (RT). RNA-PCR analysis was performed as previously described [20]. Gene-specific PCR primers for androgen receptor, Cyp19, type I and type II 5α-reductase, Abpa2, 24, and 27, and Abpbg1, 2, 21, 24, 26, and 27 were the same as those described previously [21-25]. Identity of all PCR products was validated by sequence analysis.
Real-time RNA-PCR analysis was performed for Abpa27, according to the general protocol described elsewhere for analysis of cytochrome P450 (P450) gene expression [26], with use of an ABI 7500 Fast Real-Time PCR System and SYBR Green core reagents (Applied Biosystems). The primers used for real-time PCR were: for Abpa27, 5′-agagggagattgtggccttt-3′ and 5′-cctcggtcaaggtcctatca-3′; and for glyceraldehyde 3-phosphate dehydrogenase (GAPDH), 5′-tgtgaacggatttggccgta-3′ and 5′-tcgctcctggaagatggtga-3′ (with an annealing temperature of 62°C for both transcripts). PCR products were validated by sequence analysis, and PCR specificity was confirmed by analysis of reaction products on agarose gels. One of the samples was serially diluted for construction of a standard curve. Experiments were performed in duplicate, and the results were corrected on the basis of the levels of GAPDH mRNA present in the same RNA preparation.
Immunoblot analysis
Tissues (LNG or OM) from three mice (8- to 12-week-old, male or female) were pooled for preparation of whole-cell lysate. Tissues were homogenized in the RIPA cell lysis buffer (Pierce Biotechnology, Rockford, IL), containing protease inhibitors (Halt™ Protease Inhibitor Cocktail Kit, Pierce). The homogenate was kept on ice for 10 min, and then centrifuged at 13,000 g, at 4°C, for 20 min. The supernatant fraction was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis under reducing conditions [27]; immunoblot analysis was performed with use of a rabbit anti-mouse sABP antibody [28], and an enhanced chemiluminescence kit (Amersham, Arlington Heights, IL). The optical density of detected bands was determined with a Personal Densitometer SI (Molecular Dynamics, Sunnyvale, CA).
Other methods and data analysis
Statistical significance of differences between two groups was analyzed using Student's t test. Statistical significance of differences in tissue T levels among multiple groups was analyzed using Kruskal-Wallis one-way analysis of variance on ranks; pairwise multiple comparisons were performed using Student-Newman-Keuls test. The relationship between serum and tissue T levels among individual mice tested was analyzed using Spearman rank order correlation.
Results
Tissue T levels
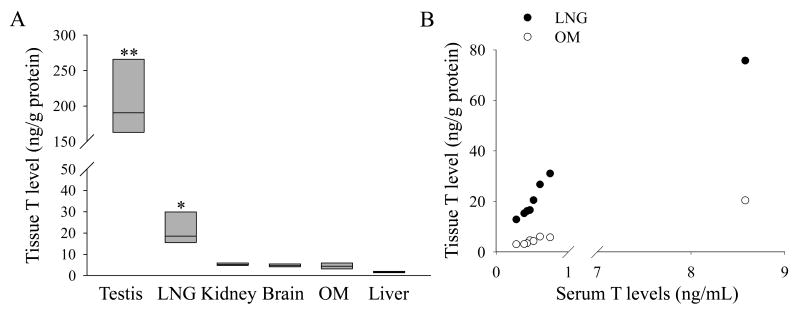
The serum and tissue levels of T were determined, with use of LC-MS/MS, for the LNG and OM of adult, male B6 mice. T levels in several other organs were also determined, for comparisons. As expected, the testis had the highest levels of T among all tissues tested; however, an unexpected finding was that T levels in the LNG (median value: 18.5 ng/g protein) were significantly higher than those in the OM (median value: 4.4 ng/g protein) for the same group of male mice (Fig. 1A). Moreover, T levels in the LNG were higher than in any of the other non-reproductive tissues examined, including brain (median value: 4.7 ng/g protein), liver (median value: 1.7 ng/g protein), and kidney (median value: 5.2 ng/g protein). Tissue T levels varied considerably among individual males tested; often (as illustrated by the example in Fig. 1), one or two mice with very high T levels exist in a group of adult males, making it difficult to compare T levels among various tissues. However, a comparison of serum and tissue T levels indicated that tissue T levels in both OM and LNG, as well as in the other tissues tested, were correlated (P < 0.001) with serum T levels, in individual adult males (Fig. 1B). The association between serum and LNG T levels was also observed during postnatal development: T was not detected in the male LNG until 8 weeks after birth, an age when serum T reached adult levels (Table 1).
Fig. 1. Serum and tissue T level in male mice.
(A). T levels in various tissues. T levels were determined for 8-week old male mice (n=8). One of the mice tested was found to have exceptionally high serum, as well as tissue, T levels, causing the data to fail the normality test. Therefore, significance of differences in T levels among the tissues was analyzed using the Kruskal-Wallis one-way analysis. The values shown are the medians, together with the 25% (lower bar) and 75% (upper bar) percentile marks. **, T levels in the testis were significantly higher than those in the other tissues (P < 0.05). *, T levels in the LNG were significantly higher than the levels in all other tissues examined, except for testis (P < 0.05).
(B). Correlation of LNG and OM T levels with serum T levels among the eight mice analyzed. Tissue and serum T levels for each mouse were plotted. The correlation coefficient (r) is 1.000 for LNG vs. serum (P < 0.001), and 0.952 for OM vs. serum (P <0.001), according to the Spearman rank order correlation test.
Table 1. Developmental changes in serum and LNG T levels in male mice.
Serum and LNG from individual mice of various ages were used for T determination. The 8-week old mice were not the same as those used for the experiment described in Figure 1. Mice with unusually high serum T level were not found in this experiment; the values shown represent means ± SD (n = 4 ∼ 5).
| Age of mouse | Testosterone level | |
|---|---|---|
| Serum | LNG | |
| pg/ml | ng/g protein | |
| 2 weeks | 53 ± 6 | < 2.5 a |
| 4 weeks | 73 ± 8 | < 2.5 a |
| 8 weeks | 610 ± 160 | 19.0 ± 0.5 |
| 12 weeks | 630 ± 70 | 21.0 ± 3.2 |
Detection limit.
In consideration of the possibility that the mice with exceptionally high T levels represent dominant males in a cage, we have also analyzed the data excluding the outlier mice. The results (not shown) confirmed the significantly higher T levels in the LNG (19.8 ± 6.7 ng/g protein; mean ± S.D.) than in the OM (4.3 ± 1.2 ng/g protein), or than in the other non-reproductive tissues examined (p<0.01; Student's t-test).
T levels were also determined for adult female B6 mice (data not shown). Serum T levels in female mice (70 ± 10 pg/ml, n = 8) were much lower than those found in adult male mice (∼600 pg/ml; Table 1). T levels in female OM and LNG were below detection limit (∼2.5 ng/g for the nasal tissues).
Microsomal T-hydroxylase activity
To understand the mechanisms underlying the higher T levels in the LNG than in the OM, we determined rates of T metabolism in the two tissues, based on the assumption that a low rate of T metabolism can lead to T accumulation in the tissue. The patterns of major hydroxy-testosterone produced in microsomal reactions were similar between the OM and the LNG, with 15α-, 15β-, and 2β-hydroxy-T being the most abundant forms, for microsomes from either male or female mice (data not shown). Rates of formation of the three major metabolites were approximately 30-fold lower in the LNG than in the OM; however, the rates were not significantly different between male and female mice (Table 2). Thus, the very low activity for T hydroxylation in the LNG may have contributed to the observed high T levels in this gland. However, the lack of differences between male and female LNG in T hydroxylase activities indicated that a very low rate of T hydroxylation is not sufficient - although it is probably necessary - for attainment of high tissue T levels.
Table 2. In vitro metabolism of T by OM and LNG microsomal preparations.
Rates of formation of three major T metabolites, 15α-hydroxy-testosterone (15α-OH-T), 15β-OH-T, and 2β-OH-T, were determined, as described in the Materials and Methods. Reaction mixtures contained 50 mM potassium phosphate buffer, PH 7.4, 10 μM T, 1 mM ascorbic acid, 0.1 mg/ml (for OM) or 0.5 mg/ml (for LNG) microsomal protein, and 1 mM NADPH. The values presented are means ± S.D. (n = 3).
| Gender | Tissue | Rates of product formation | ||
|---|---|---|---|---|
| 15α-OH-T | 15β-OH-T | 2β-OH-T | ||
| pmol/min/mg protein | ||||
| Male | OM | 3900 ± 300 | 300 ± 40 | 330 ± 50 |
| LNG | 110 ± 20 a | 8.8 ± 1.6 a | 8.8 ± 2.5 a | |
| Female | OM | 3300 ± 260 | 250 ± 30 | 260 ± 40 |
| LNG | 110 ± 10 a | 8.0 ± 0.2 a | 8.9 ± 0.7 a | |
The rates are significantly different between OM and LNG, for both sexes (P < 0.01; Student's t-test).
Tissue levels of sABP
High tissue levels of T can also result from abundant expression of molecules that are capable of binding to T. In that regard, it has been reported that the sABPs, known to be expressed in glands of the head and neck region, can bind T with high affinity [29]. A qualitative RNA-PCR analysis of Abp gene expression in the OM and LNG of male and female mice, performed with specific primers for Abpa2, 24, and 27 and those for Abpbg1, 2, 21, 24, 26, and 27 [25], detected Abpa2 and a27 transcripts in the male and female LNG; Abpa27 transcripts in the male and female OM; as well as Abpbg2 and bg27 transcripts in all tissues examined (Fig. 2A). Subsequently, a quantitative RNA-PCR analysis was performed for Abpa27, which appeared to be ubiquitously expressed in the OM and LNG; the results indicated that the level of Abpa27 mRNA was >100-fold higher in the male LNG than in the male OM (Fig. 2B).
Fig. 2. Gene expression analysis for OM and LNG.
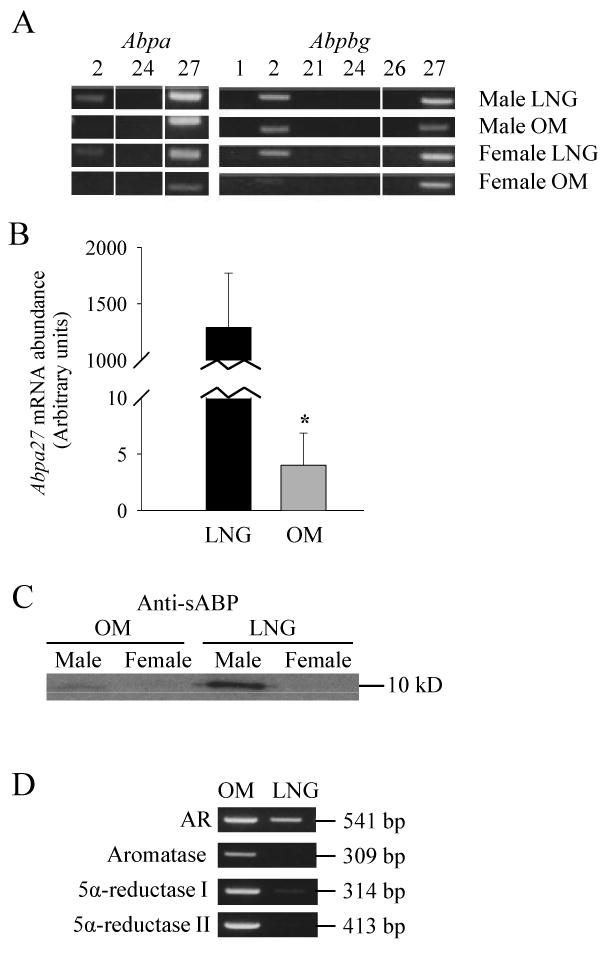
(A). Detection of various Abpa and Abpbg transcripts in the OM and LNG of male and female mice by RNA-PCR analysis. Tissues from 8- to 12-week-old mice (three per group) were pooled for total RNA preparation. PCR products were analyzed on 1% agarose gels, and visualized by staining with ethidium bromide. Numbers represent various Abpa or Abpbg transcripts. The results shown are representative of two independent determinations.
(B). Real-time RNA-PCR analysis of Abpa27 expression in the OM and LNG of male mice. The values shown represent means ± SD. *, There is a significant difference between OM (n=5) and LNG (n=4) (P < 0.01).
(C). Immunoblot analysis of sABP protein expression in the OM and LNG. Tissue homogenates from OM and LNG (30 μg protein each), pooled from three 8- to 12-week-old mice, were analyzed using a polyclonal anti-sABP antibody. The size of the sABP proteins detected is indicated. The results shown are representative of two independent determinations.
(D). RNA-PCR analysis of expression of androgen receptor (AR), aromatase, and 5α-reductase type I and type II in OM and LNG of male mice. The sizes of PCR products detected are indicated. Tissues from 8- to 12-week-old mice (three per group) were pooled for total RNA preparation. The results shown are representative of two independent determinations.
Further immunoblot analysis was performed using a polyclonal anti-sABP antibody, which was prepared against purified sABP protein [28]; it is not clear whether the anti-sABP antibody cross-reacts with all sABP isoforms, but the antibody is expected to recognize many of these highly homologous proteins. The immunoblot analysis revealed much higher levels of sABP proteins in the LNG than in the OM of male mice (Fig. 2C). A densitometric analysis of immunoblot results from two independent experiments indicated a ∼50-fold difference in the relative amounts of the sABP proteins detected, between the LNG and the OM (data not shown). In female mice, sABP proteins were detected at levels much lower than those detected in the males, in both OM and LNG (Fig. 2C). The abundant expression of sABP proteins in the male LNG supports the idea that the sABPs play a role in retaining T in this secretory gland in male mice.
Expression of androgen receptor, 5α-reductases, and aromatase
The discovery of the presence of high T levels in the LNG suggests that the LNG is possibly a target organ for T and/or related sex hormones. A qualitative RNA-PCR analysis identified mRNAs for androgen receptor in OM and in LNG, confirming that both tissues are potential biological targets of androgens (Fig. 2D). Transcripts for type I and type II 5α-reductase enzymes, which convert T to dihydrotestosterone (DHT), and for aromatase (CYP19), which converts T to estradiol, were also detected, apparently in abundance, in the OM, but such transcripts were barely (or not) detected in the LNG. In this context, 5α-reductase and aromatase enzyme activities have been reported for rat olfactory epithelium in a previous study [30], but it had not been studied whether these enzymes are also expressed in the LNG. The apparent absence of the 5α-reductases and aromatase in the LNG could have contributed to the high levels of T detected in the gland, given the known activity of these enzymes in metabolizing T. Notably, DHT, the product of 5α-reductase, is a more potent androgen than T is [31]; thus, the differential expression of the 5α-reductase in the OM and in the LNG might serve to increase the sensitivity of the OM to androgen stimulation, while protecting the LNG against overstimulation by the high levels of T present.
Discussion
The homeostasis of T in various tissues, such as the OM and the LNG, can be maintained by multiple factors, which together control the levels of free androgens that are available for carrying out biological functions. For OM and LNG, tissue T levels are clearly influenced by circulating androgen levels. Furthermore, P450-catalyzed T hydroxylation represents one of the first steps in the inactivation of T; thus, tissue T levels can be influenced by the level of steroid hydroxylase activities in that tissue. On the other hand, steroid binding proteins, such as sABP, may retard T metabolism, by sequestering T away from the metabolic enzymes. Therefore, the present finding of an unusually high T level in the LNG, a presumed non-steroidogenic organ, can be explained by the very high sABP levels (∼ 50-fold higher in the LNG than in the OM), the very low microsomal T hydroxylase activity (∼ 30-fold lower in the LNG than in the OM), and the apparent absence of the steroid 5α-reductases and the aromatase, enzymes that can convert T to other forms of steroid hormones, in this tissue.
The sABPs are known to be expressed in additional nasal tissues, such as the VNO, and other organs in the head-and-neck region, including the olfactory bulb, salivary glands, lacrimal gland, and Harderian gland [25]. Thus, we propose that T levels in these other glands or organs, in addition to the OM and LNG studied here, will differ, in a manner dictated by the relative abundances of metabolic enzymes (e.g., P450s) and steroid-binding proteins (e.g., sABP) in the particular tissue. In cells expressing sABP, T is either protein-bound or free, and the two forms exist in an equilibrium affected by the affinity of the binding and the abundances of T and sABP. In tissues with low P450 activity, free T has ample opportunity to complex with sABP, and this event leads to accumulation of T in the tissue. Contrastingly, in tissues with high P450 activity, free T is rapidly metabolized. This event leads to decreases in cellular concentrations of free T, with consequent dissociation of the sABP-T complexes, and culminates in a low steady-state level of T in the tissue.
In female mice, the level of T was below our current detection limit in both the OM and the LNG; therefore, we cannot yet determine whether a higher T level exist in the LNG than in the OM. Circulating androgen levels, as well as the levels of OM and LNG sABP, are much lower in females than in males, whereas we found microsomal T hydroxylase activities in the OM and LNG to be comparable between males and females. These patterns, when combined, predict the occurrence of very low T levels in female OM and LNG.
The high T hydroxylase activity in the OM, compared to the LNG, is believed to be related to the abundant expression of two P450 enzymes, CYP2A5 and CYP2G1, in the OM; both enzymes (in addition to being active in metabolizing numerous foreign chemicals) are efficient in the hydroxylation (and thus inactivation) of sex steroid hormones, including T, E2, and P [17,18,32]. CYP2G1 is expressed in the OM and VNO, but not in the LNG, while CYP2A5 is expressed in the OM at levels much higher than in the LNG or the VNO [9,17,32]. The role of CYP2A/2G enzymes in nasal microsomal steroid hormone metabolism has been demonstrated in in vitro studies that used P450 antibodies and chemical inhibitors [18,32].
Mouse sABPs are members of the secretoglobin family [33]. A functional sABP is a covalently linked (through a disulfide bond) heterodimer composed of one ABP α subunit (belonging to secretoglobin family B) and one ABP β or γ subunit (belonging to secretoglobin family E). The sABP proteins detected on immunoblots represent both monomers (alpha subunit, 10841 Dalton and beta subunit, 9997 Dalton), which were not distinguished by the separation, under the reducing conditions used for the gel electrophoresis; the intact sABP dimer has a molecular weight of ∼21 kD [34]. The sABP proteins are encoded by a recently expanded cluster (on mouse chromosome 7) of 30 Abpa and 34 Abpbg genes and pseudogenes, all highly similar in DNA and predicted protein sequences [35]. The biological functions of the sABPs have yet to be identified; however, these proteins are capable of binding, with high affinity, sex steroids that have the saturated A ring of T (including T, P, but not E2 or cholesterol) [29]. Based on a comparative evolutionary genomics analysis, Emes et al. [36] proposed that sABPs play a role as pheromones, or a role in modulation of odorant detection. Earlier work had suggested that sABPs function in reproductive behavior [37,38]. However, experimental evidence for a role of the sABPs in chemosensory function has yet to be obtained.
The nasal epithelium is covered with a layer of aqueous mucus, through which odorants, environmental chemicals, and pathogens must travel in order to come in contact with the underlying epithelial cells. Numerous components of the nasal mucus have been identified [see Ref. 1 for a review], including immunoglobulins, various enzymes, antioxidants (such as reduced glutathione), and mucin. Nasal mucus also contains regulatory proteins and transport proteins, such as OBP [39] and vomeromodulin [40]. The presence of the proteins in the mucus can result from either serum transudation or local synthesis and secretion [41,42]. However, there has been no report of detection of sex steroid hormones in the nasal mucus.
T is a well established regulatory molecule for a variety of cellular functions and signaling pathways [43]. Thus, our finding of an unusually high level of T in the LNG has strong physiological relevance, particularly given the known or hypothesized functions of T in the olfactory system [1]. We propose, based on the findings of the present study, that the stored androgen of the LNG can be secreted to the nasal mucus, and can thus potentially function in a paracrine fashion, in the male olfactory system. The nasal mucus is secreted by a variety of nasal glands. In rodents, secretions from the LNG can reach other parts of the nasal cavity, including the OM and VNO [13,14]. We envision that, as the LNG releases its secretory contents, the stored T is also released, perhaps carried by sABP, and distributed throughout the nasal mucus. In the OM and VNO, the bound or free T can potentially perform several functions, including: activation of membrane androgen receptors; interaction with odorant/pheromone receptors as an agonist or an antagonist; and inhibition of putative pheromone-metabolizing enzymes in the mucus. Validation of this hypothesis will likely have a significant impact on our understanding of the links between androgen homeostasis and the biology (or diseases) of the olfactory system and the upper respiratory tract in mammals.
Acknowledgments
The authors gratefully acknowledge the use of Wadsworth Center's Molecular Genetics Core facility. We thank Dr. Adriana Verschoor for reading the manuscript and Ms. Weizhu Yang for technical assistance. We are grateful to Dr. Robert Karn of the University of Arizona, College of Medicine, Tucson, AZ, for providing the anti-sABP antibody. This work was supported in part by grant ES007462 from the National Institute of Environmental Health Sciences, National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ding X, Dahl AR. Olfactory mucosa: composition, enzymatic localization, and metabolism. In: Doty RL, editor. Handbook of Olfaction and Gustation. 2nd. Marcel Dekker; New York: 2003. pp. 51–73. Chapter 3. [Google Scholar]
- 2.Moffatt CA. Steroid hormone modulation of olfactory processing in the context of socio-sexual behaviors in rodents and humans. Brain Res Brain Res Rev. 2003;43:192–206. doi: 10.1016/s0165-0173(03)00208-x. [DOI] [PubMed] [Google Scholar]
- 3.Simpson K. The role of testosterone in aggression. Mcgill J Med. 2001;6:32–40. [Google Scholar]
- 4.Balboni GC, Vannelli GB. Morphological features of the olfactory epithelium in prepubertal and postpubertal rats. In: Breiphol W, editor. Olfaction and Endocrine Regulation. IRL Press; London: 1982. pp. 285–295. [Google Scholar]
- 5.Saini KD, Breipohl W. Surface morphology in the ofactory epithelium of normal male and female rhesus monkeys. Am J Anat. 1976;147:433–445. doi: 10.1002/aja.1001470404. [DOI] [PubMed] [Google Scholar]
- 6.Balboni GC. The ultrastructure of the olfactory epithelium in the rat and its changes following castration and administration of testosterone to castrated rats. Arch Ital Anat Embriol. 1967;72:203–223. [PubMed] [Google Scholar]
- 7.Segovia S, Guillamon A. Effects of sex steroids on the development of the vomeronasal organ in the rat. Brain Res. 1982;281:209–212. doi: 10.1016/0165-3806(82)90160-2. [DOI] [PubMed] [Google Scholar]
- 8.Alekseyenko OV, Baum MJ, Cherry JA. Sex and gonadal steroid modulation of pheromone receptor gene expression in the mouse vomeronasal organ. Neuroscience. 2006;140:1349–1357. doi: 10.1016/j.neuroscience.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 9.Gu J, Dudley C, Su T, Spink DC, Zhang QY, Moss RL, Ding X. Cytochrome P450 and steroid hydroxylase activity in mouse olfactory and vomeronasal mucosa. Biochem Biophys Res Commun. 1999;266:262–267. doi: 10.1006/bbrc.1999.1807. [DOI] [PubMed] [Google Scholar]
- 10.Bojsen-Moller F. Topography of the nasal glands in rats and some other mammals. Anat Rec. 1964;150:11–24. doi: 10.1002/ar.1091500103. [DOI] [PubMed] [Google Scholar]
- 11.Moe H, Bojsen-Moller F. The fine structure of the lateral nasal gland, (Steno's gland) of the rat. J Ultrastruct Res. 1971;36:127–148. doi: 10.1016/s0022-5320(71)80093-x. [DOI] [PubMed] [Google Scholar]
- 12.Bojsen-Moller F. On the anatomy of the anterior nasal glands in man. Rev Laryngol Otol Rhinol (Bord) 1967;88(Suppl):71–75. [PubMed] [Google Scholar]
- 13.Snyder SH, Sklar PB, Pevsner J. Molecular mechanisms of olfaction. J Biol Chem. 1988;263:13971–13974. [PubMed] [Google Scholar]
- 14.Khew-Goodall Y, Grillo M, Getchell ML, Danho W, Getchell TV, Margolis FL. Vomeromodulin, a putative pheromone transporter: cloning, characterization, and cellular localization of a novel glycoprotein of lateral nasal gland. FASEB J. 1991;5:2976–2982. doi: 10.1096/fasebj.5.14.1752363. [DOI] [PubMed] [Google Scholar]
- 15.Adams DR, Deyoung DW, Griffith R. The lateral nasal gland of dog: its structure and secretory content. J Anat. 1981;132:29–37. [PMC free article] [PubMed] [Google Scholar]
- 16.Getchell ML, Mellert TK. Olfactory mucus secretion. In: Getchell TV, Doty RL, Bartoshuk LM, Snow JB, editors. Smell and Taste in Health and Disease. Raven Press; New York: 1991. pp. 83–95. [Google Scholar]
- 17.Zhuo X, Gu J, Behr MJ, Swiatek PJ, Cui H, Zhang QY, Xie Y, Collins DN, Ding X. Targeted disruption of the olfactory mucosa-specific Cyp2g1 gene: impact on acetaminophen toxicity in the lateral nasal gland, and tissue-selective effects on Cyp2a5 expression. J Pharmacol Exp Ther. 2004;308:719–728. doi: 10.1124/jpet.103.060301. [DOI] [PubMed] [Google Scholar]
- 18.Ding X, Coon MJ. Steroid metabolism by rabbit olfactory-specific P450 2G1. Arch Biochem Biophys. 1994;315:454–459. doi: 10.1006/abbi.1994.1524. [DOI] [PubMed] [Google Scholar]
- 19.Ding X, Coon MJ. Purification and characterization of two unique forms of cytochrome P-450 from rabbit nasal microsomes. Biochemistry. 1988;27:8330–8337. doi: 10.1021/bi00422a007. [DOI] [PubMed] [Google Scholar]
- 20.Zhang QY, Gu J, Su T, Cui H, Zhang X, D'Agostino J, Zhuo X, Yang W, Swiatek PJ, Ding X. Generation and characterization of a transgenic mouse model with hepatic expression of human CYP2A6. Biochem Biophys Res Commun. 2005;338:318–324. doi: 10.1016/j.bbrc.2005.08.086. [DOI] [PubMed] [Google Scholar]
- 21.Foxley GJ, Dong Q, Handelsman DJ. Quantitative reverse transcriptase polymerase chain reaction assay for mouse androgen receptor mRNA. Endocrine. 2001;15:193–198. doi: 10.1385/ENDO:15:2:193. [DOI] [PubMed] [Google Scholar]
- 22.Karolczak M, Kuppers E, Beyer C. Developmental expression and regulation of aromatase- and 5alpha-reductase type I mRNA in the male and female mouse hypothalamus. J Neuroendocrinol. 1998;10:267–274. doi: 10.1046/j.1365-2826.1998.00200.x. [DOI] [PubMed] [Google Scholar]
- 23.Dong E, Matsumoto K, Uzunova V, Sugaya I, Takahata H, Nomura H, Watanabe H, Costa E, Guidotti A. Brain 5alpha-dihydroprogesterone and allopregnanolone synthesis in a mouse model of protracted social isolation. Proc Natl Acad Sci USA. 2001;98:2849–2854. doi: 10.1073/pnas.051628598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsui D, Sakari M, Sato T, Murayama A, Takada I, Kim M, Takeyama K, Kato S. Transcriptional regulation of the mouse steroid 5alpha-reductase type II gene by progesterone in brain. Nucleic Acids Res. 2002;30:1387–1393. doi: 10.1093/nar/30.6.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laukaitis CM, Dlouhy SR, Emes RD, Ponting CP, Karn RC. Diverse spatial, temporal, and sexual expression of recently duplicated androgen-binding protein genes in Mus musculus. BMC Evol Biol. 2005;5:40. doi: 10.1186/1471-2148-5-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang X, Zhang QY, Liu D, Su T, Weng Y, Ling G, Chen Y, Gu J, Schilling B, Ding X. Expression of cytochrome p450 and other biotransformation genes in fetal and adult human nasal mucosa. Drug Metab Dispos. 2005;33:1423–1428. doi: 10.1124/dmd.105.005769. [DOI] [PubMed] [Google Scholar]
- 27.Ding X, Coon MJ. Immunochemical characterization of multiple forms of cytochrome P-450 in rabbit nasal microsomes and evidence for tissue-specific expression of P-450s NMa and NMb. Mol Pharmacol. 1990;37:489–496. [PubMed] [Google Scholar]
- 28.Dlouhy SR, Nichols WC, Karn RC. Production of an antibody to mouse salivary androgen binding protein (ABP) and its use in identifying a prostate protein produced by a gene distinct from Abp. Biochem Genet. 1986;24:743–763. doi: 10.1007/BF00499007. [DOI] [PubMed] [Google Scholar]
- 29.Karn RC. Steroid binding by mouse salivary proteins. Biochem Genet. 1998;36:105–117. doi: 10.1023/a:1018708404789. [DOI] [PubMed] [Google Scholar]
- 30.Lupo C, Lodi L, Canonaco M, Valenti A, Dessi-Fulgheri F. Testosterone metabolism in the olfactory epithelium of intact and castrated male rats. Neurosci Lett. 1986;69:259–262. doi: 10.1016/0304-3940(86)90490-8. [DOI] [PubMed] [Google Scholar]
- 31.Chung BC, Hu MC. Androgen Biosynthesis and Degradation. In: Chang C, editor. Androgens and Androgen Receptor: Mechanisms, Functions, and Clinical Applications. 1st. Kluwer Academic; Massachusetts: 2002. pp. 1–16. Chapter 1. [Google Scholar]
- 32.Hua Z, Zhang QY, Su T, Lipinskas TW, Ding X. cDNA cloning, heterologous expression, and characterization of mouse CYP2G1, an olfactory-specific steroid hydroxylase. Arch Biochem Biophys. 1997;340:208–214. doi: 10.1006/abbi.1997.9899. [DOI] [PubMed] [Google Scholar]
- 33.Laukaitis CM, Karn RC. Evolution of the secretoglobins: a genomic and proteomic view. Biol J Linnean Soc. 2005;84:493–501. [Google Scholar]
- 34.Karn RC, Laukaitis CM. Characterization of two forms of mouse salivary androgen-binding protein (ABP): implications for evolutionary relationships and ligand-binding function. Biochemistry. 2003;42:7162–7170. doi: 10.1021/bi027424l. [DOI] [PubMed] [Google Scholar]
- 35.Laukaitis CM, Heger A, Blakley TD, Munclinger P, Ponting CP, Karn RC. Rapid bursts of androgen-binding protein (Abp) gene duplication occurred independently in diverse mammals. BMC Evol Biol. 2008;8:46–62. doi: 10.1186/1471-2148-8-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Emes RD, Riley MC, Laukaitis CM, Goodstadt L, Karn RC, Ponting CP. Comparative evolutionary genomics of androgen-binding protein genes. Genome Res. 2004;14:1516–1529. doi: 10.1101/gr.2540304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Laukaitis CM, Critser ES, Karn RC. Salivary androgen-binding protein (ABP) mediates sexual isolation in Mus musculus. Evolution. 1997;51:2000–2005. doi: 10.1111/j.1558-5646.1997.tb05121.x. [DOI] [PubMed] [Google Scholar]
- 38.Talley HM, Laukaitis CM, Karn RC. Female preference for male saliva: implications for sexual isolation of Mus musculus subspecies. Evolution. 2001;55:631–634. doi: 10.1554/0014-3820(2001)055[0631:fpfmsi]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 39.Pelosi P. Perireceptor events in olfaction. J Neurobiol. 1996;30:3–19. doi: 10.1002/(SICI)1097-4695(199605)30:1<3::AID-NEU2>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 40.Krishna NS, Getchell ML, Margolis FL, Getchell TV. Differential expression of vomeromodulin and odorant-binding protein, putative pheromone and odorant transporters, in the developing rat nasal chemosensory mucosae. J Neurosci Res. 1995;40:54–71. doi: 10.1002/jnr.490400107. [DOI] [PubMed] [Google Scholar]
- 41.Ohkubo K, Baraniuk JN, Merida M, Hausfeld JN, Okada H, Kaliner MA. Human nasal mucosal carboxypeptidase: activity, location, and release. J Allergy Clin Immunol. 1995;96:924–931. doi: 10.1016/s0091-6749(95)70230-x. [DOI] [PubMed] [Google Scholar]
- 42.Ohkubo K, Okuda M, Kaliner MA. Immunological localization of neuropeptide-degrading enzymes in the nasal mucosa. Rhinology. 1994;32:130–133. [PubMed] [Google Scholar]
- 43.Mooradian AD, Morley JE, Korenman SG. Biological function of androgens. Endocr Rev. 1987;8:1–28. doi: 10.1210/edrv-8-1-1. [DOI] [PubMed] [Google Scholar]