Abstract
Superoxide Dismutase (SOD) occurs in two intracellular forms in mammals, copper-zinc SOD (CuZnSOD), found in the cytoplasm, mitochondria and nucleus, and manganese superoxide dismutase (MnSOD), in mitochondria. Changes in MnSOD expression (as compared to normal cells) have been reported in several forms of cancer, and these changes have been associated with regulation of cell proliferation, cell death, and metastasis. We have found that progestins stimulate MnSOD in T47D human breast cancer cells in a time and physiological concentration-dependent manner, exhibiting specificity for progestins and inhibition by the antiprogestin RU486. Progestin stimulation occurs at the level of mRNA, protein, and enzyme activity. Cycloheximide inhibits stimulation at the mRNA level, suggesting that progestin induction of MnSOD mRNA depends on synthesis of protein. Experiments with the MEK inhibitor UO126 suggest involvement of the MAP kinase signal transduction pathway. Finally, MnSOD-directed siRNA lowers progestin-stimulated MnSOD and inhibits progestin stimulation of migration and invasion, suggesting that up-regulation of MnSOD may be involved in the mechanism of progestin stimulation of invasive properties. To our knowledge, this is the first characterization of progestin stimulation of MnSOD in human breast cancer cells .
Keywords: Progesterone, Breast cancer, Manganese superoxide dismutase, T47D cells
1. Introduction
Superoxide dismutase (SOD) is an ubiquitously expressed protein that converts the superoxide radical (O2•−) into molecular oxygen and hydrogen peroxide [1,2]. Three forms exist in mammals: copper and zinc-containing SOD (CuZnSOD), manganese-containing SOD (MnSOD), and extracellular SOD (ECSOD), all catalyzing the following reaction:
In general, altered levels of superoxide dismutase have been found in a variety of diseases, including sickle-cell anemia [3], familial amyotropic lateral sclerosis [4–6], Alzheimer’s disease [7], and a variety of lung diseases [8,9]. In particular, altered expression of MnSOD, as compared to normal cells, has been reported in several forms of cancer, including, among others, gastric, esophageal and lung cancers [10–12]. MnSOD overexpression significantly protected Hela cervical carcinoma cells from serum starvation-induced growth suppression and death [13] and Chinese hamster ovary cells from various anticancer drugs and gamma radiation [14]. On the other hand, human melanoma cells overexpressing MnSOD lost their ability to form tumors in nude mice as well as colonies in soft agar [15], while decreased MnSOD protein and activity were observed in pancreatic adenocarcinoma cell lines [16].
In non-aggressive (MCF-7) and aggressive (BT-549 and 11-9-14) breast cancer cell lines there was an increase in MnSOD expression and enzyme activity compared to non-tumorigenic breast epithelial cell lines (MCF-12A and MCF-12F) [17]. Over-expression of MnSOD in MCF-7 cells suppressed TNF-induced caspase-3 activation and apoptosis [18]. However, others reported that MCF-7 breast cancer cells over-expressing MnSOD showed an increased expression of maspin, a proteinase inhibitor that has tumor suppression properties in breast cancer [19].
Various chemical agents and biological molecules have been shown to affect the expression of MnSOD, including phorbol-myristate 13-acetate in endothelial cells [20], lipopolysaccharide, interleukin-1, and tumor necrosis factor in pulmonary epithelial cells [21], prolactin in rat corpus luteum [22], and the steroid hormones estrogen in rat thymus [23] and progesterone in human endometrial stromal cells [24].
We have previously shown various effects of progestins in T47D human breast cancer cells, including stimulation of lactate dehydrogenase [25–28] and thymidine kinase [29] activities, and elevation of c-myc gene expression involving a progestin regulatory region [30]. In addition, we have shown that progestins increase the numbers of T47D cells by both increasing the rate of proliferation [27, 28, 31–33] and inhibiting cell death [33, 34]. Others [35,36] have also reported progestin inhibition of apoptosis in human breast cancer cell lines.
Metastasis to sites away from the primary tumor is the lethal step in cancer, and progestins have been shown by Kato et al. [37] to increase invasive properties in the human breast cancer cell line ZR-75-1, by Carnevale et al. to increase metastasis in vivo of the murine breast tumor line LM3 transfected with PR [38], and by Fu et al. to enhance invasive properties of T47D human breast cancer cells, even under conditions in which cell proliferation was prevented by ara-C [39,40].
Several authors have reported that MnSOD is involved in the invasive properties of cancer cells. Zhang et al. showed that over-expression of MnSOD in MCF-7 human breast cancer cells induced activity of a protease thought to be involved in metastatic processes, matrix metalloproteinase-2. Connor and co-workers reported that over-expression of MnSOD increased the migration and invasion of HT-1080 fibrosarcoma cells and 253J bladder tumor cells in culture and led to the development of pulmonary metastatic nodules in nude mice [42]. In the highly metastatic human breast cancer cell line MDA-MB-231, which has high endogenous levels of MnSOD, antisense RNA to MnSOD reduced invasive properties [43].
In view of the importance of the effects of progestins in breast cancer [44, 45 and references therein] and the possible role of manganese superoxide dismutase in progestin regulation of metastastic properties, we tested whether progestins would regulate this important antioxidant enzyme, and have shown that progestins indeed stimulate expression of MnSOD mRNA, protein and enzyme activity, as shown below.
2. Materials and Methods
Reagents
R5020 (17,21-dimethyl-19-nor-4,9-pregnadiene-3,20-dione) was purchased from NEN Life Science (Boston); progesterone, aldosterone, dexamethasone, estradiol-17β and testosterone from Sigma-Aldrich, and RU486 (17β-hydroxy-11β-[4-dimethyl-aminophenyl-1]-17α-[prop-1-ynil]-estra-4,9-dien-3-one) was a gift from Dr. R. Deraedt of Roussel-UCLAF (Romainville, France). U0126 was purchased from Sigma-Aldrich. Antibodies were obtained as follows: anti-human MnSOD from Upstate USA, Inc., anti-GAPDH from Chemicon, and anti-phospho-p44/42 MAP kinase and anti p44/42 MAP kinase from Cell Signaling Technology.
Cell culture
The human breast cancer cell lines T47D, MCF-7, and MDA-MB-231, obtained from the American Type Culture Collection, were grown in Corning plastic flasks (Corning, NY) in 5% CO2 in air at 37o C. Routine growth medium was minimum essential medium, powdered (autoclavable) plus non-essential amino acids, 2 mM L-glutamine, 10% fetal calf serum, 100 U/ml penicillin, 100 μg/ml streptomycin (Gibco) and 6 ng/ml insulin (Sigma). This medium contains phenol red. Cells were harvested at about 80% confluency by replacing the growth medium with splitting solution (Hank’s Balanced Salt Solution without calcium and magnesium but with 1 mM EDTA), incubating 10 minutes at 37o C, aspirating the cells and centrifuging. Cells were then washed once with PBS, centrifuged and frozen at −80o C for storage prior to making extracts. Although we have previously shown that progestins both stimulate cell proliferation and inhibit cell death in T47D cells, significant effects on cell numbers and cell density are not observable at the time points used in the experiments described herein [33, 34] . Cells in all treatment groups of each experiment were at equal viabilities at the end of the experiments.
Immunoblotting
Cells were treated with progestin to test for regulation of MnSOD both in the presence and in the absence of serum, showing progestin stimulation under both conditions. For those experiments done in the presence of serum, cells were plated into T-75 flasks at 7.5 x 106 cells per flask (~ 10 % confluency ) in twice charcoal-stripped fetal bovine serum-containing medium without phenol red, and grown for 5 days with one medium change. Following this, at zero time, medium was changed to fresh with or without hormone dissolved in ethanol so that the final hormone concentration was as indicated in figure legends and the ethanol concentration was 0.1% unless otherwise indicated. For those experiments testing the effect of hormone on MnSOD in serum-free conditions, cells were plated at about 10% confluency in routine growth medium (described above) and grown until about 80% confluent. They were then washed three times with serum-free, phenol red-free medium and treated with hormone or ethanol vehicle in serum-free, phenol red-free medium. At the appropriate time, cells were harvested and frozen at − 80o C as above and cell extracts made by sonication (3 X 10s bursts at 40 mHz with cooling on ice between bursts) in 100 microliters of NP-40 buffer (1% (V/V) Non-idet P-40, 1mM EDTA, 5 mM sodium phosphate, 1mM β-mercaptoethanol, 10% glycerol, 1% (V/V) ser/thr protein phosphatase inhibitors (Sigma), 1% (V/V) tyr protein phosphatase inhibitors (Sigma), 0.25% protease inhibitor cocktail (Sigma), pH 7.4), followed by removal of cell debris by centrifugation. After determination of protein concentration [46], equal amounts of protein for each sample were electrophoresed through 12% denaturing polyacrylamide gels and transferred to nitrocellulose membranes. Immunoblotting was then performed, using primary antibodies as listed above, appropriate horseradish peroxidase conjugated secondary antibodies and an enhanced chemiluminescence (ECL) kit from Amersham Biosciences, and X-ray film . Blots were then stripped and re-probed with antibody to GAPDH (gylceraldehyde phosphate dehydrogenase) from Chemicon International or other appropriate antibody as described in the figure legends to ensure equal gel loading and membrane transfer of each sample.
MnSOD activity assay
Cells were plated at 10% confluence in 150 cm2 flasks and grown to about 75% confluence in routine growth medium (Cell Culture). They were then washed three times with serum-free, phenol red-free medium and treated with 10nM R5020 or ethanol vehicle (0.1%) for various times in this serum-free medium. After washing three times with ice-cold PBS, cells were removed from the flask with a scraper, pelleted and frozen at −80o C. Extracts were then made and assayed for MnSOD enzyme activity essentially as previously described [47].
Real time RTPCR
Cells were plated in routine growth medium at 7 X 106 cells per dish in six 15 cm dishes and grown for 2 days, until about 80% confluent. After washing three times with serum-free, phenol red-free medium, they were treated with 10 nM R5020 or ethanol vehicle, in triplicate, in this same serum-free medium, for 24 hours. They were then harvested with trypsin-EDTA, washed twice with PBS, and frozen at −80o C. Total RNA was then isolated using the Versagene RNA Cell Kit and the Versagene RNA DNase Kit, from Gentra Systems. MnSOD mRNA relative levels were determined by quantitative RTPCR using Sybr Green PCR Master Mix for MnSOD reactions in some experiments, Taqman Universal PCR Master Mix in others and normalized with GAPDH mRNA levels using Taqman Universal PCR Master Mix (Applied Biosystems). Primers for MnSOD mRNA quantitation when using Sybr Green were: forward primer 5′ TGGCCAAGGGAGATGTTACA3′ and reverse primer 5′TGATATGACCACCACCATTGAAC3′. Optimal primer concentrations were experimentally determined to be 300nM each. The cDNA product is 72 bp long and contains sequences from exons 2 and 3 of the MnSOD gene. Total RNA concentration in the quantitative RTPCR reaction was 20 ng/μl, and the thermocycler was an ABI 7000 Sequence Detection System programmed as follows: 48o/ 30 min; 95o/ 10 min; then 40 cycles of 95o/ 15 sec & 60o/ 1 min. siRNA experiments. T47D cells were grown to 75% confluency in eight 25 cm2 flasks in medium the same as routine growth medium (above) except without antibiotics. Next, four of the cultures were transfected with 10 nM siRNA to MnSOD (# 4392420, STD) and half with 10 nM negative control siRNA (4390843) according to the manufacturer’s protocol (Applied Biosystems), using Dharmafect 1 transfection agent (Dharmacon) in fresh routine growth medium without antibiotics. After 72 hrs transfection, two of the control siRNA cultures and two of the MnSOD siRNA cultures were washed 3 times with serum-free, phenol red-free medium without antibiotics and treated 24 hrs with 10 nM R5020 or 0.1% ethanol (vehicle control) in serum-free, phenol red-free medium without antibiotics. Next, the cells were harvested and processed for immunoblotting for MnSOD and GAPDH (for normalization) as described above.
The remaining control siRNA and MnSOD siRNA-treated cultures were harvested using splitting solution as described above and single cell suspensions at 1X 106 cells per ml made in serum-free, phenol red-free medium without antibiotics. Using modified Boyden chambers with 8 micron pore inserts (Cell Biolabs, catalog # CBA-100-C), 500 microliters of twice charcoal-stripped serum-containing medium without phenol red and without antibiotics was placed in the outer wells and 300 microliters of the above cell suspension in each insert, with 10 nM R5020 or 0.1% ethanol, in triplicate, at 37o, for 24 hrs in the migration assay and 72 hrs in the invasion assay. For the migration assay, the cells have to migrate through a porous membrane, but for the invasion assay, they have to migrate first through a layer of extracellular matrix and then the membrane. The migrating and invading cells were then stained and counted (total cells in five random fields/well at 100 x magnification) as described in the manufacturer’s protocol (Cell Biolabs).
3. Results
In order to determine if progestin would affect the level of MnSOD protein in the progesterone receptor (PR)-rich human breast cancer cell line T47D, we treated cells for 24 hours with the synthetic progestin R5020, at concentrations from zero to 1 micromolar. We chose R5020 because of its stability under these conditions [48]. As shown in Figure 1A, MnSOD stimulation does not occur at the lower progestin concentrations, but appears to happen at 1 nM and becomes maximal at 10 nM through 1 micromolar. We detected no reproducible progestin stimulation of MnSOD in MCF-7 cells, which have medium levels of PR [49], even when pre-treated with estrogen, nor the PR-negative [50] MDA-MB-231 cells (data not shown).
Figure 1.
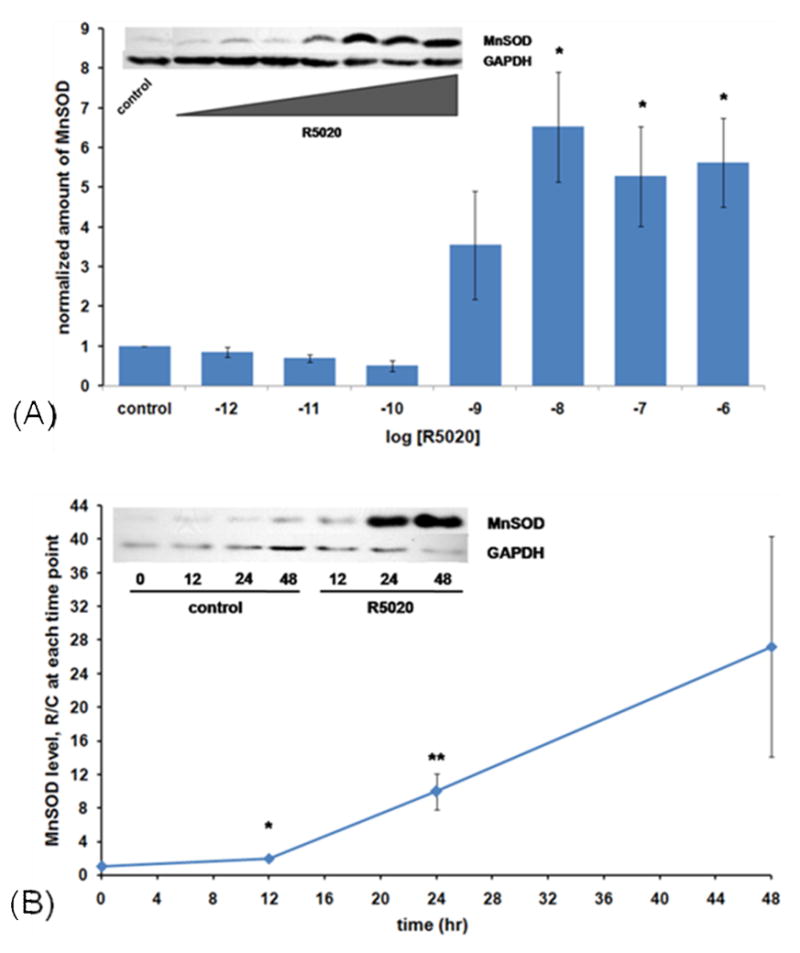
Concentration and time dependence of progestin stimulation of MnSOD protein. (A) Concentration dependence: Cells were plated and grown in 75 cm2 culture flasks in routine growth medium (Materials and Methods) to 70–80% confluency. They were then washed three times with serum-free, phenol red-free medium and treated for 24 hours with the indicated concentrations of hormone or vehicle (0.1% ethanol) in this same medium. Immunoblots were performed for both MnSOD and GAPDH, applying 120 μg protein to each electrophoretic lane, and densitometry carried out, normalizing with GAPDH levels. Results are the average +/− SEM of 4 independent experiments. *: significantly different from control, 10−12, 10−11, and 10−10 M at p<0.02 by ANOVA followed by the Student-Newman Keuls multiple comparison procedure. Inset: a representative western blot. (B) Time dependence: Cells were plated into 75 cm2 culture flasks in routine growth medium and grown for 5–6 days to 70–80 % confluency. They were then washed and treated as in Figure 1A for 24 and 48 hours with 10 nM R5020 or vehicle (0.1% ethanol). At the indicated times, cells were harvested, extracts made, and immunoblots performed for MnSOD and GAPDH as described in Materials and Methods, 80 μg of protein in each lane. Densitometry was then performed, normalizing with GAPDH as a loading and transfer control. Values are the ratio of R5020-treated to control at each time point, and are the average +/− SEM of 3 independent experiments for 12 hr and 48 hr, and 10 experiments for the 24 hr time points. *: p<0.03 compared to 12 hr control. **: p<0.001 compared to 24 hr control.
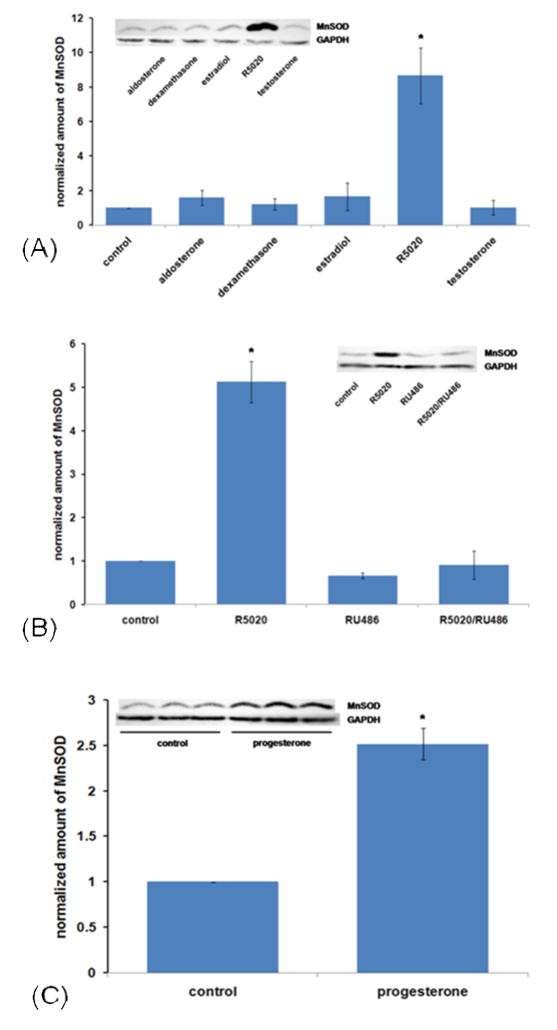
To determine the time dependency of progestin stimlation of MnSOD protein, we treated cells for 12, 24, and 48 hours with 10 nM R5020. The data in Figure1B show that this treatment, while having little effect at 12 hours, increases the level substantially by 24 hours and maintains an elevated level through 48 hours. To test whether steroid hormones other than progestins would stimulate MnSOD in T47D cells, we treated with the mineralocorticoid aldosterone, the glucocorticoid dexamethasone, the estrogen estradiol-17β, and the androgen testosterone. As shown in Figure 2A, only the progestin stimulated MnSOD. To gain information about whether progestin stimulation of MnSOD occurs through the progesterone receptor, the effect of the antiprogestin RU486 was tested. Figure 2B shows that RU486 indeed inhibits progestin stimulation of MnSOD levels, suggesting that progestin stimulation occurs through the progesterone receptor. To test whether the naturally-occurring hormone progesterone would regulate the level of MnSOD, we used 100 nM progesterone, since it has a half-life of only about 3 hours [48]. Figure 2C shows that progesterone also up-regulates MnSOD.
Figure 2.
Steroid hormone specificity and effect of the antiprogestin RU486. (A) Steroid specificity: Cells were plated and treated as in Figure 1B with 10 nM hormone or vehicle control (0.1% ethanol) for 24 hours. Cells were then harvested, extracts made, and immunoblots (inset) performed as in Materials and Methods, 160 μg of protein per electrophoretic lane. Densitometry was then performed, normalizing with GAPDH as a loading and transfer control. Results are the average +/− SEM of 3 independent experiments, except for R5020 and control, for which n = 10. *: R5020 is significantly different from all others by ANOVA followed by the Student-Newman keuls multiple comparison procedure. (B) Effect of the antiprogestin RU486 . Cells were plated, grown and treated as in Figure 1B for 24 hours with 10nM R5020, 10 nM RU486, R5020 plus RU486 or vehicle (0.2% ethanol). Immunoblots and densitometry were then performed on 180 μg of protein extracts applied to each electrophoretic lane, as shown in the graph, normalizing with GAPDH. Results are the average +/− SEM of 3 independent experiments. *: significantly different from all other values by ANOVA followed by the Student-Newman-Keuls multiple comparison procedure (p<0.001). No other differences are significant.
(C) . Stimulation of MnSOD protein by progesterone. Triplicate flasks for hormone treatment and control were plated and grown as in Figure 1B, then treated for 24 hrs with 100 nM progesterone or vehicle (0.1% ethanol) as in Figure 1B. Immunoblot and densitometry were then performed on 80 μg of protein applied to each electrophoretic lane, as shown in the graph, normalizing with GAPDH. Results are the average +/− SEM of 3 independent experiments. *: significantly different from control at p<0.001 by Student’s unpaired t test.
In order to determine if R5020 would stimulate not only the level of MnSOD protein, but also enzyme activity, we treated cells for various times with or without hormone and assayed cell extracts for enzyme activity. Table 1 shows that the progestin increased MnSOD enzyme activity about 15-fold in 48 hours. We also tested very early time points, from 10 minutes to 2 hours, and observed a very slight (~10%) but statistically significant increase at 1 and 2 hours (data not shown).
Table 1.
MnSOD Enzyme Activity (U/mg protein)
| time (hr) | ||||
|---|---|---|---|---|
| treatment | 0 | 12 | 24 | 48 |
| control | 35 | 5 | 9.1 | 5.3 |
| <2 | 2.4 | <2 | 2.9 | |
| <2 | <2 | 2.9 | <2 | |
|
| ||||
| average control (± SEM) | 1.8 (0.8) | 2.8 (1.2) | 4.3 (2.4) | 3.1 (1.2) |
| R5020 | 14 | 46 | 48 | |
| 11 | 36 | 53 | ||
| 14 | 26 | 39 | ||
|
| ||||
| average R5020 (± SEM} | 13 (1)* p=0.003 |
36 (5.8)* p=0.007 |
47 (4 1)* p<0.001 |
|
enzyme activities <2 assigned a value of 1
student’s unpaired t test
We used quantitative (real time) RTPCR to test whether progestin stimulation of MnSOD also occurs at the mRNA level. Figure 3A shows that progestin treatment elevated the level of MnSOD mRNA to about eleven times the control level in 24 hours. Figure 3B shows that this stimulation has begun by 4 hours, and is substantial by 12 hours. To determine whether progestin induction of MnSOD gene expression requires protein synthesis, we used the protein synthesis inhibitor cycloheximide. As shown in Figure 3C, cycloheximide strongly inhibits progestin stimulation of MnSOD mRNA, suggesting that it is a secondary effect, requiring protein synthesis.
Figure 3.
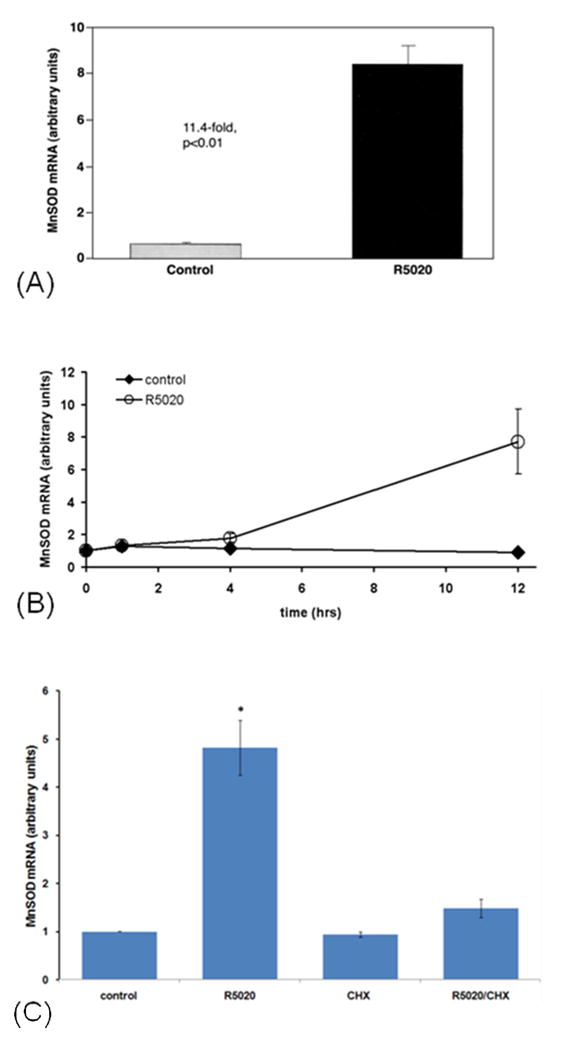
Progestin stimulation of MnSOD mRNA by real time RTPCR. (A) Cells were plated in routine growth medium (Materials and Methods) at 7 X 106 cells per dish in six 15 cm dishes and grown for 2 days, until about 80% confluent. After washing three times with serum-free, phenol red-free medium, they were treated with 10 nM R5020 or 0.1% ethanol vehicle, in triplicate, in this same serum-free medium, for 24 hours. They were then harvested with trypsin-EDTA, washed twice with PBS, and frozen at −80o C. Total RNA was then isolated using the Versagene RNA Cell Kit and the Versagene RNA DNase Kit, from Gentra Systems. MnSOD mRNA relative levels were determined by quantitative RTPCR using Sybr Green PCR Master Mix for MnSOD reactions and normalized with GAPDH mRNA levels using Taqman Universal PCR Master Mix (both from Applied Biosystems). Further details are given in Materials and Methods. Error bars refer to SEM of triplicate cultures, and the difference is statistically significant at the p<0.01 level by Student’s unpaired t test. Representative of two experiments. (B) Time dependency of progestin stimulation of MnSOD mRNA. Cells were plated in triplicate for all samples, in 75 cm2 flasks in routine growth medium, grown until about 80% confluent, and washed and treated with 10 nM R5020 or vehicle for various times as indicated. Cells were then harvested and RNA isolated, and MnSOD mRNA and GAPDH mRNA (for normalization) relative levels determined by quantitative RTPCR as in Figure 3A. Error bars are +/− SEM (of triplicates) for 1 and 4 hrs and +/− range (of duplicates) for 12 hrs. (C) Effect of the protein synthesis inhibitor cycloheximide. After growing to 80% confluency, cells were washed as above and treated, in triplicate, with 0.2% ethanol vehicle (control), 10 nM R5020, 1 mM cycloheximide (dissolved in ethanol), or both R5020 and cycloheximide, for 6 hours. Cells were then harvested, RNA isolated, and both MnSOD mRNA and GAPDH mRNA relative levels were determined by real time RTPCR as described in figure 3A. The R5020 value is statistically different from all others at the p< 0.001 level by ANOVA followed by the Student-Newman -Keuls multiple comparison procedure. All other values are statistically the same. Results are the average +/− SEM of 3 independent experiments.
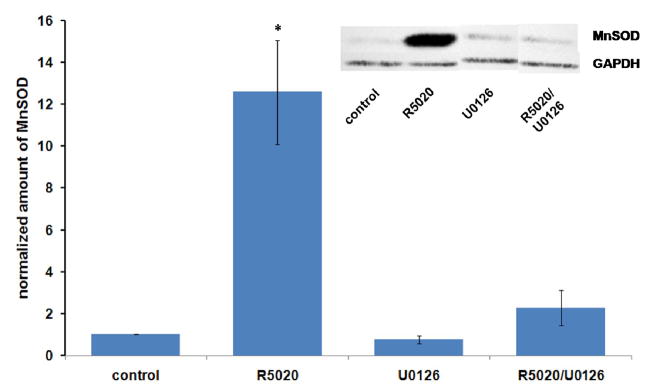
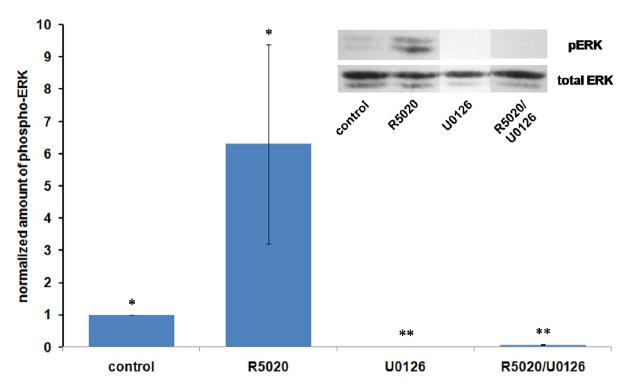
Since the mechanism of progestin action has been shown to involve not only effects at the genomic level but also signal transduction pathways mediated by components associated with the cell membrane and cytoplasm [38, 50–52], we tested an inhibitor of the MAP Kinase pathway (U0126) (53) for its effect on progestin stimulation of MnSOD expression. As shown in Figure 4, U0126 inhibited progestin stimulation of MnSOD, suggesting that stimulation requires involvement of the enzyme MAP kinase kinase (MEK). Figure 5 shows that U0126 is acting as expected to inhibit progestin –enhanced phosphorylation of MAP kinase (ERK 1 and 2) in these experiments.
Figure 4.
Effects of the MAP Kinase kinase inhibitor U0126 on progestin stimulation of MnSOD expression. T47D cells were plated, grown and treated as in Figure 1A, except that U0126 dissolved in DMSO (dimethyl sulfoxide) was added at 5 micromolar 15 minutes before R5020 ( 10 nM ) was added. Final concentrations of DMSO and ethanol were 0.1%. After 24 hours of treatment, cells were harvested, extracts made and immunoblotting performed as described in Materials and Methods. 60 μg of protein was applied to each lane. Densitometry results are the average +/− SEM of 3 independent experiments. *: different from all others at p<0.05 by ANOVA followed by the Bonferrroni t-test.
Figure 5.
UO126 inhibits progestin stimulation of phosphorylation/activation of its target proteins ERK1/2 while inhibiting stimulation of MnSOD. Extracts from the same experiments described in Figure 4 were subjected to electrophoresis and immunoblotting as described in Materials and Methods for phosphorylated ERK-1 and ERK-2 and total ERK (60 μg per lane). U0126 concentration was 5 μM and R5020 was at 10 nM. Results are the average +/− SEM of 3 independent experiments. *: different from all others at p< 0.05 by Kruskal-Wallis ANOVA followed by the Student-Newman-Keuls multiple comparison procedure. **: same as each other, different from all others at p<0.05.
As stated in the introduction, MnSOD has been reported to play a role in metastatic properties of several kinds of cancer, including breast cancer [41–43]. In addition, several laboratories have found that progestins stimulate invasive properties of human breast cancer cell lines [37–40], and Fu et al. (39) have shown that progestins stimulate migration and invasion in T47D cells even when cell division is stopped with ara-C. After confirming statistically significant progestin stimulation of migration and invasion in wild-type T47D cells (data not shown), we tested the notion that siRNA directed against MnSOD expression would inhibit progestin stimulation of migration and invasion.. The data in Figure 6 confirm progestin stimulation of migration and invasion, and that siRNA directed against MnSOD both lowers the level of progestin-stimulated MnSOD and inhibits stimulation of migration and invasion. These data are consistent with the idea that progestin stimulation of MnSOD may be part of the mechanism by which progestins stimulate invasive properties of T47D cells.
Figure 6.
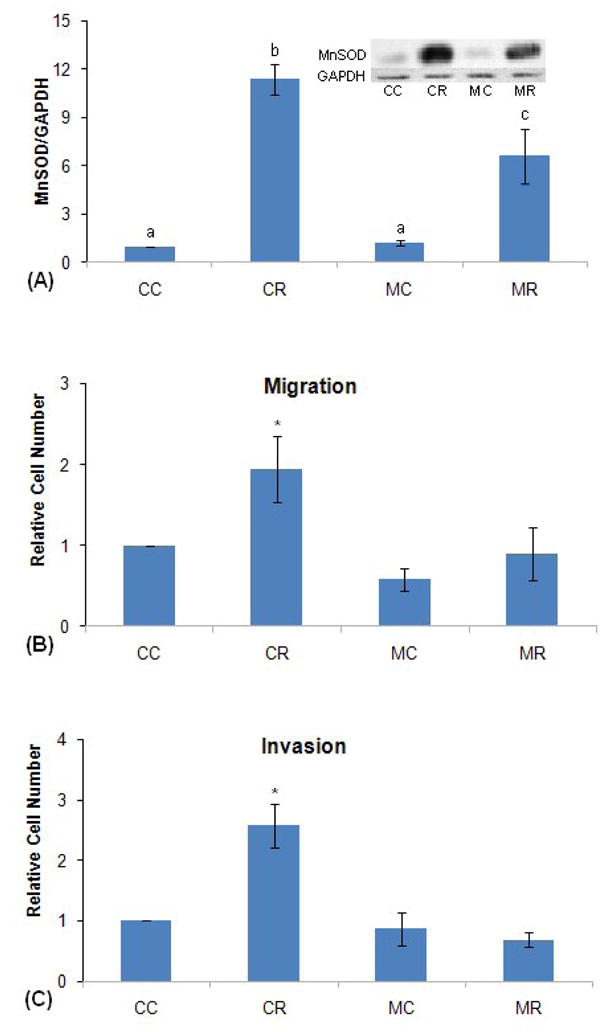
MnSOD-directed siRNA inhibits progestin stimulation of migration and invasion. (A) siRNA effect on MnSOD levels. As described in detail in Materials and Methods, T47D cells at 75% confluency were transfected with 10nM MnSOD-directed or control siRNA for 72 hrs, and then treated with 10 nM R5020 or vehicle (0.1% ethanol) for 24 hrs. The cells were then harvested and processed for immunoblotting for MnSOD and GAPDH (for normalization). The results were then analyzed by densitometry. Inset: western blot of a representative experiment. CC: Control si RNA without hormone; CR: Control si RNA with hormone; MC: MnSOD siRNA without hormone; MR: MnSOD siRNA with hormone. Densitometry: average +/− SEM of 7 independent experiments. Samples labeled with different letters are different from one another at p<0.002 by ANOVA followed by the Student-Newman-Keuls multiple comparison procedure. Those with the same letters are statistically the same.
(B) MnSOD siRNA effect on progestin stimulation of migration. As described in detail in Materials and Methods, T47D cells after transfection as above in (A) were incubated for 24 hrs with or without 10 nM R5020 in modified Boyden chambers and allowed to migrate through a polycarbonate membrane with 8 micron pores, and the migrated cells stained and counted by light microscopy, averaging 60 cells in the control (CC) samples in 7 experiments. Data are the mean +/− SEM of 7 independent experiments each done in triplicate, and were analyzed by ANOVA followed by the Student-Newman -Keuls multiple comparison procedure. CR is different from all other values at p< 0.03. No other differences are significant. CC, CR, MC, MR: same as in (A). (C) MnSOD siRNA effect on invasion. As described in detail in Materials and Methods, T47D cells after transfection as above in (A) were incubated for 72 hrs (48 hrs gave similar results) with or without 10 nM R5020 in modified Boyden chambers, passing first through a layer of extracellular matrix and then a polycarbonate membrane with 8 micron pores, and stained and counted as above, averaging 11 cells in the control (CC) samples in 4 experiments. Data are the mean +/− SEM of 4 independent experiments, each done in triplicate, and were analyzed by ANOVA followed by the Student-Newman-Keuls multiple comparison procedure. *: CR is different from all other values at p< 0.001. No other differences are significant. CC, CR, MC, MR: same as in part (A).
Thus, as shown above, our data suggest that progestin stimulation of MnSOD occurs at the level of mRNA, protein, and enzyme activity, requires protein synthesis, and involves the MAP kinase (ERK1/2) pathway. In addition, the data suggest that stimulation of MnSOD may be part of the mechanism of progestin stimulation of metastatic properties in T47D cells. To our knowledge, this is the first report characterizing progestin stimulation of MnSOD in human breast cancer cells.
4. Discussion
The concentration dependence of progestin stimulation of MnSOD is somewhat different from what we have seen for progestin effects on c-myc gene expression [K.A. Blankenship, M.R. Moore, unpublished observations], thymidine kinase activity [29], and lactate dehydrogenase activity (26), in that these are biphasic. That is, in the latter cases, stimulation occurs at the lower concentrations of progestin, increases to a maximum at higher concentrations, and then falls off in the μM range. However, stimulation of MnSOD is maintained at a maximal level even in the μM range. This may be because progestins in the μM range begin to have harmful effects on the cells, and, as stated above, various harmful substances in other systems have been shown to raise MnSOD levels [20, 21, 54]. As mentioned in the results section, we saw no progestin stimulation of MnSOD in MCF-7 cells, which was surprising since they are PR-positive. Others have shown that MnSOD protein level in MCF-7 cells is only minimally affected by tamoxifen or TNF-α alone, but in combination these two molecules dramatically stimulate MnSOD in MCF-7 cells, through an NFkB binding site in the MnSOD gene (55). Perhaps a combination of TNF-α and progestin would stimulate MnSOD expression in MCF-7 cells, although we have not tested this combination.
The naturally occurring hormone progesterone at 100 nM stimulated MnSOD to a lesser extent than 10 nM R5020. This is probably at least in part because of the short half-life (~3 hours) of progesterone in cultured human breast cancer cells as compared to that of the very stable synthetic hormone R5020 [49]. By the end of the 24 hour treatment, the progesterone would have gone through about 10 half-lives, reducing its concentration to around 0.3 nM. At any rate, the average progesterone concentration in human breast duct fluid during the menstrual cycle is around 300 nM [56].
The enzyme’s specific activity responds to progestin with a time course somewhat similar to the MnSOD protein level. The stimulation is readily observable by 12 hours and increases substantially by 24 hours. Pedram et al. have reported that estrogen stimulates MnSOD activity to about twice the control level after 1 hour of treatment in the ER-rich cell human breast cancer cell line MCF-7 [57]. As mentioned above, we also observed a slight (10%), but statistically significant progestin up-regulation of enzyme specific activity at 1 and 2 hours in T47D cells. Shiki et al. [58] and Asayama et al. [59] have demonstrated a similar time course of response of MnSOD protein to lipopolysaccharide (LPS) in bovine pulmonary artery cells and human monocytes, respectively.
Our data show that progestin stimulation of MnSOD mRNA, just as up-regulation of MnSOD protein and enzyme activity, is quite robust, mRNA level rising by 4 hours, with an approximate 11-fold stimulation occurring in 24 hours . To our knowledge, this is among the stronger stimulations so far reported for MnSOD, suggesting an important role for progestins in regulation of this antioxidant enzyme. In a calf pulmonary artery endothelial cell line, phorbol 12-myristate 13-acetate stimulated MnSOD mRNA levels around 40-fold in 6 hours [20]. Lipopolysaccharide stimulated MnSOD message levels to a similar extent in rat L2 cells, a pulmonary epithelial-like line [21]. Prolactin up-regulated MnSOD mRNA about 2-fold in hypophysectomized rat corpus luteum (in vivo) and in rat luteinized rat granulosa cells (in vitro) [22]. Tumor necrosis factor (TNF) has been shown to stimulate levels of MnSOD mRNA and protein in the human breast cancer cell line MCF-7, an effect that is enhanced by tamoxifen [55].
In order to determine whether progestin stimulation of MnSOD mRNA is a primary effect of progestins, directly up-regulating the mRNA level, or whether it requires intervening protein synthesis, we used the protein synthesis inhibitor cycloheximide. The data suggest that protein synthesis must occur between progestin treatment and enhancement of MnSOD gene expression, consistent with a secondary effect. However, the data could also result from loss of a protein with a short half-life that is required for progestin induction of the MnSOD gene, such as a transcription factor, or loss of a factor involved in MnSOD mRNA stability. Additional experiments will be required for a more definitive conclusion. This differs from the situation of phorbol myristate 13-acetate (PMA) induction of MnSOD in the SV40-transformed human lung fibroblast cell line VA-13, where cycloheximide has been shown to actually enhance MnSOD gene expression by stopping the synthesis of IκBα, allowing up-regulation of MnSOD mRNA to occur through NFκB [60].
Our data suggest that progestin stimulation of MnSOD involves the MAP kinase signal transduction pathway, as has been shown for the progestin regulation of several other effects in human breast cancer cells in culture, including stimulation of the cell cycle, expression of the genes for cyclin D1, matrix metalloproteinases -9 and -2, etc. [38, 51, 61]. What specific roles the MAP kinase signaling pathway plays in stimulation of MnSOD is unknown, although it probably involves phosphorylation of PR, since MAP kinase has been shown to phosphorylate PR [52 and references therein].
A major function of MnSOD is protection of the cell from the harmful effects of reactive oxygen species (ROS) [62]. Studies in at least three model systems demonstrate MnSOD is absolutely essential for survival in our oxygen-rich atmosphere. E. coli B cells grown under 100% O2 expressed high levels of MnSOD and were more resistant to oxygen toxicity at high pressures than cells grown under normal atmospheric conditions [63,64]. In the yeast species Saccharomyces cereviscae var. ellipsoideus, cells grown under high O2 concentrations were more resistant to oxygen toxicity at high pressures than control cells grown under a normal atmosphere [65]. Mice that are homozygous for a mutant MnSOD die within 10 days of birth with dilated cardiomyopathy, metabolic acidosis, and increased lipid levels in liver and skeletal muscle [66]. Overexpression of CuZnSOD does not prevent neonatal lethality in MnSOD-deficient mice [67], further confirming the importance of MnSOD in prevention of oxidative injury.
Previously, we have shown that progestins both stimulate proliferation [27, 28, 31–33] and inhibit apoptosis [33, 34] in the human breast cancer cell line T47D. Ory et al. [35] and Vares et al. [36] have also reported progestin inhibition of apoptosis in human breast cancer cell lines. Other workers have shown that MnSOD helps protect against cell death in various systems [13, 14, 18]; however, decreased MnSOD levels are associated with some cancers [15, 16]. During the course of the present work, Graham and co-workers, in a cDNA microarray study in T47D cells, reported that MnSOD was one of hundreds of progestin-regulated genes; however, they did not confirm by other methods or characterize the MnSOD stimulation (68). Others have reported that increased levels of MnSOD promote metastatic properties of fibrosarcoma and bladder tumor cells [42], and invasive properties of MCF-7 [41] and MDA-MB-231 human breast cancer cells [43]. Several laboratories have reported that progestins stimulate invasive properties of human breast cancer cell lines [37– 40], including the report by Fu et al. (39) which showed that this occurs in T47D cells even when cell division is stopped with ara-C, which prevents cell division but allows RNA synthesis.
Our data show that 10 nM MnSOD siRNA, while having no detectable effect on the low basal levels of MnSOD protein found in T47D cells, lowers the level of progestin- stimulated MnSOD. They also show that this lowering of MnSOD inhibits progestin stimulation of migration and invasion, suggesting that up-regulation of MnSOD may be part of the mechanism of progestin stimulation of these invasive properties. As can be seen, even though the siRNA lowers the progestin-stimulated level of MnSOD by only about 50 %, this completely blocks progestin stimulation of migration and invasion. This suggests that there may be a threshold MnSOD level which must be reached in order for progestins to enhance invasive properties of T47D human breast cancer cells. These data confirm the idea that MnSOD enhances the invasive properties of cancer cells, as Connor et al. showed by MnSOD over-expression in HT-1080 fibrosarcoma cells in culture and 253J bladder tumor cells in culture and in vivo [42]; and as Zhang et al. [41] demonstrated with MnSOD over-expression in MCF-7 human breast cancer cells and as Kattan et al [43] reported using MnSOD-specific antisense RNA in MDA-MB-231 human breast cancer cells.
In summary, we have shown that progestins significantly up-regulate MnSOD expression at the protein, enzyme activity, and mRNA levels in T47D human breast cancer cells. This effect is time and physiological concentration-dependent, occurs through the progesterone receptor, involves the MAP kinase signal transduction pathway, and is dependent on the synthesis of protein. Further, our data suggest that one physiological role of progestin stimulation of MnSOD may be enhancement of invasive properties in breast cancer, which may help explain harmful effects of progestins in hormone replacement therapy (69). To our knowledge, this is the first characterization of progestin stimulation of MnSOD in human breast cancer cells.
Acknowledgments
This work was supported by NIH grants COBRE P20 RR020180 and INBRE P20 RR016477, as well as P01-66081, P30-CA086862, R01DK49030 and R01CA100045. We thank Dr. Viroj Boonyaratanakornkit of Baylor College of Medicine for helpful discussions and acknowledge the excellent technical assistance of Amanda G. Estep and M. Allison Teter for their work at Marshall University in the siRNA experiments. We thank Umarani Pugazhenthi of the University of Colorado Cancer Center Quantitative PCR Core Laboratory for help with real-time RTPCR and Mitchell C. Coleman and the Antioxidant Enzyme Core at the University of Iowa for technical assistance with the MnSOD activity analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) J Biol Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- 2.Weisiger RA, Fridovich I. Mitochondrial superoxide dismutase. Site of synthesis and intramitochondrial localization. J Biol Chem. 1973;248:4793–4796. [PubMed] [Google Scholar]
- 3.Schacter L, Warth JA, Gordon EM, Prasad A, Klein BL. Altered amount and activity of superoxide dismutase in sickle cell anemia. Faseb J. 1998;2:237–243. doi: 10.1096/fasebj.2.3.3350236. [DOI] [PubMed] [Google Scholar]
- 4.Rothstein JD, Bristol LA, Hosler B, Brown RH, Jr, Kuncl RW. Chronic inhibition of superoxide dismutase produces apoptotic death of spinal neuron. Proc Natl Acad Sci USA. 1994;91:4155–4159. doi: 10.1073/pnas.91.10.4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hough MA, Grossman JG, Antonyuk SV, Strange RW, Doucette PA, Rodriguez JA, Whitson LJ, Hart PJ, Hayward LJ, Valentine JS, Hasnain SS. Dimer destabilization in superoxide dismutase may result in disease-causing properties: structures of motor neuron disease mutants. Proc Natl Acad Sci USA. 2004;101:5976–5981. doi: 10.1073/pnas.0305143101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rakhit R, Crow JP, Lepock JR, Kondejewski LH, Cashman NR, Chakrabartty A. Monomeric Cu, Zn-superoxide dismutase is a common misfolding intermediate in the oxidation models of sporadic and familial amyotrophic lateral sclerosis. J Biol Chem. 2004;279:15499–15504. doi: 10.1074/jbc.M313295200. [DOI] [PubMed] [Google Scholar]
- 7.Choi J, Rees HD, Weintraub ST, Levey AI, Chin LS, Li L. Oxidative modifications and aggregation of Cu/Zn superoxide dismutase associated with Alzheimer’s and Parkinson’s diseases. J Biol Chem. 2005;280:11648–11655. doi: 10.1074/jbc.M414327200. [DOI] [PubMed] [Google Scholar]
- 8.Lakari E, Pääkkö P, Pietarinen-Runtti P, Kinnula VL. Manganese superoxide dismutase and catalase are coordinately expressed in the alveolar region in chronic interstitial pneumonias and granulomatous diseases of the lung. Amer J Resp Crit Care Med. 2000;161:615–621. doi: 10.1164/ajrccm.161.2.9904091. [DOI] [PubMed] [Google Scholar]
- 9.Kinnula VL, Crapo JL. Superoxide dismutases in the lung and human lung diseases. Amer J Resp Crit Care Med. 2003;167:1600–1619. doi: 10.1164/rccm.200212-1479SO. [DOI] [PubMed] [Google Scholar]
- 10.Janssen AML, Bosman CB, van Duijn W, Oostendorp-van de Ruit MM, Kubben FJGM, Griffioen G, Lamers CB, van Krieken JH, van de Velde CJ, Verspaget HW. Superoxide dismutases in gastric and esophageal cancer and the prognostic impact in gastric cancer. Clin Cancer Res. 2000;6:3183–3192. [PubMed] [Google Scholar]
- 11.Izutani R, Asano S, Imano M, Kuroda D, Kato M, Ohyanagi H. Expression of manganese superoxide dismutase in esophageal and gastric cancers. J Gastroenterology. 1998;33:816–822. doi: 10.1007/s005350050181. [DOI] [PubMed] [Google Scholar]
- 12.Ho JC, Zheng S, Comhair SAA, Farver C, Erzurum SC. Differential expression of manganese superoxide dismutase and catalase in lung cancer. Cancer Res. 2001;6:8578–8585. [PubMed] [Google Scholar]
- 13.Palazotti B, Pani G, Colavitti R, De Leo ME, Bedogni B, Borrello S, Galeotti T. Increased growth capacity of cervical carcinoma cells over-expressing manganous superoxide dismutase. Int J Cancer. 1999;82:145–150. doi: 10.1002/(sici)1097-0215(19990702)82:1<145::aid-ijc24>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 14.Hirose K, Longo DL, Oppenheim JJ, Matsushima K. Overexpression of mitochondrial manganese superoxide dismutase promotes the survival of tumor cells exposed to interleukin-1, tumor necrosis factor, selected anticancer drugs, and ionizing radiation. Faseb J. 1993;7:61–368. doi: 10.1096/fasebj.7.2.8440412. [DOI] [PubMed] [Google Scholar]
- 15.Church SL, Grant JW, Ridnour LA, Oberley LW, Swanson PE, Meltzer PS, Trent JM. Increased manganese superoxide dismutase expression suppresses the malignant phenotype of human melanoma cells. Proc Natl AcadSci USA. 1993;90:3113–3317. doi: 10.1073/pnas.90.7.3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cullen JJ, Weydert C, Hinkhouse MM, Ritchie J, Domann FE, Spitz D, Oberley LW. The role of manganese superoxide dismutase in the growth of pancreatic adenocarcinoma. Cancer Res. 2003;63:1297–1303. [PubMed] [Google Scholar]
- 17.Mukhopadhyay S, Das SK, Mukherjee S. Expression of Mn-superoxide dismutase gene in non-tumorigenic and tumorigenic human mammary epithelial cells. J Biomed Biotech. 2004;4:195–202. doi: 10.1155/S1110724304401016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manna SK, Zhang HJ, Yan T, Oberley LW, Aggarwal BB. Over-expression of manganese superoxide dismutase suppresses tumor necrosis factor-induced apoptosis and activation of nuclear transcription factor-κB and activated protein-1. J Biol Chem. 1998;273:13245–13254. doi: 10.1074/jbc.273.21.13245. [DOI] [PubMed] [Google Scholar]
- 19.Li JJ, Colburn NH, Oberley LW. Maspin gene expression in tumor suppression induced by overexpressing manganese-containing superoxide dismutase cDNA in human breast cancer cells. Carcinogenesis. 1998;19:833–839. doi: 10.1093/carcin/19.5.833. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka T, Kurabayashi M, Aihara Y, Ohyama Y, Nagai R. Inducible expression of manganese superoxide dismutase by phorbol 12-myristate 13-acetate is mediated by Sp1 in endothelial cells. Art Throm Vasc Biol. 2000;20:392–401. doi: 10.1161/01.atv.20.2.392. [DOI] [PubMed] [Google Scholar]
- 21.Visner GA, Dougall WC, Wilson JM, Burr IA, Nick HS. Regulation of manganese superoxide dismutase by lipopolysaccharide, interleukin-1, and tumor necrosis factor: role in the acute inflammatory response. J Biol Chem. 1900;265:2856–2864. [PubMed] [Google Scholar]
- 22.Sugino N, Hirosawa-Takamori M, Zhong L, Telleria CM, Shiota K, Gibori G. Hormonal regulation of copper-zinc superoxide dismutase and manganese superoxide dismutase messenger ribonucleic acid in the rat corpus luteum: induction by prolactin and placental lactogens. Biol Reprod. 1998;59:599–605. doi: 10.1095/biolreprod59.3.599. [DOI] [PubMed] [Google Scholar]
- 23.Kasapović J, Pajović SB, Pejić S, Martinović JV. Effects of estradiol benzoate and progesterone on superoxide dismutase activity in the thymus of rats. Physiol Res. 2001;50:97–103. [PubMed] [Google Scholar]
- 24.Sugino N, Karube-Harada A, Kashida S, Takiguchi S, Kato H. Differential regulation of copper-zinc superoxide dismutase and manganese superoxide dismutase by progesterone withdrawal in human endometrial stromal cells. Mol Human Reprod. 2002;8:68–74. doi: 10.1093/molehr/8.1.68. [DOI] [PubMed] [Google Scholar]
- 25.Hagley RD, Moore MR. A progestin effect on lactate dehydrogenase in the human breast cancer cell line T-47D. Biochem Biophys Res Commun. 1985;128:520–524. doi: 10.1016/0006-291x(85)90077-4. [DOI] [PubMed] [Google Scholar]
- 26.Hagley RD, Hissom JR, Moore MR. Progestin stimulation of lactate dehydrogenase in the human breast cancer cell line T-47D. Biochim Biophys Acta. 1987;930:167–172. doi: 10.1016/0167-4889(87)90028-0. [DOI] [PubMed] [Google Scholar]
- 27.Moore MR, Hagley RD, Hissom JR. Progestin effects on lactate dehydrogenase and growth in the human breast cancer cell line T47D. In: Hankins HD, Puett D, editors. Hormones, Cell Biology and Cancer, Perspectives and Potential. Alan R. Liss, Inc; New York: 1988. pp. 61–179. [Google Scholar]
- 28.Hissom JR, Bowden RT, Moore MR. Effects of progestins, estrogens and antihormones on growth and lactate dehydrogenase in the human breast cancer cell line T47D. Endocrinology. 1989;125:418–423. doi: 10.1210/endo-125-1-418. [DOI] [PubMed] [Google Scholar]
- 29.Moore MR, Hathaway LD, Bircher JA. Progestin stimulation of thymidine kinase in the human breast cancer cell line T47D. Biochim Biophys Acta. 1991;1096:170–174. doi: 10.1016/0925-4439(91)90056-f. [DOI] [PubMed] [Google Scholar]
- 30.Moore MR, Zhou JL, Blankenship KA, Strobl JS, Edwards DP, Gentry RN. A sequence in the 5′ flanking region confers progestin responsiveness on the human c-myc gene. J Steroid Biochem Mol Biol. 1997;62:243–252. doi: 10.1016/s0960-0760(97)00036-8. [DOI] [PubMed] [Google Scholar]
- 31.Hissom JR, Moore MR. Progestin effects on growth in the human breast cancer cell line T47D--possible therapeutic implications. Biochem Biophys Res Commun. 1987;145:706–711. doi: 10.1016/0006-291x(87)91022-9. [DOI] [PubMed] [Google Scholar]
- 32.Bowden RT, Hissom JR, Moore MR. Growth stimulation of T47D human breast cancer cells by the antiprogestin RU486. Endocrinology. 1989;124:2642–2644. doi: 10.1210/endo-124-5-2642. [DOI] [PubMed] [Google Scholar]
- 33.Moore MR, Conover JL, Franks KM. Progestin effects on long-term growth, death, and Bcl-xL in breast cancer cells. Biochem Biophys Res Commun. 2000;277:650–654. doi: 10.1006/bbrc.2000.3728. [DOI] [PubMed] [Google Scholar]
- 34.Moore MR, Spence JB, Kiningham KK, Dillon JL. Progestin inhibition of cell death in human breast cancer cell lines. J Steroid Biochem Mol Biol. 2006;98:218–227. doi: 10.1016/j.jsbmb.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 35.Ory K, Lebeau J, Levalois C, Bishay K, Fouchet P, Allemand I, Therwath A, Chevillard Apoptosis inhibition mediated by medroxyprogesterone acetate treatment of breast cancer cell lines. Breast Cancer Res Treat. 2001;68:187–198. doi: 10.1023/a:1012288510743. [DOI] [PubMed] [Google Scholar]
- 36.Vares G, Ory K, Lectard B, Levalois C, Altmeyer-Morel S, Chevillard S, Lebeau J. Progesterone prevents radiation-induced apoptosis in breast cancer cells. Oncogene. 2004;23:4603–4613. doi: 10.1038/sj.onc.1207601. [DOI] [PubMed] [Google Scholar]
- 37.Kato S, Pinto M, Carvajal A, Espinoza N, Monso C, Sadarangani A, Villalon M, Brosens JJ, White JO, Richer JK, Horwitz KB, Owen GI. Progesterone Increases Tissue Factor Gene Expression, Procoagulant Activity, and Invasion in the Breast Cancer Cell Line ZR-75-1. J Clin Endo Metab. 2005;90:1181–1188. doi: 10.1210/jc.2004-0857. [DOI] [PubMed] [Google Scholar]
- 38.Carnevale RP, Proietti CJ, Salatino M, Urtreger A, Peluffo G, Edwards DP, Boonyaratanakornkit V, Charreau EH, Bal de Kier Joffe E, Schillaci R, Elizalde PV. Progestin effects on breast cancer cell proliferation, proteases activation, and in vivo development of metastatic phenotype all depend on progesterone receptor capacity to activate cytoplasmic signaling pathways. Mol Endo. 2007;21:1335–1358. doi: 10.1210/me.2006-0304. [DOI] [PubMed] [Google Scholar]
- 39.Fu XD, Giretti MS, Baldacci C, Garibaldi S, Flamini M, Sanchez AM, Gadducci A, Genazzani AR, Simoncini T. Extra-nuclear Signaling of Progesterone Receptor to Breast Cancer Cell Movement and Invasion through the Actin Cytoskeleton. PLoS ONE. 2008;3(7):e2790. doi: 10.1371/journal.pone.0002790. doi:/journal.pone.0002790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fu XD, Giretti MS, Goglia L, Flamini MI, Sanchez AM, Baldacci C, Garibaldi S, Ware R Sitruk, Genazzani AR, Simoncini T. Comparative actions of progesterone, medroxyprogesterone acetate, drospirenone and nestorone on breast cancer cell migration and invasion. BMC Cancer. 2008;8:166. doi: 10.1186/1471-2407-8-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang HJ, Zhao W, Venkataraman S, Robbins MEC, Buettner GR, Kreggel KC, Oberley LW. Activation of Matrix Metalloproteinase-2 by Over-expression of Manganese Superoxide Dismutase in Human Breast Cancer MCF-7 Cells Involves Reactive Oxygen Species. J Biol Chem. 2002;277:20919–20926. doi: 10.1074/jbc.M109801200. [DOI] [PubMed] [Google Scholar]
- 42.Connor KM, Hempel N, Nelson KK, Dabiri G, Gamarra A, Belarmino J, Van De Water L, Mian BM, Melendez JA. Manganese Superoxide Dismutase Enhances the Invasive and Migratory Activity of Tumor Cells. Cancer Res. 2007;67:10260–10267. doi: 10.1158/0008-5472.CAN-07-1204. [DOI] [PubMed] [Google Scholar]
- 43.Kattan Z, Minig V, Leroy P, Dauca M, Becuwe P. Role of manganese superoxide dismutase on growth and invasive properties of human estrogen-independent breast cancer cells. Breast Cancer Res Treat. 2007;108:203–215. doi: 10.1007/s10549-007-9597-5. [DOI] [PubMed] [Google Scholar]
- 44.Moore MR. A rationale for inhibiting progesterone-related pathways to combat breast cancer. Curr Cancer Drug Targets. 2004;4:183–189. doi: 10.2174/1568009043481515. [DOI] [PubMed] [Google Scholar]
- 45.Kariagina A, Aupperlee MD, Haslam SZ. Progesterone Receptor Isoform Functions in Normal Breast Development and Breast Cancer. Crit Rev Euk Gene Express. 2008;18:11–33. doi: 10.1615/critreveukargeneexpr.v18.i1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 47.Spitz DR, Oberley LW. An assay for superoxide dismutase activity in mammalian tissue homogenates. Anal Biochem. 1989;179:8–18. doi: 10.1016/0003-2697(89)90192-9. [DOI] [PubMed] [Google Scholar]
- 48.Horwitz KB, Mockus M, Pike AW, Fennessy PV, Sheridan RL. Progesterone receptor replenishment in T47D human breast cancer cells. Roles of protein synthesis and hormone metabolism. J Biol Chem. 1983;258:7603–7610. [PubMed] [Google Scholar]
- 49.Horwitz KB, Zava DT, Thilagar AK, Jensen EM, McGuire WL. Steroid receptor analyses of nine human breast cancer cell lines. Cancer Res. 1978;38:211–216. [PubMed] [Google Scholar]
- 50.Migliaccio A, Piccolo D, Castoria G, Di Domenico M, Bilancio A, Lombardi M, Gong W, Beato M, Auricchio F. Activation of the Src/p21ras/Erk Pathway by Progesterone Receptor via Cross-talk with Estrogen Receptor. Euro Mol Biol Org J. 1998;17:2008–2018. doi: 10.1093/emboj/17.7.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boonyaratanakornkit V, Mcgowan E, Sherman L, Mancini MA, Cheskis BJ, Edwards DP. The role of extranuclear signaling actions of progesterone receptor in mediating progesterone regulation of gene expression and the cell cycle. Mol Endo. 2007;21:359–375. doi: 10.1210/me.2006-0337. [DOI] [PubMed] [Google Scholar]
- 52.Lange CA. Integration of progesterone receptor action with rapid signaling events in breast cancer models. J Steroid Biochem Mol Biol. 2008;108:203–212. doi: 10.1016/j.jsbmb.2007.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Favata MF, Horiuchi KY, Manos EJ, Daulerio AJ, Stradley DA, Feeser WS, Van Dyk DE, Pitts WJ, Earl RA, Hobbs F, Copeland RA, Magolda RL, Scherle PA, Trzaskos JM. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J Biol Chem. 1998;273:18623–18632. doi: 10.1074/jbc.273.29.18623. [DOI] [PubMed] [Google Scholar]
- 54.Das KC, Guo X, White CW. Protein kinase Cδ-dependent induction of manganese superoxide dismutase gene expression by microtubule-active anticancer drugs. J Biol Chem. 1998;273:34639–34645. doi: 10.1074/jbc.273.51.34639. [DOI] [PubMed] [Google Scholar]
- 55.Daosukho C, Kiningham KK, Kasarkis EJ, Ittarat W, St Clair D. Tamoxifen enhancement of TNF-α induced MnSOD expression: modulation of NF-κB dimerization. Oncogene. 2002;21:3603–3610. doi: 10.1038/sj.onc.1205448. [DOI] [PubMed] [Google Scholar]
- 56.Rose DP, Tilton K, Lahti H, Wynder EL. Progesterone levels in breast duct fluid. Euro J Cancer Clin Oncol. 1986;22:111– 113. doi: 10.1016/0277-5379(86)90350-0. [DOI] [PubMed] [Google Scholar]
- 57.Pedram A, Razandi M, Wallace DC, Levin ER. Functional estrogen receptors in the mitochondria of breast cancer cells. Mol Biol Cell. 2006;17:2125–2137. doi: 10.1091/mbc.E05-11-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shiki Y, Meyrick BO, Brigham KL, Burr IM. Endotoxin increases superoxide dismutase in cultured bovine pulmonary epithelial cells. Am J Phys. 1987;252:c436–c440. doi: 10.1152/ajpcell.1987.252.4.C436. [DOI] [PubMed] [Google Scholar]
- 59.Asayama K, Janco RL, Burr IM. Selective induction of manganous superoxide dismutase in human monocytes. Am J Phys. 1985;249:393–397. doi: 10.1152/ajpcell.1985.249.5.C393. [DOI] [PubMed] [Google Scholar]
- 60.Kiningham KK, Daosukho C, St Clair DK. IκBα (inhibitory κBα) identified as labile repressor of MnSOD (manganese superoxide dismutase) expression. Biochem J. 2004;384:543–549. doi: 10.1042/BJ20040714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Faivre EJ, Lange CA. Progesterone receptors upregulate Wnt-1 to induce epidermal growth factor receptor transactivation and c-src-dependent sustained activation of erk1/2 mitogen activated protein kinase in breast cancer cells. Mol Cell Biol. 2007;27:466–480. doi: 10.1128/MCB.01539-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fridovich I. Superoxide dismutases. An adaptation to a paramagnetic gas. J Biol Chem. 1989;264:7761–7764. [PubMed] [Google Scholar]
- 63.Gregory EM, Fridovich I. Induction of superoxide dismutase by molecular oxygen. J Bacteriol. 1973;114:543–548. doi: 10.1128/jb.114.2.543-548.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gregory EM, Fridovich I. Oxygen toxicity and the superoxide dismutase. J Bacteriol. 1973;114:1193–1197. doi: 10.1128/jb.114.3.1193-1197.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gregory EM, Goscin SA, Fridovich I. Superoxide dismutase and oxygen toxicity in a eukaryote. J Bacteriol. 1974;117:456–460. doi: 10.1128/jb.117.2.456-460.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li Y, Huang TT, Carlson EJ, Melov S, Ursell PC, Olson JL, Noble LJ, Yoshimura MP, Berger C, Chan PH, Wallace DC, Epstein CJ. Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nature Genet. 1995;11:376–381. doi: 10.1038/ng1295-376. [DOI] [PubMed] [Google Scholar]
- 67.Copin JC, Gasche Y, Chan PH. Over-expression of copper/zinc superoxide dismutase does not prevent neonatal lethality in mutant mice that lack manganese superoxide dismutase. Free Rad Biol Med. 2000;28:1571–1576. doi: 10.1016/s0891-5849(00)00280-x. [DOI] [PubMed] [Google Scholar]
- 68.Graham JD, Yager ML, Hill HD, Byth K, O’Neill GM, Clarke CL. Altered progesterone receptor isoform expression remodels progestin responsiveness of breast cancer cells. Mol Endo. 2005;19:2713–2735. doi: 10.1210/me.2005-0126. [DOI] [PubMed] [Google Scholar]
- 69.Million Women Study Collaborators. Breast cancer and hormone- replacement therapy in the million women study. Lancet. 2003;362:419–427. doi: 10.1016/s0140-6736(03)14065-2. [DOI] [PubMed] [Google Scholar]