Abstract
Cross-sectional studies have found that individuals with depressive disorders or symptoms have elevated levels of inflammatory markers predictive of coronary artery disease, including interleukin-6 (IL-6) and C-reactive protein (CRP). Due to the paucity of prospective studies, however, the directionality of the depression-inflammation relationship is unclear. We evaluated the longitudinal associations between depressive symptoms and both IL-6 and CRP among 263 healthy, older men and women enrolled in the Pittsburgh Healthy Heart Project, a 6-year prospective cohort study. During the baseline and follow-up visits, participants completed the Beck Depression Inventory-II (BDI-II) to assess depressive symptoms and underwent blood draws to quantify serum IL-6 and CRP. Path analyses revealed that baseline BDI-II (β=.18, p <.01, ΔR2 =.02) was a predictor of 6-year change in IL-6, even after adjustment for demographic, biomedical, and behavioral factors as well as other negative emotions. Of all the factors examined, only body-mass index was a stronger predictor of IL-6 change than depressive symptoms. In contrast to these results, baseline IL-6 did not predict 6-year change in BDI-II. Evidence of a weak bidirectional relationship between BDI-II and CRP was also observed; however, neither of these longitudinal associations was significant. The present findings indicate that depressive symptoms may precede and augment some inflammatory processes relevant to coronary artery disease among healthy, older adults. Therefore, our results imply that depression may lead to inflammation and that inflammation may be one of the mechanisms through which depression contributes to cardiovascular risk.
Keywords: depression, inflammation, interleukin-6, C-reactive protein, coronary artery disease, prospective study
Epidemiologic evidence indicates that depression may be a risk factor for coronary artery disease (CAD), as both clinical depression and subthreshold depressive symptoms have been found to predict incident disease (Suls and Bunde, 2005). Importantly, the predictive value of depression is similar to that of many traditional cardiovascular risk factors (Rozanski et al., 2005), with relative risk ratios in the 1.5–2.7 range (Rugulies, 2002; Wulsin and Singal, 2003). Despite these findings, the mechanisms underlying this association are poorly understood. In most previous studies, depression remained a significant predictor of CAD after adjustment for known biological (e.g., hypertension) and behavioral (e.g., smoking) risk factors (Rugulies, 2002; Wulsin and Singal, 2003), suggesting that other mechanisms are involved.
One physiologic factor that has received increased attention as a potential mechanism linking depression with CAD is augmented inflammation. Several lines of evidence are consistent with this possibility. First, atherosclerosis is now considered to be a chronic inflammatory disease (Ross, 1999). Supporting this perspective is the emerging evidence that circulating levels of the proinflammatory cytokine, interleukin-6 (IL-6), and the acute-phase reactant, C-reactive protein (CRP), predict future cardiovascular events (Cesari et al., 2003; Luc et al., 2003; Pearson et al., 2003; Pradhan et al., 2002; Ridker et al., 2000). Second, evidence from cross-sectional studies indicates that depression and innate immune system activation co-occur. Specifically, depressive disorders and symptoms have been linked with elevated basal levels of IL-6 and CRP (Dentino et al., 1999; Kuo et al., 2005; Lutgendorf et al., 1999; Maes et al., 1997; Miller et al., 2002b; Penninx et al., 2003; Ranjit et al., 2007), greater stimulated production of proinflammatory cytokines (Anisman et al., 1999a, b; Lanquillon et al., 2000; Maes et al., 1991; Suarez et al., 2003; Suarez et al., 2004), and larger IL-6 responses to acute psychological stress (Pace et al., 2006). Third, although the existing literature is mixed, some investigators have found that antidepressant treatment reduces circulating levels of proinflammatory cytokines as well as decreases stimulated production of these proteins among depressed patients (Kenis et al., 2002). Finally, biologically plausible pathways – including hypothalamic-pituitary-adrenal (HPA) axis hyperactivity and autonomic nervous system (ANS) dysfunction – have been proposed to explain how depression could affect inflammatory processes (Kop and Gottdiener, 2005).
The aforementioned empirical evidence, however, is ambiguous with respect to the directionality of the depression-inflammation relationship and, therefore, is also consistent with another possibility – the macrophage theory of depression (Smith, 1991). According to this theory, inflammation plays an etiologic role in the development of depression, perhaps by altering neurotransmitter metabolism or HPA axis function (Raison et al., 2006). Other evidence is also congruent with the macrophage theory. For instance, administering proinflammatory cytokines to rats has been shown to induce sickness behavior, a syndrome characterized by affective, cognitive, and behavioral changes (e.g., anhedonia, memory deficits, decreased activity, social withdrawal, anorexia, and altered sleep patterns) similar to the symptoms of depression (Dantzer et al., 2008). Research on humans complements these animal findings, as treatment with interferon-α (a cytokine) and peripheral administration of IL-6 have been shown to increase depressive symptoms (Spath-Schwalbe et al., 1998; Wichers et al., 2002). Furthermore, recent results suggest that medications with anti-inflammatory properties, such as cyclooxygenase-2 inhibitors (Muller et al., 2006) and tumor-necrosis factor antagonists (Tyring et al., 2006), may reduce depressive symptom severity.
Because the directionality of the depression-inflammation relationship is presently unclear, prospective data are needed to ascertain whether depression is a cause or a consequence of augmented inflammation. Unfortunately, relatively few studies have examined longitudinal associations between these factors (Boyle et al., 2007; Kiecolt-Glaser et al., 2003; Matthews et al., 2007; van den Biggelaar et al., 2007), and only one investigation has simultaneously evaluated both directions of this relationship (Gimeno et al., 2009). Therefore, to address this gap in knowledge, we examined associations between depressive symptoms and two inflammatory markers relevant to CAD, serum IL-6 and CRP, over a 6-year period in a sample of healthy, older adults.
Method
Participants
Participants were 263 men and women enrolled in the Pittsburgh Healthy Heart Project (PHHP), a prospective study of healthy, community-dwelling adults aged 50–70 years. This study was approved by the University of Pittsburgh institutional review board. Participants provided written informed consent and were paid $700 for attending the baseline and 6-year visits. Details regarding recruitment and inclusion/exclusion criteria are provided elsewhere (Kamarck et al., 2007; Stewart et al., 2007). Although individuals with a history of chronic disease generally were not recruited, persons with diabetes who were not taking insulin, those with a history of cancer but no treatment in the past two years, and those with mild or moderate rheumatoid arthritis were eligible. Two hundred ninety-six (64%) of the 464 enrolled adults attended the 6-year visits, and 284 (96%) of these individuals had complete baseline and 6-year IL-6 and CRP data. Consistent with recent recommendations (Pearson et al., 2003), we excluded 21 participants who had CRP levels ≥ 10 mg/L. Table 1 shows the demographic characteristics of the remaining 263 participants. Participants in the final sample were older [t(462) = −2.86, p <.01], more educated [t(461) = −1.98, p =.05], and more likely to be white [Χ2(1, N = 463) = 9.58, p <.01] than those not in the sample; however, group differences were not observed for sex or for baseline depressive symptom severity, IL-6, or CRP.
Table 1.
Characteristics of Participants (N = 263)
| Demographic Factors | |
| Age (years) | 61.0 ± 4.8 |
| Sex, % female | 51.7 |
| Race-ethnicity, % nonwhite | 13.3 |
| Education level, % high school or less | 22.1 |
| Biomedical Factors | |
| MAP (mmHg) | 96.4 ± 9.6 |
| BMI (kg/m2) | 27.4 ± 4.3 |
| HDL cholesterol (mg/dl) | 55.0 ± 15.4 |
| Triglycerides (mg/dl) | 138.8 ± 79.0 |
| Fasting glucose (mg/dl) | 92.0 ± 11.2 |
| Fasting insulin (μU/ml) | 11.2 ± 4.4 |
| History of diabetes, % | 1.1 |
| History of rheumatoid arthritis, % | 3.4 |
| Behavioral Factors | |
| Smoking status, % current smokers | 5.7 |
| Daily alcohol intake (g/day) | 6.2 ± 9.4 |
| Physical activity level (kilocalories/week) | 969.5 ± 823.3 |
| Negative Emotions | |
| Baseline BDI-II (range: 0–63) | 3.8 ± 3.9 |
| 6-Year BDI-II (range: 0–63) | 5.2 ± 5.2 |
| Inflammatory Markers | |
| Baseline Serum IL-6 (pg/mL) | 1.8 ± 1.6 |
| 6-Year Serum IL-6 (pg/mL) | 2.7 ± 2.0 |
| Baseline Serum CRP (mg/L) | 2.2 ± 1.9 |
| 6-year Serum CRP (mg/L) | 1.5 ± 1.5 |
Note. Values are means ± standard deviations for continuous variables and percentages for categorical variables. MAP = mean arterial pressure. BMI = body-mass index. HDL = high-density lipoprotein. BDI-II = Beck Depression Inventory-II. IL-6 = interleukin-6. CRP = C-reactive protein.
Measures and Procedure
Data examined in this report were collected at the PHHP baseline and 6-year follow-up. At baseline (1998–2000), participants attended 11 visits: a medical screen, seven visits for ambulatory monitoring training and questionnaire assessments, one visit for reactivity testing, and two visits for ultrasound assessments of subclinical cardiovascular disease. An average of six years (M = 6.3, SD = 0.3) later, participants attended six follow-up visits, during which they completed a medical update, questionnaire assessments, ambulatory monitoring training, ultrasound assessments, and autonomic testing.
Depressive Symptoms
At the third baseline and follow-up visit, participants completed the Beck Depression Inventory-II (BDI-II) (Beck, 1996) on a computer (see Table 1 for descriptive statistics). The BDI-II is a widely used self-report measure of depressive symptom severity and has been shown to have high internal consistency, test-retest reliability, and construct validity (Beck, 1996; Dozois et al., 1998). Of note, participants were asked to rate the severity of their depressive symptoms over the past week instead of over the past two weeks (the usual time frame for the BDI-II). In addition to calculating the total score, we also computed two subscale scores – a cognitive-affective score (sum of items 1–3, 5–9, 13, and 14) and a somatic-vegetative score (sum of items 4, 10–12, and 15–21) (Dozois et al., 1998). BDI-II total score, cognitive-affective score, and somatic-vegetative score were each log (Xi+1) transformed to reduce positive skew.
Inflammatory Markers
Blood was drawn between 8:00 AM-1:00 PM at the first baseline and follow-up visit. Participants were instructed to fast and to avoid caffeine for 12 hours prior to these visits. Blood samples, collected in tubes with no additives, were stored at room temperature for 40 minutes and then were refrigerated until they were centrifuged within three hours of collection to isolate serum. Serum aliquots were frozen at −70°C until the time of assay. Baseline and follow-up serum samples were sent to the Laboratory for Clinical Biochemistry Research at the University of Vermont. There, IL-6 was measured using ultra-sensitive enzyme-linked immunosorbent assay kits (R&D Systems, Minneapolis, MN), which have a detection range of 0.16–12.0 pg/mL. The routine interassay coefficient of variation for this method is 6.3% at the University of Vermont. CRP was measured with a BNII nephelometer utilizing a particle-enhanced immunonephelometric assay (Dade Behring, Deerfield, IL). The detection range for this assay is 0.16–1100 mg/L, and the routine interassay coefficient of variation is 5% at the University of Vermont.
Descriptive statistics for serum IL-6 and CRP are presented in Table 1. We excluded individuals with serum CRP ≥ 10 mg/L (n = 21) at either assessment, because CRP levels above this value may be due to recent infection or trauma and thus may not be indicative of chronic inflammation (Pearson et al., 2003). In addition, we assigned persons with IL-6 levels above the upper detection limit (n = 2 at baseline; n = 3 at follow-up) a value of 12.0 pg/mL and those with CRP levels below the lower detection limit (n = 3 at baseline; n = 6 at follow-up) a value of 0.15 mg/L. Because IL-6 and CRP distributions were positively skewed, these variables were log (Xi+1) transformed.
Other Factors
Several additional factors relevant to the present study were measured during the baseline visits (see Table 1 for descriptive statistics). The following variables were assessed at the medical screen via questionnaires and interviews: age (years), sex (0 = male, 1 = female), race-ethnicity (1 = white, 2 = black, 3 = Asian, 4 = Hispanic, 5 = other), education level (1 = high school or less, 2 = technical school or some college, 3 = bachelor’s degree, 4 = master’s degree or higher), smoking status (0 = nonsmoker, 1 = current smoker), daily alcohol intake, physical activity level, and history of various medical conditions (including diabetes and rheumatoid arthritis). Race-ethnicity was coded as a binary variable (0 = white, 1 = non-white) because only five participants selected the Asian, Hispanic, or other categories. Daily alcohol intake (g/day) was calculated using the quantity-frequency method (Garg et al., 1993). Physical activity level was computed by first converting the number of blocks walked and stairs climbed per day reported on Paffenbarger Physical Activity Questionnaire (Paffenbarger et al., 1978) to kilocalories per week and then summing these two values.
A blood pressure assessment, anthropometric measurements, and a blood draw were also completed at the medical screen. Following the American Heart Association guidelines (Perloff et al., 1993), three blood pressure readings were taken at 2-min intervals using a standard mercury sphygmomanometer. Systolic (SBP) and diastolic blood pressure (DBP) were computed by averaging the last two readings, and mean arterial pressure (MAP) was calculated as DBP + (SBP − DBP)/3. Body-mass index (BMI) was computed as weight/height2 (kg/m2). Standard assays were performed to assess high density lipoprotein (HDL) cholesterol (Warnick and Albers, 1978) and triglycerides (Bucolo and David, 1973), and fasting serum glucose and insulin were measured by standard colorimetry (Bondar and Mead, 1974) and radioimmunoassay, respectively. For one participant, the glucose variable was set to missing because the observed value (214.7 mg/dl) was extreme and disconnected from the distribution.
Participants completed additional computerized questionnaires to measure other negative emotions. Specifically, the Beck Anxiety Inventory (BAI) (Beck, 1990) was administered at the third baseline visit to assess severity of anxiety symptoms, and the Cook-Medley Hostility (Ho) Scale (Cook and Medley, 1954) was administered at the fourth baseline visit to assess hostility. Because one item of the Ho Scale was accidentally omitted, the value for this item was imputed by taking the mean of the other items for each participant. We used the 27-item version of the Ho Scale instead of the 50-item version because evidence suggests that it is a stronger predictor of health outcomes (Barefoot et al., 1989). Both the BAI and Ho Scale posses good psychometric properties (Barefoot et al., 1983; Shekelle et al., 1983; Smith and Frohm, 1985; Steer and Beck, 1997). The mean scores on the BAI and Ho Scale were 5.0 (SD = 4.8) and 8.1 (SD = 4.1), respectively.
Triglycerides, fasting glucose, and fasting insulin were log transformed, daily alcohol intake and BAI scores were log (Xi+1) transformed, and physical activity level was square root transformed to reduce positive skew.
Statistical Analysis
Preliminary Analyses
To characterize the nature of the cross-sectional relationships among the factors of interest, we performed bivariate (i.e., Pearson) correlations among BDI-II, IL-6, and CRP at baseline and at 6-year follow-up.
Primary Analyses
To simultaneously evaluate (a) baseline depressive symptom severity as a predictor of change in inflammatory markers and (b) baseline inflammatory markers as predictors of change in depressive symptom severity, we performed a series of path analyses using LISREL 8.8 (Joreskog and Sorbom, 2008). Path analysis has several advantages over traditional regression, including the ability to generate more accurate parameter estimates because it is possible to predict multiple outcomes and handle missing data. To assess model fit, we used two goodness-of-fit statistics (Steiger, 1990). The chi-square statistic measures the absolute fit between the hypothesized model and the observed pattern of relationships among measured variables. Because a non-significant chi-square statistic demonstrates that there is no difference between the hypothesized and observed patterns of relationships, it indicates that the hypothesized model is acceptable. The root mean square error of approximation (RMSEA) statistic adjusts the estimate of absolute fit for the complexity of the hypothesized model. Smaller values of RMSEA indicate better model fit, with values less than.06 representing acceptable model fit (Hu and Bentler, 1999). Parameters were estimated by full information maximum likelihood, which uses all of the observed data and is superior to traditional methods of handling missing data (Enders, 2001).
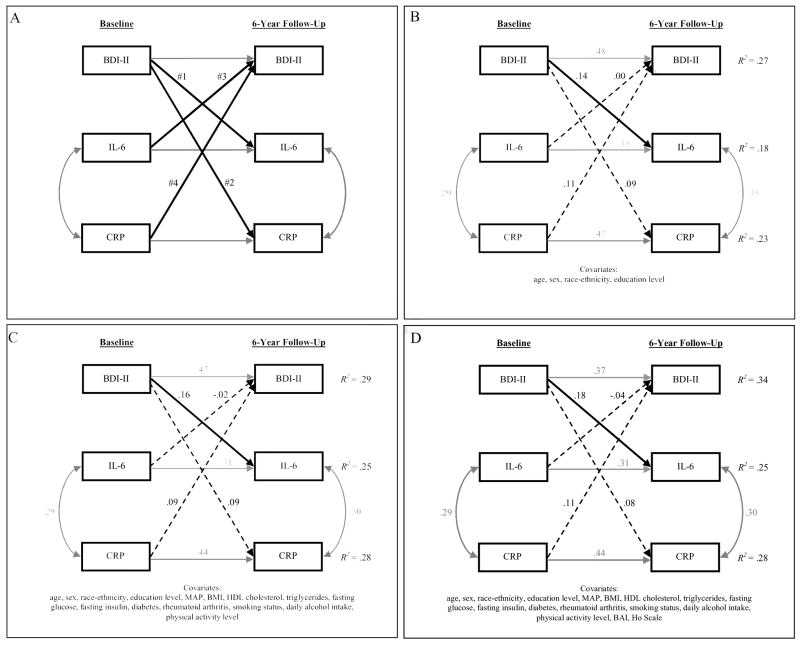
Our primary analyses consisted of three path analytic models (see Figure 1A for the conceptual model). First, a base model was created that included demographic variables (age, sex, race-ethnicity, and education level) measured at baseline and BDI-II, IL-6, and CRP assessed at both baseline and follow-up. All of the baseline variables were allowed to correlate with one another, as were IL-6 and CRP at follow-up. Because we adjusted 6-year BDI-II, IL-6, and CRP for the corresponding baseline level by including structural paths between these variables, the follow-up variables represent residualized change scores (e.g., 6-year change in IL-6). We also linked each demographic variable to each follow-up variable with a structural path, given that the demographic factors are all plausible predictors of BDI-II, IL-6, and CRP change. Finally, we included the structural paths of interest – (#1) from baseline BDI-II to IL-6 change, (#2) from baseline BDI-II to CRP change, (#3) from baseline IL-6 to BDI-II change, and (#4) from baseline CRP to BDI-II change.
Figure 1.
Models of the longitudinal associations between depressive symptoms and CAD-relevant inflammatory markers. A, Conceptual model. The four structural paths of interest are (#1) from baseline Beck Depression Inventory (BDI-II) to interleukin-6 (IL-6) change, (#2) from baseline BDI-II to C-reactive protein (CRP) change, (#3) from baseline IL-6 to BDI-II change, and (#4) from baseline CRP to BDI-II change. B, Results of the base model. Values associated with unidirectional arrows (structural paths) are standardized regression coefficients; values associated with bidirectional arrows (correlations) are Pearson correlation coefficients. Paths with significant coefficients are solid, whereas paths with nonsignficant coefficients are dashed. C, Results of the covariate model. D, Results of the full model. N = 263 for all models.
Second, to rule out potential confounders as explanations for any of the observed depression-inflammation relationships, we created a covariate model. This model was identical to the base model except that baseline biomedical (MAP, BMI, HDL cholesterol, triglycerides, fasting glucose, fasting insulin, diabetes, and rheumatoid arthritis) and behavioral (smoking status, daily alcohol intake, and physical activity level) variables were included. Because all of these control variables have been previously associated with IL-6 or CRP levels (Jenny et al., 2002; Luc et al., 2003; Pearson et al., 2003; Ridker et al., 2000; Volpato et al., 2004), we linked each of them to follow-up IL-6 and CRP with a structural path. All of the behavioral variables (Hartka et al., 1991; Korhonen et al., 2007; Strawbridge et al., 2002) and only a subset of the biomedical variables (BMI, diabetes, and rheumatoid arthritis) (Anderson et al., 2001; Dickens et al., 2002; Roberts et al., 2003) were connected to follow-up BDI-II with a structural path, given that MAP, HDL cholesterol, triglycerides, glucose, and insulin are not likely predictors of BDI-II change in the presence of the other control variables.
Third, a full model was created that was identical to the covariate model except that we included two additional baseline measures of negative emotion as control variables. This analysis was performed to determine whether the observed depression-inflammation relationships were independent of hostility and anxiety. Because measures of hostility and anxiety are at least moderately correlated with those of depression (Clark and Watson, 1991; Raynor et al., 2002) and have been found to be positively related to inflammatory markers (Graham et al., 2006; Pitsavos et al., 2006), we linked both BAI and Ho Scale to each of the follow-up variables.
Supplemental Analyses
We performed four sets of exploratory path analyses. One, to rule out subclinical cardiovascular disease as a confounder, we included log transformed carotid intima-media thickness at baseline (M = 0.77 mm, SD = 0.18 mm) in the full model as a control variable (for details, see Stewart et al., 2007). In this analysis, we also included incident cardiovascular disease (myocardial infarction, stroke/transient ischemic attack, coronary artery bypass surgery, or angioplasty reported at follow-up but not at baseline) as a control variable to determine whether further evaluation of it as a mediator of any observed associations was warranted. Thirteen individuals (4.9%) were coded as “yes” on this variable. Two, we repeated the full model after substituting BDI-II cognitive-affective and somatic-vegetative subscale scores at baseline and follow-up for the corresponding BDI-II total scores. Chi-square difference tests were conducted to determine whether the freely estimated models (coefficients of paths #1–4 were estimated separately for the BDI-II subscales) yielded better data-model fit than the constrained models (coefficients for the BDI-II subscales were set to be equal). Three, because existing evidence suggests that the link between depression and inflammation may be stronger among men than women (Ford and Erlinger, 2004), we performed multi-group path analyses to evaluate whether sex was a moderator of any of the observed relationships. Again, chi-square difference tests were conducted to determine whether the data-model fit was better for the freely estimated models (coefficients of paths #1–4 were estimated separately for men and women) than for the constrained models (coefficients for men and women were set to be equal). Four, to determine whether 6-year changes in BDI-II were associated with 6-year changes in IL-6 and CRP, we added structural paths between follow-up BDI-II and IL-6 and between follow-up BDI-II and CRP to the full model.
Results
Preliminary Analyses
Bivariate correlations performed to examine the cross-sectional relationships among the factors of interest revealed that BDI-II was not associated with IL-6 (r = −.01, p =.87) or CRP (r = −.06, p =.36) at baseline. Similarly, 6-year BDI-II was not related to 6-year IL-6 (r =.03, p =.66) or CRP (r =.05, p =.41). Consistent with previous findings (Cesari et al., 2003; Luc et al., 2003), we observed moderate positive correlations between baseline IL-6 and CRP (r =.29, p <.01) and between 6-year IL-6 and CRP (r =.39, p <.01).
Primary Analyses
We constructed three path analytic models to simultaneously evaluate baseline depressive symptom severity as a predictor of 6-year change in inflammatory markers and vice versa. The base model, which included demographic factors as control variables, showed close fit to the data, χ2 (4, N = 263) = 1.70 (p =.79), RMSEA = 0.000 (see Figure 1B). The predictor variables together explained 18%, 23%, and 27% of the variance in IL-6 change, CRP change, and BDI-II change, respectively. Baseline IL-6, CRP, and BDI-II were each strongly and positively related to their corresponding follow-up level (all ps < 0.0001). Of the demographic factors, only sex (β = −.14, p =.02) and education level (β= −.15, p =.01) were predictors of IL-6 change, as men and persons with lower education levels exhibited greater increases in IL-6 over the follow-up interval than did women and those with higher education levels. None of the demographic factors predicted CRP change or BDI-II change. Examination of the longitudinal depression-inflammation paths (#1–4) revealed that baseline BDI-II was positively associated with IL-6 change (β=.14, p = 0.01), accounting for 2% of the variance. Baseline BDI-II, however, was not a predictor of CRP change (β=.09, p = 0.12). Similarly, neither baseline IL-6 (β=.00, p = 0.98) nor CRP (β=.11, p = 0.06) were predictors of BDI-II; however, the latter regression coefficient fell just short of statistical significance.
Like the base model, the fit statistics for the covariate model indicated close data-model fit, χ2 (9, N = 263) = 8.59 (p =.48), RMSEA = 0.000 (see Figure 1C). Collectively, the biomedical and behavioral control variables explained an additional 7% of the variance in IL-6 change, 5% in CRP change, and 2% in BDI-II change beyond that accounted for the base model variables. Only BMI was a predictor of change in both IL-6 (β=.18, p =.01) and CRP (β=.14, p =.03). In the presence of the biomedical and behavioral variables, education level (β= −.14, p =.02) remained a predictor of IL-6 change but sex did not (β= −.13, p =.07). Baseline smoking (β=.14, p =.01) was a predictor of BDI-II change; smokers displayed greater BDI-II increases over time than did nonsmokers. Including the additional control variables in the model did not change the pattern of results among paths #1–4. Baseline BDI-II remained a predictor of IL-6 change (β=.16, p = 0.01), explaining 2% of the variance, and the other three paths remained nonsignficant (all ps >.13).
The full model also closely fit the data, χ2 (9, N = 263) = 9.89 (p =.36), RMSEA = 0.020, and the pattern of results was similar to that of the base and covariate models (see Figure 1D). With respect to paths #1–4, only the path from baseline BDI-II to IL-6 change (β=.18, p = 0.01) was significant in the presence of BAI and Ho Scale, indicating that this relationship is independent of other overlapping negative emotions. Once again, baseline BDI-II accounted for 2% of the variance in IL-6 change. The other three paths remained nonsignficant, although the baseline CRP to BDI change path again approached statistical significance (β=.11, p =.06). Of note, neither baseline BAI nor Ho Scale was a predictor of IL-6 change (BAI: β= −.06, p =.38; Ho Scale: β=.01, p =.84) or CRP change (BAI: β=.03, p =.69; Ho Scale: β = −.01, p =.83).
Supplemental Analyses
The first set of the exploratory analyses indicated that adjusting for baseline carotid intima-media thickness and incident cardiovascular disease (neither of which were significant predictors of IL-6 or CRP; all ps >.25) did not change the pattern of results among paths #1–4 (data not shown). This finding rules out baseline subclinical cardiovascular disease as a potential confounder and incident clinical cardiovascular disease as a possible mediator of the observed depression-inflammation relationship. Results of the second set of analyses, in which the BDI-II subscale scores were substituted for the total score, revealed that somatic-vegetative subscale (β =.15, p =.03) was a significant predictor of IL-6 change, whereas the cognitive-affective subscale (β=.08, p =.25) was not. A chi-square difference test, however, demonstrated that these coefficients were not significantly different from each other, Δχ2 (1, N = 263) = 0.22 (p =.64). In addition, neither of the BDI subscales was a predictor of CRP change (both ps >.24), and baseline IL-6 and CRP did not predict either somatic-vegetative subscale change or cognitive-affective subscale change (all ps >.07). The chi-square difference tests for these paths were all nonsignificant (all ps >.22), indicating that the coefficients for the BDI-II subscales did not significantly differ. The third set of exploratory analyses indicated that sex was not a moderator of any of the depression-inflammation relationships. Although the coefficients for paths #1–4 were not identical for men and women, the nonsignificant chi-square difference tests (all ps >.12) demonstrated that they did not vary significantly. Because our sample included only 127 men and 136 women, however, this study may have been underpowered to examine sex as a moderator. Results of the fourth set of analyses revealed that 6-year changes in BDI-II were not associated with 6-year changes in IL-6 and CRP (all ps >.38).
Discussion
The findings we report in this sample of healthy, older adults are most consistent with the notion that depression may lead to, rather than result from, augmented inflammation. Path analytic models revealed that greater depressive symptom severity at baseline was associated with larger 6-year increases in serum IL-6, even after adjustment for demographic, biomedical, and behavioral factors. Importantly, the magnitude of this relationship is not trivial; only one of the control variables, BMI, was a stronger predictor than depressive symptoms. The observed relationship also appears to be specific to depression (i.e., independent of anxiety and hostility) and driven primarily by the somatic-vegetative symptom cluster. Contrasting with these results, baseline IL-6 was not a predictor of 6-year change in depressive symptoms. We did observe evidence of a weak bidirectional relationship between depressive symptoms and serum CRP over time; however, these results should be interpreted with caution because neither direction of this relationship was significant. Taken together, our findings suggest that depressive symptoms may precede and augment some inflammatory processes implicated in the pathogenesis of CAD among healthy, older adults. Thus, our findings are concordant with a conceptual framework in which the effect of depression on CAD outcomes is mediated, in part, by augmented inflammation (Kop, 1999). It should be noted, however, that recent studies have found that inflammation accounts for only a small portion of the cardiotoxic effect of depression (Vaccarino et al., 2007; Whooley et al., 2008), suggesting that multiple mechanisms are at work.
Depression-related dysfunction in two systems that normally exert anti-inflammatory effects could explain how depression might increase inflammatory activity over time, as was observed in this study. Clinical depression and subthreshold depressive symptoms have both been linked with hyperactivity of the HPA axis, as indicated by elevated glucocorticoid levels (Plotsky et al., 1998; van Eck et al., 1996), and diminished activation of the parasympathetic nervous system, as indicated by reduced heart rate variability (Carney et al., 2005; Thayer et al., 1998). Although glucocorticoids do suppress inflammation in the short-term (Guyton and Hall, 2000), sustained elevations over time may bring about downregulation or desensitization of the macrophage glucocorticoid receptors, which could result in blunted anti-inflammatory responses to these hormones (Leonard, 2001; Miller et al., 2002a). Similar to glucocorticoids, existing evidence indicates that parasympathetic activation also curbs inflammation (Tracey, 2002). As an example, recent investigations have shown that reduced heart rate variably is associated with increased levels of IL-6 and CRP (Sajadieh et al., 2004; Sloan et al., 2007).
To our knowledge, five previous studies have evaluated prospective associations between depression and inflammation: three examined the relationship from depression to future inflammation (Boyle et al., 2007; Kiecolt-Glaser et al., 2003; Matthews et al., 2007), one examined the relationship from inflammation to future depression (van den Biggelaar et al., 2007), and one examined both directions of this association (Gimeno et al., 2009). Paralleling our findings, Boyle and colleagues (2007) reported that a composite score – representing the shared variance among depression, hostility, and anger – predicted 10-year increases in another CAD-relevant inflammatory marker, the third complement protein (C3). It is worth noting that secondary analyses revealed that each individual negative emotion predicted C3 change as well. Similar to the current study, the relationship between the negative emotion composite score and C3 was not present at baseline but instead emerged over time. Also consistent with our observations, Matthews et al. (2007) did not detect an association between baseline depressive symptoms and change in CRP over a 5-year period. Furthermore, Gimeno and colleagues’ (2009) finding that the cognitive symptoms of depression did not predict 12-year changes in IL-6 and CRP is in line with our BDI-II subscale analyses, in which we observed that the somatic-vegetative subscale, but not the cognitive-affective subscale, predicted IL-6 change.
Kiecolt-Glaser et al. (2003), however, reported findings that contradict ours; they found that baseline depressive symptom severity was not associated with 6-year change in IL-6. Of relevance, these researchers administered the 13-item BDI (Beck and Beck, 1972), which primarily assesses the cognitive-affective symptom cluster (Beck et al., 1988). In addition to this investigation, results of the two studies examining inflammatory markers as predictors of future depressive symptoms conflict with our findings. Specifically, in the study conducted by van den Biggelaar et al. (2007), baseline CRP and stimulated production of interleukin-1 beta (IL-1β) were positively associated with 5-year change in depressive symptoms, although the production of other proinflammatory cytokines (including IL-6) was not. Similarly, Gimeno and colleagues (2009) found that baseline IL-6 and CRP were predictive of 12-year change in the cognitive symptoms of depression; however, the effects were small (both βs = 0.046) and, consequently, may only be detected in samples much larger than the present one.
In total, the available evidence suggests that relationship between depression and inflammation is bidirectional and complex. Although our findings indicate that the depression-to-inflammation link might be stronger than the inflammation-to-depression link, it seems likely that the magnitude of either direction of this association may depend on other factors, such as the depressive symptom clusters examined, the inflammatory markers assessed, and the populations studied. For instance, our results, as well as those of Kiecolt-Glaser et al. (2003) and Gimeno et al. (2009), suggest that the cognitive-affective symptoms of depression may be weakly or not related to changes in IL-6 or CRP over time. Our findings additionally indicate that the somatic-vegetative symptoms may be significantly related to longitudinal increases in these inflammatory markers. Of note, because only the cognitive-affective symptoms of depression were assessed in the two previous studies (Gimeno et al., 2009; Kiecolt-Glaser et al., 2003), those results cannot help to evaluate this latter possibility. Considering the mechanisms thought to account for the influence of depression on immune system function, one might also expect depression to be a stronger predictor of inflammatory markers more proximal to the HPA axis and the autonomic nervous system (e.g., proinflammatory cytokines) than those more distal (e.g., acute-phase reactants). This is the pattern of results observed in our sample, as well as in a recent meta-analysis of cross-sectional studies examining the associations between depression and IL-1 (d = 0.35), IL-6 (d = 0.25), and CRP (d = 0.15) (Howren et al., 2009).
With respect to the populations studied, it is possible that the inflammation-to-depression link is stronger and, therefore, more likely to be detected in patient versus community samples. Although van den Biggelaar and colleagues’ (2007) investigation was a population-based study of adults aged 85 years and older, it is reasonable to characterize their cohort as a patient sample, as approximately 85% of the participants had a history of chronic disease when arthritis is included (Mass et al., 2009). In the other study that detected an inflammation-to-depression association (Gimeno et al., 2009), the cohort consisted of generally healthy adults; however, the effects of IL-6 and CRP on change in the cognitive symptoms of depression were small (albeit significant due to the large sample size). Altogether, these findings raise the possibility that the elevations in inflammatory markers observed among healthy adults (like the present sample) may be too slight to induce measurable changes in depression, except in large samples. This may not be the case among patients with chronic diseases. Examination of baseline CRP levels of the existing studies supports this notion, as the level reported by van den Biggelaar et al. (median = 4.0 mg/L) is more than twice that observed in Gimeno and colleagues’ study (M = 0.8–0.9 mg/L) and in our investigation (M = 2.2 mg/L). Because these ideas are speculative, future prospective studies of community and patient samples, with repeated measures of both depressive symptom clusters and multiple inflammatory markers, are needed to determine whether the depression-inflammation relationship is moderated by the aforementioned factors.
Because little is known about predictors of longitudinal changes in inflammatory marker, several results not central to our objective are worth noting. Of all the control variables, only baseline BMI was a predictor of IL-6 and CRP change, which supports the notion that increased body mass promotes systemic inflammation (Visser et al., 1999). Consistent with a recent review (Nazmi and Victora, 2007), we also found that education level, a marker of socioeconomic status, was inversely associated with IL-6 change. We were surprised by the number of nonsignificant paths, given that all of the selected biomedical and behavioral factors had been previously associated with IL-6 or CRP levels in cross-sectional analyses. The lack of longitudinal associations for most of these factors raises the possibility that some may be a consequence of inflammation instead of a cause. Similar to our results, Kiecolt-Glaser and colleagues (2003) reported that factors cross-sectionally associated with inflammatory markers (e.g., gender and ethnicity) did not predict 6-year change in IL-6. Unlike the present study, however, these researchers found that alcohol consumption was positively related and exercise was inversely related to IL-6 change. We also found that baseline carotid intima-media thickness was not a predictor of IL-6 or CRP change. This finding is inconsistent with the risk marker model (Pearson et al., 2003), which proposes that basal inflammatory marker level merely reflects the extent of underlying atherosclerosis.
Although our investigation has many strengths, such as the longitudinal design and the simultaneous evaluation of both directions of the depression-inflammation relationship, there are some important limitations. First, because our sample consisted of healthy volunteers, the range of BDI-II scores was somewhat restricted, which may have resulted in the underestimation of effect sizes of the relationships involving depression. Second, we obtained only a single measurement of depressive symptoms and each inflammatory marker at baseline and follow-up. Due to the within-person variation of depressive symptoms (Beck et al., 1988) and inflammatory markers (Sakkinen et al., 1999), the reliability of our baseline and follow-up estimates would have been enhanced had we obtained and averaged two separate measurements at both time points (Pearson et al., 2003). However, in many of the epidemiologic studies demonstrating a link between depressive symptoms or inflammatory markers and cardiovascular risk, estimates of baseline level were also based on just one measurement (Cesari et al., 2003; Luc et al., 2003; Pradhan et al., 2002; Ridker et al., 2008; Suls and Bunde, 2005). Third, we included only two waves of data, baseline and 6-year follow-up, in our analyses. In the PHHP, depressive symptoms and inflammatory markers were also assessed at a 3-year follow-up. Unfortunately, due to a clerical error, the BDI-II instructions given at year three (rate your symptom severity during the past two weeks) were not identical to those given at the baseline and year six (rate your symptom severity during the past week). Although including a third wave of data is preferable in most situations, in this instance it would have reduced our confidence in the results, given that we would have been unable to determine whether changes in depressive symptom severity from baseline to year three and from year three to year six were due to true growth or a modification to the scale. Finally, because our sample consisted of only 35 (13.3%) non-white adults, our findings may not generalize to other racial or ethic groups.
In summary, we have shown that depressive symptoms precede and predict subsequent increases in serum IL-6, a CAD-relevant inflammatory marker, among healthy, older adults. Our results imply that (a) depression may lead to augmented inflammation and that (b) inflammation may be one of the mechanisms that accounts for the deleterious effect of depression on cardiovascular health. The present findings also lead us to speculate that anti-inflammatory approaches may be an effective avenue to reduce the excessive CAD morbidity and mortality of individuals with clinical depression or elevated depressive symptoms.
Acknowledgments
This research was supported by the National Heart, Lung, and Blood Institute Grant HL56346 (TK, PI), Program Project Grant HL040962, and the Pittsburgh Mind-Body Center Grants HL076852 and HL076858. For their assistance with data collection, we thank the entire project staff of the Pittsburgh Healthy Heart Project.
Footnotes
Conflict of Interest Statement: All authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care. 2001;24:1069–1078. doi: 10.2337/diacare.24.6.1069. [DOI] [PubMed] [Google Scholar]
- Anisman H, Ravindran AV, Griffiths J, Merali Z. Endocrine and cytokine correlates of major depression and dysthymia with typical or atypical features. Molecular Psychiatry. 1999a;4:182–188. doi: 10.1038/sj.mp.4000436. [DOI] [PubMed] [Google Scholar]
- Anisman H, Ravindran AV, Griffiths J, Merali Z. Interleukin-1 beta production in dysthymia before and after pharmacotherapy. Biological Psychiatry. 1999b;46:1649–1655. doi: 10.1016/s0006-3223(99)00211-5. [DOI] [PubMed] [Google Scholar]
- Barefoot JC, Dahlstrom WG, Williams RB., Jr Hostility, CHD incidence, and total mortality: a 25-year follow-up study of 255 physicians. Psychosomatic Medicine. 1983;45:59–63. doi: 10.1097/00006842-198303000-00008. [DOI] [PubMed] [Google Scholar]
- Barefoot JC, Dodge KA, Peterson BL, Dahlstrom WG, Williams RB., Jr The Cook-Medley hostility scale: item content and ability to predict survival. Psychosomatic Medicine. 1989;51:46–57. doi: 10.1097/00006842-198901000-00005. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA. Manual for the Beck Anxiety Inventory. Psychological Corporation; San Antonio, TX: 1990. [Google Scholar]
- Beck AT, Beck RW. Screening depressed patients in family practice: A rapid technique. Postgraduate Medicine. 1972;52:81–85. doi: 10.1080/00325481.1972.11713319. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Garbin MG. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clinical Psychology Review. 1988;8:77–100. [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory. The Psychological Corporation; San Antonio, TX: 1996. [Google Scholar]
- Bondar RJ, Mead DC. Evaluation of glucose-6-phosphate dehydrogenase from Leuconostoc mesenteroides in the hexokinase method for determining glucose in serum. Clinical Chemistry. 1974;20:586–590. [PubMed] [Google Scholar]
- Boyle SH, Jackson WG, Suarez EC. Hostility, anger, and depression predict increases in C3 over a 10-year period. Brain, Behavior, & Immunity. 2007;21:816–823. doi: 10.1016/j.bbi.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucolo G, David H. Quantitive determination of serum triglycerides by the use of enzymes. Clinical Chemistry. 1973;19:476–482. [PubMed] [Google Scholar]
- Carney RM, Freedland KE, Veith RC. Depression, the autonomic nervous system, and coronary heart disease. Psychosomatic Medicine. 2005;67:S29–S33. doi: 10.1097/01.psy.0000162254.61556.d5. [DOI] [PubMed] [Google Scholar]
- Cesari M, Penninx BW, Newman AB, Kritchevsky SB, Nicklas BJ, Sutton-Tyrrell K, Rubin SM, Ding J, Simonsick EM, Harris TB, Pahor M. Inflammatory markers and onset of cardiovascular events: results from the Health ABC study. Circulation. 2003;108:2317–2322. doi: 10.1161/01.CIR.0000097109.90783.FC. [DOI] [PubMed] [Google Scholar]
- Clark LA, Watson D. Tripartite model of anxiety and depression: psychometric evidence and taxonomic implications. Journal of Abnormal Psychology. 1991;100:316–336. doi: 10.1037//0021-843x.100.3.316. [DOI] [PubMed] [Google Scholar]
- Cook WW, Medley DM. Proposed hostility and pharisaic-virtue scales for the MMPI. Journal of Applied Psychology. 1954;38:414–418. [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW, Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dentino AN, Pieper CF, Rao MK, Currie MS, Harris T, Blazer DG, Cohen HJ. Association of interleukin-6 and other biologic variables with depression in older people living in the community. Journal of the American Geriatrics Society. 1999;47:6–11. doi: 10.1111/j.1532-5415.1999.tb01894.x. [DOI] [PubMed] [Google Scholar]
- Dickens C, McGowan L, Clark-Carter D, Creed F. Depression in rheumatoid arthritis: a systematic review of the literature with meta-analysis. Psychosomatic Medicine. 2002;64:52–60. doi: 10.1097/00006842-200201000-00008. [DOI] [PubMed] [Google Scholar]
- Dozois DJA, Dobson KS, Ahnberg JL. A psychometric evaluation of the Beck Depression Inventory-II. Psychological Assessment. 1998;10:83–89. [Google Scholar]
- Enders CK. The performance of the full information maximum likelihood estimator in multiple regression models with missing data. Educational and Psychological Measurement. 2001;61:713. [Google Scholar]
- Ford DE, Erlinger TP. Depression and C-reactive protein in US adults: data from the Third National Health and Nutrition Examination Survey. Archives of Internal Medicine. 2004;164:1010–1014. doi: 10.1001/archinte.164.9.1010. [DOI] [PubMed] [Google Scholar]
- Garg R, Wagener DK, Madans JH. Alcohol consumption and risk of ischemic heart disease in women. Archives of Internal Medicine. 1993;153:1211–1216. [PubMed] [Google Scholar]
- Gimeno D, Kivimäkia M, Brunner EJ, Elovainio M, De Vogli R, Steptoe A, Kumari M, GDOL, Rumley A, Marmot MG, Ferrie JE. Associations of C-reactive protein and interleukin-6 with cognitive symptoms of depression: 12-year follow-up of the Whitehall II study. Psychological Medicine. 2009;39:413–423. doi: 10.1017/S0033291708003723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham JE, Robles TF, Kiecolt-Glaser JK, Malarkey WB, Bissell MG, Glaser R. Hostility and pain are related to inflammation in older adults. Brain, Behavior, & Immunity. 2006;20:389–400. doi: 10.1016/j.bbi.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Guyton AC, Hall JE. Textbook of Medical Physiology. W.B. Saunders; Philadelphia: 2000. [Google Scholar]
- Hartka E, Johnstone B, Leino EV, Motoyoshi M, Temple MT, Fillmore KM. A meta-analysis of depressive symptomatology and alcohol consumption over time. British Journal of Addiction. 1991;86:1283–1298. doi: 10.1111/j.1360-0443.1991.tb01704.x. [DOI] [PubMed] [Google Scholar]
- Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: A meta-analysis. Psychosomatic Medicine. 2009;71:171–186. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling. 1999;6:1–55. [Google Scholar]
- Jenny NS, Tracy RP, Ogg MS, Luong le A, Kuller LH, Arnold AM, Sharrett AR, Humphries SE. In the elderly, interleukin-6 plasma levels and the -174G>C polymorphism are associated with the development of cardiovascular disease. Arteriosclerosis, Thrombosis & Vascular Biology. 2002;22:2066–2071. doi: 10.1161/01.atv.0000040224.49362.60. [DOI] [PubMed] [Google Scholar]
- Joreskog KG, Sorbom D. LISREL (Version 8.8) Scientific Software International; Chicago, IL: 2008. [Google Scholar]
- Kamarck TW, Muldoon MF, Shiffman SS, Sutton-Tyrrell K. Experiences of demand and control during daily life are predictors of carotid atherosclerotic progression among healthy men. Health Psychology. 2007;26:324–332. doi: 10.1037/0278-6133.26.3.324. [DOI] [PubMed] [Google Scholar]
- Kenis G, Maes M, Kenis G, Maes M. Effects of antidepressants on the production of cytokines. International Journal of Neuropsychopharmacology. 2002;5:401–412. doi: 10.1017/S1461145702003164. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Preacher KJ, MacCallum RC, Atkinson C, Malarkey WB, Glaser R. Chronic stress and age-related increases in the proinflammatory cytokine IL-6. Proc Natl Acad Sci U S A. 2003;100:9090–9095. doi: 10.1073/pnas.1531903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kop WJ. Chronic and acute psychological risk factors for clinical manifestations of coronary artery disease. Psychosomatic Medicine. 1999;61:476–487. doi: 10.1097/00006842-199907000-00012. [DOI] [PubMed] [Google Scholar]
- Kop WJ, Gottdiener JS. The role of immune system parameters in the relationship between depression and coronary artery disease. Psychosomatic Medicine. 2005;67:S37–S41. doi: 10.1097/01.psy.0000162256.18710.4a. [DOI] [PubMed] [Google Scholar]
- Korhonen T, Broms U, Varjonen J, Romanov K, Koskenvuo M, Kinnunen T, Kaprio J. Smoking behaviour as a predictor of depression among Finnish men and women: a prospective cohort study of adult twins. Psychological Medicine. 2007;37:705–715. doi: 10.1017/S0033291706009639. [DOI] [PubMed] [Google Scholar]
- Kuo HK, Yen CJ, Chang CH, Kuo CK, Chen JH, Sorond F. Relation of C-reactive protein to stroke, cognitive disorders, and depression in the general population: systematic review and meta-analysis. Lancet Neurology. 2005;4:371–380. doi: 10.1016/S1474-4422(05)70099-5. [DOI] [PubMed] [Google Scholar]
- Lanquillon S, Krieg JC, Bening-Abu-Shach U, Vedder H. Cytokine production and treatment response in major depressive disorder. Neuropsychopharmacology. 2000;22:370–379. doi: 10.1016/S0893-133X(99)00134-7. [DOI] [PubMed] [Google Scholar]
- Leonard BE. The immune system, depression and the action of antidepressants. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2001;25:767–780. doi: 10.1016/s0278-5846(01)00155-5. [DOI] [PubMed] [Google Scholar]
- Luc G, Bard JM, Juhan-Vague I, Ferrieres J, Evans A, Amouyel P, Arveiler D, Fruchart JC, Ducimetiere P, Group PS. C-reactive protein, interleukin-6, and fibrinogen as predictors of coronary heart disease: the PRIME Study. Arteriosclerosis, Thrombosis & Vascular Biology. 2003;23:1255–1261. doi: 10.1161/01.ATV.0000079512.66448.1D. [DOI] [PubMed] [Google Scholar]
- Lutgendorf SK, Garand L, Buckwalter KC, Reimer TT, Hong SY, Lubaroff DM. Life stress, mood disturbance, and elevated interleukin-6 in healthy older women. Journals of Gerontology Series A-Biological Sciences & Medical Sciences. 1999;54:M434–439. doi: 10.1093/gerona/54.9.m434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes M, Bosmans E, De Jongh R, Kenis G, Vandoolaeghe E, Neels H. Increased serum IL-6 and IL-1 receptor antagonist concentrations in major depression and treatment resistant depression. Cytokine. 1997;9:853–858. doi: 10.1006/cyto.1997.0238. [DOI] [PubMed] [Google Scholar]
- Maes M, Bosmans E, Suy E, Vandervorst C, DeJonckheere C, Raus J. Depression-related disturbances in mitogen-induced lymphocyte responses and interleukin-1 beta and soluble interleukin-2 receptor production. Acta Psychiatrica Scandinavica. 1991;84:379–386. doi: 10.1111/j.1600-0447.1991.tb03163.x. [DOI] [PubMed] [Google Scholar]
- Mass DW, van der Mast RC, de Craen AJ. Increased C-reactive protein is not associated with apathy: The Leiden 85-Plus Study. International Journal of Geriatric Psychiatry. 2009 doi: 10.1002/gps.2242. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Matthews KA, Schott LL, Bromberger J, Cyranowski J, Everson-Rose SA, Sowers MF. Associations between depressive symptoms and inflammatory/hemostatic markers in women during the menopausal transition. Psychosomatic Medicine. 2007;69:124–130. doi: 10.1097/01.psy.0000256574.30389.1b. [DOI] [PubMed] [Google Scholar]
- Miller GE, Cohen S, Ritchey A. Chronic psychological stress and the regulation of pro-inflammatory cytokines: A glucocorticoid-resistance model. Health Psychology. 2002a;21:531–541. doi: 10.1037//0278-6133.21.6.531. [DOI] [PubMed] [Google Scholar]
- Miller GE, Stetler CA, Carney RM, Freedland KE, Banks WA. Clinical depression and inflammatory risk markers for coronary heart disease. American Journal of Cardiology. 2002b;90:1279–1283. doi: 10.1016/s0002-9149(02)02863-1. [DOI] [PubMed] [Google Scholar]
- Muller N, Schwarz MJ, Dehning S, Douhe A, Cerovecki A, Goldstein-Muller B, Spellmann I, Hetzel G, Maino K, Kleindienst N, Moller HJ, Arolt V, Riedel M, Muller N, Schwarz MJ, Dehning S, Douhe A, Cerovecki A, Goldstein-Muller B, Spellmann I, Hetzel G, Maino K, Kleindienst N, Moller HJ, Arolt V, Riedel M. The cyclooxygenase-2 inhibitor celecoxib has therapeutic effects in major depression: results of a double-blind, randomized, placebo controlled, add-on pilot study to reboxetine. Molecular Psychiatry. 2006;11:680–684. doi: 10.1038/sj.mp.4001805. [DOI] [PubMed] [Google Scholar]
- Nazmi A, Victora CG. Socioeconomic and racial/ethnic differentials of C-reactive protein levels: a systematic review of population-based studies. BMC Public Health. 2007;7:212. doi: 10.1186/1471-2458-7-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace TW, Mletzko TC, Alagbe O, Musselman DL, Nemeroff CB, Miller AH, Heim CM. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. American Journal of Psychiatry. 2006;163:1630–1633. doi: 10.1176/ajp.2006.163.9.1630. [DOI] [PubMed] [Google Scholar]
- Paffenbarger RS, Wing AL, Hyde RT. Physical activity as an index of heart attack risk in college alumni. American Journal of Epidemiology. 1978;108:161–175. doi: 10.1093/oxfordjournals.aje.a112608. [DOI] [PubMed] [Google Scholar]
- Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, 3rd, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, Rifai N, Smith SC, Jr, Taubert K, Tracy RP, Vinicor F Centers for Disease, C., Prevention, American Heart, A. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- Penninx BW, Kritchevsky SB, Yaffe K, Newman AB, Simonsick EM, Rubin S, Ferrucci L, Harris T, Pahor M. Inflammatory markers and depressed mood in older persons: results from the Health, Aging and Body Composition study. Biological Psychiatry. 2003;54:566–572. doi: 10.1016/s0006-3223(02)01811-5. [DOI] [PubMed] [Google Scholar]
- Perloff D, Grim C, Flack J, Frohlich ED, Hill M, McDonald M, Morgenstern BZ. Human blood pressure determination by sphygmomanometry. Circulation. 1993;88:2460–2470. doi: 10.1161/01.cir.88.5.2460. [DOI] [PubMed] [Google Scholar]
- Pitsavos C, Panagiotakos DB, Papageorgiou C, Tsetsekou E, Soldatos C, Stefanadis C. Anxiety in relation to inflammation and coagulation markers, among healthy adults: the ATTICA study. Atherosclerosis. 2006;185:320–326. doi: 10.1016/j.atherosclerosis.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Owens MJ, Nemeroff CB. Psychoneuroendocrinology of depression: Hypothalamic-pituitary-adrenal axis. Psychiatric Clinics of North America. 1998;21:293–307. doi: 10.1016/s0193-953x(05)70006-x. [DOI] [PubMed] [Google Scholar]
- Pradhan AD, Manson JE, Rossouw JE, Siscovick DS, Mouton CP, Rifai N, Wallace RB, Jackson RD, Pettinger MB, Ridker PM. Inflammatory biomarkers, hormone replacement therapy, and incident coronary heart disease: prospective analysis from the Women’s Health Initiative observational study. JAMA. 2002;288:980–987. doi: 10.1001/jama.288.8.980. [DOI] [PubMed] [Google Scholar]
- Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranjit N, Diez-Roux AV, Shea S, Cushman M, Seeman T, Jackson SA, Ni H. Psychosocial factors and inflammation in the multi-ethnic study of atherosclerosis. Archives of Internal Medicine. 2007;167:174–181. doi: 10.1001/archinte.167.2.174. [DOI] [PubMed] [Google Scholar]
- Raynor DA, Pogue-Geile MF, Kamarck TW, McCaffery JM, Manuck SB. Covariation of psychosocial characteristics associated with cardiovascular disease: Genetic and environmental influences. Psychosomatic Medicine. 2002;64:191–203. doi: 10.1097/00006842-200203000-00002. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Danielson E, Fonseca FAH, Genest J, Gotto AM, Jr, Kastelein JJP, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Rifai N, Stampfer MJ, Hennekens CH. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation. 2000;101:1767–1772. doi: 10.1161/01.cir.101.15.1767. [DOI] [PubMed] [Google Scholar]
- Roberts RE, Deleger S, Strawbridge WJ, Kaplan GA. Prospective association between obesity and depression: evidence from the Alameda County Study. International Journal of Obesity. 2003;27:514–521. doi: 10.1038/sj.ijo.0802204. [DOI] [PubMed] [Google Scholar]
- Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- Rozanski A, Blumenthal JA, Davidson KW, Saab PG, Kubzansky L. The epidemiology, pathophysiology, and management of psychosocial risk factors in cardiac practice: the emerging field of behavioral cardiology. Journal of the American College of Cardiology. 2005;45:637–651. doi: 10.1016/j.jacc.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Rugulies R. Depression as a predictor for coronary heart disease. a review and meta-analysis. American Journal of Preventive Medicine. 2002;23:51–61. doi: 10.1016/s0749-3797(02)00439-7. [DOI] [PubMed] [Google Scholar]
- Sajadieh A, Nielsen OW, Rasmussen V, Hein HO, Abedini S, Hansen JF. Increased heart rate and reduced heart-rate variability are associated with subclinical inflammation in middle-aged and elderly subjects with no apparent heart disease. European Heart Journal. 2004;25:363–370. doi: 10.1016/j.ehj.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Sakkinen PA, Macy EM, Callas PW, Cornell ES, Hayes TE, Kuller LH, Tracy RP. Analytical and biologic variability in measures of hemostasis, fibrinolysis, and inflammation: Assessment and implications for epidemiology. American Journal of Epidemiology. 1999;149:261–267. doi: 10.1093/oxfordjournals.aje.a009801. [DOI] [PubMed] [Google Scholar]
- Shekelle RB, Gale M, Ostfeld AM, Paul O. Hostility, risk of coronary heart disease, and mortality. Psychosomatic Medicine. 1983;45:109–114. doi: 10.1097/00006842-198305000-00003. [DOI] [PubMed] [Google Scholar]
- Sloan RP, McCreath H, Tracey KJ, Sidney S, Liu K, Seeman T, Sloan RP, McCreath H, Tracey KJ, Sidney S, Liu K, Seeman T. RR interval variability is inversely related to inflammatory markers: the CARDIA study. Molecular Medicine. 2007;13:178–184. doi: 10.2119/2006-00112.Sloan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RS. The macrophage theory of depression. Medical Hypotheses. 1991;35:298–306. doi: 10.1016/0306-9877(91)90272-z. [DOI] [PubMed] [Google Scholar]
- Smith TW, Frohm KD. What’s so unhealthy about hostility? Construct validity and psychosocial correlates of the Cook and Medley Ho scale. Health Psychology. 1985;4:503–520. doi: 10.1037//0278-6133.4.6.503. [DOI] [PubMed] [Google Scholar]
- Spath-Schwalbe E, Hansen K, Schmidt F, Schrezenmeier H, Marshall L, Burger K, Fehm HL, Born J. Acute effects of recombinant human interleukin-6 on endocrine and central nervous sleep functions in healthy men. Journal of Clinical Endocrinology & Metabolism. 1998;83:1573–1579. doi: 10.1210/jcem.83.5.4795. [DOI] [PubMed] [Google Scholar]
- Steer RA, Beck AT. Beck Anxiety Inventory. In: Zalaquett CP, Wood RJ, editors. Evaluating stress: A book of resources. Scarecrow Press, Inc; Lanham, MD: 1997. pp. 23–40. [Google Scholar]
- Steiger JH. Structural model evaluation and modification: An interval estimation approach. Multivariate Behavioral Research. 1990;25:173–180. doi: 10.1207/s15327906mbr2502_4. [DOI] [PubMed] [Google Scholar]
- Stewart JC, Janicki DL, Muldoon MF, Sutton-Tyrrell K, Kamarck TW. Negative emotions and 3-year progression of subclinical atherosclerosis. Archives of General Psychiatry. 2007;64:225–233. doi: 10.1001/archpsyc.64.2.225. [DOI] [PubMed] [Google Scholar]
- Strawbridge WJ, Deleger S, Roberts RE, Kaplan GA. Physical activity reduces the risk of subsequent depression for older adults. American Journal of Epidemiology. 2002;156:328–334. doi: 10.1093/aje/kwf047. [DOI] [PubMed] [Google Scholar]
- Suarez EC, Krishnan RR, Lewis JG. The relation of severity of depressive symptoms to monocyte-associated proinflammatory cytokines and chemokines in apparently healthy men. Psychosomatic Medicine. 2003;65:362–368. doi: 10.1097/01.psy.0000035719.79068.2b. [DOI] [PubMed] [Google Scholar]
- Suarez EC, Lewis JG, Krishnan RR, Young KH. Enhanced expression of cytokines and chemokines by blood monocytes to in vitro lipopolysaccharide stimulation are associated with hostility and severity of depressive symptoms in healthy women. Psychoneuroendocrinology. 2004;29:1119–1128. doi: 10.1016/j.psyneuen.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Suls J, Bunde J. Anger, anxiety, and depression as risk factors for cardiovascular disease: the problems and implications of overlapping affective dispositions. Psychological Bulletin. 2005;131:260–300. doi: 10.1037/0033-2909.131.2.260. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Smith M, Rossy LA, Sollers JJ, Friedman BH. Heart period variability and depressive symptoms: gender differences. Biological Psychiatry. 1998;44:304–306. doi: 10.1016/s0006-3223(98)00008-0. [DOI] [PubMed] [Google Scholar]
- Tracey KJ. The inflammatory reflex. Nature. 2002;420:853–859. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- Tyring S, Gottlieb A, Papp K, Gordon K, Leonardi C, Wang A, Lalla D, Woolley M, Jahreis A, Zitnik R, Cella D, Krishnan R, Tyring S, Gottlieb A, Papp K, Gordon K, Leonardi C, Wang A, Lalla D, Woolley M, Jahreis A, Zitnik R, Cella D, Krishnan R. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367:29–35. doi: 10.1016/S0140-6736(05)67763-X. [DOI] [PubMed] [Google Scholar]
- Vaccarino V, Johnson BD, Sheps DS, Reis SE, Kelsey SF, Bittner V, Rutledge T, Shaw LJ, Sopko G, Bairey Merz CN National Heart LaBI. Depression, inflammation, and incident cardiovascular disease in women with suspected coronary ischemia: the National Heart, Lung, and Blood Institute-sponsored WISE study. Journal of the American College of Cardiology. 2007;50:2044–2050. doi: 10.1016/j.jacc.2007.07.069. [DOI] [PubMed] [Google Scholar]
- van den Biggelaar AH, Gussekloo J, de Craen AJ, Frolich M, Stek ML, van der Mast RC, Westendorp RG. Inflammation and interleukin-1 signaling network contribute to depressive symptoms but not cognitive decline in old age. Experimental Gerontology. 2007;42:693–701. doi: 10.1016/j.exger.2007.01.011. [DOI] [PubMed] [Google Scholar]
- van Eck MM, Berkhof H, Nicolson N, Sulon J. The effects of perceived stress, traits, mood states and stressful daily events on salivary control. Psychosomatic Medicine. 1996;58:447–458. doi: 10.1097/00006842-199609000-00007. [DOI] [PubMed] [Google Scholar]
- Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB. Elevated C-reactive protein levels in overweight and obese adults. JAMA. 1999;282:2131–2135. doi: 10.1001/jama.282.22.2131. [DOI] [PubMed] [Google Scholar]
- Volpato S, Pahor M, Ferrucci L, Simonsick EM, Guralnik JM, Kritchevsky SB, Fellin R, Harris TB. Relationship of alcohol intake with inflammatory markers and plasminogen activator inhibitor-1 in well-functioning older adults: the Health, Aging, and Body Composition study. Circulation. 2004;109:607–612. doi: 10.1161/01.CIR.0000109503.13955.00. [DOI] [PubMed] [Google Scholar]
- Warnick GR, Albers JJ. A comprehensive evaluation of the heparin-manganese precipitation procedure for estimating high density lipoprotein cholesterol. J Lipid Res. 1978;19:65–76. [PubMed] [Google Scholar]
- Whooley MA, de Jonge P, Vittinghoff E, Otte C, Moos R, Carney RM, Ali S, Dowray S, Na B, Feldman MD, Schiller NB, Browner WS. Depressive symptoms, health behaviors, and risk of cardiovascular events in patients with coronary heart disease. JAMA. 2008;300:2379–2388. doi: 10.1001/jama.2008.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichers M, Maes M, Wichers M, Maes M. The psychoneuroimmuno-pathophysiology of cytokine-induced depression in humans. International Journal of Neuropsychopharmacology. 2002;5:375–388. doi: 10.1017/S1461145702003103. [DOI] [PubMed] [Google Scholar]
- Wulsin LR, Singal BM. Do depressive symptoms increase the risk for the onset of coronary disease? A systematic quantitative review. Psychosomatic Medicine. 2003;65:201–210. doi: 10.1097/01.psy.0000058371.50240.e3. [DOI] [PubMed] [Google Scholar]