Abstract
While the role of left prefrontal cortex in reasoning tasks has long been recognized, the role of right prefrontal cortex remains unclear. One patient study (Goel et al., 2007) has suggested that right prefrontal cortex plays an essential role in resolving indeterminate relations. To test this hypothesis, and to identify the involvement of specific regions within right prefrontal cortex we scanned 17 normal volunteers with fMRI while they engaged in a transitive inference task involving determinate and indeterminate relations. The results show a nice crossover interaction such that, right ventrolateral prefrontal cortex (BA 47) is selectively activated by the processing of indeterminate trials with no belief-bias cues, while left lateral prefrontal cortex (BA 45) is selectively activated by the processing of indeterminate trials containing belief-bias cues. These results are not only consistent with, but also amplify, the lesion data by identifying specific regions within right and left prefrontal cortex.
Keywords: Reasoning, hemispheric asymmetry, neuroimaging, frontal lobes, uncertainty
INTRODUCTION
Research with split-brain patients provides considerable evidence for left hemisphere involvement (even dominance) in the critical domains of reasoning, decision-making, and problem solving (Gazzaniga, 2000; Gazzaniga, Ivry, & Mangun, 1998; Wolford, Miller, & Gazzaniga, 2000) while limiting the role of the right hemisphere, and in particular the right prefrontal cortex (PFC) to little more than visual organization (Corballis, 2003). This conclusion has been reinforced by recent neuroimaging and patient studies of logical reasoning, where numerous studies have implicated left PFC in logical reasoning tasks (Acuna, Eliassen, Donoghue, & Sanes, 2002; Canessa et al., 2005; Fangmeier, Knauff, Ruff, & Sloutsky, 2006; Goel, Buchel, Frith, & Dolan, 2000; Goel & Dolan, 2001b, 2003, 2004; Goel, Gold, Kapur, & Houle, 1997, 1998; Goel, Shuren, Sheesley, & Grafman, 2004; Heckers, Zalesak, Weiss, Ditman, & Titone, 2004; Knauff, Fangmeier, Ruff, & Johnson-Laird, 2003; Knauff, Kassubek, Mulack, & Greenlee, 2000; Knauff, Mulack, Kassubek, Salih, & Greenlee, 2002; Noveck, Goel, & Smith, 2004).
However, this traditional view is being modified. Several neuroimaging studies have activated right PFC in specific conditions of logical reasoning tasks. Two such conditions include the introduction of unfamiliar or nonspecific material (lacking conceptual content) and conflict detection/resolution situations. In the former condition, resolving logical arguments such as “All A are B; all B are C; all A are C” activates bilateral prefrontal cortex (left BA 44 & 45, right BA 45), whereas resolving equivalent logical forms containing materials we have beliefs about, e.g. “All apples are fruits; all fruits are nutritious; all apples are nutritious” activates only left prefrontal cortex (BA 44) (Goel et al., 2000; Goel & Dolan, 2003). In the latter condition, the presence of conflict, be it belief-logic conflict, where the logical conclusion conflicts with our beliefs (e.g. all apples are fruit; all fruit are poisonous; all apples are poisonous), or even the simple presence of inverted relations (e.g. John is taller than Mary; Mary is taller than George; George is shorter than John), results in activation in right prefrontal cortex (BA 45) (Goel et al., 2000; Goel & Dolan, 2003; Prado & Noveck, 2007). Such findings have led Goel et al. (2005) to speculate that the left prefrontal cortex is necessary and sometimes sufficient for logical reasoning, while the right prefrontal cortex is sometimes necessary but not sufficient. However, these roles may not explain the full range of right PFC involvement in logical reasoning.
A recent patient study (Goel et al., 2007) provides another interesting role for right PFC in terms of processing indeterminate logical relations. Such relations occur when information in the premises underdetermines the conclusion. As an example, consider transitive arguments of the form A> B; B>C; A>C. Such arguments can be either valid or invalid. Valid arguments (e.g. A>B; B>C; A>C) are determinate. For example, given A>B and B>C the relationship between A and C is absolutely determined by the information provided: it follows that A>C. No other relationship is possible between A and C. Invalid arguments can be either determinate or indeterminate. Determinate invalid arguments (e.g. A>B; B>C; C>A) are inconsistent. For example, given A>B and B>C the relationship between A and C is absolutely determined NOT to be C>A. This possibility contradicts the information provided in the premises and therefore must be false. In indeterminate arguments (e.g. A>B; A>C; B>C), on the other hand, not enough information is provided to determine the relationship between B and C. It is possible that B>C, or C>B, or B=C. Such arguments are also invalid but for different reasons than the inconsistent ones. They are invalid because there is no fact of the matter as to the relationship between B and C; it is truly indeterminate.
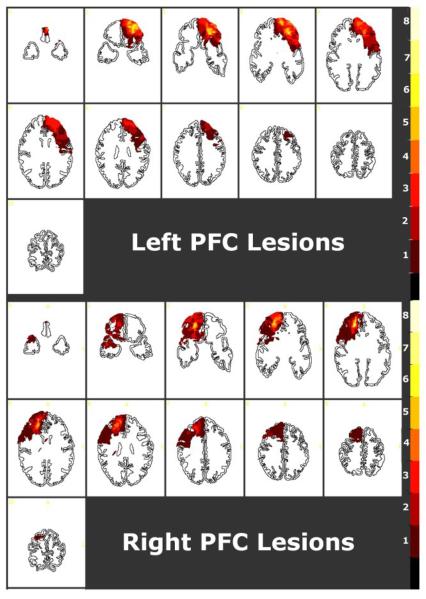
Goel et al. (2007) administered such problems to patients with focal, unilateral, frontal lobe lesions, and age and education-matched normal controls. They reported a hemisphere by determinacy interaction such that, patients with focal lesions to left prefrontal cortex showed specific impairment on determinate trials compared to normal controls and patients with focal lesions to right prefrontal cortex, whereas patients with focal lesions to right prefrontal cortex showed specific impairment on indeterminate trials compared to normal controls and patients with focal lesions to left prefrontal cortex. The lesions in these patients encompassed large areas in the prefrontal cortices including, BAs 10, 8, 9, 11, 44, 45, 6 & 47, and to a lesser extent BAs 24, 25, 32 & 46 (see Figure 1). Therefore it was not possible for this study to isolate specific regions of left and right prefrontal cortex were involved in the effect.
Figure 1.
Lesion overlay maps for 15 patients (transverse slices, R=L), displayed on a template transformed to Talairach dimensions. The 11 slices (7-11 mm thickness) are 17 degrees relative to the inferior orbitomeatal line, and correspond closely to the Damasio and Damasio (1989) transverse brain template. The lesions in these patients encompassed large areas in the prefrontal cortices including, BAs 10, 8, 9, 11, 44, 45, 6 & 47, and to a lesser extent BAs 24, 25, 32 & 46. Patients with focal lesions to left prefrontal cortex showed specific impairment on determinate trials compared to normal controls and patients with focal lesions to right prefrontal cortex, whereas patients with focal lesions to right prefrontal cortex showed specific impairment on indeterminate trials compared to normal controls and patients with focal lesions to left prefrontal cortex. Reproduced from Goel et al.(2007).
To determine the specific regions of right PFC involved in the resolution of indeterminate trials, and to further articulate the role of background knowledge in dealing with indeterminacy, we carried out an event-related fMRI study in which normal healthy volunteers engaged in transitive reasoning while being scanned. A factorial design was used to control for determinacy of logical form and familiarity of content. We reasoned that the belief-bias effect resulting from familiarity of content (Evans, Barston, & Pollard, 1983; Goel & Dolan, 2003) would modulate perceived indeterminacy in logical form. That is, propositions containing familiar content that subjects have beliefs about will result in increased responses consistent with subjects' beliefs (due to belief-bias effects) thus reducing the impact of logical indeterminacy. Propositions containing unfamiliar content would not be susceptible to belief-bias effects and would allow for increased levels of sensitivity to logical indeterminacy. At the level of functional anatomy, the former condition would result in greater activation in left prefrontal cortex while the latter condition would result in greater activation in right prefrontal cortex in regions encompassed by the lesions noted in Figure 1.
METHOD
Participants
Seventeen right-handed participants (8 females, 9 males) aged 21-36 years (mean 27.3, SD = 4.1), with 16.8 (SD = 3.2) years of education and without any history of psychiatric or neurological disorder, were scanned. All participants gave informed consent in accordance with the NINDS Internal Review Board.
Stimuli & Experimental Design
The stimuli consisted of 80 three-term transitive arguments involving nonspatial relations (reasoning trials) and 55 baseline trials. The reasoning trials were organized into a 3 × 2 factorial design, whereby the first factor was Determinacy (determinate valid, determinate inconsistent, indeterminate) and the second factor was Familiarity (familiar content, unfamiliar content) (see Table 1). Determinacy is a function of logical form and is explained, with examples, in the introduction. To balance for the number of “valid” and “invalid” responses 40 determinate valid trials, 20 determinate invalid trials (i.e. inconsistent), and 20 indeterminate invalid trials were used.
Table 1.
Reasoning Problem Types
| Determinate (40) | Indeterminate (20) | ||
|---|---|---|---|
| Valid (20) | Inconsistent (20) | ||
| Familiar (40) | Africa is bigger then Europe. Asia is bigger than Africa. Europe is not as big as Asia. |
Africa is bigger than Europe. Asia is bigger than Africa. Asia is not as big as Europe |
The Earth is dimmer than the sun. The sun is brighter than Mars. The Earth is brighter than Mars. |
|
Unfamiliar Content (40) |
Ralph is braver then Celia. Celia is braver than Tim. Ralph is braver than Tim. |
Ralph is braver than Celia. Celia is braver than Tim. Tim is braver than Ralph. |
Bert is older than Tom. Bert is older than Sal. Tom is not as old as Sal. |
The Familiarity factor manipulates subjects' familiarity or knowledge of the truth or falsity of the content of the propositions. Familiar content trials contained propositions that subjects would have beliefs about (e.g. Hawks are more dangerous than pigeons) while unfamiliar content trials contained propositions about nonspecific entities that subjects could not have beliefs about (e.g. Ralph is braver than Celia). Subjects' beliefs about the content of reasoning trials are known to have a major impact on reasoning processes (Evans et al., 1983; Goel & Dolan, 2003) and were tested with a post scan questionnaire. Example items from each condition appear in Table 1.
As in several previous studies (Goel et al., 2000; Goel & Dolan, 2001b), baseline trials were created by pairing the first two sentences of one argument with the conclusion of a different argument in the study (e.g. Lucy is more romantic than Barry; Lucy is less romantic than Victor; ∴ Ralph is braver than Tim.). (See rationale below.) Baseline trials were used only to generate the main effect of reasoning to validate that it was indeed consistent with previous studies using a similar paradigm. They were not utilized in the key analyses of interest.
Stimuli Presentation
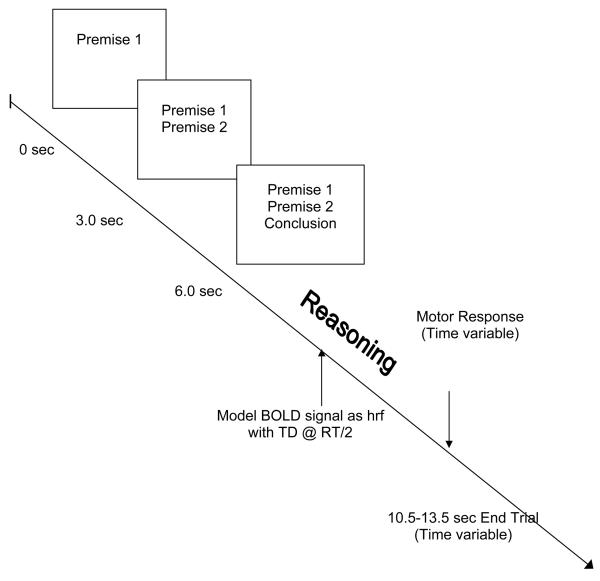
Stimuli were presented randomly in an event-related design (see Figure 2). The beginning of each trial was signalled by an “*” at 0 ms. The first sentence appeared on the screen at 500 ms, the second at 3500 ms, and the third at 6500 ms. All sentences remained on the screen until the end of the trial. The length of trials varied from 10.5 seconds to 13.5 seconds (to allow for 1 TR jitter, see below), leaving subjects 4.5 - 7.5 seconds (after the presentation of the third sentence) to respond. Participants responded by pressing one of two keys on a button box. Half the participants were instructed to respond with their left hand and the other half with the right hand, to control for neural activation associated with motor response.
Figure 2.
Time course of stimuli presentation. See text.
Task
The task in all conditions was the same. Subjects were required to determine whether the conclusion followed logically from the premises (i.e. whether the argument was valid) irrespective of the truth or falsity of the conclusion. The concept of “necessarily follows” was illustrated with various example arguments before scanning. All subjects readily recognized the concept and clearly understood the task. Subjects further reviewed example (not actual) stimuli from each condition prior to being scanned to ensure to that they understood the task.
In (baseline) trials, where the first two sentences were related, subjects would need to begin to integrate the premises and construct a representation of the problem, but when the third, unrelated, sentence appeared they could immediately disengage the task and respond ‘no’. In (reasoning) trials where the three sentences constituted an argument, subjects would need to continue with the reasoning component of the task after the presentation of the third sentence. The difference between completing the reasoning task and disengaging after the presentation of the third sentence isolates the reasoning components of interest. Subjects were instructed to respond as quickly as possible and move to the next trial if the stimuli advanced before they could respond. Subjects responded by pressing one of two keys on a button box, corresponding with either ‘valid’ or ‘invalid’, after the presentation of the third sentence.
fMRI Scanning Technique
A General Electric 3.0T whole body MR scanner was used to acquire 256×256×124 3D SPGR volumes with voxel size of 0.9375 × 0.9375 mm × 1.5 mm. Functional (fMRI) volumes were acquired using EPI (echo planar imaging) with a TR of 3000 ms and a TE of 40 ms. Each brain volume was obtained using two excitation pulses and consisted of 22 interleaved slices that were 6 mm thick with no gap. The in-plane matrix was 64x64, with a voxel dimension of 3.75×3.75 mm. Data were recorded during a single acquisition period. A total of 558 volume images were acquired over three sessions (186 volumes per session). The first six volumes in each session were discarded (leaving 180 volumes per session) to allow for T1 equilibration effects.
Trials from all conditions were randomly presented in a single-event design. The mean trial time was 12000 ms +/− 1500 ms (TR) with a random jitter. Trials thus varied from 10.50 seconds to 13.50 seconds. There were 45 event presentations during a session for a total of 135 over the three sessions. Each session lasted 9.02 minutes.
Data Analysis
Data were analyzed using Statistical Parametric Mapping (SPM2) (Friston et al., 1995). All volumes in a session were spatially realigned to the first volume of the session and temporally realigned to the AC-PC slice, to account for different sampling times of different slices. A mean image created from the realigned volumes was co-registered with the structural T1 volume. The structural volumes were then spatially normalized to the Montreal Neurological Institute brain template (Evans et al., 1993) using nonlinear basis functions (Ashburner & Friston, 1999). The derived spatial transformation was then applied to the realigned T2* volumes, which were then spatially smoothed with a 12 mm FWHM isotropic Gaussian kernel (in order to make comparisons across subjects and to permit application of random field theory for corrected statistical inference) (Worsley & Friston, 1995). The resulting time series across each voxel were high-pass filtered with a cut-off of 120 sec, using cosine functions to remove section-specific low frequency drifts in the BOLD signal. Global means were normalized by proportional scaling to a Grand Mean of 100.
All events were modeled in the design matrix, but the event of interest was the neural activity following presentation of the conclusion, corresponding to the reasoning component of the task. The BOLD signal corresponding to this the event was modeled as a canonical hemodynamic response function with time derivative at the midway point between the presentation of the third sentence and the motor response (on a trial by trial, subject by subject basis). The other events were modeled as events of no interest (with canonical hemodynamic response functions).
Condition effects at each voxel were estimated according to the general linear model and regionally specific effects compared using linear contrasts. Each contrast produced a statistical parametric map of the t-statistic for each voxel, which was subsequently transformed to a unit normal Z-distribution.
We report the contrast associated with the main effect of reasoning (to relate our results to previous studies) and the main effects of familiarity and determinacy, but are primarily interested in the determinacy by familiarity interaction comparisons. As we had a priori hypotheses about regions of activation in the former case from previous imaging studies (Goel, 2007; Goel & Dolan, 2001b; Goel, Makale, & Grafman, 2004), and a priori hypotheses about regions of activation in the latter contrasts from a patient study (Goel et al., 2007) (see Figure 1), we used a voxel-level intensity threshold of p<.001 (uncorrected). Activations outside these regions are reported if they survived a voxel-level intensity threshold of p<.05 corrected for multiple comparisons.
RESULTS
Behavioral Results
Behavioral scores are reported in Table 2. Within-subject repeated-measures analyses for both reaction time and accuracy were performed for: (1) Determinacy (3 levels: Valid, Inconsistent, Indeterminate) and (2) Familiarity (2 levels: Familiar and Unfamiliar). Analysis of accuracy indicated a significant interaction between Determinacy and Content Familiarity [F(2,32)= 3.82, p=0.032]. Pairwise comparisons reveal the interaction was driven by reduced accuracy scores for indeterminate trials with familiar content compared to indeterminate trials with unfamiliar content (66.71 % versus 76.88% respectively, p < 0.001). There were no significant differences between familiar and unfamiliar content for valid trials or for inconsistent trials. Analysis of reaction time revealed that there was no main effect of Determinacy or Familiarity and no Determinacy by Familiarity interaction (p>0.05).
Table 2.
Behavioral Results: Average Accuracy and Reaction Times (standard deviation).
| Familiar | Unfamiliar | |
|---|---|---|
| Valid | 72.94% (11.04) | 76.23% (9.63) |
| 3276 msec (660) | 3264 msec (723) | |
| Inconsistent | 71.94% (27.36) | 70.94% (24.55) |
| 3573 msec (873) | 3498 msec (801) | |
| Indeterminate | 66.71% (16.46) | 76.88% (18.34) |
| 3317 msec (808) | 3609 msec (1025) |
fMRI Results
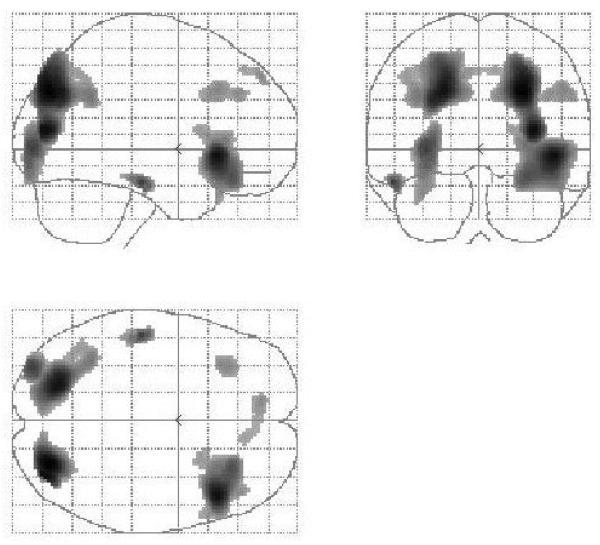
The fMRI results are reported in Table 3. To compare our results with previous studies we began our analysis with the main effect of reasoning (reasoning minus baseline trials; see Figure 3). Consistent with previous studies (Goel, 2007) this comparison revealed activation in bilateral PFC (BA 45, 47, 9, 8), superior parietal lobule (BA 7), temporal cortex (BA 21/22) and occipital cortex (BA 18).
Table 3.
Results of fMRI analysis. See text.
| MNI Coordinates |
||||
|---|---|---|---|---|
| Location | X | Y | Z | Z-score |
| Main Effect Reason (Reason – Baseline) | ||||
| Right Inferior PFC (BA 45/47) | 46 | 24 | −4 | 4.50 |
| Right ventrolateral PFC (BA 47) | 30 | 32 | −16 | 3.61 |
| Left ventrolateral PFC (BA 47) | −32 | 30 | −20 | 2.98 |
| Middle Frontal Gyrus (BA 9) | 52 | 24 | 36 | 2.99 |
| Left Superior Frontal Gyrus (BA9) | −10 | 50 | 44 | 2.98 |
| Middle/Inferior Temporal Gyrus (BA 20/21) |
−52 | −22 | −20 | 3.79 |
| Left Superior Parietal Lobule (BA 7) | 26 | −78 | 34 | 4.80 |
| Right Superior Parietal Lobule (BA 7) | −22 | −72 | 34 | 4.44 |
| Inferior Occipital Gyrus (BA18) | −32 | −88 | 0 | 3.81 |
| Fusiform Gyrus (BA 18) | −32 | −90 | −14 | 3.15 |
| Main Effect of Familiarity | ||||
| Familiar trials – unfamiliar trials | ||||
| Left lateral PFC (BA 45) | −54 | 26 | 8 | 3.58 |
| Right superior parietal lobule (BA 7) | 34 | −60 | 64 | 3.38 |
| Left superior occipital gyrus (BA 19) | −30 | − 86 | − 24 | 3.14 |
| Unfamiliar trials – familiar trials | ||||
| Posterior cingulated gyrus (BA 30) | −10 | −42 | 18 | 4.03 |
| Right orbital prefrontal cortex (BA 25) | 14 | 8 | − 20 | 3.47 |
| Main Effect of Determinacy | ||||
| Indeterminate trials – determinate trials | ||||
| Right dorsolateral PFC (BA 9) | 42 | 30 | 32 | 3.49 |
| Right ventral lateral PFC (BA 47) | 36 | 28 | −22 | 3.01 |
| Determinate trials – indeterminate trials | ||||
| Right inferior parietal lobule (BA 40) | 44 | − 42 | 28 | 2.99 |
| Determinacy × Familiarity Interaction Analysis | ||||
|
([indeterminate unfamiliar - indeterminate familiar] - [determinate unfamiliar - determinate familiar]) |
||||
| Right Ventrolateral PFC (BA 47) | 38 | 10 | −10 | 4.24 |
| (extending into insula) | 52 | 16 | −10 | 3.45 |
|
([indeterminate familiar - indeterminate unfamiliar] - [determinate familiar - determinate unfamiliar]) |
||||
| Right Parietal Cortex (BA 7) & precuneus | 24 | −56 | 32 | 4.77 |
| Left ventrolateral PFC (BA 47) | −34 | 24 | −8 | 3.01 |
|
Determinacy × Familiarity interaction analysis (restricted to inconsistent trials) | ||||
|
([indeterminate unfamiliar - indeterminate familiar] - [inconsistent unfamiliar - inconsistent familiar]) |
||||
| Right Ventrolateral PFC (BA 47) | 36 | 28 | −22 | 3.46 |
| (extending into insula) | 42 | 12 | −10 | 3.55 |
|
([indeterminate familiar - indeterminate unfamiliar] - [inconsistent familiar - inconsistent unfamiliar]) |
||||
| Right Parietal Cortex (BA 7) & precuneus | 24 | −56 | 32 | 4.81 |
| Left lateral PFC (BA 45) | −50 | 36 | 2 | 3.17 |
Figure 3.
Sagittal, coronal, and transverse views of the statistical parametric map (SPM) rendered into standard stereotactic space and projected onto a glass brain. The main effect of reasoning (all reasoning trials minus baseline trials) activates a bilateral frontal, parietal, occipital, and left temporal network similar to that seen in previous studies (see Goel, 2007 for summary).
An examination of the main effects revealed that reasoning about familiar or specific material, versus reasoning about unfamiliar or nonspecific material, resulted in activation in left lateral prefrontal cortex (BA 45)1, right superior parietal lobule (BA 7), and left superior occipital gyrus (BA 19). The reverse comparison, reasoning trials with unfamiliar, or nonspecific material, versus reasoning trials with familiar, or specific material, resulted in activation in posterior cingulate gyrus (BA 30) and right orbital PFC (BA 25).
The main effect of determinacy revealed that indeterminate minus determinate trials activated right dorsolateral prefrontal cortex (BA 9), right ventral lateral prefrontal cortex (BA 47; cluster size = 608). (There was also some corresponding activation in left lateral prefrontal cortex (BA 47) (−34, 22, −8; Z. = 2.65; cluster size = 207), but it did not read significance.) The reverse comparison, determinate trials versus indeterminate trials, resulted in activation only in right inferior parietal lobule (BA 40).
Further, to directly test the prediction from the lesion data, with which we began, we restricted ourselves to trials involving unfamiliar or nonspecific material, and compared indeterminate trials with determinate trials (indeterminate – determinate). The results revealed bilateral activation in lateral prefrontal cortex, with right prefrontal cortex (BA 45/47) (42, 20, −8; Z. = 4.29; cluster size = 833 voxels) showing greater involvement than left prefrontal cortex (BA 45/47) (−34, 22 −8; Z. = 3.91; cluster size = 210).
However, given the potential for interaction between familiarity and indeterminacy, discussed above, and to show a dissociation in the involvement of left and right prefrontal cortex, we focused our analysis on the interaction. A comparison of the difference between indeterminate unfamiliar trials versus indeterminate familiar trials with the difference between determinate unfamiliar trials versus determinate familiar trials ([indeterminate unfamiliar - indeterminate familiar] - [determinate unfamiliar - determinate familiar]) revealed activation in right ventrolateral PFC (BA 47) (extending into insula), and a corresponding nonsignificant activation in left ventrolateral PFC (BA 47) (−34, 24, −10; Z. = 2.86). The reverse comparison, the difference between indeterminate familiar trials versus indeterminate unfamiliar trials with the difference between determinate familiar trials versus determinate unfamiliar trials, ([indeterminate familiar - indeterminate unfamiliar] - [determinate familiar - determinate unfamiliar]), resulted in activation in left inferior prefrontal cortex (BA 45) and right parietal cortex (BA 7) and precuneus.
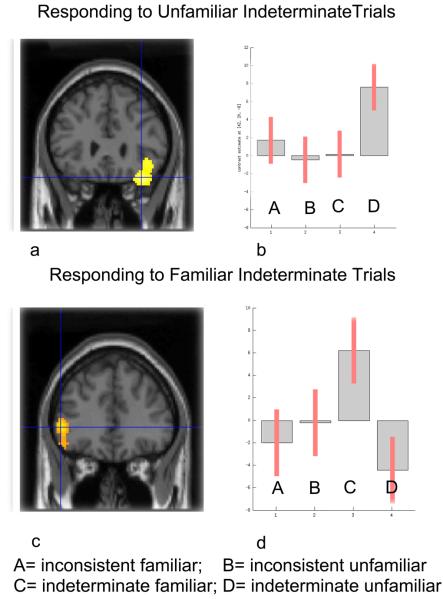
There is, however, a potential confound in this analysis. The determinate trials contain both valid and invalid (inconsistent) items whereas the indeterminate trials consist of only invalid items. To discount for the possibility that the above results are an artifact of an imbalance of “yes” and “no” responses in the determinate and indeterminate conditions, we again carried out a determinacy by familiarity interaction analysis, but this time using only the invalid subset of determinate trials (i.e. inconsistent trials). The results were very similar to those reported in the above analysis. A comparison of the difference between indeterminate unfamiliar trials versus indeterminate familiar trials with the difference between inconsistent unfamiliar trials versus inconsistent familiar trials, ([indeterminate unfamiliar - indeterminate familiar] - [inconsistent unfamiliar - inconsistent familiar]), results in activation in right ventrolateral PFC (BA 47) extending into insula (see Figure 4a). The reverse comparison, the difference between indeterminate familiar trials minus indeterminate unfamiliar trials versus inconsistent familiar minus inconsistent unfamiliar trials ([indeterminate familiar - indeterminate unfamiliar] - [inconsistent familiar - inconsistent unfamiliar]) resulted in activation in left inferior PFC (BA 45) and right parietal cortex (BA 7) and precuneus (see Figure 4b).
Figure 4.
A statistical parametric map (SPM) rendered into standard stereotactic space and superimposed on to cronal sections of a magnetic resonance image (MRI) which is itself in standard space. (a) A comparison of the difference between indeterminate unfamiliar trials versus indeterminate familiar trials with the difference between determinate unfamiliar trials versus determinate familiar trials ([indeterminate unfamiliar - indeterminate familiar] - [inconsistent unfamiliar - inconsistent familiar]) results in activation in right ventrolateral PFC (36, 28, −22; z= 3.65) (BA 47), extending into insula. (b) Condition specific parameter (beta) estimates show that the right PFC is selectively responding to indeterminate unfamiliar trials, where the argument is logically indeterminate and there is no belief-bias information in the conclusion to queue a response. (c) The reverse comparison, the difference between determinate unfamiliar trials minus determinate familiar trials versus indeterminate unfamiliar minus indeterminate familiar trials, ([inconsistent unfamiliar - inconsistent familiar] - [indeterminate unfamiliar - indeterminate familiar]), resulted in activation in left inferior prefrontal cortex (−50, 36, 2; z = 3.17) (BA 45). (d) Condition specific parameter (beta) estimate show that the left PFC is selectively responding to indeterminate familiar trials, where the argument is logically indeterminate but there is belief-bias information in the conclusion on which to base (potentially incorrect) responses.
DISCUSSION
Both the behavioral and fMRI results are consistent with our a priori predictions. Subjects performed significantly above chance on all reasoning trials but were selectively impaired on the indeterminate familiar content trials. The explanation of this selective reduction in accuracy is that subjects' have beliefs about the content of the material they are reasoning about and these beliefs are facilitating a response that will be incorrect half of the time. That is, any belief laden conclusion that leads subjects to respond in the affirmative to indeterminate items (e.g. “diamonds are more expensive than apples”) will reduce accuracy in these trials. The subject's attempt to maintain the indeterminacy of the conclusion will be frequently overcome by belief-bias effects. The MRI data shows us the functional anatomy of this process.
Specifically, modulating towards indeterminate unfamiliar trials ([indeterminate unfamiliar - indeterminate familiar] - [determinate unfamiliar - determinate familiar]) results in activation in right ventral lateral PFC (BA 47), extending into the insula. Parameter estimates show that right prefrontal cortex is selectively responding to indeterminate unfamiliar trials, where the conclusion is logically indeterminate and there is no belief-bias information in the conclusion to queue a response that would undermine the indeterminacy. The reverse comparison, modulating towards indeterminate familiar trials ([indeterminate familiar - indeterminate unfamiliar] - [determinate familiar - determinate unfamiliar]) results in activation in left inferior PFC (BA 45/47) and right parietal cortex (BA 7/31). Parameter estimates show that the left prefrontal cortex is selectively responding to indeterminate familiar trials where the conclusion is logically indeterminate, but there is belief-bias information in the conclusion on which to base a (incorrect) response. These results are consistent with the lesion data that formed the basis of our a priori hypothesis.
We explain these results in terms of an interplay between the left PFC's propensity to overinterpret information (Gazzaniga, 2000) and the right PFC's capacity to inhibit or temper this overinterpretation by maintaining ambiguous or indeterminate mental representations of the problem/situation (Goel & Vartanian, 2005). We are continually sampling the world for information. The information that we receive is always insufficient to predict future states and is further limited by our perspective and sensory apparatus. The function of the left hemisphere “interpreter” is to complete the pattern (often prematurely and incorrectly). Thus, in the case of trials with indeterminate logical form but with familiar content that subjects will have beliefs about, the left PFC generates (incorrect) responses based upon beliefs rather than logical analysis. The functions of the right-hemisphere seem to include (1) inhibiting or preventing the left hemisphere from completing patterns prematurely (i.e. drawing premature conclusions), and (2) maintaining the indeterminate/incomplete pattern so it can be further evaluated. Thus, in the case of trials with indeterminate logical form but with unfamiliar content (that subjects can have no beliefs about), there are no content cues/patterns for the left PFC to complete. In these cases, the right PFC is more successful in maintaining logical indeterminacy.
These results serve to highlight the importance of thinking in terms of hemispheric specialization rather than left hemisphere dominance for reasoning, and more generally, cognitive processes. While the left prefrontal cortex is critical to reasoning processes, our data suggest that this is only half of the story. The right hemisphere, more particularly the right ventrolateral prefrontal cortex has critical roles to play in reasoning processes that go beyond “the perception of visual information” (Corballis, 2003). One of these roles is to actively maintain indeterminate/incomplete patterns and prevent the left prefrontal cortex from premature completion, until they can be fully evaluated.
The highlighting of indeterminacy of information as a modulating factor between hemispheres is a new proposal within the neuropsychology literature. Traditional hemispheric distinctions have been modality based, typically involving linguistic (left hemisphere) versus visuospatial (right hemisphere) processing of information (Springer & Deutsch, 1998). Our proposal is consistent with more recent studies that emphasize the importance of specific processing demands, rather than the modality of information in determining hemispheric lateralization (Stephan et al., 2003). For example, processing of literal, concrete sentences involves the left hemisphere while the processing of more abstract, figurative language also engages right-hemisphere systems (Beeman, Bowden, & Gernsbacher, 2000; Goel & Dolan, 2001a; Wapner, Hamby, & Gardner, 1981). Indeed, one can argue the more abstract, figurative language is less precise and determinate than more concrete, literal language.
It may be possible to reconcile the issue of determinacy of information that we claim serves to modulate processing across left and right prefrontal cortex with the more traditional claims of linguistic and visuospatial processing. One might argue that linguistic propositions typically have specific truth values while pictorial or visuospatial representations typically do not (Fodor, 1975). For example, the proposition “John is wearing a red shirt” can be either true or false. It has a determinant truth value associated with it. A pictorial representation of John wearing a red shirt is also a representation of John sitting down, being 5′4″ tall, wearing a hat, standing on his head, etc.. There is no one specific truth value associated with it. The pictorial representation may be considered indeterminate by virtue of having multiple propositional contents. If this proposal is correct, it may be possible to reinterpret some of the classic left-right hemisphere findings in terms of determinate and indeterminate information or propositional content (Goel, 1995).
It is also worth noting that there are a growing number of studies in the decision-making literature involving uncertainty (Hsu, Bhatt, Adolphs, Tranel, & Camerer, 2005; Huettel, 2006; Huettel, Song, & McCarthy, 2005). This notion of uncertainty typically involves gambling or economic scenarios with quantifiable risk/reward trade-offs, and show more complex patterns of activation, but include right prefrontal cortex and insula (Huettel et al., 2005). Our notion of indeterminacy is different, or perhaps just simpler, in that, while there is a lack of information (literally no fact of the matter), there is no quantification or risk-reward trade-off involved.
Finally, our results also serve to explain some discrepancies in the involvement of left and right prefrontal cortex in logical reasoning tasks as reported by various neuroimaging studies (Goel, 2007). Some studies report only left PFC activation (Goel et al., 1997, 1998; Noveck et al., 2004), others report only right PFC activation (Parsons & Osherson, 2001), while still others report bilateral PFC activation (Acuna et al., 2002; Canessa et al., 2005; Goel et al., 2000; Goel, Makale et al., 2004; Knauff et al., 2002; Prado & Noveck, 2007). We already know that some of these discrepancies can be accounted for by the nature of the stimuli, presence of conflict detection and/or resolution and content familiarity (Goel, 2007). The current results further suggest that not controlling for determinate and indeterminate trials across conditions, may also introduce unintended confounds.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
There was also corresponding activation in right lateral prefrontal cortex (BA 45) (54, 36, 6; Z = 2.89), but it did not reach threshold.
References
- Acuna BD, Eliassen JC, Donoghue JP, Sanes JN. Frontal and Parietal Lobe Activation during Transitive Inference in Humans. Cerebral Cortex. 2002;12(12):1312–1321. doi: 10.1093/cercor/12.12.1312. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Nonlinear Spatial Normalization using Basis Functions. Human Brain Mapping. 1999;7(4):254–266. doi: 10.1002/(SICI)1097-0193(1999)7:4<254::AID-HBM4>3.0.CO;2-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeman MJ, Bowden EM, Gernsbacher MA. Right and left hemisphere cooperation for drawing predictive and coherence inferences during normal story comprehension. Brain & Language. 2000;71(2):310–336. doi: 10.1006/brln.1999.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canessa N, Gorini A, Cappa SF, Piattelli-Palmarini M, Danna M, Fazio F, Perani D. The effect of social content on deductive reasoning: an fMRI study. Human Brain Mapping. 2005;26(1):30–43. doi: 10.1002/hbm.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corballis PM. Visuospatial processing and the right-hemisphere interpreter. Brain & Cognition. 2003;53(2):171–176. doi: 10.1016/s0278-2626(03)00103-9. [DOI] [PubMed] [Google Scholar]
- Damasio H, Damasio AR. Lesion Analysis in Neuropsychology. Oxford University Press; Oxford: 1989. [Google Scholar]
- Evans AC, Collins DL, Mills SR, Brown ED, Kelly RL, Peters TM. 3D Statistical Neuroanatomical Models from 305 MRI Volumes; Proc. IEEE-Nuclear Science Symposium and Medical Imaging Conference; 1993. pp. 1813–1817. [Google Scholar]
- Evans JSBT, Barston J, Pollard P. On the conflict between logic and belief in syllogistic reasoning. Memory and Cognition. 1983;11:295–306. doi: 10.3758/bf03196976. [DOI] [PubMed] [Google Scholar]
- Fangmeier T, Knauff M, Ruff CC, Sloutsky V. FMRI evidence for a three-stage model of deductive reasoning. Journal of Cognitive Neuroscience. 2006;18(3):320–334. doi: 10.1162/089892906775990651. [DOI] [PubMed] [Google Scholar]
- Fodor JA. The Language of Thought. Harvard University Press; Cambridge, Massachusetts: 1975. [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JB, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: A general approach. Human Brain Mapping. 1995;2:189–210. [Google Scholar]
- Gazzaniga MS. Cerebral specialization and interhemispheric communication: does the corpus callosum enable the human condition? Brain. 2000;123(Pt 7):1293–1326. doi: 10.1093/brain/123.7.1293. [DOI] [PubMed] [Google Scholar]
- Gazzaniga MS, Ivry RB, Mangun GR. Cognitive Neuroscience: The Biology of the Mind. W. W. Norton; NY: 1998. [Google Scholar]
- Goel V. Sketches of Thought. MIT Press; Cambridge, MA: 1995. [Google Scholar]
- Goel V. Cognitive Neuroscience of Deductive Reasoning. In: Holyoak K, Morrison R, editors. Cambridge Handbook of Thinking and Reasoning. Cambridge University Press; Cambridge: 2005. pp. 475–492. [Google Scholar]
- Goel V. Anatomy of deductive reasoning. Trends in the Cognitive Sciences. 2007;11(10):435–441. doi: 10.1016/j.tics.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Goel V, Buchel C, Frith C, Dolan RJ. Dissociation of Mechanisms Underlying Syllogistic Reasoning. NeuroImage. 2000;12(5):504–514. doi: 10.1006/nimg.2000.0636. [DOI] [PubMed] [Google Scholar]
- Goel V, Dolan RJ. Functional Neuroanatomy of Humor: Segregating Cognitive & Affective Components. Nature Neuroscience. 2001a;4(3) doi: 10.1038/85076. [DOI] [PubMed] [Google Scholar]
- Goel V, Dolan RJ. Functional Neuroanatomy of Three-Term Relational Reasoning. Neuropsychologia. 2001b;39(9):901–909. doi: 10.1016/s0028-3932(01)00024-0. [DOI] [PubMed] [Google Scholar]
- Goel V, Dolan RJ. Explaining modulation of reasoning by belief. Cognition. 2003;87(1):B11–22. doi: 10.1016/s0010-0277(02)00185-3. [DOI] [PubMed] [Google Scholar]
- Goel V, Dolan RJ. Differential involvement of left prefrontal cortex in inductive and deductive reasoning. Cognition. 2004;93(3):B109–121. doi: 10.1016/j.cognition.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Goel V, Gold B, Kapur S, Houle S. The Seats of Reason: A Localization Study of Deductive & Inductive Reasoning using PET (O15) Blood Flow Technique. NeuroReport. 1997;8(5):1305–1310. doi: 10.1097/00001756-199703240-00049. [DOI] [PubMed] [Google Scholar]
- Goel V, Gold B, Kapur S, Houle S. Neuroanatomical Correlates of Human Reasoning. Journal of Cognitive Neuroscience. 1998;10(3):293–302. doi: 10.1162/089892998562744. [DOI] [PubMed] [Google Scholar]
- Goel V, Makale M, Grafman J. The hippocampal system mediates logical reasoning about familiar spatial environments. Journal of Cognitive Neuroscience. 2004;16(4):654–664. doi: 10.1162/089892904323057362. [DOI] [PubMed] [Google Scholar]
- Goel V, Shuren J, Sheesley L, Grafman J. Asymmetrical involvement of frontal lobes in social reasoning. Brain. 2004;127(Pt 4):783–790. doi: 10.1093/brain/awh086. [DOI] [PubMed] [Google Scholar]
- Goel V, Tierney M, Sheesley L, Bartolo A, Vartanian O, Grafman J. Hemispheric specialization in human prefrontal cortex for resolving certain and uncertain inferences. Cerebral Cortex. 2007;17(10):2245–2250. doi: 10.1093/cercor/bhl132. [DOI] [PubMed] [Google Scholar]
- Goel V, Vartanian O. Dissociating the roles of right ventral lateral and dorsal lateral prefrontal cortex in generation and maintenance of hypotheses in set-shift problems. Cereb Cortex. 2005;15(8):1170–1177. doi: 10.1093/cercor/bhh217. [DOI] [PubMed] [Google Scholar]
- Heckers S, Zalesak M, Weiss AP, Ditman T, Titone D. Hippocampal activation during transitive inference in humans. Hippocampus. 2004;14(2):153–162. doi: 10.1002/hipo.10189. [DOI] [PubMed] [Google Scholar]
- Hsu M, Bhatt M, Adolphs R, Tranel D, Camerer CF. Neural systems responding to degrees of uncertainty in human decision-making. Science. 2005;310(5754):1680–1683. doi: 10.1126/science.1115327. [DOI] [PubMed] [Google Scholar]
- Huettel SA. Behavioral, but not reward, risk modulates activation of prefrontal, parietal, and insular cortices. Cognitive Affective Behavioural Neuroscience. 2006;6(2):141–151. doi: 10.3758/cabn.6.2.141. [DOI] [PubMed] [Google Scholar]
- Huettel SA, Song AW, McCarthy G. Decisions under uncertainty: probabilistic context influences activation of prefrontal and parietal cortices. Journal of Neuroscience. 2005;25(13):3304–3311. doi: 10.1523/JNEUROSCI.5070-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knauff M, Fangmeier T, Ruff CC, Johnson-Laird PN. Reasoning, models, and images: behavioral measures and cortical activity. Journal of Cognitive Neuroscience. 2003;15(4):559–573. doi: 10.1162/089892903321662949. [DOI] [PubMed] [Google Scholar]
- Knauff M, Kassubek J, Mulack T, Greenlee MW. Cortical activation evoked by visual mental imagery as measured by fMRI. Neuroreport. 2000;11(18):3957–3962. doi: 10.1097/00001756-200012180-00011. [DOI] [PubMed] [Google Scholar]
- Knauff M, Mulack T, Kassubek J, Salih HR, Greenlee MW. Spatial imagery in deductive reasoning: a functional MRI study. Brain Research Cognitive Brain Research. 2002;13(2):203–212. doi: 10.1016/s0926-6410(01)00116-1. [DOI] [PubMed] [Google Scholar]
- Noveck IA, Goel V, Smith KW. The neural basis of conditional reasoning with arbitrary content. Cortex. 2004;40(45):613–622. doi: 10.1016/s0010-9452(08)70157-6. [DOI] [PubMed] [Google Scholar]
- Parsons LM, Osherson D. New Evidence for Distinct Right and Left Brain Systems for Deductive versus Probabilistic Reasoning. Cerebral Cortex. 2001;11(10):954–965. doi: 10.1093/cercor/11.10.954. [DOI] [PubMed] [Google Scholar]
- Prado J, Noveck IA. Overcoming perceptual features in logical reasoning: a parametric functional magnetic resonance imaging study. Journal of Cognitive Neuroscience. 2007;19(4):642–657. doi: 10.1162/jocn.2007.19.4.642. [DOI] [PubMed] [Google Scholar]
- Springer SP, Deutsch G. Left Brain, Right Brain. 5th ed. W. H. Freeman; San Francisco, CA: 1998. [Google Scholar]
- Stephan KE, Marshall JC, Friston KJ, Rowe JB, Ritzl A, Zilles K, Fink GR. Lateralized cognitive processes and lateralized task control in the human brain. Science. 2003;301(5631):384–386. doi: 10.1126/science.1086025. [DOI] [PubMed] [Google Scholar]
- Wapner W, Hamby S, Gardner H. The Role of the Right Hemisphere in the Apprehension of Complex Linguistic Materials. Brain And Language. 1981;14:15–33. doi: 10.1016/0093-934x(81)90061-4. [DOI] [PubMed] [Google Scholar]
- Wolford G, Miller MB, Gazzaniga M. The left hemisphere's role in hypothesis formation. Journal of Neuroscience. 2000;20(6):1–4. doi: 10.1523/JNEUROSCI.20-06-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsley KJ, Friston KJ. Analysis of fMRI time-series revisited - again. NeuroImage. 1995;2:173–181. doi: 10.1006/nimg.1995.1023. [DOI] [PubMed] [Google Scholar]