Abstract
This study examined the utility of structural and functional MRI at 1.5 and 3 Tesla (T) in the pre-surgical evaluation and prediction of post-surgical cognitive outcome in temporal lobe epilepsy (TLE). Forty-nine patients undergoing presurgical evaluation for temporal lobe (TL) resection and twenty-five control subjects were studied. Patients completed standard pre-surgical evaluations including, intracarotid amobarbital test (IAT) and neuropsychological testing. During functional imaging, subjects performed a complex visual scene-encoding task. High-resolution structural MRI scans were used to quantify hippocampal volumes. Both structural and functional imaging successfully lateralized the seizure focus and correlated with IAT memory lateralization, with improvement for functional imaging at 3T as compared to 1.5T. Ipsilateral structural and functional MRI data was related to cognitive outcome and greater functional asymmetry was related to earlier age of onset. These findings support continued investigation of the utility of MRI and fMRI in the presurgical evaluation of TLE.
Keywords: Temporal Lobe Epilepsy, Epilepsy Surgery, fMRI, Neuropsychological Outcome, Wada, IAT
Introduction
Approximately 30 to 40 percent of patients with mesial temporal lobe epilepsy (TLE) continue to have disabling seizures despite treatment with antiepileptic medicines. Anterior temporal lobe resective surgery dramatically improves seizure control in these medically refractory patients, with 60–70 percent of patients becoming seizure free, with the exception of simple partial seizures [1]. However, a frequent complication of the resection is memory deficit [2, 3]. Accordingly, to try to optimize the risks and benefits of temporal lobectomy, a variety of techniques are used during the presurgical evaluation to confirm seizure lateralization and attempt to predict cognitive sequelae (i.e. language and memory outcome) of the procedure. Intracarotid Amobarbital Testing (IAT), neuropsychological testing, structural MRI and more recently functional MRI all contribute to the pre-surgical evaluation, lateralization of cognitive functions, and counseling of the patient as to the expected cognitive outcome with respect to language and memory.
Several studies have shown that hippocampal volumetric asymmetry in TLE corresponds to the side of seizure origin, and that hippocampal volume is reduced compared to normal controls [4–7]. Some authors have suggested that hippocampal volume also predicts functional outcomes [8–10]. Neuropsychological deficits have been well-defined in the literature for both pre-surgical [11, 12] and post-surgical cognitive functioning [13–16] revealing subgroups that exhibit increases and decreases (more so in left TLE patients) in verbal memory, which are frequently independent of post-surgical seizure control.
Neuropsychological functioning before and after surgery is also affected by the developmental stage during which the seizures began. Earlier onset has been associated with lower scores on retrograde memory measures [17] and generalized compromise of cognitive functioning [18] in the TLE population, while later onset has been associated with post-surgical naming difficulty [19], but also good post-surgical seizure control [20]. Early onset may be associated with neural reorganization which can result in use of brain areas that are not optimized for certain cognitive functions, but may also contribute to the better post-surgical outcome due to reduced risk of removing functioning brain tissue. Likewise, later onset may be associated with less reorganization with resulting better pre-surgical performance, but also a greater post-surgical decline due to resection of functional brain regions [18, 21, 22]. Establishing a relationship between these presurgical variables and structural and functional imaging measures would help to establish the role imaging can play in contributing to the pre-surgical evaluation.
IAT lateralization is widely accepted as the “gold-standard” for pre-surgical lateralization of language and memory functioning. This procedure has a number of limitations, including risk of medical complications, cost and personnel requirements, inability to retest, somnolence, limited time for testing, a small stimulus set, and inter-site procedural differences [For a review see: 23]. Additionally, IAT memory measures have been shown to be less reliable than IAT language measures [24]. For these reasons, there is an increasing interest in supplementing or replacing the IAT with a test that is safer, more valid and more reliable. A growing number of epilepsy centers are now investigating the possible replacement of the IAT with non-invasive imaging [25]. In the investigation of language, fMRI has been demonstrated to successfully lateralize function, correlate with IAT [26], and provide good predictive power [26–28] and reliability [29]. However, assessing lateralization of memory functions and predicting post-surgical memory outcome has been less successful.
Prior studies have demonstrated activation in the TL (posterior hippocampus, parahippocampus, and fusiform gyrus) of healthy controls during explicit memory encoding of novel complex scenes during fMRI at 1.5 Tesla (1.5T) [30] and at 3 Tesla (3T) [31]. Studies in nonclinical populations have revealed differing activation patterns in the anterior and posterior aspects of the hippocampus, relational processing (associative, conceptual or semantic tasks) eliciting more anterior and novelty eliciting more posterior activation [32]. In the TLE patient population, TL activation during memory tasks has been shown to contribute to the prediction of seizure outcome [33] and several measures of memory outcome, including scene memory [34], navigation memory [35] and verbal memory [36, 37]. Additionally, several studies have shown concordance between TL activation and IAT memory testing [38–40] and have begun to optimize procedures for obtaining more reliable TL fMRI activation and tailoring analyses to patient factors [41].
The overwhelming majority of memory fMRI studies in TLE to date have been completed using 1.5T MRI scanners. However, over the past several years clinical MRI at 3T has become widely available. Only one published study has examined TLE patients (nine total; six with post-surgical data) at 3T, showing good concordance between IAT and memory asymmetry in 50% of subjects [42]. The three main objectives of this study are: 1) to examine the relationship between commonly used pre-surgical clinical data (seizure laterality, age of onset, neuropsychological functioning, IAT) and fMRI memory data, 2) to use a large patient cohort to determine whether structural and functional asymmetries provide predictive value for memory outcome following temporal lobectomy and 3) to assess the value of increased sensitivity and increased spatial resolution of MRI at a 3T as compared to 1.5T.
Methods
Subjects
Patients with refractory nonlesional or lesional TLE undergoing presurgical evaluation for temporal lobectomy were recruited from the Penn Epilepsy Center at the Hospital of the University of Pennsylvania and the Comprehensive Epilepsy Center at Thomas Jefferson University. A combination of clinical MRI findings, EEG, IAT, and neuropsychological testing was used to lateralize the side of seizure. Patients with brain tumors, traumatic brain injuries, vascular lesions involving the temporal lobe, extratemporal epilepsy, prior temporal lobectomy, or contraindications to MRI were excluded. Patients with severe mental retardation who were likely to be unable to cooperate with the MRI examination were also excluded.
Twenty patients completed the study at 3T and twenty-nine patients at 1.5T. Side of seizure, age, gender and handedness are summarized in table 1. The subjects with bitemporal TLE did not undergo surgery and were excluded from the analysis. The 1.5T cohort was limited to those participants from a previous study who had structural images with spatial resolution suitable for hippocampal volumetry [34]. Thirty-five patients repeated the scene-encoding paradigm behavioral task approximately six to twelve months after surgery.
Table 1.
Subject Demographic Data
| Group | N | Age (mean ± S.D.) |
Gender | Handedness | Years with Seizures (mean ± S.D.) |
Seizure Laterality |
IAT Memory Dominance |
Structural Asymmetry (> ± 2 S.D. from mean control asymmetry) |
Seizure Free Outcome (≥ 1 year post- surgery) |
|---|---|---|---|---|---|---|---|---|---|
| 1.5T | 29 | 37.3 ± 9.7* |
*Male: 12 | Right: 26 | 20.9 ± 11.3 | Right: 16 | Right: 7 | Rt > Lt: 6 | Class I: 18 |
| (23 underwent, surgery) |
Female: 17 | Left: 3 | Left: 11 | Left: 15 | Lt > Rt: 9 | Class II: 2 | |||
| Bilateral: 2 | Bilateral: 1 | Class III: 1 | |||||||
| No IAT: 6 | Class IV: 0 | ||||||||
| No Sx.: 6 | |||||||||
| Unknown: 2 | |||||||||
| 3T | 20 | 40.0 ± 13.5 |
Male: 4 | Right: 16 | 23.3 ± 14.8 | Right: 8 | Right: 6 | Rt > Lt: 7 | Class I: 10 |
| (16 underwent surgery) |
Female: 16 | Left: 3 | Left: 10 | Left: 6 | Lt > Rt: 3 | Class II: 4 | |||
| Ambidextrous: 1 | Bilateral: 2 | Bilateral: 3 | Class III: 0 | ||||||
| No IAT: 4 | Class IV: 2 | ||||||||
| Incomplete IAT: 1 | No Sx.: 4 | ||||||||
No difference between the 1.5T and 3T group on age, years with seizures and gender distribution.
Twenty-five control subjects (10 scanned at 1.5T and 15 scanned at 3T) were recruited from the University of Pennsylvania community. Subjects with a history of neurologic illness, psychiatric disorders or contraindications to MRI were excluded. All subjects provided written informed consent for the study procedures in accordance with the guidelines of the Institutional Review Board at the University of Pennsylvania.
fMRI Data Acquisition
All scanning protocols involved a brief localizer scan, high-resolution T1-weighted 3D anatomical imaging, and gradient-echo echo-planar imaging for BOLD contrast fMRI. Adjustable pads were used to restrict head motion during MRI scanning. Imaging was conducted on a 3T Siemens Trio MRI scanner (Siemens, Germany) or a 1.5T GE Signa MRI scanner (General Electric, USA), both using a product T/R head coil. High-resolution anatomical MRI scans (3T: 0.98×0.98×1mm; 1.5T: 0.94×0.94×1.5mm) were collected both for volumetric measurement and for localization of functional data. Functional data were collected using a standard BOLD imaging sequence. At 3T, TR = 3000ms, TE = 30 ms, 40 axial slices and 3 mm isotropic voxels (27 mm3 voxel volume). At 1.5T, TR = 2000ms, TE = 50ms, 20 axial slices 3.75× 3.75×5.0 mm voxels (70.3 mm3 voxel volume).
fMRI Memory Task
Memory for complex scenes was assessed using a block-design experiment, with seven blocks of visual scenes alternating with seven blocks of a repeated control scene. Scenes were selected from a Photodisc® (PhotoDisc, Inc, Seattle WA) thumbnail archive to be difficult to encode using an exclusively verbal strategy. A single randomly retiled scene was shown during the control condition. Novel visual scenes were presented for 3600ms, with a 400ms interstimulus interval, for 9 scenes per 36-second block, for a total of 63 images. At 1.5T, 6 blocks of novel visual scenes (alternating with 6 control blocks) were presented for 3500ms, with a 500ms interstimulus interval, for 10 scenes per 40-second block, for a total of 60 images [34]. Minor changes in timing between the task used in the 1.5T and 3T scanner were implemented to match the stimulus presentation time with the image acquisition timing. Stimulus presentation was performed during BOLD scanning and the recognition test was performed in the scanner (but without concurrent image acquisition) at 3T or outside the scanner at 1.5T. The behavioral task paradigm was implemented in E-prime (Psychology Software Tools, Pittsburgh, PA) running on a PC laptop. In scanner responses were made using a fiberoptic response system (FORP, Current Designs, Philadelphia PA). Images were back-projected onto a Mylar screen that the subject views through a mirror mounted on the head coil.
Subjects were instructed to remember the novel scenes for a subsequent recognition test, and to passively view the scrambled scene. Subjects performed a self-paced forced-choice recognition task after stimulus presentation, indicating whether or not they had previously viewed the target scene. Memory function was calculated using a discrimination score for the scene recognition task, which was defined by the Two-High Threshold Theory: [(# correct/total possible correct) − (# false positives/total possible false positives)] [43]. Thirty-five subjects returned post-surgery to complete the scene memory task and recognition test. The post-surgical testing included an alternative version of the complex scene-encoding stimulus in order to avoid practice effects. Change in discrimination score from pre to post-surgery (post – pre) was calculated as an index of memory outcome.
MRI Data Analysis
Hippocampal Volumetry
The hippocampal head, body, and tail were segmented manually in ITK-SNAP [44]. Hippocampal volumes were manually determined from structural MRI by two authors (DMH and JP) with training provided by a neuroradiologist and an epileptologist (RW and SG, respectively). Anatomical boundaries were determined using published guidelines [45, 46]. Neuroanatomical atlases were used to verify boundaries during segmentation [47, 48]. Volumetric asymmetry ratios (ARs) were calculated with the following formula: [(contralateral volume−ipsilateral volume)/(contralateral volume+ipsilateral volume)].
Hippocampal Segmentation Reliability
Inter-rater reliability for the hippocampal segmentations was calculated using the Dice Similarity Coefficient and was comparable to previously reported reliability for hippocampal segmentation [49, 50]. In subjects with TLE, the Dice Similarity Coefficient was 81% for ipsilateral hippocampal volumes and 81% for contralateral hippocampal volumes. The Dice Similarity Coefficient for the normal control subjects was an average of 83% for the right hippocampus and 84% for the left hippocampus.
Functional Activation
fMRI data were analyzed using VoxBo (www.voxbo.org), according to the methods outlined in previously published data [31, 34]. A simple boxcar function was used to perform the cognitive subtraction analysis (task condition - control condition). Statistical parametric maps of activation associated with complex scene encoding were generated for each patient using a general linear model. Because both the absolute fMRI signal change and the t-score for task-correlated activity may be highly variable across subjects, we used the suprathreshold volume of activation for t>0 within each ROI as the primary measure of BOLD activation. Parallel analyses performed by weighting each activated voxel by its t-value [51] did not alter the findings.
Region of interest (ROI) analysis based on a published regional atlas in Talairach space [52] was used to quantify fMRI activation in individual subjects. Two ROIs were used: the hippocampus alone (H), and the hippocampus as well as the parahippocampal gyrus and fusiform gyrus (HPF). A paired samples t-test examining contralateral-ipsilateral functional asymmetry scores obtained with hand-drawn hippocampal masks (M=0.13, SD=0.22) and template hippocampal masks (M=0.10, SD=0.14) revealed no significant differences [t(14)=1.05, p=0.31].
For each ROI two asymmetry ratios were then calculated. First, the ROI activation in the hemisphere ipsilateral to seizure focus was subtracted from that of the hemisphere contralateral to the seizure focus, and then divided by the sum of both values [(contralateral − ipsilateral)/(contralateral + ipsilateral)]. Second, a left-right AR [(left hemisphere − right hemisphere)/(left hemisphere + right hemisphere)] was calculated for comparison of patient fMRI asymmetry with both control fMRI asymmetry and patient IAT lateralization.
Finally, based on our prior experience comparing fMRI results to clinical indices [34], we also examined absolute fMRI signal in the ipsilateral and contralateral hippocampal and HPF ROIs separately. A fractional activation ratio was calculated for the ipsilateral and contralateral hippocampi and HPF region. This was calculated by dividing the number of voxels with a positive activation by the number of voxels with activation data within the ROI.
The extent of coverage and the presence of a positive signal at each field strength was compared at 1.5T and 3T. The percentage of the area within the ROI (hippocampal and HPF) with detectable BOLD activation was greater in the hippocampal ROI at 1.5T than at 3T [ipsilateral, t(12)=3.1, p=0.009; contralateral, t(12)=3.1, p=0.009, equal variance not assumed], but equivalent within the HPF ROI. Despite this, there were more voxels with positive activation in the contralateral HPF region [t(34)=−2.6, p=0.025] at 3T.
Neuropsychological Testing
Neuropsychological testing was carried out before and after surgery, in many cases as part of the patient’s routine clinical evaluation. The neuropsychological testing data set was limited by different clinical practice standards across sites. For this reason all subjects did not receive the same pre and post-surgical tests, effectively reducing the number of subjects included in the analysis.
The memory tests included in this battery assessed a broad range of material-specific memory domains. Visuospatial memory functioning was examined using pre- to post-surgical change scores from the immediate and delayed Faces and Visual Reproduction subtests of the Wechsler Memory Scale III (WMS-III). Verbal memory functioning was examined using pre to post-surgical change scores from the immediate and delayed Logical Memory subtest of the WMS-III and the total learning and long delay of the California Verbal Learning Test-Version II (CVLT-II). Change scores were calculated by subtracting pre- from post-surgical performance. These tests were chosen from a database of neuropsychological tests in order to represent verbal and visual memory functioning. Additionally, these tests were completed on the highest percentage of included subjects, 20–30 out of a total of the 36, depending on the measure.
Reliable change indices (RCI) for subtests of the WMS-III (LMI, LMII, Faces I, Faces II) and the CVLT-II, were used to assess global change in memory [53, 54]. Each score was designated as −1 (decrease), 0 (no change), or 1 (increase) according to published RCIs. An RCI ratio was created for each subject who had at least five cognitive change scores. This ratio ranged from −1 (a decrease on all cognitive measures) to 1 (an increase on all cognitive measures).
To study the effect of age of onset on pre and post-surgical cognition and imaging variables, patients were divided into two groups. The early onset group (EO) was defined by onset of continuous seizures at or before age nine and the late onset group (LO) was defined by onset of continuous seizures after age nine, in accordance with the use of this variable in the epilepsy literature [21].
Statistical Analysis
Statistical analyses comparing structural and functional ROI data to other indices were carried out in SPSS (SPSS, Inc., Chicago, IL, USA). For functional data, the suprathreshold voxel counts and asymmetry scores for hippocampal and HPF ROIs were entered into the analyses. Independent samples t-tests were used to examine group differences on the scene memory test, structural and functional variables and demographic data. A one-way ANOVA was performed to compare structural and functional ARs for right and left-sided seizure subjects and controls. Paired t-tests compared pre to post-surgical change on the neuropsychological measures. Linear regression was used to examine the prediction of cognitive change with pre-surgical neuropsychological measures and pre-surgical imaging variables. Correlations were computed to compare structural and functional ARs and absolute activation measures with IAT laterality (Spearman’s rank) and measures of cognitive change.
Results
Scene Memory and Neuropsychological data
Behavioral performance on the scene memory task was examined in patients and controls. Comparisons of means revealed that patients performed worse than controls at both the pre-surgical [t(68)=−2.96, p=0.004] and post-surgical [t(57)=−4.32, p<0.001, equal variances not assumed] time points. Comparisons between patients with right-sided and left-sided seizures did not show differences on pre-surgical, post-surgical or change scores for the scene memory test.
Patients with left-sided seizure had a mean decline after left anterior temporal lobectomy on the four measures of verbal memory, WMS-III Logical Memory immediate (LM-I) and delayed (LM-II) and the CVLT long delay (CVLT-LD) and CVLT total learning (CVLT-TOT), although the group differences only reached significance for LM-I [t(23)=2.17, p=0.04] and CVLT-TOT [t(20)=2.26, p=0.036, equal variances not assumed]. Patients with right-sided seizure had a mean decline on the four measures of visual memory, WMS-III Visual Reproduction immediate (VR-I) and delay (VR-II) and Facial recognition immediate (Faces I) and delay (Faces II), although the group differences only reached significance on Faces II [t(18)=−2.09, p=0.05] (see Table 2).
Table 2.
Neuropsychological Change from Pre to Post-Surgery
| Neuropsychological Measure |
N | Left-sided Seizure | Right-sided Seizure | |||
|---|---|---|---|---|---|---|
| Mean | S.D. | Mean | S.D. | |||
| Tests of Verbal Memory |
Logical Memory I |
25 | −4.15* | 9.28 | 3.33 | 7.85 |
| Logical Memory II |
27 | −2.64 | 7.93 | 2.31 | 9.21 | |
| CVLT-Long Delay |
30 | −4.00 | 5.16 | −1.59 | 2.81 | |
| CVLT-Total | 28 | −15.15* | 15.08 | −4.20 | 9.54 | |
| Tests of Visual Memory |
Faces I | 20 | −3.82 | 6.06 | −1.00 | 6.78 |
| Faces II | 20 | 1.00 | 6.21 | −5.22* | 7.10 | |
| Visual Reproduction I |
26 | −0.77 | 18.12 | −1.31 | 17.09 | |
| Visual Reproduction II |
25 | 4.92 | 23.31 | −4.62 | 16.58 | |
Significant difference between left and right, p<0.05
Linear regression models were calculated for each of the eight cognitive measures. The pre-surgical score was entered as the predictor variable and the change score was entered as the dependent variable. The models for LM-I [F(1,24)=6.25, p=0.02], LM-II [F(1,26)=6.13, p=0.02], VR-I [F(1,25)=23.3, p<0.001] and VR-II [F(1,24)=7.43, p=0.012], all showed a significant prediction of cognitive change, with higher presurgical scores predicting greater post-surgical decline (see figure 1). The R2 adj values ranged from 17% to 47%. The model for the CVLT-LD only explained 7% of the variance in the change score [F(1,29)=3.09, p=0.09]. The model for Faces I and II, as well as CVLT-TOT, did not explain variance in the change score.
Figure 1.
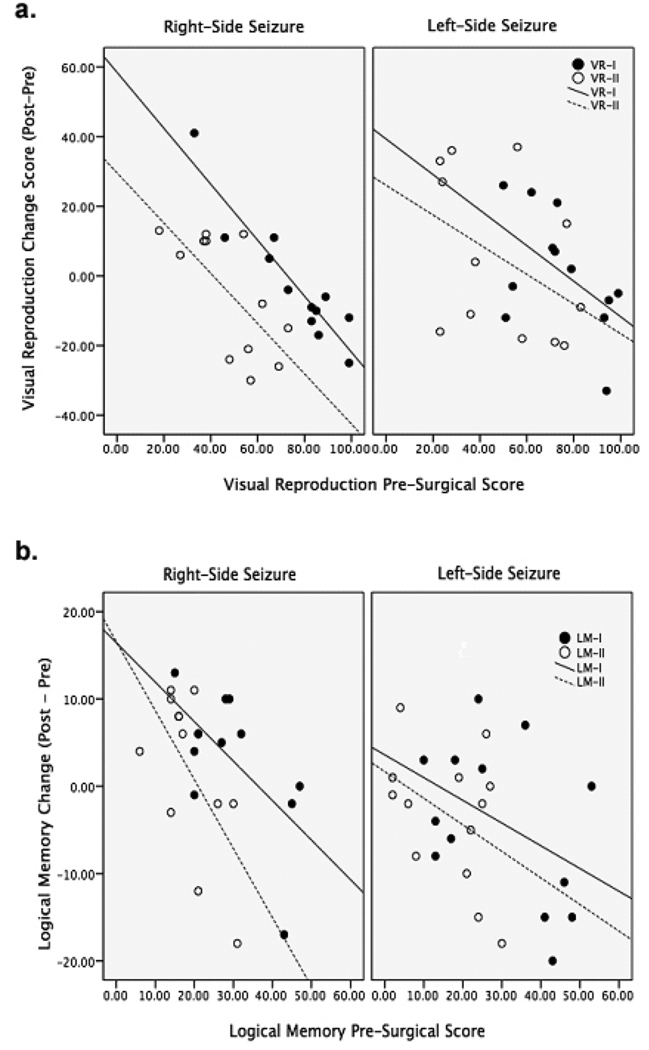
Structural and Functional Data
Unless otherwise noted, data is reported for the 1.5T and 3T patients, combined. When a separate comparison at each field strength revealed a different and significant result compared to the combined group, the results are presented. The patients scanned at 1.5T and 3T did not differ significantly by age [t(44)= −1.47, p=0.15], years of education [t(24)=1.28, p=0.213], age of onset [t(39)=−0.076, p=0.94], years with seizures [t(39)=−0.55, p=0.55] or gender [x2(2)=1.48, p=0.48].
Comparing patient and control data
Patients had significantly more asymmetric hippocampi than control subjects [F(2,66)=27.4, p<0.001], see table 3 and figure 2. Ipsilateral hippocampi were smaller than contralateral hippocampi in the patient group [t(38)=−5.0 p<0.001]. Additionally, both hippocampi were smaller in the patient groups when compared to a control hippocampus [right: F(2,63)=30.9, p<0.001; left: F(2,63)=46.4, p=<0.001].
Table 3.
Structural and functional asymmetry ratios at 1.5T and 3T.
| 1.5 T | 3T | |||||
|---|---|---|---|---|---|---|
|
Right Side Seizure |
Left Side Seizure |
Control |
Right Side Seizure |
Left Side Seizure |
Controls | |
| Structural AR | M= 0.12* | M= −0.18† | M= −0.03 | M=0.13* | M= −0.14*† | M= −0.01 |
| SD= 0.16 | SD= 0.16 | SD= 0.05 | SD=0.16 | SD= 0.15 | SD= 0.03 | |
| Hippocampal Functional AR |
M= 0.08 | M= 0.07 | M= 0.09 | M= 0.01 | M= −0.18*† | M= −0.003 |
| SD= 0.37 | SD= 0.50 | SD= 0.17 | SD= 0.12 | SD= 0.18 | SD= 0.04 | |
| HPF Functional AR |
M= −0.02 | M= −0.03 | M=0.05 | M= −0.05 | M= −0.25*† | M= −0.04 |
| SD= 0.25 | SD= 0.24 | SD=0.08 | SD= 0.08 | SD= 0.14 | SD= 0.05 | |
Asymmetry ratio calculation = (left−right)/(left+right)
Significant difference between patients and controls, p≤0.05.
Significant difference between left and right-sided seizure patients, p<0.05.
Figure 2.
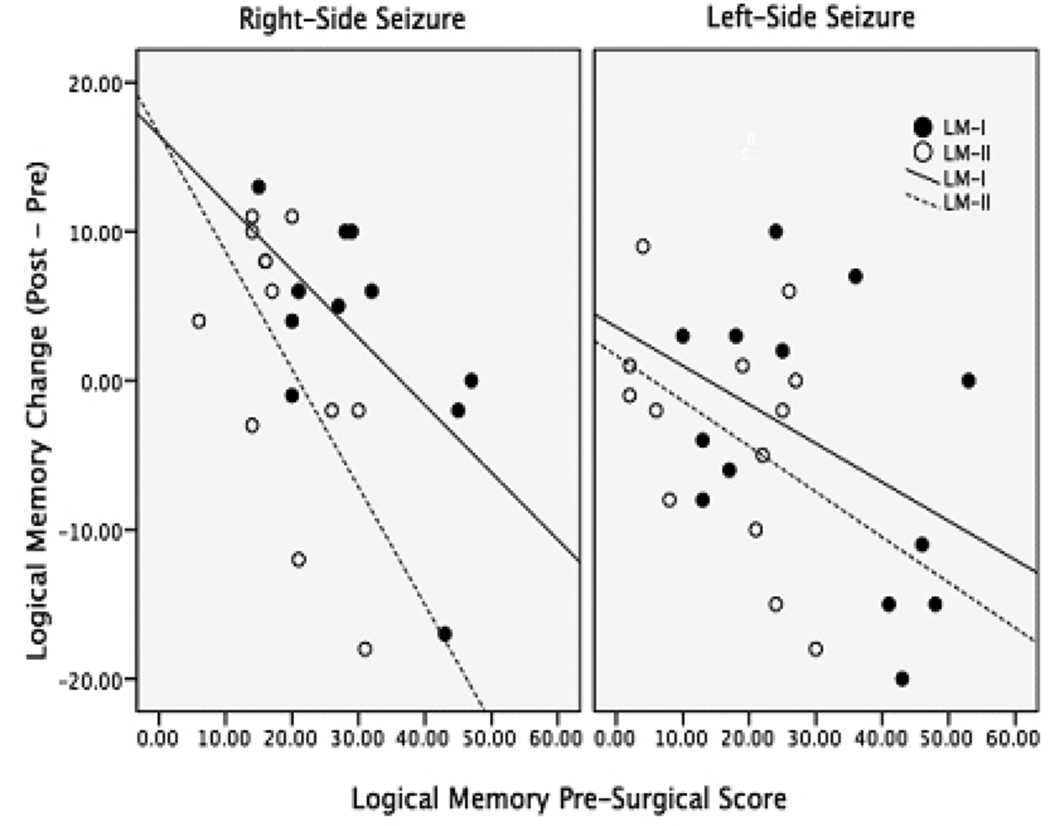
Hippocampal and HPF function was not more asymmetric in patients than controls for the combined group [H: F(2,67)=0.82, p=0.45; HPF: F(2,69)=3.1, p=0.052], but was significantly more asymmetric in patients than controls for the 3T group [H: F(2,27)=8.1, p=0.002; HPF: F(2,27)=14.8, p<0.001], see table 3. In this group, Bonferroni post hoc tests revealed a significant difference between the right and left-side seizure groups [CI95= 0.04 (lower) 0.36 (upper) p=0.01] and between the left-side seizure group and controls [CI95= −0.31 (lower) −0.06 (upper) p=0.003] for hippocampal function. There was also a difference between right and left-sided seizure groups [CI95= 0.07 (lower) 0.33 (upper) p=0.002] and between the left-side seizure group and controls [CI95=0.12 (lower) 0.31 (upper) p<0.001] for HPF function.
Comparing imaging to standard pre-surgical data
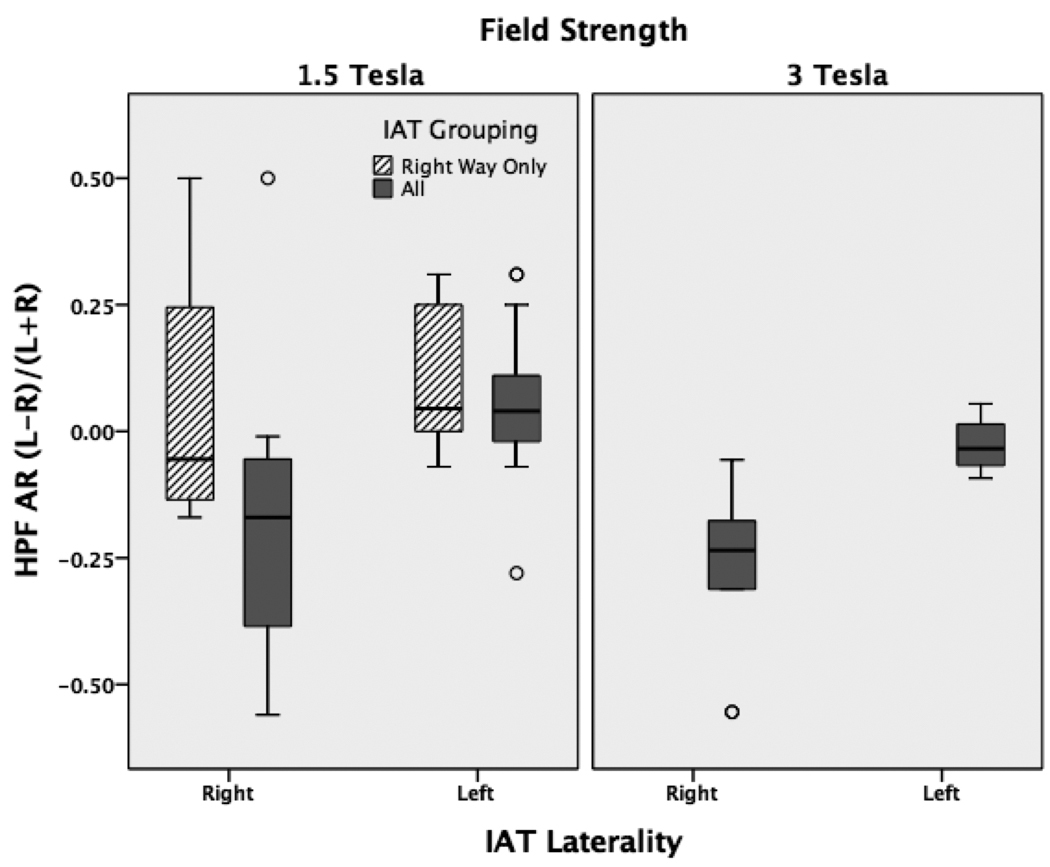
When compared to IAT laterality, hippocampal structural AR and hippocampal functional AR showed medium sized correlations without reaching significance (r=0.33, p=0.075; r=0.36, p=0.06), while HPF AR showed a high correlation (r=0.59, p=0.001), see figure 3. Examining the 1.5T and 3T groups separately revealed higher correlation values in the 3T group on all measures (H structure: r=0.64, p=0.04; H function: r=0.69, p=0.04; HPF: r=0.78, p=0.01). The 1.5T group had lower correlations and higher p-values on all measures (H structure: r=0.1, p=0.69; H function: r=0.2, p=0.50; HPF: r=0.46, p=0.04).
Figure 3.
Comparing groups with early and late age-of-onset of seizure, differences in both structure and function are evident (table 4). Patients with onset before age nine had a smaller ipsilateral hippocampus than those with later onset [t(34.9)= −2.89, p=0.007, equal variances not assumed]. In the early onset group, functional activation was lower within the ipsilateral hippocampus [t(36)= −2.40, p=0.022] and HPF region [t(36)= −2.14, p=0.039] and there was a greater asymmetry ratio in both the hippocampus [t(36)=3.37, p=0.002] and the HPF region [t(36)=2.13, p=0.04].
Table 4.
Age of Onset: Differences on Imaging Variables
| ≤ 9 years of age | > 9 years of age | |||
|---|---|---|---|---|
| Mean | S.D. | Mean | S.D. | |
| Ipsilateral hippocampal volume |
1142 * | 345 | 1559 | 534 |
| Contralateral hippocampal volume |
1720 | 348 | 1793 | 366 |
| Hippocampal structural AR |
0.317 | 0.33 | −0.066 | 0.34 |
| Ipsilateral hippocampal function |
0.477 * | 0.29 | 0.719 | 0.30 |
| Contralateral hippocampal function |
0.753 | 0.30 | 0.666 | 0.35 |
| Hippocampal functional AR |
0.328 * | 0.33 | −0.063 | 0.35 |
| Ipsilateral HPF function |
0.518 * | 0.23 | 0.702 | 0.26 |
| Contralateral HPF function |
0.711 | 0.23 | 0.707 | 0.27 |
| HPF functional AR | 0.150 * | 0.21 | −0.016 | 0.24 |
Significant difference between age of onset groups, p<0.05.
Pre-surgical imaging and post-surgical cognitive outcome
Comparisons were only made for the combined 1.5T and 3T group since structural measures were comparable and the combined group maintained a larger sample size.
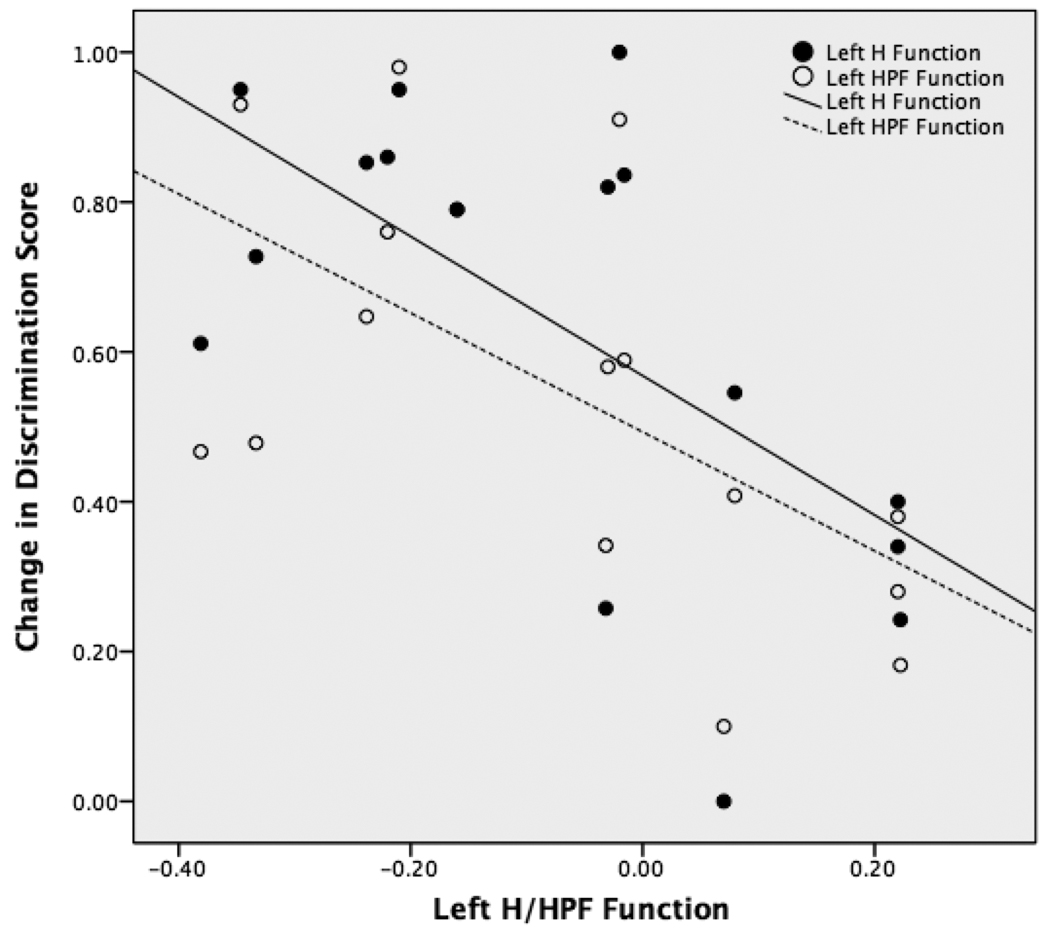
The change in discrimination score on the scene memory task was correlated with the hippocampal and HPF function on the left in patients with left-sided seizures (figure 4). As hippocampal and HPF function increased on the left, there was a greater decrease in the scene memory score post-surgically [left H function: r=−0.62, p=0.01; left HPF: r=−0.60, p=0.01]. There was no relationship between the imaging variables and change in scene memory for the patients with right-sided seizure.
Figure 4.
For subjects with left-sided seizure, the larger the ipsilateral hippocampal volume, the greater the post-surgical decline on all three measures of verbal memory (LM-I: r=−0.66, p=0.01; LM-II: r=−0.84, p<0.001; CVLT-LD: r=−0.57, p=0.04). For subjects with right-sided seizure, the larger the ipsilateral hippocampal volume, the less post-surgical decline on one measure of visual memory, Faces-I (r=0.87, p=0.01). Neither right or left-sided seizure patients showed a relationship between functional activation ipsilateral or contralateral to the seizure focus and changes in neuropsychological measures. Regression models that entered pre-surgical neuropsychological measures followed by structural or functional variables did not improve the level of prediction of neuropsychological outcome beyond the models that only included the pre-surgical neuropsychological measure.
The RCI ratio, a global measure of cognitive change, was significantly correlated with the structural AR and the ipsilateral hippocampal volume, indicating that greater asymmetry (r=0.55, p=0.02) and a larger ipsilateral hippocampus (r= −0.56, p=0.02) was associated with decline in post-surgical cognition. The functional ARs did not correlate with the RCI ratio.
Discussion
This study examined hippocampal structure and function obtained using MRI and neuropsychological functioning in TLE patients who were subsequently treated with surgical resection of the affected mesial temporal lobe. As expected, hippocampal structural asymmetry was more pronounced for TLE patients than for control subjects and TLE patients as a group had significantly smaller hippocampi ipsilateral to the seizure focus. These findings are consistent with the literature [4, 5] and support the use of quantitative hippocampal volumetry for lateralization of the seizure focus in mesial temporal lobe epilepsy at either field strength. Although the correlations between structural asymmetry and seizure lateralization were roughly comparable in 1.5T and 3T data, supporting prior work [55], group differentiation using functional asymmetry and concordance between imaging data and IAT memory lateralization were improved for the higher resolution 3T data.
Cognitive functioning in our sample was comparable to previous reports of right and left-sided TLE patients [2]. Left-sided seizure patients had a significant post-surgical decline on measures of verbal memory, while right-sided TLE patients had significant post-surgical decline on measures of visual memory. Material specificity of the cognitive change was evident in many cases although RCIs revealed individuals who had both verbal and visual memory decline or had memory decline that is not typically linked to the lateralization of their seizure focus. Additionally, regardless of the side of seizure, pre-surgical scores on almost all neuropsychological measures were significant predictors of post-surgical neuropsychological change, again supporting the use of this sample as representative of the TLE population [56].
Functional asymmetry of the hippocampus and HPF region in the TLE population was significantly different from the control group at 3T, but not at 1.5T. This relationship between group and asymmetry was significant even though there were fewer subjects at 3T than at 1.5T. Although several differences existed for data acquisition parameters at 1.5T and 3T, these findings suggest that 3T is superior to 1.5T for fMRI of memory encoding in TLE. The increased sensitivity for memory fMRI at 3T suggests that functional asymmetry may contribute to seizure lateralization, as has previously been demonstrated for PET imaging [57] and IAT [58].
Functional asymmetries can also reveal differences in neural organization within different populations of TLE patients. We found greater functional asymmetries within the hippocampus and HPF region in TLE patients with an early age of onset, possibly due to greater reorganization of function in this group compared with patients with later onset. Similar reorganization patterns have been reported for language lateralization with fMRI, which has shown more right-sided and bilateral language representation in patients with TLE, particularly in those who began having seizures early in childhood [59].
IAT remains the accepted “gold standard” for lateralization of language and memory. In our cohort, IAT laterality was significantly correlated with hippocampal structural asymmetry at 3T, but not at 1.5T. IAT laterality was also either significantly correlated with HPF functional asymmetry at 1.5T and 3T and hippocampal functional asymmetry at 3T, with higher correlation values for the 3T group. IAT and fMRI are fundamentally different measures. fMRI measures endogenous activation with both hemispheres functioning normally, while IAT measures residual function after the injected hemisphere is deafferented. Accordingly, the correlations between IAT and fMRI for memory lateralization may be expected to be less than perfect and it may be most useful to consider IAT and fMRI as complementary approaches for memory lateralization. Further studies with larger cohorts will examine the contribution of each measure to pre-surgical evaluation in the individual patient and determine when a combined approach or single diagnostic measure would be more useful in the initial evaluation and prediction of outcome.
Post-surgical cognitive change was examined using the scene memory task, standard neuropsychological measures, and a global composite score to account for change on multiple tests across domains (RCI ratio). We found the strongest relationships between ipsilateral hippocampal structure and hippocampal and HPF function for patients with left sided seizures, such that a larger and more active left hippocampus or left HPF region was associated with post-surgical cognitive decline. Decline on multiple measures, using RCIs, was associated with a larger ipsilateral hippocampus and greater volumetric asymmetry between hippocampi for all TLE patients. These results support the importance of assessing ipsilateral hippocampal volume and hippocampal and HPF function to assist in predicting post-surgical changes in memory functioning.
The literature on post-surgical memory outcome refers to relationships between both the side ipsilateral and the side contralateral to the seizure focus and neuropsychological outcomes, suggesting that the integrity of both sides contribute to the prediction of post-surgical memory outcome. These relationships were addressed by Chelune [60] and labeled the functional reserve and hippocampal adequacy models. The functional reserve model associates intact contralateral hippocampal function with good post-surgical memory outcome and the hippocampal adequacy model associates the integrity of ipsilateral hippocampal function with a decline in post-surgical memory outcome. There is support for both the functional reserve model [61, 62] and the hippocampal adequacy models in the literature [41, 63]. Our results provide additional support for the hippocampal adequacy model.
The significant correlations found between functional activation in the HPF region and change in memory support earlier work implicating the contribution of extra-hippocampal structures to both memory encoding [30, 64] and memory outcome following surgical intervention [65, 66]. The importance of including these extra-hippocampal regions is also underscored by reports of entorhinal and perirhinal damage and involvement in the pathophysiology of TLE [4, 67, 68]. Additionally, hippocampal-sparing temporal lobe resections have resulted in verbal memory deficits when the perirhinal and entorhinal cortices were included in the resection [69]. Taken together, these findings suggest a complementary future role for structural and functional MRI in planning tailored resections to maximize seizure control while minimizing neuropsychological deficits.
Based on our findings of higher correlations between imaging measurements, lateralization of seizures and IAT at 3T, improvements in the sensitivity of memory fMRI at 3T may allow for more stringent thresholding, a greater range of asymmetries, and more robust correlations with clinical indices. Several opportunities exist for further methodological improvements in both data acquisition and analysis. BOLD fMRI signal loss due to static susceptibility gradients can be reduced with smaller voxel sizes [42], though the optimal voxel size has yet to be determined. Use of perfusion fMRI with arterial spin labeling can also reduce sensitivity to static susceptibility gradients [70, 71] and a recent comparison between ASL and BOLD fMRI during scene encoding showed that ASL provides better sensitivity for TL activation than BOLD using 3mm isotropic voxels, as were used in this data set [72]. These data were also collected using a standard volume coil whereas improved sensitivity may be obtained in future studies using a multicoil array. Better data analysis techniques that rely on spatial correspondence based analysis of the hippocampus may improve sensitivity and allow for more accurate investigation of pre-surgical hippocampal integrity [73].
In comparing 1.5T to 3T fMRI, a higher signal-to-noise ratio at 3T is typically expected to translate into an improved quality of functional data [74, 75]. While we also conclude that 3T offers advantages over 1.5T data, there are several variables that complicate a direct comparison and make it difficult to attribute the gain at 3T to a single factor. BOLD fMRI sensitivity is primarily dependent on temporal SNR rather than static SNR, and temporal SNR can be compromised by an increase in physiological noise as field strength increases, resulting in some attenuation of the potential gain in SNR at 3T [76, 77]. Our 3T fMRI data was also obtained using a smaller voxel size than our 1.5T data, which can reduce both intravoxel dephasing due to susceptibility gradients and partial volume effects. The latter is of particular interest in small anatomic structures such as the hippocampus, and our finding of increased hippocampal activation at 3T despite reduced hippocampal signal coverage suggests that this was the primary benefit of reduced voxel sizes in our 3T data. It is therefore likely that an interaction of increased BOLD sensitivity and decreased voxel size leads to improved data quality.
The current study has several limitations. First, three different neuropsychologists, using different stimuli and testing procedures, completed the IAT procedures. Controlling these variables for the IAT procedure would allow for more reliable comparisons. Additionally, not all patients received the same neuropsychological testing before and after surgery, reducing the sample size in these analyses. A more standardized neuropsychological battery might allow for more extensive and detailed correlations between imaging results and cognitive outcome. Second, different scanning platforms were used to acquire the 1.5T (prior generation GE Signa) and 3T (current generation Siemens Trio) data. Thus, while field strength is probably the most significant difference between the 1.5T and 3T datasets, some platform- or generation-specific effects cannot be excluded. Third, although this study offers data on the largest cohort of TLE patients imaged at 3T, increasing the patient cohort would allow for greater statistical power and increased predictive ability. At the present time, a variety of tasks and paradigms are used for fMRI of memory. However, as evidence accumulates demonstrating the utility of fMRI of memory in TLE, and the relative benefits of specific fMRI approaches, support for multi-site clinical trials to demonstrate its efficacy in much larger series of patients should follow. Finally, effective use of memory fMRI in clinical practice will require robust procedures that provide reliable results in individual patients for use in clinical decision-making and perhaps ultimately surgical planning.
The results of this study support our prior results [34] demonstrating the feasibility of presurgical memory lateralization using fMRI for lateralizing seizure focus and predicting memory outcome at the group level. While structural asymmetries were comparable between 1.5T and 3T data, functional asymmetries showed improved lateralization with seizure focus in the 3T cohort. Significant correlations observed between fMRI and both IAT and changes in neuropsychological performance lend further support to the use of fMRI as a complementary presurgical memory assessment tool in TLE. Additionally, relatively greater reorganization of memory function was observed in patients with an early age of seizure onset. Finally, the observed contributions of ipsilateral TL function to predicting memory outcome provides additional support to the hippocampal adequacy model. These findings add to the literature on pre-surgical evaluation and organization of memory function in TLE.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Engel J, Jr, Wiebe S, French J, Sperling M, Williamson P, Spencer D, et al. Practice parameter: temporal lobe and localized neocortical resections for epilepsy: report of the Quality Standards Subcommittee of the American Academy of Neurology, in association with the American Epilepsy Society and the American Association of Neurological Surgeons. Neurology. 2003;60:538–547. doi: 10.1212/01.wnl.0000055086.35806.2d. [DOI] [PubMed] [Google Scholar]
- 2.Gleissner U, Helmstaedter C, Schramm J, Elger CE. Memory outcome after selective amygdalohippocampectomy in patients with temporal lobe epilepsy: one-year follow-up. Epilepsia. 2004;45:960–962. doi: 10.1111/j.0013-9580.2004.42203.x. [DOI] [PubMed] [Google Scholar]
- 3.Trenerry MR, Jack CR, Jr, Cascino GD, Sharbrough FW, So EL. Bilateral magnetic resonance imaging-determined hippocampal atrophy and verbal memory before and after temporal lobectomy. Epilepsia. 1996;37:526–533. doi: 10.1111/j.1528-1157.1996.tb00604.x. [DOI] [PubMed] [Google Scholar]
- 4.Bernasconi N, Bernasconi A, Caramanos Z, Antel SB, Andermann F, Arnold DL. Mesial temporal damage in temporal lobe epilepsy: a volumetric MRI study of the hippocampus, amygdala and parahippocampal region. Brain. 2003;126:462–469. doi: 10.1093/brain/awg034. [DOI] [PubMed] [Google Scholar]
- 5.Cendes F, Andermann F, Gloor P, Evans A, Jones-Gotman M, Watson C, et al. MRI volumetric measurement of amygdala and hippocampus in temporal lobe epilepsy. Neurology. 1993;43:719–725. doi: 10.1212/wnl.43.4.719. [DOI] [PubMed] [Google Scholar]
- 6.Seidenberg M, Kelly KG, Parrish J, Geary E, Dow C, Rutecki P, et al. Ipsilateral and contralateral MRI volumetric abnormalities in chronic unilateral temporal lobe epilepsy and their clinical correlates. Epilepsia. 2005;46:420–430. doi: 10.1111/j.0013-9580.2005.27004.x. [DOI] [PubMed] [Google Scholar]
- 7.Keller SS, Mackay CE, Barrick TR, Wieshmann UC, Howard MA, Roberts N. Voxel-based morphometric comparison of hippocampal and extrahippocampal abnormalities in patients with left and right hippocampal atrophy. Neuroimage. 2002;16:23–31. doi: 10.1006/nimg.2001.1072. [DOI] [PubMed] [Google Scholar]
- 8.Martin RC, Sawrie SM, Knowlton RC, Bilir E, Gilliam FG, Faught E, et al. Bilateral hippocampal atrophy: consequences to verbal memory following temporal lobectomy. Neurology. 2001;57:597–604. doi: 10.1212/wnl.57.4.597. [DOI] [PubMed] [Google Scholar]
- 9.Martin RC, Kretzmer T, Palmer C, Sawrie S, Knowlton R, Faught E, et al. Risk to verbal memory following anterior temporal lobectomy in patients with severe left-sided hippocampal sclerosis. Archives of neurology. 2002;59:1895–1901. doi: 10.1001/archneur.59.12.1895. [DOI] [PubMed] [Google Scholar]
- 10.Kneebone AC, Lee GP, Wade LT, Loring DW. Rey Complex Figure: figural and spatial memory before and after temporal lobectomy for intractable epilepsy. J Int Neuropsychol Soc. 2007;13:664–671. doi: 10.1017/S1355617707070828. [DOI] [PubMed] [Google Scholar]
- 11.O'Brien CE, Bowden SC, Bardenhagen FJ, Cook MJ. Neuropsychological correlates of hippocampal and rhinal cortex volumes in patients with mesial temporal sclerosis. Hippocampus. 2003;13:892–904. doi: 10.1002/hipo.10128. [DOI] [PubMed] [Google Scholar]
- 12.Jokeit H, Ebner A. Long term effects of refractory temporal lobe epilepsy on cognitive abilities: a cross sectional study. J Neurol Neurosurg Psychiatry. 1999;67:44–50. doi: 10.1136/jnnp.67.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wachi M, Tomikawa M, Fukuda M, Kameyama S, Kasahara K, Sasagawa M, et al. Neuropsychological changes after surgical treatment for temporal lobe epilepsy. Epilepsia. 2001;42 Suppl 6:4–8. [PubMed] [Google Scholar]
- 14.Rausch R, Kraemer S, Pietras CJ, Le M, Vickrey BG, Passaro EA. Early and late cognitive changes following temporal lobe surgery for epilepsy. Neurology. 2003;60:951–959. doi: 10.1212/01.wnl.0000048203.23766.a1. [DOI] [PubMed] [Google Scholar]
- 15.Baxendale S, Thompson P. Defining meaningful postoperative change in epilepsy surgery patients: measuring the unmeasurable? Epilepsy Behav. 2005;6:207–211. doi: 10.1016/j.yebeh.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 16.Seidenberg M, Hermann B, Wyler AR, Davies K, Dohan FC, Jr, Leveroni C. Neuropsychological outcome following anterior temporal lobectomy in patients with and without the syndrome of mesial temporal lobe epilepsy. Neuropsychology. 1998;12:303–316. doi: 10.1037//0894-4105.12.2.303. [DOI] [PubMed] [Google Scholar]
- 17.Lah S, Lee T, Grayson S, Miller L. Effects of temporal lobe epilepsy on retrograde memory. Epilepsia. 2006;47:615–625. doi: 10.1111/j.1528-1167.2006.00476.x. [DOI] [PubMed] [Google Scholar]
- 18.Hermann B, Seidenberg M, Bell B, Rutecki P, Sheth R, Ruggles K, et al. The neurodevelopmental impact of childhood-onset temporal lobe epilepsy on brain structure and function. Epilepsia. 2002;43:1062–1071. doi: 10.1046/j.1528-1157.2002.49901.x. [DOI] [PubMed] [Google Scholar]
- 19.Schwarz M, Pauli E, Stefan H. Model based prognosis of postoperative object naming in left temporal lobe epilepsy. Seizure. 2005;14:562–568. doi: 10.1016/j.seizure.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 20.Clusmann H, Kral T, Fackeldey E, Blumcke I, Helmstaedter C, von Oertzen J, et al. Lesional mesial temporal lobe epilepsy and limited resections: prognostic factors and outcome. Journal of neurology, neurosurgery, and psychiatry. 2004;75:1589–1596. doi: 10.1136/jnnp.2003.024208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Griffin S, Tranel D. Age of seizure onset, functional reorganization, and neuropsychological outcome in temporal lobectomy. J Clin Exp Neuropsychol. 2007;29:13–24. doi: 10.1080/13803390500263568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hermann BP, Seidenberg M, Haltiner A, Wyler AR. Relationship of age at onset, chronologic age, and adequacy of preoperative performance to verbal memory change after anterior temporal lobectomy. Epilepsia. 1995;36:137–145. doi: 10.1111/j.1528-1157.1995.tb00972.x. [DOI] [PubMed] [Google Scholar]
- 23.Simkins-Bullock J. Beyond speech lateralization: a review of the variability, reliability, and validity of the intracarotid amobarbital procedure and its nonlanguage uses in epilepsy surgery candidates. Neuropsychol Rev. 2000;10:41–74. doi: 10.1023/a:1009044630227. [DOI] [PubMed] [Google Scholar]
- 24.Loddenkemper T, Morris HH, Lineweaver TT, Kellinghaus C. Repeated intracarotid amobarbital tests. Epilepsia. 2007;48:553–558. doi: 10.1111/j.1528-1167.2007.00982.x. [DOI] [PubMed] [Google Scholar]
- 25.Baxendale S. The role of functional MRI in the presurgical investigation of temporal lobe epilepsy patients: a clinical perspective and review. J Clin Exp Neuropsychol. 2002;24:664–676. doi: 10.1076/jcen.24.5.664.1005. [DOI] [PubMed] [Google Scholar]
- 26.Binder JR, Swanson SJ, Hammeke TA, Morris GL, Mueller WM, Fischer M, et al. Determination of language dominance using functional MRI: a comparison with the Wada test. Neurology. 1996;46:978–984. doi: 10.1212/wnl.46.4.978. [DOI] [PubMed] [Google Scholar]
- 27.Rutten GJ, Ramsey NF, van Rijen PC, Alpherts WC, van Veelen CW. FMRI-determined language lateralization in patients with unilateral or mixed language dominance according to the Wada test. Neuroimage. 2002;17:447–460. doi: 10.1006/nimg.2002.1196. [DOI] [PubMed] [Google Scholar]
- 28.Gaillard WD, Balsamo L, Xu B, McKinney C, Papero PH, Weinstein S, et al. fMRI language task panel improves determination of language dominance. Neurology. 2004;63:1403–1408. doi: 10.1212/01.wnl.0000141852.65175.a7. [DOI] [PubMed] [Google Scholar]
- 29.Rutten GJ, Ramsey NF, van Rijen PC, van Veelen CW. Reproducibility of fMRI-determined language lateralization in individual subjects. Brain Lang. 2002;80:421–437. doi: 10.1006/brln.2001.2600. [DOI] [PubMed] [Google Scholar]
- 30.Stern CE, Corkin S, Gonzalez RG, Guimaraes AR, Baker JR, Jennings PJ, et al. The hippocampal formation participates in novel picture encoding: evidence from functional magnetic resonance imaging. Proc Natl Acad Sci U S A. 1996;93:8660–8665. doi: 10.1073/pnas.93.16.8660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Narayan VM, Kimberg DY, Tang KZ, Detre JA. Experimental design for functional MRI of scene memory encoding. Epilepsy Behav. 2005;6:242–249. doi: 10.1016/j.yebeh.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 32.Binder JR, Bellgowan PS, Hammeke TA, Possing ET, Frost JA. A comparison of two FMRI protocols for eliciting hippocampal activation. Epilepsia. 2005;46:1061–1070. doi: 10.1111/j.1528-1167.2005.62004.x. [DOI] [PubMed] [Google Scholar]
- 33.Killgore WD, Glosser G, Casasanto DJ, French JA, Alsop DC, Detre JA. Functional MRI and the Wada test provide complementary information for predicting post-operative seizure control. Seizure. 1999;8:450–455. doi: 10.1053/seiz.1999.0339. [DOI] [PubMed] [Google Scholar]
- 34.Rabin ML, Narayan VM, Kimberg DY, Casasanto DJ, Glosser G, Tracy JI, et al. Functional MRI predicts post-surgical memory following temporal lobectomy. Brain. 2004 doi: 10.1093/brain/awh281. [DOI] [PubMed] [Google Scholar]
- 35.Janszky J, Jokeit H, Kontopoulou K, Mertens M, Ebner A, Pohlmann-Eden B, et al. Functional MRI predicts memory performance after right mesiotemporal epilepsy surgery. Epilepsia. 2005;46:244–250. doi: 10.1111/j.0013-9580.2005.10804.x. [DOI] [PubMed] [Google Scholar]
- 36.Powell HW, Richardson MP, Symms MR, Boulby PA, Thompson PJ, Duncan JS, et al. Preoperative fMRI predicts memory decline following anterior temporal lobe resection. Journal of neurology, neurosurgery, and psychiatry. 2008;79:686–693. doi: 10.1136/jnnp.2007.115139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Richardson MP, Strange BA, Thompson PJ, Baxendale SA, Duncan JS, Dolan RJ. Pre-operative verbal memory fMRI predicts post-operative memory decline after left temporal lobe resection. Brain. 2004;127:2419–2426. doi: 10.1093/brain/awh293. [DOI] [PubMed] [Google Scholar]
- 38.Deblaere K, Backes WH, Tieleman A, Vandemaele P, Defreyne L, Vonck K, et al. Lateralized anterior mesiotemporal lobe activation: semirandom functional MR imaging encoding paradigm in patients with temporal lobe epilepsy--initial experience. Radiology. 2005;236:996–1003. doi: 10.1148/radiol.2363040780. [DOI] [PubMed] [Google Scholar]
- 39.Golby AJ, Poldrack RA, Illes J, Chen D, Desmond JE, Gabrieli JD. Memory lateralization in medial temporal lobe epilepsy assessed by functional MRI. Epilepsia. 2002;43:855–863. doi: 10.1046/j.1528-1157.2002.20501.x. [DOI] [PubMed] [Google Scholar]
- 40.Jokeit H, Okujava M, Woermann FG. Memory fMRI lateralizes temporal lobe epilepsy. Neurology. 2001;57:1786–1793. doi: 10.1212/wnl.57.10.1786. [DOI] [PubMed] [Google Scholar]
- 41.Richardson MP, Strange BA, Duncan JS, Dolan RJ. Memory fMRI in left hippocampal sclerosis: optimizing the approach to predicting postsurgical memory. Neurology. 2006;66:699–705. doi: 10.1212/01.wnl.0000201186.07716.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Szaflarski JP, Holland SK, Schmithorst VJ, Dunn RS, Privitera MD. High-resolution functional MRI at 3T in healthy and epilepsy subjects: hippocampal activation with picture encoding task. Epilepsy Behav. 2004;5:244–252. doi: 10.1016/j.yebeh.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 43.Snodgrass JG, Corwin J. Pragmatics of measuring recognition memory: applications to dementia and amnesia. J Exp Psychol Gen. 1988;117:34–50. doi: 10.1037//0096-3445.117.1.34. [DOI] [PubMed] [Google Scholar]
- 44.Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, et al. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage. 2006;31:1116–1128. doi: 10.1016/j.neuroimage.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 45.Jack CR, Jr, Theodore WH, Cook M, McCarthy G. MRI-based hippocampal volumetrics: data acquisition, normal ranges, and optimal protocol. Magn Reson Imaging. 1995;13:1057–1064. doi: 10.1016/0730-725x(95)02013-j. [DOI] [PubMed] [Google Scholar]
- 46.Watson C, Andermann F, Gloor P, Jones-Gotman M, Peters T, Evans A, et al. Anatomic basis of amygdaloid and hippocampal volume measurement by magnetic resonance imaging. Neurology. 1992;42:1743–1750. doi: 10.1212/wnl.42.9.1743. [DOI] [PubMed] [Google Scholar]
- 47.Duvernoy HM. The Human Hippocampus: Functional Anatomy, Vascularization, and Serial Sections with MRI. New York: Springer; 2005. [Google Scholar]
- 48.Mai J, Paxinos G, Assheuer J. Atlas of the Human Brain. Second Edition. Academic Press; 2003. [Google Scholar]
- 49.Haller JW, Banerjee A, Christensen GE, Gado M, Joshi S, Miller MI, et al. Three-dimensional hippocampal MR morphometry with high-dimensional transformation of a neuroanatomic atlas. Radiology. 1997;202:504–510. doi: 10.1148/radiology.202.2.9015081. [DOI] [PubMed] [Google Scholar]
- 50.Carmichael OT, Aizenstein HA, Davis SW, Becker JT, Thompson PM, Meltzer CC, et al. Atlas-based hippocampus segmentation in Alzheimer's disease and mild cognitive impairment. Neuroimage. 2005;27:979–990. doi: 10.1016/j.neuroimage.2005.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Branco DM, Suarez RO, Whalen S, O'Shea JP, Nelson AP, da Costa JC, et al. Functional MRI of memory in the hippocampus: Laterality indices may be more meaningful if calculated from whole voxel distributions. Neuroimage. 2006;32:592–602. doi: 10.1016/j.neuroimage.2006.04.201. [DOI] [PubMed] [Google Scholar]
- 52.Kikinis R, Gleason PL, Moriarty TM, Moore MR, Alexander E, 3rd, Stieg PE, et al. Computer-assisted interactive three-dimensional planning for neurosurgical procedures. Neurosurgery. 1996;38:640–649. discussion 9–51. [PubMed] [Google Scholar]
- 53.Woods SP, Delis DC, Scott JC, Kramer JH, Holdnack JA. The California Verbal Learning Test--second edition: test-retest reliability, practice effects, and reliable change indices for the standard and alternate forms. Arch Clin Neuropsychol. 2006;21:413–420. doi: 10.1016/j.acn.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 54.Martin R, Sawrie S, Gilliam F, Mackey M, Faught E, Knowlton R, et al. Determining reliable cognitive change after epilepsy surgery: development of reliable change indices and standardized regression-based change norms for the WMS-III and WAIS-III. Epilepsia. 2002;43:1551–1558. doi: 10.1046/j.1528-1157.2002.23602.x. [DOI] [PubMed] [Google Scholar]
- 55.Scorzin JE, Kaaden S, Quesada CM, Muller CA, Fimmers R, Urbach H, et al. Volume determination of amygdala and hippocampus at 1.5 and 3.0T MRI in temporal lobe epilepsy. Epilepsy Res. 2008;82:29–37. doi: 10.1016/j.eplepsyres.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 56.Baxendale S, Thompson P, Harkness W, Duncan J. Predicting memory decline following epilepsy surgery: a multivariate approach. Epilepsia. 2006;47:1887–1894. doi: 10.1111/j.1528-1167.2006.00810.x. [DOI] [PubMed] [Google Scholar]
- 57.Manno EM, Sperling MR, Ding X, Jaggi J, Alavi A, O'Connor MJ, et al. Predictors of outcome after anterior temporal lobectomy: positron emission tomography. Neurology. 1994;44:2321–2326. doi: 10.1212/wnl.44.12.2321. [DOI] [PubMed] [Google Scholar]
- 58.Cohen-Gadol AA, Westerveld M, Alvarez-Carilles J, Spencer DD. Intracarotid Amytal memory test and hippocampal magnetic resonance imaging volumetry: validity of the Wada test as an indicator of hippocampal integrity among candidates for epilepsy surgery. J Neurosurg. 2004;101:926–931. doi: 10.3171/jns.2004.101.6.0926. [DOI] [PubMed] [Google Scholar]
- 59.Springer JA, Binder JR, Hammeke TA, Swanson SJ, Frost JA, Bellgowan PS, et al. Language dominance in neurologically normal and epilepsy subjects: a functional MRI study. Brain. 1999;122(Pt 11):2033–2046. doi: 10.1093/brain/122.11.2033. [DOI] [PubMed] [Google Scholar]
- 60.Chelune GJ. Hippocampal adequacy versus functional reserve: predicting memory functions following temporal lobectomy. Arch Clin Neuropsychol. 1995;10:413–432. [PubMed] [Google Scholar]
- 61.Sabsevitz DS, Swanson SJ, Morris GL, Mueller WM, Seidenberg M. Memory outcome after left anterior temporal lobectomy in patients with expected and reversed Wada memory asymmetry scores. Epilepsia. 2001;42:1408–1415. doi: 10.1046/j.1528-1157.2001.38500.x. [DOI] [PubMed] [Google Scholar]
- 62.Chiaravalloti ND, Glosser G. Material-specific memory changes after anterior temporal lobectomy as predicted by the intracarotid amobarbital test. Epilepsia. 2001;42:902–911. doi: 10.1046/j.1528-1157.2001.02500.x. [DOI] [PubMed] [Google Scholar]
- 63.Kneebone AC, Chelune GJ, Dinner DS, Naugle RI, Awad IA. Intracarotid amobarbital procedure as a predictor of material-specific memory change after anterior temporal lobectomy. Epilepsia. 1995;36:857–865. doi: 10.1111/j.1528-1157.1995.tb01628.x. [DOI] [PubMed] [Google Scholar]
- 64.Kohler S, Crane J, Milner B. Differential contributions of the parahippocampal place area and the anterior hippocampus to human memory for scenes. Hippocampus. 2002;12:718–723. doi: 10.1002/hipo.10077. [DOI] [PubMed] [Google Scholar]
- 65.Paglioli E, Palmini A, Portuguez M, Paglioli E, Azambuja N, da Costa JC, et al. Seizure and memory outcome following temporal lobe surgery: selective compared with nonselective approaches for hippocampal sclerosis. J Neurosurg. 2006;104:70–78. doi: 10.3171/jns.2006.104.1.70. [DOI] [PubMed] [Google Scholar]
- 66.Morino M, Uda T, Naito K, Yoshimura M, Ishibashi K, Goto T, et al. Comparison of neuropsychological outcomes after selective amygdalohippocampectomy versus anterior temporal lobectomy. Epilepsy Behav. 2006;9:95–100. doi: 10.1016/j.yebeh.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 67.Siegel AM, Wieser HG, Wichmann W, Yasargil GM. Relationships between MR-imaged total amount of tissue removed, resection scores of specific mediobasal limbic subcompartments and clinical outcome following selective amygdalohippocampectomy. Epilepsy Res. 1990;6:56–65. doi: 10.1016/0920-1211(90)90009-k. [DOI] [PubMed] [Google Scholar]
- 68.Bonilha L, Kobayashi E, Rorden C, Cendes F, Li LM. Medial temporal lobe atrophy in patients with refractory temporal lobe epilepsy. J Neurol Neurosurg Psychiatry. 2003;74:1627–1630. doi: 10.1136/jnnp.74.12.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Weintrob DL, Saling MM, Berkovic SF, Reutens DC. Impaired verbal associative learning after resection of left perirhinal cortex. Brain. 2007;130:1423–1431. doi: 10.1093/brain/awm013. [DOI] [PubMed] [Google Scholar]
- 70.Fernandez-Seara MA, Wang Z, Wang J, Rao HY, Guenther M, Feinberg DA, et al. Continuous arterial spin labeling perfusion measurements using single shot 3D GRASE at 3 T. Magn Reson Med. 2005;54:1241–1247. doi: 10.1002/mrm.20674. [DOI] [PubMed] [Google Scholar]
- 71.Wang Z, Wang J, Connick TJ, Wetmore GS, Detre JA. Continuous ASL (CASL) perfusion MRI with an array coil and parallel imaging at 3T. Magn Reson Med. 2005;54:732–737. doi: 10.1002/mrm.20574. [DOI] [PubMed] [Google Scholar]
- 72.Fernandez-Seara MA, Wang J, Wang Z, Korczykowski M, Guenther M, Feinberg DA, et al. Imaging mesial temporal lobe activation during scene encoding: Comparison of fMRI using BOLD and arterial spin labeling. Hum Brain Mapp. 2007 doi: 10.1002/hbm.20366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yushkevich PA, Detre JA, Mechanic-Hamilton D, Fernandez-Seara MA, Tang KZ, Hoang A, et al. Hippocampus-specific fMRI group activation analysis using the continuous medial representation. Neuroimage. 2007;35:1516–1530. doi: 10.1016/j.neuroimage.2007.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Meindl T, Born C, Britsch S, Reiser M, Schoenberg S. Functional BOLD MRI: comparison of different field strengths in a motor task. Eur Radiol. 2008;18:1102–1113. doi: 10.1007/s00330-008-0869-1. [DOI] [PubMed] [Google Scholar]
- 75.van der Zwaag W, Francis S, Head K, Peters A, Gowland P, Morris P, et al. fMRI at 1.5, 3 and 7 T: Characterising BOLD signal changes. Neuroimage. 2009 doi: 10.1016/j.neuroimage.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 76.Bodurka J, Ye F, Petridou N, Murphy K, Bandettini PA. Mapping the MRI voxel volume in which thermal noise matches physiological noise--implications for fMRI. Neuroimage. 2007;34:542–549. doi: 10.1016/j.neuroimage.2006.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fera F, Yongbi MN, van Gelderen P, Frank JA, Mattay VS, Duyn JH. EPI-BOLD fMRI of human motor cortex at 1.5 T and 3.0 T: sensitivity dependence on echo time and acquisition bandwidth. J Magn Reson Imaging. 2004;19:19–26. doi: 10.1002/jmri.10440. [DOI] [PubMed] [Google Scholar]


