Abstract
Yamoa™ (ground bark of Funtumia elastica tree) is marketed and sold as a dietary supplement with anecdotal therapeutic effects in the treatment of asthma and hay fever. We determined that Yamoa™ and Yamoa™-derived polysaccharides affected innate immunity, in part, by priming γδ T cells. Gene expression patterns in purified bovine γδ T cells and monocytes induced by Yamoa™ were similar to those induced by ultrapure lipopolysaccharide (uLPS). In the presence of accessory cells, Yamoa™ had priming effects that were similar to those of LPS on bovine and murine γδ T cells, but much more potent than LPS on human γδ T cells. The bioactive component of Yamoa™ was delineated to a complex polysaccharide fraction (Yam-I). Intraperitoneal injection of Yamoa™ and Yam-I in mice induced rapid increases in peritoneal neutrophils directed by changes in chemokine expression. In support of a unique agonist found in Yam-I, similar peritonitis responses were also observed in TLR4- and MyD88- deficient mice. Therapeutic treatment with Yam-I resulted in decreased bacterial counts in feces from mice with Salmonella enterica serotype Typhimurium (ST)-induced enterocolitis. This characterization of the immune stimulatory properties of polysaccharides derived from Yamoa™ suggests mechanisms for the anecdotal positive effects of its ingestion and that these polysaccharides show potential for application in innate protection from disease.
Keywords: γδT cells, Yamoa™, lipopolysaccharide, priming, peritonitis, dietary supplement
Introduction
Herbal products have been used as effective treatments for millennia and their use is increasing rapidly, yet in many cases their safety and specific mechanisms of action are not well defined. We have recently focused on characterization of active components of common herbal products that have purported immune benefits [1,2]. Our goal was to define compounds that stimulated the innate immune system, but unlike most studies of innate immunity, which focus primarily on myeloid cells, we sought compounds that affected innate lymphocytes, specifically γδ T cells. We defined a unique response by γδ T cells to a water soluble fraction derived from Yamoa™, the ground bark of the Funtumia elastica tree, currently marketed as an asthma treatment [1]. In addition to defining novel stimulatory adjuvants for γδ T cells, it is of critical interest to characterize both potentially beneficial immune stimulatory capacity and potential risks of dietary supplements currently in use by the public.
γδ T cells have the capacity to rapidly mediate a large array of effector activities and are recognized as an important cell of the innate immune system [3]. γδ T cells are potent cytolytic cells [4,5], and produce an array of cytokines that enhance the activities of macrophages, neutrophils, and other lymphocytes [6–8]. γδ T cells can also present antigen [9,10], induce or suppress inflammation [11–13], and are important to the health of epithelial cell monolayers [14,15]. γδ T cells are found in all mucosal surfaces [16] and have been shown to respond and participate in host defense responses in a variety of pathogen-induced diseases [3]. As such, after epithelial cells, γδ T cells are one of the first cell populations to encounter pathogens that invade through the gut epithelial lining [16] and are well positioned to respond to agonists in dietary supplements. Health benefits of tea consumption have been linked to stimulation of the anti-microbial response of human γδ T cells [17,18]. Also, some fruit and vegetable juices expand γδ T cells following consumption [19,20]. Based on these and related studies, novel therapeutic protocols that focus on increasing the functional activity of this T cell population are being pursued in the clinic to combat infection and cancers [21–23].
We recently defined a transitional activation state of γδ T cells, antigen-independent priming, that is characterized by increased expression of a few cytokines, such as interleukin 8 (IL-8) and granulocyte-macrophage colony-stimulating factor (GM-CSF), cell surface proteins, such as interleukin-2 receptor α (IL-2Rα, CD25) and CD69, and increased responsiveness to downstream stimuli [24,25]. Priming of γδ T cells is induced by bacterial pathogen associated molecular patterns (PAMPs), [26,27]. However, whereas monocyte/macrophages are very sensitive to bacterial PAMPs, priming of γδ T cells requires a much higher concentration of PAMPs, and results in a much more subtle priming response. In contrast, potent γδ T cell priming activity at low concentrations was identified in extracts of common commercially available plant-derived products with purported immune modulating activity, one example of which was polyphenolic tannins [1]. γδ T cell priming may contribute to the roles of these cells in innate protection from infection [24].
The intent of this study was to characterize the bioactivity found in the product Yamoa™ that is currently in use by the public. It was also of interest to compare γδ T cell responses to agonists in Yamoa™ with responses to bacterial PAMPs [26]. Yamoa™ had a potent in vitro effect on γδ T cells from bovine calves, humans, and mice in the presence of accessory cells. Bovine γδ T cell gene expression changes in response to Yamoa™ were similar to, but more robust than, responses to ultrapure lipopolysaccharide (uLPS). The active components in Yamoa™ were delineated to a polysaccharide fraction called Yam-I. Very low concentrations of Yam-I drove neutrophil recruitment (peritonitis) in mice that was partially tolllike receptor 4 (TLR4)- and myeloid differentiation primary response gene (88) (MyD88)-independent, suggesting a mechanism unique from that of LPS. Therapeutic treatment with Yam-I provided increased innate protection from mucosal infection with Salmonella enterica serotype Typhimurium (ST). Thus, Yamoa™ and its derived polysaccharides represent novel γδ T cell agonists and may provide benefit as innate immunomodulators.
Materials and Methods
Yamoa™
Yamoa™ was prepared by suspending Yamoa™ powder, from either The Official Yamoa Powder Website (yamoapowder.com) or Bouncing Bear Pharmaceuticals (herbalfire.com) at approximately 0.3 g/ml in sterile water, mixing to resuspend, centrifugation, and filtration of the supernatant fluids containing water soluble fractions. Multiple lots from the two Yamoa™ suppliers were tested.
Microarray experiments
Microarray experiments were performed essentially as previously described [24,28] to determine the response of γδ T cells to Yamoa™ compared to uLPS. Bovine peripheral blood mononuclear cells (PBMCs) were collected from 3 different calves and monocytes were isolated using cross-reacting human anti-CD14 microbeads and the MACS system, according to the manufacturer’s instructions (Miltenyi). CD14-negative cells were then stained with GD3.8 (pan γδ T cell) and CC21 (pan B cell). γδ and non-B cell/non-γδ T cells (essentially αβ T cells with a few NK cells) were then sorted using a FACS Vantage (BD) to γδ T cell purities of greater than 97%. Sorted cells were rested overnight, then stimulated with either phosphate buffered saline (PBS), uLPS (Invivogen, 10 µg/ml), or Yamoa™ (32.6 µg/ml) for 4 hours. RNA was extracted and analyzed on an Agilent Bioanalyzer, and only high quality RNA was used for microarray analysis. RNA was then amplified and used to probe the Genechip® Bovine Genome Arrays (Affymetrix), as we have previously described [24,28]. Microarrays were used to determine the γδ T cell responses to PBS, uLPS and Yamoa™ treatment using cells from 3 different calves (9 arrays). For general comparison, the responses of monocytes and non-γδ T cells to PBS, uLPS and Yamoa™ were determined using pooled RNA from the 3 different calves on 1 array per treatment (6 arrays). Hybridization, staining and scans were performed as previously described, and analysis was done using GeneSpring (Silicon Genetics) with robust multi array (RMA) preprocessing and Microsoft Excel. All data was normalized to the median and filtered on expression levels (>100 raw in at least 1 sample) and fold change. The microarray data have been deposited in NCBIs Gene Expression Omnibus (GEO, http://www.ncbi.nlm.nih.gov/geo/) and are accessible through GEO Series accession number GSE13320.
CFSE assay for proliferation of human cells
The carboxyfluorescein succinimidyl ester (CFSE) assay for human cells has been recently described [1]. Briefly PBMCs from human donors were labeled with 2.5 µM CFSE for 5 minutes at 37°C, rinsed twice with Hank’s balanced salt solution (HBSS) and suspended at 1×106 cells per ml in XVIVO serum-free medium (Cambrex). Yamoa™, uLPS (each at various concentrations) or PBS were added to the media with IL-15 (20 ng/ml) and incubated at 37° C for 5 days. Cells were then stained for γδ TCR and read on a BD FACSCalibur flow cytometer equipped with a high-throughput screening (HTS) loader.
In vitro priming of mouse spleen γδ T cells
Spleens were harvested from 8–12-week-old TCRα-deficient mice (on a C57/B6 background) that have a greater number of γδ T cells than WT animals [29] and homogenized. Red blood cells (RBCs) were lysed with ACK (0.15 M ammonium chloride, 1 mM potassium carbonate, 0.1 mM EDTA disodium salt) buffer, nitex filtered, and leukocytes isolated using a percoll (Sigma) gradient (40%/60%). Cells were plated (2.5×106 cells/ml) in 96-well round bottom tissue culture plates, and the priming assay was performed as previously described [24] with agonist (Yamoa™ or Yam-I, various concentrations) or LPS from Salmonella (ST LPS, Sigma) or uLPS (10 µg/ml) stimulation for 48 hours followed by IL-2 (1 ng/ml) addition and 72-hour incubation. Data were collected on a BD FACSCalibur flow cytometer equipped with an HTS loader.
Polysaccharide isolation from Yamoa™
Polysaccharides were isolated from Yamoa™ essentially as described elsewhere [30,31]. Briefly, 450 g Yamoa™ powder (from either source) was extracted with 5L boiling distilled H2O for 1 hour. The aqueous extract was centrifuged at 2,000 × g for 15 minutes, and a 4-fold volume of ethanol was added to the supernatant to precipitate polysaccharides overnight at 4° C. The precipitate was pelleted by centrifugation, re-dissolved in distilled H2O, sonicated for 10 minutes, and centrifuged at 2,000 × g for 15 minutes. The supernatant fluid was then filtered through a 0.22 µm filter and concentrated in an Amicon concentrator with a 5 kDa Amicon PM5 membrane (Millipore, Billerica, MA) to obtain the crude polysaccharide extract (YamPS, recovery of 0.6 % by weight or 2.8 g total weight). YamPS was fractionated using ion-exchange chromatography on a DEAE-cellulose column equilibrated with 0.05 M Tris-HCl buffer (pH 8.0). Bound material was sequentially eluted with 2M NaCl (Yam-I fraction, yield 0.7g) and 0.3 N NaOH (Yam-II fraction, yield 1.25 g). The presence of polysaccharides or other high molecular weight material (>5 kDa) in the unbound fraction was minimal (<0.1% of total bound fraction). Both fractions induced increases in IL-2Rα on bovine γβ T cells (data not shown), but the Yam-I fraction retained activity at lower dilutions compared to the Yam-II fraction, and the NaCl eluent (Yam-I) more readily yielded to further fractionation, so this was pursued. Yam-I was further fractionated by size-exclusion chromatography (SEC) on a Sepharose-6B column (2.5×92 cm) equilibrated with 0.01 M Tris-HCl buffer (pH 7.2) containing 0.15 M NaCl and eluted with the same buffer at a flow rate of 22 ml/hour. The carbohydrate elution profile was determined by the phenol-sulfuric acid (H2SO4) method [32], modified to a microplate format, and absorbance was measured at 488 nm using a SpectraMax Plus microplate reader (Molecular Devices, Palo Alto, CA). The relevant fractions were pooled and concentrated. Three fractions were obtained, designated as Yam-I-1 to Yam-I-3. These fractions were analyzed by HPLC, and elution was monitored with a refractive index detector as described previously [30]. Approximate molecular weights were determined after calibration with pullulan standards as follows: Yam-I-1 ~404,000 Da, Yam-I-2 ~35,000 Da, and Yam-I-3 ~5,900 Da. Thus, related polysaccharides of various sizes comprise the Yam-I fraction. These smaller fractions, which were difficult to generate, were used for limited in vitro assays. For analysis of biological activity, these fractions were diluted in PBS to a concentration of 5 mg/ml and filtered through sterile 0.22 µm filters. Each of the fractions described above was applied at various concentrations to bovine PBMCs plated at 2×106 cells/ml. After 24 hours, the cells were labeled for γδ TCR and IL2Rα and analyzed by flow cytometry as previously described [1].
Physical and chemical analysis of polysaccharide fractions from Yamoa™
For 1H-nuclear magnetic resonance (1H-NMR) analysis, samples (6 mg) were dissolved in 0.6 mL D2O, filtered through 0.2 µm filters and the spectra were recorded on a Bruker DRX-600 spectrometer (Bruker BioSpin, Billerica, MA) at 20 °C using 3-(trimethylsilyl)-propionic 2,2,3,3,-d4 acid sodium salt as an internal reference.
The presence of arabinogalactan in the samples was detected by single radial gel diffusion in a 1% agarose gel containing 100 µg/ml β-glucosyl Yariv reagent, which selectively interacts with and precipitates compounds containing type II arabinogalactan structures. Four µl of each polysaccharide fraction (10 mg/ml) was loaded into the wells, and the samples were incubated at room temperature for 24 h in a humid atmosphere. A positive reaction was identified by a reddish circle around the well, and arabic gum (4 mg/ml) served as a positive control.
For monosaccharide composition analysis, the samples were hydrolyzed at 100 °C for 6 hours with 3 M trifluoroacetic acid, and the resulting samples were separated by thin-layer chromatography (TLC) on Whatman silica gel 60 plates with monosaccharide standards for reference. The TLC plates were developed with butanol/acetic acid/water (3:1:1), and the bands were visualized by spraying the plates with aniline– diphenylamine reagent (2% aniline, 2% diphenylamine and 8.5% H3PO4 acid in acetone) and heated at 100 °C for 10 minutes. Individual monosaccharide bands were scraped from the plate, extracted with H2O and quantified using a colorimetric method with monosaccharide standards. Briefly, the extracts were mixed with anthrone reagent (0.2% anthrone and 1% thiourea in H2SO4). After heating at 100 °C for 10 min, the absorbance was measured at 620 nm.
LPS removal from Yam-I
In attempt to remove potential contaminating bacterial LPS from Yam-I, 1 ml of 100 µg/ml solution of Yam-I was mixed with 0.5 ml of BioRad AffiPrep Polymyxin Support beads. The mixture was rotated at 4° C for 4 hours, then support beads were collected by centrifugation at 100 × g for 10 minutes. The supernatant fluid was removed and the process was repeated with fresh Polymyxin Support beads.
Peritonitis model
As suggested by the bovine gene expression data, experiments were conducted to analyze movement of innate immune cells in vivo in response to Yamoa™. Briefly, mice were injected with Yamoa™ (250 µg, optimal dose and timing were empirically determined), Yam-I (varying concentrations), or PBS. After 3 hours, blood was collected in Microvette® 200 tubes (Sarstedt) and plasma was separated by centrifugation (12,000 × g for 10 minutes). RBCs were lysed for 10 minutes in ACK buffer, cells were washed in HBSS, and leukocytes were analyzed by flow cytometry. After euthanasia, peritoneal cavities were washed with 8 ml sterile HBSS (injected and retrieved). Cells were pelleted at 500 × g for 5 minutes and similarly subjected to flow cytometric analysis using standard techniques. Blood and peritoneal cells were stained with directly conjugated RB6–8C5-FITC (recognizes Ly6G; bright on all neutrophils, dull on some macrophages), anti-CD45.2-APC (all leukocytes-disregards RBCs) and anti-CD11b-PE (bright on neutrophils and macrophages) and read on a BD FACSCalibur equipped with an HTS loader. Of the viable leukocytes, based on forward- /side-scatter (FSC/SSC) profiles and positive CD45 staining, the percentage of neutrophils (RB6bright/CD11bbright) was determined.
Salmonella enterica serotype Typhimurium (ST) enterocolitis model
We received the virulent streptomycin-resistant strain of ST (SL1344, a kind gift from Dr. Bradley Jones, University of Iowa) for use in an enterocolitis model of ST infection involving pretreatment with streptomycin [33]. Briefly, food was withdrawn from 6–12 week old BALB/c mice for 4 hours prior to pretreatment with 20 mg streptomycin by oral gavage. Twenty-four hours later, food again was withdrawn from mice 4 hours prior to infection with approximately 1×106 colony forming units (CFU)/mouse by oral gavage. Seven hours post-infection, mice were treated by i.p. injection with Yamoa™ (250 µg), Yam-I (varying concentrations) in 200 µl PBS or PBS alone. Twenty-four hours after infection, mice were euthanized and fecal pellets were collected. Fecal pellets were weighed, ground, diluted, and plated on Luria Bertani (LB) plates (with 50 µg/ml streptomycin) to determine CFU counts.
All experiments were performed in accordance with National Institutes of Health guidelines and approved by the Institutional Animal Care and Use Committee (animal experiments) and the Institutional Review Board (human blood) of Montana State University (Bozeman, MT).
Where presented, statistical significance was calculated using an unpaired, one-tailed t-test in Prism 5 (GraphPad, Inc.).
Results
Gene expression changes in purified bovine γδ T cells
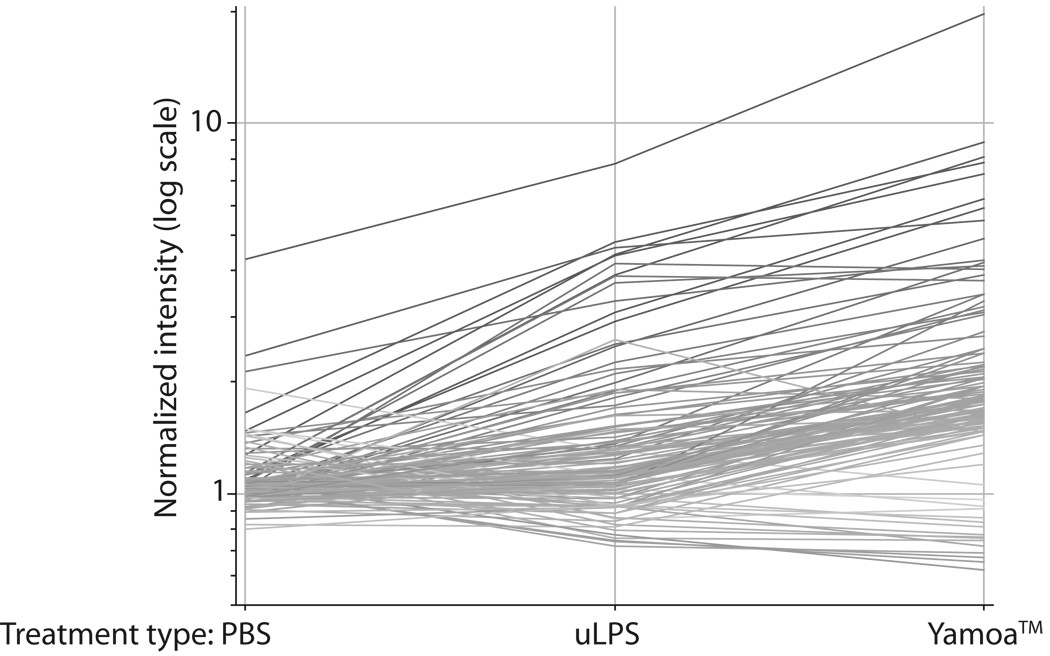
A robust agonist with γδ T cell-specific activity was identified in Yamoa™ using high-throughput assays that measured increased IL-2 receptor α (IL-2Rα) expression or proliferation using bovine PBMC [1]. To better understand the specific effects of Yamoa™ on immune cells, microarray experiments were performed. Gene expression profiles suggested that Yamoa™ stimulation of bovine cell subsets was slightly more potent, but very similar to that of uLPS, which we have shown also primes γδ T cells [26]. In response to uLPS, expression of 29 genes was increased by ≥1.5-fold in purified bovine γδ T cells, whereas expression of all but 5 of these genes plus an additional 81 (105 genes total) genes was increased after stimulation with Yamoa™ (Table I, Figure 1). Notably, six genes that changed the most in γδ T cells in response to Yamoa™ represented forms of the chemokines CXCL1 and CXCL2, suggesting that a change in neutrophil trafficking may be an in vivo effect of Yamoa™ stimulation of γδ T cells. These results suggested that the effect of Yamoa™ on gene expression in γδ T cells was similar to, but more robust than that of uLPS.
Table I.
Annotated genes that changed at least 2-fold (a subset of the genes depicted in Figure 1) in Yamoa™-treated, sorted bovine γδ T cells compared to fold change in cells treated with uLPS.
| Fold Change Relative to PBS | ||
|---|---|---|
| uLPS | Yamoa™ | Description |
| 3.396 | 7.093 | chemokine (C-X-C motif) ligand 2 (CXCL2) |
| 3.439 | 6.958 | chemokine (C-X-C motif) ligand 1 (CXCL1) |
| 2.825 | 5.726 | GRO-beta mRNA, complete cds. |
| 2.605 | 5.253 | GRO-beta mRNA, complete cds. |
| 2.522 | 4.956 | MI2A_HUMAN Macrophage inflammatory protein-2-alpha precursor |
| 2.964 | 4.926 | interleukin 8 (neutrophil activating peptide 1) (IL8) |
| 2.876 | 4.731 | (granulocyte chemotactic protein 2) (CXCL6) |
| 1.812 | 4.586 | thrombospondin (THBS) |
| 1.979 | 4.192 | interleukin 1, beta (IL1B) |
| 3.444 | 3.816 | chemokine (C-C motif) ligand 3-like 1 (CCL3L1) |
| <1.5 | 3.488 | interleukin 6 (interferon, beta 2) (IL6) |
| 2.242 | 3.459 | plasminogen activator inhibitor-2 mRNA, partial cds. |
| 2.179 | 3.335 | prostaglandin-endoperoxide synthase 2 (PTGS2) |
| 3.384 | 3.284 | IF5A_HUMAN Initiation factor 5A |
| 1.898 | 3.19 | interleukin-1 alpha mRNA, complete cds. |
| 1.884 | 3.15 | interleukin 1-beta (IL-1-beta) mRNA, complete cds. |
| 2.104 | 3.083 | interleukin 1, alpha (IL1A) |
| <1.5 | 2.638 | coagulation factor III (thromboplastin, tissue factor) (F3) |
| <1.5 | 2.345 | transcription factor NF-kappa-B2, p100 splice form – human |
| <1.5 | 2.333 | pir:A37251 (H.sapiens) A37251 probable nuclear hormone receptor NAK1 - human |
| 1.962 | 2.315 | mRNA for thrombospondin (partial) 2162 bp. |
| <1.5 | 2.275 | TR18_HUMAN Tumor necrosis factor receptor superfamily member 18 precursor |
| 1.842 | 2.242 | sp:P08567 (H.sapiens) PLEK_HUMAN Pleckstrin |
| <1.5 | 2.214 | sp:P43354 (H.sapiens) NR42_HUMAN Orphan nuclear receptor NURR1 |
| <1.5 | 2.191 | nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha |
| 1.734 | 2.166 | superoxide dismutase 2, mitochondrial (SOD2) |
| 1.532 | 2.133 | ref:NP_071342.1 (H.sapiens) chemokine |
| <1.5 | 2.048 | sp:Q9UK39 (H.sapiens) NOCT_HUMAN Nocturnin |
| <1.5 | 2.04 | A54025 transcription factor ATF3 – human |
| 1.599 | 2.02 | ref:NP_071342.1 (H.sapiens) chemokine |
Figure 1.
Microarray analysis of gene changes in bovine γδ T cells induced by Yamoa™, ultrapure LPS and PBS. Lines in Figure 2 represent the average relative gene expression levels of genes that changed at least 1.5-fold in Yamoa™- stimulated (right) bovine γδ T cells. The average was based on expression in treated cells from 3 different calves and compared to their expression after uLPS (middle) and PBS (left) treatment. Lines are shaded based on expression levels induced by Yamoa™ (darker shades represent greater change).
Non-γδ T lymphocytes and monocytes responded similarly whether they were stimulated with uLPS or Yamoa™ as determined by microarray analysis. These data were derived from single microarrays per treatment with mixed RNA from 3 different calves, which precluded strict interpretation. Nevertheless, in the non-γδ T lymphocyte population that included αβ T cells and NK cells, uLPS and Yamoa™ induced increased expression (≥2-fold) of similar numbers of genes, 35 and 38 genes respectively, and the responses were largely overlapping between the two agonists. The same was true in purified bovine monocytes, in which uLPS and Yamoa™ induced increased expression of similar numbers of genes, 604 and 618 respectively, and approximately 75% of genes increased in expression by one agonist were also increased with the other. Thus, the results of this global analysis of the effect of Yamoa™ on bovine cell subsets suggested that the effect of Yamoa™ on γδ T cells was enhanced compared to uLPS, but the two adjuvant materials had somewhat overlapping effects on other peripheral bovine cell subsets. Similarities in gene expression with uLPS suggested that either compounds in Yamoa™ may utilize similar pathways as those utilized by uLPS, or that LPS itself or some similar form could be found in Yamoa™. These gene expression data provided critical clues in the initial investigation of phenotypic changes induced by Yamoa™ on human and mouse cells, the effects of Yamoa™ in vivo, and the structural definition of the specific agonist in Yamoa™ and its potential pathways.
Analyses of human and murine γδ cells in vitro
Although Yamoa™ was originally identified as an agonist for bovine γδ T cells, secondary screens were performed using cells derived from human subjects and mice to determine the extent of the conservation of the priming effect. Human γδ T cells from multiple donors proliferated in response to Yamoa™ in the presence of IL-15 in mixed cultures and non-γδ lymphocytes did not respond (Figure 2A). The effect could be titrated to very low concentrations and was still measurable using approximately 100 ng/ml of Yamoa™ on cells from most human donors. In contrast, human cells had only a very limited response to much greater concentrations of uLPS, or other LPS sources in similar assays, clearly distinguishing agonists in Yamoa™ from LPS. Both major human γδ T cell subsets; the mucosal phenotype Vδ1 γδ T cell subset, and the more inflammatory Vδ2 γδ T cell subset, responded to Yamoa™ (data not shown). These results indicate that human γδ T cells responded globally to Yamoa™ and this response was clearly distinct from their response to LPS.
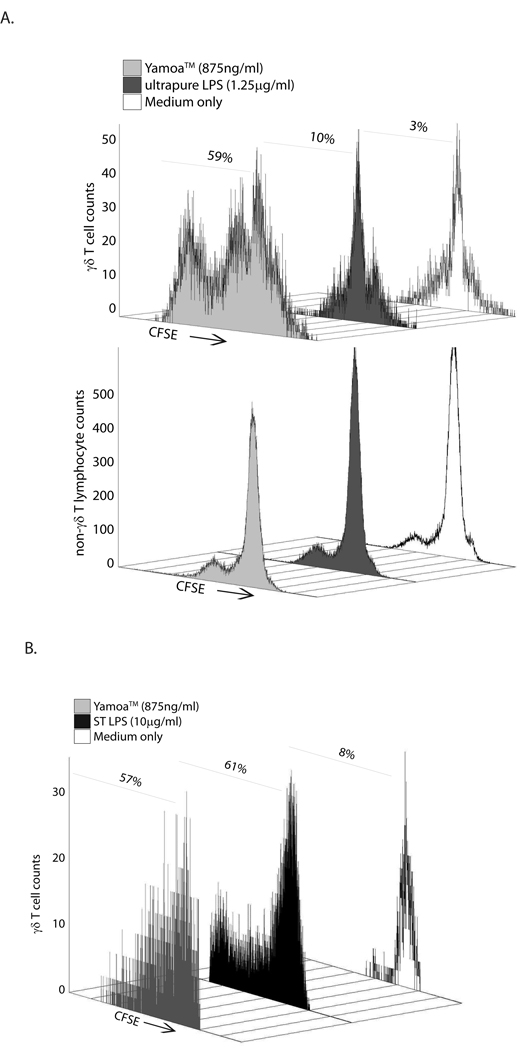
Figure 2.
γδ T cells are primed by agonists in Yamoa™. A. Human cells were stained with CFSE, cultured with 20 ng/ml IL-15 and treated with Yamoa ™, ultrapure LPS or media only control. After 5 days, the cells were analyzed by two-color flow cytometry. Yamoa™ was effective at priming human γδ T cells (top panel), and not other lymphocytes (lower panel), to proliferate as evidenced by a reduction in CFSE staining on the x-axis at a wide range of effective concentrations, whereas LPS had very little effect. Representative of at least 3 experiments with different donors. B. Mouse spleen cells were stained with CFSE, and treated with Yamoa ™, Salmonella LPS, or media only control for 48 hours then IL-2 (1 ng/ml) was added for 72 hours and the cells were analyzed by flow cytometry. Yamoa™ was effective at priming mouse γδ T cells (front panel), to proliferate as evidenced by a reduction in CFSE staining on the x-axis, at a wide range of effective concentrations, similar to LPS. Percentage values represent live lymphocytes having divided at least once. Representative of at least 3 experiments.
The in vitro effect of Yamoa™ was also demonstrated to be conserved in mice. CFSE-labeled mouse spleen cells from at least 3 αβ TCR-deficient mice were pre-treated (primed) with the extract for 48 hours, then interleukin-2 (IL-2) was added for an additional 72 hours. As shown in Figure 2B, murine γδ T cells were primed to proliferate in response to IL-2 after treatment with Yamoa™. In contrast to human cells, mouse γδ T cells also responded to ST LPS and uLPS. In contrast to human and bovine cells, in which the Yamoa™ response was γδ T cell specific, non-γδ T cell populations in these αβ TCR-deficient mouse spleens also responded to Yamoa™ (data not shown). Similar to the assays in human cells, very low concentrations of Yamoa™ were effective. Consistent responses of γδ T cells from bovine calves, humans and mice to Yamoa™ underscored the conserved effect of Yamoa™ and the utility of mouse models for elucidation of the mechanisms of Yamoa™-derived innate adjuvants.
Determination of the active fraction in Yamoa™
Preliminary experiments were performed to determine the chemical composition of the γδ T cell agonists in Yamoa™. First, we found that multiple lots from two different commercial sources exhibited similar bioactivity, suggesting that a random contaminant likely did not account for our results. Because other γδ T cell agonists were shown to be polyphenolic tannins [1,34], Yamoa™ was first tested for the presence of tannins. Binding of Yamoa™ to PVPP (insoluble polyvinylpolypyrrolidone), which binds free polyphenolic tannins [35], did not remove adjuvant activity, based on IL2Rα expression on bovine γδ T cells [1]. Enzymatic treatments suggested protein- and prenylated phosphate-containing compounds were not required for the bioactivity, and nucleic acids could not be detected (Supplemental Figure S1). SDS-PAGE analysis of concentrated preparations of Yamoa™ revealed a dominant large molecular mass species and the characteristic “laddering” properties attributed to LPS [36,37] were not observed. Thus, we considered whether the active component was present in the polysaccharide fraction, as polysaccharides derived from various medicinal plants have been shown previously to have immunomodulatory activity and can be large in size [38].
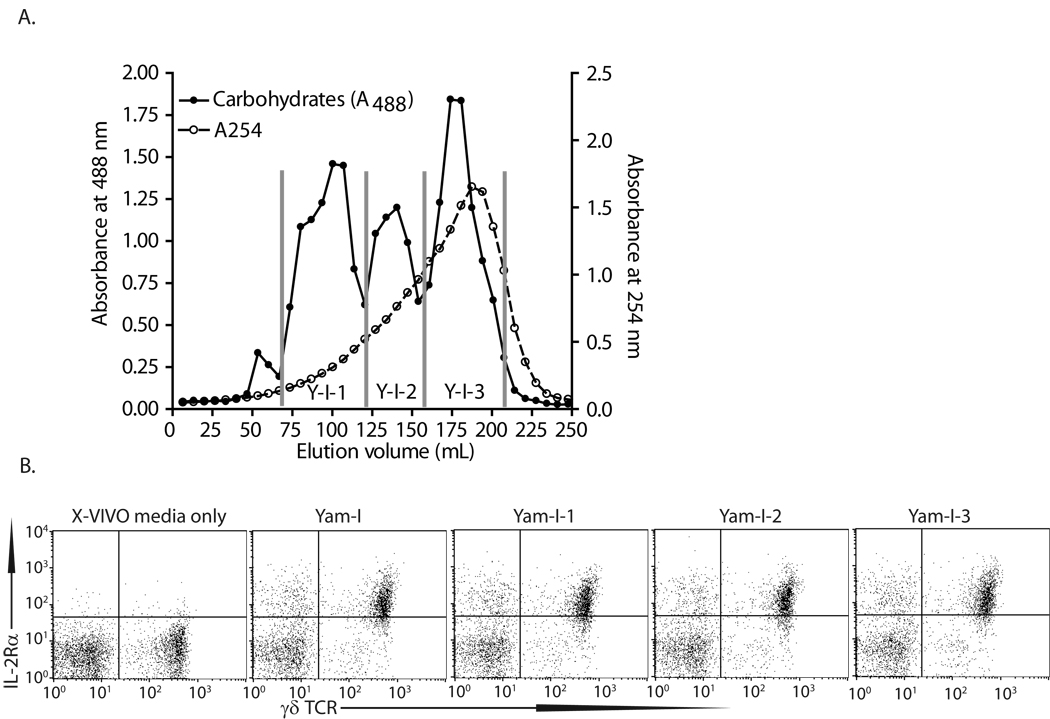
Polysaccharides were extracted from Yamoa™, fractionated as previously described [31], and analyzed for biological activity. Crude Yamoa™, and fractions YamPS (total polysaccharides), Yam-I (NaCl elution), Yam-I-1, Yam-I-2, Yam-I-3 (specific sub-fractions of Yam-I, Figure 3A) were tested in assays designed to measure changes in IL2Rα expression by bovine γδ T cells, as previously described [1]. The Yam-I fraction enhanced IL2Rα expression, but further fractionation of Yam-I did not increase specific activity in these assays (Figure 3B). Analysis of Yam-I by NMR confirmed the polysaccharide composition of Yam-I and indicated that, if LPS were present, it would have to be a minor component of the purified preparation (Supplemental Figure S2). Sugar composition analysis revealed that polysaccharides in all Yamoa™ fractions, except for fraction Yam-II, consisted primarily of galactose (Gal), glucose (Glc), rhamnose (Rha), arabinose (Ara), glucosamine (GlcA), and galactosamine (GalA), with Glc and Gal being the dominant monosaccharides. In contrast, fraction Yam-II contained traces of Rha, Glc, Ara, and GlcA, but had a much higher level of Gal in molecular % than all other fractions (Table II). Analysis of the Yamoa™ fractions using the Yariv test showed that all fractions, except for fractions Yam-II and Yam-I-3, contained type II arabinogalactan (Table II). Polysaccharides from Yamoa™ did not induce hemagglutination of human or murine erythrocytes, as do carbohydrates isolated from other barks [39], thus they did not contain lectin-like activity. The Yam-I fraction was similarly isolated from 4 different lots of Yamoa™ from the two suppliers with similar results. These results suggested that the polysaccharide fractions isolated from Yamoa™ were responsible for the observed biological activity.
Figure 3.
Isolation and characterization of Yamoa™-derived polysaccharides. A. Beginning with the NaCl buffer elution fraction from the ion-exchange DEAE-cellulose column (Yam-I), three major fractions were eluted from a Sepharose-6B column by SEC and designated as Yam-I-1, −2 and −3. These fractions were analyzed by HPLC and approximate molecular weights were determined as follows: Yam-I-1, ~404,000Da, Yam-I-2,~35,000Da, Yam-I-3~5,900Da. B. Each resulting fraction was applied to bovine PBMC cultures for 24 hours. Cells were stained and analyzed by flow cytometry for IL2Rα surface expression.
Table II.
Monosaccharide composition (molecular %) of Yamoa™-derived fractions were identified and quantified based on TLC analysis of known standards
| Yam-I | Yam-II | Yam-I-1 | Yam-I-2 | Yam-I-3 | |
|---|---|---|---|---|---|
| Rhamnose | 12 | Trace | 9 | 14 | 17 |
| Glucose | 27 | Trace | 30 | 25 | 29 |
| Arabinose | 6 | Trace | 4 | 7 | 3 |
| Galactose | 40 | 93 | 41 | 44 | 32 |
| Glucuronic acid | 5 | Trace | 8 | 4 | 3 |
| Galacturonic acid | 10 | 7 | 9 | 6 | 15 |
| Yariv test | Positive | Negative | Positive | Positive | Negative |
In vivo responses to Yamoa™ and Yam-I
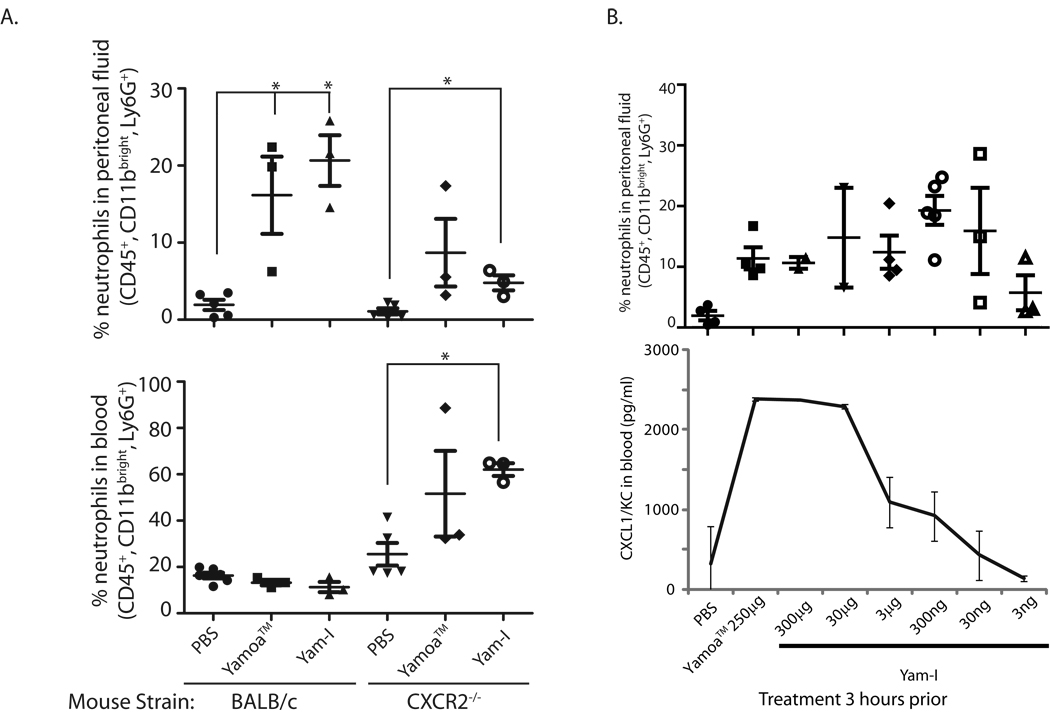
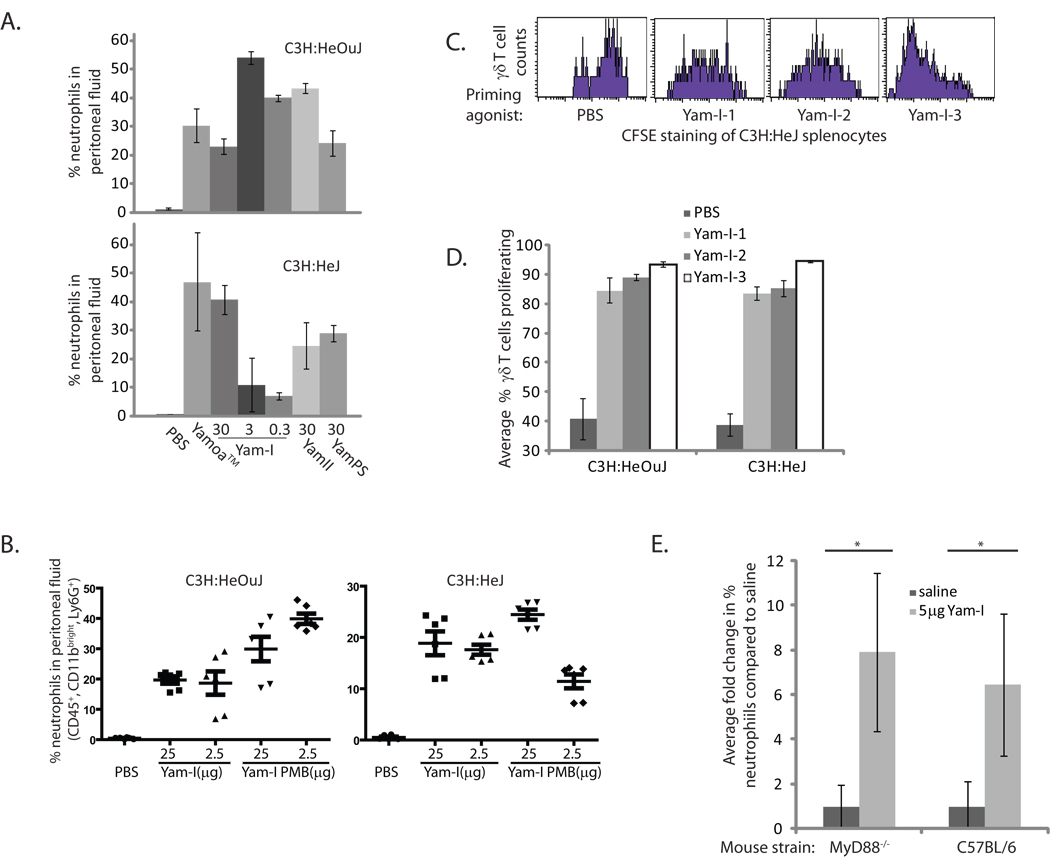
Given that chemokine transcript expression was induced by Yamoa™ in bovine γδ T cells, we began to assess the possible in vivo activities of Yamoa™ and Yam-I in mice. The murine chemokines functionally equivalent to IL-8 (CXCL1/KC and CXCL2/Mip-2) have well characterized roles in neutrophil recruitment [40–42], thus their expression following Yamoa™ and Yam-I injection was evaluated in mice. After empirical determination of dose and timing, we found that intraperitoneal injection of 250 µg of Yamoa™ or 30 µg Yam-I resulted in a substantial increase in the percentage of neutrophils that accumulated in the peritoneum of BALB/c mice after 3 hours. Presuming a critical role for the chemokines CXCL1/2 in the peritonitis response to Yamoa™ and Yam-I, this induction of peritonitis was also analyzed in mice deficient in CXCR2 (on BALB/c background), a receptor that directs neutrophil trafficking in response to CXCL chemokines. As shown in Figure 4A, both crude preparations and Yam-I effectively directed neutrophil influx into the peritoneum in BALB/c (WT) mice, and this response was diminished in CXCR2-deficient mice. CXCR2-deficient mice had a higher baseline percentage of neutrophils in the blood that increased in response to Yamoa™ and Yam-I, in contrast to peripheral (blood) neutrophils in WT mice, which did not increase following stimulation. CXCL1 (KC) levels were measured by ELISA in the peritoneal fluid and sera and were comparable in both mouse strains. Thus, CXCR2 sensing of induced chemokine changes was critical for neutrophil recruitment to the peritoneum, but dispensable for mobilization of neutrophils into the blood, in response to Yamoa™ and Yam-I. Yam-I activity was titrated in the peritonitis model in BALB/c mice to determine an optimal dose for induction of systemic effects by intraperitoneal delivery. Very low concentrations of Yam-I (30–300 ng/mouse) were sufficient to induce a local increase in peritoneal neutrophils. Optimal induction of CXCL1/KC in the serum, a more systemic effect, occurred at a Yam-I dose approximately 10-fold less than that of Yamoa™ (Fig. 4B). The in vivo activity of low concentrations of Yam-I were consistent with the low functional concentrations found in in vitro assays, suggesting that the Yam-I fraction is a concentrated source of the bioactive agonists.
Figure 4.
Neutrophil trafficking induced by Yamoa™-derived polysaccharides. A. BALB/c (WT) and CXCR2-deficient mice (at least 3 per group) were treated by intraperitoneal injection with PBS, 250 µg of Yamoa™ or 30 µg Yam-I per mouse, and the number of neutrophils that accumulated in the peritoneum (top panel) and blood (bottom panel) was measured after 3 hours. Error bar denotes SEM, *p<0.01. B. Yam-I contains a concentrated source of the active component of Yamoa™. Yamoa™ (250 µg) and lower concentrations of Yam-I were tested for induction of peritonitis in mice (at least 3 per group) by analysis of neutrophil recruitment (top panel, error bar denotes SEM) and serum CXCL1/KC by ELISA (error bar denotes SD), 3 hours after intraperitoneal injection.
To better characterize the distinction between Yam-I and LPS or other potential bacterial contaminants, we measured the peritonitis response in mice deficient for TLR4 and MyD88. Both C3H:HeJ (TLR4-deficient) and C3H:HeOuJ (TLR4-competent) mice responded to intraperitoneal injection of all isolated fractions of Yamoa™; however, the optimal concentrations differed between the two mouse strains (Figure 5A). Specifically, TLR4-deficient mice had greater neutrophil influx responses to higher doses of Yam-I, and TLR4-competent mice responded better to lower doses, more similar to BALB/c mice. In an effort to address the possible contamination of Yam-I with LPS that may be contributing to the TLR4-dependent response, we attempted to remove any contaminating LPS from Yam-I using Polymyxin B (PMB). Yam-I obtained from before and after two sequential rounds of PMB treatment was tested in the peritonitis model using C3H:HeJ and C3H:HeOuJ mice. The results shown in Figure 5B indicated that the response in TLR4-competent mice was not decreased by PMB treatment of Yam-I. Similarly, the TLR4-deficient mice had a slightly improved response to the 25 µg injection of PMB treated Yam-I, and a slightly decreased response to 2.5 µg, consistent with their diminished responses to lower doses. To better characterize potential TLR4-dependent and -independent components in the Yam-I sub-fractions (Yam-I-1, −2 and −3), a murine cell priming assay, as described for Figure 2B, was conducted in these mouse strains. Slight differences were detected between the 3 sub-fractions. The specific subsets of γδ T cells that proliferated in C3H:HeJ and C3H:HeOuJ mice in response to Yam-I-1 and Yam-I-2 were similar, but the magnitude of the response was slightly greater in TLR4-competent mice, suggesting again, some contribution of TLR4 in the response to these fractions (Figure 5C, D). In contrast, the strongest γδ T cell response was detected after stimulation with Yam-I-3 and the response was similar whether the cells were derived from C3H:HeOuJ or C3H:HeJ mice (Figure 5C, D). Thus, the γδ T cell specific fraction (Yam-I-3) appeared to be predominantly TLR4-independent. Finally, to address the concern that the TLR4-independent agonist(s) may be dependent on TLR2, or other MyD88-dependent PAMP receptors [43], we confirmed that MyD88-deficient mice (on C57BL/6 background) responded after injection of 5 µg of Yam-I in the same peritonitis model (Figure 5E). Thus, Yam-I clearly contains the relevant bioactivity in Yamoa™ in these assays and it appears to be a distinct agonist for γδ T cells that is not completely dependent on TLR4 or MyD88.
Figure 5.
Responses of TLR-deficient mice to Yamoa™-derived polysaccharides. A. In a peritonitis assay, C3H:HeJ (TLR4-deficient) and C3H:HeOuJ (TLR4-competent) mice (at least 3 per group) were injected i.p. with Yamoa™ (250 µg) and polysaccharide fractions derived from it (30, 3 and 0.3 µg Yam-I, or 30 µg Yam-II or YamPS). After 3 hours, neutrophil recruitment into the peritoneum was measured by flow cytometry. Error bars represent SD. B. Yam-I was treated with two rounds of treatment with PMB beads, 2.5 and 25 µg doses of pre- and post- treatment Yam-I were injected into C3H:HeJ and C3H:HeOuJ mice (5 mice per group), and peritonitis was similarly measured. Thin bar indicates mean, thick error bars represent SEM. C. Spleen cells from C3H:HeJ mice were treated with the 3 sub-fractions of Yam-I in a priming assay, and proliferation was measured using CFSE staining in flow cytometry. Histograms show cells gated based on forward and side scatter (lymphocytes) and γδ T cell staining. D. Comparison of the percent of γδ T cells that proliferated in a priming assays described for 5C using spleen cells from C3H:HeOuJ or C3H:HeJ mice (at least 3 per group). Error bars represent SD. E. The fold change in % neutrophil in MyD88-deficient mice (on a C57/BL6 background) was compared to C57/BL6 mice in the peritonitis assay (as in 5A) after injection of 5 µg Yam-I or sterile saline. (3 mice per treatment, error bar denotes SD, *p<0.01).
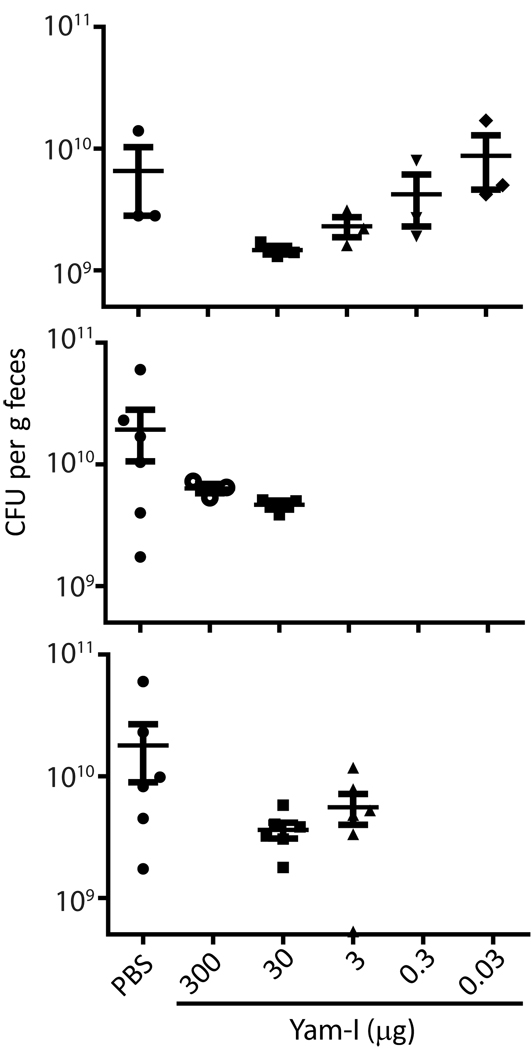
To determine the potential for therapeutic treatment with Yamoa™ and Yam-I in a setting with relevance to human infectious disease, we utilized a mouse model of ST-induced enterocolitis [33]. Mice were pre-treated with streptomycin and infected with a streptomycin-resistant strain of ST. Eight hours after infection, mice were treated with varying doses of Yam-I by i.p. injection or PBS. At 24 hours after infection, mice were euthanized, and ST CFU counts in feces were determined. As shown in Figure 6, a 30 µg dose of Yam-I was the optimal effective dose for reduction of fecal ST CFU counts, and the difference between groups of mice treated with this optimal dose of Yam-I and vehicle only control was statistically significant (p=0.0234). It was also apparent that treatment with Yam-I at many doses greatly reduced variability between animals; however, wide variation between PBS-treated animals precluded statistical significance within experiments. These data suggest that Yam-I or Yamoa™ treatment may be effective as therapeutic innate immunostimulants in ST infection and potentially other infectious disease settings.
Figure 6.
Therapeutic treatment with Yam-I of mice with ST-induced enterocolitis reduced ST CFU counts in the feces. BALB/c mice (3–6 mice per treatment group) were infected with ST (1×106 CFU/mouse) and 8 hours later injected with the indicated doses of Yam-I, or vehicle only (PBS). At 24 hours after infection, fecal pellets were collected for assessment of bacterial load on LB agar containing 50 µg/ml streptomycin (CFU/g feces). Three separate experiments are shown, thin bar indicates mean, thick error bars represent SEM.
Discussion
Both prophylactic and post-exposure strategies involving innate immune stimulation by adjuvants have been shown to prevent or ameliorate infections [44,45]. Unlike many studies of innate immunostimulants that target monocytic lineage cells, we have focused on specific stimulation of γδ T cells. These cells localize to mucosal surfaces, where they play roles in innate protection and maintenance of homeostasis [3,46]. As a major population of intraepithelial lymphocytes in the gastrointestinal tract, γδ T cells are also uniquely positioned to respond to agonists ingested in food and dietary supplements. Here, we report that agonists in Yamoa™, a supplement with anecdotal immune benefits, specifically primed γδ T cells. Consistent with the responses of leukocytes to other plant-derived polysaccharides [38,47], monocytes, as well as other non-γδ T lymphocytes, such as B cells, also responded to Yamoa™. Yamoa™ can stimulate γδ T cells directly as evidenced by gene expression changes in purified bovine γδ T cells that were similar to, but greater in number and magnitude, than those induced by LPS. The changes in chemokine transcript expression induced in γδ T cells were consistent with in vivo changes (peritonitis) observed in mice. Interestingly, in models very similar to the peritonitis response we describe, such as an experimental sepsis model [48] and an E. coli i.p. infection model [49], a critical role for mouse γδ T cells in inflammatory IL-17 expression has been demonstrated. A similar role was also demonstrated for lung γδ T cells [50]. Thus, despite representing a very small cell population, the response of γδ T cells to innate adjuvants may have global consequences in vivo.
The role of microbial contaminants in plant-derived products has been the focus of recent studies [43,51]. Of note, Yam-I demonstrated a strong positive result in a standard limulus amoebocyte lysate (LAL) assay that was not altered by PMB treatment, but this is likely complicated by β-glucans or similar component(s) of the polysaccharides in Yam-I, which may also cause a positive result in the LAL assay. Although Yam-I appeared functionally similar to LPS in a number of assays, there were clear differences between Yam-I and bacterial LPS, suggesting that Yamoa™ contains novel polysaccharide agonists. The most obvious difference was the observation that human γδ T cells proliferated in response to Yamoa™, whereas treatment with LPS elicited minimal responses. Furthermore, TLR4-deficient mice responded to Yamoa™ and all fractions derived from it, though higher concentrations were required compared to those required for wild-type mice. MyD88-deficient mice also responded to low doses of Yam-I. Thus, although there appears to be TLR4-dependent components in Yam-I, it also contains MyD88 and TLR4-independent components. Even though TLR2 signaling is dependent on MyD88, results from assays measuring neutrophil trafficking and γδ T cell priming in response to Yam-I performed in TLR2-deficient mice confirmed data from MyD88-deficient mice (data not shown). The use of a non-TLR receptor may, in part, explain the enhanced activity of Yamoa™ compared to LPS on γδ T cells, on which expression of TLRs is difficult to demonstrate. In contrast, monocytes responded similarly to Yamoa™ and LPS, based on gene expression data, suggesting that TLR4 may be more relevant for the response of these cells. Yamoa™ did not display the characteristic laddering effects in SDS-PAGE analysis seen with LPS, PMB treatment did not remove the relevant γδ T cell agonist activity from the isolated polysaccharide preparation, LPS signatures were not detected in NMR analyses and similar results were obtained from multiple lots from two different Yamoa™ suppliers, all of which argue against random bacterial contamination in the Yamoa™ preparations. Collectively, our data supports the existence of novel polysaccharide γδ T cell agonists in Yamoa™, which represents the first report of such activity in a plant-derived product.
Our data does not preclude the possibility that the relevant agonists in the plant bark are derived from commensal microbes (endophytes) associated with the plant. However, there is precedence for novel non-LPS, plant-derived polysaccharides that interact with TLR4 to drive monocyte responses and that result in non-TLR4-dependent T cell responses. Tsuji et al. determined that TLR4/MyD88-dependent responses to high molecular weight poly galactans (λ-carrageenans) isolated from red algae were dominant for monocyte-driven stimulation of innate immunity, but TLR4/MyD88-independent responses were most notable in IFN-γ production in T cell cultures and induction of oral tolerance [47]. Similarly, our results suggest that a TLR4-independent component (Yam-I-3) may have particular specificity for γδ T cells. Clearly, Yam-I functionally overlaps with LPS to some extent but the intestinal mucosa is constantly exposed to LPS from food, environment and normal flora. Furthermore, in our oral Salmonella model, extensive additional exposure to bacterial LPS (and other PAMPs) occurred due to the bacterial infection. Despite this exposure, therapeutic addition of Yam-I induced rapid and robust beneficial cellular responses at the intestinal surface resulting in decreased CFU counts in feces. Thus, whether the agonist activity found in Yamoa™ is plant or endophytic microbe derived, or a perhaps inseparable combination of both, it clearly contains unique capacity for innate stimulation. Despite the specific source of the agonists in Yamoa™, it is ingested by the public, thus thorough studies of changes induced by its ingestion are warranted to fully understand its mechanisms and appreciate its potential.
The only published characterizations of Funtumia elastica describes the isolation of steroidal alkaloids from its leaves and bark with antiplasmodial [52] or antifungal activity [53]. Steroids may also be one component involved in the anecdotal effect of Yamoa™ on asthma, but our data suggesting immunostimulatory effects of Yamoa™ are inconsistent with anti-inflammatory effects attributed to steroids. Asthma is considered a TH2 cell-mediated disease, and treatments that increase TH1 responses can be effective in alleviating symptoms [54]. The TH1 response is the signature response of γδ T cells and also a known result of LPS signaling. Whereas some studies suggest a role for γδ T cells in negative regulation of airway responsiveness [55,56], others using γδ T cell-deficient mice implicate them as contributory to lung pathology [11,57,58]. Recently specific production of IFN-γ by γδ T cells was shown to result in reduced airway responses in rats [59]. Clearly outcomes may depend on the various models of study, and activity of specific subsets of γδ T cells, that may not necessarily translate directly to asthma in humans. Specific stimulation of human γδ T cells with Yamoa™ or derived polysaccharides is potentially relevant in asthma and may be warranted as a novel approach in the field.
Yamoa™ contains an effective innate adjuvant that appears safe and may have potential for application in disease settings. Analyses of polysaccharide fractions isolated from Yamoa™ showed that Yam-I was effective in vitro and in vivo at much lower concentrations compared to Yamoa™, suggesting it contained a concentrated source of the active component in Yamoa™. Specific activity at low doses may support potential for relevant systemic effects after ingestion of Yamoa™. To our knowledge this is the first report of specific stimulation of γδ T cells by a plant derived product that is attributable to a polysaccharide fraction. These preliminary studies and the anecdotal reports of use in humans suggest that adjuvants contained in Yamoa™ may have similar stimulatory activity on immune cells as do other innate adjuvants, with no evidence of toxic or other adverse effects. Our data suggest that Yamoa™ contains polysaccharides with unique innate stimulatory capacity.
Supplementary Material
Acknowledgements
We thank Diana Buckner, Andrew Ramstead and Gang Xie for technical support. MyD88−/− mice were kindly provided by Dr. Kieren A. Marr, Division of Infectious Diseases, Oregon Health and Science University, Portland, OR. We would also like to thank Dr Scott Busse (Montana State University, Bozeman, MT) for help in running the NMR samples. This study was supported by funding from the National Institutes of Health (NIH) (NCCAM AT0004986-01), NIAID Contract (HHSN26620040009C/N01-AI-40009), NIH COBRE (P20 RR020185), M.J. Murdock Charitable Trust and The Montana State University Agricultural Experimental Station.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Holderness J, Jackiw L, Kimmel E, Kerns HMM, Radke M, Hedges JF, Petrie C, McCurley P, Glee PM, Palecanda A, Jutila MA. Select plant tannins induce IL-2Rα up-regulation and augment cell division in γδ T cells. J Immunol. 2007;179:6468–6478. doi: 10.4049/jimmunol.179.10.6468. [DOI] [PubMed] [Google Scholar]
- 2.Holderness J, Hedges JF, Daughenbaugh KF, Kimmel E, Graff JC, Freedman B, Jutila MA. Response of γδ T cells to plant-derived tannins. Crit Rev Immunol. 2008;28:377–402. doi: 10.1615/critrevimmunol.v28.i5.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hayday AC. [gamma][delta] cells: a right time and a right place for a conserved third way of protection. Ann Rev Immunol. 2000;18:975–1026. doi: 10.1146/annurev.immunol.18.1.975. [DOI] [PubMed] [Google Scholar]
- 4.Rivas A, Koide J, Cleary ML, Engleman EG. Evidence for involvement of the gamma, delta T cell antigen receptor in cytotoxicity mediated by human alloantigen-specific T cell clones. J Immunol. 1989;142:1840–1846. [PubMed] [Google Scholar]
- 5.Ciccone E, Viale O, Bottino C, Pende D, Casorati G, Tambussi G, Moretta A, Moretta L. Antigen recognition by human T cell receptor gamma-positive lymphocytes. Specific lysis of allogeneic cells after activation in mixed lymphocyte culture. J Exp Med. 1988;167:1517-:22. doi: 10.1084/jem.167.4.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Born W, Cady C, Jones-Carson J, Migone N, Mukasa A, Lahn M, O'Brien R. Immunoregulatory functions of gamma delta T cells. Adv Immunol. 1999;71:77–144. [PubMed] [Google Scholar]
- 7.Mak TW, Ferrick DA. The gamma delta T-cell bridge: linking innate and acquired immunity. Nat Med. 1998;4:764–765. doi: 10.1038/nm0798-764. [DOI] [PubMed] [Google Scholar]
- 8.Ferrick DA, Schrenzel MD, Mulvania T, Hsieh B, Ferlin WG, Lepper H. Differential production of interferon-gamma and interleukin-4 in response to Th1- and Th2-stimulating pathogens by gamma delta T cells in vivo. Nature. 1995;373:255–257. doi: 10.1038/373255a0. [DOI] [PubMed] [Google Scholar]
- 9.Collins RA, Werling D, Duggan SE, Bland AP, Parsons KR, Howard CJ. Gammadelta T cells present antigen to CD4+ alpha beta T cells. J Leukoc Biol. 1998;63:707–714. doi: 10.1002/jlb.63.6.707. [DOI] [PubMed] [Google Scholar]
- 10.Brandes M, Willimann K, Moser B. Professional antigen-presentation function by human gamma delta T Cells. Science. 2001 Feb. 16291(5507):1289–1292. doi: 10.1126/science.1110267. 2005;309:264-8. [DOI] [PubMed] [Google Scholar]
- 11.Zuany-Amorim C, Ruffie C, Haile S, Vargaftig BB, Pereira P, Pretolani M. Requirement for gamma delta T cells in allergic airway inflammation. Science. 1998;280:1265–1267. doi: 10.1126/science.280.5367.1265. [DOI] [PubMed] [Google Scholar]
- 12.O'Brien RL, Yin X, Huber SA, Ikuta K, Born WK. Depletion of a gamma delta T cell subset can increase host resistance to a bacterial infection. J Immunol. 2000;165:6472–6479. doi: 10.4049/jimmunol.165.11.6472. [DOI] [PubMed] [Google Scholar]
- 13.Egan PJ, Carding SR. Down modulation of the inflammatory response to bacterial infection by gamma delta T cells cytotoxic for activated macrophages. J Exp Med. 2000;191:2145–2158. doi: 10.1084/jem.191.12.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boismenu R, Havran WL. Modulation of epithelial cell growth by intraepithelial γδ T cells. Science. 1994;266:1253–1255. doi: 10.1126/science.7973709. [DOI] [PubMed] [Google Scholar]
- 15.Jameson J, Ugarte K, Chen N, Yachi P, Fuchs E, Boismenu R, Havran WL. A role for skin gamma delta T cells in wound repair. Science. 2002;296:747–749. doi: 10.1126/science.1069639. [DOI] [PubMed] [Google Scholar]
- 16.Ferrick DA, King DP, Jackson KA, Braun RK, Tam S, Hyde DM, Beaman BL. Intraepithelial gamma delta T lymphocytes: sentinel cells at mucosal barriers. Springer Semin Immunopathol. 2000;22:283–296. doi: 10.1007/s002810000047. [DOI] [PubMed] [Google Scholar]
- 17.Kamath AB, Wang L, Das H, Li L, Reinhold VN, Bukowski JF. Antigens in tea-beverage prime human Vgamma 2Vdelta 2 T cells in vitro and in vivo for memory and nonmemory antibacterial cytokine responses. PNAS. 2003;100:6009–6014. doi: 10.1073/pnas.1035603100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bukowski JF, Morita CT, Brenner MB. Human gamma delta T cells recognize alkylamines derived from microbes, edible plants, and tea: implications for innate immunity. Immunity. 1999;11:57–65. doi: 10.1016/s1074-7613(00)80081-3. [DOI] [PubMed] [Google Scholar]
- 19.Nantz MP, Rowe CA, Nieves C, Jr, Percival SS. Immunity and Antioxidant Capacity in Humans Is Enhanced by Consumption of a Dried, Encapsulated Fruit and Vegetable Juice Concentrate. J Nutr. 2006;136:2606–2610. doi: 10.1093/jn/136.10.2606. [DOI] [PubMed] [Google Scholar]
- 20.Percival SS, Bukowski JF, Milner J. Bioactive Food Components that Enhance {gamma}{delta} T Cell Function May Play a Role in Cancer Prevention. J Nutr. 2008;138:1–4. doi: 10.1093/jn/138.1.1. [DOI] [PubMed] [Google Scholar]
- 21.Sicard H, Ingoure S, Luciani B, Serraz C, Fournie JJ, Bonneville M, Tiollier J, Romagne F. In vivo immunomanipulation of V{gamma}9V{delta}2 T cells with a synthetic phosphoantigen in a preclinical nonhuman primate model. J Immunol. 2005;175:5471–5480. doi: 10.4049/jimmunol.175.8.5471. [DOI] [PubMed] [Google Scholar]
- 22.Dieli F, Gebbia N, Poccia F, Caccamo N, Montesano C, Fulfaro F, Arcara C, Valerio MR, Meraviglia S, Di Sano C, Sireci G, Salerno A. Induction of gamma delta T-lymphocyte effector functions by bisphosphonate zoledronic acid in cancer patients in vivo. Blood. 2003;102:2310–2311. doi: 10.1182/blood-2003-05-1655. [DOI] [PubMed] [Google Scholar]
- 23.Casetti R, Perretta G, Taglioni A, Mattei M, Colizzi V, Dieli F, D'Offizi G, Malkovsky M, Poccia F. Drug-induced expansion and differentiation of V{gamma}9V{delta}2 T cells in vivo: The role of exogenous IL-2. J Immunol. 2005;175:1593–1598. doi: 10.4049/jimmunol.175.3.1593. [DOI] [PubMed] [Google Scholar]
- 24.Hedges JF, Buckner DL, Rask KM, Kerns HMM, Jackiw LO, Trunkle TC, Pascual DW, Jutila MA. Mucosal lymphatic-derived γδ T cells respond early to experimental Salmonella enterocolits by increasing expression of IL-2Rα. Cell Immunol. 2007;246:8–16. doi: 10.1016/j.cellimm.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jutila MA, Holderness J, Graff JC, Hedges JF. Antigen Independent priming: A transitional response of bovine γδ T cells to infection. Anim Health Res Rev. 2007;9:47–57. doi: 10.1017/S1466252307001363. [DOI] [PubMed] [Google Scholar]
- 26.Hedges JF, Lubick KJ, Jutila MA. γδ T cells respond directly to pathogen associated molecular patterns. J Immunol. 2005;174:6045–6053. doi: 10.4049/jimmunol.174.10.6045. [DOI] [PubMed] [Google Scholar]
- 27.Kerns HMM, Jutila MA, Hedges JF. The distinct response of γδ T cells to the Nod2 agonist, muramyl dipeptide. Cell Immunol. 2009;257:38–43. doi: 10.1016/j.cellimm.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lahmers KK, Hedges JF, Jutila MA, Deng M, Abrahamsen MS, Brown WC. Comparative gene expression by WC1+ {gamma}{delta} and CD4+ {alpha}{beta} T lymphocytes, which respond to Anaplasma marginale, demonstrates higher expression of chemokines and other myeloid cell-associated genes by WC1+ {gamma}{delta} T cells. J Leukoc Biol. 2006;80:939–952. doi: 10.1189/jlb.0506353. [DOI] [PubMed] [Google Scholar]
- 29.Hughes DP, Hayday A, Craft JE, Owen MJ, Crispe IN. T cells with gamma/delta T cell receptors (TCR) of intestinal type are preferentially expanded in TCR-alpha-deficient lpr mice. J Exp Med. 1995;182:233–241. doi: 10.1084/jem.182.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xie G, Schepetkin IA, Siemsen DW, Kirpotina LN, Wiley JA, Quinn MT. Fractionation and characterization of biologically-active polysaccharides from Artemisia tripartita. Phytochemistry. 2008;69:1359–1371. doi: 10.1016/j.phytochem.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xie G, Schepetkin IA, Quinn MT. Immunomodulatory activity of acidic polysaccharides isolated from Tanacetum vulgare L. Int Immunopharm. 2007;7:1639–1650. doi: 10.1016/j.intimp.2007.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dubois M, Gilles K, Hamilton JK, Rebers PA, Smith F. A colorimetric method for the determination of sugars. Anal Chem. 1956;28:350–356. doi: 10.1038/168167a0. [DOI] [PubMed] [Google Scholar]
- 33.Barthel M, Hapfelmeier S, Quintanilla-Martinez L, Kremer M, Rohde M, Hogardt M, Pfeffer K, Russmann H, Hardt WD. Pretreatment of mice with streptomycin provides a Salmonella enterica serovar Typhimurium colitis model that allows analysis of both pathogen and host. Infect Immun. 2003;71:2839–2858. doi: 10.1128/IAI.71.5.2839-2858.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Graff JC, Jutila MA. Differential regulation of CD11b on γδ T cells and monocytes in response to unripe apple tannins. J.Leukoc.Biol. 2007;82:603–607. doi: 10.1189/jlb.0207125. [DOI] [PubMed] [Google Scholar]
- 35.Toth GB, Pavia H. Removal of dissolved brown algal phlorotannins using insoluble polyvinylpolypyrrolidone (PVPP) J Chem Ecol. 2001;27:1899–1910. doi: 10.1023/a:1010421128190. [DOI] [PubMed] [Google Scholar]
- 36.Amaro C, Biosca EG, Fouz B, Garay E. Electrophoretic analysis of heterogeneous lipopolysaccharides from various strains of Vibrio vulnificus biotypes 1 and 2 by silver staining and immunoblotting. Curr Microbiol. 1992;2:99–104. doi: 10.1007/BF01570967. [DOI] [PubMed] [Google Scholar]
- 37.Firoozkoohi J, Zandi H, Olsen I. Comparison of lipopolysaccharides from Bacteroides, Porphyromonas, Prevotella, Campylobacter and Wolinella spp. by tricine-SDS-PAGE. Endod Dent Traumatol. 1997;13:13–18. doi: 10.1111/j.1600-9657.1997.tb00003.x. [DOI] [PubMed] [Google Scholar]
- 38.Schepetkin IA, Quinn MT. Botanical polysaccharides: Macrophage immunomodulation and therapeutic potential. Int Immunopharm. 2006;6:317–333. doi: 10.1016/j.intimp.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 39.Singh T, Wu JH, Peumans WJ, Rougé P, Van Damme EJM, Wu AM. Recognition profile of Morus nigra agglutinin (Morniga G) expressed by monomeric ligands, simple clusters and mammalian polyvalent glycotopes. Mol Immunol. 2007;44:451–462. doi: 10.1016/j.molimm.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 40.Wengner AM, Pitchford SC, Furze RC, Rankin SM. The co-ordinated action of G-CSF and ELR+CXC chemokines in neutrophil mobilisation during acute inflammation. Blood. 2008;111:42–49. doi: 10.1182/blood-2007-07-099648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McColl SR, Clark-Lewis I. Inhibition of murine neutrophil recruitment in vivo by CXC chemokine receptor antagonists. J Immunol. 1999;163:2829–2835. [PubMed] [Google Scholar]
- 42.Tanimoto N, Terasawa M, Nakamura M, Kegai D, Aoshima N, Kobayashi Y, Nagata K. Involvement of KC, MIP-2, and MCP-1 in leukocyte infiltration following injection of necrotic cells into the peritoneal cavity. Biochem Biophys Res Commun. 2007;361:533–536. doi: 10.1016/j.bbrc.2007.07.060. [DOI] [PubMed] [Google Scholar]
- 43.Pugh ND, Tamta H, Balachandran P, Wu X, Howell J, Dayan FE, Pasco DS. The majority of in vitro macrophage activation exhibited by extracts of some immune enhancing botanicals is due to bacterial lipoproteins and lipopolysaccharides. Int Immunopharmacol. 2008;8:1023–1032. doi: 10.1016/j.intimp.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dittmer U, Olbrich AR. Treatment of infectious diseases with immunostimulatory oligodeoxynucleotides containing CpG motifs. Curr Opin Microbiol. 2003;6:472–477. doi: 10.1016/j.mib.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 45.Amlie-Lefond C, Paz DA, Connelly MP, Huffnagle GB, Dunn KS, Whelan NT, Whelan HT. Innate immunity for biodefense: A strategy whose time has come. J Allergy Clin Immunol. 2005;116:1334–1342. doi: 10.1016/j.jaci.2005.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jameson J, Havran WL. Skin gammadelta T-cell functions in homeostasis and wound healing. Immunol Rev. 2007;215:114–122. doi: 10.1111/j.1600-065X.2006.00483.x. [DOI] [PubMed] [Google Scholar]
- 47.Tsuji RF, Hoshino K, Noro Y, Tsuji NM, Kurokawa T, Masuda T, Akira S, Nowak B. Suppression of allergic reaction by lambda-carrageenan: toll-like receptor 4/MyD88-dependent and -independent modulation of immunity. Clin Exp.Allergy. 2003;33:249–258. doi: 10.1046/j.1365-2222.2003.01575.x. [DOI] [PubMed] [Google Scholar]
- 48.Flierl MA, Rittirsch D, Gao H, Hoesel LM, Nadeau BA, Day DE, Zetoune FS, Sarma JV, Huber-Lang MS, Ferrara JLM, Ward PA. Adverse functions of IL-17A in experimental sepsis. FASEB J. 2008;22:2198–2205. doi: 10.1096/fj.07-105221. [DOI] [PubMed] [Google Scholar]
- 49.Shibata K, Yamada H, Hara H, Kishihara K, Yoshikai Y. Resident V{delta}1+ {gamma}{delta} T Cells Control Early Infiltration of Neutrophils after Escherichia coli Infection via IL-17 Production. J Immunol. 2007;178:4466–4472. doi: 10.4049/jimmunol.178.7.4466. [DOI] [PubMed] [Google Scholar]
- 50.Lockhart E, Green AM, Flynn JL. IL-17 Production Is Dominated by {gamma}{delta} T Cells rather than CD4 T Cells during Mycobacterium tuberculosis Infection. J Immunol. 2006;177:4662–4669. doi: 10.4049/jimmunol.177.7.4662. [DOI] [PubMed] [Google Scholar]
- 51.Tamta H, Pugh ND, Balachandran P, Moraes R, Sumiyanto J, Pasco DS. Variability in in vitro macrophage activation by commercially diverse bulk echinacea plant material is predominantly due to bacterial lipoproteins and lipopolysaccharides. J Agric Food Chem. 2008;56:10552–10556. doi: 10.1021/jf8023722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zirihi GN, Grellier P, Guede-Guina F, Bodo B, Mambu L. Isolation, characterization and antiplasmodial activity of steroidal alkaloids from Funtumia elastica (Preuss) Stapf. Bioorg Med Chem Lett. 2005;15:2637–2640. doi: 10.1016/j.bmcl.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 53.Adekunle AA, Ikumapayi AM. Antifungal property and phytochemical screening of the crude extracts of Funtumia elastica and Mallotus oppositifolius. West Indian Med J. 2006;55:219–223. doi: 10.1590/s0043-31442006000400003. [DOI] [PubMed] [Google Scholar]
- 54.Zosky GR, Sly PD. Animal models of asthma. Clin Exp.Allergy. 2007;37:973–988. doi: 10.1111/j.1365-2222.2007.02740.x. [DOI] [PubMed] [Google Scholar]
- 55.Lahn M, Kanehiro A, Takeda K, Joetham A, Schwarze J, Kohler G, O'Brien R, Gelfand EW, Born W, Kanehio A. Negative regulation of airway responsiveness that is dependent on gamma delta T cells and independent of alphabeta T cells. Nat Med. 1999;5:1150–1156. doi: 10.1038/13476. [DOI] [PubMed] [Google Scholar]
- 56.Lahn M, Kanehiro A, Takeda K, Terry J, Hahn YS, Aydintug MK, Konowal A, Ikuta K, O'Brien RL, Gelfand EW, Born WK. MHC class I-dependent Vgamma4+ pulmonary T cells regulate alpha beta T cell-independent airway responsiveness. PNAS. 2002;99:8850–8855. doi: 10.1073/pnas.132519299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Svensson L, Lilliehook B, Larsson R, Bucht A. gammadelta T cells contribute to the systemic immunoglobulin E response and local B-cell reactivity in allergic eosinophilic airway inflammation. Immunology. 2003;108:98–108. doi: 10.1046/j.1365-2567.2003.01561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schramm CM, Puddington L, Yiamouyiannis CA, Lingenheld EG, Whiteley HE, Wolyniec WW, Noonan TC, Thrall RS. Proinflammatory roles of T-cell receptor (TCR)gamma delta and TCRalpha beta lymphocytes in a murine model of asthma. Am J Respir Cell Mol.Biol. 2001 Jul. 25(1):125–131. doi: 10.1165/ajrcmb.22.2.3620. 2000;22:218-25. [DOI] [PubMed] [Google Scholar]
- 59.Isogai S, Athiviraham A, Fraser RS, Taha R, Hamid Q, Martin JG. Interferon-gamma-dependent inhibition of late allergic airway responses and eosinophilia by CD8+gammadelta T cells. Immunology. 2007;122:230–238. doi: 10.1111/j.1365-2567.2007.02632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.