Abstract
Recent research indicates that glial cells control complex functions within the nervous system. For example, it has been shown that glial cells contribute to the development of pathological pain, the process of long-term potentiation, and the formation of memories. These data suggest that glial cell activation exerts both adaptive and pathological effects within the CNS. To extend this line of work, the present study investigated the role of glia in spinal learning and spinal learning deficits using the spinal instrumental learning paradigm. In this paradigm rats are transected at the second thoracic vertebra (T2) and given shock to one hind limb whenever the limb is extended (controllable shock). Over time these subjects exhibit an increase in flexion duration that reduces net shock exposure. However, when spinalized rats are exposed to uncontrollable shock or inflammatory stimuli prior to testing with controllable shock, they exhibit a learning deficit. To examine the role of glial in this paradigm, spinal glial cells were pharmacologically inhibited through the use of fluorocitrate. Our results indicate that glia are involved in the acquisition, but not maintenance, of spinal learning. Furthermore, the data indicate that glial cells are involved in the development of both shock and inflammation-induced learning deficits. These findings are consistent with prior research indicating that glial cells are involved in both adaptive and pathological processes within the spinal cord.
Keywords: Spinal Instrumental Learning, Fluorocitrate, LPS, Inflammation, Microglia, Astrocytes, Spinal Plasticity, Recovery of Function, Spinal Cord Injury, Pain
1. Introduction
Early accounts of glial cell function focused primarily upon their role in neuronal support. However, recent research has revealed that neuronal support is only one facet of glial cell activity, and it has become apparent that glia can modulate many aspects of central nervous system (CNS) function. For example, Meller and colleagues (1994) demonstrated that glial cell inhibition attenuates inflammation-induced hyperalgesia. Research has also shown that glial cells underlie the hyperalgesic states induced by HIV-1 gp120 (Milligan et al., 2001) and formalin (Watkins et al., 1997), as well as mirror image pain (Milligan et al., 2003) and spinal nerve transection neuropathic pain (Sweiter et al., 2001; Takeda et al., 2004). Not only is glial cell activation necessary for these pathological pain states, it has been shown that an injection of activated microglia is sufficient to set off a cascade that leads to the development of pain in the absence of tissue damage (Narita et al., 2006).
There is also growing evidence that glial cells influence learning and memory. It has been demonstrated that glial cells proliferate (Jahanshahi et al., 2007, 2008), hypertrophy (Kleim et al., 2007), and undergo changes in gene expression (Hydén & Egyházi, 1963) in response to learning and memory tasks. Glial inhibition can also disrupt memory consolidation in single-trial aversion learning tasks (Gibbs et al., 2006a, 2006b). Furthermore, long-term potentiation (LTP) and long-term depression (LTD), which are thought to be the neural basis of learning and memory, appear to require glial cell activity (Ikeda & Murase, 2004; Ma & Zhao, 2002). This suggests that glial cells are actively involved in both adaptive and pathological processes within the CNS.
To extend this line of work, the present study investigated the role of glia in modulating spinal plasticity using the spinal instrumental learning paradigm, a simple instrumental (response-outcome) learning task (Grau et al., 1998, 2006). Prior research has demonstrated that the isolated spinal cord is capable of encoding the relationship between leg position and shock. If transected rats receive shock to the tibialis anterior muscle whenever their leg is extended (controllable shock), they learn to maintain their leg in a flexed position and, consequently, minimize net shock exposure. The increase in flexion duration is not merely an artifact of shock, given that transected rats that receive shock independent of leg position (uncontrollable shock) fail to exhibit an increase in flexion duration. Furthermore, exposure to uncontrollable shock undermines future learning, such that rats previously exposed to uncontrollable shock fail to exhibit an increase in flexion duration when later tested with controllable shock (Crown et al., 2002; Grau et al., 1998). This learning deficit can be induced by just 6-min of uncontrollable shock to the leg or tail and lasts up to 48 h (Crown et al., 2002).
It has been suggested that uncontrollable stimulation acts to disrupt learning by inducing a state comparable to central sensitization. In support of this hypothesis it has been demonstrated that stimuli that induce allodynia (e.g., carrageenan and capsaicin) can induce spinal instrumental learning deficits (Ferguson et al., 2006; Hook et al., 2008). For example, Young and colleagues (2007) demonstrated that peripherally administered lipopolysaccharide (LPS), which results in inflammation, glial activation, and allodynia, induces a spinal instrumental learning deficit. Furthermore, it was demonstrated that this deficit is mediated by LPS-induced inflammation. Based on these data the authors hypothesized that shock and LPS exert detrimental effects on learning, in part, through the activation of glial cells.
While evidence suggests that glial cell activation leads to central inflammatory processes that can impair learning, the application of this work to spinal learning is complicated by a number of observations. First, both controllable and uncontrollable aversive events can activate glial cells and initiate inflammatory processes (Blandino et al., 2006; Deak et al., 2005; Frank et al., 2007; Nair & Bonneau, 2006; O’Connor et al., 2003; Sugama et al., 2007). Second, in some cases low levels of inflammatory cytokines appear to enhance, rather than disrupt, learning (Avital et al., 2003; Bohme et al., 1993, 1991; Brennan et al., 2003; Goshen et al., 2007; Lu et al., 1999; Malen and Chapman, 1997; Pollmächer et al., 2002; Zhuo et al., 1993). Finally, glial cell activation can lead to the release of factors, such as BDNF, that enhance learning (Gómez-Pinilla et al., 2007; Yamada et al., 2002). Though complicating the derivation of experimental predictions, these observations lend further evidence that glial cells modulate plasticity and highlight the need for further research.
The present study examined the role of glial cells in the acquisition and maintenance of spinal instrumental learning and the acquisition of the spinal learning deficit. It was hypothesized that glial cell inhibition would disrupt the acquisition and maintenance of spinal instrumental learning. It was also hypothesized that blocking glial cell activation prior to uncontrollable shock or LPS administration would block the learning deficit as it blocks the development of experimentally induced pain. Glial cells were inhibited through the use of fluorocitrate. Fluorocitrate inhibits aconitase, a necessary product of the tricarboxylic acid (TCA) cycle of glial cells and, thereby, disrupts energy dependent transmitter up-take and release. When administered centrally at a 1 nmol dose, fluorocitrate’s actions are fast-acting, selective to glia, and reversible within 24 h (Paulsen et al., 1987). Our results provide further evidence that glial cells are involved in adaptive as well as pathological processes within the spinal cord.
2. Materials and Methods
2.1 Subjects
Male Sprague-Dawley rats from Harlan (Houston, TX) were used in all experiments (N = 134). At the time of testing, the rats were approximately 100–120 days old and weighed between 350–410 g. They were individually housed on a 12 h light-dark cycle with food and water available ad libitum. All procedures performed were approved by the Texas A&M University Laboratory Animal Care Committee.
2.2 Transection, Catheter Placement, and Post-Operative Care
In Experiment 1, surgical anesthesia was achieved by administration of 50 mg/kg pentobarbital. In all remaining experiments, subjects were anesthetized with 5% isoflurane gas, and once a surgical plane of anesthesia was reached, a maintenance concentration of 2% isoflurane was used. Once anesthetized, the shoulders were shaved and sterilized with iodine. A 1.5 cm anterior-posterior incision was made over the protuberance of the 2nd thoracic vertebra (T2) and the tissue above T2 was cleared to expose the spinal cord. Cauterization was used to transect the cord and the cavity was filled with Gelfoam (Harvard Apparatus, Holliston, MA). An intrathecal (i.t.) catheter, consisting of a polyethylene tube (PE-10, VWR International, Bristol, CT), was inserted to allow for i.t. drug delivery. The catheter was cut to 25 cm, fitted with a 0.09 mm stainless steel wire (Small Parts, Inc., Miami Lakes, FL), and inserted 9 cm down the dorsal surface of the spinal cord via the subarachnoid space. The incision was then closed with Michel Clips (Fine Science Tools, Forest City, CA), the catheter was secured with Super Glue, and the wire guide was removed.
To facilitated collection of our dependent measure of learning, the subjects’ hind legs were shaved and secured to their body in a natural flexed position with porous tape (Ortholetic 1.3 cm width). Immediately following surgery, rats were given a 5 mL intraperitoneal (i.p.) injection of 0.9% sterile saline and were allowed to recover in a temperature-controlled room (26.7°C) with food and water available ad libitum. Because the surgery results in a loss of bladder function, bladder expression occurred twice daily, along with supplemental i.p. injections of saline (5 mL). Upon completion of all necessary testing, rats were euthanized with 100 mg/kg of pentobarbital.
2.3 Instrumental Shock Apparatus
Instrumental learning was assessed using controllable leg shock (as described by Grau et al., 1998). In this procedure subjects were loosely restrained in a Plexiglas tube (8 cm diameter × 23.5 cm length) that was notched to allow subjects’ legs to hang freely. A shock generator (BRS/LVE Model SG-903, Laurel, MD) was used to administer shock. Two leg electrodes were connected to a computer-controlled relay that regulated the administration of shock. The first electrode consisted of stainless steel wire that was inserted through the subject’s skin over the tibia, 1.5 cm above the tarsals. The second electrode was constructed from a stainless steel pin inserted into the tibialis anterior muscle 1.7 cm above the first electrode. Shock delivery was achieved by attaching leads from an AC (60 Hz) shock generator (BRS/LVE, Model SG-903, Laurel, MD) to each electrode.
The shock intensity was adjusted to produce a flexion force of 0.4 Newton (N). In order to determine flexion force, a plastic line (4 lb test Stren: Dupont, Wilmington, DE) was connected to the subject’s foot immediately behind the planter protuberance. The line passed through the eyelet directly under the foot of the rat and attached to a strain gauge (Fort-100, World Precision Instruments, New Haven, CT) fastened to a ring stand. The ring stand was then positioned directly behind the subject and the line was stretched taut, engaging the strain gauge. A 0.3 s shock was administered to determine flexion force and voltage was adjusted to produce a 0.4 force. Once the necessary voltage was determined, the line was removed and the flexion force apparatus was put aside.
To monitor leg position, a plastic dish containing a salt solution was placed below the rat’s hind leg. A contact electrode, fashioned from a stainless steel rod (7cm long, 0.46 diameter) with the proximal end insulated with 2.5 cm of heat shrink tubing and attached to a fine wire (0.01 sq mm [36 AWG], [1] × 20 cm) connected to a digital input monitored by a computer, was secured with porous tape to the plantar surface of the foot just distal to the planter protuberance. To determine the resting position of the electrode, three short (0.15 s) shocks were delivered and the level of solution in the dish was adjusted so that the distal end of the electrode was submerged 4 mm below the surface of the salt solution. A wire ground was placed in the solution. During testing, when the electrode was in contact with the solution, a circuit was completed and a shock was administered to the tibialis anterior muscle. This shock induced a sufficient flexion force to draw the electrode out of the solution, which terminated the shock. A computer monitored the state of the circuit at a rate of 30 Hz.
Three behavioral measures were collected during testing: time in solution, response number, and response duration. These measures were used to assess the capacity of the subject to perform an instrumental response. Performance on these measures was assessed in 1 min bins for the duration of instrumental testing. The response number increased each time the contact electrode left the solution. Our primary index of learning, response duration, was derived for each bin using the following equation: Response Duration = (60 s − time in solution)/(response number + 1).
2.4 Uncontrollable Tail Shock
Uncontrollable tail shock was administered while the subject was loosely restrained in a Plexiglas tube (8 cm diameter × 23.5 cm length). Shock was delivered to the tail using an electrode constructed from a modified fuse clip. The clip was coated with electrocardiogram (ECG) gel (Harvard Apparatus, Holliston, MA) and secured 6 cm behind the base of the tail with porous tape. A 660-V transformer administered constant-current 1.5 mA shocks on a computer controlled random schedule. Shocks were administered using the method of Crown and colleagues (2002) in which 6 min of 80 ms shocks were administered on a variable inter-shock interval (range = 0.2 – 3.8 s; mean = 2 s). This procedure has been shown to reliably undermine future learning.
2.5 Drug Administration
Low doses of fluorocitrate (Sigma-Aldrich) selectively and reversibly disrupt the TCA cell cycle within glia and, thereby, disrupt energy dependent activity (Paulsen et al., 1987). Fluorocitrate was dissolved in 1 μL of saline to reach final concentrations of 0.0078, 0.0625, 0.5, and 4 nmol. LPS from E. coli 0111:B4, Sigma-Aldrich, St. Louis, MO) is an endotoxin derived from the cell wall of gram-negative bacteria. When administered to an organism LPS activates glial cells and leads to the release of proinflamamtory cytokines (Herber et al., 2006; Ledeboer et al., 2002; Sugaya et al., 1998; Terrazzino et al., 1997). LPS was dissolved in 10 μL of saline to produce a final concentration of 1, 10, and 100 μg. The drug or an equal volume of vehicle (sterile saline) was administered through the implanted catheter at the appropriate time point, followed by a 20 μL saline flush.
2.6 Experimental Designs
2.6.1. Experiment 1: Effect of fluorocitrate on spinal instrumental learning response acquisition
To determine if fluorocitrate would disrupt spinal instrumental learning subjects received complete spinal transactions 24 h prior to receiving intrathecal (i.t.) infusions of fluorocitrate or vehicle (n=6). Fluorocitrate was given in concentrations of 0.0078, 0.0625, 0.5, or 4 nmol (1 μL volume) 20 min prior to testing with 30 min of controllable shock.
2.6.2. Experiment 2: Effect of fluorocitrate on maintenance of the spinal instrumental response
We also assessed the role of glial cells in the maintenance of an already acquired instrumental response. Spinally transected subjects were randomly assigned to fluorocitrate or control groups (n=8). All subjects underwent 60 min of instrumental training. Twenty minutes into the 60 min training session subjects received either 0.5 nmol of fluorocitrate or vehicle (1 μL volume). Analysis was conducted on the last 30 min of testing, once fluorocitrate was given time to take effect.
2.6.3. Experiment 3: Effect of fluorocitrate administration on the expression of the shock-induced learning deficit
Subjects were randomly assigned to the fluorocitrate or vehicle group as well as the uncontrollable shock or no shock condition. Spinally transected subjects were given i.t. infusions of 0.5 nmol of fluorocitrate or vehicle (1 μL) twenty minutes prior to delivery of 6 min or 0 min of uncontrollable tailshock (n=6). Twenty-four hours later all subjects were tested with 30 min of controllable shock.
2.6.4. Experiment 4: Effect of i.t. LPS on spinal instrumental learning
In order to verify that centrally administered LPS would induce a learning deficit, spinally transected subjects were administered 1, 10, or 100 μg LPS or vehicle (10 μL volume) 2.5 h prior to testing with 30 min of controllable shock (n=6). Next, to determine if this deficit, like the shock-induced deficit, persists at least 24 h, subjects were administered 100 μg of LPS or vehicle (10 μL volume) 24 h prior to testing (n=6). Although a relatively high dose of LPS, this dose of i.t. LPS has been shown to facilitate wind up and enhance the acute C-fiber response (Reeve et al., 2000). Furthermore, a 150 μg dose of i.t. LPS has been shown to induce thermal hyperalgesia (Meller et al., 1994).
2.6.5. Experiment 5: Effect of fluorocitrate administration on the expression of the LPS-induced learning deficit
Finally, to determine the necessity of glia in the LPS-induced learning deficit, spinally transected subjects were administered 0.5 nmol of fluorocitrate or vehicle in a 1 μL volume 20 min prior to administration of 100 μg of LPS in a10 μL volume (n=6). Subjects were tested 24 h later with 30 min of controllable shock.
2.7 Statistics
The effect of experimental treatment was analyzed using repeated measures analysis of variance (ANOVA). A Tukey’s honestly significant difference (HSD) post hoc test was performed where appropriate. For all analyses, p < 0.05 was considered significant.
2.8 Baseline Measures of Behavioral Reactivity
Initial response durations and shock intensities required to produce a 0.4 N flexion force were analyzed across groups to ensure that drug and shock treatment had no impact on subjects’ capacity to make the necessary behavioral response. One-way ANOVAs performed on each measure in each experiment concluded that experimental conditions did not influence baseline measures of behavioral reactivity, all Fs < 2.86, ps > 0.05.
3. Results
3.1. Fluorocitrate dose-dependently inhibits acquisition of spinal instrumental learning
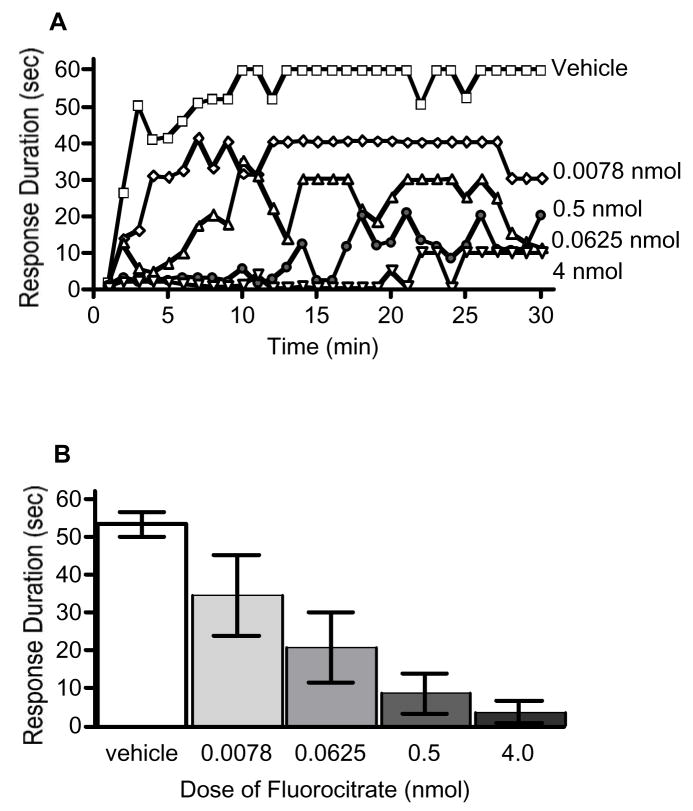
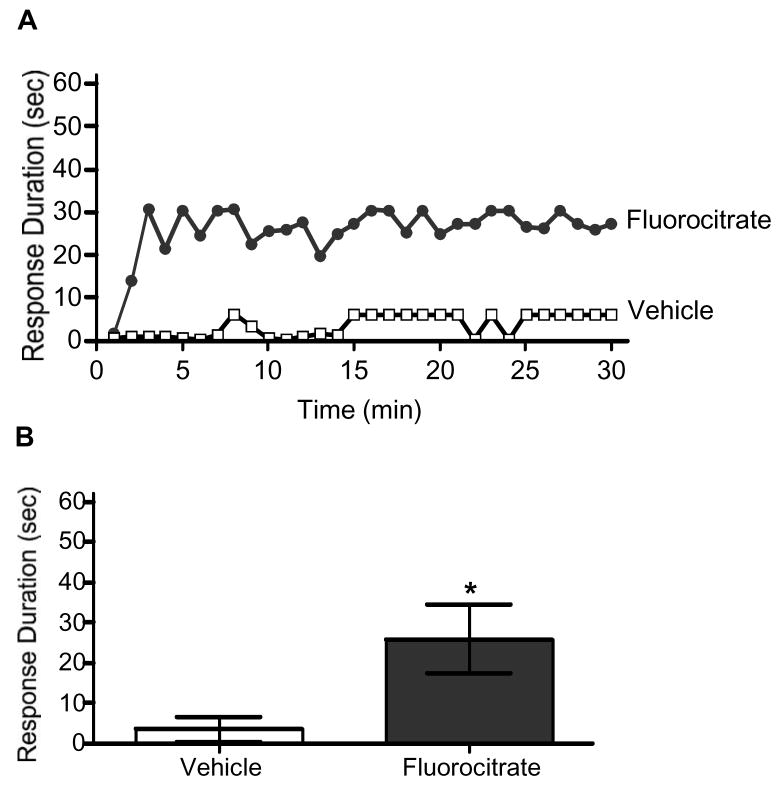
Previous research suggests that fluorocitrate can disrupt learning and memory consolidation (Gibbs et al., 2006a, 2006b; Hertz et al., 1996; O’Dowd et al., 1994) as well as spinally mediated pathological pain processing (Meller et al., 1994; Milligan et al., 2003; Obata et al., 2006; Watkins et al., 1997). To determine if fluorocitrate would interfere with spinally mediated instrumental learning, subjects were administered vehicle, 0.0078, 0.0625, 0.5, or 4 nmol fluorocitrate 20 min prior to testing. Subjects administered vehicle alone exhibited a progressive increase in response duration across the 30 min of training (Fig. 1). Conversely, administration of fluorocitrate prior to instrumental testing significantly attenuated subjects’ capacity to maintain a prolonged flexion response. An ANOVA revealed significant main effects of time, F(29,725) = 6.77, p < 0.001, and dose, F(4,25) = 9.21, p < 0.05. The ANOVA also indicated there was a significant time X dose interaction, F(116,725) = 1.34, p < 0.05. Post hoc analysis of group means showed that subjects administered vehicle maintained significantly longer response durations than subjects in the 0.0625, 0.5, and 4 nmol fluorocitrate dose groups, p < 0.05.
Fig. 1.
Fluorocitrate inhibits spinal instrumental learning in a dose dependent fashion. Spinally transected rats subjects received vehicle (open squares), 0.0078 (diamonds), 0.0625 (light triangles), 0.5 (circles), or 4 (dark triangles) nmol of fluorocitrate 20-min prior to the onset of controllable shock. Changes in response duration across time (A) and mean (±SEM) response durations (B) are depicted.
3.2. Fluorocitrate does not disrupt the maintenance of spinal instrumental learning
Prior research suggests that some manipulations, such as antagonizing NMDA receptors, not only disrupt the acquisition of spinal instrumental responses but also the maintenance of an already acquired response (Joynes et al., 2004). Furthermore, there is evidence that glial cell inhibition can not only act to prevent the development of pathological pain states, but also act to attenuate and/or reverse some already acquired pathological pain states (Hains & Waxman, 2006; Lan et al., 2007; Milligan et al., 2003; Obata et al., 2006; Zhuang et al., 2006). Therefore, we sought to determine if spinal glial inactivation could also disrupt the maintenance of the spinal instrumental response.
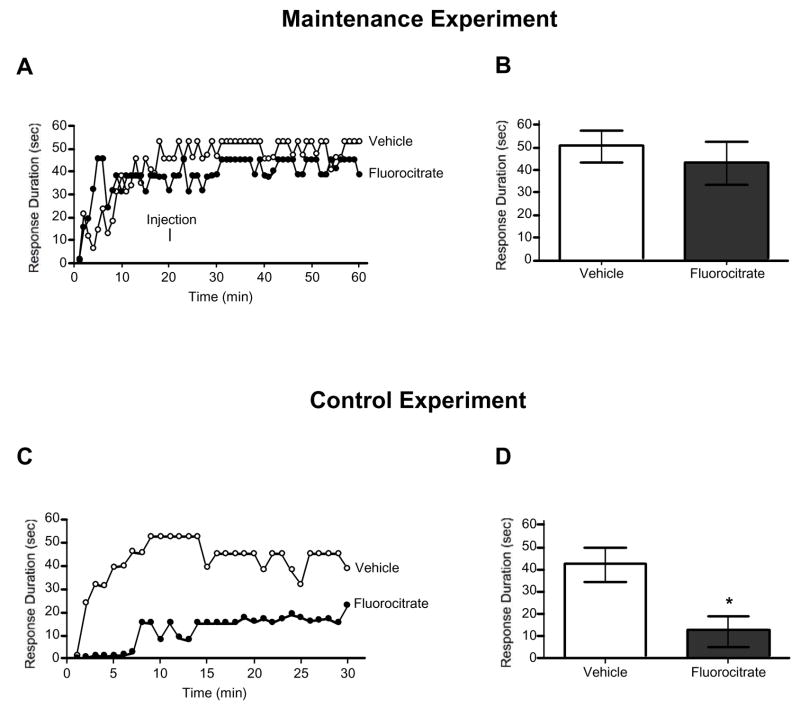
Subjects were administered 0.5 nmol of fluorocitrate or vehicle 20 min into a 60 min spinal instrumental learning testing session, sufficient time for subjects to master the task. The final 30 min of the testing session was analyzed to determine if fluorocitrate administration disrupted performance. Pretrained groups, however, continued to exhibit a prolonged flexion response regardless of drug condition, all Fs < 1.06, p > 0.05 (Fig. 2A). Given the null results, additional controls were included to verify the efficacy of fluorocitrate. Subjects were loosely restrained in testing tubes for 60 min, no shock was administered during the first 30 min, subjects were given vehicle or 0.5 nmol of fluorocitrate 20 min into the 60 min session, and at 30 min into the 60 min session instrumental testing began. As anticipated, subjects administered vehicle exhibited a progressive increase in response duration, while those administered fluorocitrate did not (Fig. 2B). An ANOVA revealed a significant main effect of time, F(29,406) = 3.88, p < 0.001, and dose, F(1,14) = 8.38, p < 0.05, as well as a significant time X dose interaction, F(29,406) = 1.70, p < 0.05. This interaction emerged because subjects administered vehicle exhibited significantly greater response durations over test trials, when compared to subjects administered fluorocitrate.
Fig. 2.
Fluorocitrate does not affect the maintenance of spinal instrumental learning. Subjects received 0.5 nmol fluorocitrate or vehicle 20-min into a 60-min test session. Changes in response duration across time (A) and mean (±SEM) response durations for the last 30-min of testing (B) are depicted. In the control experiment transected subjects received 0.5 nmol fluorocitrate or vehicle 10-min prior to 30-min of testing to verified the potency of the drug. Changes in response duration across time (C) and mean (±SEM) response durations for the last 30-min of testing (D) are depicted. An asterisk (*) indicates there was a statistically significant difference (p < .05).
3.3. Fluorocitrate protects against the development of the shock induced learning deficit
Prior exposure to uncontrollable shock inhibits the ability of subjects to learn about controllable shock (Crown et al., 2002; Grau et al., 1998). Acquisition of this deficit can be prevented by many substances that prevent pathological pain, such as NK-1 receptor antagonists and 5-HT agonists (Baumabauer et al., 2007; Crown & Grau, 2005). Given that glial cell inhibition can prevent pathological pain (Guo et al., 2007; Hua et al., 2005; Ledeboer et al., 2005; Mika et al., 2007; Milligan et al., 2003; Obata et al., 2006; Qin et al., 2006), we sought to determine if glial inhibition would also prevent the spinal instrumental learning deficit. Therefore, subjects were administered 0.5 nmol of fluorocitrate or vehicle 20 min prior to the delivery of 0 min or 6 min of uncontrollable shock.
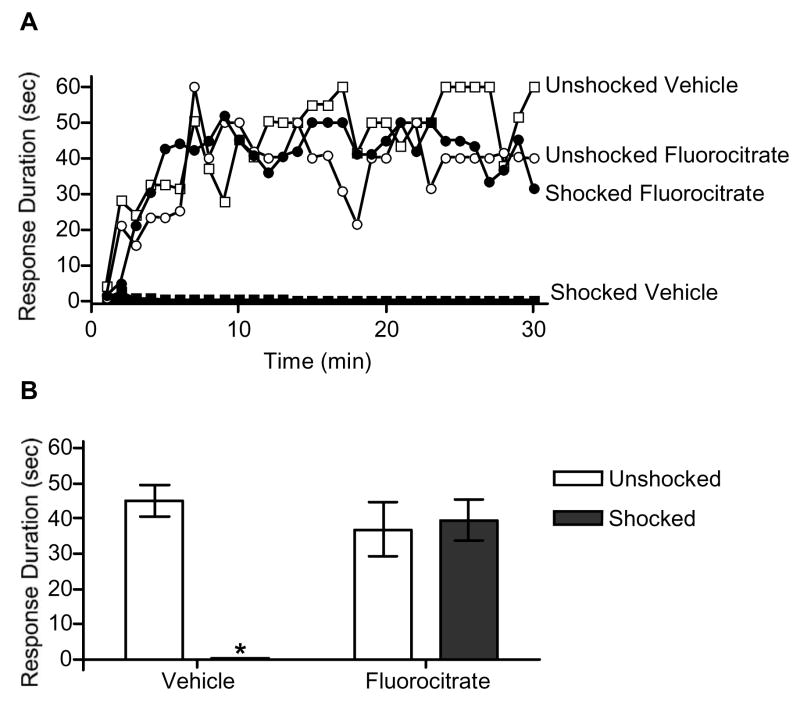
When tested 24 h later, unshocked subjects exhibited a progressive increase in response duration, irrespective of drug condition (Fig. 3). Conversely, shocked rats administered vehicle exhibited a learning deficit. Interestingly, pre-treatment with fluorocitrate prevented the induction of the shock induced learning deficit. An ANOVA revealed a significant main effect of time, F(29,580) = 5.40, p < 0.001, shock, F(1,20) = 13.05, p < 0.01, and drug, F(1,20) = 7.21, p < 0.05. The ANOVA also revealed significant time X shock, F(29,580) = 1.50, p < 0.05, and drug X shock, F(1,20) = 16.42, p <0.01, interactions. Post hoc analysis of group means indicate that subjects given saline had significantly shorter response durations than subjects in all other conditions, p < 0.05.
Fig. 3.
Fluorocitrate protects against the development of the learning deficit. Subjects received 0.5 nmol fluorocitrate or vehicle 20-min prior to the onset of controllable shock. Changes in response duration across time (A) and mean (±SEM) response durations (B) are depicted. An asterisk (*) indicates that a statistically significant difference was observed (p < .05).
3.4. Intrathecal LPS induces a spinal instrumental learning deficit
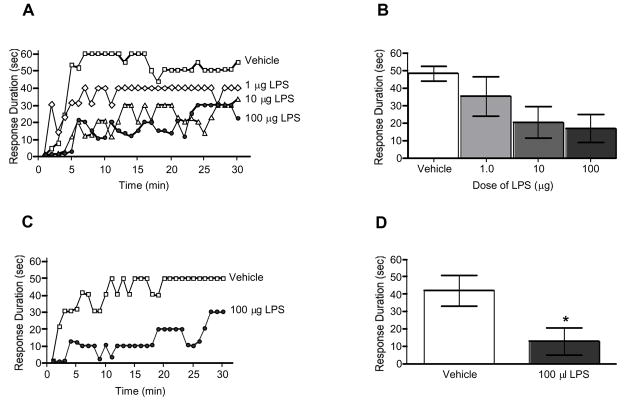
Previous research has shown that presenting spinally transected rats with an immune challenge using systemic LPS results in a shock-like learning deficit that can be reversed with an interleukin-1 (IL-1) receptor antagonist (Young et al., 2007). Given the important role that glial cells play in the release of IL-1 and other proinflammatory cytokines, it is likely that spinal glial cells are involved in the production of this learning deficit. In an attempt to reduce systemic inflammation and to more specifically target central inflammatory processes, subjects were administered 1, 10, or 100 μg LPS or vehicle intrathecally (i.t.) 2.5 h prior to instrumental testing. An ANOVA confirmed that rats administered LPS had shorter response durations over time than rats administered vehicle, F(87,580) = 1.37, p < 0.05 (Fig. 4A). Next we sought to determine if i.t. LPS produced a long lasting learning deficit. Although, much of the inflammation induced by LPS administration would have resolved within 24 h, research suggests that LPS induces long lasting changes in glial cell activation (Sugaya et al., 1998) as well as in genes involved in learning and memory (Bonow et al., 2008). Therefore, subjects were administered 100 μg LPS or vehicle 24 h prior to instrumental testing. We found that subjects administered LPS had significantly shorter response durations compared to vehicle controls, F(1,10) = 22.08, p < 0.01 (Fig. 4B). This suggests that 100 μg i.t. LPS induces a learning deficit that lasts for at least 24 h.
Fig. 4.
Intrathecal LPS induces a learning deficit. Transected subjects received 0, 1, 10, or 100 μg LPS or vehicle 150-min (A and B) or 24-hr (C and D) prior to the onset of controllable shock. An asterisk (*) indicates that a statistically significant difference was observed (p < .05).
3.5. Fluorocitrate attenuates the LPS-induced learning deficit
Finally, we assessed the role of spinal glia in the LPS-induced learning deficit. To accomplish this subjects were administered 0.5 nmol of i.t. fluorocitrate or vehicle 20 min prior to receiving 100 μg of i.t. LPS. As anticipated, subjects given vehicle and LPS exhibited a learning deficit (Fig. 5). As hypothesized, blocking glial activation with fluorocitrate prior to LPS treatment attenuated the LPS-induced learning deficit. An ANOVA revealed significant main effects of time, F(29,522) = 2.43, p < 0.001, and dose, F(1,18) = 6.00, p < 0.05, confirming that subjects receiving fluorocitrate prior to LPS had significantly longer response durations than those administered vehicle prior to LPS.
Fig. 5.
Fluorocitrate attenuates the LPS-induced learning deficit. Subjects received 0.5 nmol fluorocitrate or vehicle 20-min prior to LPS administration (100 μg). Changes in response duration across time (A) and mean (±SEM) response durations (B) are depicted. An asterisk (*) indicates that a statistically significant difference was observed (p < .05).
4. Discussion
There is mounting evidence that glial cells modulate plasticity within the central nervous system. Most notably, glial cells within the spinal cord (Meller et al., 1994; Milligan et al., 2001; 2003; Sweiter et al., 2001; Takeda et al., 2004; Watkins et al., 1997), and more recently the ventral posterolateral nucleus (Zhao et al., 2007) and the rostral ventromedial medulla (Wei et al., 2008), have been shown to be involved in the development and maintenance of various experimentally induced pathological pain states. Given the parallels between the spinal instrumental learning deficit and central sensitization, we hypothesized that glial cells would also modulate spinal instrumental learning deficits induced by shock or inflammatory stimuli. The experiments were also motivated by studies implicating glia in other forms of neural plasticity, such as brain-dependent memory consolidation and LTP within the hippocampus (Gibbs et al., 2006a, 2006b; Ikeda & Murase, 2004; Ma & Zhao, 2002). Therefore, we also hypothesized that glial cells may be necessary for spinal instrumental learning. We found that fluorocitrate inhibited response acquisition (Exp. 1), but did not interfere with response maintenance (Exp. 2), of spinal instrumental learning. We also found that fluorocitrate protected against the detrimental effects of uncontrollable shock (Exp. 3) and attenuated the detrimental effects of LPS (Exp. 4 and 5).
This research provides further evidence for the role of glia cells in both adaptive and pathological processes within the spinal cord. Furthermore, it suggests that controllable and uncontrollable shock lead to vastly different outcomes and that both effects involve glial cells. Future research will attempt to identify points of contrast between controllably and uncontrollably shocked glial cells by examining their morphology, cytokine release, and changes in gene expression.
Our findings provide further evidence that inflammation can disrupt learning, as reported by others (Pugh et al., 1998; Sell et al., 2001; Shaw et al., 2001; Thomson & Sutherland, 2005). It must be acknowledged, however, that cytokines do not invariably induce a learning deficit and, in some cases, can enhance learning (Avital et al., 2003; Bohme et al., 1993, 1991; Brennan et al., 2003; Goshen et al., 2007; Lu et al., 1999; Malen and Chapman, 1997; Pollmächer et al., 2002; Zhuo et al., 1993). Furthermore, in brain-dependent tasks, some changes in performance may be attributable to inflammation-induced alterations in motivation or affect. Our data provide additional evidence that high levels of inflammation disrupt spinal learning and implicate glial cells in the production of this deficit. Because the inflammatory stimulus was administered directly to the spinal cord, and because the spinal cord was surgically disconnected from the brain, these findings suggest inflammation can impact plasticity independent of its effect on brain-dependent motivational/affective systems. Fluorocitrate did not, however, completely reverse the LPS-induced learning deficit. This may have occurred because a high dose of LPS was used, one known to produce a robust allodynia and C-fiber activation (Meller et al., 1994; Reeve et al., 2000). At this dose, the effect of LPS could have extended beyond the effective reach of fluorocitrate treatment.
The present study provides further evidence that spinal glia modulate both adaptive and pathological processes within the spinal cord (Chen et al., 2008; Hains & Waxman, 2006; Meller et al., 1994; Milligan et al., 2001, 2003; Narita et al., 2006; Sweiter et al., 2001; Watkins et al., 1997). Furthermore, the results suggest that the spinal instrumental learning paradigm provides an excellent model to explore the mechanisms that mediate these effects. At the same time, we must acknowledge some limitations to this work. First, although fluorocitrate has been shown to be selective for glial cells and reversible at low doses (less than 1 nmol), drug selectivity is an inherent concern when using a pharmacological strategy. Therefore, future research verifying the effectiveness of fluorocitrate in the inhibition of glial cells and the use of other glial inhibitors to provide converging lines of evidence are planned. Another limitation concerns the potential role of factors known to impact the expression of learned behavior, such as state-dependent learning and performance effects. Although our paradigm effectively isolates spinal tissue from direct, brain-dependent, alterations in motivation and affect, indirect influences are possible. Further, some experimental manipulations may disrupt the performance of our target response, which is why care is taken to assess behavioral responsiveness at the start of testing.
As the mechanisms by which glial cells modulate plasticity within the spinal cord are elucidated, novel treatment targets will be uncovered. Future research needs to dissociate the contribution to adaptive and pathological processes. The long-term goal of this work is to identify cellular manipulations that selectively promote adaptive plasticity and inhibit destructive processes that foster pathological pain and undermine function (Gómez-Pinilla et al., 2007; Grau et al., 2004; Hook et al, 2008; Young et al., 2007). This information may improve treatment options for those suffering from spinal cord injuries, pathological pain, and diseases characterized by heightened levels of glial cell activation, such as multiple sclerosis, ALS (amyotrophic lateral sclerosis), and Alzheimer’s disease.
Acknowledgments
This work was in partial fulfillment of the Master of Science degree requirements for EGV at Texas A&M University. This work was supported by NIH grants NS041548 and ND058412 to JWG and NS060822 awarded to MWM, as well as an NSF GRF to EGV. Thanks also to Dr. Erin E. Young for her comments on early drafts of this manuscript.
Footnotes
Conflicts of Interest Statement: All authors declare that there are no conflicts of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Avital A, Goshen I, Kamsler A, Segal M, Iverfeldt K, Richter-Levin G, Yirmiya R. Impaired interleukin-1 signaling is associated with deficits in hippocampal memory processes and neural plasticity. Hippocampus. 2003;13:826–834. doi: 10.1002/hipo.10135. [DOI] [PubMed] [Google Scholar]
- Baumbauer KM, Young EE, Hoy KC, Joynes RL. Neurokinin receptors modulate the impact of uncontrollable stimulation on adaptive spinal plasticity. Behav Neurosci. 2007;121:1082–1094. doi: 10.1037/0735-7044.121.5.1082. [DOI] [PubMed] [Google Scholar]
- Blandino P, Barnum CJ, Deak T. The involvement of norepinephrine and microglia in hypothalamic and splenic IL-1beta responses to stress. J Neuroimmunol. 2006;173:87–95. doi: 10.1016/j.jneuroim.2005.11.021. [DOI] [PubMed] [Google Scholar]
- Böhme GA, Bon C, Lemaire M, Reibaud M, Piot O, Stutzmann JM, Doble A, Blanchard JC. Altered synaptic plasticity and memory formation in nitric oxide synthase inhibitor-treated rats. Proc Natl Acad Sci U S A. 1993;90:9191–9194. doi: 10.1073/pnas.90.19.9191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhme GA, Bon C, Stutzmann JM, Doble A, Blanchard JC. Possible involvement of nitric oxide in long-term potentiation. Eur J Pharmacol. 1991;199:379–381. doi: 10.1016/0014-2999(91)90505-k. [DOI] [PubMed] [Google Scholar]
- Brennan FX, Beck KD, Servatius RJ. Low doses of interleukin-1beta improve the leverpress avoidance performance of sprague-dawley rats. Neurobiol Learn Mem. 2003;80:168–171. doi: 10.1016/s1074-7427(03)00060-1. [DOI] [PubMed] [Google Scholar]
- Chen Y, Zhang X, Wang C, Li G, Gu Y, Huang LY. Activation of P2X7 receptors in glial satellite cells reduces pain through downregulation of P2X3 receptors in nociceptive neurons. Proc Natl Acad Sci U S A. 2008;105:16773–16778. doi: 10.1073/pnas.0801793105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crown ED, Ferguson AR, Joynes RL, Grau JW. Instrumental learning within the spinal cord: IV. Induction and retention of the behavioral deficit observed after noncontingent shock. Behav Neurosci. 2002;116:259–267. doi: 10.1037//0735-7044.116.6.1032. [DOI] [PubMed] [Google Scholar]
- Crown ED, Grau JW. Evidence that descending serotonergic systems protect spinal cord plasticity against the disruptive effect of uncontrollable stimulation. Exp Neurol. 2005;196:164–176. doi: 10.1016/j.expneurol.2005.07.016. [DOI] [PubMed] [Google Scholar]
- Deak T, Bordner KA, McElderry NK, Barunm CJ, Blandino P, Deak MM, Tammariello SP. Stress-induced increases in hypothalamic IL-1: A systemic analysis of multiple stressor paradigms. Brain Res Bull. 2005;64:541–556. doi: 10.1016/j.brainresbull.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Ferguson AR, Crown ED, Grau JW. Nociceptive plasticity inhibits adaptive learning in the spinal cord. Neuroscience. 2006;141:421–431. doi: 10.1016/j.neuroscience.2006.03.029. [DOI] [PubMed] [Google Scholar]
- Frank MG, Baratta MV, Sprunger DB, Watkins LR, Maier SF. Microglia serve as a neuroimmune substrate for stress-induced potentiation of CNS pro-inflammatory cytokine responses. Brain Behav Immun. 2007;21:47–59. doi: 10.1016/j.bbi.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Gibbs ME, O’Dowd BS, Hertz E, Hertz L. Astrocytic energy metabolism consolidates memory in young chicks. Neuroscience. 2006a;141:9–13. doi: 10.1016/j.neuroscience.2006.04.038. [DOI] [PubMed] [Google Scholar]
- Gibbs ME, Anderson DG, Hertz L. Inhibition of glycogenolysis in astrocytes interrupts memory consolidation in young chickens. Glia. 2006b;54:214–222. doi: 10.1002/glia.20377. [DOI] [PubMed] [Google Scholar]
- Gómez-Pinilla F, Huie JR, Ying Z, Ferguson AR, Crown ED, Baumbauer KM, Edgerton VR, Grau JW. BDNF and learning: Evidence that instrumental training promotes learning within the spinal cord by up-regulating BDNF expression. Neuroscience. 2007;148:893–906. doi: 10.1016/j.neuroscience.2007.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshen I, Kreisel T, Ounallah-Saad H, Renbaum P, Zalzstein Y, Ben-Hur T, Levy-Lahad E, Yirmiya R. A dual role for interleukin-1 in hippocampal-dependent memory processes. Psychoneuroendocrinology. 2007;32:1106–1115. doi: 10.1016/j.psyneuen.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Grau JW, Hook MA. Instrumental learning within the spinal cord: Underlying mechanisms and implications for recovery after injury. Behav Cogn Neurosci Rev. 2006;5:191–239. doi: 10.1177/1534582306289738. [DOI] [PubMed] [Google Scholar]
- Grau JW, Barstow DG, Joynes RL. Instrumental learning within the spinal cord: I. Behavioral properties. Behav Neurosci. 1998;112:1366–1386. doi: 10.1037//0735-7044.112.6.1366. [DOI] [PubMed] [Google Scholar]
- Grau JW, Washburn SN, Hook MA, Ferguson AR, Crown ED, Garcia G, Bolding KA, Miranda RC. Uncontrollable nociceptive stimulation undermines recovery after spinal cord injury. J Neurotrauma. 2004;21:1795–1817. doi: 10.1089/neu.2004.21.1795. [DOI] [PubMed] [Google Scholar]
- Guo W, Wang H, Watanabe M, Shimizu K, Zou S, LaGraize SC, Wei F, Dubner R, Ren K. Glial-cytokine-neuronal interactions underlying the mechanisms of persistent pain. J Neurosci. 2007;27:6006–6018. doi: 10.1523/JNEUROSCI.0176-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hains BC, Waxman SG. Activated microglia contribute to the maintenance of chronic pain after spinal cord injury. J Neurosci. 2006;26:4308–4317. doi: 10.1523/JNEUROSCI.0003-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herber DL, Maloney JL, Roth LM, Freeman MJ, Morgan D, Gordon MN. Diverse microglial responses after intrahippocampal administration of lipopolysaccharide. Glia. 2006;53:382–391. doi: 10.1002/glia.20272. [DOI] [PubMed] [Google Scholar]
- Hertz L, Gibbs ME, O’Dowd BS, Sedman GL, Robinson SR, Sykova E, Hajeck I, Hertz E, Peng L, Huang R, Ng KT. Astrocyte-neuron interaction during one-trial aversive learning in the neonate chick. Neurosci Biobehav Rev. 1996;20:537–551. doi: 10.1016/0149-7634(95)00020-8. [DOI] [PubMed] [Google Scholar]
- Hook MA, Huie JR, Grau JW. Peripheral inflammation undermines the plasticity of the isolated spinal cord. Behav Neurosci. 2008;122:233–249. doi: 10.1037/0735-7044.122.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua XY, Svensson CI, Matsui T, Fitzsimmons B, Yaksh TL, Webb M. Intrathecal minocycline attenuates peripheral inflammation-induced hyperalgesia by inhibiting p38 MAPk in spinal microglia. Eur J Neurosci. 2005;22:2431–2440. doi: 10.1111/j.1460-9568.2005.04451.x. [DOI] [PubMed] [Google Scholar]
- Hydén H, Egyházi E. Glial RNA changes during a learning experiment in rats. Proc Natl Acad Sci U S A. 1963;49:618–624. doi: 10.1073/pnas.49.5.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda H, Murase K. Glial nitric oxide-mediated long-term presynaptic facilitation revealed by optical imaging in rat spinal dorsal horn. J Neurosci. 2004;24:9888–9896. doi: 10.1523/JNEUROSCI.2608-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda H, Kiritoshi T, Murase K. Effect of excitatory and inhibitory agents and a glial inhibitor on optically-recorded primary-afferent excitation. Mol Pain. 2008;4:39. doi: 10.1186/1744-8069-4-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahanshahi M, Sadeghi Y, Hosseini A, Naghdi N. The effect of spatial learning on the number of astrocytes in rat dentate gyrus. Neuroanatomy. 2007;6:51–53. [Google Scholar]
- Jahanshahi M, Sadeghi Y, Hosseini A, Naghdi N, Marjani A. The effect of spatial learning on the number of astrocytes in the CA3 subfield of the rat hippocampus. Singapore Med J. 2008;49:388–391. [PubMed] [Google Scholar]
- Joynes RL, Janjua K, Grau JW. Instrumental learning within the spinal cord: VI. The NMDA receptor antagonist, AP5, disrupts acquisition and maintenance of an acquired flexion response. Behav Brain Res. 2004;154:431–438. doi: 10.1016/j.bbr.2004.03.030. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Markham JA, Vij K, Freese JL, Ballard DH, Greenough WT. Motor learning induces astrocytic hypertrophy in the cerebellar cortex. Behav Brain Res. 2007;178:244–249. doi: 10.1016/j.bbr.2006.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan L, Yuan H, Duan L, Cao R, Gao B, Shen J, Xiong Y, Chen LW, Rao ZR. Blocking the glial function suppresses subcutaneous formalin-induced nociceptive behavior in the rat. Neurosci Res. 2007;57:112–119. doi: 10.1016/j.neures.2006.09.014. [DOI] [PubMed] [Google Scholar]
- Ledeboer A, Binnekade R, Breve JJ, Bol JG, Tilders FJ, Van Dam AM. Site-specific modulation of LPS-induced fever and interleukin-1 beta expression in rats by interleukin-10. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1762–R1772. doi: 10.1152/ajpregu.00766.2001. [DOI] [PubMed] [Google Scholar]
- Ledeboer A, Sloane EM, Milligan ED, Frank MG, Mahony JH, Maier SF, Watkins LR. Minocycline attenuates mechanical allodynia and proinflammatory cytokine expression in rat models of pain facilitation. Pain. 2005;115:71–83. doi: 10.1016/j.pain.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Lu YF, Kandel ER, Hawkins RD. Nitric oxide signaling contributes to late-phase LTP and CREB phosphorylation in the hippocampus. J Neurosci. 1999;19:10250–10261. doi: 10.1523/JNEUROSCI.19-23-10250.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma JY, Zhao ZQ. The involvement of glia in long-term plasticity in the spinal dorsal horn of the rat. Neuroreport. 2002;13:1781–1784. doi: 10.1097/00001756-200210070-00017. [DOI] [PubMed] [Google Scholar]
- Malen PL, Chapman PF. Nitric oxide facilitates long-term potentiation, but not long-term depression. J Neurosci. 1997;17:2645–2651. doi: 10.1523/JNEUROSCI.17-07-02645.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meller ST, Dykstra C, Grzybycki D, Murphy S, Gebhart GF. The possible role of glia in nociceptive processing and hyperalgesia in the spinal cord of the rat. Neuropharmacology. 1994;33:1471–1478. doi: 10.1016/0028-3908(94)90051-5. [DOI] [PubMed] [Google Scholar]
- Mika J, Osikowicz M, Makuch W, Przewlocka B. Minocycline and pentoxifylline attenuate allodynia and hyperalgesia and potentiate the effects of morphine in rat and mouse models of neuropathic pain. Eur J Pharmacol. 2007;560:142–149. doi: 10.1016/j.ejphar.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Milligan ED, O’Connor KA, Armstrong CB, Hansen MK, Martin D, Tracey KJ, Maier SF, Watkins LR. Systemic administration of CNI-1493, a p38 mitogen-activated protein kinase inhibitor, blocks intrathecal human immunodeficiency virus-1 gp120-induced enhanced pain states in rats. J Pain. 2001;2:326–333. doi: 10.1054/jpai.2001.26174. [DOI] [PubMed] [Google Scholar]
- Milligan ED, Twining C, Chacur M, Biedenkapp J, O’Connor K, Poole S, Tracey K, Martin D, Maier SF, Watkins LR. Spinal glia and proinflammatory cytokines mediate mirror-image neuropathic pain in rats. J Neurosci. 2003;23:1026–1040. doi: 10.1523/JNEUROSCI.23-03-01026.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair A, Bonneau RH. Stress-induced elevation of glucocorticoids increases microglia proliferation through NMDA receptor activation. J Neuroimmunol. 2007;171:72–85. doi: 10.1016/j.jneuroim.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Narita M, Yoshida T, Nakajima M, Miyatake M, Takagi T, Yajima Y, Suzuki T. Direct evidence for spinal cord microglia in the development of a neuropathic pain-like state in mice. J Neurochem. 2006;97:1337–1348. doi: 10.1111/j.1471-4159.2006.03808.x. [DOI] [PubMed] [Google Scholar]
- O’Connor KA, Johnson JD, Hansen MK, Wieseler Frank JL, Maksimova E, Watkins LR, Maier SF. Peripheral and central proinflammatory cytokine response to a severe acute stressor. Brain Res. 2003;991:123–132. doi: 10.1016/j.brainres.2003.08.006. [DOI] [PubMed] [Google Scholar]
- O’Dowd BS, Gibbs ME, Sedman GL, Ng KT. Astrocytes implicated in the energizing of intermediate memory processes in neonate chicks. Brain Res Cogn Brain Res. 1994;2:537–551. doi: 10.1016/0926-6410(94)90006-x. [DOI] [PubMed] [Google Scholar]
- Obata H, Eisenach JC, Hussain H, Bynum T, Vincler M. Spinal glial activation contributes to postoperative mechanical hypersensitivity in the rat. J Pain. 2006;7:816–822. doi: 10.1016/j.jpain.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Paulsen RE, Contestabile A, Villani L, Fonnum F. An in vivo model for studying function of brain tissue temporarily devoid of glial cell metabolism: The use of fluorocitrate. J Neurochem. 1987;48:1377–1385. doi: 10.1111/j.1471-4159.1987.tb05674.x. [DOI] [PubMed] [Google Scholar]
- Pollmächer T, Haack M, Schuld A, Beichenberg A, Yirmiya R. Low levels of circulating inflammatory cytokines—do they affect human brain functions? Brain Behav Immun. 2002;16:525–532. doi: 10.1016/s0889-1591(02)00004-1. [DOI] [PubMed] [Google Scholar]
- Pugh CR, Kumagawa K, Fleshner M, Watkins LR, Maier SF, Rudy JR. Selective effects of peripheral lipopolysaccharide administration on contextual and auditory-cue fear conditioning. Brain Behav Immun. 1998;12:212–229. doi: 10.1006/brbi.1998.0524. [DOI] [PubMed] [Google Scholar]
- Qin M, Wang JJ, Cao R, Zhang H, Duan L, Gao B, Xiong YF, Chen LW, Rao ZR. The lumbar spinal cord glial cells actively modulate subcutaneous formalin induced hyperalgesia in the rat. Neurosci Res. 2006;55:442–450. doi: 10.1016/j.neures.2006.04.017. [DOI] [PubMed] [Google Scholar]
- Reeve AJ, Patel S, Fox A, Walker K, Urban L. Intrathecally administered endotoxin or cytokines produce allodynia, hyperalgesia, and changes in spinal cord neuronal response to nociceptive stimuli in the rat. Eur J Pain. 2000;4:247–257. doi: 10.1053/eujp.2000.0177. [DOI] [PubMed] [Google Scholar]
- Rubio N. Mouse astrocytes store and deliver brain-derived neurotrophic factor using the non-catalytic gp95TrkB receptor. Eur J Neurosci. 1997;9:1847–1853. doi: 10.1111/j.1460-9568.1997.tb00751.x. [DOI] [PubMed] [Google Scholar]
- Sell KM, Crowe SF, Kent S. Lipopolysaccharide induces memory-processing deficits in day-old chicks. Pharmacol Biochem Behav. 2001;68:497–502. doi: 10.1016/s0091-3057(01)00463-4. [DOI] [PubMed] [Google Scholar]
- Shaw KN, Commins S, O’Mara SM. Lipopolysaccharide causes deficits in spatial learning in the watermaze but not in BDNF expression in the rat denate gyrus. Behav Brain Res. 2001;124:47–54. doi: 10.1016/s0166-4328(01)00232-7. [DOI] [PubMed] [Google Scholar]
- Sugama S, Fujita M, Hashimoto M, Conti B. Stress induced morphological microglial activation in the rodent brain: involvement of interleukin-18. Neuroscience. 2007;146:1388–1399. doi: 10.1016/j.neuroscience.2007.02.043. [DOI] [PubMed] [Google Scholar]
- Sugaya K, Chou S, Xu SJ, McKinney M. Indicators of glial activation and brain oxidative stress after intraventricular infusion of endotoxin. Brain Res Mol Brain Res. 1998;58:1–9. doi: 10.1016/s0169-328x(97)00365-3. [DOI] [PubMed] [Google Scholar]
- Sweitzer SM, Schubert P, DeLeo JA. Propentofylline, a glial modulating agent, exhibits antiallodynic properties in a rat model of neuropathic pain. J Pharmacol Exp Ther. 2001;297:1210–1217. [PubMed] [Google Scholar]
- Takeda K, Sawamura S, Sekiyama H, Tamai H, Hanaoka K. Effect of methylprednisolone on neuropathic pain and spinal glial activation in rats. Anesthesiology. 2004;100:1249–1257. doi: 10.1097/00000542-200405000-00029. [DOI] [PubMed] [Google Scholar]
- Terrazzino S, Perego C, De Luigi A, De Simoni MG. Interleukin-6, tumor necrosis factor and corticosterone induction by central lipopolysaccharide in aged rats. Life Sci. 1997;61:695–701. doi: 10.1016/s0024-3205(97)00534-1. [DOI] [PubMed] [Google Scholar]
- Thomson LM, Sutherland RJ. Systemic administration of lipopolysaccharide and interleukin-1beta have different effects on memory consolidation. Brain Res Bull. 2005;67:24–29. doi: 10.1016/j.brainresbull.2005.05.024. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Martin D, Ulrich P, Tracey KJ, Maier SF. Evidence for the involvement of spinal cord glia in subcutaneous formalin induced hyperalgesia in the rat. Pain. 1997;71:225–235. doi: 10.1016/s0304-3959(97)03369-1. [DOI] [PubMed] [Google Scholar]
- Wei F, Guo W, Zou S, Ren K, Dubner R. Supraspinal glial-neuronal interactions contribute to descending pain facilitation. J Neurosci. 2008;28:10482–10495. doi: 10.1523/JNEUROSCI.3593-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K, Mizuno M, Nabeshima T. Role for brain-derived neurotrophic factor in learning and memory. Life Sci. 2002;70:735–744. doi: 10.1016/s0024-3205(01)01461-8. [DOI] [PubMed] [Google Scholar]
- Young EE, Baumbauer KM, Elliot A, Joynes RL. Lipopolysaccharide induces a spinal learning deficit that is blocked by IL-1 receptor antagonism. Brain Behav Immun. 2007;21:748–757. doi: 10.1016/j.bbi.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Zhuang ZY, Wen YR, Zhang DR, Borsello T, Bonny C, Strichartz GR, Decosterd I, Ji RR. A peptide c-Jun N-terminal kinase (JNK) inhibitor blocks mechanical allodynia after spinal nerve ligation: Respective roles of JNK activation in primary sensory neurons and spinal astrocytes for neuropathic pain development and maintenance. J Neurosci. 2006;26:3551–3560. doi: 10.1523/JNEUROSCI.5290-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao P, Waxman SG, Hains BC. Modulation of thalamic nociceptive processing after spinal cord injury through remote activation of thalamic microglia by cysteine-cysteine chemokine ligand 21. J Neurosci. 2007;27:8893–8902. doi: 10.1523/JNEUROSCI.2209-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo M, Small SA, Kandel ER, Hawkins RD. Nitric oxide and carbon monoxide produce activity-dependent long-term synaptic enhancement in hippocampus. Science. 1993;260:1946–1950. doi: 10.1126/science.8100368. [DOI] [PubMed] [Google Scholar]