Abstract
Angioedema is a potentially life-threatening adverse effect of angiotensin-converting enzyme inhibitors. Bradykinin and substance P, substrates of angiotensin-converting enzyme, increase vascular permeability and cause tissue edema in animals. Studies indicate that amino-terminal degradation of these peptides, by aminopeptidase P and dipeptidyl peptidase IV, may be impaired in individuals with angiotensin-converting enzyme inhibitor–associated angioedema. This case-control study tested the hypothesis that dipeptidyl peptidase IV activity and antigen are decreased in sera of patients with a history of angiotensin-converting enzyme inhibitor–associated angioedema. Fifty subjects with a history of angiotensin-converting enzyme inhibitor–associated angioedema and 176 angiotensin-converting enzyme inhibitor–exposed control subjects were ascertained. Sera were assayed for angiotensin-converting enzyme activity, aminopeptidase P activity, aminopeptidase N activity, dipeptidyl peptidase IV activity, and antigen and the ex vivo degradation half-lives of bradykinin, des-Arg9-bradykinin, and substance P in a subset. The prevalence of smoking was increased and of diabetes decreased in case versus control subjects. Overall, dipeptidyl peptidase IV activity (26.6±7.8 versus 29.6±7.3 nmol/mL per minute; P=0.026) and antigen (465.8±260.8 versus 563.1±208.6 ng/mL; P=0.017) were decreased in sera from individuals with angiotensin-converting enzyme inhibitor–associated angioedema compared with angiotensin-converting enzyme inhibitor–exposed control subjects without angioedema. Dipeptidyl peptidase IV activity (21.5±4.9 versus 29.8±6.7 nmol/mL per minute; P=0.001) and antigen (354.4±124.7 versus 559.8±163.2 ng/mL; P=0.003) were decreased in sera from cases collected during angiotensin-converting enzyme inhibition but not in the absence of angiotensin-converting enzyme inhibition. The degradation half-life of substance P correlated inversely with dipeptidyl peptidase IV antigen during angiotensin-converting enzyme inhibition. Environmental or genetic factors that reduce dipeptidyl peptidase IV activity may predispose individuals to angioedema.
Keywords: angiotensin-converting enzyme inhibitors, angioneurotic edema, antigens, CD26, substance P, neuropeptides, bradykinin
Angiotensin-converting enzyme (ACE) inhibitors (ACEis) are widely used to treat hypertension, congestive heart failure, and renal insufficiency. ACEi-associated angioedema is rare, occurring in 0.1% to 0.7% of exposed individuals.1,2 Reactions range from mild swelling of the tongue, lips, other areas of the face, hands, feet, or bowel to life-threatening airway compromise. Although many cases of ACEi-associated angioedema occur soon after the initiation of ACEi treatment, the reaction may occur years after the initiation of therapy.3 Given that 40 million people are currently taking ACEis, the number of people at risk for this adverse effect is substantial.
Although bradykinin has been implicated in the pathogenesis of hereditary forms of angioedema,4 the mechanism of ACEi-associated angioedema is unknown. Clinical factors associated with an increased risk of ACEi-associated angioedema include black race and female gender, whereas smoking is a risk factor for ACE/neutral endopeptidase (NEP) inhibitor–associated angioedema.5–7 Black Americans have an incidence of ACEi-associated angioedema ≈4 to 5 times that of white Americans.5 In addition, immunosuppressed cardiac and renal transplant patients have an increased incidence of ACEi-associated angioedema.8 In contrast, diabetic subjects were protected against both ACEi-associated and ACE/NEP inhibitor–associated angioedema in the Omapatrilat Cardiovascular Treatment Versus Enalapril Trial.6,7
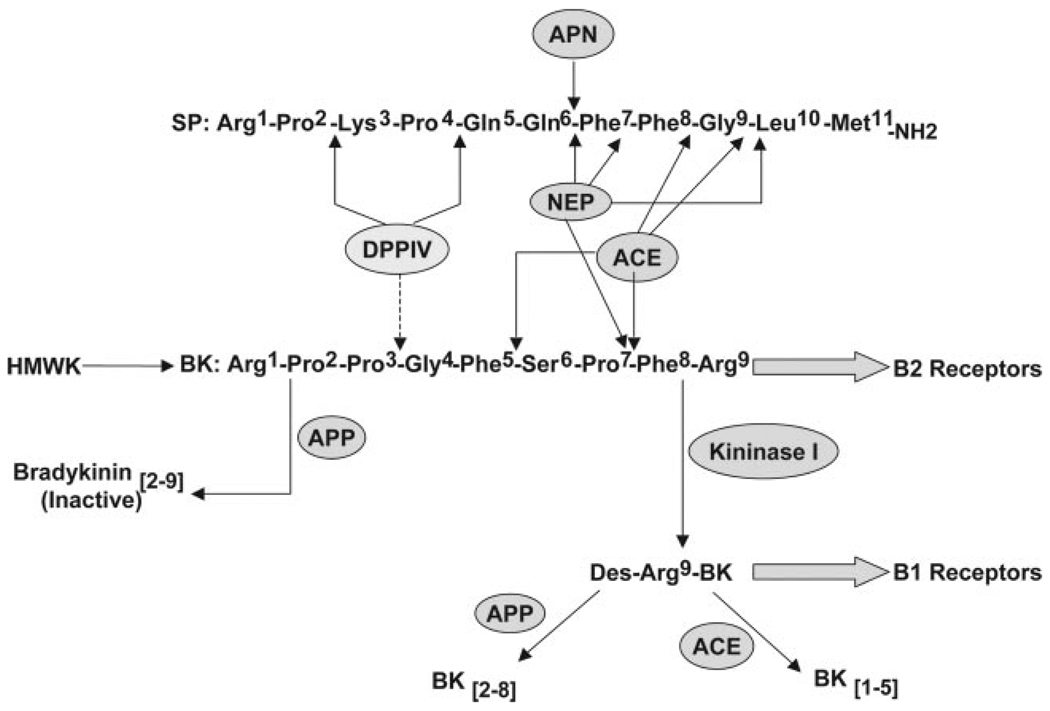
Bradykinin and substance P are 2 substrates of ACE that produce tracheal edema in animals.9,10 Bradykinin is degraded primarily by ACE (EC 3.4.15.1; Figure 1); however, during ACE inhibition, other enzymes, including neutral endopeptidase (NEP-24.11; EC 3.4.24.11), aminopeptidase P (APP; EC 3.4.11.9), and kininase I (carboxypeptidase N; EC 3.4.17.3), assume greater importance in the metabolism of bradykinin.11 Cleavage of bradykinin by kininase I yields the active metabolite des-Arg9-bradykinin, which is inactivated primarily by APP.
Figure 1.
Schematic of the degradation of bradykinin (BK), des-Arg9-bradykinin (des-Arg9-BK), and substance P (SP). See text. HMWK indicates high molecular weight kininogen; APN, APN or aminopeptidase M. Dashed line shows cleavage of an inactive bradykinin fragment.
The degradation of bradykinin by kininase I is enhanced in sera from patients with ACEi-associated angioedema compared with that from control subjects.12 This finding implicates a defect in a nonkininase I-nonkininase II pathway of bradykinin degradation in patients with ACEi-associated angioedema. Supporting this notion, APP activity and des-Arg9-bradykinin degradation are decreased in the sera of angioedema patients of European origin compared with control subjects.13,14 However, APP activity has not been found to be decreased in all of the case subjects with angioedema compared with control subjects.12,15 In addition, the frequency of decreased APP activity in the population (18%) exceeds the incidence of angioedema in ACEi-exposed patients.16 Moreover, whereas the gene encoding for membrane-bound APP, the source of circulating APP activity, is X linked,17,18 ACEi-associated angioedema is more common in women than in men. Taken together, these data suggest that defects in additional pathways may contribute to ACEi-associated angioedema.
In addition to increasing vascular permeability directly via its B2 receptor, bradykinin stimulates the release of substance P from sensory nerves, which causes increased vascular permeability by acting at the NK1 receptor.19 ACE also degrades substance P; however, in the setting of ACE inhibition, dipeptidyl peptidase IV (DPPIV or CD26; EC 3.4.14.5) sequentially degrades substance P to substance P 5–11, which is susceptible to further degradation by aminopeptidase N (APN or CD13, 3.4.11.2).20 DPPIV is a multifunctional enzyme that plays a role in immune function, HIV pathogenesis, metastasis of several malignancies, and regulation of blood glucose.21 The current study derives from a previous finding of our laboratory that serum DPPIV activity is diminished during acute ACEi-associated angioedema.15 This study tested the hypothesis that decreased DPPIV antigen concentration or activity is associated with ACEi-associated angioedema in a validation population. In addition, the study tested the hypothesis that decreased DPPIV antigen or activity is associated with increased serum substance P degradation half-life.
Methods
Identification of Case and Control Subjects
The study was approved by the Vanderbilt University Institutional Review Board, and all of the subjects provided written informed consent. Case subjects were identified either at the time of presentation for medical care or by an institutional review board–approved search of the electronic medical chart at a tertiary care hospital, Vanderbilt University Medical Center, using the term “angioedema.” Subjects identified via the electronic record were contacted through their primary care physicians. Individuals were defined as having ACEi-associated angioedema if they had swelling of the lips, pharynx, or face while taking an ACEi but had never had any other episodes of angioedema. Control subjects who had been treated with an ACEi for ≥6 months without experiencing angioedema were also identified by institutional review board–approved search of the electronic medical chart. In keeping with the racial and gender makeup of previously ascertained case subjects, control subjects were prespecified to be 50% black American, 50% white American, and 50% female. To avoid enriching the control population with individuals with a history of ACEi-induced cough or with sufficiently mild hypertension to allow discontinuation of their ACEi, control subjects who were currently taking an ACEi, as well as those whose ACEi had been stopped for some reason other than angioedema, were included. Medical history, including the history of angioedema, was confirmed by a research nurse or physician using a detailed case report form. Subjects were considered diabetic if they were taking oral antidiabetic medications or insulin or, in the case of 3 patients, by review of blood glucose and hemoglobin A1C values.
Laboratory Analysis
Blood for biochemical assays was collected in the absence of anticoagulant, centrifuged immediately, and stored at −80°C until assay. DPPIV antigen concentration was determined using a commercially available ELISA kit (CD 26, Bender MedSystems). DPPIV activity was assayed by incubating sera with a colorimetric substrate, l-glycyl-l-prolyl p-nitroanilide (Sigma), at 37°C, as described previously.15 Additional assays were completed as serum volume permitted. Please see the data supplement at http://hyper.ahajournals.org for details of APN and APP activity assay methodology.
The degradation half-lives of bradykinin, des-Arg9-bradykinin, and substance P were determined by adding known concentrations of exogenous bradykinin or substance P to serum at 37°C.16 Please see http://hyper.ahajournals.org for additional details of bradykinin, des-Arg9-bradykinin, and substance P assays.
Statistical Analysis
Data are presented as mean±SD. Between-group comparisons were made using χ2 testing for categorical variables and Student’s t or Mann Whitney U testing, as appropriate, for continuous variables. To account for the different proportions of case and control subjects exposed to ACEi at the time of blood collection, analyses were performed after stratification for ACEi use at the time of blood collection, except as indicated otherwise in the text. The independent effects of DPPIV activity, current smoking status, and diabetes on case-control status were determined using logistic regression. The relationship between enzyme activity and the log-transformed degradation half-life of substance P was analyzed using the Pearson correlation coefficient. A 2-sided P<0.05 was considered significant. Statistical analysis was performed using SPSS 15.0 (SPSS Inc).
Results
Fifty subjects with a history of ACEi-associated angioedema and 176 ACEi-exposed control subjects were ascertained (Table 1). Age was similar in subjects with ACEi-associated angioedema and ACEi-exposed control subjects. By design, ≈50% of control subjects were white American, 50% were black American, and 50% were female. All of the subjects had hypertension. The prevalence of diabetes was significantly lower in case subjects compared with control subjects. Conversely, the prevalence of smoking was significantly higher in case subjects than in control subjects. Sera were available for measurement of DPPIV activity or antigen from 38 subjects with angioedema and 170 of the control subjects. For the remaining subjects, serum had been assayed previously and was no longer available.15 In 8 of the angioedema subjects, serum was obtained at the time of presentation with angioedema and, thus, during ACE inhibition (data on serum DPPIV activity, but not antigen, were reported previously for 2 of these subjects15). These subjects were taking lisinopril (n=4), enalapril (n=2), ramipril (n=1), and fosinopril (n=1). A total of 152 control subjects were taking an ACEi at the time of the blood draw.
Table 1.
Characteristics of Case and Control Subjects
| All Participants |
Participants With Serum Available |
|||||
|---|---|---|---|---|---|---|
| Characteristic | ACEi-Associated Angioedema (n=50) | ACEi-Exposed Control Subjects (n=176) | P | ACEi-Associated Angioedema (n=38) | ACEi-Exposed Control Subjects (n=170) | P |
| Black:white, n | 20:30 | 88:88 | 0.21 | 13:25 | 87:83 | 0.06 |
| Male:female, n | 24:26 | 85:91 | 0.97 | 16:22 | 83:87 | 0.45 |
| Age, mean±SD, y | 58.6±14.4 | 58.0±11.1 | 0.78 | 59.7±14.7 | 58.0±11.1 | 0.52 |
| Nonsmoker:smoker:unknown, n | 32:16:2 | 149:27:0 | 0.005 | 27:11 | 144:26 | 0.047 |
| Nondiabetic:diabetic:unknown, n | 40:9:1 | 112:64:0 | 0.02 | 32:6 | 60:110 | 0.02 |
| ACEi:off ACEi, n | 15:35 | 158:18 | <0.001 | 8:30 | 152:18 | <0.001 |
Table 2 shows the biochemical characteristics of case and control subjects. Among both case and control subjects, ACE activity was significantly lower, and the half-lives of degradation of bradykinin and its active metabolite des-Arg9-bradykinin were significantly prolonged in individuals taking an ACEi compared with those not taking an ACEi. Similarly, APN activity was lower in ACEi-exposed control subjects than in control subjects not taking an ACEi. However, there was no difference in ACE activity, APP activity, APN activity, bradykinin degradation half-life, or des-Arg9-bradykinin degradation half-life between case and control subjects either in the presence or absence of ACE inhibition. Likewise, endogenous bradykinin concentrations were comparable in sera collected from 6 case subjects and 17 ACEi-exposed control subjects (please see the data supplement).
Table 2.
Biochemical Characterization of ACEi-Associated Angioedema Case and Control Subjects
| ACEi-Associated Angioedema, Mean±SD (n) |
ACEi-Exposed Control Subjects, Mean±SD (n) |
|||
|---|---|---|---|---|
| Serum Measurement | ACEi | Off ACEi | ACEi | Off ACEi |
| ACE activity, nmol/min per mL | 6.0±9.5 (5)* | 54.4±18.2 (24) | 2.6±2.5 (98)* | 48.1±13.6 (16) |
| APP activity, pmol/min per mL | 184.0±181.5 (7) | 180.6±166.3 (23) | 175.5±170.9 (93) | 242.5±142.1 (15) |
| APN activity, nmol/min per mL | 31.2±6.3 (5) | 37.1±10.5 (23) | 29.3±10.4† (98) | 36.2±9.5 (16) |
| DPPIV activity, nmol/mL per min | 21.5±4.9 (8)†‡ | 28.0±7.9 (30) | 29.8±6.7 (149) | 27.7±11.5 (16) |
| DPPIV antigen, ng/mL | 354.4±124.7 (6)§ | 488.8±276.7 (29) | 559.8±163.2 (152) | 591.7±441.8 (18) |
| T1/2 bradykinin, seconds | 157.2±128.3 (6)† | 41.1±19.5 (18) | 262.8±217.7 (51)* | 52.1±20.0 (14) |
| T1/2 des-Arg9-bradykinin, seconds | 1261.8±772.9 (6)† | 456.0±299.8 (18) | 2020.7±1674.6 (52)* | 507.2±235.3 (14) |
| T1/2 substance P, seconds | 4004.5±2004.5 (4) | 2844.1±1882.3 (19) | 4020.1±2367.7 (49) | 4037.6±3097.4 (14) |
T1/2 indicates half-life.
P<0.001 vs off ACEi
P<0.05 vs off ACEi
P=0.001 vs control subjects
P<0.01 vs control subjects.
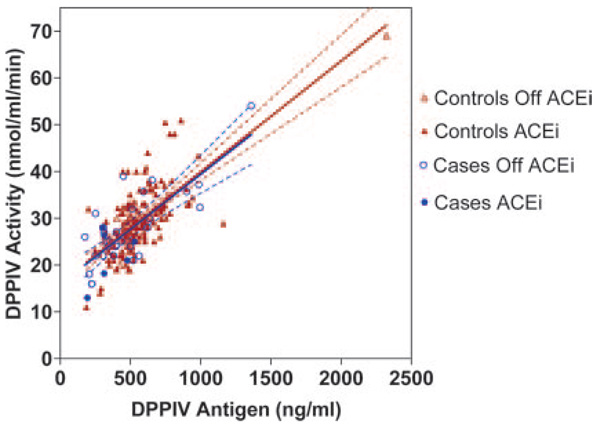
DPPIV antigen accounted for 76% of the variability in DPPIV activity in the absence of ACEi (R2=0.758; P<0.001) and 38% of the variability during ACEi (R2=0.377; P<0.001). Nevertheless, ACEi use did not alter the slope of the relationship between DPPIV activity and antigen (Figure 2). DPPIV antigen and activity were similar in whites and blacks, regardless of ACEi use. DPPIV, ACE, APP, and APN enzyme activities and the degradation half-lives of peptides were similar in men and women. Among subjects taking an ACEi, DPPIV activity was lower in smokers compared with nonsmokers (27.1±8.0 versus 29.8±.6.6 nmol/mL per minute; P=0.04) and tended to be higher in diabetic subjects compared with nondiabetic subjects (30.6±7.1 versus 28.6±6.6 nmol/mL per minute; P=0.07).
Figure 2.
Relationship between DPPIV activity and soluble DPPIV antigen level in angioedema case and control subjects. Regression line and 95% confidence intervals were calculated separately for case and control subjects. For control subjects, R2=0.48; for case subjects, R2=0.60.
When analyzed without stratification for current ACEi use, DPPIV activity (26.6±7.8 versus 29.6±7.3 nmol/mL per minute for all case subjects versus all control subjects; P=0.026) and DPPIV antigen (465.8±260.8 versus 563.1±208.6 ng/mL for all case subjects versus all control subjects; P=0.017) were significantly decreased in individuals with a history of ACEi-associated angioedema compared with control subjects. When analyzed separately in subjects currently taking an ACEi and those no longer taking an ACEi, DPPIV activity and antigen were lower in case subjects than in control subjects only among subjects exposed to ACEi at the time of blood collection (Table 2). There was no correlation between ACE activity and DPPIV activity among angioedema patients when analyzed during ACE inhibition (P=0.71) or in the absence of ACE inhibition (P=0.11). To determine whether DPPIV activity predicted case-control status independent of smoking and diabetes, we conducted binary logistic regression. During ACE inhibition, DPPIV activity was a significant predictor of case-control status after controlling for smoking and diabetes (odds ratio: 0.81; 95% CI: 0.70 to 0.93; P=0.004).
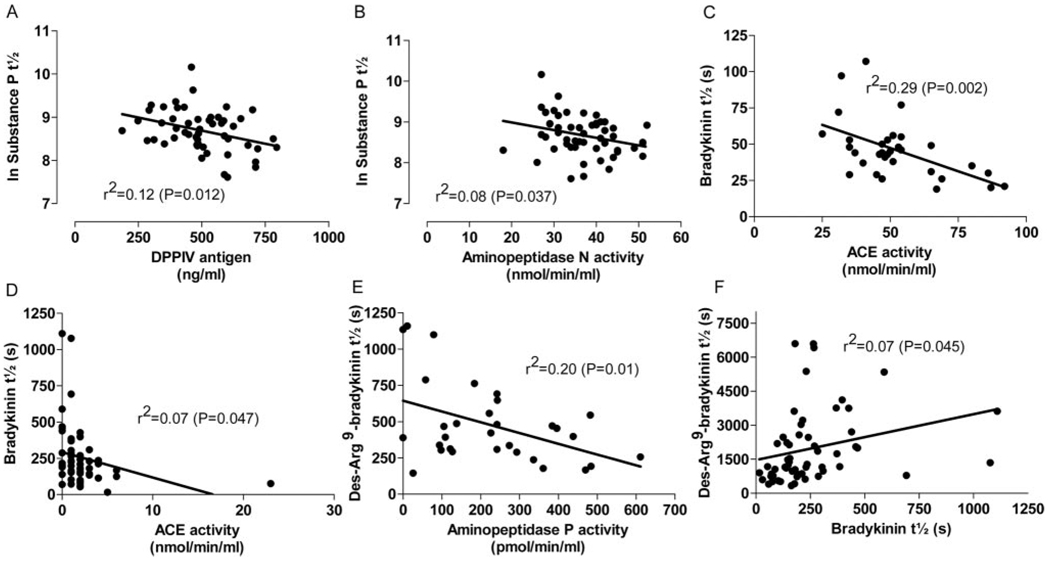
We next examined the relationship between DPPIV and the ex vivo degradation half-life of substance P. In the absence of ACEi, the half-life of substance P degradation did not statistically significantly correlate inversely with ACE activity (r=−0.293; P=0.098). During ACEi, substance P degradation half-life correlated inversely with DPPIV antigen (r=−0.345; P=0.012; Figure 3) and with APN activity (r=−0.288; P=0.037). Bradykinin half-life correlated inversely with ACE activity in the sera in the absence of ACEi (r=−0.535; P=0.002) and as well as with ACE activity in sera from subjects taking an ACEi (r=−0.259; P=0.047). Des-Arg9-bradykinin half-life correlated inversely with APP activity (r=−0.445; P=0.01) in the absence of ACEi but not during ACE inhibition. Rather, during ACEi, des-Arg9-bradykinin half-life correlated with bradykinin half-life (r=0.266; P=0.045).
Figure 3.
The relationship between log-transformed substance P degradation half-life and (A) DPPIV antigen and (B) APN activity during ACE inhibition. The relationship between bradykinin half-life and ACE activity in the absence (C) and presence (D) of an ACEi. Relationship between des-Arg9-bradykinin and (E) APP activity in the absence of ACE inhibition and (F) bradykinin half-life in the setting of ACE inhibition.
Discussion
This study tested the hypothesis that DPPIV antigen concentration or activity is decreased in ACEi-associated angioedema. In addition, the study tested the hypothesis that decreased DPPIV activity is associated with increased serum substance P degradation half-life. As reported previously,15 DPPIV activity was decreased in the sera during ongoing ACEi-associated angioedema but not after recovery from angioedema. Moreover, the serum concentration of soluble DPPIV antigen was similarly decreased during ACEi-associated angioedema, indicating that angioedema is associated with a reduction in DPPIV enzyme protein rather than with a decrease in its specific activity.
DPPIV contributes to the amino-terminal degradation of both the kinins and of substance P, vasodilator peptide substrates of ACE that have been implicated in animal models of angioedema.9,10 However, whereas bradykinin and des-Arg9-bradykinin are inactivated by APP before cleavage by DPPIV,22 studies in rodents indicate that DPPIV contributes significantly to the inactivation of substance P.20,23 In this study, we found that, in the absence of pharmacological ACE inhibition, the degradation half-life of exogenous substance P and serum ACE activity tended to correlate inversely, but this correlation did not reach statistical significance. However, during ACE inhibition, substance P degradation correlated inversely with DPPIV antigen and APN activity. These data suggest that the relative contribution of DPPIV to the degradation of substance P is increased in the setting of ACE inhibition.
Although this study does not specifically address the mechanism underlying the association between decreased DPPIV activity and antigen and ACEi-associated angioedema, substance P contributes to the development of ACEi-associated angioedema in rodent models. Sulpizio et al10 reported that infusion of bradykinin or substance P induced tracheal edema in a rat model. Emanueli et al9 reported that either bradykinin receptor antagonism or NK1 antagonism attenuates plasma extravasation in tracheal and other tissues of mice treated with captopril. Likewise, we have observed recently that DPPIV-deficient Fisher 344 rats demonstrate increased susceptibility to ACEi-induced peritracheal edema through an NK1 receptor–dependent mechanism.24 Ultimately, studies using bradykinin and NK1-receptor antagonists are needed to determine the contributions of endogenous bradykinin and substance P, respectively, to ACEi-associated angioedema in humans.25
Based on the data in DPPIV-deficient rats, we hypothesized that the degradation of exogenous substance P would be diminished in the sera of individuals with ACEi-associated angioedema. However, contrary to our hypothesis, the ex vivo degradation half-life of substance P was not prolonged in these subjects. This suggests the need to identify additional mechanisms whereby decreased DPPIV activity and antigen concentrations associate with ACEi-associated angioedema but also highlights the limitations of small sample size and serum measurements. The degradation half-life of substance P correlated with DPPIV antigen only during ACE inhibition; however, the majority of individuals with ACEi-associated angioedema were no longer taking an ACEi at the time that their blood was collected. In addition, we measured soluble DPPIV antigen and serum activity, but DPPIV is a cell-surface–associated peptidase.21 Although serum DPPIV activity correlates with the T-cell membrane–bound antigen,26 membrane-bound DPPIV in connective tissue stroma has a 10-fold lower Michaelis constant than does soluble DPPIV.27 The regulation of DPPIV expression seems to be tissue specific,28,29 and soluble antigen concentrations may reflect not only expression but also cleavage from the cell surface. Similarly, the ex vivo degradation of substance P may not reflect its local in vivo metabolism.
Black race and female gender have been associated with an increased risk of ACEi-associated angioedema. Nevertheless, we did not detect an effect of either race or gender on enzyme activity or peptide degradation. Smoking has been associated with an increased risk of ACEi-associated angioedema,30 and we also found an increased prevalence of smoking among case subjects compared with control subjects. Interestingly, serum DPPIV activity was significantly decreased in the sera of smokers who were taking an ACEi. At least 1 previous study reported lower DPPIV activity in bronchial alveolar lavage fluid from smokers compared with fluid from non-smokers.31 As reported previously as well,6 the prevalence of diabetes was decreased among individuals with ACEi-associated angioedema compared with controls. Here, DPPIV activity tended to be increased in the sera of diabetic subjects compared with that of nondiabetic subjects. Likewise, DPPIV activity has been associated with increased glucose concentrations in diabetic subjects.32
As expected, the degradation half-lives of bradykinin and des-Arg9-bradykinin were increased during ACEi, in either case subjects or control subjects. Des-Arg9-bradykinin acts as a proinflammatory agonist at the B1 receptor and is degraded primarily via APP.22 In the current study, the degradation half-life of des-Arg9-bradykinin correlated inversely with APP activity in the sera in the absence of ACEi. During ACEi, an increased proportion of bradykinin is degraded to des-Arg9-bradykinin via the kininase I pathway12 (Figure 1). The finding that the des-Arg9-bradykinin half-life correlated with the bradykinin half-life in the sera of ACEi-treated subjects likely reflects continued formation of des-Arg9-bradykinin from exogenous bradykinin under these conditions. Unexpectedly, we found no difference in APP activity or des-Arg9-bradykinin concentrations between case and control subjects. This contrasts with data from a largely European population13 and may reflect differences in ethnicity or in concurrent ACEi use. For example, in the current study, among subjects not taking an ACEi at the time that their blood was collected, there was a nonsignificant trend toward higher APP activity in the sera of control subjects compared with case subjects. Moreover, given that bradykinin stimulates the release of substance P,33 it is plausible that either decreased APP activity, leading to decreased inactivation of bradykinin and des-Arg9-bradykinin with consequent stimulation of substance P release, or decreased DPPIV activity, leading to decreased inactivation of substance P, could result in the common phenotype of ACEi-associated angioedema.
The findings of this study may have important implications for the use of DPPIV inhibitors in the treatment of diabetes. In addition to degrading bradykinin and substance P, DPPIV degrades the incretins glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1 (7–36 amide).34 Clinical studies of DPPIV inhibitors indicate that this class of drugs improves glycemic control in patients with type 2 diabetes mellitus.35–37 The data from the present study suggest that individuals treated with this class of drugs, while concurrently taking an ACEi, may be at increased risk for the development of angioedema. Published trials have either not included subjects taking ACEi or have not commented on the inclusion of subjects exposed to ACEis.35–39 However, studies report an increased incidence of nasopharyngitis in subjects randomly assigned to DPPIV inhibitor35,38,40; in 1 study, 1 of 175 sitagliptin-treated subjects was hospitalized for angioedema.41 Review of the Food and Drug Administration Adverse Events Reporting System revealed 10 cases of angioedema or angioedema-related symptoms associated with sitagliptin exposure from November 2006 through July 2, 2007 (Food and Drug Administration Adverse Event Reporting System Freedom of Information Report, July 2, 2007). Given the variable temporal relationship between the first use of an ACEi and onset of angioedema, the impact of DPPIV inhibition on the risk of angioedema in ACEi-exposed patients may be difficult to discern.
Perspectives
In summary, DPPIV activity is decreased in individuals with a history of ACEi-associated angioedema, as is DPPIV antigen. Genetic or environmental factors that decrease DPPIV activity may predispose individuals to ACEi-associated angioedema.
Supplementary Material
Acknowledgments
Sources of Funding
This work was supported in part by grant M01 RR-00095 from the National Center for Research Resources, National Institutes of Health, as well as by National Institutes of Health grants R01-HL079184 and R01-HL065193. J.B.B. received support from a National Institutes of Health Cardiovascular Pharmacology Training Grant (R01-HL076133).
Footnotes
Reprints: Information about reprints can be found online at http://www.lww.com/reprints
Disclosures
N.J.B. is a consultant for Novartis, one of several companies developing a dipeptidyl peptidase IV inhibitor. She holds a patent for a method of identifying susceptibility to angiotensin-converting enzyme inhibitor–associated angioedema. The remaining authors report no conflicts.
References
- 1.Slater EE, Merrill DD, Guess HA, Roylance PJ, Cooper WD, Inman WH, Ewan PW. Clinical profile of angioedema associated with angiotensin converting-enzyme inhibition. JAMA. 1988;260:967–970. [PubMed] [Google Scholar]
- 2.Israili ZH, Hall WD. Cough and angioneurotic edema associated with angiotensin-converting enzyme inhibitor therapy. A review of the literature and pathophysiology. Ann Intern Med. 1992;117:234–242. doi: 10.7326/0003-4819-117-3-234. [DOI] [PubMed] [Google Scholar]
- 3.Brown NJ, Snowden M, Griffin MR. Recurrent angiotensin-converting enzyme inhibitor–associated angioedema. JAMA. 1997;278:232–233. doi: 10.1001/jama.278.3.232. [DOI] [PubMed] [Google Scholar]
- 4.Nussberger J, Cugno M, Amstutz C, Cicardi M, Pellacani A, Agostoni A. Plasma bradykinin in angio-oedema. Lancet. 1998;351:1693–1697. doi: 10.1016/S0140-6736(97)09137-X. [DOI] [PubMed] [Google Scholar]
- 5.Brown NJ, Ray WA, Snowden M, Griffin MR. Black Americans have an increased rate of angiotensin converting enzyme inhibitor-associated angioedema. Clin Pharmacol Ther. 1996;60:8–13. doi: 10.1016/S0009-9236(96)90161-7. [DOI] [PubMed] [Google Scholar]
- 6.Kostis JB, Kim HJ, Rusnak J, Casale T, Kaplan A, Corren J, Levy E. Incidence and characteristics of angioedema associated with enalapril. Arch Intern Med. 2005;165:1637–1642. doi: 10.1001/archinte.165.14.1637. [DOI] [PubMed] [Google Scholar]
- 7.Food and Drug Administration. [Accessed August 21, 2007];Advisory Committee briefing document: omapatrilat. Available at: http://www.fda.gov/ohrms/dockets/ac/02/briefing/3877B2_01_BristolMeyersSquibb.pdf.
- 8.Abbosh J, Anderson JA, Levine AB, Kupin WL. Angiotensin converting enzyme inhibitor-induced angioedema more prevalent in transplant patients. Ann Allergy Asthma Immunol. 1999;82:473–476. doi: 10.1016/S1081-1206(10)62723-8. [DOI] [PubMed] [Google Scholar]
- 9.Emanueli C, Grady EF, Madeddu P, Figini M, Bunnett NW, Parisi D, Regoli D, Geppetti P. Acute ACE inhibition causes plasma extravasation in mice that is mediated by bradykinin and substance P. Hypertension. 1998;31:1299–1304. doi: 10.1161/01.hyp.31.6.1299. [DOI] [PubMed] [Google Scholar]
- 10.Sulpizio AC, Pullen MA, Edwards RM, Brooks DP. The effect of acute angiotensin-converting enzyme and neutral endopeptidase 24.11 inhibition on plasma extravasation in the rat. J Pharmacol Exp Ther. 2004;309:1141–1147. doi: 10.1124/jpet.103.064105. [DOI] [PubMed] [Google Scholar]
- 11.Moreau ME, Garbacki N, Molinaro G, Brown NJ, Marceau F, Adam A. The kallikrein-kinin system: current and future pharmacological targets. J Pharmacol Sci. 2005;99:6–38. doi: 10.1254/jphs.srj05001x. [DOI] [PubMed] [Google Scholar]
- 12.Blais C, Jr, Rouleau JL, Brown NJ, Lepage Y, Spence D, Munoz C, Friborg J, Geadah D, Gervais N, Adam A. Serum metabolism of bradykinin and des-Arg9-bradykinin in patients with angiotensin-converting enzyme inhibitor-associated angioedema. Immunopharmacology. 1999;43:293–302. doi: 10.1016/s0162-3109(99)00133-2. [DOI] [PubMed] [Google Scholar]
- 13.Adam A, Cugno M, Molinaro G, Perez M, Lepage Y, Agostoni A. Aminopeptidase P in individuals with a history of angio-oedema on ACE inhibitors. Lancet. 2002;359:2088–2089. doi: 10.1016/S0140-6736(02)08914-6. [DOI] [PubMed] [Google Scholar]
- 14.Molinaro G, Cugno M, Perez M, Lepage Y, Gervais N, Agostoni A, Adam A. Angiotensin-converting enzyme inhibitor-associated angioedema is characterized by a slower degradation of des-arginine(9)-bradykinin. J Pharmacol Exp Ther. 2002;303:232–237. doi: 10.1124/jpet.102.038067. [DOI] [PubMed] [Google Scholar]
- 15.Lefebvre J, Murphey LJ, Hartert TV, Jiao SR, Simmons WH, Brown NJ. Dipeptidyl peptidase IV activity in patients with ACE-inhibitor-associated angioedema. Hypertension. 2002;39:460–464. doi: 10.1161/hy0202.103054. [DOI] [PubMed] [Google Scholar]
- 16.Cyr M, Lepage Y, Blais C, Jr, Gervais N, Cugno M, Rouleau JL, Adam A. Bradykinin and des-Arg(9)-bradykinin metabolic pathways and kinetics of activation of human plasma. Am J Physiol Heart Circ Physiol. 2001;281:H275–H283. doi: 10.1152/ajpheart.2001.281.1.H275. [DOI] [PubMed] [Google Scholar]
- 17.Sprinkle TJ, Stone AA, Venema RC, Denslow ND, Caldwell C, Ryan JW. Assignment of the membrane-bound human aminopeptidase P gene (XPNPEP2) to chromosome Xq25. Genomics. 1998;50:114–116. doi: 10.1006/geno.1998.5302. [DOI] [PubMed] [Google Scholar]
- 18.Duan QL, Nikpoor B, Dube MP, Molinaro G, Meijer IA, Dion P, Rochefort D, Saint-Onge J, Flury L, Brown NJ, Gainer JV, Rouleau JL, Agostoni A, Cugno M, Simon P, Clavel P, Potier J, Wehbe B, Benarbia S, Marc-Aurele J, Chanard J, Foroud T, Adam A, Rouleau GA. A variant in XPNPEP2 is associated with angioedema induced by angiotensin I-converting enzyme inhibitors. Am J Hum Genet. 2005;77:617–626. doi: 10.1086/496899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Campos MM, Calixto JB. Neurokinin mediation of edema and inflammation. Neuropeptides. 2000;34:314–322. doi: 10.1054/npep.2000.0823. [DOI] [PubMed] [Google Scholar]
- 20.Russell JS, Chi H, Lantry LE, Stephens RE, Ward PE. Substance P and neurokinin A metabolism by cultured human skeletal muscle myocytes and fibroblasts. Peptides. 1996;17:1397–1403. doi: 10.1016/s0196-9781(96)00201-x. [DOI] [PubMed] [Google Scholar]
- 21.Lambeir AM, Durinx C, Scharpe S, DeMeester I. Dipeptidyl-peptidase IV from bench to bedside: an update on structural properties, functions, and clinical aspects of the enzyme DPP IV. Crit Rev Clin Lab Sci. 2003;40:209–294. doi: 10.1080/713609354. [DOI] [PubMed] [Google Scholar]
- 22.Pesquero JB, Jubilut GN, Lindsey CJ, Paiva AC. Bradykinin metabolism pathway in the rat pulmonary circulation. J Hypertens. 1992;10:1471–1478. doi: 10.1097/00004872-199210120-00006. [DOI] [PubMed] [Google Scholar]
- 23.Ahmad S, Wang L, Ward PE. Dipeptidyl(amino)peptidase IV and aminopeptidase M metabolize circulating substance P in vivo. J Pharmacol Exp Ther. 1992;260:1257–1261. [PubMed] [Google Scholar]
- 24.Byrd JB, Shreevatsa A, Putlur P, Foretia D, McAlexander L, Sinha T, Does MD, Brown NJ. Dipeptidyl peptidase IV deficiency increases susceptibility to angiotensin-converting enzyme inhibitor-induced peritracheal edema. J Allergy Clin Immunol. 2007;120:403–408. doi: 10.1016/j.jaci.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 25.Bork K, Frank J, Grundt B, Schlattmann P, Nussberger J, Kreuz W. Treatment of acute edema attacks in hereditary angioedema with a bradykinin receptor-2 antagonist (Icatibant) J Allergy Clin Immunol. 2007;119:1497–1503. doi: 10.1016/j.jaci.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 26.Elgun S, Keskinege A, Akan H, Karaca L. Serum dipeptidyl peptidase IV activity correlates with the T-cell CD26 antigen. Clin Chem Lab Med. 1999;37:839–840. doi: 10.1515/CCLM.1999.126. [DOI] [PubMed] [Google Scholar]
- 27.Pereira DA, Gomes L, El Cheikh MC, Borojevic R. Dipeptidyl peptidase IV (CD26) activity in the hematopoietic system: differences between the membrane-anchored and the released enzyme activity. Braz J Med Biol Res. 2003;36:567–578. doi: 10.1590/s0100-879x2003000500003. [DOI] [PubMed] [Google Scholar]
- 28.Abbott CA, Baker E, Sutherland GR, McCaughan GW. Genomic organization, exact localization, and tissue expression of the human CD26 (dipeptidyl peptidase IV) gene. Immunogenetics. 1994;40:331–338. doi: 10.1007/BF01246674. [DOI] [PubMed] [Google Scholar]
- 29.Bohm SK, Gum JR, Jr, Erickson RH, Hicks JW, Kim YS. Human dipeptidyl peptidase IV gene promoter: tissue-specific regulation from a TATA-less GC-rich sequence characteristic of a housekeeping gene promoter. Biochem J. 1995;311:835–843. doi: 10.1042/bj3110835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morimoto T, Gandhi TK, Fiskio JM, Seger AC, So JW, Cook EF, Fukui T, Bates DW. An evaluation of risk factors for adverse drug events associated with angiotensin-converting enzyme inhibitors. J Eval Clin Pract. 2004;10:499–509. doi: 10.1111/j.1365-2753.2003.00484.x. [DOI] [PubMed] [Google Scholar]
- 31.Van DV V, Naber BA, Van Hal PT, Overbeek SE, Hoogsteden HC, Versnel MA. Peptidase activities in serum and bronchoalveolar lavage fluid from allergic asthmatics–omparison with healthy non-smokers and smokers and effects of inhaled glucocorticoids. Clin Exp Allergy. 1999;29:813–823. doi: 10.1046/j.1365-2222.1999.00550.x. [DOI] [PubMed] [Google Scholar]
- 32.Mannucci E, Pala L, Ciani S, Bardini G, Pezzatini A, Sposato I, Cremasco F, Ognibene A, Rotella CM. Hyperglycaemia increases dipeptidyl peptidase IV activity in diabetes mellitus. Diabetologia. 2005;48:1168–1172. doi: 10.1007/s00125-005-1749-8. [DOI] [PubMed] [Google Scholar]
- 33.Kopp UC, Farley DM, Smith LA. Bradykinin-mediated activation of renal sensory neurons due to prostaglandin-dependent release of substance P. Am J Physiol. 1997;272:R2009–R2016. doi: 10.1152/ajpregu.1997.272.6.R2009. [DOI] [PubMed] [Google Scholar]
- 34.Mentlein R, Gallwitz B, Schmidt WE. Dipeptidyl-peptidase IV hydrolyses gastric inhibitory polypeptide, glucagon-like peptide-1(7–36)amide, peptide histidine methionine and is responsible for their degradation in human serum. Eur J Biochem. 1993;214:829–835. doi: 10.1111/j.1432-1033.1993.tb17986.x. [DOI] [PubMed] [Google Scholar]
- 35.Ahren B, Simonsson E, Larsson H, Landin-Olsson M, Torgeirsson H, Jansson PA, Sandqvist M, Bavenholm P, Efendic S, Eriksson JW, Dickinson S, Holmes D. Inhibition of dipeptidyl peptidase IV improves metabolic control over a 4-week study period in type 2 diabetes. Diabetes Care. 2002;25:869–875. doi: 10.2337/diacare.25.5.869. [DOI] [PubMed] [Google Scholar]
- 36.Ahren B, Gomis R, Standl E, Mills D, Schweizer A. Twelve- and 52-week efficacy of the dipeptidyl peptidase IV inhibitor LAF237 in metformin-treated patients with type 2 diabetes. Diabetes Care. 2004;27:2874–2880. doi: 10.2337/diacare.27.12.2874. [DOI] [PubMed] [Google Scholar]
- 37.Mari A, Sallas WM, He YL, Watson C, Ligueros-Saylan M, Dunning BE, Deacon CF, Holst JJ, Foley JE. Vildagliptin, a dipeptidyl peptidase-IV inhibitor, improves model-assessed beta-cell function in patients with type 2 diabetes. J Clin Endocrinol Metab. 2005;90:4888–4894. doi: 10.1210/jc.2004-2460. [DOI] [PubMed] [Google Scholar]
- 38.Pi-Sunyer FX, Schweizer A, Mills D, Dejager S. Efficacy and tolerability of vildagliptin monotherapy in drug-naive patients with type 2 diabetes. Diabetes Res Clin Pract. 2007;76:132–138. doi: 10.1016/j.diabres.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 39.Rosenstock J, Baron MA, Camisasca RP, Cressier F, Couturier A, Dejager S. Efficacy and tolerability of initial combination therapy with vildagliptin and pioglitazone compared with component monotherapy in patients with type 2 diabetes. Diabetes Obes Metab. 2007;9:175–185. doi: 10.1111/j.1463-1326.2006.00698.x. [DOI] [PubMed] [Google Scholar]
- 40.Charbonnel B, Karasik A, Liu J, Wu M, Meininger G. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes inadequately controlled with metformin alone. Diabetes Care. 2006;29:2638–2643. doi: 10.2337/dc06-0706. [DOI] [PubMed] [Google Scholar]
- 41.Rosenstock J, Brazg R, Andryuk PJ, Lu K, Stein P. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing pioglitazone therapy in patients with type 2 diabetes: a 24-week, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther. 2006;28:1556–1568. doi: 10.1016/j.clinthera.2006.10.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.