Abstract
We explored whether the M2 muscarinic receptor in the guinea pig ileum elicits a highly potent, direct-contractile response, like that from the M3 muscarinic receptor knockout mouse. First, we characterized the irreversible receptor-blocking activity of 4-DAMP mustard in ileum from muscarinic receptor knockout mice to verify its M3 selectivity. Then, we used 4-DAMP mustard to inactivate M3 responses in the guinea pig ileum to attempt to reveal direct, M2 receptor-mediated contractions. The muscarinic agonist, oxotremorine-M, elicited potent contractions in ileum from wild-type, M2 receptor knockout, and M3 receptor knockout mice characterized by negative log EC50 (pEC50) values ± SEM of 6.75 ± 0.03, 6.26 ± 0.05, and 6.99 ± 0.08, respectively. The corresponding Emax values in wild-type and M2 receptor knockout mice were approximately the same, but that in the M3 receptor knockout mouse was only 36% of wild type. Following 4-DAMP mustard treatment, the concentration–response curve of oxotremorine-M in wild-type ileum resembled that of the M3 knockout mouse in terms of its pEC50, Emax, and inhibition by selective muscarinic antagonists. Thus, 4-DAMP mustard treatment appears to inactivate M3 responses selectively and renders the muscarinic contractile behavior of the wild-type ileum similar to that of the M3 knockout mouse. Following 4-DAMP mustard treatment, the contractile response of the guinea pig ileum to oxotremorine-M exhibited low potency and a competitive-antagonism profile consistent with an M3 response. The guinea pig ileum, therefore, lacks a direct, highly potent, M2-contractile component but may have a direct, lower potency M2 component.
Keywords: Ileum, Guinea pig, Muscarinic receptor knockout mice, 4-DAMP mustard, M2 muscarinic receptor, M3 muscarinic receptor
Introduction
Subtype selective antagonists inhibit muscarinic contractions of gastrointestinal and urinary bladder smooth muscle in a manner that agrees with an M3 receptor mechanism (Eglen et al. 1996). This behavior is consistent with the known coupling of the M3 receptor to Gq/11 (Noronha-Blob et al. 1989; Candell et al. 1990; Roffel et al. 1990), which is often involved in Ca2+ mobilization. The details of how M3-Gq/11 signaling leads to contraction are unclear, however, because contraction of the guinea pig ileum depends mainly on an extracellular source of Ca2+ (Bolger et al. 1983).
The M2 muscarinic receptor is also expressed in smooth muscle, and it outnumbers the M3 by a factor of at least four (Eglen et al. 1996). The apparent lack of a role of the M2 receptor in contraction can be explained by the nature of its known signaling mechanisms in smooth muscle (see Table 1). Stimulation of the M2 receptor activates a nonselective cation conductance; however, the conductance depends on Ca2+ (Bolton 1979; Inoue 1991; Sakamoto et al. 2007). This explains why there is little increase in conductance unless both the M2 and the Ca2+-mobilizing M3 receptor are activated simultaneously. The M2 receptor is also known to inhibit Ca2+-activated K+ channels, which release smooth muscle from inhibitory K+ currents and enhance contraction (Kotlikoff et al. 1992). Like the cation conductance, however, this mechanism also requires Ca2+ mobilization by another receptor to activate the K+ current before the M2 receptor can inhibit it. Finally, the M2 receptor inhibits adenylate cyclase in smooth muscle. This inhibition opposes the relaxant effect of receptors that increase cyclic adenosine monophosphate (cAMP; e.g., β-adrenoceptor; Thomas et al. 1993; Thomas and Ehlert 1996; Sawyer and Ehlert 1998; Ehlert et al. 2005), and this effect requires Ca2+ mobilization in the first place; otherwise, there is no contraction to be relaxed by the β-adrenoceptor and no β-adrenoceptor response for the M2 receptor to inhibit. In trachea, M2 receptor activation opposes forskolin- but not isoproterenol-induced relaxation, which is consistent with the postulate that the β2-adrenoceptor mediates relaxation through a non-cAMP mechanism in the trachea (Ostrom and Ehlert 1998; 1999).
Table 1.
Summary of the types of contractions elicited by M2 and M3 muscarinic receptors in smooth muscle and their putative mechanisms
| Receptor | Type of contraction | Tissue and species | Putative mechanism |
|---|---|---|---|
| M2 | Direct contraction | Mouse: ileum, trachea, and urinary bladder | Unknown, Gi mobilization of extracellular Ca2+ |
| Conditional inhibition of cAMP-mediatied relaxation | Mouse: ileum, trachea, and urinary bladder | Gi-mediated inhibition of adenylate cyclase | |
| Guinea pig: ileum, trachea, colon, and esophagus | |||
| Conditional enhancement of M3-receptor-mediated contraction | Mouse: ileum, urinary bladder, and uterus | Gi stimulation of Icat; Gi inhibition of BKCa | |
| Guinea pig: colon and ileum | |||
| M3 | Direct contraction | Widespread in guinea pig and mouse smooth muscle | Major: unknown Gq-mediated influx of extracellular Ca2+ |
| Minor: Gq-mediated phosphoinositide hydrolysis and release of intracellular Ca2+ |
Contraction is defined as direct if activation of the indicated receptor by itself is sufficient to cause contraction. If activation of the receptor subtype by itself has no effect on contraction but elicits or enhances contraction when other receptors are activated, then the muscarinic contraction is defined as conditional. Further details are described in the text.
Icat nonselective cation conductance, BKCa Ca2+-activated potassium channel
It may seem that these M2 effects would be manifest in standard pharmacological antagonism studies. However, we have shown that the competitive antagonism of a response mediated through M2–M3 receptor interactions resembles the profile of the directly acting receptor (i.e., the M3) and not that of the conditionally acting receptor (i.e., the M2; Ehlert 2003). Thus, the M3 antagonism profile of standard muscarinic contractions of the ileum and bladder is not inconsistent with the postulate that both M2 and M3 receptors interact to elicit contraction.
Studies on the mouse uterus are consistent with a conditional role for the M2 receptor in contraction (Kitazawa et al. 2008). The competitive antagonism of the muscarinic contractile response of wild-type uterus resembles an M3 profile, but the Emax for contraction is inhibited by about 50% in uterus from the M2 knockout (KO) mouse. In the M3 KO mouse, muscarinic contractions are absent. Thus, the M2 receptor is unable to elicit direct contraction of the mouse uterus, but is able to enhance M3 receptor-mediated contractions.
Following inactivation of M3 receptors in guinea pig ileum and colon, we have identified two types of muscarinic contractile responses for the M2 receptor. One is a highly potent M2 receptor-mediated inhibition of forskolin- and β-adrenoceptor-mediated relaxation, and the other is a less potent M2 receptor-mediated enhancement of M3 receptor contractile signaling (Ehlert 2003). Circumstantial evidence suggests that the latter is involved in heterologous desensitization, which requires activation of both M2 and M3 receptors and is potently antagonized by M2 selective antagonists (Griffin et al. 2004).
Studies on muscarinic receptor knockout mice are consistent with these observations but have revealed an additional, highly potent, direct-contractile mechanism for the M2 receptor. In M3 KO mice, the M2 receptor elicits a highly potent contractile response in ileum and trachea, although the maximum of this response is only about 40% that of the muscarinic contraction in wild-type and M2 KO tissue (Matsui et al. 2000; Matsui et al. 2002).
In the present study, we have investigated whether a similar, highly potent, direct-M2-receptor-mediated contraction occurs in the guinea pig ileum. We show that treatment of the wild-type mouse ileum with 4-DAMP mustard (N-2-chloroethyl-4-piperidinyl diphenylacetate) uncovers a highly potent, direct-M2-contractile mechanism and converts its pharmacological behavior into that of the M3 KO mouse. In contrast, treatment of the guinea pig ileum with 4-DAMP mustard caused a large, 56-fold reduction in agonist potency, and the residual muscarinic response exhibited an M3-pharmacological profile. Thus, the guinea pig ileum appears to lack the highly potent direct-contractile-M2 mechanism observed in the mouse. Our data also illustrate that ileal smooth muscle from whole-body M3 KO mice accurately displays the contractile activity of the M2 receptor in wild-type mice and that 4-DAMP mustard is a useful tool for inactivating the M3 responses selectively.
Methods
Animals M2 muscarinic receptor knockout (M2−/−; M2 KO) and M3 muscarinic receptor knockout (M3−/−; M3 KO) mice were generated as described by Matsui et al. (2002).
Contractile assays in isolated ileal tissue Contractile measurements were made on ileum from male Hartley guinea pigs (300–400 g) and C57Bl-6 mice (25–30 g) as described previously (Griffin et al. 2004). The medium was Krebs–Ringer bicarbonate (KRB) buffer (124 mM NaCl, 5 mM KCl, 1.3 mM MgCl2, 26 mM NaHCO3, 1.2 mM KH2PO4, 1.8 mM CaCl2, 10 mM glucose) containing indomethacin (1 μM) and maintained at 37°C and gassed with O2/CO2 (19:1). Tissues were allowed to incubate for at least an hour and were subsequently challenged with KCl (50 mM) three times, followed by the measurement of a cumulative concentration–response curve to oxotremorine-M. This was done to speed up the equilibration of the ileum, which undergoes a time-dependent increase in contractile activity. After appropriate washing, the ileum was incubated for another 30 min prior to the collection of the data presented under “Results”. Contractile responses to oxotremorine-M were measured using a cumulative technique. The Emax value of oxotremorine-M increased with an increase in age or body weight of each strain of mouse. In addition, the ileal contraction to KCl (50 mM) increased with an increase in body weight but was similar in mice of equivalent body weights across the different strains. All contractions to oxotremorine-M, therefore, were normalized relative to that elicited by KCl (50 mM).
4-DAMP mustard treatment A solution of 4-DAMP mustard was first cyclized in 10 mM phosphate buffer, pH 7.4, for 30 min at 37°C to allow formation of the aziridinium ion (Thomas et al. 1992). The solution was placed on ice and used as soon as possible. Ilea were incubated with 4-DAMP mustard (40 nM) in combination with the M2 selective antagonist AF-DX 116 (4 μM; [[2-2(diethyl-amino)methyl]-1-piperidinyl]-acetyl]-5,11-dihydro-6H-pyrido[2,3b][1,4] benzodiazepine-6-one) for one or two 1-h time periods, in a final volume of 50 ml of KRB buffer. This minimum volume of medium is essential because 4-DAMP mustard is inactivated by tissue nucleophiles, particularly in substantial tissues like the guinea-pig ileum. After these incubations, the tissue was washed three times and incubated for 30 min prior to contractile measurements.
Analysis of concentration–response curves Concentration–response curves were analyzed using GraphPad Prism software (GraphPad Software, Inc., La Jolla, CA, USA) using the variable slope concentration–response curve function. The negative log dissociation constants of antagonists (pKB) were estimated from experiments in which their ability to shift the agonist concentration–response curve to the right was measured (Arunlakshana and Schild 1959):
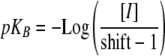 |
In this equation, [I] denotes the molar concentration of antagonist, and shift denotes EC50 value of the agonist measured in the presence of the antagonist divided by that measured in its absence. The Log shift and pKB values were determined for individual experiments and averaged. The significance of differences were evaluated using the unpaired Student's t test with the overly conservative Bonferroni adjustment of the critical value of P where appropriate.
Drugs and chemicals The reagents used in this study were obtained from the following sources: AF-DX 116, Boehringer Ingelheim Pharmaceutical, Ridgefield, CT, USA; oxotremorine-M and indomethacin, Sigma RBI, Natick, MA, USA; 4-DAMP was synthesized using a method similar to that described by Barlow et al. (1976), and 4-DAMP mustard was synthesized as described previously (Thomas et al. 1992).
Results
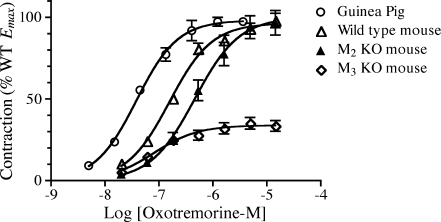
Contractile activity of oxotremorine-M in guinea pig and mouse ileum Oxotremorine-M potently elicited contractions in ilea from both the mouse and guinea pig. The average negative log EC50 (pEC50) ± SEM and Emax ± SEM values of oxotremorine-M were both greater in guinea pig (7.48 ± 0.05 and 58.2 ± 4.5 mN) than in wild-type mouse (6.75 ± 0.03 and 12.2 ± 0.5 mN). The average responses and their associated SEM values in guinea pig and wild-type mouse were normalized relative to the average Emax value of its respective group (i.e., guinea pig or wild-type mouse), and the normalized data are plotted in Fig. 1. The higher potency of oxotremorine-M in the guinea pig is readily apparent from the figure. The contractile activity of oxotremorine-M was also investigated in ilea from M2 and M3 KO mice. The responses in each mouse ileum were first normalized relative to the contraction elicited by KCl (50 mM) as described under “Methods.” The mean contractile responses and their respective SEM values were then normalized relative to the Emax value of the wild-type mouse. The average Emax value ± SEM of oxotremorine-M in the M2 KO mouse ileum (101.2 ± 7.5%) was similar to that of wild type, whereas that measured in the M3 KO mouse was substantially smaller (35.7 ± 3.5%). Normalization of the muscarinic responses in the mouse to that of KCl did not significantly change the relationship among the Emax values in the M2 KO and M3 KO strains relative to wild type but did cause a modest reduction in the variance of the mean estimates in wild-type and M2 KO mice. The potency of oxotremorine-M in the M2 KO mouse (pEC50 = 6.26 ± 0.05) was about one third that of the wild-type mouse, whereas that in the M3 KO mouse (pEC50, 6.99 ± 0.08) was 1.7-fold greater than wild type. These differences in pEC50 were significant as indicated in the summary of these data in the legend to Table 2. Since prior reports have shown that the ileum from the M2/M3 double KO mouse lacks a muscarinic contractile response, the data in Fig. 1 are consistent with the postulate that the muscarinic contractile response in the wild-type mouse ileum includes a major M3-receptor component as well as a minor, but more potent, M2-receptor component. Matsui et al. (2000; 2002) have reached a similar conclusion .
Fig. 1.
Contractile activity of oxotremorine-M in ilea from the guinea pig and from wild type, M2 KO, and M3 KO mice. The data represent the mean values ± SEM from experiments on 13 guinea pigs, 39 wild-type mice, 25 M2 KO mice, and 25 M3 KO mice. The responses in the guinea-pig ileum have been normalized relative to Emax and those in mice to the Emax in wild type
Table 2.
Contractile activity of oxotremorine-M in guinea pig ileum and in wild-type, M2 KO, and M3 KO mouse ileum
| pEC50 | Eamax (% wild type) | Hill slope | |
|---|---|---|---|
| Guinea pig (13) | 7.48 ± 0.05b | 100 ± 7.7 | 1.38 ± 0.20 |
| Mouse | |||
| Wild type (39) | 6.75 ± 0.03 | 100 ± 4.1 | 1.14 ± 0.06 |
| M2 KO (25) | 6.26 ± 0.05c | 101 ± 7.5 | 1.18 ± 0.06 |
| M3 KO (25) | 6.99 ± 0.08c | 35.7 ± 3.5 d | 1.18 ± 0.18 |
The data are from Table 1 and represent the mean values ± SEM. The number of experiments is indicated in parentheses
aThe Emax and SEM values have been normalized relative to the average wild-type Emax value for each species.
bSignificantly different from the pEC50 value of wild-type mouse ileum (P = 4.3 × 10−16)
cOne-way analysis of variance showed highly significant differences among the pEC50 values measured in wild type, M2 KO, and M3 KO mouse ilea (F2,86 = 44.14; P = 6.4 × 10−14). Using the unpaired Student's t test, the P values for differences among the mean pEC50 values of wild type and M2 KO (P = 3.3 × 10−11), wild type and M3 KO (P = 0.0054), and M2 KO and M3 KO (P = 2.1 × 10−10) were all less than Bonferroni's adjusted 0.05 value of P for three comparisons (0.0167).
dSignificantly different from the Emax of wild-type ileum (P = 6.1 × 10−19)
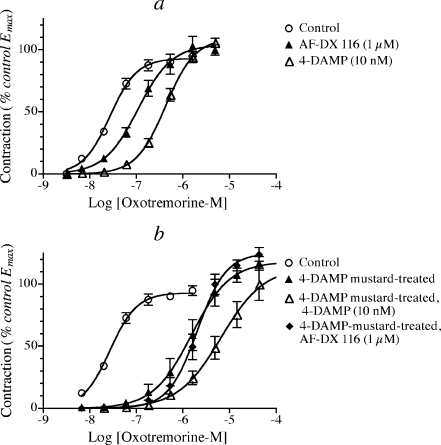
Antagonism of the muscarinic response in mouse ileum We investigated two muscarinic antagonists (AF-DX 116 and 4-DAMP (N,N-dimethyl-4-piperidinyl diphenylacetate)) with known selectivity for receptor subtypes to determine if their inhibitory action in the mouse ileum is consistent with the picture of M2 and M3 receptor function described above in connection with our studies on KO mice. The binding affinities (pKD, negative log dissociation constant) of AF-DX 116 for the human M2 and M3 receptor subtypes are 7.27 ± 0.05 and 6.10 ± 0.06, and those of 4-DAMP are 7.87 ± 0.03 and 8.81 ± 0.05, respectively (Esqueda et al. 1996; Griffin et al. 2004). Thus, AF-DX 116 exhibits about 15-fold higher affinity for the M2 receptor relative to M3, whereas 4-DAMP exhibits an opposite tenfold selectivity. 4-DAMP actually exhibits high affinity for all subtypes of the muscarinic receptor except the M2. We tested each antagonist at a concentration approximately equal to the greater of its two KD values for M2 and M3 receptors. With this strategy, the M2 selective AF-DX 116 and the M3-selective 4-DAMP should only cause about twofold shifts in the concentration–response curve of an agonist for eliciting M3 and M2 responses, respectively, but much greater ten- to 15-fold shifts in responses mediated by the receptors for which they exhibit selectivity (i.e., M2 and M3, respectively).The results of antagonism studies using AF-DX 116 (1 μM) in the mouse ileum from wild-type, M2 KO, and M3 KO mice are show in Fig. 2a–c. In ileum from the M3 KO mouse (Fig. 2c), AF-DX 116 caused a 10.5-fold shift in the concentration response curve of oxotremorine-M, which yielded an estimated pKB value of (6.97 ± 0.03) similar to its binding affinity for the M2 receptor (pKD = 7.27 ± 0.05). In contrast, AF-DX 116 only caused 2.6- and 2.7-fold shifts in the concentration response curves of oxotremorine-M in wild-type and M2 KO mice, respectively. 4-DAMP exhibited the opposite selectivity (Fig. 2a–c). It caused a 6.8-fold shift in the concentration–response curve of oxotremorine-M in the M2 KO mouse (Fig. 2b), which yields an estimated pKB value (8.74 ± 0.19) similar to its binding affinity for the M3 receptor (8.81 ± 0.05, Griffin et al. 2004). A similar shift of 14-fold was observed in the wild-type mouse (Fig. 2a), whereas a much smaller shift (1.5-fold) was measured in ilea from the M3 KO mouse (Fig. 2c). These results are summarized in Table 3 and are consistent with the postulate mentioned above that the muscarinic contractile response in the mouse ileum includes major and minor, directly acting M3 and M2 components, respectively. We also investigated the effects of 4-DAMP mustard on the muscarinic contractile response of the mouse ileum (Fig. 3). At neutral pH, 4-DAMP mustard forms an aziridinium ion that binds covalently to muscarinic receptors. When used at a concentration of 40 nM in the presence of AF-DX 116 (4 μM) for 1 h at 37°C, 4-DAMP mustard inactivates 96% of human M3 receptors expressed in CHO cells but only 22% of human M2 receptors (Griffin et al. 2003). Isolated ilea from wild-type, M2 KO, and M3 KO mice were incubated, with 4-DAMP mustard (40 nM) in combination with AF-DX 116 (4 μM) for a total time of 2 h, and washed extensively (see “Methods”). This treatment reduced the Emax value of oxotremorine-M in wild-type mouse ileum to only 43% of control while having little effect on EC50. As shown in Fig. 3a, the residual response in wild-type ileum after 4-DAMP mustard treatment was nearly identical to that measured in the untreated ileum from the M3 KO mouse. Similar results were obtained when the incubation with 4-DAMP mustard only lasted 1 h (Table 4). Treatment with 4-DAMP mustard (2 h) caused a large inhibition in the response to oxotremorine-M in the M2 KO mouse ileum (Fig. 3b), but had little effect on the response in the M3 KO ileum (Fig. 3c). The results are consistent with the postulate that 4-DAMP mustard treatment selectively inactivates M3 responses over M2, thereby converting the muscarinic behavior of the wild-type ileum into that of the M3 KO ileum. These results are summarized in Table 4. To obtain further support for this hypothesis, we characterized the pharmacological profile of the muscarinic response in 4-DAMP mustard-treated wild-type ileum using the competitive antagonists, 4-DAMP and AF-DX 116. After 4-DAMP mustard treatment, AF-DX 116 (1 μM) and 4-DAMP (10 nM) caused 9.8- and 3.0-fold shifts in the concentration response curve to oxotremorine-M in wild-type mouse ileum, yielding pKB estimates of 6.90 ± 0.13 and 8.30 ± 0.28, respectively (Fig. 3d). This profile of antagonism is similar to that described above for the ileum from M3 KO mice (Fig. 2c).
Fig. 2.
Competitive antagonism of the response to oxotremorine-M by AF-DX 116 (1 μM) and 4-DAMP (10 nM) in ilea from wild type (a), M2 KO (b), and M3 KO (c) mice. The data are normalized relative to the Emax of control and represent the mean values ± SEM from four to seven experiments
Table 3.
Effects of AF-DX 116 and 4-DAMP on the contractile response to oxotremorine-M in mouse ileum
| AF-DX 116 (1 μM) | 4-DAMP (10 nM) | |||
|---|---|---|---|---|
| Log shifta | pKB | Log shifta | pKB | |
| Wild type (7, 7) | 0.42 ± 0.06 | 6.17 ± 0.14 | 1.16 ± 0.13 | 9.12 ± .14 |
| M2 KO (4, 6) | 0.43 ± 0.18 | 5.93 ± 0.42 | 0.83 ± 0.19 | 8.74 ± .19 |
| M3 KO (7, 4) | 1.02 ± 0.02 | 6.97 ± 0.03 | 0.18 ± 0.17 | n.d.b |
The data are from Fig. 1 and represent the mean values ± SEM. The two numbers in parentheses beside each mouse strain denote the number of experiments done with AF-DX 116 and 4-DAMP, respectively
aThe Log shift denotes the logarithm of the ratio of the EC50 value measured in the presence of the antagonist divided by that measured in its absence
bThe pKB was not determined because of the low log shift value
Fig. 3.
Effects of 4-DAMP mustard treatment on contractions elicited to oxotremorine-M in mouse ileum. a Responses were measured in ilea from the M3 KO mouse (open triangles) and from wild-type ileum before (open circles) and after (closed triangles) treatment with 4-DAMP mustard (40 nM) in combination with AF-DX 116 (4 μM) for 2 h followed by washing as described under “Methods.” b Responses were measured in ilea from the M2 KO mouse before (open circles) and after (closed triangles) treatment with 4-DAMP mustard as described in a. c Same as b except that responses were measured in ilea from the M3 KO mouse. d All responses were measured in ilea from wild-type mice that had been treated with 4-DAMP mustard as described in a. After this treatment, responses were measured in the absence (open circles) and presence of AF-DX 116 (1 μM; closed triangles) or 4-DAMP (10 nM; open triangles). Mean values ± SEM from five to seven experiments are plotted in a–d
Table 4.
Effect of 4-DAMP mustard treatment (40 nM) in combination with AF-DX 116 (4 μM) on the contractile activity of oxotremorine-M in mouse ileum
| Control | 4-DAMP mustard | ||
|---|---|---|---|
| pEC50 | pEC50 | Emax (%)a | |
| Wild type | |||
| 1 h treatment (9) | 6.59 ± 0.08 | 6.62 ± 0.17 | 34 ± 8 |
| 2 h treatment (7) | 6.96 ± 0.06 | 6.89 ± 0.14 | 43 ± 9 |
| M2 KO | |||
| 2 h treatment (6) | 6.39 ± 0.09 | 5.80 ± 0.16 | 33 ± 9 |
| M3 KO | |||
| 2 h treatment (5) | 7.15 ± 0.08 | 7.36 ± 0.09 | 74 ± 3 |
The data are from Fig. 2 and represent the mean values ± SEM. The numbers in parentheses beside each mouse strain denote the number of experiments. The Emax values in 4-DAMP mustard-treated ileum are normalized relative to that measured under control conditions
aEmax values and their SEM have been normalized relative to the average Emax value of control
Characterization of the muscarinic contractile response in guinea pig ileum The effects of AF-DX 116 (1 μM) and 4-DAMP (10 nM) on the contractile response to oxotremorine-M in the guinea pig ileum are shown in Fig. 4a. These two antagonists caused shifts of 3.1- and tenfold, respectively, in the concentration–response curve. This behavior is consistent with the well-known M3 profile of this tissue, yielding pKB ± SEM values of 6.28 ± 0.10 and 9.00 ± 0.06 for AF-DX 116 and 4-DAMP, respectively, in excellent agreement with the binding affinity for the human M3 receptor. Treatment of the guinea pig ileum with 4-DAMP mustard (40 nM) in combination with AF-DX 116 (4 μM) for 2 h followed by washing caused a 56-fold dextral shift in the concentration–response curve to oxotremorine-M, with a small increase in its Emax value (Fig. 4b). This small effect can be attributed to time, since we observed time-dependent increases in Emax with repetitive measurement of concentration–response curves to oxotremorine-M. Unlike the behavior observed in wild-type mouse ileum, treatment of the guinea pig ileum with 4-DAMP mustard did not uncover a direct, highly potent contraction with a low Emax value. Following 4-DAMP mustard treatment, the effects of AF-DX 116 (1.3-fold dextral shift) and 4-DAMP (4.3-fold dextral shift) on the EC50 value of oxotremorine-M in the guinea pig ileum where qualitatively similar to those measured before 4-DAMP mustard treatment and, hence, suggestive of a direct M3 mechanism. Control experiments showed that the potency of oxotremorine-M increased 1.45-fold 1 h after 4-DAMP mustard treatment, suggesting that the measured antagonist-induced shifts were underestimated. Correcting these measured shifts by a factor of 1.45 yields theoretical shifts of 1.89 and 6.21 for AF-DX 116 (1 μM) and 4-DAMP (10 nM), respectively, which yield pKB values of 5.94 and 8.72 for these antagonists.
Fig. 4.
Effects of AF-DX 116 (1 μM) and 4-DAMP (10 nM) before (a) and after (b) 4-DAMP mustard treatment on contractile responses to oxotremorine-M in the guinea pig ileum. a Responses were measured in the absence (open symbols) and presence of AF-DX 116 (closed triangles) or 4-DAMP (open triangles). b Responses were first measured under control conditions (open circles). After 4-DAMP mustard treatment and washing, responses were measured in the absence (closed triangles) and presence of AF-DX 116 (closed diamonds) or 4-DAMP (open triangles). Ilea were treated with 4-DAMP mustard (40 nM) for 1 h in the presence of AF-DX 116 (4 μM) and were then washed three times as described under “Methods.” The data in a and b represent the mean response values ± SEM from ten to 13 experiments
Discussion
Muscarinic agonists elicit contraction in isolated ileum, trachea, and urinary bladder from many mammals, including the mouse. This function undergoes small, large, and complete losses in the M2 KO, M3 KO, and M2/M3 double KO mice, respectively (Matsui et al. 2000; Matsui et al. 2002), showing that M2 and M3 receptors account for contraction and that, in the absence of other agents, the latter contributes more to the response than the former. Contractions to efficacious muscarinic agonists are insensitive to tetrodotoxin, indicating that the relevant M2 and M3 receptors are located postjunctionally (Unno et al. 2005). Muscarinic agonists display high potency for eliciting contraction through the M2 receptor in smooth muscle from the M3 KO mouse, yet compared to that measured in wild-type tissue, their Emax values are only 10% in urinary bladder and 30–40% in ileum and trachea (Matsui et al. 2000). Direct M2 receptor-mediated contractions in the ileum from the M3 KO mouse have been reported to be evanescent (Unno et al. 2005), although we have found them to be reasonably stable over the time required to measure data for a cumulative, concentration–response curve. These contractions are pertussis toxin-sensitive and inhibited completely by the voltage-dependent Ca2+ antagonist, nicardipine (Unno et al. 2005). In contrast, M3 receptor-mediated contractions are pertussis toxin-insensitive and are partially inhibited by nicardipine in mouse (Unno et al. 2005) but nearly completely inhibited by voltage-sensitive Ca2+ channel blockers in guinea pig (Bolger et al. 1983).
The first report (Matsui et al. 2000) of a directly mediated M2 contraction in the M3 KO mouse was surprising because prior studies on the guinea pig had not uncovered such a role, although clear evidence for conditional M2 responses—that is, those dependent on other receptors—had been observed. It might be argued that the direct M2 effect had gone unnoticed in guinea pigs because the antagonists used to characterize contraction lacked the requisite selectivity for muscarinic receptor subtypes to detect a small M2 effect. This raises the question of whether the direct contractile role of the M2 receptor was missed in the guinea pig or whether guinea pigs simply differ from mice in their lack of this potent M2 function. For these reasons, we investigated whether it is possible to convert the muscarinic response of the ileum from the wild-type mouse into that of the M3 KO using 4-DAMP mustard and, if so, whether this treatment reveals a direct M2 receptor-mediated contraction in the guinea pig.
The compound, 4-DAMP mustard, is a nitrogen mustard derivative that cyclizes spontaneously into a reactive aziridinium ion nearly identical to the competitive muscarinic antagonist 4-DAMP except for its lack of two hydrogen atoms (Barlow et al. 1990). The latter compound only exhibits about tenfold higher affinity for the M3 receptor over the M2. At 100% receptor occupancy, 4-DAMP mustard alkylates M2 and M3 receptors at a similar rate (rate constant, 0.1 min−1; half time, 7 min), but selectivity for the M3 receptor can be achieved at the cost of a slower rate of alkylation by using a lower concentration of the aziridinium ion or by adding a competitive, M2-selective antagonist (e.g., AF-DX 116) to the incubation (Thomas et al. 1992). Using 4-DAMP mustard (40 nM) in combination with AF-DX 116 (4 μM) for 1 h, we showed that it is possible to alkylate 96% of a population of the human M3 receptor expressed in CHO cells, while only inactivating 22% of human M2 receptors (Griffin et al. 2003).
Treatment of the wild-type mouse ileum with 4-DAMP mustard reduced the Emax of the contractile response to oxotremorine-M by about 60% while having little effect on EC50. The residual concentration–response curve resembled that measured in the M3 KO mouse, in terms of its EC50, Emax, and antagonism by AF-DX 116 and 4-DAMP. These compounds had pKB values of 6.90 and 8.30, respectively, that differed by only about 0.4 log units from their binding affinities (pKD) for human M2 receptors (7.27 and 7.87 (Esqueda et al. 1996; Griffin et al. 2004)). The difference may be ascribed to recycling of muscarinic receptors after 4-DAMP mustard treatment as discussed below. In contrast, the pKB values of the same compounds in wild-type mouse ileum (6.17 and 9.12) are similar to their respective binding affinities (pKD) for the M3 receptor (6.10 and 8.81 (Esqueda et al. 1996; Griffin et al. 2004)). The direct M2-component of contraction in the wild-type mouse does not significantly perturb the antagonism profile of the wild-type response from that expected for a pure M3 response, illustrating the inability of these antagonists to resolve a minor receptor component of the response. 4-DAMP mustard treatment had little effect on muscarinic contractions in the M3 KO mouse. Our data suggest that 4-DAMP mustard treatment selectively inactivated M3 receptors in the wild-type ileum to unmask direct M2-receptor-mediated contractions that behaved similarly to those of the M3 KO mouse.
In contrast, 4-DAMP mustard treatment completely eliminated the high-potency response of the guinea pig ileum to oxotremorine-M. Only low-potency contractions to oxotremorine-M remained after 4-DAMP mustard treatment, for the concentration–response curve shifted to the right about 56-fold with no decline in Emax. These low-potency contractions were antagonized by AF-DX 116 and 4-DAMP in a manner qualitatively resembling that expected for an M3 response. The shift in the concentration–response curve caused by the M2-selective AF-DX 116 was only one fortieth of that expected for an M2 response, and that caused by the M3-selective 4-DAMP was threefold greater than expected for an M2 response. Both shifts, however, were about threefold smaller than that expected for an M3 response. This decrement in antagonism may be explained, in part, by the trafficking of new muscarinic receptors to the plasma membrane after 4-DAMP mustard treatment because control experiments showed about a 1.5-fold increase in the potency of oxotremorine-M during the same time period.
The guinea pig ileum is exquisitely sensitive to muscarinic agonists, and only a fraction of 1% of the muscarinic receptor population is required for the response at EC50 (Ringdahl 1984). A recovery of such a small amount of receptors seems plausible after 4-DAMP mustard treatment and before the response to oxotremorine-M was measured in the presence of antagonist (45–60 min). This time was used for washing residual agonist from the tissue and incubating with antagonist. In tissue homogenates, there is no recovery of muscarinic receptor binding after a few hours following 4-DAMP mustard treatment (Thomas et al. 1992), although the error in this measurement is at the same level as that capable of causing a small leftward shift in the concentration–response curve (i.e., about 2% of the receptor population).
In contrast to that of the guinea pig, the M3 response of the mouse ileum is much less sensitive to oxotremorine-M. Based on our prior work, it requires approximately 30% receptor occupancy by oxotremorine-M to elicit a 50% contractile response (Tran et al. 2009). This difference in the sensitivities of the mouse and guinea pig ileum can explain why it was possible to reduce the Emax value of oxotremorine-M in both the wild-type and M2 KO ileum, while the same treatment did not affect the Emax in the guinea pig ileum.
Our inability to detect direct, M2-receptor-mediated contractions in the guinea pig ileum does not rule them out; our point is that if they exist, they must be mediated by oxotremorine-M with much less potency than in the mouse or that it requires an agonist with much greater efficacy than oxotremorine-M to detect them. Since relative efficacy of oxotremorine-M is similar to or greater than that of acetylcholine at the M2 receptor (Ehlert 1985; Tran et al. 2009), our data show that highly potent, direct M2-receptor-mediated contractions are not mediated by acetylcholine physiologically. Thus, although the M2 receptor of the guinea pig ileum mediates a high potency inhibition of relaxation and a low potency enhancement of M3 receptor-mediated contractions (Ehlert 2003), it does not mediate a high potency direct contraction like that of the mouse ileum.
Acknowledgments
This work was supported by grant number HL079166 from the National Institutes of Health (RSO).
Open Access This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Arunlakshana O, Schild HO (1959) Some quantitative uses of drug antagonists. Brit J Pharmacol 14:48–58 [DOI] [PMC free article] [PubMed]
- Barlow RB, Berry KJ, Glenton PA, Nilolaou NM, Soh KS (1976) A comparison of affinity constants for muscarine-sensitive acetylcholine receptors in guinea-pig atrial pacemaker cells at 29°C and in ileum at 29°C and 37°C. Brit J Pharmacol 58:613–620 [DOI] [PMC free article] [PubMed]
- Barlow RB, Shepherd MK, Veale MA (1990) Some differential effects of 4-diphenylacetoxy-N-(2-chloroethyl)-piperidine hydrochloride on guinea-pig atria and ileum. J Pharm Pharmacol 42:412–418 [DOI] [PubMed]
- Bolger GT, Gengo P, Klockowski R, Luchowski E, Siegel H, Janis RA, Triggle AM, Triggle DJ (1983) Characterization of binding of the Ca++ channel antagonist, [3H]nitrendipine, to guinea-pig ileal smooth muscle. J Pharmacol Exp Ther 225:291–309 [PubMed]
- Bolton TB (1979) Mechanisms of action of transmitters and other substances on smooth muscle. Physiol Rev 59:606–718 [DOI] [PubMed]
- Candell LM, Yun SH, Tran LL, Ehlert FJ (1990) Differential coupling of subtypes of the muscarinic receptor to adenylate cyclase and phosphoinositide hydrolysis in the longitudinal muscle of the rat ileum. Mol Pharmacol 38:689–697 [PubMed]
- Eglen RM, Hegde SS, Watson N (1996) Muscarinic receptor subtypes and smooth muscle function. Pharmacol Rev 48:531–565 [PubMed]
- Ehlert FJ (1985) The relationship between muscarinic receptor occupancy and adenylate cyclase inhibition in the rabbit myocardium. Mol Pharmacol 28:410–421 [PubMed]
- Ehlert FJ (2003) Pharmacological analysis of the contractile role of M2 and M3 muscarinic receptor in smooth muscle. Receptors Channels 9:261–277 [DOI] [PubMed]
- Ehlert FJ, Griffin MT, Abe DM, Vo TH, Taketo MM, Manabe T, Matsui M (2005) The M2 muscarinic receptor mediates contraction through indirect mechanisms in mouse urinary bladder. J Pharmacol Exp Ther 313:368–378 [DOI] [PubMed]
- Esqueda EE, Gerstin EH Jr, Griffin MT, Ehlert FJ (1996) Stimulation of cyclic AMP accumulation and phosphoinositide hydrolysis by M3 muscarinic receptors in the rat peripheral lung. Biochem Pharmacol 52:643–658 [DOI] [PubMed]
- Griffin MT, Hsu JC-H, Shehnaz D, Ehlert FJ (2003) Comparison of pharmacological antagonism of M2 and M3 muscarinic receptors expressed in isolation and in combination. Biochem Pharmacol 65:1227–1241 [DOI] [PubMed]
- Griffin MT, Matsui M, Shehnaz D, Ansari KZ, Taketo MM, Manabe T, Ehlert FJ (2004) Muscarinic agonist-mediated heterologous desensitization in isolated ileum requires activation of both muscarinic M2 and M3 receptors. J Pharmacol Exp Ther 308:339–349 [DOI] [PubMed]
- Inoue R (1991) Ion channels involved in responses to muscarinic receptor activation in smooth muscle. In: Sperelakis NKuriyama H (ed) Ion channels of vascular smooth muscle cells and endothelial cells. Elsevier, New York, pp 81–91
- Kitazawa T, Hirama R, Masunaga K, Nakamura T, Asakawa K, Cao J, Teraoka H, Unno T, Komori S, Yamada M, Wess J, Taneike T (2008) Muscarinic receptor subtypes involved in carbachol-induced contraction of mouse uterine smooth muscle. Naunyn Schmiedebergs Arch Pharmacol 377:503–513 [DOI] [PubMed]
- Kotlikoff MI, Kume H, Tomasic M (1992) Muscarinic regulation of membrane ion channels in airway smooth muscle cells. Biochem Pharmacol 43:5–10 [DOI] [PubMed]
- Matsui M, Motomura D, Karasawa H, Fujikawa T, Jiang J, Komiya Y, Takahashi S, Taketo MM (2000) Multiple functional defects in peripheral autonomic organs in mice lacking muscarinic acetylcholine receptor gene for the M3 subtype. Proc Natl Acad Sci U S A 97:9579–9584 [DOI] [PMC free article] [PubMed]
- Matsui M, Motomura D, Fujikawa T, Jiang J, Takahashi S, Manabe T, Taketo MM (2002) Mice lacking M2 and M3 muscarinic acetylcholine receptors are devoid of cholinergic smooth muscle contractions but still viable. J Neurosci 22:10627–10632 [DOI] [PMC free article] [PubMed]
- Noronha-Blob L, Lowe V, Patton A, Canning B, Costello D, Kinnier WJ (1989) Muscarinic receptors: relationships among phosphoinositide breakdown, adenylate cyclase inhibition, in vitro detrusor muscle contractions and in vivo cystometrogram studies in guinea pig bladder. J Pharmacol Exp Ther 249:843–851 [PubMed]
- Ostrom RS, Ehlert FJ (1998) M2 muscarinic receptors inhibit forskolin- but not isoproterenol-mediated relaxation in bovine tracheal smooth muscle. J Pharmacol Exp Ther 286:234–242 [PubMed]
- Ostrom RS, Ehlert FJ (1999) Comparison of functional antagonism between isoproterenol and M2 muscarinic receptors in guinea pig ileum and trachea. J Pharmacol Exp Ther 288:969–976 [PubMed]
- Ringdahl B (1984) Determination of dissociation constants and relative efficacies of oxotremorine analogs at muscarinic receptors in the guinea-pig ileum by pharmacological procedures. J Pharmacol Exp Ther 229:199–206 [PubMed]
- Roffel AF, Meurs H, Elzinga CR, Zaagsma J (1990) Characterization of the muscarinic receptor subtype involved in phosphoinositide metabolism in bovine tracheal smooth muscle. Brit J Pharmacol 99:293–296 [DOI] [PMC free article] [PubMed]
- Sakamoto T, Unno T, Kitazawa T, Taneike T, Yamada M, Wess J, Nishimura M, Komori S (2007) Three distinct muscarinic signalling pathways for cationic channel activation in mouse gut smooth muscle cells. J Physiol 582:41–61 [DOI] [PMC free article] [PubMed]
- Sawyer GW, Ehlert FJ (1998) Contractile role of the M2 and M3 muscarinic receptors in the guinea pig colon. J Pharmacol Exp Ther 284:269–277 [PubMed]
- Thomas EA, Ehlert FJ (1996) Involvement of the M2 muscarinic receptor in contractions of the guinea pig trachea, guinea pig esophagus and rat fundus. Biochem Pharmacol 51:779–788 [DOI] [PubMed]
- Thomas EA, Hsu HH, Griffin MT, Hunter AL, Luong T, Ehlert FJ (1992) Conversion of N-(2-chloroethyl)-4-piperidinyl diphenylacetate (4-DAMP mustard) to an aziridinium ion and its interaction with muscarinic receptors in various tissues. Mol Pharmacol 41:718–726 [PubMed]
- Thomas EA, Baker SA, Ehlert FJ (1993) Functional role for the M2 muscarinic receptor in smooth muscle of guinea pig ileum. Mol Pharmacol 44:102–110 [PubMed]
- Tran JA, Chang A, Matsui M, Ehlert FJ (2009) Estimation of relative microscopic affinity constants of agonists for the active state of the receptor in functional studies on M2 and M3 muscarinic receptors. Mol Pharmacol 75:381–396 [DOI] [PMC free article] [PubMed]
- Unno T, Matsuyama H, Sakamoto T, Uchiyama M, Izumi Y, Okamoto H, Yamada M, Wess J, Komori S (2005) M(2) and M(3) muscarinic receptor-mediated contractions in longitudinal smooth muscle of the ileum studied with receptor knockout mice. Br J Pharmacol 146:98–108 [DOI] [PMC free article] [PubMed]