Abstract
Purpose
To phenotype a family with RHO (Asp190Asn or D190N) dominantly inherited retinitis pigmentosa (RP) and to describe an approach to surveying affected families.
Methods
Four patients from a family with a history of autosomal dominant RP had complete clinical examinations and underwent full-field electroretinography (ERG), fundus autofluorescence (AF) imaging, and genetic testing. One patient had microperimetry (MP) mapping.
Results
The patients’ ages ranged from 6 years old to 47 years old. The proband, the father, had fundoscopic findings typical of RP. A small, hyperfluorescent ring centered at the fovea was apparent on AF. MP showed preservation of central 7 degrees of visual field within this ring. The three children were all asymptomatic with visual acuity of 20/15 in each eye. One child had mild retinal pigment epithelium migration on fundoscopy; the other two children had normal fundoscopic examinations. Two children showed increased parafoveal AF. In the two affected children, average ERG b-wave implicit times were delayed in scotopic conditions and maximal ERG tracings had abnormal waveforms. Genetic analysis confirmed that two of three asymptomatic children carried the D190N allele.
Conclusions
Patients with RHO (D190N) adRP can show classic signs of RP on fundus examination and may be able to maintain good central visual acuity into adulthood. By combining clinical examination with AF imaging and electrophysiology, it is possible to offer presymptomatic clinical evaluation to families with this RP.
Keywords: autosomal dominant, retinitis pigmentosa, rhodopsin, D190N, autofluorescence, electroretinogram, genetic testing, gene therapy, children, presymptomatic
Introduction
Retinitis pigmentosa (RP), with an incidence of 1/4000 in the United States, is a generalized rod-cone degeneration that begins with decreased peripheral vision and night blindness and progresses to central vision loss 1, 2. The inheritance pattern of non-syndromic RP is autosomal dominant in about 20% of patients 3. In a survey of 200 families with autosomal dominant retinitis pigmentosa (adRP), the genetic defect was known in 54% of patients and mutations in rhodopsin (RHO) accounted for approximately half of these cases 4.
RHO is a G-protein-coupled receptor that initiates the phototransduction cascade in rods 5–10. The substitution of aspartic acid by asparagine (Asp190Asn or D190N) is one of four thermally unstable RHO mutations (Asp190Asn, Thr94Ile, Gly51Val, and Ser186Trp) that cause RP 11, 12. Photoreceptor degeneration in both vitamin A deficiency and these thermally unstable RHO mutations is a consequence of false signaling between RHO and phosphodiesterase (PDE) in the dark 13–15. In a normal retina, low RHO dark noise level allows rod photoreceptors to detect a single photon.
RP can be difficult to diagnose in children because it is slowly progressive and presents in different ways. Autosomal dominant RP is typically diagnosed later and people can seem unaffected for most of their childhood. In this report, we genotype and phenotype a family with adRP at varying stages of disease.
Materials and methods
Patients were enrolled with the approval of the Institutional Review Board of Columbia University (protocol IRB-AAAB6560). The tenets of the Declaration of Helsinki were followed. All patients had a full history and dilated ophthalmic examination by a retina attending. Old records were reviewed when indicated.
Fundus autofluorescence (AF) imaging was obtained using scanning laser ophthalmoscopy (HRA2, Heidelberg Engineering, Heidelberg, Germany) by illuminating the fundus with argon laser light (488 nm) and viewing the resultant fluorescence through a band pass filter with a short wavelength cut off at 495 nm 16–19.
MP-1 microperimetry (MP, Nidek Technologies, Padova, Italy) under photopic conditions was done with a 1-degree fixation target using a 10-2 pattern to test macular sensitivity. For each test point location, light sensitivity threshold values were recorded with a white background light and luminance set at 1.27 cd/m2. Units of sensitivity were recorded from 0 dB (400 asb, 127 cd/m2) to 20 dB (4 asb, 1.27 cd/m2).
Pupils were dilated using tropicamide 1% and phenylephrine hydrochloride 2.5% prior to electrophysiological testing. Full-field electroretinogram (ERG) to test retinal function was performed using extended testing protocols using the International Society for Clinical Electrophysiology of Vision (ISCEV) standards 20. The protocol included rod-specific and standard bright flash ERG, both recorded after a minimum of 20 minutes dark adaptation. Following 10 minutes light adaptation, the photopic 30 Hz Flicker cone and transient photopic cone ERG were recorded20.
Deoxyribonucleic acid (DNA) was extracted from serum blood of three patients. Genetic testing was done with direct sequencing of blood as previously described 16, 21. Extracted genomic DNAs were assessed by denaturing high pressure liquid chromatography (DHPLC) and direct sequencing of polymerase chain reaction (PCR) products from all the coding exons including splice junctions.
Primers (5’-3’) used for RHO screening are as follows: exon 1 ctgcagcggggattaatatg (forward), ggacaggagaagggagaagg (reverse); exon 2 ggttgccttcctagctaccc (forward), ctaccctgagtgggcatttg (reverse); exons 3,4 gaatgtgaagccccagaaag (forward), ccctgggaagtagcttgtcc (reverse), exon 5-1 tcactaacgtgccagttcc (forward); exon 5-2 ttaaaaacctgccccaaggt (reverse), exon 5-3 ttactatgattatcacctcc (forward); exon 5-4 agaggtgacaaagcttgttt (reverse). Both DNA single strands were sequenced to confirm base pair change.
Results
Case 1
The proband was a 47 year old man who had a history of poor vision and night blindness for twenty-five years. His mother had a history of nyctalopia and vision loss more severe and of earlier onset than his own. He had been enrolled in the National Institute of Health’s Randomized Clinical Trial for Retinitis Pigmentosa and was known to carry a RHO, D190N mutation. The patient had been taking oral vitamin A supplements (15,000 IU/day) for 20 years. The family was most interested in finding out if any of three children were affected with adRP.
The patient also had a history of herpes simplex keratitis in his left eye which was treated with topical steroids. He then developed steroid induced glaucoma and underwent a trabeculectomy. On exam, his vision was 20/25 OD and 20/80 OS. Intraocular pressures were 14 mmHg OS and 5 mmHg OD. Slit lamp exam showed an elevated filtering bleb in the superotemporal quadrant of the left eye with a posterior intraocular lens.
Dilated fundus exam showed optic nerve pallor with attenuated arterioles and retinal pigment epithelium (RPE) migration in the mid-periphery of both eyes (Figure 1A). His right eye had an incidental choroidal nevus along the inferior temporal arcade. AF showed a small ring of high-density hyperfluorescence (Figure 2A), which correlated to a central field threshold elevation as measured by MP (Figure 3).
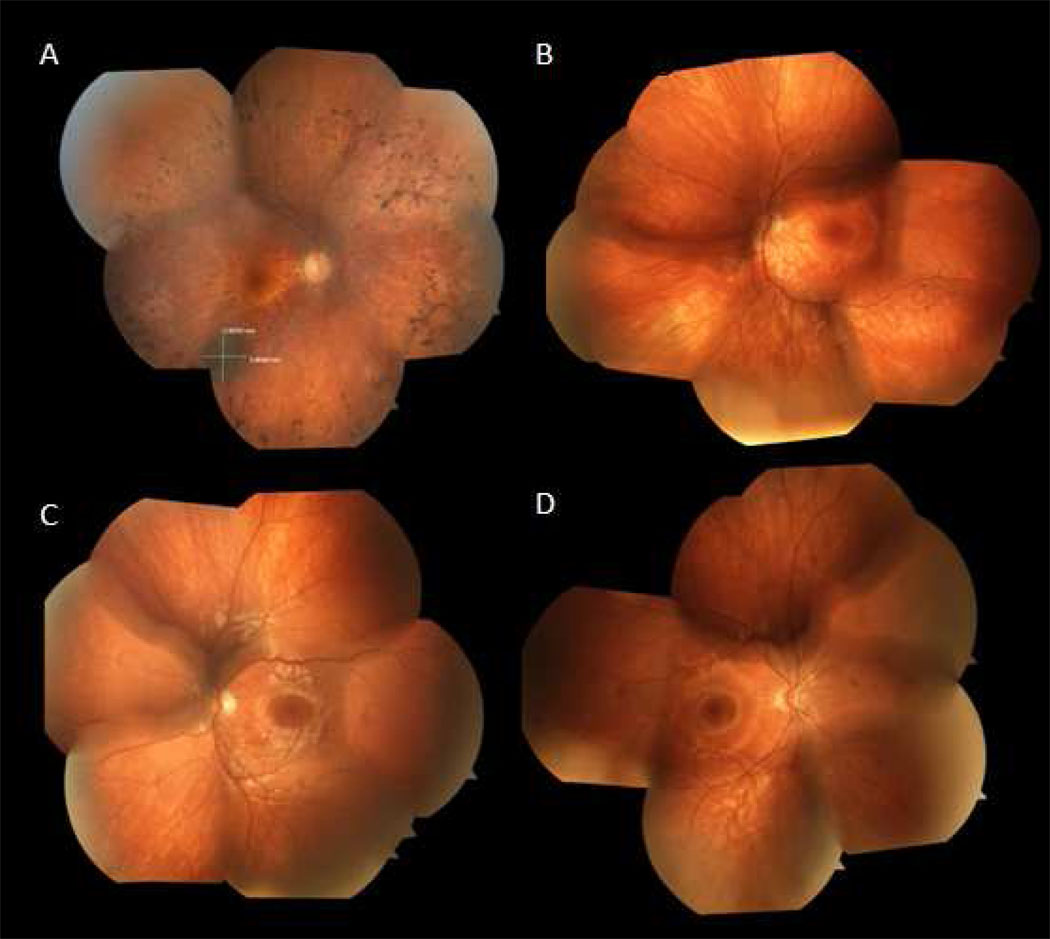
Figure 1. Color fundus photographs.
A. 47 year old man with D190N adRP, right eye. Optic nerve has mild pallor and there is extensive intraretinal pigment migration in the mid-periphery. Note the incidental choroidal nevus 3.9 × 3.36 mm along the inferotemporal arcade.
B. 11 year old son affected with adRP, left eye. Note the mild RPE migration inferonasally.
C. 9 year old son without RP, left eye. Optic nerve without pallor and macula with good foveal reflex.
D. 7 year old son affected with adRP, right eye. Optic nerve without pallor and macula with good foveal reflex, clinically indistinguishable from panel C.
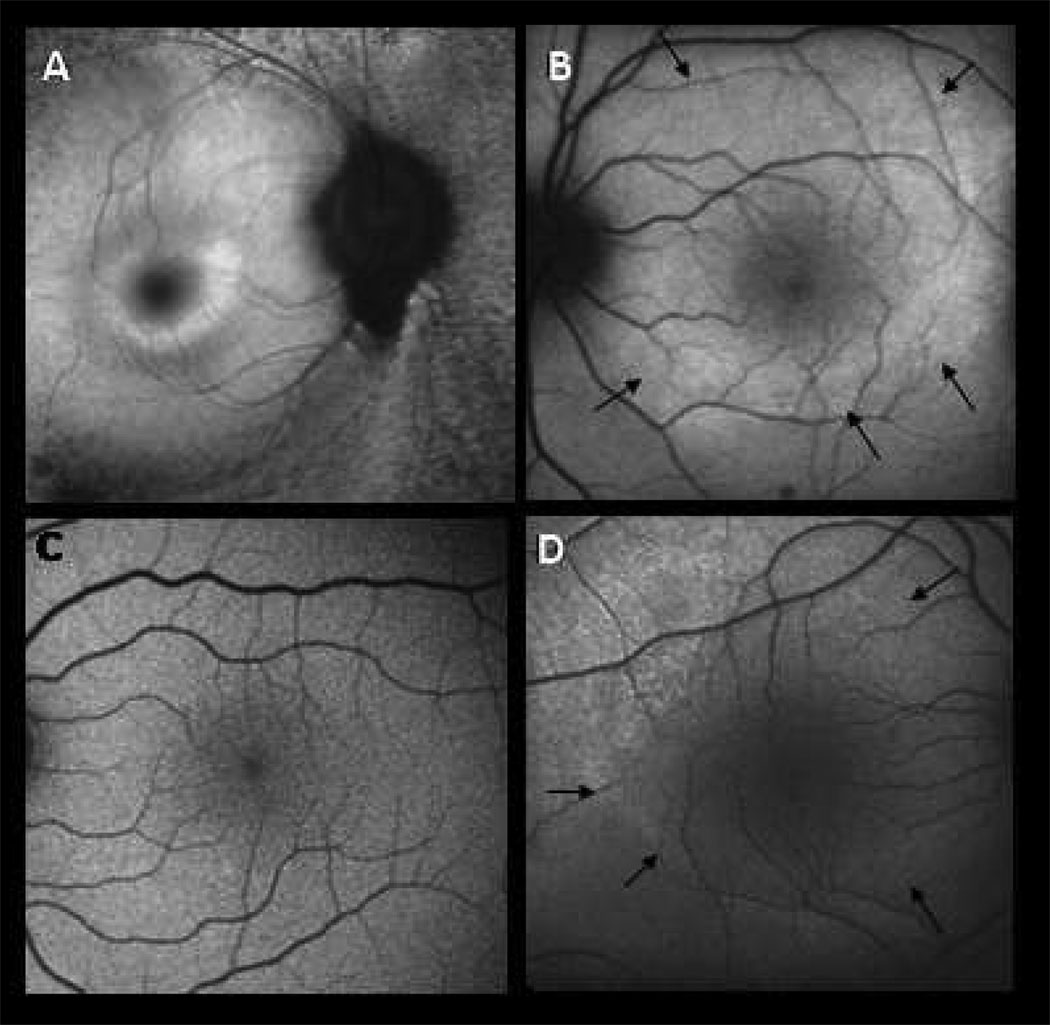
Figure 2. Autofluorescence imaging.
A. 47 year old man with D190N adRP, right eye. Macula with small hyperfluorescent ring.
B. 11 year old son affected with adRP, left eye. Macula with large hyperfluorescent ring (black arrows.)
C. 9 year old son without RP, left eye. Macular autofluorescence normal.
D. 7 year old son affected with adRP, right eye. Macula with early, large hyperfluorescent ring (black arrows) similar to panel B.
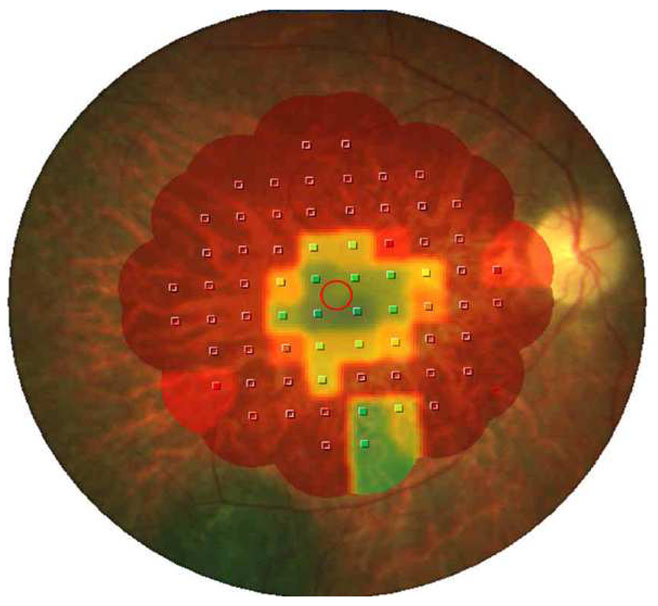
Figure 3.
Microperimetry 10-2 mapping of right eye in case 1. Red shows scotoma and green (seven degrees of field) is retina which is well functioning.
Full-field ERG, followed regularly since 1985, showed that rod responses were more severely decreased than cone responses, consistent with late stage rod-cone dystrophy. When compared over time, his photopic 30-Hz flicker ERG decreased by about an average of 3% per year. Amplitudes (in microvolts) of his photopic 30-Hz flicker ERGs were 19.4 in 1985, 8.54 in 1994, 13.7 in 1996, 14.51 in 1997, 15.1 in 1998, 10.3 in 1999, 10.8 in 2000, and 10.2 in 2001.
Case 2
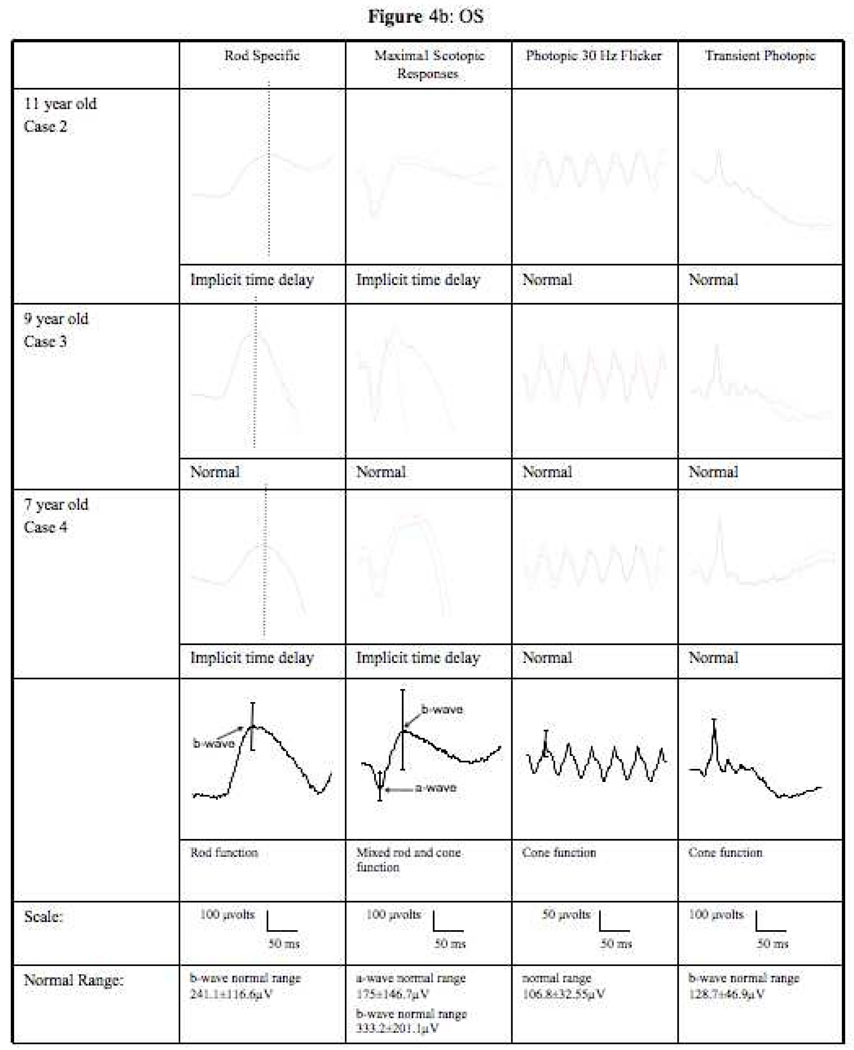
The 11 year old son of the above patient was asymptomatic. Visual acuity was 20/15 in each eye. Eyes were orthophoric with cover testing. Anterior exam was unremarkable. Dilated fundus exam showed attenuated arterioles and mild, early retinal pigment migration in the mid-peripheral retina (Figure 1B). Mean ERG from both eyes showed a delayed b-wave implicit time at 99.5 ms (normal=96 ms) under scotopic conditions with no delay under photopic conditions (Table 1). The maximal scotopic b-wave had an abnormally broad peak (Figure 4). AF imaging revealed large hyperfluorescent rings in both maculas (Figure 2B). These areas did not correlate to any fundoscopic abnormalities. Genetic testing was positive for D190N (Figure 5).
Table 1.
Quantitative ERG results, average of both eyes. Amplitudes and implicit times of rod specific, maximal mixed rod cone responses (max). photopic 30 Hz flicker, and transient photopic tracings.
| Amplitude | Implicit Time | |||
|---|---|---|---|---|
| mV | ms | |||
| a-wave | b-wave | a-wave | b-wave | |
| Rod Specific | ||||
| Case 2 | 236 (124.4*) | 99.5 (96.0**) | ||
| Case 3 | 420 (124.4*) | 84.5 (96.0**) | ||
| Case 4 | 195.5 (124.4*) | 110 (96.0**) | ||
| Max ERG | ||||
| Case 2 | 218.50 (28.3*) | 244.5 (132.1*) | 15 (18.88*) | 50 (57.87*) |
| Case 3 | 299 (28.3*) | 488.5 (132.1*) | 15 (18.88*) | 45.5 (57.87*) |
| Case 4 | 155 (28.3*) | 346.5 (132.1*) | 18 (18.88*) | 73.5 (57.87*) |
| Photopic 30 Hz flicker | ||||
| Case 2 | 108 (74.3*) | 26 (26.0**) | ||
| Case 3 | 140 (74.3*) | 25 (26.0**) | ||
| Case 4 | 91 (74.3*) | 26 (26.0**) | ||
| Transient photopic | ||||
| Case 2 | 50 (26.14*) | 175.5 (81.8*) | 14.5 (13.89*) | 30 (30.63*) |
| Case 3 | 40.5 (26.14*) | 278.5 (81.8*) | 14 (13.89*) | 30 (30.63*) |
| Case 4 | 42 (26.14*) | 155 (81.8*) | 15 (13.89*) | 31.5 (30.63*) |
indicates the lower threshold of normal amplitude, defined as two standard deviations below the mean of normal controls.
indicates the mean of normal implicit times in controls (10 to 40 years of age).
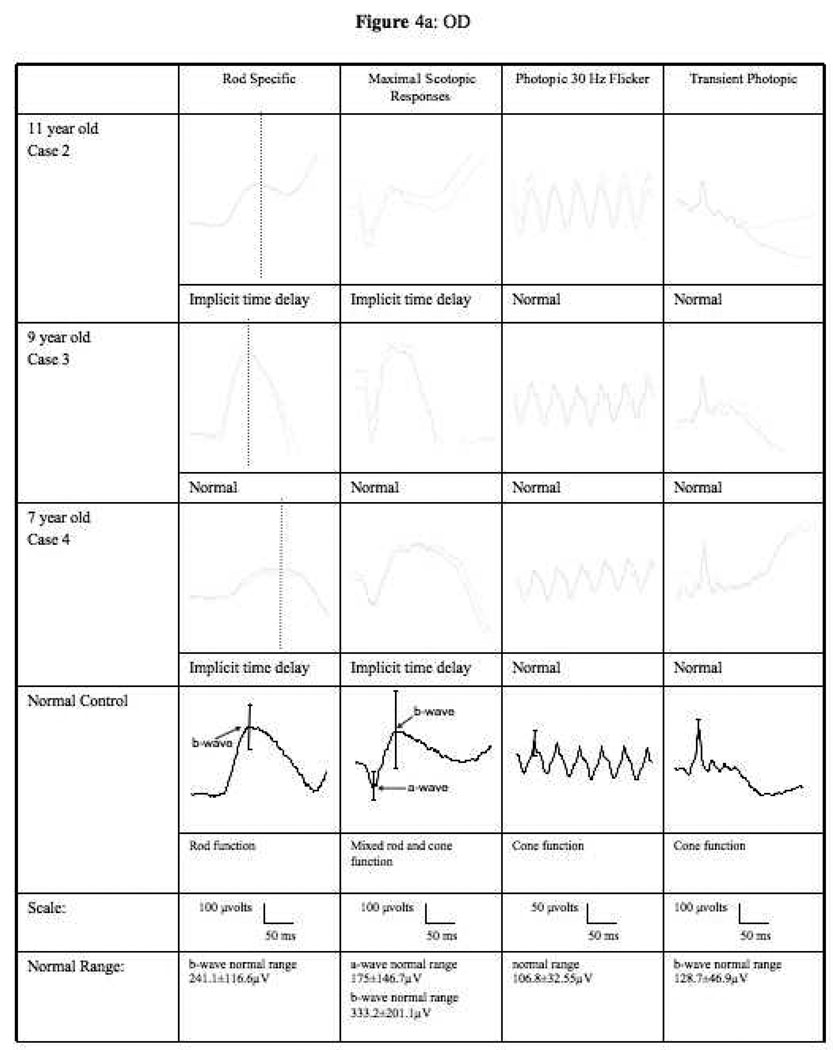
Figure 4.
ERG tracings of cases 2, 3, and 4 under rod specific, maximum scotopic, photopic 30Hz flicker and transient photopic conditions. (a) right eye (b) left eye. Scales and normal range indicated below the tracings.
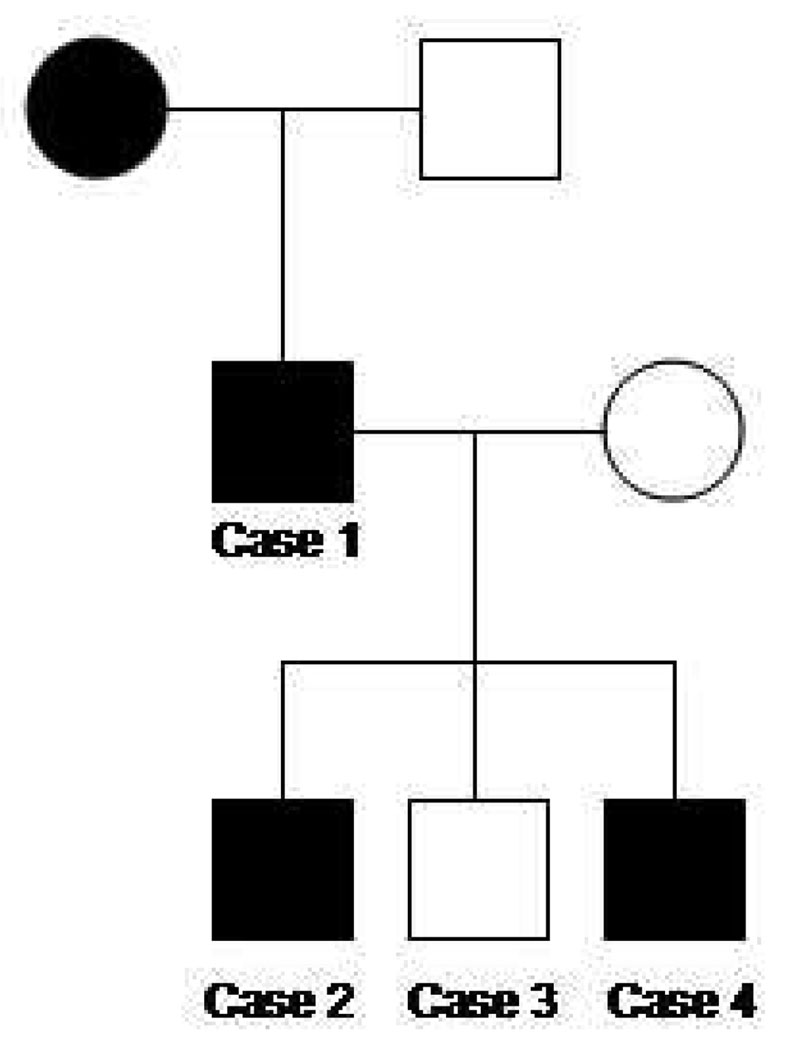
Figure 5.
Three generation pedigree of the family with known RHO, D190N affected individuals. Note autosomal dominant inheritance is supported by the presence of consecutive affected generations and male-to-male transmission.
Case 3
The 9 year old son had visual acuity of 20/15 in each eye. Eyes were orthophoric with cover testing. Anterior segment was normal and dilated fundus exam revealed a healthy foveal reflex and well appearing optic nerves (Figure 1C). ERG had normal implicit times in both scotopic and photopic conditions with normal waveforms (Table 1, Figure 4). AF imaging was also normal (Figure 2C). Genetic testing was negative for D190N (Figure 5).
Case 4
The 7 year old son was asymptomatic. Visual acuity was 20/15 in each eye. Eyes were orthophoric with cover testing. Anterior exam was unremarkable and dilated exam showed a healthy foveal reflex without optic nerve pallor (Figure 1D). However, mean ERG from both eyes showed a delayed b-wave scotopic implicit time of 110 ms (normal= 96 ms) and normal b-wave photopic implicit time (Table 1). Furthermore, the maximal scotopic b-wave had an abnormal waveform (Figure 4). AF imaging showed a large high-density hyperfluorescent ring (Figure 2D) similar to case 2. Genetic testing was positive for D190N (Figure 5).
Discussion
This is the first report to make genotype-phenotype correlations in patients with the RHO, D190N mutation. We studied four members of a family with adRP over two generations and across three decades.
Presymptomatic diagnosis may be important for families of children with retinal dystrophies as the uncertainty of disease may be more frustrating than knowing and being able to prepare. In this study, all children were asymptomatic, and the eldest son had a small amount of intraretinal pigment migration on fundoscopic examination. However, the diagnosis was made in two of three children based on AF and ERG. They had parafoveal rings of increased fluorescence on AF 22. Case 4 is unique in that standard history and clinical examination were both unremarkable. The diagnosis of disease was only suspected based on family history and imaging with AF.
It is often challenging to obtain reliable ERG results in children with retinal dystrophies, partly because the normative values of healthy, cooperative children are not readily available in most diagnostic centers. Our study suggests that AF imaging can be more child-friendly in diagnosing and prognosticating young, asymptomatic children with RP. One consistent finding in our affected patients, as proven with genetic testing, is that there was a delay in b-wave implicit times under scotopic conditions with normal b-wave implicit times under photopic conditions when compared with normative adult values (Table 1). In addition, the two affected children have abnormal waveforms as demonstrated 23, 24
We confirmed our clinical impressions with DNA sequencing analyses of the RHO gene. In current practice, most cases of RP are diagnosed solely on clinical methods without the benefit of molecular confirmation. The recently developed microarray gene chip is attempting to make molecular diagnosis of RP more efficient and cost-effective 25. In the era of emerging gene therapy for retinal dystrophies 26, 27, the molecular diagnosis of RP for every patient becomes more clinically relevant. Genotype-phenotype correlations as described in this paper will contribute to more cost-efficient genotyping in the future.
The differential diagnosis for AF rings includes rod-cone dystrophies, cone-rod dystrophies, and macular toxicity such as with hydroxychloroquine 28. In patients with retinal dystrophies, the hyperfluorescent rings may be due to early cellular dysfunction and overproduction of lipofuscin 29, 30. Autofluorescence can be useful to monitor disease progression in patients with retinal dystrophies 30. In this family the affected sons have large rings and are asymptomatic. The father has a 4-degree hyperfluorescent ring which corresponds to a constricted visual field as demonstrated by MP.
This is the first report to phenotype D190N patients whose rate of progression appears to be similar to other individuals with RHO mutations but milder than individuals with simplex RP 24, 31–34. The proband’s 30-Hz flicker ERG showed a decline of 3% per year rather than the 10% per year decline that has been observed in most other RP patients 12, 32, 35. Mutations that affect RHO transport tend to manifest as more severe degenerations than D190N, which prolongs RHO signaling 11, 12. It has also been suggested that cytoplasmic and extracellular mutations in RHO such as D190N have less severe pheontypes than transmembrane mutations 36.
In summary, our report phenotypes a spectrum of adRP due to RHO, D190N in a family with three affected members. Our case series also highlights the importance of using AF with ERG in evaluating young, presymptomatic children with RP.
Acknowledgements
We are grateful to Henry Perry-Friedman, Vivienne C. Greenstein and the Photography Department of Columbia University for their support. SHT is a Burroughs-Wellcome Program in Biomedical Sciences Fellow, and is supported by the Charles Culpeper Scholarship, Foundation Fighting Blindness, Hirschl Trust, Schneeweiss Stem Cell Fund, Joel Hoffmann Foundation, Donald and Barbara Jonas Family Fund, Jahnigen-Hartford-American Geriatrics Society, Eye Surgery Fund, Bernard Becker-Association of University Professors in Ophthalmology-Research to Prevent Blindness (RPB), and Jules Stein-RPB Omics Laboratory.
References
- 1.Berson EL. Retinitis Pigmentosa: The Friedenwald Lecture. Invest Opthal Vis Sci. 1993;34:1655–1676. [PubMed] [Google Scholar]
- 2.Bunker CH, Berson EL, Bromley WC, Hayes RP, Roderick TH. Prevalence of retinitis pigmentosa in Maine. American J of Opthalmol. 1984;97:357–365. doi: 10.1016/0002-9394(84)90636-6. [DOI] [PubMed] [Google Scholar]
- 3.Daiger SP, Bowne SJ, Sullivan LS. Perspective on genes and mutations causing retinitis pigmentosa. Arch Ophthalmol. 2007;125:151–158. doi: 10.1001/archopht.125.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sullivan LS, Bowne SJ, Birch DG, et al. Prevalence of disease-causing mutations in families with autosomal dominant retinitis pigmentosa: a screen of known genes in 200 families. Invest Ophthalmol Vis Sci. 2006;47:3052–3064. doi: 10.1167/iovs.05-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsang SH, Gouras P. Chapter 2. Molecular Physiology and Pathology of the Retina. In: Tasman W, Jaeger EA, editors. Duane's Clinical Opthalmology. Philadelphia: J.B. Lippincott; 1996. [Google Scholar]
- 6.Tsang SH, Gouras P. Photoreceptors and photoreceptor dysfunctions. In: Adelman G, Smith B, editors. Encyclopedia of Neuroscience. Amsterdam: Elsevier Science; 2004. pp. 1633–1644. [Google Scholar]
- 7.Tsang SH, Burns ME, Calvert PD, et al. Role of the Target Enzyme in Deactivation of Photoreceptor G Protein in Vivo. Science. 1998;282:117–121. doi: 10.1126/science.282.5386.117. [DOI] [PubMed] [Google Scholar]
- 8.Tsang SH, Woodruff ML, Janisch KM, Cilluffo MC, Farber DB, Fain GL. Removal of Phosphorylation Sites of Gamma Subunit of Phosphodiesterase6 Alters Rod Light Response. J Physiol. 2006;14:14. doi: 10.1113/jphysiol.2006.121772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsang SH, Woodruff ML, Chen CK, et al. GAP-independent termination of photoreceptor light response by excess gamma subunit of the cGMP-phosphodiesterase. J Neurosci. 2006;26:4472–4480. doi: 10.1523/JNEUROSCI.4775-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Woodruff ML, Janisch KM, Peshenko IV, Dizhoor AM, Tsang SH, Fain GL. Modulation of phosphodiesterase6 turnoff during background illumination in mouse rod photoreceptors. J Neurosci. 2008;28:2064–2074. doi: 10.1523/JNEUROSCI.2973-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Janz JM, Fay JF, Farrens DL. Stability of dark state rhodopsin is mediated by a conserved ion pair in intradiscal loop E-2. J Biol Chem. 2003;278:16982–16991. doi: 10.1074/jbc.M210567200. [DOI] [PubMed] [Google Scholar]
- 12.Yan EC, Kazmi MA, Ganim Z, et al. Retinal counterion switch in the photoactivation of the G protein-coupled receptor rhodopsin. Proc Natl Acad Sci U S A. 2003;100:9262–9267. doi: 10.1073/pnas.1531970100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woodruff M, Wang Z, Chung HY, Redmond T, Fain GL, Lem J. Spontaneous activity of opsin apoprotein is a cause of Leber congenital amaurosis. Nat Genet. 2003 September;35:21. doi: 10.1038/ng1246. 2003. [DOI] [PubMed] [Google Scholar]
- 14.Lisman J, Fain G. Support for the equivalent light hypothesis for RP. Nat Med. 1995;1:1254–1255. doi: 10.1038/nm1295-1254. [DOI] [PubMed] [Google Scholar]
- 15.Fain GL, Lisman JE. Light, Ca2+, and photoreceptor death: new evidence for the equivalent-light hypothesis from arrestin knockout mice [In Process Citation] Invest Ophthalmol Vis Sci. 1999;40:2770–2772. [PubMed] [Google Scholar]
- 16.Tsang SH, Vaclavik V, Bird AC, Robson AG, Holder GE. Novel phenotypic and genotypic findings in X-linked retinoschisis. Arch Ophthalmol. 2007;125:259–267. doi: 10.1001/archopht.125.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robson AG, Saihan Z, Jenkins SA, et al. Functional characterisation and serial imaging of abnormal fundus autofluorescence in patients with retinitis pigmentosa and normal visual acuity. Br J Ophthalmol. 2006;90:472–479. doi: 10.1136/bjo.2005.082487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robson AG, Egan C, Holder GE, Bird AC, Fitzke FW. Comparing rod and cone function with fundus autofluorescence images in retinitis pigmentosa. Adv Exp Med Biol. 2003;533:41–47. doi: 10.1007/978-1-4615-0067-4_6. [DOI] [PubMed] [Google Scholar]
- 19.von Ruckmann A, Fitzke FW, Bird AC. Distribution of fundus autofluorescence with a scanning laser ophthalmoscope. Br J Ophthalmol. 1995;79:407–412. doi: 10.1136/bjo.79.5.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marmor M, Holder G, Seeliger M, Yamamoto S. Standard for clinical electroretinography. 2003 doi: 10.1023/b:doop.0000036793.44912.45. www.ISCEV.org. [DOI] [PubMed] [Google Scholar]
- 21.Tsang SH, Woodruff ML, Jun L, et al. Transgenic mice carrying the H258N mutation in the gene encoding the beta-subunit of phosphodiesterase-6 (PDE6B) provide a model for human congenital stationary night blindness. Human Mutation. 2007:243–254. doi: 10.1002/humu.20425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wabbels B, Demmler A, Paunescu K, Wegscheider E, Preising MN, Lorenz B. Fundus autofluorescence in children and teenagers with hereditary retinal diseases. Graefes Arch Clin Exp Ophthalmol. 2006;244:36–45. doi: 10.1007/s00417-005-0043-2. [DOI] [PubMed] [Google Scholar]
- 23.Berson EL, Gouras P, Gunkel RD. Rod responses in retinitis pigmentosa, dominantly inherited. Arch Opthalmology. 1968;80:355–388. doi: 10.1001/archopht.1968.00980050060009. [DOI] [PubMed] [Google Scholar]
- 24.Berson EL. Ocular findings in a form of retinitis pigmentosa with a rhodopsin gene defect. Trans Am Ophthalmol Soc. 1990;88:355–388. [PMC free article] [PubMed] [Google Scholar]
- 25.Koenekoop RK, Lopez I, den Hollander AI, Allikmets R, Cremers FP. Genetic testing for retinal dystrophies and dysfunctions: benefits, dilemmas and solutions. Clin Experiment Ophthalmol. 2007;35:473–485. doi: 10.1111/j.1442-9071.2007.01534.x. [DOI] [PubMed] [Google Scholar]
- 26.Bainbridge JW, Smith AJ, Barker SS, et al. Effect of gene therapy on visual function in Leber's congenital amaurosis. N Engl J Med. 2008;358:2231–2239. doi: 10.1056/NEJMoa0802268. [DOI] [PubMed] [Google Scholar]
- 27.Maguire AM, Simonelli F, Pierce EA, et al. Safety and efficacy of gene transfer for Leber's congenital amaurosis. N Engl J Med. 2008;358:2240–2248. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kellner U, Renner AB, Tillack H. Fundus autofluorescence and mfERG for early detection of retinal alterations in patients using chloroquine/hydroxychloroquine. Invest Ophthalmol Vis Sci. 2006;47:3531–3538. doi: 10.1167/iovs.05-1290. [DOI] [PubMed] [Google Scholar]
- 29.Fleckenstein M, Charbel Issa P, Fuchs HA, et al. Discrete arcs of increased fundus autofluorescence in retinal dystrophies and functional correlate on microperimetry. Eye. 2008 doi: 10.1038/eye.2008.59. [DOI] [PubMed] [Google Scholar]
- 30.Robson AG, Michaelides M, Saihan Z, et al. Functional characteristics of patients with retinal dystrophy that manifest abnormal parafoveal annuli of high density fundus autofluorescence; a review and update. Doc Ophthalmol. 2008;116:79–89. doi: 10.1007/s10633-007-9087-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilson JH, Wensel TG. The nature of dominant mutations of rhodopsin and implications for gene therapy. Mol Neurobiol. 2003;28:149–158. doi: 10.1385/MN:28:2:149. [DOI] [PubMed] [Google Scholar]
- 32.Mendes HF, van der Spuy J, Chapple JP, Cheetham ME. Mechanisms of cell death in rhodopsin retinitis pigmentosa: implications for therapy. Trends Mol Med. 2005;11:177–185. doi: 10.1016/j.molmed.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 33.Neidhardt J, Barthelmes D, Farahmand F, Fleischhauer JC, Berger W. Different amino acid substitutions at the same position in rhodopsin lead to distinct phenotypes. Invest Ophthalmol Vis Sci. 2006;47:1630–1635. doi: 10.1167/iovs.05-1317. [DOI] [PubMed] [Google Scholar]
- 34.Oh KT, Oh DM, Weleber RG, et al. Genotype-phenotype correlation in a family with Arg135Leu rhodopsin retinitis pigmentosa. Br J Ophthalmol. 2004;88:1533–1537. doi: 10.1136/bjo.2004.043653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berson EL. Long-term visual prognoses in patients with retinitis pigmentosa: the Ludwig von Sallmann lecture. Exp Eye Res. 2007;85:7–14. doi: 10.1016/j.exer.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schuster A, Weisschuh N, Jagle H, et al. Novel rhodopsin mutations and genotype-phenotype correlation in patients with autosomal dominant retinitis pigmentosa. Br J Ophthalmol. 2005;89:1258–1264. doi: 10.1136/bjo.2004.063933. [DOI] [PMC free article] [PubMed] [Google Scholar]