Abstract
Background
The associations among income, total knee arthroplasty, and underlying rates of knee osteoarthritis are not well understood. We study whether high income Medicare recipients are more likely to have a knee arthroplasty and less likely to suffer from knee osteoarthritis.
Methods
Two data sources were used; the 2000 Medicare claims data measuring the incidence of total knee arthroplasty by race, ethnicity, ZIP code income, and region (N = 27.5 million), and the National Health and Nutrition Examination Survey (NHANES III) for ages 60 and over ((N = 1926) with variables measuring radiographic and clinical evidence of osteoarthritis. Logistic regression methods were used to adjust for covariates.
Results
At the national level, age-adjusted rates of total knee arthroplasty in the high-income quintile were no higher than in the low-income group (odds ratio 0.98, 95% C.I. 0.96 – 1.00). Within regions, access to care was better for high-income groups (odds ratio 1.19, 95% C.I., 1.16 – 1.20). Racial disparities in arthroplasty were significant (p < .001); the odds ratio for Black men was 0.36 (95% C.I., 0.34 – 0.38) and for Asian women 0.45 (95% C.I., 0.41 – 0.49). There was no evidence of an income gradient for most clinical and radiographic measures of arthritis. The exception was a significant (p < .05) negative association between income and pain on passive motion.
Conclusions
High-income Medicare enrollees are no less likely to experience osteoarthritis than low-income enrollees, but experience somewhat better access to care. Racial disparities are more important than those attributable to socioeconomic status.
Introduction
There are large disparities by race and ethnicity in the use of knee replacements in the United States,1–4 but the evidence on how these rates vary by income is either several decades old 5 or arrives at conflicting conclusions 1, 6, 7 The association between income and health outcomes is important for two reasons. First, some studies have found that racial disparities in the prevalence of disease disappear after controlling for socioeconomic status (SES).8, 9 It is therefore important to measure whether racial differences observed in previous studies reflect in part the fact that African-American and Hispanic Medicare enrollees are, on average, in lower income categories than whites.
Second, dramatic increases in Part B Medicare premiums for high income households were enacted as part of the 2003 drug benefit legislation, and are scheduled to begin their phase-in by 2007.10 Currently all Medicare enrollees pay 25 percent of the cost of Part B benefits, but the goal of the current legislation is for high income Medicare enrollees to pay as much as 80 percent of average Part B benefits, or more than a tripling of current premiums. This in part stems from the widespread view that higher income groups utilize Medicare program benefits more intensively than lower income groups.11 While there remains some controversy regarding the link between socioeconomic status and total Medicare expenditures,12,13 we focused instead on how income affects the use of a particularly cost-effective procedures such as knee arthroplasty.14,15 To test this hypothesis, we used a 2000 sample of nearly every enrollee in the Medicare program (N = 27.5 million) to estimate the incidence of total knee arthroplasty conditional on race, ethnicity, and income measured at the ZIP code level.
It is difficult to make inferences about disparities in the general population without a better understanding of how the disease burden differs by income or race.4 While there is good evidence that women, and African American and Hispanic women in particular, experience substantially higher rates of knee osteoarthritis,16–19 there is no evidence on such differences by income groups. As well, there is increasing evidence that the functional aspects of disease may differ across income or education groups, even after controlling for objective clinical measures.20 Radiographic, clinical, and self-reported pain measures from the NHANES III (National Health and Nutrition Examination Survey) were also used to test the hypothesis that the prevalence of osteoarthritis differs by income and race. Our hypothesis is that higher income Medicare recipients are less likely to experience osteoarthritis but more likely to have a total knee arthroplasty.
Data and Methods
Incidence Rates of Knee Osteoarthritis in the Medicare Claims Data
The 2000 Medicare claims data comprised every Medicare enrollee over the age of 65 in the fee-for-service program. Individuals were excluded if enrolled in a Medicare Health Maintenance Organization (HMO). The claims data were used to construct rates of knee arthroplasty based on the International Classification of Disease (ICD-9) code 81.54 (total knee replacement), which did not include revisions. These data were then matched by ZIP code to median income in the ZIP code using data from the 2000 Census. There are important issues in the measurement of race and ethnicity in the Medicare claims data, largely arising from the piecemeal approaches in years past where just a few racial categories were collected.21, 22 In recent years, the sample sizes of Hispanic and Asian groups have expanded, allowing for four racial or ethnic groups: African-American, Hispanic, Asian, and all others, who for purposes of exposition we denote as white. The sensitivity of racial categorization was generally high, meaning that if people reported themselves as Hispanic, they generally were, but the specificity was lower, meaning that not all self-identified Hispanics were identified as such in the Medicare data.22
Income was measured by matching Medicare claims by ZIP code to U.S. 2000 Census data on median income. In the regression, the logarithmic transformation of income is used. This implies that a percentage increase in income will have similar effects on health status whether the change occurs at low or high-income levels. The income measures are also used to create income quintiles, ranging from Quintile 1 (the 20 percent of the Medicare population with the lowest ZIP code income) to Quintile 5 (the 20 percent with the highest ZIP code incomes).
In the statistical analysis, we estimated the shape of the income gradient using two approaches: one that did not adjust for where the patient lived, and another that did. 1, 23 The former income gradient reflects the overall association in the U.S. between income and TKA utilization rates. The latter reflects “access to care,” or the influence of income on TKA utilization rates within a region, for example because of better access to local orthopedic surgeons in higher income neighborhoods. These two rates could differ if, for example, high-income Medicare enrollees are more likely to live in regions with higher overall rates of TKA for both low- and high-income patients.
We used the 306 Hospital Referral Regions (HRRs) from the Dartmouth Atlas of Health Care. An HRR was defined to be a region centered on a hospital or group of hospitals that offered cardiovascular and neurosurgical procedures in 1992/93, so that each HRR included at least one large tertiary hospital. Nearly every ZIP code in the United States was assigned to an HRR based on the observed migration patterns of hospital use among the elderly population.24, 25
The statistical analysis was performed using logistic regression analysis in STATA 9.0 that adjusts for racial and ethnic identification, sex, 5-year age categorical variables, and (in some cases) regional categorical variables. In interpreting incidence rates of TKA, estimated odds ratios can be interpreted as relative risks, since the underlying incidence rates were so small.26
Prevalence of Osteoarthritis and Knee Pain in NHANES III
The NHANES III survey was conducted from 1988 through 1994 and included information on self-reported health and the results of a physician’s clinical examination of the knees for the population age 60 and over. In 2001, data on radiographic measures of knee osteoarthritis for NHANES III were released by the Centers for Disease Control.
Three dependent variables were considered. The first was a Kellgren-Lawrence (KL) score of 2 or more for at least one knee. A score of 2 reflected minimal osteophytes, with possible joint space narrowing, cysts and sclerosis, 3 measured moderate osteophytes and narrowing, while 4 indicated severe osteophytes with definite narrowing.27 The second measure combined radiographic and clinical measures: a KL score of 2 or more in at least one knee plus evidence of at least one of the following: a clinical examination of crepitus, swelling, or maximum limitation on passive motion less than 115 degrees. The third measure, pain on passive motion, was self-reported and we hypothesize that the response to this question may be mediated by factors such as socioeconomic status.
Independent variables are age (in five year intervals), sex, and race. Because there were so few Hispanic and Asian respondents in the sample, we considered just three groups: Black, white, and other reported racial or ethnic categories. Quartiles of Body-mass index (BMI) were used to allow for a nonlinear association between BMI and the prevalence of osteoarthritis and knee pain. As well, region of residence was included (South, Northeast, Midwest, and West). All variables are listed in Table 2.
Table 2.
Summary Statistics from the NHANES III Survey
| Independent Variables | Mean Value | Standard Deviation |
|---|---|---|
| Age | 70.40 | 7.54 |
| % Black | 8.20 | 27.44 |
| % Other Non – White | 3.73 | 18.95 |
| % White | 88.08 | 32.41 |
| % Female | 57.34 | 49.47 |
| % Income > $35,000 | 32.33 | 46.79 |
| % Income $15,000 – $35,000 | 38.54 | 48.68 |
| % Income < $15,000 | 29.12 | 45.44 |
| BMI (average) | 27.17 | 5.06 |
| South | 26.02 | 43.88 |
| Midwest | 28.42 | 45.11 |
| Northeast | 21.98 | 41.42 |
| West | 23.58 | 42.46 |
| Dependent Variables | ||
| Evidence of Crepitus for at least one knee | 49.81 | 50.01 |
| Maximum Limitation on Knee Motion < 115 Degrees | 4.60 | 20.96 |
| Evidence of swelling for at least one knee | 4.27 | 20.23 |
| Kellgren-Lawrence Score ≥ 2 for at least one knee | 37.36 | 48.39 |
| KL ≥ 2 and one of the following: crepitus, limitation of knee motion, or swelling |
24.20 | 42.84 |
| Pain on passive motion for at least one knee | 16.22 | 36.87 |
Notes: Sample Size = 1926. Percentages in each of the three shaded areas sum to 100%.
Income was reported in intervals, and for primary results was converted to logarithmic form using interval midpoints. This assumption was relaxed by the use of approximate income quintiles (20th percentiles) defined in the NHANES data: <$12,000, $12–19,000, $19–28,000, $28–50,000, and $50,000+. A logistic regression was used to estimate the models with each of the three dependent variables using both population weights and sampling strata using the SVY and LOGISTIC commands in STATA version 9.0; odds ratios were converted into probabilities using the ADJUST command.
Results
Incidence Rates of Total Knee Arthroplasty in the Medicare Claims Data
Of the 35,035,220 total Medicare enrollees in July 2000 aged 65 and over, 6,802,284 were excluded because of enrollment in an HMO for any month during 2000, while 738,277 were dropped because they could not be linked to Census ZIP code data, leaving 27,494,659 individuals in the sample. In this group there were 136,449 people who in 2000 had at least one TKA, or a national rate of 4.96 per 1,000 enrollees. Sample sizes by gender and for each racial or ethnic group are shown in the first column of Table 1.
Table 1.
Knee Arthroplasty Rates By Race, Ethnicity, and Income in the Elderly Medicare Population: 2000
|
Overall Rates: Adjusting for Age, Sex, Racial or Ethnic Identification, and Income |
Access to Care: Adjusting for Age, Sex, Racial Ethnic Identification, Income, and Region |
|||||||
|---|---|---|---|---|---|---|---|---|
| Sample Size | Crude TKA Rate (Per 1,000) |
Odds Ratio |
95% Lower C.I. |
95% Upper C.I. |
Odds Ratio |
95% Lower C.I. |
95% Upper C.I. |
|
| Income (Log) | 0.980 | 0.964 | 0.995 | 1.192 | 1.168 | 1.216 | ||
| Black Men | 828,302 | 1.603 | 0.359 | 0.339 | 0.379 | 0.416 | 0.394 | 0.440 |
| Hispanic Men | 179,922 | 3.174 | 0.674 | 0.620 | 0.732 | 0.796 | 0.732 | 0.866 |
| Asian Men | 160,762 | 1.275 | 0.278 | 0.242 | 0.319 | 0.364 | 0.317 | 0.418 |
| White Men | 10,103,053 | 4.463 | 1.000 | 1.000 | ||||
| Black Women | 1,351,520 | 4.325 | 1.015 | 0.987 | 1.043 | 1.188 | 1.155 | 1.223 |
| Hispanic Women | 249,953 | 4.693 | 1.028 | 0.970 | 1.089 | 1.282 | 1.207 | 1.361 |
| Asian Women | 216,825 | 2.039 | 0.449 | 0.409 | 0.493 | 0.586 | 0.533 | 0.645 |
| White Women | 14,404,322 | 5.679 | 1.342 | 1.326 | 1.357 | 1.350 | 1.334 | 1.365 |
N =27,494,659. The national TKA rate is 4.963 per 1,000 population. The odds ratios reported here can be interpreted as showing the relative risk of a person in a given category having received a TKA in 2000. For example, compared to the reference group of white men, Asian men are roughly 28 percent as likely to receive a TKA (not controlling for region). Age variables at five-year intervals (65–69, 70–74, 75–79, 80–84, 85+) included in both regressions but not reported.
The incidence of knee arthroplasty by income and race or ethnicity is shown in Table 1, for just the crude rate (Column 2), and after adjusting for race, income, and age (Column 3). The odds ratio of TKA was 0.36 for Black men (95% C.I. 0.34 – 0.38), 0.28 for Asian men (95% C.I. 0.24 – 0.32), and 0.45 for Asian women (95% C.I. 0.41 – 0.49). In this Table, the reported coefficients can be interpreted as relative risk; thus Asian women, for example, were just 45 percent as likely to receive a TKA as white men. Rates for Black women (1.02, 95% C.I. 0.99 – 1.04) are below those for white women (1.34, 95% C.I. 1.33 – 1.36). Separate analyses stratified by each income quintile yielded similar results to those above. Finally, there was little association between rates of total knee replacement and income; the odds ratio was 0.98, a very modest association significant (p < .05) solely because of the large sample size.
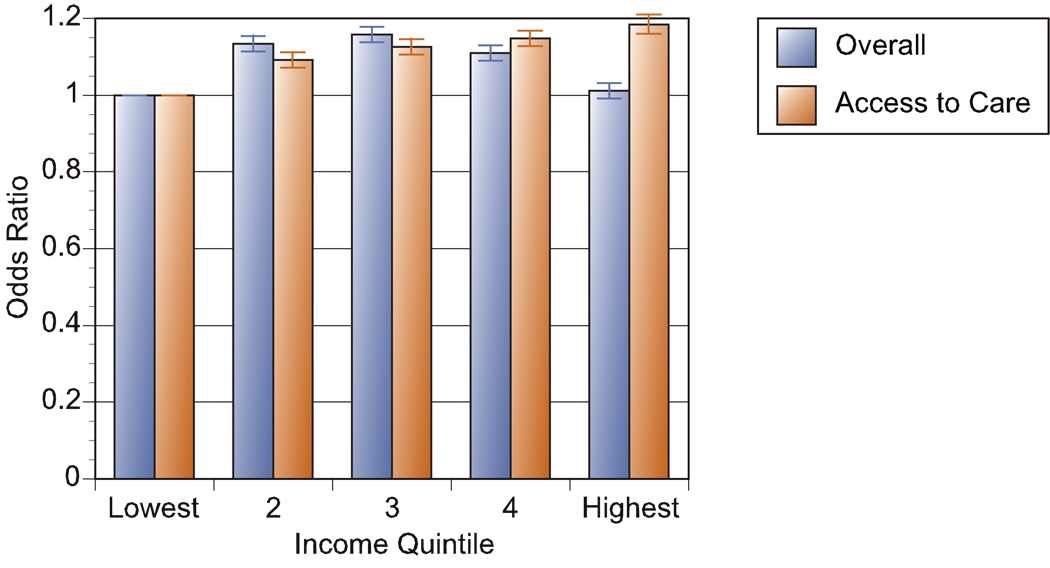
The association between TKA and income was stronger after adjusting for the hospital referral region (HRR); the odds ratio was 1.19 (95% C.I. 1.17 – 1.22). Adjusting for the HRR means that the estimated association occurs solely because of variations in income within the region – Beverly Hills versus South Central Los Angeles, for example. In this type of comparison, a 10 percent increase in income within a region was associated with a 1.9 percent increased likelihood of knee arthroplasty. Figure 1 illustrates the association between income and TKA by income using these two different approaches to measuring the income gradient: the overall effect (adjusting for age and racial or ethnic identification but not region) and “access to care” which includes all covariates including the region of residence. As in the previous regression analysis, the “access to care” measure yields a pronounced income gradient, with the highest quintile experiencing rates 18.5% higher than those in the lowest quintile.
Figure 1. Odds Ratio of Total Knee Replacement in the Medicare Population by Income Quintile.
The overall odds-ratio of total knee replacement is shown for each ZIP code income quintile in the year 2000, ranging from the lowest 20 percent of Medicare enrollees in Quintile 1, to the highest 20 percent in Quintile 5. Ninety-five percent confidence intervals are also shown. The “Overall” regression shows how knee arthroplasty rates vary by income with controls for age, sex, and racial or ethnic identification. It describes a shallow inverted U shape, with slightly higher rates in the middle income quintile (16 percent above the reference group), but with nearly identical rates in the bottom and top income quintiles. The “Access to Care” regression adjusts for the previous factors, but includes as well the region of residence, so this can be interpreted as the effect of income within the same region. Access to care is better for higher income households, with an 18.5 percent greater chance of TKR in the highest income quintile.
Prevalence of Osteoarthritis and Knee Pain in NHANES
Of the 20,050 adult people in the NHANES sample, there were 2,589 people over age 60 with radiographic measures of knee osteoarthritis. Of these, 431 were excluded because they reported congestive heart failure, cancer, and possible active infections. In addition, respondents were excluded because of missing data on Body-Mass Index (BMI) (N = 7), race (N = 1), radiographic measures (N = 161), and questions about knee pain and functioning (N = 63), leaving a sample of 1,926. A description of the variables and summary statistics are reported in Table 1. Thirty-eight percent of respondents were found to have a KL score of 2 or more in at least one knee (N=807), and one-quarter had both clinical and radiographic evidence of OA: a KL score of 2 or more, and either crepitus, limitation of knee motion, or swelling in the knee (N=570).
Table 3 considers the association between income and a diagnosis of OA. In the first column, the dependent variable is a binary value for whether the KL score is 2 or above. The odds ratio for the logarithm of income was 1.10 (95% C.I., 0.95 – 1.27), meaning there was no significant association between income and the KL score. There were differences by sex and racial group; for white women (with white men the reference group), the odds ratio was 1.52 (95% C.I., 1.11 – 2.09) for Black women, the odds ratio was 3.60 (95% C.I., 2.33 – 5.56) and for Black men the odds ratio was 1.20 (95% C.I., 1.43 – 3.38). BMI was also an important risk factor for OA (p < .001). Similar results were obtained for the more restrictive measure of OA: a KL score equal to 2 or more plus evidence of either crepitus, limitations on knee movement, or swelling (Table 3). There was no association between this measure and income; as is shown in Figure 2 there was a slight positive association between evidence of OA and income quintile, although the results were not significant given the limited sample size.
Table 3.
Regression Analysis: NHANES III Survey
| Dependent Variable |
Kellgren-Lawrence (KL) Score ≥ 2 |
KL Score ≥ 2 and Evidence of Crepitus, Swelling, or Limits on Knee Movement |
||||
|---|---|---|---|---|---|---|
| Odds Ratio |
95% Lower C.I. |
95% Upper C.I. |
Odds Ratio |
95% Lower C.I. |
95% Upper C.I. |
|
| Income (Log) | 1.099 | 0.949 | 1.273 | 1.084 | 0.925 | 1.269 |
| Men: White | 1.000 | 1.000 | ||||
| Men: Black | 2.197 | 1.427 | 3.383 | 2.115 | 1.348 | 3.317 |
| Men: Other | 1.820 | 0.496 | 6.680 | 1.801 | 0.278 | 11.651 |
| Women: White | 1.523 | 1.108 | 2.094 | 1.379 | 0.979 | 1.942 |
| Women: Black | 3.601 | 2.332 | 5.560 | 3.574 | 2.296 | 5.563 |
| Women: Other | 4.930 | 1.300 | 18.687 | 1.448 | 0.516 | 4.068 |
| BMI (Quartile 1) | 1.000 | 1.000 | ||||
| BMI (Quartile 2) | 2.126 | 1.391 | 3.251 | 1.832 | 1.144 | 2.935 |
| BMI (Quartile 3) | 4.007 | 2.613 | 6.144 | 4.618 | 2.960 | 7.203 |
| BMI (Quartile 4) | 9.088 | 5.867 | 14.079 | 8.641 | 5.524 | 13.517 |
| South | 1.000 | 1.000 | ||||
| Northeast | 0.870 | 0.594 | 1.274 | 0.700 | 0.457 | 1.073 |
| Midwest | 1.356 | 0.972 | 1.890 | 1.069 | 0.739 | 1.547 |
| West | 1.021 | 0.665 | 1.568 | 0.712 | 0.448 | 1.133 |
Notes: The dependent variable is a Kellgren-Lawrence Score of 2, 3, or 4 (the first column) or the combination of both a KL Score of 2 , 3, or 4 and the presence on examination of either crepitus, swelling, or limits on knee motion. N = 1926. All estimates adjusted for population weights and sampling strata. Age variables included in regression but not reported. For these two measures (KL ≥ 2, or KL ≥ 2 with evidence of crepitus, swelling, or limitation of knee movement), the estimated odds ratio cannot be interpreted as a relative risk.26
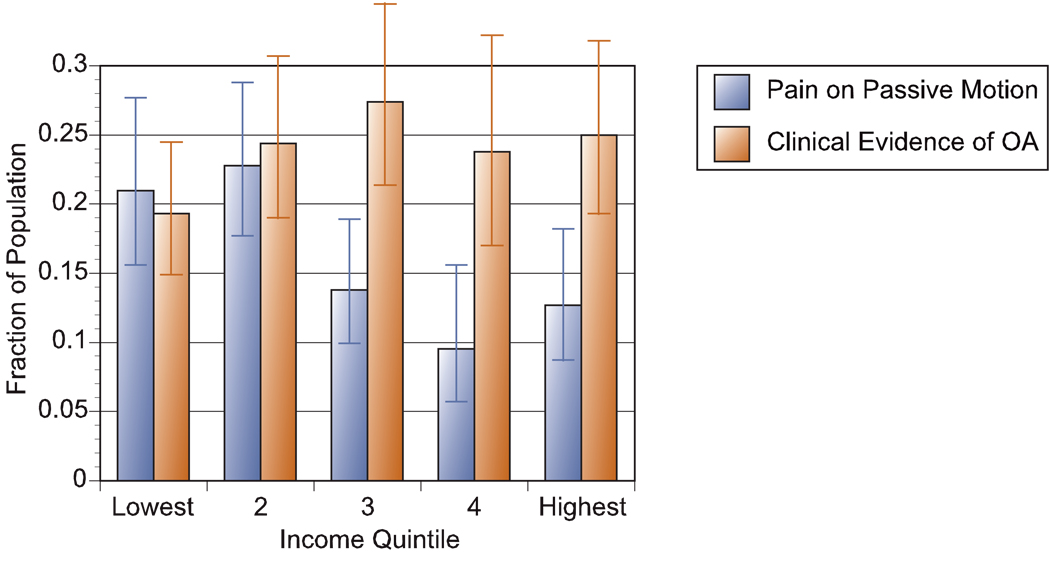
Figure 2. Clinical Evidence of Osteoarthritis and Pain on Passive Motion by Income.
Results from two separate regressions using the NHANES III data set (N = 1926). The logistic regression analysis was adjusted for population weight and strata, and controls for age, sex, race, region, and BMI, with odds ratios converted to sample probabilities using the ADJUST command in STATA 9.0. Clinical evidence of osteoarthritis (OA) is the presence of a KL score of 2 or more and evidence on examination of crepitus, swelling, or limitation of movement. Ninety-five percent confidence intervals are also shown. There is no significant difference between the lowest and highest quintile with regard to clinical evidence of OA, but there is a significant difference between the lowest and highest quintiles for pain on passive motion (p = .04).
Pain on passive motion was also considered. In this logistic regression (results not reported), there was no significant difference in pain by race or ethnicity. However, there was significantly greater pain on passive motion for Black and White women (p < .01). Figure 2 demonstrates the strong negative association between income and knee pain on passive motion (p < .05), even after adjusting for race, gender, BMI, and region. Overall rates of knee pain range from 20 percent in the bottom income quintile to 9 percent in the second-highest income quintile. Knee pain was also a more sensitive indicator of OA for high income respondents; 78 percent in the top quintile who reported pain on passive motion also were diagnosed with a KL score of 2 or more, contrasting with 66 percent in the lowest income group.
Discussion
Across the U.S., there was little association between socioeconomic status and rates of total knee arthroplasty. Nor was there an association between socioeconomic status and clinical evidence of knee osteoarthritis. This first result is in contrast to results reported by Mahomed et. al.7 who found that the “dually eligible” who were enrolled in both Medicare and Medicaid (and thus presumably reflecting poverty status) were less likely to receive a TKA. However, eligibility for Medicaid is often predicated on poor health or nursing home residence, which could by itself reduce the appropriateness of a TKA.
One puzzling result from the statistical analysis is why access to care is greater for high-income Medicare enrollees within a region, but the overall association between income and utilization is essentially zero. The solution to the puzzle requires an understanding of the geographic patterns of TKA in the United States, where there are large documented variations in utilization rates.1, 25 For example, the average rate of TKA for white women in Manhattan (New York City) during 1998–2000 was 2.9 per 1,000 enrollees. In Little Rock, Arkansas the corresponding rate was 6.6 per 1,000, and in McAllen Texas the rate was 8.8 per 1,000.1 Suppose that high-income residents within the regions of Manhattan, Little Rock, and McAllen were 18.5 percent more likely to receive a TKA (the result we find on average across the U.S.). Were we to then compare rates of TKA among high income people in the United States -- without adjusting for region -- a different pattern would emerge: even the wealthiest residents of Manhattan would be less likely to receive a TKA than the lower-income residents of McAllen. One measure is not better than the other; they each provide an answer to a different question.23
This study demonstrates that total knee replacement (TKA) among minority groups are substantially lower than for whites, and this result holds after controlling for income. Thus observed racial disparities, which are consistent with earlier studies,16, 19, 28, 29 are not the consequence of socioeconomic class, but persist even when comparing Black, Asian, or white Medicare enrollees with similar economic status. Nor can these differences be explained by variation in underlying rates of osteoarthritis, since these rates were higher still among Black men and women.
The work of Ibrahim et. al. supports the idea that among Black patients with OA, there is much greater belief in the efficacy of physical therapy, Tylenol, herbal medicine, massage, and prayer, and considerably less belief in the efficacy of surgery.30 Less well understood is our finding that Asian men and women also experience very low rates of TKA. One potential explanation could be the poor quality of patient-doctor interactions. One study found Asian-Americans (N = 621) far less likely than Black (N = 1037), Hispanic (N = 1153), or white (N = 3488) respondents to understand what their doctor told them, and only 49 percent strongly agreed with the statement that the “Doctor understands my background/values” in contrast to 60 percent for Black respondents and 61 percent for whites.31
Pain on passive motion is sometimes used as a symptom for late-stage OA, 32 and is shown in this paper to be a sensitive indicator of radiographic evidence for OA for people over age 60. Unlike other clinical indicators of OA, pain on passive motion was negatively associated with income, a result also shown by education in an earlier study. 33 This finding raises the possibility that other factors may mediate the response to passive motion. The issue of non-organic signs has been considered in lower back pain, 34 but little is known about such factors in knee OA.
One important limitation of the study is the use of ZIP code or neighborhood income instead of individual income. One advantage, however, is that ZIP code income avoids errors arising from survey data, where income measures are often measured with error, and better captures housing or financial wealth, often an important source of financial support among elderly people. Previous researchers using data sources with both individual and ZIP code income have shown ZIP code income to be a valid measure of socioeconomic status.35
These results have potential implications for surgical practice They suggest that minority patients or those with lower income levels (within a given region) are currently less likely to undergo TKA, despite similar or greater prevalence of osteoarthritis. In part, lower utilization rates may reflect financial burdens for lower income households without supplemental insurance, but even in the Canadian system where financial barriers are minimal, Hawker et. al. found fewer than one in five deemed medically appropriate expressed an interest in having a joint replacement, with rates among lower income groups even lower (p < .10). The real challenge is in creating an environment where patients are able to make well-informed decisions that overcome past biases, language barriers, and distrust of the health care system, 36 for example the use of decision aids to help patients understand benefits and risks of surgery.37
Our finding also has implications for recent legislation mandating a tripling of Part B premiums for the highest income Medicare enrollees. Based on our findings, higher Part B premiums cannot be justified because higher income taxpayers have access to one common surgical procedure, total knee arthroplasty. Whether these patterns hold for other surgical procedures is less well understood.
Acknowledgments
This research was supported by NIAMS MCRC P60-AR048094 and NIA PO1-AG19783. We are grateful to three anonymous reviewers, the Editor, and Tamara Morgan for substantial improvements to the manuscript.
References
- 1.Skinner J, J W, Sporer M, Wennberg J. Racial, ethnic, and geographic disparities in rates of knee arthroplasty among Medicare patients. New England Journal of Medicine. 2003;349-14:1350–1359. doi: 10.1056/NEJMsa021569. [DOI] [PubMed] [Google Scholar]
- 2.Escalante A, Espinosa-Morales R, del Rincon I, Arroyo R, Older S. Recipients of hip replacement for arthritis are less likely to be Hispanic, independent of access to health care and socioeconomic status. Arthritis & Rheumatism. 2000;43-2:390–399. doi: 10.1002/1529-0131(200002)43:2<390::AID-ANR20>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 3.Ibrahim S, Siminoff L, Burant C, Kwoh C. Understanding ethnic differences in the utilization of joint replacement for osteoarthritis. Medical Care. 2002;40-1(Suppl):I44–I51. doi: 10.1097/00005650-200201001-00006. [DOI] [PubMed] [Google Scholar]
- 4.Wilson M, May D, Kelly J. Racial differences in the use of total knee arthroplasty for osteoarthritis among older Americans. Ethnicity & Disease. 1994;4:57–67. [PubMed] [Google Scholar]
- 5.Gittelsohn A, Halpern J, Sanchez R. Income, race, and surgery in Maryland. Am J Pub Health. 1991;81:1435–1441. doi: 10.2105/ajph.81.11.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Westert GP, Smits JPJM, Poler JJ, Mackenbach JP. Community Income and Surgical Rates in the Netherlands. Journal of Epidemiology and Community Health. 2003;57:519–522. doi: 10.1136/jech.57.7.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mahomed NN, Barrett J, Katz JN, Baron JA, Wright J, Losina E. Epidemiology of Total Knee Replacement in the United States Medicare Population. Journal of Bone and Joint Surgery. 2005;87A-6:1223–1228. doi: 10.2106/JBJS.D.02546. [DOI] [PubMed] [Google Scholar]
- 8.Bradley CJ, Given CW, Roberts C. Race, Socioeconomic Status, and Breast Cancer Treatment and Survival. J Natl Cancer Inst. 2002;94:490–496. doi: 10.1093/jnci/94.7.490. [DOI] [PubMed] [Google Scholar]
- 9.Barbeau EM, Krieger N, Soobader M-J. Working Class Matters: Socioeconomic Disadvantage, Race/Ethnicity, Gender, and Smoking in NHIS 2000. American Journal of Public Health. 2004;94-2:269–278. doi: 10.2105/ajph.94.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lueck S, Rogers D. The Wall Street Journal Online. New York: 2003. Medicare Test May Prove Moot. [Google Scholar]
- 11.Shaviro D. Who Should Pay for Medicare? Chicago: University of Chicago Press; 2004. [Google Scholar]
- 12.McClellan M, Skinner J. The Incidence of Medicare. Journal of Public Economics. 2006;90-1-2:257–276. [Google Scholar]
- 13.Battacharya J, Lakdawalla D. Does Medicare Benefit the Poor? New Answers to an Old Question. Journal of Public Economics. 2006;90-1-2:277–294. [Google Scholar]
- 14.Hirsch H. Total joint replacement: a cost-effective procedure for the 1990s. Med Health R I. 1998;81-5:162–164. [PubMed] [Google Scholar]
- 15.Lavernia C, Guzman J, Gachupin-Garcia A. Cost-effectiveness and quality of life in knee arthroplasty. Clinical Orthopaedics & Related Research. 1997;345:134–139. [PubMed] [Google Scholar]
- 16.Anderson JJ, Felson DT National Health and Nutrition Examination Survey (NHANES I) Factors associated with osteoarthritis of the knee in the first. American Journal of Epidemiology. 1988;128-1:179–189. doi: 10.1093/oxfordjournals.aje.a114939. [DOI] [PubMed] [Google Scholar]
- 17.Hawker GA, Wright JG, Coyte PC, Williams JI, Harvey B, Blazier R, Badley EM. Differences between men and women in the rate of use of hip and knee arthroplasty. NEJM. 2000;342-14:1016–1022. doi: 10.1056/NEJM200004063421405. [DOI] [PubMed] [Google Scholar]
- 18.Katz J, Wright E, et al. Differences between men and women undergoing major orthopedic surgery for degenerative arthritis. Arthritis & Rheumatism. 1994;37-5:687–694. doi: 10.1002/art.1780370512. [DOI] [PubMed] [Google Scholar]
- 19.Hirsch R, Cheng X, Grigorian M, Stork A, Schaffler G, et al. Radiographic Knee Osteoarthritis Prevalence in Older Adults in the United States. Arthritis and Rheumatism. 2001 Sept.(449 Suppl):S225. (Abstract No. 1033) [Google Scholar]
- 20.Deyo RA, Tsui-Wu Y-J. Functional Disability Due to Back Pain: A Population-Based Study Indicating the Importance of Socioeconomic Factors. Arthritis and Rheumatism. 1987;30-11:1247–1253. doi: 10.1002/art.1780301107. [DOI] [PubMed] [Google Scholar]
- 21.Lauderdale D, Goldberg J. The expanded racial and ethnic codes in the Medicare data files: Their Completeness of Coverage and Accuracy. American Journal of Public Health. 1996 May;86-5:712–716. doi: 10.2105/ajph.86.5.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arday S, Arday D, Monroe S, Zhang J. HCFA's Racial and Ethnic Data: Current Accuracy and Recent Improvements. Health Care Financing Review. 2000;21-4:107–116. [PMC free article] [PubMed] [Google Scholar]
- 23.Chandra A, Skinner J. Geography and Racial Disparities in Health. In: Anderson NB, Bulatao RA, editors. Critical Perspectives on Racial and Ethnic Differences in Health in Late Life. Washington DC: National Academy Press; 2004. [PubMed] [Google Scholar]
- 24.Wennberg J, Cooper M, editors. The Dartmouth Atlas of Health Care. First ed. Chicago, IL: American Hospital Publishing, Inc.; 1997. [PubMed] [Google Scholar]
- 25.Weinstein J, editor. The Dartmouth Atlas of Musculoskeletal Health Care. Chicago, IL: American Hospital Association Press; 2000. [PubMed] [Google Scholar]
- 26.Schwartz L, Wolosin S, Welch H. Misunderstandings About the Effects of Race and Sex on Physician's Referrals for Cardiac Catheterization. NEJM. 1999;341-4:279–283. doi: 10.1056/NEJM199907223410411. [DOI] [PubMed] [Google Scholar]
- 27.Kellgren J, Lawrence J. Radiological assessment of osteoarthrosis. Annals of the Rheumatic Diseases. 1957;16:494–501. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leigh J, Fries J. Occupation, income, and education as independent covariates of arthritis in four national probability samples. Arthritis and Rheumatism. 1991;34-8:984–995. doi: 10.1002/art.1780340808. [DOI] [PubMed] [Google Scholar]
- 29.Leigh J, Fries J. Correlations between education and arthritis in the 1971–1975 NHANES I. Social Science and Medicine. 1994;38-4:575–583. doi: 10.1016/0277-9536(94)90254-2. [DOI] [PubMed] [Google Scholar]
- 30.Ibrahim S, Siminoff L, Burant C, Kwoh C. Variations in perceptions of treatment and self-care practices in elderly with osteoarthritis: A comparison between African American and white patients. Arthritis and Rheumatism. 2001;45-4:340–345. doi: 10.1002/1529-0131(200108)45:4<340::AID-ART346>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 31.Saha S, Arbelaez JJ, Cooper LA. Patient-physician relationships and racial disparities in the quality of health care. American Journal of Public Health. 2003;93-10:1713–1719. doi: 10.2105/ajph.93.10.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beers M, Berkow R, editors. The Merck Manual of Diagnosis and Therapy Vol. Section 5, Chapter 52. 2005. www.merck.com/mrkshared/mmanual/section5/chapter52/52a.jsp ed. [Google Scholar]
- 33.Hannan MT, Anderson JJ, Pincus T, Felson DT. Educational attainment and osteoarthritis: Differential associations with radiographic changes and symptom reporting. Journal of Clinical Epidemiology. 1992;45-2:139–147. doi: 10.1016/0895-4356(92)90006-9. [DOI] [PubMed] [Google Scholar]
- 34.Fishbain D, Code B, Cutler R, Lewis J, Rosomoff H, Rosomoff R. A structured evidence-based review on the meaning of nonorganic physical signs: Waddell signs. Pain Medicine. 2003;4-2:141–181. doi: 10.1046/j.1526-4637.2003.03015.x. [DOI] [PubMed] [Google Scholar]
- 35.Geronimus A, Bound J, Neidert On the Validity of Using Census Geocode Characteristics to Proxy Individual Socioeconomic Characteristics. Journal of the American Statistical Association. 1996;91:529–537. [Google Scholar]
- 36.Katz JN. Patient preferences and health disparities. Jama. 2001;286-12:1506–1509. doi: 10.1001/jama.286.12.1506. [DOI] [PubMed] [Google Scholar]
- 37.O'Connor AM, Llewellyn-Thomas HA, Flood AB. Modifying Unwarranted Variations in Health Care: Shared Decision Making Using Patient Decision Aids. Health Affairs. 2004;(Variations Suppl):63–72. doi: 10.1377/hlthaff.var.63. [DOI] [PubMed] [Google Scholar]