Abstract
A goal of personalized medicine as applied to oncology is to identify compounds that exploit a defined molecular defect in a cancerous cell. A compound called procaspase-activating compound 1 (PAC-1) was reported that enhances the activity of procaspase-3 in vitro and induces apoptotic death in cancer cells in culture and in mouse xenograft models. Experimental evidence indicates that PAC-1 activates procaspase-3 in vitro through chelation of inhibitory zinc ions. Described herein is the synthesis and biological activity of a family of PAC-1 derivatives where key functional groups have been systematically altered. Analysis of these compounds reveals a strong correlation between the in vitro procaspase-3 activating effect and their ability to induce death in cancer cells in culture. Importantly, we also show that a fluorescently-labeled version of PAC-1 co-localizes with sites of caspase-3 activity in cancer cells. The data presented herein further bolster the hypothesis that PAC-1 induces apoptosis in cancer cells through the direct activation of procaspase-3, has implications for the design and discovery of next-generation procaspase-3 activating compounds, and sheds light on the anti-apoptotic role of cellular zinc.
Keywords: PAC-1, apoptosis, microscopy, fluorescence, zinc
INTRODUCTION
Cancer recently overtook heart disease as the number one killer in the United States for individuals under 85 years of age.1 Despite intensive research efforts towards targeted anti-cancer therapies, most modern chemotherapeutic regimens still rely on general cytotoxins that indiscriminately induce death in rapidly dividing cell types.2 As resistance to apoptosis is a hallmark of cancer,3–5 an intriguing approach to anti-cancer drug discovery is the re-activation of apoptotic cascades in cancer cells.6 Indeed, burgeoning interest in apoptotic proteins as anti-cancer targets has led to the discovery of several promising classes of small molecules,6–9 including those that bind Bcl-2,10 MDM2,11, 12 XIAP,13, 14 and those that supress expression of survivin.15, 16
The caspase enzymes are cysteine proteases that both initiate and execute the apoptotic program.17, 18 The caspases are produced as low activity zymogens (proenzymes) that can be activated by various mechanisms.19, 20 For example, procaspase-9 is converted to caspase-9 through formation of a multimeric protein complex called the apoptosome,21 while procaspase-8 is converted to caspase-8 through a proximity model.22, 23 Active caspase-9 and caspase-8 can in turn directly proteolyze procaspase-3 and procaspase-7, forming active caspase-3 and caspase-7.24, 25 Caspase-3 is the key “executioner” caspase in the apoptotic cascade, and once activated this enzyme cleaves scores of cellular substrates, committing the cell to apoptotic death.26, 27 Based on mouse knockout studies and other work, procaspase-7/caspase-7 may have a less important function relative to procaspase-3/caspase-3 in the “demolition phase” of apoptosis, although caspase-7 likely has some distinct cellular substrates.28, 29 We reported the identification of a small molecule, called PAC-1 (compound 1 in Figure 1), which enhances the activity of procaspase-3 in vitro and induces apoptotic death in cancer cell lines and cells from primary tumor isolates.30 PAC-1 also showed efficacy in mouse models of lung and renal cancer and thus has promise as an anti-cancer agent.30 PAC-1 is the first small molecule discovered that induces the in vitro autoactivation of a proenzyme protease, and as such could serve as a prototype for other protease activating compounds.
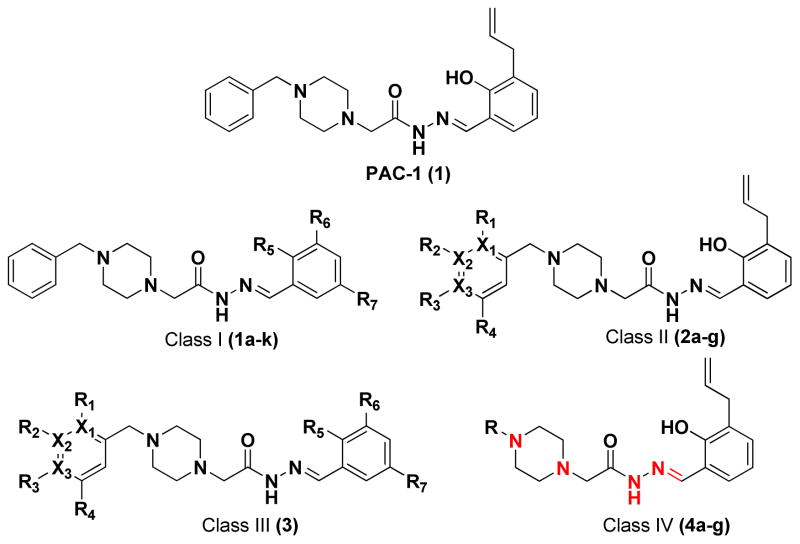
Figure 1.
PAC-1, and four classes of PAC-1 derivatives. Class I compounds (1a-k) have modifications on the phenolic ring, Class II compounds(2a-g) have modification on the benzyl ring, Class III compounds (3) have modifications on both the phenolic and benzyl rings, and Class IV compounds (4a-g) have modifications to the key heteroatoms and bonds indicated in red.
PAC-1 derivatives lacking the phenolic hydroxyl do not activate procaspase-3 in vitro and do not induce death of cancer cells in culture.30 This information, combined with the known tendency of ortho-hydroxy N-acyl hydrazones to bind metal ions,31 led us to suspect metal ion binding as part of the mechanism of action of PAC-1. As caspase-332 and procaspase-333 are known to be inhibited by zinc, we focused on zinc and recently determined that PAC-1 binds to zinc in a 1:1 stoichiometry with a dissociation constant of 52 nM.33 In addition, we discovered that PAC-1 enhances the activity of procaspase-3, caspase-3, procaspase-7, and caspase-7 in vitro through the sequestration of inhibitory zinc ions.33 Described herein is the use of derivative synthesis and biochemical analysis to determine the exact PAC-1 functional groups responsible for caspase activation, zinc chelation, and cell death. Importantly, the results of these experiments show a strong correlation between the ability of a compound to bind zinc, activate caspase-3 in vitro, and induce death in cancer cells in culture. Based on this structure-activity relationship, a novel fluorescent derivative of PAC-1 was designed and synthesized. Subsequent confocal microscopy experiments indicate that fluorescently-labeled PAC-1 co-localizes with sites of caspase-3 activity in the cell. Overall, the results described herein support the hypothesis that PAC-1 induces death in cancer cells through direct activation of procaspase-3 via sequestration of inhibitory zinc ions, implicate zinc as an endogenous anti-apoptotic agent, and suggest a shared mode of action for certain zinc-chelating anti-cancer compounds.
RESULTS
Design and synthesis of PAC-1 derivatives
In an effort to fully define the structural requirements of PAC-1 for procaspase-3/caspase-3 activation and cell death induction, we designed a series of derivatives with systematically altered functional groups that would allow a detailed structure-activity relationship (SAR) for PAC-1 to be constructed. This SAR would in turn allow the design of a fluorescent version of PAC-1 that could be used to determine the subcellular localization of this compound and its ability to co-localize with sites of caspase-3 activity in the cell. Thus, the twin goals of the SAR study were the determination of functional groups important for the biological activity of PAC-1, and the discovery of regions on the PAC-1 scaffold that could be altered with minimal loss in activity.
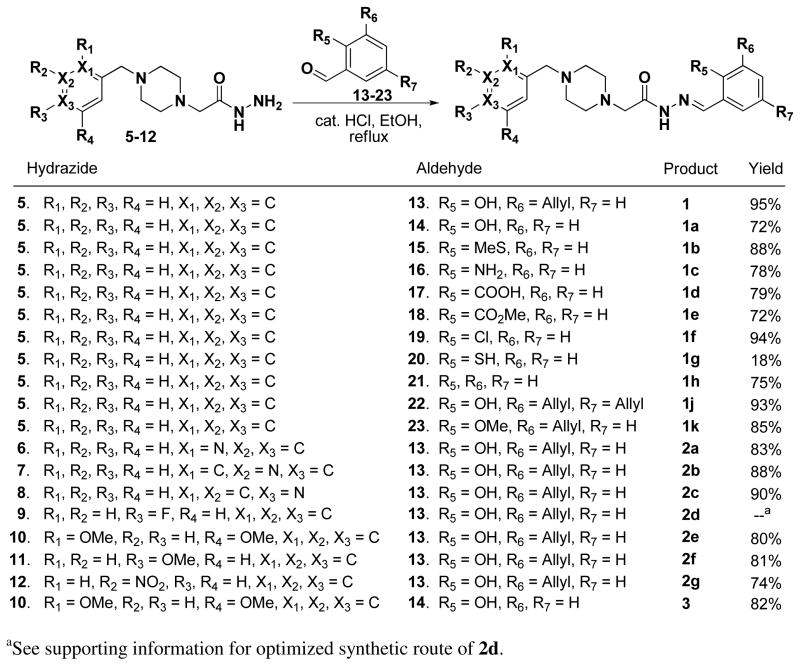
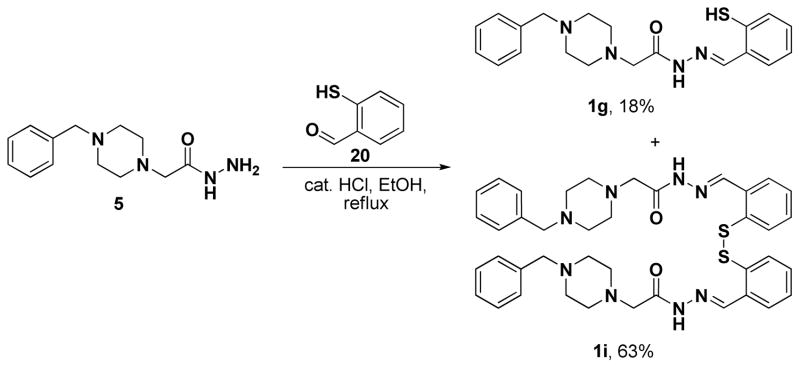
Four classes of PAC-1 derivatives were envisioned (Figure 1): alterations on the phenolic ring (Class I, 1a-k), on the benzyl ring (Class II, 2a-g), simultaneous modification of both the benzyl and phenolic rings (Class III, 3), and alterations to the PAC-1 core (Class IV, 4a-g, with modifications of the indicated atoms and bonds made in red). The majority of the Class I-III compounds were synthesized as shown in Scheme 1, via condensation of the appropriate hydrazides (5–12) and aldehydes (13–23). As shown in Scheme 1, yields for this condensation were between 72–95%, the exception being 1g, which spontaneously forms a disulfide dimer resulting in a diminished yield (Scheme 2). Through such condensation reactions we constructed eleven Class I derivatives (1a-k), seven Class II derivatives (2a-g), and one Class III derivative (3). The hydrazide building blocks in Scheme 1 were synthesized through reaction of various benzyl chlorides with piperazine, alkylation of the resulting products with ethyl chloroacetate, followed by reaction of the resulting ester with hydrazine (see Supporting Information for details). The aldehyde building blocks were either purchased or synthesized from simple starting materials (see Supporting Information for details).
Scheme 1.
The condensation of hydrazides and aldehydes to create PAC-1 derivatives of Class I-III.
Scheme 2.
The condensation of hydrazide 5 and aldehyde 20 provides 1g and its dimer, 1i.
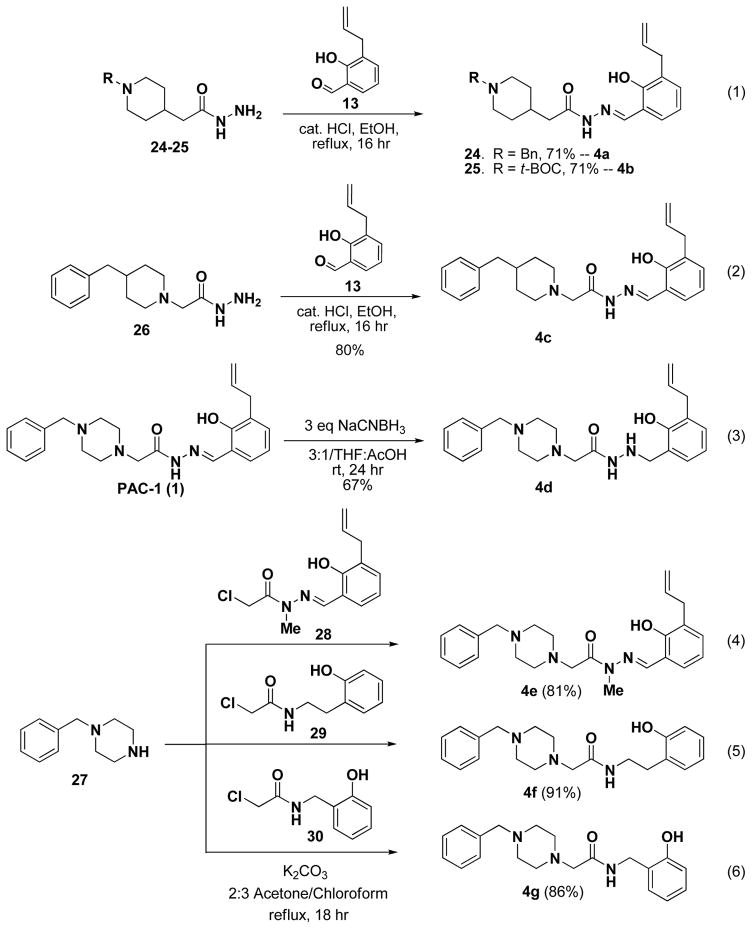
Three Class IV derivatives could also be synthesized through analogous condensation reactions. Thus, compounds lacking one of two piperazine nitrogens (4a-c) were synthesized from the corresponding hydrazides and aldehyde as shown in Scheme 3, equations 1 and 2. However, a number of the derivatives in Class IV could not be synthesized via analogous condensation reactions and required alternative synthetic routes. Thus, compound 4d was synthesized by reduction of the hydrazone in PAC-1 using NaCNBH3 (Scheme 3, equation 3). Compounds 4e-g were synthesized through reaction of 27 with the appropriate chloride (28–30) (Scheme 3, equations 4–6).
Scheme 3.
Syntheses of Class IV derivatives.
Evaluation of PAC-1 derivatives
With 26 derivatives of PAC-1 in hand, all compounds were evaluated for their ability to induce death in cancer cells, to relieve zinc-mediated inhibition of caspase-3 in vitro, and to bind zinc. Assessment of all compounds in these three assays provides a comprehensive data set used to formulate a structure-activity relationship for PAC-1; brief descriptions of these assays are provided below.
The induction of cell death was determined using the U-937 (human lymphoma) cell line. U-937 cells grow in culture as a suspension and previous studies with PAC-1 have been conducted with these cells,30, 33 thus they provide a convenient benchmark for assessment of PAC-1 analogues. For these experiments, cells were exposed to a range of compound concentrations for 72 hours, cell death was quantitated via a sulforhodamine B assay,34 and IC50 values were calculated from logistical dose-response curves.
We had previously shown that zinc inhibits both active caspase-3 and procaspase-3, and that PAC-1 is able to enhance the activity of both of these enzymes.33 Because of the low catalytic activity of procaspase-3 (~200-fold less active than caspase-3),33, 35 we used the activation of caspase-3 as the method to evaluate and compare PAC-1 and its derivatives. Therefore, all 27 compounds (PAC-1 and its 26 derivatives) were tested at multiple concentrations with caspase-3 and zinc to determine their ability to relieve zinc-mediated inhibition of caspase-3 in vitro. The percent activation of caspase-3 at a compound concentration of 10 μM, the concentration at which most compounds showed maximal activity, is reported in Table 1. For these enzyme activation assays, all buffers were first treated with Chelex resin to remove divalent metal cations.
Table 1.
Evaluation of PAC-1 and its derivatives
| U937 (IC50, μM) | Caspase-3 (%Activation at 10 μM) | Zinc Binding (Kd, nM) | U937 (IC50, (μM) | Caspase-3 (% Activation at 10 μM) | Zinc Binding (KD, nM) | ||
|---|---|---|---|---|---|---|---|
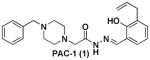 PAC-1 (1) |
4.8 ±1.0 | 45.8 ± 4.8 | 52 ±2 |
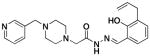 2b |
14 ± 2 | 20.6 ± 1.6 | 62 ±9 |
|
1a |
15.3 ±2.0 | 30 ± 2 | 77 ±2 |
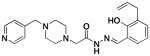 2c |
21 ± 6 | 26.3 ±0.1 | 80 ± 14 |
|
1b |
61 ±22 | 0 | >1 ×104 |
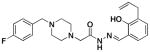 2d |
2.0 ± 0.2 | 24.2 ± 1.7 | 48 ±3 |
|
1c |
>100 | 0 | >1 ×104 |
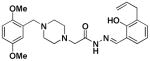 2e |
1.0 ± 0.1 | 31 ±3.6 | 39 ± 3 |
|
1d |
>100 | 0 | >1 ×104 |
2f |
2.7 ±0.8 | 24 ± 3.4 | 47 ±5 |
|
1e |
>100 | 0 | >1 ×104 |
2g |
4.6 ± 1.0 | 19.5 ±1.8 | 45 ±13 |
|
1f |
57 ±5 | 0 | >1 ×104 |
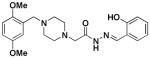 3 |
12 ± 2 | 30.7 ±1.2 | 65 ± 4 |
|
1g |
>100 | 0 | >1 ×104 |
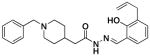 4a |
2.8 ± 1.1 | 0a | 44 ± 2 |
|
1h |
>100 | 0 | >1 ×104 |
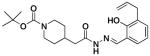 4b |
6.5 ± 3.6 | 0a | 48 ± 7 |
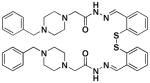 1i |
>100 | 0 | >1 ×104 |
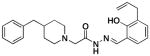 4c |
2.7 ± 1.1 | 36 ±3 | 49 ± 2 |
|
4d |
59 ± 14 | 17.1 ±1.2 | > 1 × 104 | ||||
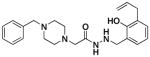 1j |
1.8 ± 0.4 | 59 ± 5 | 43 ±9 |
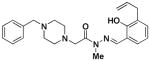 4e |
22 ± 3 | 0 | > 1 × 104 |
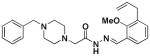 1k |
32 ±12 | 0 | >1 ×104 |
4f |
>100 | 4.1 ±1.0 | > 1 ×104 |
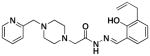 2a |
9.5 ±1.8 | 48 ± 3 | 68 ±10 |
 4g |
>100 | 4.4 ± 0.9 | > 1 ×104 |
All errors represent standard error of the mean, n=3.
These compounds exhibit a significant inhibitory effect at 10 μM, masking any activation (see text).
The affinity of each compound for zinc was determined through used of an EGTA titration according to the method of Zhu and co-workers.36 For these experiments, PAC-1 or derivatives were added in a Hepes buffer containing a large excess of EGTA, followed by incremental addition of zinc, and the fluorescence of the small molecule—zinc complex was read (excitation at 410 nm, emission at 475 nm). The resulting increase of fluorescence intensity was plotted against the calculated free zinc concentration in accord with similar protocols.36 This plot constitutes a formation curve from which binding constants were calculated.
The results of these cell death, caspase-3 activation, and zinc binding experiments are shown in Table 1 (see supporting information Figures S1–S3 for individual data sets). As shown, there is a strong correlation between the ability of compounds to activate caspase-3 in vitro, bind zinc, and induce death in cancer cells in culture. Universally, compounds that do not activate caspase-3 in vitro do not bind zinc or induce significant death of the cancer cells in culture. For example, removal or alkylation of the ortho-hydroxyl (compounds 1h and 1k) results in compounds that do not bind zinc, activate caspase-3, or kill cells to any large degree. Likewise, replacement of this –OH with a thioether (1b), amine (1c), carboxylic acid (1d), methyl ester (1e), chloride (1f), or sulfhydryl (1g) results in compounds that are basically inactive in all three assays. However, if the ortho-hydroxyl is maintained, a variety of substitution patterns are tolerated on the phenolic ring. For example, (1j) contains allyl groups at two positions on this ring and is a potent caspase-3 activator, zinc binder, and cell death inducer, while the compound lacking any allyl groups (1a) is also reasonably potent in these assays.
The derivatives in which the nature of the benzyl ring was altered (Class II derivatives, 2a-g) were also assessed. As shown in Table 1, these compounds universally retained the ability to induce death in U-937 cells, to activate caspase-3 in vitro, and bind zinc. The one Class III derivative (3) was also active in all three assays.
Compounds in Class IV were constructed to systematically probe the importance of the ortho-hydroxy N-acylhydrazone motif and the piperazine nitrogens on biological activity. Specifically, compounds were created in which the piperazine nitrogens were replaced with CH (compounds 4a-c), that have the C-N double bond reduced (4d), that have an N-methylation (4e), and that have the hydrazone linkage replaced with an amide (4f-g). Assays reveal that all compounds with alterations to the N-acylhydrazone motif were inactive. No zinc binding could be detected for compounds 4d-g; these four compounds also have no/low capacity for caspase-3 activation in vitro, and they do not strongly induce death in U-937 cells in culture (Table 1). In contrast, compound 4c, in which one of the piperazine nitrogens is changed to a CH, showed cell death induction, caspase-3 activation, and zinc binding activity almost identical to PAC-1.
Clear SAR trends are thus apparent from the data in Table 1: 1) PAC-1 derivatives that are unable to bind zinc do not activate caspase-3 in vitro, and do not appreciably induce death in U-937 cells in culture. This information suggests the zinc binding capacity of PAC-1 is important for its cell death inducing properties. 2) The ortho-hydroxy N-acylhydrazone motif is critical for zinc binding. 3) Virtually all compounds that bind zinc activate caspase-3 in vitro and induce death in U-937 cells in culture. Only compounds 4a and 4b, which show no in vitro caspase-3 activation but are still cytotoxic to U-937 cells, are exceptions to this trend. Interestingly, we find that these two compounds (at a concentration of 10 μM) are reasonably potent inhibitors of caspase-3 enzymatic activity, thus any activating effect of the compound may be masked by this inhibition (See Supporting Figure S4). This inhibition effect is consistent with previous data showing that PAC-1 will also inhibit procaspase-3/caspase-3 at high compound concentrations.33
Design, synthesis, and biochemical characterization of a fluorescent version of PAC-1
Based on the information gleaned from the SAR study, above, a fluorescent version of PAC-1 was designed that was predicted to retain the ability to bind zinc, activate caspase-3, and induce cell death. Specifically, as the SAR indicates that modifications on the benzyl ring are well-tolerated, a modified benzyl ring was used to conjugate PAC-1 with a fluorophore.
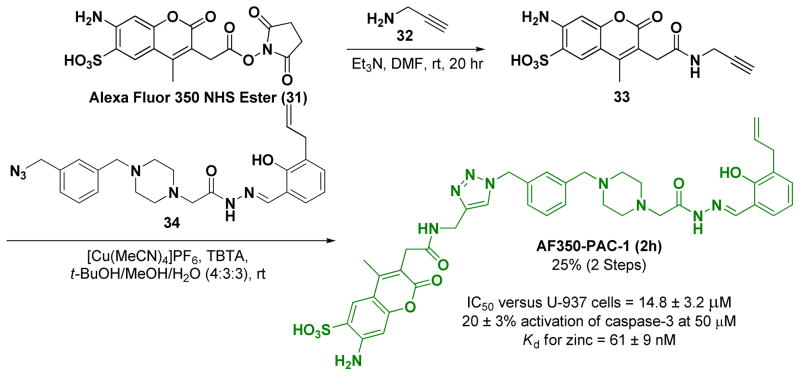
To ensure orthogonal fluorescent signals in our proposed co-localization experiments, we choose a fluorophore that we could excite at 350 nm to conjugate to PAC-1, and of the possible dyes in this category, Alexa Fluor 350 was selected. To synthesize the fluorophore-conjugated PAC-1, Alexa Fluor 350 (activated as its N-hydroxysuccinimide (NHS) ester), 31, was first treated with amine 32 to create alkynyl-Alexa Fluor 350 (compound 33, Scheme 4). The Cu(I)-catalyzed variant of the Huisgen [3+2] cycloaddition reaction37 was then used to link azido-PAC-1 (compound 34, whose synthesis is described in the Supporting Information) with 33 to form 2h (Alexa Fluor 350-conjugated PAC-1 or AF350-PAC-1, Scheme 4). 2h was characterized by HRMS and 1H NMR (see Supporting Information). Once in hand 2h was evaluated for its ability to induce death in U-937 cells in culture, activate caspase-3 in vitro, and bind zinc. 2h has activity similar to PAC-1 in these assays (IC50 versus U-937 cells = 14.8 ± 3.2 μM, caspase-3 activation at 10 μM = 6.3 ± 1.6% and at 50 μM = 20 ± 3%, Kd for zinc = 61 ± 9 nM, full details in Supporting Information Figure S5)
Scheme 4.
The synthesis of 2h, a fluorescent version of PAC-1.
Once it was determined that 2h retained similar activity to PAC-1 in these three assays, confocal microscopy experiments were conducted. The goals of these experiments were 1) to determine the subcellular localization of 2h; 2) to determine if 2h binds zinc in the cell, 3) to determine if 2h can activate procaspase-3/-7 to caspase-3/-7 inside the cell, and 4) to determine if 2h colocalizes with sites of caspase-3/-7 activity inside the cell.
Subcellular localization of 2h in fixed cells
Toward the first and second goals, live cell imaging was used with 2h and SK-MEL-5 (human melanoma) cells; these adherent cells are a convenient cell line for microscopy studies. SK-MEL-5 cells were treated with 25 μM 2h for 1 hour, washed with media lacking phenol red, and further incubated for 2 hours. Cells were then imaged using a Leica multiphoton confocal microscope. Cells treated with 2h show clear fluorescence in the cytoplasm, with little staining observed in the nucleus (Figure 2); the DNA in this experiment was stained with the SYBR green nucleic acid dye and pseudo-colored blue in Figure 2. The image in Figure 2 is representative of wider field images (see Supporting Information Figure S6) that show a similar staining pattern in ~50% of cells. The other ~50% of cells are either dead or lack staining all together. Furthermore, a punctate cytoplasmic staining pattern is observed for 2h (Figure 2). This punctate staining is similar to the localization pattern observed by other fluorescent zinc chelators,36, 38–41 and is hypothesized to be a visualization of the “zincosome”.42–45 The punctate staining pattern indicates that 2h readily crosses the cell membrane in a short period of time (<1 hour) and suggests that 2h chelates zinc in the cell.
Figure 2.
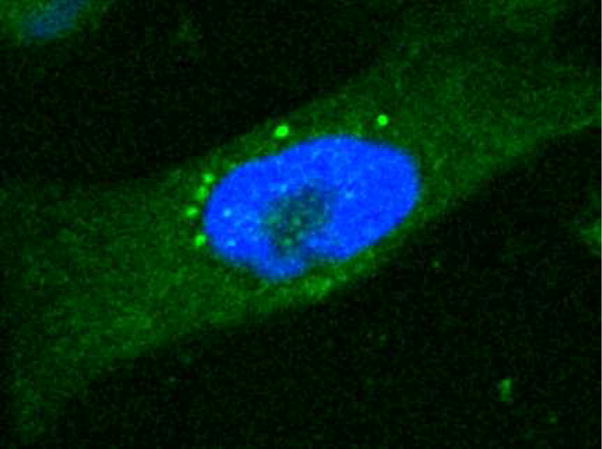
2h (pseudo-colored green) localizes to the cytoplasm in SK-MEL-5 cells and gives punctate staining similar to other fluorescent zinc chelators (see text). DNA was stained with SYBR green and pseudo-colored blue.
Visualization of PAC-1-induced activation of procaspase-3 in live cells
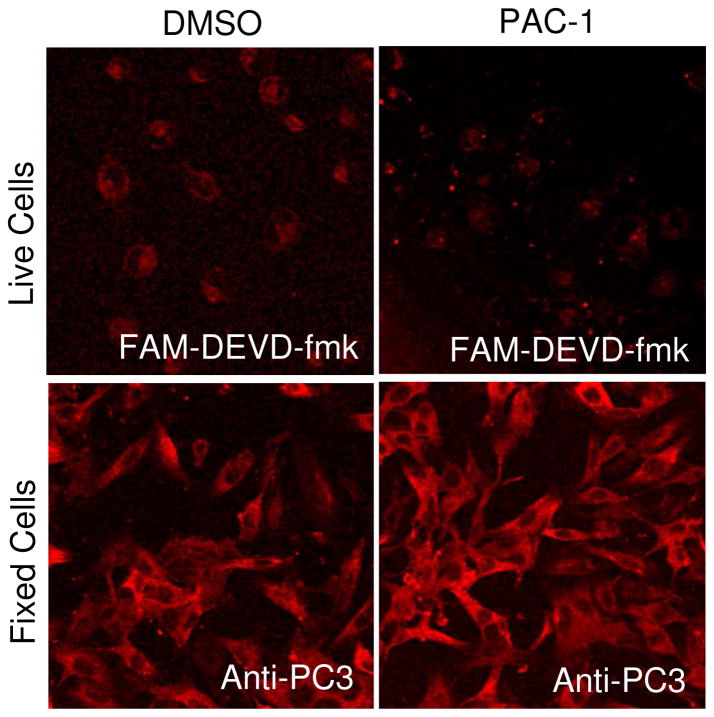
Because PAC-1 is known to relieve zinc-mediated inhibition of both procaspase-3/7 and active caspase-3/7 in vitro,33 we sought to investigate this phenomenon in cell culture. To determine the effect of PAC-1 treatment on the activity of procaspase-3/-7 and caspase-3/-7 in the cell, PAC-1 (not 2h) was utilized in conjunction with a fluorescently-labeled, irreversible peptidic inhibitor of caspase-3 and -7, known as FAM-DEVD-fmk. FAM-DEVD-fmk is a cell permeable carboxyfluorescein conjugated peptide that irreversibly alkylates the active site cysteine in active caspase-3 and -7 via its fluoromethyl ketone (fmk) warhead,46–48 thus staining with this reagent provides a means to visualize caspase-3/-7 activity in the cell. In the experiment, SK-MEL-5 cells were treated with PAC-1 (25 μM) and FAM-DEVD-fmk (25 μM). After incubation with these compounds for 1 hour, the cells were washed with media lacking phenol red and further incubated for 2 hours. Cells were then imaged using a Leica multiphoton confocal microscope. In these live cell images the active caspase-3/-7 population within cells can be observed with minimal interference from the inactive procaspase-3/-7. As shown in Figure 3 (top), in vehicle treated cells only minimal staining is apparent; however, upon treatment with PAC-1, the cells exhibit spots of intense fluorescence, indicative of caspase-3 activity. This result indicates that the procaspase-3 in these areas has been activated to caspase-3.
Figure 3.
(Top) Activity of caspase-3 in vehicle-treated and PAC-1 treated SK-MEL-5 cells. Cells were treated with either DMSO or PAC-1 (25 μM), and then treated with FAM-DEVD-fmk (25 μM), a caspase-3/-7 inhibitor that will covalently append a fluorophore to enzymes that possess caspase-3/-7 activity. (Bottom) Visualization of the entire procaspase-3/caspase-3 population of SK-MEL-5 cells using an antibody to caspase-3.
To determine how these localized areas of caspase-3 activity compared to the total population of procaspase-3, we utilized a rabbit anti-caspase-3 antibody that recognizes both active caspase-3 and procaspase-3. SK-MEL-5 cells were treated with either DMSO or 25 μM PAC-1 for 1 hour. The cells were then washed with media lacking phenol red and allowed to incubate further for 2 hours. At this point the cells were fixed and stained with the anti-caspase-3 primary antibody, followed by an anti-rabbit Alexa Fluor 647 secondary antibody, and mounted on slides. All cells were imaged on a Leica multi-photon confocal microscope. In the immunofluorescent images, procaspase-3/caspase-3 localization is confined to the cytoplasm (Figure 3, bottom) as is consistent with results in the literature.49 Thus, upon short treatments with PAC-1 there is no obvious change in the amounts or localization of procaspase-3/caspase-3. Analysis of the images in Figure 3 suggests that a sub-population of the procaspase-3 is being activated to caspase-3 in the PAC-1 treated cells.
Cellular co-localization of 2h with sites of caspase-3 activity
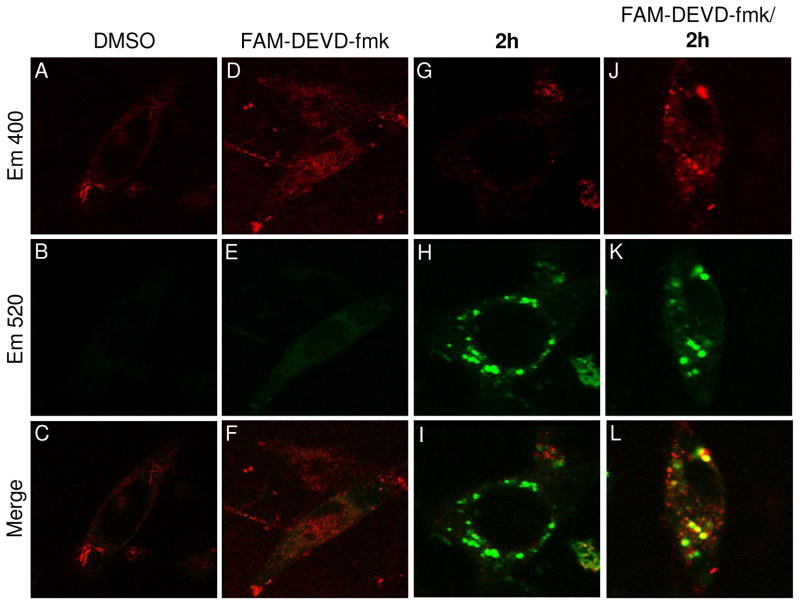
To determine if the spots of caspase-3/7 activity observed in the image in Figure 3 are due to direct induction of procaspase-3/-7 activition by PAC-1, we sought to determine if 2h will co-localize with caspase-3/-7 activity inside the cell. SK-MEL-5 cells were treated with DMSO or 2h and/or FAM-DEVD-fmk for 1 hour. Cells were then washed in media lacking phenol red and incubated for an additional 2 hours before confocal imaging. Visualization at 400 nm (pseudo colored green) and 520 nm (pseudo colored red) reports on the cellular localization of 2h and FAM-DEVD-fmk, respectively, and representative images are shown from the red channel, green channel, and a merge of the two (top, middle, bottom, respectively in Figure 4). Cells treated with vehicle alone showed minimal staining in the green and red channels (Figure 4A, B, C). In cells that are stained with the FAM-DEVD-fmk caspase-3/-7 inhibitor, there is very little additional staining observed over background (Figure 4D, E, F). Cells that were treated with 2h showed punctate cytoplasmic staining in the green channel with no bleed-through to the red channel, consistent with data in Figure 3 (Figure 4G, H, I). However, when cells are treated with both 2h and FAM-DEVD-fmk, staining corresponding to active caspase-3/-7 is observed in a similar staining pattern to the 2h staining (Figure 4J, K, L). In fact, the majority of this staining overlaps in the merged image (Figure 4L) – this overlap was quantified as 52% using Image J software over 12 cells (see Supporting Information Figure S7).50 Combined, the results suggests that there is low/no basal cellular caspase-3/-7 activity in these cells, but that procaspase-3/-7 becomes rapidly activated in the presence of 2h. In addition, the sites of intense cellular caspase-3/-7 enzymatic activity overlap considerably with the sub-cellular localization of AFC350-PAC-1, suggesting that AFC350-PAC-1 is responsible for procaspase-3/-7 activation at these sites.
Figure 4.
Colocalization of 2h with sites of caspase-3/-7 enzymatic activity. SK-MEL-5 cells treated with DMSO show background levels of staining in both the red and green channels (A–C). Cells treated only with FAM-DEVD-fmk do not show any staining above background levels (D–F). Cells treated only with 2h shows punctate staining in the cytoplasm with no increase in the emission at 400 nm (G–I). Cells treated concurrently with 2h and FAM-DEVD-fmk show spots of intense caspase-3/-7 activity (pseudo-colored red) and punctate staining of 2h (pseudo-colored green), which exhibit good co-localization in the merged image (J–L).
Induction of apoptosis by PAC-1 derivatives
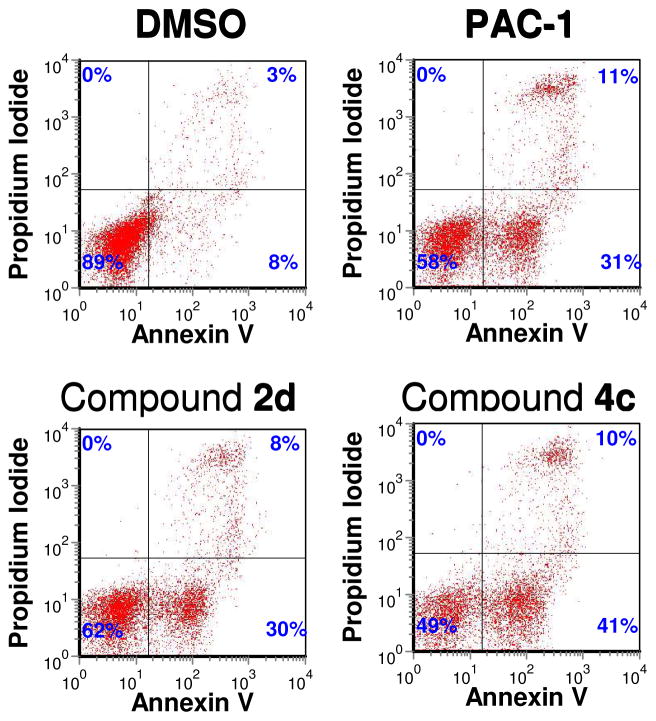
To assess if PAC-1 derivatives induce apoptotic death, the effect of two of the more potent compounds (2d and 4c) was evaluated versus U-937 cells. For these experiments, each compound (at 50 μM) was incubated with U-937 cells for 12 hours, at which point cells were co-stained with propidium iodide (PI) and FITC-labeled Annexin V. Cells that are negative for PI staining, but positive for Annexin V are considered to be in the early stages of apoptosis.51 Such dual staining experiments were previously used to show that PAC-1 induces apoptotic death in cancer cells in culture.30 The result of this PI/Annexin V experiment with the PAC-1 derivatives clearly show that each compound induces apoptotic death (Figure 5).
Figure 5.
Assessment of apoptotic induction by PAC-1 derivatives. U-937 cells were treated with PAC-1, 2d, and 4c (50 μM each) or vehicle control for 12 hours, then stained with propidium iodide (PI) and FITC-labeled Annexin V. All three compounds induce apoptosis as assessed by the Annexin V positive/PI negative populations. Results shown are representative of three replicate experiments with each compound.
DISCUSSION
PAC-1 is a novel experimental therapeutic that has shown efficacy in several mouse models of cancer.30 It was recently discovered that PAC-1 enhances the activity of procaspase-3, procaspase-7, caspase-3, and caspase-7 in vitro through the chelation of inhibitory zinc ions.33 To probe the relevance of this in vitro mechanism to the anti-cancer activity of PAC-1 in cell culture, we determined a full structure-activity relationship for PAC-1 with respect to its ability to induce death in cancer cells in culture, its ability to activate caspase-3 in vitro, and its ability to chelate zinc. We then used this information to construct a fluorescent derivative of PAC-1 that could be used to track sub-cellular localization. The information obtained through these experiments not only sheds light on the mechanism by which PAC-1 induces death in cancer cells, but also on the role of cellular zinc as an endogenous anti-apoptotic agent.
Evaluation of 26 PAC-1 derivatives suggests an intimate link between the metal chelating properties of the compounds and their ability to induce caspase-3 activation in vitro and to induce death in U-937 cells in culture. Significantly, any alteration to the ortho-hydroxy N-acylhydrazone motif results in compounds with no ability to bind zinc and low/no activity in the caspase-3 activation and cell death assays. This data is consistent with a large body of literature showing that ortho-hydroxy N-acylhydrazones are metal chelators.31, 52–58 Conversely, other sites on PAC-1 could be altered without losing activity in these three assays. Minimally, this data suggests that the metal chelating moiety of PAC-1 is critical to the anti-cancer properties of this compound.
Procaspase-3 is known to reside in the cytoplasm49, 59 where it remains in an inactive form until it is cleaved by upstream apoptotic caspases.18 Several studies have shown that procaspase-3 is capable of autoactivation in vitro,19, 33, 35 although the relevance of this mode of activation in vivo is unclear. Our hypothesis for the mode of action of PAC-1 induced cell death is that it relieves the zinc-mediated inhibition of procaspase-3, allowing procaspase-3 to use its enzymatic activity to cleave another molecule of procaspase-3, resulting in the active caspase-3.33 Once this occurs, the process accelerates – any caspase-3 generated can then cleave procaspase-3 (this is known to occur in vitro35) in a feed-forward cycle. We conducted a series of experiments designed to investigate if PAC-1 directly induces caspase-3 activity in the cell, and if PAC-1 does so through the direct activation of procaspase-3.
In an initial experiment, the population of active caspase-3 in PAC-1 treated cells was examined relative to vehicle treated controls, and relative to the population of cellular procaspase-3. As might be expected, we found that there is very little caspase-3 activity in actively growing SK-MEL-5 cells in culture (as assessed by the fluorescent caspase-3/-7 substrate). In contrast, the cytoplasm shows considerable procaspase-3 (as assessed with antibody staining). These results are shown in the left panels of Figure 3. Treatment of cells with PAC-1 for 1 hr induced strong localized spots of caspase-3/-7 activity (Figure 3, top right), indicating that PAC-1 causes the rapid elevation of caspase-3/-7 enzymatic activity. By employing a fluorescently-labeled version of PAC-1 (compound 2h) we were then able to determine that 2h co-localizes to the spots of intense caspase-3/-7 activity (Figure 4). This co-localization data is perhaps the strongest evidence to date supporting the notion that PAC-1 directly induces procaspase-3 activation inside the cancer cell.
Interestingly, our recent data show that PAC-1 will activate both procaspase-3 and caspase-3,33 thus another possibility for the PAC-1 mode of action would be that PAC-1 relieves the zinc-mediated inhibition of caspase-3. As the FAM-DEVD-fmk inhibitor only reports on active caspase-3, the experiments described in this manuscript do not directly rule out this possibility. However, this scenario seems less likely given that cancer cell procaspase-3:caspase-3 ratios are typically strongly weighted in favor of procaspase-3,60–62 although in some primary cancer cell types and cell lines there does appears to be significant levels of caspase-3.63–66
The data in this manuscript also suggest a prominent role for zinc as an endogenous anti-apoptotic agent, and there is much literature to support this notion.42, 67–69 It is estimated that as much as 10% of cellular zinc exists loosely bound to various cellular proteins or contained in the “zincosomes”. Perhaps most provocatively, zinc has been shown to co-localize with procaspase-3,70, 71 and we have shown that zinc inhibits procaspase-3 enzymatic activity in vitro.33
Thus, the weight of the available evidence suggests the following mechanism for the PAC-1 mediated induction of apoptosis in cancer cells: PAC-1 rapidly enters the cell where, with a dissociation constant of ~50 nM,33 it competes favorable for the loosely bound zinc pool. Enzymes whose activity was previously inhibited by zinc, such as procaspase-3, are now freed to carry out their catalytic function. Even with an enzymatic rate 200-fold less than caspase-3, procaspase-3 is active enough to catalyze the hydrolysis of other molecules of procaspase-3, thus activating them to caspase-3. Active caspase-3 can cleave its >100 cellular substrates to execute the apoptotic program, and it can also cleave more procaspase-3, further stimulating the feed-forward cycle.
This model for the cytotoxicity of PAC-1 is supported by several lines of evidence: Rapid caspase-3/-7 activity is seen in cells treated with PAC-1, both in whole cell experiments where caspase-3 activity is measured with chromogenic substrates,30 and in microscopy experiments where caspase-3 activity can be assessed with a fluorescent inhibitor (Figure 3). In addition, caspase-3 activity is observed before mitochondrial membrane depolarization in PAC-1 treated cells.30 Also, exogenous zinc inhibits the apoptosis-inducing effect of PAC-1,33 and zinc co-localizes with cellular procaspase-3.70, 71 Finally and perhaps most significantly, a fluorescent version of PAC-1 co-localizes with sites of cellular caspase-3 activity (Figure 4).
Relative to other zinc chelators, PAC-1 appears to have the proper combination of moderate affinity for zinc while still retaining the ability to rapidly penetrate cells – modest zinc affinity allowing for chelation of the loosely bound zinc pool, with sufficient hydrophilicity to promote cell permeability. It is unlikely that PAC-1 is special in this regard; other cell-permeable, moderate-affinity zinc chelators are likely to induce death through a similar mechanism as PAC-1. It is also unlikely that PAC-1 removes zinc only from procaspase-3. The full effect of chelating the loosely-bound cellular zinc pools remains to be uncovered; indeed, the results of PAC-1 may explain the mode of action of other known zinc-chelating anti-cancer agents.72, 73
In summary, the SAR we have established herein for PAC-1 paves the way for the development of even more potent versions of this compound. The design, synthesis, and evaluation of 2h has allowed us to gather further data supporting the hypothesis that PAC-1 directly activates cellular procaspase-3 through the chelation of inhibitory zinc ions. As PAC-1 is the first small molecule reported to directly activate procaspase-3, the data reported herein is critical for the continued understanding and advancement of procaspase-3 activation as an anti-cancer strategy. The development and in vivo testing of more potent versions of PAC-1 and further investigations into the anti-apoptotic role of cellular zinc are underway in our laboratories, and these results will be presented in due course.
Supplementary Material
Acknowledgments
We are grateful to the National Institutes of Health (R01-CA120439) and the Susan G. Komen Foundation for the Cure (BCTR0707325) for support of this work. Q.P.P. was partially supported by a Chemistry-Biology Interface Training Grant from the National Institutes of Health (Ruth L. Kirschstein National Research Service Award 1 T32 GM070421 from the NIGMS). We thank Joseph Sandhorst and Valerie Fako (UIUC) for preliminary studies toward the synthesis of PAC-1 derivatives.
ABBREVIATIONS
- PAC-1
procaspase-activating compound 1
- SAR
structure-activity relationship
- NHS
N-hydroxysuccinimide
- PI
propidium iodide
Footnotes
Supporting Information Available. Full experimental protocols and compound characterization data. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, Feuer EJ, Thun MJ. Cancer statistics. CA Cancer J Clin. 2005;55:10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- 2.Malhotra V, Perry MC. Classical chemotherapy: mechanisms, toxicities and the therapeutic window. Cancer Biol Ther. 2003;2:S2–4. [PubMed] [Google Scholar]
- 3.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 4.Igney FH, Krammer PH. Death and anti-death: tumor resistance to apoptosis. Nature Rev Cancer. 2002;2:277–288. doi: 10.1038/nrc776. [DOI] [PubMed] [Google Scholar]
- 5.Zornig M, Hueber AO, Baum W, Evan G. Apoptosis regulators and their role in tumorigenesis. Biochim Biophys Acta. 2001;1551:F1–F37. doi: 10.1016/s0304-419x(01)00031-2. [DOI] [PubMed] [Google Scholar]
- 6.Fesik SW. Promoting apoptosis as a strategy for cancer drug discovery. Nat Rev Cancer. 2005;5:876–885. doi: 10.1038/nrc1736. [DOI] [PubMed] [Google Scholar]
- 7.Bremer E, van Dam G, Kroesen BJ, de Leij L, Helfrich W. Targeted induction of apoptosis for cancer therapy: current progress and prospects. Trends Mol Med. 2006;12:382–393. doi: 10.1016/j.molmed.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 8.Green DR, Kroemer G. Pharmacological manipulation of cell death: clinical applications in sight? . J Clin Invest. 2005;115:2610–2617. doi: 10.1172/JCI26321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Denicourt C, Dowdy SF. Targeting apoptotic pathways in cancer cells. Science. 2004;305:1411–1413. doi: 10.1126/science.1102974. [DOI] [PubMed] [Google Scholar]
- 10.Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, Bruncko M, Deckwerth TL, Dinges J, Hajduk PJ, Joseph MK, Kitada S, Korsmeyer SJ, Kunzer AR, Letai A, Li C, Mitten MJ, Nettesheim DG, Ng S, Nimmer PM, O’Connor JM, Oleksijew A, Petros AM, Reed JC, Shen W, Tahir SK, Thompson CB, Tomaselli KJ, Wang B, Wendt MD, Zhang H, Fesik SW, Rosenberg SH. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 11.Vassilev LT, Vu BT, Graves B, Carvajal D, Podlaski F, Filipovic Z, Kong N, Kammlott U, Lukacs C, Klein C, Fotouhi N, Liu EA. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science. 2004;303:844–848. doi: 10.1126/science.1092472. [DOI] [PubMed] [Google Scholar]
- 12.Tovar C, Rosinski J, Filipovic Z, Higgins B, Kolinsky K, Hilton H, Zhao X, Vu BT, Qing W, Packman K, Myklebost O, Heimbrook DC, Vassilev LT. Small-molecule MDM2 antagonists reveal aberrant p53 signaling in cancer: Implications for therapy. Proc Natl Acad Sci U S A. 2006;103:1888–1893. doi: 10.1073/pnas.0507493103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li L, Thomas RM, Suzuki H, De Brabander JK, Wang X, Harran PG. A small molecule Smac mimic potentiates TRAIL- and TNFalpha-mediated cell death. Science. 2004;305:1471–1474. doi: 10.1126/science.1098231. [DOI] [PubMed] [Google Scholar]
- 14.Sun H, Nikolovska-Coleska Z, Yang CY, Qian D, Lu J, Qiu S, Bai L, Peng Y, Cai Q, Wang S. Design of small-molecule peptidic and nonpeptidic Smac mimetics. Acc Chem Res. 2008;41:1264–1277. doi: 10.1021/ar8000553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakahara T, Takeuchi M, Kinoyama I, Minematsu T, Shirasuna K, Matsuhisa A, Kita A, Tominaga F, Yamanaka K, Kudoh M, Sasamata M. YM155, a novel small-molecule survivin suppressant, induces regression of established human hormone-refractory prostate tumor xenografts. Cancer Res. 2007;67:8014–8021. doi: 10.1158/0008-5472.CAN-07-1343. [DOI] [PubMed] [Google Scholar]
- 16.Satoh T, Okamoto I, Miyazaki M, Morinaga R, Tsuya A, Hasegawa Y, Terashima M, Ueda S, Fukuoka M, Ariyoshi Y, Saito T, Masuda N, Watanabe H, Taguchi T, Kakihara T, Aoyama Y, Hashimoto Y, Nakagawa K. Phase I study of YM155, a novel survivin suppressant, in patients with advanced solid tumors. Clin Cancer Res. 2009;15:3872–3880. doi: 10.1158/1078-0432.CCR-08-1946. [DOI] [PubMed] [Google Scholar]
- 17.Boatright KM, Salvesen GS. Mechanisms of caspase activation. Curr Opin Cell Biol. 2003;15:725–731. doi: 10.1016/j.ceb.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 18.Earnshaw WC, Martins LM, Kaufmann SH. Mammalian caspases: structure, activation, substrates, and functions during apoptosis. Annu Rev Biochem. 1999;68:383–424. doi: 10.1146/annurev.biochem.68.1.383. [DOI] [PubMed] [Google Scholar]
- 19.Feeney B, Pop C, Swartz P, Mattos C, Clark AC. Role of loop bundle hydrogen bonds in the maturation and activity of (Pro)caspase-3. Biochemistry. 2006;45:13249–63. doi: 10.1021/bi0611964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pop C, Feeney B, Tripathy A, Clark AC. Mutations in the procaspase-3 dimer interface affect the activity of the zymogen. Biochemistry. 2003;42:12311–12320. doi: 10.1021/bi034999p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Acehan D, Jiang X, Morgan DG, Heuser JE, Wang X, Akey CW. Three-dimensional structure of the apoptosome: implications for assembly, procaspase-9 binding, and activation. Mol Cell. 2002;9:423–432. doi: 10.1016/s1097-2765(02)00442-2. [DOI] [PubMed] [Google Scholar]
- 22.Chao Y, Shiozaki EN, Srinivasula SM, Rigotti DJ, Fairman R, Shi Y. Engineering a dimeric caspase-9: a re-evaluation of the induced proximity model for caspase activation. PLoS Biol. 2005;3:e183. doi: 10.1371/journal.pbio.0030183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keller N, Mares J, Zerbe O, Grutter MG. Structural and biochemical studies on procaspase-8: new insights on initiator caspase activation. Structure. 2009;17:438–448. doi: 10.1016/j.str.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 24.Stennicke HR, Jurgensmeier JM, Shin H, Deveraux Q, Wolf BB, Yang X, Zhou Q, Ellerby HM, Ellerby LM, Bredesen D, Green DR, Reed JC, Froelich CJ, Salvesen GS. Pro-caspase-3 is a major physiologic target of caspase-8. J Biol Chem. 1998;273:27084–27090. doi: 10.1074/jbc.273.42.27084. [DOI] [PubMed] [Google Scholar]
- 25.Yin Q, Park HH, Chung JY, Lin SC, Lo YC, da Graca LS, Jiang X, Wu H. Caspase-9 holoenzyme is a specific and optimal procaspase-3 processing machine. Mol Cell. 2006;22:259–268. doi: 10.1016/j.molcel.2006.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luthi AU, Martin SJ. The CASBAH: a searchable database of caspase substrates. Cell Death Differ. 2007;14:641–650. doi: 10.1038/sj.cdd.4402103. [DOI] [PubMed] [Google Scholar]
- 27.Timmer JC, Salvesen GS. Caspase substrates. Cell Death Differ. 2007;14:66–72. doi: 10.1038/sj.cdd.4402059. [DOI] [PubMed] [Google Scholar]
- 28.Walsh JG, Cullen SP, Sheridan C, Luthi AU, Gerner C, Martin SJ. Executioner caspase-3 and caspase-7 are functionally distinct proteases. Proc Natl Acad Sci U S A. 2008;105:12815–12819. doi: 10.1073/pnas.0707715105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Slee EA, Adrain C, Martin SJ. Executioner caspase-3, -6, and -7 perform distinct, non-redundant roles during the demolition phase of apoptosis. J Biol Chem. 2001;276:7320–7326. doi: 10.1074/jbc.M008363200. [DOI] [PubMed] [Google Scholar]
- 30.Putt KS, Chen GW, Pearson JM, Sandhorst JS, Hoagland MS, Kwon JT, Hwang SK, Jin H, Churchwell MI, Cho MH, Doerge DR, Helferich WG, Hergenrother PJ. Small molecule activation of procaspase-3 to caspase-3 as a personalized anticancer strategy. Nature Chem Biol. 2006;2:543–550. doi: 10.1038/nchembio814. [DOI] [PubMed] [Google Scholar]
- 31.Charkoudian LK, Pham DM, Franz KJ. A pro-chelator triggered by hydrogen peroxide inhibits iron-promoted hydroxyl radical formation. J Am Chem Soc. 2006;128:12424–12425. doi: 10.1021/ja064806w. [DOI] [PubMed] [Google Scholar]
- 32.Stennicke HR, Salvesen GS. Biochemical characteristics of caspases-3, -6, -7, and -8. J Biol Chem. 1997;272:25719–25723. doi: 10.1074/jbc.272.41.25719. [DOI] [PubMed] [Google Scholar]
- 33.Peterson QP, Goode DR, West DC, Ramsey KN, Lee J, Hergenrother PJ. PAC-1 Activates Procaspase-3 in Vitro through Relief of Zinc-Mediated Inhibition. J Mol Biol. 2009:144–158. doi: 10.1016/j.jmb.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vichai V, Kirtikara K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat Protoc. 2006;1:1112–1116. doi: 10.1038/nprot.2006.179. [DOI] [PubMed] [Google Scholar]
- 35.Bose K, Pop C, Feeney B, Clark AC. An uncleavable procaspase-3 mutant has a lower catalytic efficiency but an active site similar to that of mature caspase-3. Biochemistry. 2003;42:12298–12310. doi: 10.1021/bi034998x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang S, Clark RJ, Zhu L. Highly sensitive fluorescent probes for zinc ion based on triazolyl-containing tetradentate coordination motifs. Org Lett. 2007;9:4999–5002. doi: 10.1021/ol702208y. [DOI] [PubMed] [Google Scholar]
- 37.Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. A stepwise Huisen cycloaddition process: copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew Chem Int Ed. 2002;41:2596–2599. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 38.Haase H, Beyersmann D. Intracellular zinc distribution and transport in C6 rat glioma cells. Biochem Biophys Res Commun. 2002;296:923–928. doi: 10.1016/s0006-291x(02)02017-x. [DOI] [PubMed] [Google Scholar]
- 39.Fahrni CJ, O’Halloran TV. Aqueous coordination chemistry of quinoline-based fluorescence probes for the biological chemistry of zinc. J Am Chem Soc. 1999;121:11448–11458. [Google Scholar]
- 40.Truong-Tran AQ, Ruffin RE, Zalewski PD. Visualization of labile zinc and its role in apoptosis of primary airway epithelial cells and cell lines. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1172–L1183. doi: 10.1152/ajplung.2000.279.6.L1172. [DOI] [PubMed] [Google Scholar]
- 41.Zalewski PD, Forbes IJ, Betts WH. Correlation of apoptosis with change in intracellular labile Zn(II) using zinquin [(2-methyl-8-p-toluenesulphonamido-6-quinolyloxy)acetic acid], a new specific fluorescent probe for Zn(II) Biochem J. 1993;296 (Pt 2):403–408. doi: 10.1042/bj2960403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beyersmann D, Haase H. Functions of zinc in signaling, proliferation and differentiation of mammalian cells. BioMetals. 2001;14:331–341. doi: 10.1023/a:1012905406548. [DOI] [PubMed] [Google Scholar]
- 43.Devirgiliis C, Murgia C, Danscher G, Perozzi G. Exchangeable zinc ions transiently accumulate in a vesicular compartment in the yeast Saccharomyces cerevisiae. Biochem Biophys Res Commun. 2004;323:58–64. doi: 10.1016/j.bbrc.2004.08.051. [DOI] [PubMed] [Google Scholar]
- 44.Wellenreuther G, Cianci M, Tucoulou R, Meyer-Klaucke W, Haase H. The ligand environment of zinc stored in vesicles. Biochem Biophys Res Commun. 2009;380:198–203. doi: 10.1016/j.bbrc.2009.01.074. [DOI] [PubMed] [Google Scholar]
- 45.Wong PF, Abubakar S. High intracellular Zn2+ ions modulate the VHR, ZAP-70 and ERK activities of LNCaP prostate cancer cells. Cell Mol Biol Lett. 2008;13:375–390. doi: 10.2478/s11658-008-0009-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bedner E, Smolewski P, Amstad P, Darzynkiewicz Z. Activation of caspases measured in situ by binding of fluorochrome-labeled inhibitors of caspases (FLICA): correlation with DNA fragmentation. Exp Cell Res. 2000;259:308–313. doi: 10.1006/excr.2000.4955. [DOI] [PubMed] [Google Scholar]
- 47.Smolewski P, Bedner E, Du L, Hsieh TC, Wu JM, Phelps DJ, Darzynkiewicz Z. Detection of caspases activation by fluorochrome-labeled inhibitors: Multiparameter analysis by laser scanning cytometry. Cytometry. 2001;44:73–82. doi: 10.1002/1097-0320(20010501)44:1<73::aid-cyto1084>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 48.Smolewski P, Grabarek J, Phelps DJ, Darzynkiewicz Z. Stathmo-apoptosis: arresting apoptosis by fluorochrome-labeled inhibitor of caspases. Int J Oncol. 2001;19:657–663. doi: 10.3892/ijo.19.4.657. [DOI] [PubMed] [Google Scholar]
- 49.Kamada S, Kikkawa U, Tsujimoto Y, Hunter T. Nuclear translocation of caspase-3 is dependent on its proteolytic activation and recognition of a substrate-like protein(s) J Biol Chem. 2005;280:857–860. doi: 10.1074/jbc.C400538200. [DOI] [PubMed] [Google Scholar]
- 50.Bolte S, Cordelieres FP. A guided tour into subcellular colocalization analysis in light microscopy. J Microsc. 2006;224:213–232. doi: 10.1111/j.1365-2818.2006.01706.x. [DOI] [PubMed] [Google Scholar]
- 51.van Engeland M, Nieland LJ, Ramaekers FC, Schutte B, Reutelingsperger CP. Annexin V-affinity assay: a review on an apoptosis detection system based on phosphatidylserine exposure. Cytometry. 1998;31:1–9. doi: 10.1002/(sici)1097-0320(19980101)31:1<1::aid-cyto1>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 52.Buss JL, Neuzil J, Gellert N, Weber C, Ponka P. Pyridoxal isonicotinoyl hydrazone analogs induce apoptosis in hematopoietic cells due to their iron-chelating properties. Biochem Pharmacol. 2003;65:161–172. doi: 10.1016/s0006-2952(02)01512-5. [DOI] [PubMed] [Google Scholar]
- 53.Sivaramaiah S, Reddy PR. Direct and Derivative Spectrophotometric Determination of Zinc with 2,4-Dihydroxybenzaldehyde Isonicotinoyl Hydrazone in Potable Water and Pharmaceutical Samples. J Anal Chem. 2005;60:828–832. [Google Scholar]
- 54.Hershko C, Abrahamov A, Konijn AM, Breuer W, Cabantchik IZ, Pootrakul P, Link G. Objectives and methods of iron chelation therapy. Bioinorg Chem Appl. 2003:151–168. doi: 10.1155/S1565363303000128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gulea A, Poirier D, Roy J, Stavila V, Bulimestru I, Tapcov V, Birca M, Popovschi L. In vitro antileukemia, antibacterial and antifungal activities of some 3d metal complexes: chemical synthesis and structure - activity relationships. J Enzyme Inhib Med Chem. 2008;23:806–818. doi: 10.1080/14756360701743002. [DOI] [PubMed] [Google Scholar]
- 56.Abou-Melha KS. Transition metal complexes of isonicotinic acid (2-hydroxybenzylidene)hydrazide. Spectrochim Acta A Mol Biomol Spectrosc. 2008;70:162–170. doi: 10.1016/j.saa.2007.07.023. [DOI] [PubMed] [Google Scholar]
- 57.Koh LL, Kon OL, Loh KW, Long YC, Ranford JD, Tan AL, Tjan YY. Complexes of salicylaldehyde acylhydrazones: cytotoxicity, QSAR and crystal structure of the sterically hindered t-butyl dimer. J Inorg Biochem. 1998;72:155–162. doi: 10.1016/s0162-0134(98)10075-2. [DOI] [PubMed] [Google Scholar]
- 58.Borkow G, Fletcher RS, Barnard J, Arion D, Motakis D, Dmitrienko GI, Parniak MA. Inhibition of the ribonuclease H and DNA polymerase activities of HIV-1 reverse transcriptase by N-(4-tert-butylbenzoyl)-2-hydroxy-1-naphthaldehyde hydrazone. Biochemistry. 1997;36:3179–3185. doi: 10.1021/bi9624696. [DOI] [PubMed] [Google Scholar]
- 59.Shikama YUM, Miyashita T, Yamada M. Comprehensive studies on subcellular localizations and cell death-inducing activities of eight GFP-tagged apoptosis-related caspases. Exp Cell Res. 2001;264:315–325. doi: 10.1006/excr.2000.5153. [DOI] [PubMed] [Google Scholar]
- 60.Roy S, Bayly CI, Gareau Y, Houtzager VM, Kargman S, Keen SLC, Rowland K, Seiden IM, Thornberry NA, Nicholoson DW. Maintenance of caspase-3 proenzyme dormancy by an intrinsic “safety catch” regulatory tripeptide. Proc Natl Acad Sci US A. 2001;98:6132–6137. doi: 10.1073/pnas.111085198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Krepela E, Prochazka J, Liul X, Fiala P, Kinkor Z. Increased expression of Apaf-1 and procaspase-3 and the functionality of intrinsic apoptosis apparatus in non-small cell lung carcinoma. Biol Chem. 2004;385:153–168. doi: 10.1515/BC.2004.034. [DOI] [PubMed] [Google Scholar]
- 62.Kania J, Konturek SJ, Marlicz K, Hahn EG, Konturek PC. Expression of survivin and caspase-3 in gastric cancer. Dig Dis Sci. 2003;48:266–271. doi: 10.1023/a:1021915124064. [DOI] [PubMed] [Google Scholar]
- 63.Grigoriev MY, Pozharissky KM, Hanson KP, Imyanitov EN, Zhivotovsky B. Expression of caspase-3 and -7 does not correlate with the extent of apoptosis in primary breast carcinomas. Cell Cycle. 2002;1:337–342. [PubMed] [Google Scholar]
- 64.O’Donovan N, Crown J, Stunell H, Hill AD, McDermott E, O’Higgins N, Duffy MJ. Caspase 3 in breast cancer. Clin Cancer Res. 2003;9:738–742. [PubMed] [Google Scholar]
- 65.Yang S, Liu J, Thor AD, Yang X. Caspase expression profile and functional activity in a panel of breast cancer cell lines. Oncol Rep. 2007;17:1229–1235. [PubMed] [Google Scholar]
- 66.Persad R, Liu C, Wu TT, Houlihan PS, Hamilton SR, Diehl AM, Rashid A. Overexpression of caspase-3 in hepatocellular carcinomas. Mod Pathol. 2004;17:861–867. doi: 10.1038/modpathol.3800146. [DOI] [PubMed] [Google Scholar]
- 67.Aiuchi T, Mihara S, Nakaya M, Masuda Y, Nakajo S, Nakaya K. Zinc ions prevent processing of caspase-3 during apoptosis induced by geranylgeraniol in HL-60 cells. J Biochem (Tokyo) 1998;124:300–303. doi: 10.1093/oxfordjournals.jbchem.a022111. [DOI] [PubMed] [Google Scholar]
- 68.Chai F, Truong-Tran AQ, Ho LH, Zalewski PD. Regulation of caspase activation and apoptosis by cellular zinc fluxes and zinc deprivation: A review. Immunol Cell Biol. 1999;77:272–278. doi: 10.1046/j.1440-1711.1999.00825.x. [DOI] [PubMed] [Google Scholar]
- 69.Chimienti F, Seve M, Richard S, Mathieu J, Favier A. Role of cellular zinc in programmed cell death: temporal relationship between zinc depletion, activation of caspases, and cleavage of Sp family transcription factors. Biochem Pharmacol. 2001;62:51–62. doi: 10.1016/s0006-2952(01)00624-4. [DOI] [PubMed] [Google Scholar]
- 70.Truong-Tran AQ, Grosser D, Ruffin RE, Murgia C, Zalewski PD. Apoptosis in the normal and inflamed airway epithelium: role of zinc in epithelial protection and procaspase-3 regulation. Biochem Pharmacol. 2003;66:1459–1468. doi: 10.1016/s0006-2952(03)00498-2. [DOI] [PubMed] [Google Scholar]
- 71.Carter JE, Truong-Tran AQ, Grosser D, Ho L, Ruffin RE, Zalewski PD. Involvement of redox events in caspase activation in zinc-depleted airway epithelial cells. Biochem Biophys Res Commun. 2002;297:1062–1070. doi: 10.1016/s0006-291x(02)02292-1. [DOI] [PubMed] [Google Scholar]
- 72.Adler M, Shafer H, Hamilton T, Petrali JP. Cytotoxic actions of the heavy metal chelator TPEN on NG108–15 neuroblastoma-glioma cells. Neurotoxicology. 1999;20:571–582. [PubMed] [Google Scholar]
- 73.Zhao R, Planalp RP, Ma R, Greene BT, Jones BT, Brechbiel MW, Torti FM, Torti SV. Role of zinc and iron chelation in apoptosis mediated by tachpyridine, an anti-cancer iron chelator. Biochem Pharmacol. 2004;67:1677–1688. doi: 10.1016/j.bcp.2003.12.036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.