Abstract
Background
The growing numbers of survivors of innovative cancer treatments such as hematopoietic stem cell transplantation (HSCT) often report subsequent cognitive difficulties. The purpose of this study is to evaluate and compare neurocognitive changes in patients with chronic myelogenous leukemia (CML) or primary myelodysplastic syndrome (MDS) after allogeneic HSCT or other therapies.
Methods
Prospective cohort study employing serial evaluations of attention, concentration, memory, mood and quality of life in a consecutive sample of 106 eligible patients with CML (n=91) or MDS (n=15) at enrollment, and then 12 and 18 months after HSCT or other therapy.
Results
The three evaluations were completed by 98%, 95%, and 89% of surviving participants, respectively. Among all patients, there was significant improvement in memory over 18 months. For example, the 45 people receiving HSCT (42 with CML, 3 with MDS) compared favorably to those who had other treatment on most measures of neuropsychological function, except they had improved mental health (p=.034), worse physical function (p=.049), and more difficulty with coordination and fine motor speed bilaterally (dominant, p=.005, and non-dominant hands, p=.0019). CML patients overall had improved phonemic fluency (p=.014).
Conclusions
Time and diagnosis may be important factors when assessing neurocognitive and other changes. Complaints about “chemobrain” following HSCT merit further study, as deficits may actually pre-date initiation of treatment and then subsequently improve. Study results could reassure prospective HSCT recipients since it compares favorably to other treatments when mental status side effects are considered.
Keywords: Chemobrain, HSCT, Chronic Myelogenous Leukemia, Quality of life, Memory, Myelodysplastic Syndrome
Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) is an established treatment of hematologic malignancies and other immunohematopoietic disorders.1, 2 Most studies of long-term adjustment after HSCT demonstrate that recipients have good- to excellent quality of life despite deficits in the physical, psychological, social, and cognitive functioning domains. 3, 4, 5
Elucidation of the memory and attention concerns expressed by HSCT survivors is an area of active inquiry. Some investigators have described cognitive impairment in patients evaluated either before or after the procedure only.6, 7 Others, using baseline and follow-up assessment, have generally shown impairment prior to transplant, decline in some areas shortly after transplant, and improvement without complete return to baseline levels of cognitive function 12 months after HSCT.
The effect of allogeneic HSCT on cognition has also been difficult to judge as previous large studies have included subjects with a mix of diagnoses or with a mix of treatments that may differentially affect the central nervous system. A large number of those tested before transplant may not have been participants after transplant. Two recent studies with initial sample sizes greater than 100 are illustrative. For example, one study randomized 476 patients to one of three testing schedules, with only one group tested prior to HSCT, and then 6 and 12 months later; the other 2 groups were tested only after HSCT. The participants in this study received either autologous (79%) or allogeneic (21%) transplants and had a wide variety of diseases including solid malignancy (63% multiple myeloma, 10% non-Hodgkin lymphoma, 7% acute myelogenous leukemia, 6% breast cancer, 3% Hodgkin lymphoma, and 11% other). While these researchers found improvement in cognitive function over 12 months, there was 70% attrition in the group with pre-HSCT evaluations. 8 Another study that had an initial sample size of 142 adult recipients of allogeneic HSCT included patients with total body irradiation (63%) and not; prior cranial irradiation or intrathecal chemotherapy (12%); and also pooled results for patients with a variety of hematologic malignancies (42% chronic phase CML, 8% accelerated CML, 14% AML in remission, 7% AML new or relapsed, 13% myelodysplasias, 11% lymphoma, 4% multiple myeloma, 2% chronic lymphocytic leukemia). 54, or 38% of the 142 participants, were tested at all time points.9 The majority of the other published studies that have sought to complete serial evaluation of cognitive changes have had smaller initial sample sizes (25 to 71), with follow-up evaluations at time points ranging from 1-3 days after HSCT to up to one year later.10, 11, 12, 13, 14, 15, 16
Thus, more remains to be learned about cognitive changes after allogeneic HSCT. Data about allogeneic HSCT are confounded by samples that include autologous recipients, patients with breast cancer, or patients with prior cranial radiation, or intrathecal treatment. Autologous HSCT recipients may have fewer central nervous system complications than recipients with allogeneic HSCT.17 Neuropsychological consequences of breast cancer patients differ from those with hematologic disorders.14 Prior cranial radiation or intrathecal treatment may exert an adverse impact on subsequent cognitive performance thus obscuring the true consequences of HSCT. A high attrition rate in participants tested before and after HSCT may not be random; for example, perhaps only those with better function return to studies.
Patients are now more concerned about the threat of cancer treatments to cognition. They may have heard about “chemobrain,” which has been characterized as cognitive slowing and difficulties with concentration, memory, and multitasking. While some previous neurocognitive findings related to HSCT were compared to normative data, most did not compare results to other treatment options. Comparison of cognitive changes in patients undergoing allogeneic HSCT to patients receiving other treatment options is timely and can address the fears that may influence treatment decisions. 18
The purpose of this prospective cohort study was to evaluate and compare the neurocognitive changes in people with either chronic myelogenous leukemia (CML) or primary myelodysplastic syndrome (MDS) as they underwent treatment for their illness. The two diseases were chosen since neither typically involves the central nervous system. Their treatments are similar, with allogeneic HSCT as a curative possibility.19, 20 Participants were asked to complete three evaluations over the course of the study so that the effects of time, treatment (allogeneic HSCT or other), and disease (CML or MDS) on neurocognitive function could be measured. Participants were evaluated prior to HSCT, and then 12 and 18 months later to extend our knowledge about the longer-term effects. It was hypothesized that individuals receiving allogeneic HSCT would experience more neuropsychological changes than those getting other treatments, but the duration and extent of such changes would be revealed over the course of the project.
Patients and Methods
A prospective cohort study of 108 individuals with chronic myelogenous leukemia or primary myelodysplastic syndrome was planned. Sample size was based on an estimated enrollment of 36 subjects a year for three years with an 1:1 ratio of HSCT to non-transplant patients, and an estimated attrition rate of 20%. Eligibility criteria included agreement to participate in 3 neuropsychological evaluations over the course of 18 months, reading and listening comprehension of English, and diagnosis within the past year or a new treatment plan that included HSCT within the next year. Exclusion criteria included history of significant head injury (resulting in loss of consciousness), stroke, epilepsy, or other central nervous system pathology requiring radiation, surgery, or past/ current intrathecal medication, and current alcohol or substance abuse or dependence, all factors which could influence performance on neuropsychological tests.
Participants were recruited from the Dana Farber /Brigham and Women's Cancer Care in Boston, MA (84.3%), with others coming from the Massachusetts General Hospital and other practices (6.5%). The remainder responded to web-based and other study advertisements (9.2%). All were to receive either allogeneic transplantation or other treatment as directed by their physicians.
Participants completed a baseline assessment interview, which consisted of: 1) a patient profile to include education, usual occupation, past medical and psychiatric history, and medication history. Usual occupation was coded according to the 1989 General Social Survey (GSS) prestige scales; 21 2) the Shipley Institute of Living Scale to estimate a full-scale intelligence quotient (IQ); 22, 23 3) Medical Outcomes Study 36 Item Short Form (SF-36) to evaluate the physical and mental health of the participant;26 and 4) the Brief Profile of Mood States (POMS), where participants were asked to rate how each adjective from a list of 11 that described how they felt in the past week with each item scored from zero (not at all) to four (extremely), for a total ranging from 0 to 44. 24 As an estimate of “confusion” or “cognitive difficulties,” two items (bewildered and muddled) from the POMS were highlighted with a score ranging from zero (none) to eight (most). To confirm current alcohol and drug diagnoses, participants completed the Alcohol and Drug Modules from the Structured Clinical Interview for DSM-IV.25
Participants were also administered a battery of neuropsychological tests. Test selection was based on previous research on patients with hematological malignancies, and incorporated measures of attention, executive function, memory, and motor speed. 26, 27 Attention and concentration were evaluated with the Trail Making Test and the Verbal Fluency Test.28, 29 The Trail Making Test has two parts, A and B, and measures visuomotor speed, attention, and mental flexibility. Part A involves connecting lines from 1 to 25 that are randomly printed on a sheet of paper. It primarily assesses attention and motor speed. Part B incorporates letters and numbers, and the subject alternates between numbers and letters, in order. The Verbal Fluency Test measures activation retrieval that is phonemic (letters) and semantic (animals). It is a measure of rapid word retrieval under restricted search conditions. Individuals are asked to generate as many words as they can in one-minute trials. Anterograde learning and memory were assessed with the six-trial Buschke Selective Reminding Test. A list of 12 words is read to the subject who is instructed to remember as many words as possible. On subsequent trials of recollection, the subject is selectively reminded of omitted words. Two measures from the Buschke are emphasized in this study: total recall and long-term storage (LTS).30 The Grooved Pegboard was used to test coordinated and fine motor speed by asking participants to place pegs in a board with their dominant and non-dominant hands as quickly as possible.31
Participants completed repeated assessments 12 and 18 months after the initial one. Each repeat assessment included the SF-36, Brief POMS, and the Grooved Pegboard Test. In order to mitigate possible learning effects on the neuropsychological testing, subjects were given randomly alternate forms of the Verbal Fluency Test, Trail Making Test, and Buschke Selective Reminding Test. The reliability and validity of these tests are well documented.39, 32, 33
Neuropsychological tests were scored according to established procedures.34, 35, 36, 37 Raw scores were converted to Z scores from age, sex, and /or education corrected normative data. A Z score between −1.0 and +1.0 is between the 16th and 84th percentile for the general population. The SF-36 items and scales were scored as required and where a higher score indicates a better state of health.38 Both the Physical Component Summary (PCS) and Mental Component Summary (MCS) have a mean of 50 and a standard deviation of 10.
Participants gave written informed consent. They received an honorarium of $50 for each assessment. This study was reviewed and approved by the Partners Institutional Review Board, which is responsible for the review and approval of all human subject research conducted by the staff of Brigham and Women's Hospital and other Partners affiliated hospitals.
Data Analysis
Data were analyzed using the SAS statistical package (version 9.1, SAS Institute, Cary, NC). Descriptive results are reported as counts with percentages or means with standard deviations. Fisher exact tests or chi-square test of significance were used to compare categorical baseline participant demographic and clinical characteristics as appropriate between disease and treatment subgroups. T-tests were used to compare age, occupational prestige, and estimated total IQ.
The primary purposes of this study were to quantify neuropsychological and mental status at the start of therapy and again after 12 and 18 months, and to determine whether there were any significant changes over time. Our analyses were based on the 77 participants who had completed at least two evaluations and we used a longitudinal repeated measures linear regression to look for improvement or worsening over time. The SAS Mixed procedure was used to adjust for correlation between serial measurements on patients, and an unstructured correlation matrix was used to allow the correlation to diminish with time. One of our primary concerns, however, was the possibility that the course of neurocognitive changes might depend on the type of disease (CML or MDS) or treatment regimen (HSCT or other treatment). Therefore, for each outcome variable we ran two preliminary regression models. The first model included a time by disease interaction in order to check whether neurocognitive changes were different in CML patients than in MDS patients. The second model included a time by treatment interaction in order to check whether neurocognitive changes were different in HSCT treated patients than in patients receiving other treatments. The two interaction terms were not included in the same preliminary model to avoid collinearity. If either (or both) interaction term was found to be statistically significant, it was then included in a final model which had our primary predictor, time (as a categorical variable), as well as covariates for disease type and treatment regimen. We emphasize though, that our purpose was not to compare outcomes between patient subgroups, or to compare outcomes between treatments in a non-randomized setting. We simply wanted to insure that if the time course were different in a particular subgroup of patients, that we would be able to show the results separately for each relevant subgroup.
Results
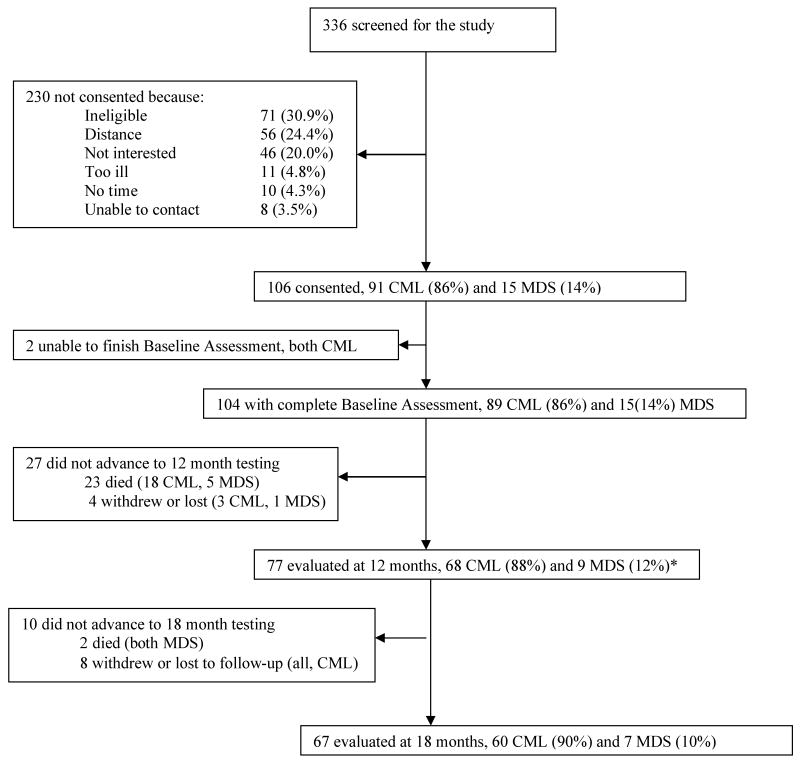
Of the 336 individuals who were screened for study enrollment, 106 (31.5%) were eligible, gave informed consent, and are described in Table 1. 104 of the 106 (98%) completed the baseline assessment; two with CML were unable to finish and were excluded. The baseline assessment was completed at a median of 5.6 months after the participants' diagnosis date; or 6.1 months for the 91 people diagnosed with CML and 3.8 months for the 15 diagnosed with MDS. With respect to HSCT, the baseline assessment was completed an average of 67 days (SD=106) before the procedure. 77 participants completed the second assessment, which occurred an average of 400 (SD=77) days after the baseline assessment. Most loss at this time was due to death (23 of 104 cohort participants died) leading to a follow-up rate of 95% of survivors (or 73% of those who gave initial consent). These 77 subjects with at least 2 serial measures are included in all of our analyses of longitudinal change. Between 12 and 18 months, 2 participants died and 8 were lost to follow-up. 67 of the 75 (89%) surviving participants completed the final, 18-month evaluation (average of 185 days (SD=59) after the 12 month evaluation). The 67 participants represent 63% of those who gave initial consent. The study began in January 2002 and ended in January 2007. Figure 1 summarizes the overall study flow.
Table 1. Subject Characteristics at Enrollment.
| Variable | By Disease CML, n=91 | MDS, n=15 | p-value | By Treatment1 HSCT, n=45 | Other Tx, n=58 | p-value |
|---|---|---|---|---|---|---|
| Mean age years (SD) | 45.2 (11.9) | 64.8 (9.3) | <0.0001 | 40.6 (10.6) | 53.5 (12.9) | p<0.0001 |
| Sex | ||||||
| male (%) | 47 (52) | 11(73) | 0.12 | 21 (47) | 35 (60) | 0.17 |
| female (%) | 44 (48) | 4 (27) | 24 (53) | 23 (40) | ||
| Racial/Ethnic Background | ||||||
| Caucasian/white (%) | 78 (86) | 15 (100) | 0.29 | 38 (84) | 52 (90) | 0.08 |
| African-Am/black (%) | 7 (8) | 0 | 5 (11) | 2 (3) | ||
| Other (%) | 6 (6) | 0 | 2 (5) | 4 (7) | ||
| Marital Status | ||||||
| Married (%) | 53 (58) | 12 (80) | 0.08 | 26 (58) | 38 (65) | 0.14 |
| Other (%) | 38 (42) | 3 (20) | 19 (42) | 20 (35) | ||
| Education | ||||||
| High School (%) | 23 (25) | 3 (20) | 0.11 | 11 (24) | 15 (25) | 0.25 |
| Partial College (%) | 26 (28.6) | 2 (13) | 16 (36) | 11 (20) | ||
| 4 year College (%) | 27 (29.7) | 3 (20) | 11 (24) | 17(30) | ||
| Graduate School (%) | 15 (16) | 6 (40) | 7 (16) | 15 (25) | ||
| Occupational Prestige | ||||||
| Mean (SD) | 48.8 (20.7) | 44.8 (25.0) | 0.64 | 44.3 (24.5) | 50.5(18.9) | 0.26 |
| Total Estimated IQ (SD) | 106 (10) | 113 (12) | 0.06 | 105 (10) | 108 (11) | 0.34 |
| HSCT (%) | 43 (47) | 3 (20) | 0.053 | 45 (100) | 0 | N/A |
| CML (%) | 91 (100) | 0 | N/A | 42 (93) | 46 (80) | 0.053 |
3 participants did not receive treatment
Figure 1. Study Flow Chart.
* The study analysis is based on the 77 people who had at least 2 evaluations
The individuals with Chronic Myelogenous Leukemia (CML, n=91) and Myelodysplastic Syndrome (MDS, n=15) were similar in terms of sex, racial and ethnic background, education, occupational prestige (e.g., 48.72=management related occupation, 44.47=actuary), and total estimated IQ at enrollment. People with MDS were significantly older (p < .0001) but this age difference was accounted for in the analyses by the use of z-scores that are corrected for age, gender, and education. Recipients of allogeneic HSCT were significantly younger (mean = 40.6 (SD=10.6) years versus 53.5 (SD=12.9) years, p< .0001). None of the participants satisfied diagnostic criteria for current alcohol or drug abuse or dependence diagnoses.
There was a trend that a higher proportion of individuals with CML received an allogeneic HSCT (47% or n=42) when compared to those with MDS (20% or n=3, p=.053). Graft versus host disease prophylaxis consisted of tacrolimus and methotrexate administered using the standard techniques for those receiving HSCT.39 About half of the HSCT (53%) used stem cells from a donor related to the patient. Only 3 of the 45 allogeneic HSCT were nonmyeloablative; 94% of the HSCT patients received total body irradiation (total dose 14 Gy, in 7 fractions). Among the 48 people with CML who received other treatment, the majority was treated with imantinib mesylate (85.4%); the remainder received hydroxyurea (10.4%) or interferon (4.2%). Treatment for the 12 people with MDS who did not receive an allogeneic HSCT was as follows: hydroxyurea (n=2, 17%); supportive treatment (n=3, 25%); erythropoietin (n=4, 33%); and azactidine (n=5, 42%); with some receiving more than one of the listed treatments simultaneously. Table 1 summarizes subject characteristics at enrollment.
Time, Treatment, and Disease Effects on Outcome Measures
Outcome measures were differentially affected by time, disease, and treatment. In general, when significant time effects were found to be present, they were in the direction of improvement relative to baseline. Table 2 summarizes the least square means with standard errors for estimating the time effects on the outcome measures.
Table 2. Significant Time Effects on Measures.
| Least Square Means (SE) at 3 Timepoints | ||||
|---|---|---|---|---|
| Measure | Baseline | 12 Months | 18 Months 1 | P2 |
| Total Recall | -.53 (.15) | -.18 (.15) | -.077 (.16) | .0048 |
| Compared to baseline | p=.0055 | p=.0016 | ||
| Long Term Storage | -.41 (.15) | -.22 (.15) | .0018 (.15) | .011 |
| Compared to baseline | p=.144 | p=.0028 | ||
| Significant Time Effects by Treatment | ||||
| Least Square Means (SE) at 3 Timepoints | ||||
| Measure | Baseline | 12 Months | 18 Months | P2 |
| SF-36, Mental Component Scale (MCS) | .034 | |||
| HSCT | 43.62 (2.32) | 49.40 (1.87) | 50.26 (1.78) | |
| Compared to baseline | p=.013 | p=.0097 | ||
| Other treatment | 48.16 (1.47) | 48.57 (1.61) | 49.64 (1.53) | |
| Compared to baseline | p=.69 | p=.21 | ||
| Significant Time Effects by Diagnosis | ||||
| Least Square Means (SE) at 3 Timepoints | ||||
| Measure | Baseline | 12 Months | 18 Months | P2 |
| Phonemic Fluency | .014 | |||
| CML | -.13 (.11) | .10 (.13) | .065 (.12) | |
| Compared to baseline | p=.009 | p=.033 | ||
| MDS | -.039 (.29) | -.47 (.20) | -.033 (.26) | |
| Compared to baseline | p=.16 | p=.98 | ||
| Pegboard, Dominant | .041 | |||
| CML | -.72 (.20) | -.55 (.16) | -.46 (.18) | |
| Compared to baseline | p=.38 | p=.094 | ||
| MDS | -.1.79 (.50) | -1.41 (.54) | -2.32 (.58) | |
| Compared to baseline | p=.46 | p=.092 | ||
The difference between the 18 and 12 month values were not statistically significant, with p>0.05.
P-values for the Total Recall and Long-Term Storage are for time effects across all patients. P-values for other outcomes are for the time-by-treatment or time-by diagnosis interaction.
Time Effects on Neuropsychological Measures
Overall, participants' Total Recall measured by the Buschke Selective Reminding Test improved over time, with significant gains from baseline to 12-month (p=.0055) and from baseline to 18 month (p=.0016) evaluations. Similarly, there was improvement in Long-Term Storage, with the greatest improvement from baseline to 18-month evaluation (p=.0028). There were neither treatment nor diagnosis effects on either of these two measures of memory. (See Table 2)
Time by diagnosis interactions were identified for two neurocognitive measures, phonemic fluency (p=.014) and Grooved Pegboard for the dominant hand (p=.04). For phonemic fluency, patients with CML had improved scores at 12 months (p=.009) and 18 months (p=.033), both compared to baseline. In contrast, those with MDS had no improvement compared to baseline at either 12 or 18 months. For the Grooved Pegboard, dominant hand, those with CML improved from baseline to 18 months (p=.094), but those with MDS declined in the same time period (p=.092).
Disease Effects on Neuropsychological Measures
Across all time points, CML participants had better performance than those with MDS on several neurocognitive measures. Specifically, those with CML had better performance on the Grooved Pegboard test of motor function for the non-dominant (Effect estimate=1.47 (SE=.50), p=.0042) hand. CML patients were also about 1 SD better than those with MDS for the dominant hand grooved pegboard test, but the magnitude of difference varied with time, as shown in Table 2. Similarly, there was a significant difference by diagnosis on performance for both Part A and Part B of the Trailmaking Test. Overall, subjects with CML had better scores on Part A (Effect estimate= 0.69, (SE=0.32), p=0.034) and Part B (Effect estimate=2.09 (SE=0.50), p=.0002) when compared to those with MDS. Table 3 summarizes these selected neuropsychological results.
Table 3. Selected Neuropsychological Measures.
| Baseline Mean (SD) |
12 months Mean (SD) |
18 months Mean (SD) |
Estimate (SE) | P-value1 | |
|---|---|---|---|---|---|
| TRAILS B | |||||
| CML | .37 (1.14) | .49 (.94) | .58 (.99) | .69 (.32) | .034 |
| MDS | -.36 (1.77) | -.33 (1.32) | -.074 (.90) | ||
| HSCT | .0069 (1.33) | .33 (.84) | .59 (.86) | -.11 (.21) | .60 |
| Other Tx | .422 (1.17) | .43 (1.10) | .48 (1.07) | ||
| TRAILS B | |||||
| CML | -.38 (2.53) | .069 (1.61) | .12 (1.66) | 2.09 (.53) | .0002 |
| MDS | -1.42 (2.46) | -2.20 (2.21) | -1.36 (2.33) | ||
| HSCT | -.40 (1.96) | -.48 (1.83) | -.16 (1.22) | -.57 (.35) | .11 |
| Other Tx | -.55 (2.80) | -.053 (1.82) | .048 (2.00) | ||
| PEGBOARD, Dominant | |||||
| CML | -.71 (1.73) | -.55 (1.45) | -.45 (1.65) | N/A2 | |
| MDS | -1.74 (1.36) | -1.41 (1.55) | -2.44 (1.74) | ||
| HSCT | -1.36 (2.20) | -1.32 (1.52) | -1.40 (1.99) | -1.13 (.31) | .0005 |
| Other Tx | -.57 (1.37) | -.31 (1.35) | -.23 (1.47) | ||
| PEGBOARD, non-Dominant | |||||
| CML | -.77 (1.69) | -.66 (1.52) | -.54 (1.66) | 1.47 (.50) | .0042 |
| MDS | -1.71 (1.42) | -1.86 (2.13) | -2.40 (2.24) | ||
| HSCT | -1.20 (2.21) | -1.46 (1.68) | -1.55 (1.99) | -1.08 (.33) | .0019 |
| Other Tx | -.70 (1.33) | -.45 (1.51) | -.27 (1.52) | ||
| SEMANTIC FLUENCY | |||||
| CML | .089 (1.02) | .21 (1.16) | .14 (1.11) | -.16 (.34) | .64 |
| MDS | .23 (.84) | .13 (1.12) | .79 (.62) | ||
| HSCT | -.038 (1.01) | .27 (1.08) | -.053 (.66) | -.21 (.23) | .36 |
| Other Tx | .18 (.99) | .16 (1.19) | .33 (1.26) | ||
P-values are for the main effects of diagnosis and treatment, across all time points.
Because of the significant time-by-diagnosis interaction shown in Table 2, the difference between patients with CML and MDS varies with time and cannot be reported as a single estimate.
Treatment Effects on Neuropsychological Measures
HSCT recipients had worse performance on tests of motor function (Grooved Pegboard) for both the dominant (Effect estimate=-1.13 (SE= 0.31), p=.0005) and non-dominant hands (Effect estimate=-1.08, (SE=0.33), p=.0019). These results are summarized in Table 3.
Other Neuropsychological Measures
There were no time, treatment, or disease effects on semantic fluency, which generally appeared to stay within normal limits. The Z-scores for this measure are by disease and treatment are listed in Table 3.
Other Measures
SF-36, Mental and Physical Component Summary Scores
Significant time by treatment effects for the mental component summary score (MCS) of the SF-36 were identified (p=.034). HSCT recipients had low initial MCS scores (LSM=46.6) but demonstrated statistically significant gains at the time of 12 (∼+5.8, p=.013) and 18 (∼+6.6, p=.01) month follow-up when compared to baseline. In contrast, other treatment patients had no significant changes in their mean MCS scores from the time of initial evaluation to 18- month follow-up, with all scores being less than 50. (see Table 2).
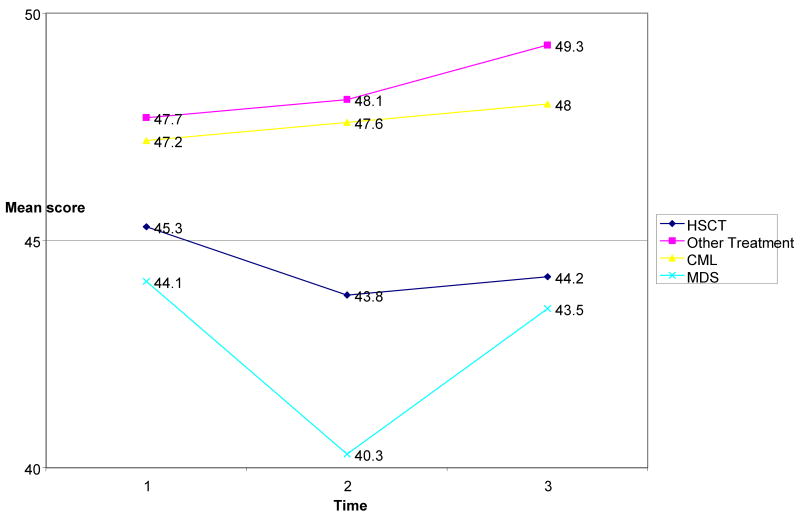
HSCT recipients had poorer average physical component summary scores from the SF-36 by nearly 4 points (p=.049). The deficit was present at the pre-treatment baseline evaluation and through follow-up, and should not be attributed to HSCT therapy. Figure 2 shows the PCS scores for participants by disease and treatment.
Figure 2. SF- 36 Physical Component Summary Score by Treatment and Disease.
POMS
Table 4 lists the means with standard deviations for the POMS and the two “confused” items. The results for the POMS did not differ by disease (Effect estimate= 2.56, (SE=2.65), p=0.34) or treatment (Effect estimate=-0.34 (SE=1.80), p=0.85). Overall, participants did not endorse many items consistent with depression. Similarly, there were no differences on the two “confused” items by disease (Effect estimate=0.18, (SE=.50), p=.72) or treatment (Effect estimate=-0.23, (SE=0.34), p=0.51).
Table 4. Measures of Mood, Means (Standard Deviation).
| POMS | Baseline | 12 months | 18 months |
|---|---|---|---|
| CML | 8.79 (9.15) | 7.46 (8.57) | 7.37 (8.47) |
| MDS | 5.00 (3.94) | 4.11 (4.22) | 7.42 (7.34) |
| HSCT | 9.77 (7.98) | 7.16 (8.54) | 6.53 (6.42) |
| no HSCT | 7.60 (9.14) | 7.02 (8.16) | 7.82 (9.21) |
| Confused | Baseline | 12 months | 18 months |
| CML | 1.16 (1.72) | 1.16 (1.73) | 0.95 (1.64) |
| MDS | 0.55 (1.01) | 0.55 (.73) | 1.71 (1.80) |
| HSCT | 1.11 (1.53) | 0.68 (1.14) | 0.84 (1.28) |
| no HSCT | 1.08 (1.74) | .94 (1.64) | 1.12 (1.19) |
| POMS | Baseline Means, (SD) | 12 months Means, (SD) | 18 months Means, (SD) |
| CML | 8.79 (9.15) | 7.46 (8.57) | 7.37 (8.47) |
| MDS | 5.00 (3.94) | 4.11 (4.22) | 7.42 (7.34) |
| HSCT | 9.77 (7.98) | 7.16 (8.54) | 6.53 (6.42) |
| no HSCT | 7.60 (9.14) | 7.02 (8.16) | 7.82 (9.21) |
| Confused | Baseline | 12 months | 18 months |
| CML | 1.16 (1.72) | 1.16 (1.73) | 0.95 (1.64) |
| MDS | 0.55 (1.01) | 0.55 (.73) | 1.71 (1.80) |
| HSCT | 1.11 (1.53) | 0.68 (1.14) | 0.84 (1.28) |
| no HSCT | 1.08 (1.74) | .94 (1.64) | 1.12 (1.19) |
Discussion
The main finding of this prospective cohort study is that time effects on measures of neurocognitive function are important. There was general improvement from the time of treatment initiation to 18 months later for measures of memory (total recall, p=.005, and long-term storage, p=.01). Hence, complaints about “chemobrain” following HSCT merit further study, as deficits appear to pre-date initiation of that treatment and then subsequently improve. Indeed, participants did not strongly endorse the two POMS items suggestive of confusion or difficulties with mental acuity (feeling bewildered or muddled) and in addition, did not endorse many items consistent with depressed mood. This is important because earlier, influential studies suggested that cancer patients who reported concentration and memory problems, in fact were more likely to be clinically depressed or anxious, than to have such deficits confirmed on objective testing. 40 Strengths of the study include a low attrition rate, focus on allogeneic HSCT for two hematologic diseases and exclusion criteria that eliminated prior central nervous system trauma and treatment, all of which could have obscured results. Several other findings are highlighted.
First, people treated with HSCT compared favorably to those who received other treatment with regards to their performance on most measures of neuropsychological function and mood. When compared to people who received other types of treatment, those who underwent HSCT had worse physical function (p=.049), and had more difficulty with coordination and fine motor speed in both the dominant (p=.005) and non-dominant hands (p=.0019). Possible explanations for the lower PCS among HSCT recipients include greater severity or acuity of hematologic malignancy. Greater difficulty in coordination and fine motor speed in HSCT recipients may be accounted for the late effects of total body irradiation and chemotherapy, or by graft-versus-host disease or the medications for its treatment.
Second, diagnosis or disease may be an important factor when assessing neurocognitive and other changes because there may be disease specific features. For example, patients with CML show significantly more improvement over time on phonemic fluency and motor tasks than patients with MDS.
Several potential limitations to the generalizability of findings should be noted. Since patients were not randomized to treatment, any differences between treatment groups could as easily be ascribed to the patient selection process because of his/ her disease, or as to treatment itself. The number of subjects with MDS was small relative to the number of those with CML. The IQ of the sample was average to high average; so it is possible that people with higher cognitive reserve participated. Subjects were given modest honoraria, which may have influenced rates of participation in either direction. HSCT was naturalistically compared to other treatment, of which imantinib mesylate was the most common. This medication is a molecularly targeted therapy for the treatment of cancer and may be associated with fewer side effects than conventional chemotherapy. Use of two items from the POMS to capture self-assessment of confusion is a novel application and needs further study. Finally, it was impossible to evaluate the neurocognitive function of participants before their diagnosis. Future, ideal studies would address some of these limitations, if possible.
These considerations notwithstanding, the results from this study support findings from other research, and extend our knowledge about the trajectory of neurocognitive recovery. Like other investigations, a general improvement in the 12 months after HSCT is observed, with some impairments pre-HSCT. 8-16 Moreover, there appears to be further improvement at 18 months. Since allogeneic HSCT is a therapy for a wide variety of diseases, these results suggest that it may be important to evaluate neurocognitive changes in the context of baseline disease as well.
Early detection and improved treatment of cancer, such as HSCT, have resulted in growing numbers of cancer survivors, now over 10 million.41 This large group has entered a new phase of medical care that will need to take into account the long-term physical and psychological effects of their cancer treatment.42 While there appear to be some differences in neurocognitive function after HSCT compared to other treatments, particularly with regards to fine motor function, there was no apparent increase in adverse effects for other aspects of executive function that were evaluated. The PCS and MCS scores for the study sample were lower than the norms established for a healthy US population with no chronic conditions (median PCS=56.86 and median MCS=54.26), but comparable to those for cancer (except skin cancer) in the US (median PCS=41.49 and median MCS=51.32).35 Thus, the results from this study could reassure prospective HSCT recipients in that this particular treatment compares favorably to other treatments when mental status side effects are considered. Indeed, the overall pattern of measures of neurocognitive function indicate that there is improvement over time.
Acknowledgments
Alyson Lavigne-Dolan and Christina Breigleb were the lead research assistants.
This study was supported in part by Grant Number RSG-01-246-01-PBP from the American Cancer Society and K24 AA 000289 from the NIAAA (both, GC).
Footnotes
Results based on this study were presented in a poster session at the American Academy of Clinical Neuropsychology, Boston, MA, June 19-21, 2008.
The authors have no conflicts of interest to report.
References
- 1.Aschan J. Allogeneic haematopoietic stem cell transplantation: current status and future outlook. Br Med Bull. 2006;77 and 78:23–36. doi: 10.1093/bmb/ldl005. [DOI] [PubMed] [Google Scholar]
- 2.Ringden O, LeBlanc K. Allogeneic hematopoietic stem cell transplantation: state of the art and new perspectives. APMIS. 2005;113:813–830. doi: 10.1111/j.1600-0463.2005.apm_336.x. [DOI] [PubMed] [Google Scholar]
- 3.Tierney DK, Facione N, Padilla G, et al. Response shift: a theoretical exploration of quality of life following hematopoietic cell transplantation. Cancer Nurs. 2007;30:125–138. doi: 10.1097/01.NCC.0000265002.79687.af. [DOI] [PubMed] [Google Scholar]
- 4.Syrjala KL, Langer SL, Abrams JR, et al. Late effects of hematopoietic cell transplantation among 10-year adult survivors compared with case-matched controls. J Clin Oncol. 2005;23:6596–6606. doi: 10.1200/JCO.2005.12.674. [DOI] [PubMed] [Google Scholar]
- 5.Andrykowski MA, Bishop MM, Hahn EA, et al. Long-term health-related quality of life, growth, and spiritual well-being after hematopoietic stem cell transplantation. J Clin Oncol. 2005;23:599–608. doi: 10.1200/JCO.2005.03.189. [DOI] [PubMed] [Google Scholar]
- 6.Harder H, Van Gool AR, Cornelissen JJ, et al. Assessment of pre-treatment cognitive performance in adult bone marrow haemopoeitic stem cell transplantation patients: a comparative study. Eur J Cancer. 2005;41:1007–1016. doi: 10.1016/j.ejca.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 7.Booth-Jones M, Jacobsen PB, Ransom S, et al. Characteristics and correlates of cognitive functioning following bone marrow transplantation. Bone Marrow Transplant. 2005;36:695–702. doi: 10.1038/sj.bmt.1705108. [DOI] [PubMed] [Google Scholar]
- 8.Jacobs SR, Small BJ, Booth-Jones M, et al. Changes in cognitive functioning in the year after hematopoietic stem cell transplantation. Cancer. 2007;110:1560–7. doi: 10.1002/cncr.22962. [DOI] [PubMed] [Google Scholar]
- 9.Syrjala KL, Dikmen S, Langer SL, et al. Neuropsychologic changes from before transplantation to 1 year in patients receiving myeloablabtive allogeneic hematopoietic cell transplant. Blood. 2004;104:3386–92. doi: 10.1182/blood-2004-03-1155. [DOI] [PubMed] [Google Scholar]
- 10.Schulz-Kindermann F, Mehnert A, Scherwath A, et al. Cognitive function in the acute course of allogeneic hematopoietic stem cell transplantation for hematological malignancies. Bone Marrow Transplant. 2007;39:789–799. doi: 10.1038/sj.bmt.1705663. [DOI] [PubMed] [Google Scholar]
- 11.Beglinger LJ, Duff K, Van Der Heiden S, et al. Neuropsychological and psychiatric functioning pre- and posthematopoietic stem cell transplantation in adult cancer patients: a preliminary study. J Intl Neuropsychol. 2007;13:172–177. doi: 10.1017/S1355617707070208. [DOI] [PubMed] [Google Scholar]
- 12.Harder H, Duivenvoorden HJ, Van Gool AR, et al. Neurocognitive functions and quality of life in haematological patients receiving haematopoietic stem cell grafts: a one-year follow-up pilot study. J Clin Exp Neuropsychol. 2006;28:283–293. doi: 10.1080/13803390490918147. [DOI] [PubMed] [Google Scholar]
- 13.Sostak P, Padovan CS, Yousry TA, et al. Prospective evaluation of neurological complications after allogeneic bone marrow transplantation. Neurology. 2003;60:842–848. doi: 10.1212/01.wnl.0000046522.38465.79. [DOI] [PubMed] [Google Scholar]
- 14.Ahles TA, Tope DM, Furstenberg C, et al. Psychologic and neuropsychologic impact of autologous bone marrow transplantation. J Clin Oncol. 1996;14:1457–1462. doi: 10.1200/JCO.1996.14.5.1457. [DOI] [PubMed] [Google Scholar]
- 15.Meyers CA, Weitzner M, Byrne K, et al. Evaluation of the Neurobehavioral Functioning of Patients Before, During, and After Bone Marrow Transplantation. J Clin Oncol. 1994;12:820–826. doi: 10.1200/JCO.1994.12.4.820. [DOI] [PubMed] [Google Scholar]
- 16.Parth P, Dunlap WP, Kennedy RS, et al. Motor and cognitive testing of bone marrow transplant patients after chemoradiotherapy. Percept Mot Skills. 1989;68:1227–1241. doi: 10.2466/pms.1989.68.3c.1227. [DOI] [PubMed] [Google Scholar]
- 17.Rizzo JD, Wingard JR, Tichelli A, et al. Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation: joint recommendations of the European Group for Blood and Marrow Transplantation, the Center for International Blood and Marrow Transplant Research, and the American Society of Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2006;12:138–151. doi: 10.1016/j.bbmt.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 18.Hurria A, Somlo G, Ahles T. Renaming “chemobrain”. Cancer Invest. 2007;25:373–7. doi: 10.1080/07357900701506672. [DOI] [PubMed] [Google Scholar]
- 19.Quintas-Cardama A, Cortes JE. Chronic myeloid leukemia: diagnosis and treatment. Mayo Clin Proc. 2006;81:973–988. doi: 10.4065/81.7.973. [DOI] [PubMed] [Google Scholar]
- 20.Steensma DP, Bennett JM. The myelodysplastic syndromes: diagnosis and treatment. Mayo Clin Proc. 2006;81:104–30. doi: 10.4065/81.1.104. [DOI] [PubMed] [Google Scholar]
- 21.Nakao K, Treas J. Computing 1989 prestige scores. GSS Methodological Report No. 70. Chicago, IL: NORC; 1990. [Google Scholar]
- 22.Mason CF, Lemmon D, Wayne KS, et al. Shipley Institute of Living Scale: formulas for abstraction quotients from a normative sample of 580. Sex and socioeconomic status considered as additional moderating variables. Psychol Assess. 1991;3:412–417. [Google Scholar]
- 23.McHorney CA, Ware JE, Raczek AE. The MOS 36-item, short-form health survey (SF-36): psychometric and clinical uses of validity in measuring physical and mental constructs. Med Care. 1993;331:247–263. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 24.Cella DR, Jacobsen PB, Orav EJ, et al. A brief POMS measure of distress for cancer patients. J Chron Dis. 1987;40:939–942. doi: 10.1016/0021-9681(87)90143-3. [DOI] [PubMed] [Google Scholar]
- 25.First MB, Spitzer RL, Gibbon M, et al. Structured clinical interview for axis I DSM-IV disorders Patient edition (SCID-I/P), version 2.0) New York: Biometrics Research Department, New York State Psychiatric Institute; 1994. [Google Scholar]
- 26.Harder H, Corenlissen JJ, van Gool AR, et al. Cognitive functioning and Quality of Life in Long-term Adult Survivors of Bone Marrow Transplantation. Cancer. 2002;95:183–192. doi: 10.1002/cncr.10627. [DOI] [PubMed] [Google Scholar]
- 27.Andrykowski M, Schmitt FA, Gregg ME, et al. Neuropsychologic impairment in adult bone marrow transplant candidates. Cancer. 1992;70:2288–2297. doi: 10.1002/1097-0142(19921101)70:9<2288::aid-cncr2820700913>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 28.Strauss E, Sherman EMS, Spreen O. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. Third Edition. New York: Oxford University Press; 2006. [Google Scholar]
- 29.Strub RL, Black FW. The mental status examination in neurology. Philadelphia, PA: FA Davis Company; 1980. [Google Scholar]
- 30.Buschke H. Selective Reminding Test. Psychol Assess. 1995;1040-35907:177–82. [Google Scholar]
- 31.Grooved Pegboard. Psychological Assessment Resources, Inc; 2000. [Google Scholar]
- 32.Lezak MD, Howieson DB, Loring DW. Neuropsychological Assessment. Fourth Edition. New York: Oxford University Press; 2004. [Google Scholar]
- 33.Mitrushina M, Boone KB, Razani J, et al. Handbook of Normative Data for Neuropsychological Assessment. Second Edition. New York: Oxford University Press; 2005. [Google Scholar]
- 34.Larrabee GJ, Trahan DE, Levin HS. Normative data for a six-trial administration of the verbal selective reminding test. Clin Neuropsychol. 2000;14:110–118. doi: 10.1076/1385-4046(200002)14:1;1-8;FT110. [DOI] [PubMed] [Google Scholar]
- 35.Ruff RM, Parker SB. Gender- and age-specific changes in motor speed and eye-hand coordination in adults: Normative values for the finger tapping and grooved pegboard test. Percept Mot Skills. 1993;62:407–416. doi: 10.2466/pms.1993.76.3c.1219. [DOI] [PubMed] [Google Scholar]
- 36.Tombaugh TN. Trail making test A and B: Normative data stratified by age and education. Arch Clin Neuropsychol. 2004;19:203–214. doi: 10.1016/S0887-6177(03)00039-8. [DOI] [PubMed] [Google Scholar]
- 37.Tombaugh TN, Kozak J, Rees L. Normative data stratified by age and education for two measures of verbal fluency: FAS and animal naming. Arch Clin Neuropsychol. 1999;14:167–177. [PubMed] [Google Scholar]
- 38.Ware JE, Kosinski M. SF-36 Physical and Mental Health Summary Scales: A Manual for Users of Version 1. Second. Lincoln, RI: Quality Metric Incorporated; 2001. [Google Scholar]
- 39.Nash RA, Antin JH, Karanes C, et al. Phase 3 study comparing methotrexate and tacrolimus with methotrexate and cyclosporine for prophylaxis of acute graft versus host disease after marrow transplantation from unrelated donors. Blood. 2000;96:2062–8. [PubMed] [Google Scholar]
- 40.Cull A, Hay C, Love SB, et al. What do patients mean when they complain of concentration and memory problems? British Journal of Cancer. 1996;74:1674–9. doi: 10.1038/bjc.1996.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stein KD, Syrjala KL, Andrykowski MA. Physical and psychological long-term and late effects of cancer. Cancer. 2008;112:2577–92. doi: 10.1002/cncr.23448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rowland JH, Hewitt M, Ganz PA. Cancer survivorship: a new challenge in delivering quality cancer care. J Clin Oncol. 2006;24:5101–5104. doi: 10.1200/JCO.2006.09.2700. [DOI] [PubMed] [Google Scholar]