Summary
Synaptic vesicle (SV) exo- and endocytosis are tightly coupled to sustain neurotransmission in presynaptic terminals, and both are regulated by Ca2+. Ca2+ influx triggered by voltage-gated Ca2+ channels is necessary for SV fusion. However, extracellular Ca2+ has also been shown to be required for endocytosis. The intracellular Ca2+ levels (< 1μM) that trigger endocytosis are typically much lower than those (> 10μM) needed to induce exocytosis, and endocytosis is inhibited when the Ca2+ level exceeds 1μM. Here we identify and characterize a transmembrane protein associated with SVs, which upon SV fusion, localizes at periactive zones. Loss of Flower results in impaired intracellular resting Ca2+ levels and impaired endocytosis. Flower multimerizes and is able to form a channel to control Ca2+ influx. We propose that Flower functions as a Ca2+ channel to regulate synaptic endocytosis and hence couples exo- with endocytosis.
Keywords: Ca2+ channel, endocytosis, coupling exo-endocytosis, tetraspanin, synaptic transmission, Flower
Introduction
At presynaptic nerve terminals, synaptic vesicle (SV) exocytosis and endocytosis are temporally and spatially coupled to sustain transmitter release. At rest, intracellular Ca2+ concentrations are estimated to be between ~40-80 nM (Guo et al., 2005; Xiong et al., 2002). However, in response to nerve stimuli, membrane depolarizations activate voltage-gated Ca2+ channels (VGCCs) localized at active zones (Catterall and Few, 2008; Lisman et al., 2007) leading to local intracellular peaks of ~10-100 μM to trigger SV fusion (Adler et al., 1991; Heidelberger et al., 1994; Schneggenburger and Neher, 2000). This high local Ca2+ level decays very rapidly to allow a new stimulus to evoke a new round of release within milliseconds.
Ca2+ is also thought to play a critical role in SV endocytosis (Cousin and Robinson, 1998; Gad et al., 1998; Guatimosim et al., 1998; Klingauf et al., 1998; Marks and McMahon, 1998; Neale et al., 1999; Sankaranarayanan and Ryan, 2001), although the intracellular Ca2+ concentration ([Ca2+]i) that initiates endocytosis is much lower than that which triggers exocytosis (Cousin and Robinson, 2000; Marks and McMahon, 1998). Moreover, endocytosis is abolished above 1μM [Ca2+]i in synaptic terminals (von Gersdorff and Matthews, 1994). Hence, endocytosis seems to be triggered in a narrow and low [Ca2+]i range.
Massive exocytosis can be induced by black widow spider venom (Henkel and Betz, 1995), caffeine (Zefirov et al., 2006), and veratridine (Kuromi and Kidokoro, 2002) in the absence of extracellular Ca2+. However, extracellular Ca2+ is necessary to initiate endocytosis in these paradigms, suggesting that a Ca2+ influx independent of VGCCs is critical to trigger endocytosis. Some data indicate that Calmodulin may function as a high affinity sensor required for endocytosis (Cousin, 2000), and that the Ca2+/Calmodulin-dependent phosphatase, Calcineurin, forms a complex with Dynamin I at ~1 μM Ca2+ to promote endocytosis (Lai et al., 1999). Finally, Kuromi et al. proposed that a Ca2+ channel must be present at Drosophila NMJs to promote endocytosis (Kuromi et al., 2004). These observations suggest that an unknown Ca2+ channel may specifically regulate SV retrieval. However, how endocytosis is coupled to exocytosis remains a major question in the SV cycle.
Here we report the identification of a protein that has not been previously characterized in any species and whose loss affects endocytosis. It contains three or four transmembrane domains and is associated with SVs. Upon fusion of the SVs with the presynaptic membrane, the protein is present in the periactive zones where endocytosis is known to occur. The protein contains a 9 amino acid motif in the second transmembrane domain with homology to selectivity filters described in VGCCs and Transient Receptor Potential channels (TRPV5 and 6). We present evidence that it mediates Ca2+ entry in heterologous salivary gland cells and radioactive 45Ca2+ influx in reconstituted proteoliposomes. In addition, its loss also affects resting Ca2+ levels in presynaptic terminals.
Results
Identification of a new complementation group that affects synaptic transmission
To identify novel proteins that affect synaptic transmission in Drosophila, we performed an unbiased forward genetic screen using ethyl methanesulfonate (EMS) on chromosome 3L. We utilized the eyFLP/FRT system in combination with electroretinogram (ERG) recordings to isolate mutants in which there is a loss of “On-Off” transients (Figure 1A) (Stowers and Schwarz, 1999). We identified several complementation groups from about 49,017 flies screened for this defect (Ohyama et al., 2007), including the one discussed here.
Figure 1. 3L5 corresponds to CG6151, which encodes three alternatively spliced isoforms with three or four TM domains.
(A) The genotypes of flies and their ERG traces. Homozygous DB25 or DB56 flies are rescued by expression of CG6151-RA or -RB under the control of GMR-GAL4 or by a genomic rescue construct. Positions of “On” and “Off” transients of ERGs are marked by a red arrowhead. Impaired “On” and “Off” transients are marked by green circles. (B-D) Axons and nerve terminals of R7 and R8 in the medulla of the adult brain are stained for Chaoptin (red). Stereotypic R7 and R8 axon terminals are labeled with white dots. Scale bars: 20 μm. (E) Genomic organization of CG6151 which includes seven exons producing three isoforms (RA, RB, and RC) with distinct C-termini. A P element, P[EPgy2]CG6151 (P), is integrated in the 5′UTR of CG6151. The 8.2 kb genomic rescue construct is shown below the gene. Positions and types of tagged epitopes in two tagged genomic constructs (FLAG/HA/MYC and YFP/GFP/RFP) are shown. (F) Three protein isoforms (PA, PB, and PC) contain three or four TM domains, depending on the transmembrane prediction program used (Figure S2B). The position of the mutations in DB25 and DB56 are indicated. (G) The CG6151 orthologs from Drosophila melanogaster (Dm), Caenorhabditis elegans (Ce), Anopheles gambiae (Ag), Xenopus tropicalis (Xt), Mus musculus (Mm), and Homo sapiens (Hs) are aligned. The amino acids mutated in DB25 and DB56 are marked with red stars. Note that G128 is conserved in all species. (H) Complementation tests combined with lethal phase assays: first instar larvae (L1), second instar larvae (L2), late pupae (LP), and adult (A). Df is deficiency Df(3L)X-21.2. ® indicates that the lethal combination is rescued by the genomic rescue transgene.
Mutants of one lethal complementation group (3L5) consisting of two alleles, DB25 and DB56, are homozygous lethal. Mutant eyes induced by eyFLP/FRT are morphologically normal (data not shown). However, they display severely impaired “On-Off” transients in ERGs and slightly reduced depolarizations (Figure 1A). A defect in neurotransmission as indicated by loss of “On-Off” transients could be due to either a developmental or a transmitter release defect. We therefore determined if the mutant photoreceptors (R) exhibit axon guidance defects by staining 3L5 eyFLP mutant adult optic lobes for Chaoptin (Zipursky et al., 1984), a photoreceptor membrane protein. As shown in Figures 1B-D, the synaptic patterns of R7 and R8 photoreceptor subtypes in the wild-type control and mutant medulla are indistinguishable, suggesting that mutations in the 3L5 group do not affect neuronal development and axon guidance. To examine if the 3L5 alleles affect the ultrastructure of the R1-R6 photoreceptor axon terminals, we carried out transmission electron microscopy (TEM) in the lamina. The data are summarized in Figure S1. The key observation is that the number of SVs is decreased in 3L5 mutants. In addition, we observed aberrant sizes and numbers of capitate projections. Capitate projections (CP) are glial invaginations that have been implicated in endocytosis in R terminals (Fabian-Fine et al., 2003). A reduced number of SVs in R terminals have so far only been described in the endocytic mutant shibirets (Fabian-Fine et al., 2003). These data suggest that endocytosis may be impaired in the 3L5 mutant photoreceptors.
3L5 corresponds to CG6151, a gene encoding a protein with three or four transmembrane domains
To identify the gene, we mapped the molecular lesions in 3L5 mutants (Figure S2A)(Zhai et al., 2003). We identified a P element, P{EPgy2}CG6151EY08496 (P) (Bellen et al., 2004), integrated in the 5′UTR of CG6151 which causes semi-lethality and fails to complement the lethality of both 3L5 alleles, suggesting that 3L5 corresponds to CG6151 (Figures 1E and 1H). CG6151 encodes three potential alternatively spliced isoforms, CG6151-RA, -RB, and -RC (Tweedie et al., 2009), encoding proteins about 190 amino acids long. They are predicted to contain three or four transmembrane (TM) domains with distinct C-termini (Figures 1E-F and Figure S2B). CG6151 is evolutionarily conserved from worm to human and exhibits 39% identity and 61% similarity between amino acids 25 and 150 of the fly and human homologues (Figure 1G).
The DB25 allele contains a G128D mutation whereas the DB56 allele contains a premature stop codon, Q24* (Figures 1F-G). These mutations are responsible for the lethality and the phenotypic defects discussed below, as an 8.2 kb genomic construct covering 1 kb upstream of the 5′UTR and 0.5 kb downstream of the 3′UTR rescues the lethality and the ERG defects of homozygous or transheterozygous mutants (Figures 1A, 1E, and 1H). Complementation tests combined with lethal phase assays suggest the following allelic series: P < DB25 < DB56 = Df (Figure 1H). Note that the lethality of all allelic combinations shown in Figure 1H was rescued by the genomic rescue construct. The DB56 premature stop codon is likely the most severe allele as it causes second instar lethality.
The protein encoded by CG6151 localizes to SVs and periactive zones
CG6151 is expressed in the central nervous system (CNS) starting at embryonic stage 13 (data not shown and Figures S2C-D). To determine which isoforms are expressed and required in the nervous system, we expressed the RA, RB, and RC isoforms under the control of the pan-neuronal driver, elav-GAL4. We assessed their ability to rescue the lethality and ERG defects associated with the 3L5 mutants. The RA and RB isoforms, but not the RC isoform, are able to efficiently rescue lethality, suggesting that they are mainly required in the nervous system. Expression of RA and RB using the photoreceptor-specific driver, GMR-GAL4, also rescue the ERG defects (Figure 1A) indicating that CG6151 is expressed and necessary presynaptically in photoreceptor cells.
To determine the subcellular localization of the three isoforms, we tagged a single genomic rescue construct with three different tags at the different carboxy termini (FLAG for RA; HA for RB; MYC for RC). Another genomic rescue construct was tagged with three fluorescent tags (YFP for RA; GFP for RB; RFP for RC) (Figure 1E). These two genomic constructs rescued the lethality associated with 3L5 mutants. We only observed expression of the protein B form tagged with HA (PB-HA) and GFP (PB-GFP) in the neuropils of embryonic, larval, adult CNS, and R1-R6 terminals (Figures S2E-L and data not shown) but we did not detect staining for the PA and PC tagged isoforms (data not shown). These data suggest that the PB isoform encoded by CG6151 is widely expressed in the nervous system at most stages of development and in adults.
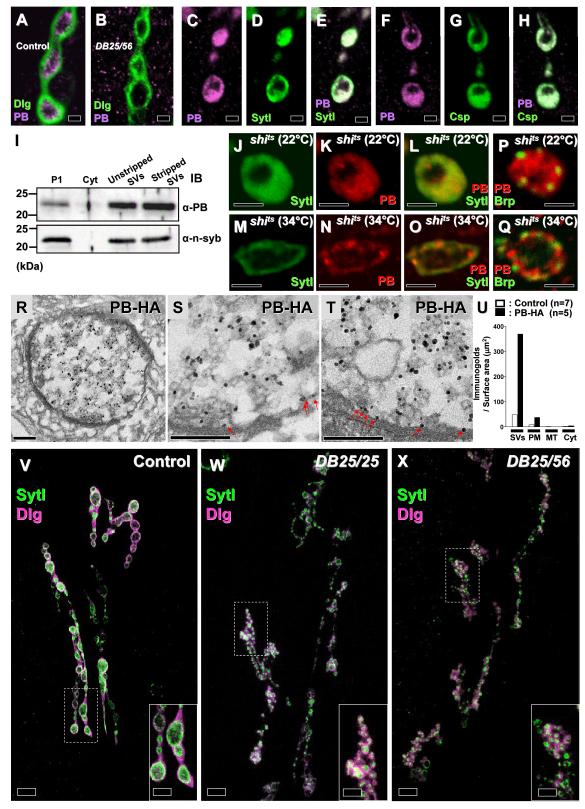
We also generated two different guinea pig (gp115 and 116) antibodies that specifically identify the PB isoform. As shown in Figure 2A, the PB protein is localized presynaptically at NMJs, which are outlined by the postsynaptic marker, Discs large (Dlg) (Lahey et al., 1994). The anti-PB antibody fails to detect protein in third instar DB25/DB56 animals (Figure 2B). As shown in Figures 2C-H, the PB protein colocalizes with the SV markers Synaptotagmin I (SytI) (Littleton et al., 1993) and Cysteine string protein (Csp) (Zinsmaier et al., 1994). Similarly, the PB-HA protein colocalizes with SytI (Figures S2M-O). These data suggest that the PB protein is associated with SVs and presynaptic membranes. The association with SVs was corroborated by probing partially purified and base stripped SVs from adult fly brains (Figure 2I), revealing an abundant 21 kDa protein, in agreement with the estimated molecular weight. Hence, the data indicate that the protein is a previously unidentified, integral membrane protein of SVs.
Figure 2. The 3L5 protein is a SV- and presynaptic membrane-associated protein, and mutants display flowery NMJ boutons.
(A-B) NMJ boutons of wild-type control third instar larvae (A) are stained with an antibody against the PB isoform (gp115; magenta). The boutons are outlined by staining for Dlg (green). The signal is absent in mutant boutons (B). (C-H) The PB isoform (magenta) colocalizes with the SV proteins SytI (green in D and E) and Csp (green in G and H), respectively. (I) In immunoblots (IB), the PB isoform is detected by the gp116 antibody in P1 (total exact), unstripped SV, and stripped SV, but not in cytoplasmic (Cyt) fractions. As a control, a SV protein, neuronal synaptobrevin (n-syb), is present in the same fractions. (J-Q) NMJ boutons of shits1 third instar larvae at permissive (J-L and P) and non-permissive (M-O and Q) temperatures are costained for the PB isoform (red) and a SV marker, SytI (green) and an active zone marker, Bruchpilot (Brp) (green). (R-U) Immunogold-EM of NMJ boutons. The nanogold signals were obtained by using an anti-HA antibody with silver enhancement. (R-T) Flies that carry the genomic PB-HA transgene (genotype: w1118; P{ w+ gen res [Flag/HA/Myc]}/ P{ w+ gen res [Flag/HA/Myc]}; FRT80B DB56/FRT80B DB56) display many positive signals in presynaptic regions. Higher magnification (S and T) shows that the PB-HA protein is localized to SVs as well as the synaptic bouton membrane (red arrow). Quantification (U): synaptic veiscles (SVs), plasma membrane (PM), mitochrondria (MT), cytoplasm (Cyt). The number of NMJ type Ib boutons counted is indicated. (V-X) NMJ boutons of third instar muscle 6/7 of the wild-type control (V), DB25/DB25 (W), and DB25/DB56 (X) were stained for the synaptic vesicle marker, SytI (green), and the postsynaptic marker, Dlg (magenta). 3L5 mutant boutons are clustered and flowery as shown in insets in V-X. Scale bars: 2 μm in A-H and J-Q; 200 nm in R-T; 10 μm in V-X; 5 μm in insets of V-X.
If the PB protein is a SV-associated protein, vesicle release in the absence of endocytosis should lead to its redistribution to the presynaptic membrane. The temperature-sensitive mutant shibire (shits1), which encodes Dynamin, is essential for SV retrieval but not for vesicle fusion (Koenig and Ikeda, 1989) at the non-permissive temperature (34°C). Hence, a SV-associated protein such as SytI should be redistributed from SVs to the presynaptic membrane at 34°C in shits1 mutants. This effect is indeed observed (Figures 2J and 2M). Similarly, under the same conditions, most PB protein is redistributed to the plasma membrane (Figures 2K-L and 2N-O) and localized surrounding active zones which are identified with the anti-Bruchpilot (Brp) antibody (nc82) (Figures 2P-Q) (Wagh et al., 2006). Hence, the PB isoform is a SV-associated protein that, upon SV exocytosis, becomes much enriched in periactive zones where endocytosis occurs (Marie et al., 2004).
We further visualized the localization of the PB-HA protein by immunogold EM labeling using an anti-HA antibody with silver enhancement (Koh et al., 2007). Control flies that do not contain the genomic PB-HA transgene (Figure S2P) show very few scattered positive signals when compared to flies (DB56/DB56) that carry the transgene and are rescued (Figures 2R-T). The vast majority of the gold particles are clearly associated with SVs within the NMJ boutons (Figures 2R-U). However, some of the gold particles are also observed near the plasma membrane (red arrows). These data show that PB is a SV protein that can also localize to the plasma membrane, consistent with all the other data presented in Figure 2.
3L5 mutants are impaired in SV endocytosis
The reduced number of SVs and aberrant size and shape of CPs in R1-6 terminals as well as the protein localization at nerve terminals suggests a possible role in endocytosis. Mutations in genes that affect endocytosis including endophilin, synaptojanin, lap, dynamin, eps15, and dap160 cause an increase in bouton number at NMJs. However, the number of additional satellite boutons varies widely among the different endocytic mutants and does not necessarily correlate with the severity of the endocytic defect (Dickman et al., 2006; Koh et al., 2007). We therefore determined if the number of boutons at mutant third instar larvae is altered. As shown in Figures 2V-X and S3A-C, the mutants display numerous extra boutons which are often small, clustered, and flowery in nature. We therefore named the gene flower. However, the total number of active zones as measured with nc82 (Brp) is similar in mutants and wild-type controls (Figures S3D-F), suggesting that the number of release sites per muscle is not altered in the mutants. Moreover, the total surface area of the NMJ boutons are also similar in mutants (2104 ± 191 μm2 in muscle 6, n = 6) and wild-type controls (1656 ± 184 μm2 in muscle 6, n = 6). The defect in bouton number in flower mutants is rather severe when compared to most endocytic mutants.
Endocytic mutants such as endophilin, synaptojanin, eps15, and dap160 exhibit qualitatively similar defects in synaptic transmission in flies: they display normal excitatory junctional potentials (EJPs) at low stimulation frequencies (0.2-1Hz) and a run-down when stimulated at 10Hz (Dickman et al., 2005; Koh et al., 2007; Koh et al., 2004; Verstreken et al., 2002; Verstreken et al., 2003). We therefore recorded evoked EJPs at 0.2 Hz in 0.5 mM or 1 mM extracellular Ca2+ ([Ca2+]o). At these [Ca2+]o, the EJP amplitudes from wild-type controls and mutants are similar (Figures 3A-E). At 10 Hz in 0.5 mM [Ca2+]o, wild-type controls display facilitation (relative amplitude = 120) which is maintained, whereas mutants display a run-down of EJPs (relative amplitude = 65; Figure 3F). In addition, SV release in wild-type controls exhibits a post-tetanic potententiation (PTP) immediately after a 1 s pause and shift to 0.2 Hz, probably triggered by elevated [Ca2+]i. This PTP is severely impaired in mutants, and the EJPs recover slowly, similar to other endocytic mutants (Dickman et al., 2005). Hence, these data indicate that flower mutants exhibit a defect in endocytosis.
Figure 3. flower mutants display normal exocytosis but impaired endocytosis.
(A-C) EJP traces obtained from wild-type controls and mutants at 0.2 Hz in 0.5 mM [Ca2+]o. (D-E) The mean EJP amplitude at 0.2 Hz stimulation in 0.5 mM [Ca2+]o (D) and 1 mM [Ca2+]o (E). (F) At 10 Hz in 0.5 mM [Ca2+]o, wild-type controls (blue) display facilitation whereas mutants show a run-down of EJPs. After shifting to 0.2 Hz, wild-type controls show a PTP response. In contrast, mutants display an impaired PTP response and very slow recovery. (G) Same as in F but in 1 mM [Ca2+]o. (H) Cumulative probability and quantification of mEJPs. Amplitudes of mEJPs in mutants are larger than wild-type controls. (I) The mean frequency of mEJPs in 0.25 mM [Ca2+]o with 5 μM TTX in flower mutants is reduced. (J and K) The junctional quantal content (JQC) at 0.2 Hz in 0.5 mM and 1 mM [Ca 2+]o is reduced in mutants. (L-N) FM1-43 uptake was performed by stimulating filleted third instar larvae with 1.5 mM [Ca2+]o and 90 mM [K+]o for 1 min. Total fluorescence of FM1-43 in the muscle 6 is counted and normalized with the JQC in 1 mM [Ca2+]o (see Supplemental experimental procedures for details). The normalized level of FM1-43 fluorescence is significantly reduced in mutants when compared to controls (O). The number of muscles 6 in A3 or A4 segments analyzed is indicated. For electrophysiology, each recording is obtained from one animal (we recorded from a total of 52 animals). For FM1-43 assay, at least 6 animals were used in both wild-type controls and mutants. P value: *, P<0.05; **, P<0.01. Error bars indicate SEM. Scale bars: 10 μm in L and M.
To determine if the endocytic defect is sensitive to different [Ca2+]o, we also recorded at 1mM [Ca2+]o instead of 0.5mM (Figure 3G). Upon a 10 Hz stimulation for 10 min in 1 mM [Ca2+]o, control animals sustain transmission better than the two flower mutant combinations. However, the difference between the flower mutants and the wild type control in 0.5 mM [Ca2+]o is 60% whereas in 1mM [Ca2+]o the difference is 20%. More importantly, the recovery after a shift to 0.2 Hz in flower mutants is significantly faster in 1 mM [Ca2+]o than in 0.5 mM [Ca2+]o suggesting that endocytosis is less defective in these conditions. These results indicate that an increased extracellular Ca2+ concentration can partially rescue the endocytic defects in the flower mutants.
An increase in the SV diameter and quantal size has been reported for many endocytic mutants, including synaptojanin, lap, dap160, eps15, and stonedB mutants (Dickman et al., 2005; Fergestad et al., 1999; Koh et al., 2007; Koh et al., 2004; Zhang et al., 1998), possibly because the aberrant recruitment of clathrin and/or an impaired timing of fission SV size. The neurotransmitter content of SVs is increased in these mutants. As shown in Figure 3H we also observed an increase in the quantal size in flower mutants. In addition, the frequency of mEJPs is reduced (Figure 3I). Given the increase in quantal size and similar EJP amplitudes, the junctional quantal content estimated from dividing EJP by mEJP in flower mutants is decreased by ~29% (Figures 3J-K). Note however that the total amount of SV membranes that fuse in flower mutants is estimated to be 109% of wild-type controls as the SVs are significantly larger in flower mutants (see next section).
To establish if there is a defect in endocytosis, we performed FM1-43 dye uptake assays to label newly formed SVs. We incubated NMJs with FM1-43 dye in 90 mM [K+]o plus 1.5 mM [Ca2+]o and measured total fluorescence of NMJ boutons of the entire muscle 6. As shown in Figures 3L-N, the mutants exhibit a 57% reduction of dye uptake as compared to wild-type controls. Since the QC is reduced by ~29% in the mutants (Figure 3K), we estimated the recycling efficiency of SVs by normalizing the fluorescence with the total number of SVs released during stimulation and the total number of release sites in both wild-type controls and mutants (see supplemental experimental procedures). After normalization, flower mutant boutons exhibit a 41% reduction in dye uptake when compared to wild-type controls (Figure 3O). These data indicate that flower mutants are defective in endocytosis.
flower mutants display a reduced number of SVs, increased SV size, and an increased number of endocytic intermediates at NMJs
Endocytic mutants exhibit a reduced number of SVs. In addition, several mutants also exhibit an increased heterogeneity in SV size, and an increased number of endocytic intermediates (Dickman et al., 2005; Koh et al., 2007; Koh et al., 2004; Verstreken et al., 2002; Verstreken et al., 2003; Zhang et al., 1998). Consistent with this, transmission electron microscopy (TEM) of mutant boutons at rest reveals a significant reduction in SV density (Figures 4A-B and 4I). Furthermore, when neurons were stimulated, control boutons still contained numerous SVs (Figures 4C and 4I) whereas flower mutants displayed a severe reduction in the number of SVs (Figure 4D and 4I). In addition, most of the residual SVs are distributed at active zones similar to what was observed in endophilin, synaptojanin, and dap160 mutants (Dickman et al., 2005; Koh et al., 2004; Verstreken et al., 2002; Verstreken et al., 2003) (Figures 4E-F). We also found that SVs are more heterogeneous in size and their diameter increased in mutants when compared to wild-type control boutons (Figures 4A-F and 4J and Figures S4A-D) consistent with the increase in the mEJP amplitude (Figure 3H). Finally, we observed significantly more endocytic intermediates, such as omega structures and collared pits, after stimulation (Figures 4G-H and 4K). In summary, flower mutants have ultrastructural defects at NMJs that are consistent with defects in SV retrieval. All the previous data combined with the TEM data provide strong evidence that flower plays an important role in endocytosis.
Figure 4. NMJ boutons of flower mutants exhibit typical endocytic phenotypes at the EM level.
(A-H) TEM of type Ib boutons of NMJs in A2 or A3 segments in wild-type controls (A, C, and E) and mutants (B, D, F, G, and H) at rest (A, B, E, and F) or stimulated (C, D, G, and H). Mutant boutons exhibit a significant depletion in the number of SVs (compare A and B; compare E and F). Most of the residual SVs are associated with active zones in mutants (B and F). Mutant terminals display omega structures (G) and collared pits (H) which are rarely observed in wild-type control boutons. (I-K) Quantification of the number of SVs (I), the size of SVs (J), and SV intermediates (K). Type Ib boutons of NMJs in third instar wild-type controls (n=20), DB25/DB25 (n=23), DB25/DB56 (n=20), wild-type controls (Stimulated) (n=21), and DB25/DB56 (Stimulated) (n=26) were analyzed. At least three animals for each genotype were used. P value: *, P<0.05; **, P<0.01; ***, P<0.001. Error bars indicate SEM. Scale bar: 200 nm in A-F; 20 nm in G and H.
Flower displays properties of Ca2+ channels
Flower and its homologues display the highest homology in the TM domains. Interestingly, an EAP motif (a.a. 79-81) in the second predicted TM segment is present in all homologues (Figure 1G). In VGCCs, an acidic amino acid [glutamic (E) or aspartic (D) acid] in one of the TM domains plays a key role in ion selectivity. Four TM domains line the pore and each provides a negatively charged amino acid. For example, the L-type VGCC Cav1.2 pore domains display four glutamic acid (EEEE) residues (Owsianik et al., 2006; Sather and McCleskey, 2003). Other Ca2+ channels such as the Ca2+-gating transient receptor potential channels, TRPV5 and 6, contain six TM domains. To generate a pore with four negatively charged amino acids, the TRPV5 and TRPV6 proteins form homo- or heterotetramers (Hoenderop et al., 2003). In addition, similar to Flower, the CRACM/Orai1 Ca2+ channel has four TM domains, and two of the TM domains carry a negatively charged amino acid (Prakriya et al., 2006). The CRACM/Orai1 proteins form dimers or tetramers, and each monomer contributes one TM domain to the pore (Gwack et al., 2007; Vig et al., 2006). Interestingly, the second TM domain of Flower displays sequence similarity with the ion selectivity filters of TRPV5 and 6 (Figure 5A). Since Flower has only a single evolutionarily conserved E in the second TM domain, and since at least 4 negatively charged amino acids should line the pore, we first tested whether Flower was able to form a multimeric protein complex.
Figure 5. Flower forms multimers and is required to maintain resting intracellular Ca2+ in presynaptic terminals.
(A) Top: alignment of the pore region containing the selectivity filters of human voltage-gated L-type (CaV1.2; accession number Q13936) and T-type (Cav3.1; accession number O43497) Ca2+ channels and the fly VGCC (Cac; accession number AAX52486) with fly and mouse Flower TM2 segments. Bottom: alignment of the selectivity filters of human TRPV5 (accession number Q9NQA5) and TRPV6 (accession number Q9H1D0) with the fly and mouse Flower TM2 segments. The glutamic (E) or aspartic (D) acids are indicated by red dotted rectangles. The residue Proline (P) in fly and mouse Flower TM2 and TRPV5 and 6 are indicated by black dotted rectangles. Proline has a ring structure similar to that of Tryptophan (W). Hence, the putative selectivity filter of Flower seems to be a hybrid between VGCCs and TRPV 5 and 6 channels. (B) Immunoblots of flies that are coexpressing Flower tagged with GFP and Flower tagged with HA. The proteins were immunoprecipitated with the anti-HA antibody and detected with the anti-GFP antibody (left) or the reverse (right). The PB-GFP protein is coimmunoprecipitated by the PB-HA protein, and vice versa. In the lower right blot, the black arrow identifies the PB-HA protein and the red arrow indicates non-specific bands. (C) Head extracts of Canton S or rescued flies expressing PB-HA were incubated with anti-HA antibody beads. The IP complexes were eluted with HA peptides and the eluates were warmed at 37°C for 10 min with 2% SDS and 2.5% β-mercaptoethanol β and resolved in a 10% SDS PAGE gel. Monomers (M), dimers (D), trimers (Tr), and tetramers (Te) can easily be identified. Higher oligomers (*) were also observed. (D) Top: Experimental paradigm to measure [Ca2+]i upon release of most SVs in the presynaptic membrane. All recordings were performed on muscle 13 in segments A2 to A4 segments. ΔF/F0 of GCaMP1.6 in shits1 is significantly increased when compared to wild-type controls. The number of type Ib NMJ boutons analyzed is indicated. At least four animals of each genotype were used. (E) Measuring resting [Ca2+]i using GCaMP1.6 in NMJ boutons of muscle 13 in segments A2 to A4. The X-axis represents different bouton size areas based on pixel number. The number of NMJ boutons analyzed is indicated. At least six animals were used for each genotype. P value: ns, not significant; *, P<0.05; **, P<0.01; ***, P<0.001. Error bars indicate SEM.
We introduced two distinct tagged constructs into null mutant animals (DB56/DB56): one in which the PB isoform is tagged with HA whereas the other is tagged with GFP (Figure 1E). Upon coimmunoprecipitation (CoIP), the proteins were eluted with 2% SDS and 2.5 % β-mercaptoethanol, boiled for 5 min, and resolved in a 10% SDS-PAGE gel. CoIP of PB-HA with anti-HA antibody allows detection of the PB-GFP protein by western blotting, and vice versa (Figure 5B), suggesting that Flower has the ability to form at least a homodimer. To test whether Flower can form higher multimers, the PB-HA proteins from head extracts of PB-HA-rescued adult flies were immunoprecipitated with anti-HA antibody. To limit the dissociation of possible multimers, CoIP complexes were eluted with the HA peptide, heated in 2% SDS and 2.5 % β-mercaptoethanol at 37 °C for 10 min, and resolved on a 10% SDS PAGE gel. As shown in Figure 5C, we observed bands consistent with dimers, trimers, tetramers, and higher multimers. Our results indicate that Flower can indeed form higher order multimers, consistent with the possibility that it may form a channel.
Flower is required to maintain the resting intracellular Ca2+ level in presynaptic terminals
Flower localizes to endocytic zones upon vesicle fusion in shits1 terminals (Figure 2N and 2Q). If Flower mediates Ca2+ influx, one would anticipate that these terminals would exhibit a higher resting [Ca2+]i after most or all SVs have been released, and most of the Flower proteins has been integrated in the plasma membrane. To assess resting [Ca2+]i in presynaptic terminals of shits1, we monitored [Ca2+]i by expressing a genetically encoded Ca2+ indicator transgene, GCaMP1.6, in NMJ boutons. GCaMP1.6 has a high affinity for Ca2+ (Kd = 146 nM) and gives a prominent change in fluorescence when bound to Ca2+ (Ohkura et al., 2005; Reiff et al., 2005). The experimental paradigm is shown in Figure 5D: third instar larvae were dissected at 21°C and the temperature was gradually increased to 34°C to block endocytosis (Delgado et al., 2000; Ramaswami et al., 1994). Once at 34°C, we measured baseline fluorescence (F0). We then stimulated nerve axons for 6 min at 30 Hz to promote SV release and waited one minute to allow [Ca2+]i to return to resting levels (Macleod et al., 2004) and measured the increase in fluorescence, ΔF (FR-F0). As shown in Figure 5D, wild-type boutons display a minor increase in ΔF/F0. However, shits1 mutant show a robust 4-fold increase in fluorescence when compared to wild-type controls, indicating that [Ca2+]i is significantly increased under these conditions. We then attempted to create double mutants of shits1 and flower to determine if loss of Flower can suppress [Ca2+]i and reverse the phenotype. Unfortunately, we were unable to recover double mutants at 18°C, 21°C or room temperature. These data again suggest that Flower plays a role in endocytosis, since other endocytic mutants in combination with shits1 also exhibit synthetic lethality (Petrovich et al., 1993). Unfortunately, they did not allow us to assess if the increase in [Ca2+]i was controlled by flower.
Since Flower is localized to the plasma membrane in resting boutons (Figure 2R-U), it may also affect resting [Ca2+]i levels in presynaptic terminals. We therefore compared the resting fluorescence level of GCaMP1.6 in NMJ boutons of wild-type controls and mutants. We stained and quantified the level of the GCaMP1.6 protein for normalization. As shown in Figure 5E, we observed significantly decreased [Ca2+]i in mutant terminals when compared to wild-type controls and animals that are rescued with the genomic rescue transgene. These data provide strong evidence that Flower affects [Ca2+]i levels at presynaptic terminals. It is also likely that the release of SVs and incorporation of Flower into the presynaptic membrane modulates the resting [Ca2+]i levels, as these [Ca2+]i levels are increased when most vesicles are integrated into the presynaptic membrane (Figure 5D).
Heterologous expression of Flower allows Ca2+ influx in salivary glands
Expression of Flower in heterologous cells such as S2 cells, HEK293 cells, or Xenopus oocytes does not lead to the localization of the protein to the plasma membrane (data not shown). Hence, to test whether Flower is able to promote Ca2+ influx we expressed an HA-tagged Flower (Figure S5A) in active secretory Drosophila salivary gland (SG) cells (Farkas and Sutakova, 1998). These cells do not normally express Flower. As shown in Figures S5C-E, a significant fraction of the Flower protein localizes to the plasma membrane in these SG cells. The SG cells were immobilized on L-polysine-coated coverslips. We then monitored fluorescence changes upon incubation in 4 μM Fluo 4-AM, a calcium indicator, in the presence of [Ca2+]o (10 and 100 μM). As shown in Figures 6A-B and D-E, expression of Flower leads to an increase in fluorescence dependent on the calcium concentration. Similarly, we coexpressed Flower and GCaMP1.6 to examine the kinetics of [Ca2+]i at various extracellular [Ca2+]o. As shown in Figure 6F, SGs were dissected and bathed sequentially in different [Ca2+]o ( 0.1 mM, 1 mM, and 0 mM plus 10 mM EGTA). F0 was defined as the fluorescence level of GCaMP1.6 in 0 mM [Ca2+]o and 10 mM EGTA at 90 min. Similar to the results of Fluo 4-AM, expression of Flower caused a significant increase in fluorescence when compared to expression of the control transgene lacZ. These data suggest that Flower is sufficient to mediate Ca2+ entry in SGs when ectopically expressed.
Figure 6. Heterologous expression of Flower allows Ca2+ influx.
(A-C) Images of Drosophila SGs captured over 1 hr period. SGs derived from third instar larvae expressing wild-type Flower-PB or Flower-PB with an E79Q substitution were loaded with 4 μM Fluo 4-AM and 100 μM [Ca2+]o. (D) Quantification of the Fluo 4-AM fluorescence level in A-C. (E) Similar as D but incubation in 10 μM [Ca2+]o. (F) SGs coexpressing Flower-PB and GCaMP1.6 or lacZ (as a control transgene) and GCaMP1.6 were bathed sequentially in various [Ca2+]o (0.1 mM, 1 mM, and 0 mM plus 10 mM EGTA). GCaMP1.6 fluorescence in 0 mM plus 10 mM EGTA at 90 min was selected as F0. Quantification of ΔF/F0 of GCaMP1.6 is shown. The number of SGs analyzed is indicated. Scale bars: 50 μm in A-C. Error bars indicate SEM.
Given the functional dependence of cation-selective channels on the conserved negatively charged residues (Owsianik et al., 2006; Sather and McCleskey, 2003), we hypothesized that a mutation which alters glutamic acid (E) in the second TM domain should affect Ca2+ entry. We therefore expressed the E79Q mutant in SGs. As the E79Q mutant protein is less abundant than the wild-type protein in 10/10 different transgenes, we used three copies of the E79Q transgene to adjust for protein expression levels. We first determined that the protein was present at SG membranes and at similar levels as the wild-type Flower protein (Figures S5B and S5F-H’). As shown in Figures 6C-D, the fluorescence associated with expression of the E79Q protein is similar to the negative controls. These data indicate that Flower is sufficient to allow Ca2+ influx in heterologous cells.
Purified Flower conducts Ca2+ in proteoliposomes
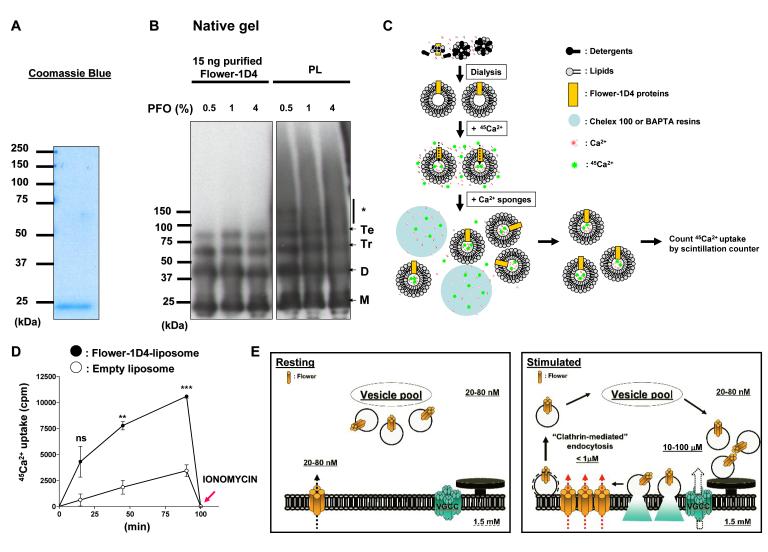
As the function of Flower cannot easily be assessed using conventional cells, we determined whether the Flower protein can form a Ca2+-permeable cationic channel in proteoliposomes. We purified the protein and reconstituted proteoliposomes with the Flower protein to determine if radioactive 45Ca2+ can enter these liposomes. The Drosophila tagged Flower protein was overexpressed in yeast and purified from membrane fractions by antibody affinity chromagraphy. As shown in Figure 7A, a single ~22 kDa band was observed on a Coomassie stained gel. The purified protein is able to form homo-multimers, including tetramers (Figure S6), similar to the endogenous Flower protein, and different crosslinkers enrich the levels of the higher multimers (Figure S6). Similarly, these multimers can be resolved on a native gel (Figure 7B) (Ramjeesingh et al., 1999).
Figure 7. Flower can form a Ca2+-permeable cationic channel.
(A) The purified Drosophila Flower-1D4 protein was boiled for 5 min in the presence of 2% SDS and 2.5% β-mercaptoethanol, separated on a 10% SDS-PAGE gel, and stained with Coomassie blue. (B) Purified Flower-1D4 proteins or Flower-containing proteoliposomes (PL) were incubated with 0.5, 1, or 4% perfluoro-octanoic acid (PFO) sample buffers and run on a 4-12% gradient Tris/glycine precast native gel. Multimeric Flower complexes were observed. Monomers (M), dimers (D), trimers (Tr), tetramers (Te), higher oligomers (*). (C and D) Experimental paradigm for the radioactive 45Ca2+ influx assay. Quantification is shown in D. Three independent experiments were used for quantification. P value: ns, not significant; **, P<0.01; ***, P<0.001. Error bars indicate SEM. (E) Proposed model. Flower, a SV- and presynaptic membrane-associated Ca2+ channel, fuses with the presynaptic membrane upon exocytosis. It then permits a Ca2+ influx which regulates endocytosis as well as resting intracellular Ca2+ levels. Upon endocytosis the majority of the Flower protein is removed, as is typically observed for SV-associated proteins. The remainder of the protein probably affects the resting Ca2+ levels in the presynaptic terminals.
To carry out the radioactive 45Ca2+ influx assay, we used the procedure outlined in Figure 7C. Purified Flower protein was mixed with detergent-solubilized soybean azolectin and then dialyzed to remove the detergents used to solubilize the yeast extracted Flower protein and lipids. Under these standard conditions, homogeneous proteoliposomes containing purified Flower protein are formed (Moiseenkova-Bell et al., 2008). As shown in Figures 7B, Flower proteins incorporated into proteoliposomes (PL) also form multimers under non-denaturing conditions. Upon proteoliposome formation, 1 mM [Ca2+]o containing radioactive 45Ca2+ was added to create an ion gradient to mimic physiological conditions. After incubation for 15, 45, and 90 min, [Ca2+]o was dramatically reduced to low nM levels using the Ca2+ sponges Chelex 100 and BAPTA resins (See Experimental procedures). 45Ca2+ uptake by the liposomes was then estimated. As shown in Figures 7D, 45Ca2+ uptake by Flower-containing liposomes significantly increases over time when compared to empty liposomes. To release the 45Ca2+ from the proteoliposomes at the end of the 90 min incubation, they were perforated with ionomycin. After this treatment, 45Ca2+ activity returns to baseline, showing that the entrapped 45Ca2+ is releasable from the vesicles. These results show that a multimeric Flower protein can indeed form a homomultimeric channel that conducts Ca2+.
Discussion
Ca2+ influx triggers both SV exo- and endocytosis. Since SV retrieval requires much lower Ca2+ levels than those that elicit release, it was proposed that the Ca2+ levels needed to initiate endocytosis derived from diffusion of VGCC-dependent Ca2+ influxes from active zones. However, several reports have documented a requirement for extracellular Ca2+ during endocytosis (Henkel and Betz, 1995; Kuromi and Kidokoro, 2002; Zefirov et al., 2006). Furthermore, Kuromi et al. (2004) proposed that a specific Ca2+ channel is required for SV endocytosis in Drosophila. In the present study, we identify and characterize a SV- and presynaptic membrane-associated protein with three or four transmembrane domains that is evolutionarily conserved but has not been previously characterized in any species. Animals that lack Flower display the typical features of endocytic mutants. These include supernumerary boutons, a low number of SVs in boutons at rest, a severe depletion of SVs upon stimulation (except at active zones), enlarged SVs, a decrease in FM1-43 uptake, a run-down in neurotransmitter release upon repetitive stimulation, and an accumulation of endocytic intermediates.
flower mutants exhibit impaired Ca2+ handling, even when the boutons are at rest. This argues that Flower plays a role in Ca2+ homeostasis at rest as well as during endocytosis. Since Flower is associated with SVs and the presynaptic membrane, a reduction in Flower levels may cause lower resting Ca2+ levels because Ca2+ efflux from SVs or Ca2+ influx from the extracellular compartment is impaired. However, a role for Flower in SVs is unlikely. Firstly, experimental evidence suggests that SVs have no or a very limited role in the sequestration of Ca2+ at NMJs in Drosophila (Macleod et al., 2004). Secondly, although single SV fusions with the plasma membrane in flower mutants elicit larger amplitudes than in wild-type animals, our data suggest that this is due to the fact that the SVs in flower mutants are larger than wild-type. Indeed, there is a near perfect correlation between the SV size and the size of the mEJPs, as observed in some other endocytic mutants (Koh et al., 2004; Zhang et al., 1998). This suggests that the Flower protein present in the presynaptic membrane affects the resting Ca2+ levels, but does not exclude a role for the protein in SVs.
The second TM domain of Flower contains a 9 amino acid motif similar to the selectivity filters identified in Ca2+-gating TRPV channels (Owsianik et al., 2006). TRPV5 and 6 channels are homo- or multimeric channels that have been shown to form a pore lined by four negatively charged amino acids, similar to VGCCs (Hoenderop et al., 2003). We show that Flower can form tetramers and higher order multimers and that its heterologous expression in salivary gland cells promotes Ca2+ influx. In addition, substitution of a conserved, negatively-charged glutamate (E) residue in the second TM domain with a neutral amino acid (Q) abolishes this Ca2+ influx. Furthermore, the purified Flower protein in proteoliposomes can form a Ca2+-permeable cationic channel. We therefore propose a simple model, depicted in Figure 7E, to account for our data. SV exocytosis is triggered by VGCCs located at active zones. Subsequently, SVs, and hence Flower proteins, are integrated in the presynaptic membrane where they mostly localize to periactive zones, known sites for endocytosis (Marie et al., 2004). A homo-multimeric Flower complex then promotes Ca2+ influx which triggers clathrin-mediated endocytosis. This is also supported by the observation that higher extracellular Ca2+ alleviates the endocytic defect (Figure 3G). We propose that endocytosis removes most, but not all, of the Flower protein, thereby removing a key trigger for endocytosis. Thus, Flower may perform a simple autoregulatory role for itself during endocytosis. This model also addresses how exo-endocytosis coupling may be mediated at presynatic terminals. The remainder of the Flower protein that is not endocytosed may help to regulate basal Ca2+ levels.
Experimental procedures
Drosophila strains and genetics
Control animals for most experiments are the y w; P{ry+ neoFRT}80Bisogenized flies that were used to induce the DB25 and DB56 mutations (Ohyama et al., 2007). For mapping, P element stocks and deficiencies were obtained from the Bloomington Drosophila Stock Center (Zhai et al., 2003). The screen on chromosome 3L and ERG recordings were performed as described previously (Ohyama et al., 2007).
Biochemistry
Synaptic vesicle and cytosolic fractions were prepared as described previously (Schulze et al., 1995; Verstreken et al., 2003). For coimmunoprecipitation, adult head extracts were prepared as described previously (Verstreken et al., 2003). For details see Supplemental data.
Calculation of added membrane
Amount of added membrane (μm2) = JQC × 4πr2, where ‘JQC’ is the junctional quantal content, and 4πr2, is the surface of a sphere (r = SV radius).
FM1-43 dye uptake
FM1-43 dye uptake experiments were performed as described (Verstreken et al., 2008). For details see Supplemental data.
Calcium imaging
NMJs
Third instar larvae of wild-type controls and mutants were dissected at 21°C in HL6 solution (Macleod et al., 2002) with 1 mM CaCl2 and 7 mM glutamate, which desensitizes the glutamate receptors and reduces muscle contractions. To determine resting [Ca2+]i, the resting fluorescence of GCaMP1.6 in NMJ boutons of muscle 13 from hemisegments A2 to A4 were measured. The mean fluorescence density of GCaMP1.6 in boutons of similar sizes in controls and mutants was counted and compared. The amount of GCaMP1.6 protein in boutons was stained and quantified by using a rabbit anti-GFP Ab (1:200, Invitrogen). Samples were fixed in 3.7% formaldehyde for 20 min, mounted, and imaged in the same set-up using a Zeiss 510 confocal microscope. Equidistant confocal slices were scanned. All fluorescent signals were obtained by Z-axis projection and counted using Image J. Background in the muscle was subtracted from bouton labeling. The relative values shown in Figure 5E are the fluorescence signal of GCaMP1.6 normalized by the amount of the GCaMP1.6 protein. To determine the fluorescence kinetics of GCaMP1.6 in shits1 terminals, samples were gradually heated for ~ 6 min from 21°C to 34°C, and, once the temperature was stabilized at 34°C for 1 min, F0 was measured. The nerve axon was taken up by the HL6-filled electrode and stimulated at 30 Hz for 6 min, which causes a fusion of all SVs into the plasma membrane in shits1 terminals. After a 1 min rest period, ΔF was determined. The mean fluorescent density of GCaMP1.6 in type Ib boutons of muscle 13 from hemisegments A2 to A4 was measured and quantified. Fluorescent images were captured through a Zeiss Optical objective (model ACHROPLAN 40X water lens, Zeiss) and an AxioCam MRm CCD camera and processed using AxioVision 4.6.3 (Zeiss). In addition, images from different genotypes were captured with a fixed exposure time of 150 ms. All GCaMP1.6 fluorescence signals were processed using Image J (National Institutes of Health). Background in the muscle was subtracted from bouton’s signal.
Salivary glands
All calcium imaging experiments were performed at room temperature (20–22°C), in modified HL-3 solution (HL-3M) (64 mM NaCl, 5 mM KCl, 20 mM MgCl2, 16 mM NaHCO3, 0.65 mM NaH2PO4, 5 mM trehalose, 113 mM sucrose). Different concentrations of Ca2+ were added. Solutions were always oxygenated with a mixture of 95% O2-5% CO2 to maintain the pH at 7.2. All procedures were performed in the dark. Third instar salivary glands were dissected in cold Schneider’s S2 medium and placed on 25mm poly-D-lysine (0.1 mg/ml) coated coverslips. Loading of the Ca2+ indicator into salivary glands was achieved by incubating tissues in a HL-3M solution with 4 μM Fluo-4 AM (final DMSO concentration is 0.4%), the membrane-permeant form of the fluorescent Ca2+ indicator (F-14201, Molecular Probes). For GCaMP1.6 experiments, salivary glands were dissected similarly and incubated with HL-3M in various Ca2+ concentrations. Fluorescence images were captured through a Zeiss Optical objective (model Plan-NeoFLUAR 2.5X, Zeiss) and an AxioCam MRm CCD camera and processed using AxioVision 4.6.3 (Zeiss). Images were captured every 10 min during dye loading with fixed exposure time of 2 s. Fluorescence intensity from images was calculated by subtracting background in the medium and quantified using Image J (National Institutes of Health).
Protein expression and purification
The purification procedure of the FlowerWT-1D4 fusion protein was described previously (Moiseenkova-Bell et al., 2008). The coding sequence of flower-1D4 was PCR-amplified and subcloned into the yeast expression plasmid YEpHIS (Figler et al., 2000) with a strong constitutive PMA1 promoter. A 1D4 immunoaffinity tag (TETSQVAPA) was added at the C-terminus to enable purification. The protease-deficient S. cerevisiae yeast strain BJ5457 was used for overexpression. Overexpression and plasma membrane isolation were performed as described (Moiseenkova et al., 2003). Plasma membrane derived from a 5L culture of yeast transformed with flower-1D4 were solubilized at 4°C with 15 mM Foscholine-14 (Anatrace) in 25 mM Tris-Cl (pH 8.0), 200 mM NaCl, 15 mM EDTA, and 1 mM PMSF for 2 h. Insoluble membranes were removed by centrifugation at 100,000 × g for 40 min, using the Type 45 Ti rotor (Beckman Coulter). The supernatant containing the Flower-1D4 protein was added to an affinity column of CNBr-activated Sepharose 4B coupled to the 1D4 antibody (3 ml, 3–4 mg of Ab/ml)(Yu and Oprian, 1999). After washing with 25 mM Tris-Cl (pH 8.0), 200 mM NaCl, 15 mM EDTA, 1 mM PMSF, and 1.2 mM Foscholine-14, the protein was eluted by adding 1 mg/ml of 1D4 peptide into the washing buffer. Purified protein was resolved by electrophoresis on a 10% SDS-PAGE gel and detected by Coomassie Blue staining. For crossing experiments, purified proteins or proteoliposomes were incubated with different crosslinkers on ice for 1 hr, and the reaction was terminated by adding 100 mM Tris-Cl (pH 7.5) on ice for 15 min. Samples were incubated with 2% SDS sample buffer (without β-mercaptoethanol) and run on a 10% SDS gel. All crosslinkers were obtained from Invitrogen. Method for the PFO/PAGE is described as previously (Ramjeesingh et al., 1999). Multimers were observed on western blots with the anti-1D4 antibody.
Radioactive 45Ca2+ influx assay
Phospholipids (soybean azolectin, Sigma) were solubilized with 400 mM n-octyl-β-D-glycopyranoside (Anatrace), 25 mM Tris-Cl (pH 8.0), and 200 mM NaCl. For preparation of proteoliposomes, 1 mg of detergent-solubilized phospholipids were mixed with 5 μg of purified Flower-1D4 protein with a final volume of 200 μl in 25 mM Tris-Cl (pH 8.0) and 200 mM NaCl, such that the ratio of purified Flower-1D4 protein to phospholipids was 1 to 200. For preparation of empty liposomes, 1 mg of phospholipids was mixed with the appropriate volume of elution buffer used to purify proteins. Detergent was removed by dialyzing at 4°C for 4 days with 25 mM Tris-Cl (pH 8.0) and 200 mM NaCl to prepare unilamellar vesicles. The dialysis buffer was changed daily. After liposome formation, unincorporated proteins were precipitated by centrifuging at 12,000 × g for 10 min. Phospholipids from supernatant were quantified as inorganic phosphate after hydrolysis. The concentration of phospholipids from empty liposomes (3.5 μg/μl) and the Flower-1D4 containing liposomes (3.8 μg/μl) is similar. A 40 μl aliquot (~140 μg of lipids) was mixed with 360 μl of assay buffer (25 mM Tris-Cl (pH 8.0), 198.3 mM NMDG-Cl, 1.11 mM CaCl2 including 25 μCi 45CaCl2/ml. The final concentration of [Na+]o is 20 mM, which may create a driving force for Ca2+ permeability if Flower functions as Ca2+ channels that are permeable for monovalent ions (Owsianik et al., 2006). In addition, the gradient between 1 mM [Ca2+]o and low μM [Ca2+]i is close to a physiological condition. Liposomes were incubated for different time periods (15, 45, and 90 min). Furthermore, at the end of the 90 min incubation, the liposomes were perforated using 2.5 μM ionomycin to release all their entrapped Ca2+ contents. [Ca2+]o was dramatically reduced to low nM levels by sequentially mixing liposomes with the Ca2+ sponges Chelex 100 (Bio-rad) resins for 5 min twice and BAPTA resins for 5 min once (Invitrogen) (Meyer et al., 1990). The resins were spun down, and supernatants containing liposomes were mixed with scintillation liquid. The 45Ca2+ uptake by liposomes was calculated by scintillation counter. The value remaining after 90 min ionomycin treatment was considered as residual [Ca2+]o that is not removed after treatment with Ca2+ sponges. As shown in Figure 7D, the uptake data was normalized by subtracting the residual [Ca2+]o.
Statistical analysis
Student’s t-test was used in all statistical analyses and performed using GraphPad Prism (GraphPad Software, Inc.).
Supplementary Material
Acknowledgements
We thank Yuchun He and Hongling Pan for creating transgenic animals and thank Yu Cao and Zhihua Wang for technical assistance. We thank Ben Tobe, Julie Vargas, Frank T. Horrigan for helping radioactive isotope experiments. We thank Karen L. Schulze for editing the manuscript. We thank Christian Rosenmund, Huda Zoghbi, Patrik Verstreken, Robin Hiesinger, Paul Pfaffinger, Ellen Lumpkin, Mingshan Xue, and Nikos Giagtzoglou for comments and discussions. We thank the Bloomington Stock Center and the Developmental Studies Hybridoma Bank for reagents. C.V.L. was supported by an NRSA from the NINDS (F30NS056520). Confocal microscopy was supported by the BCM Intellectual and Developmental Disabilities Research Center. H.J.B. is an investigator of the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adler EM, Augustine GJ, Duffy SN, Charlton MP. Alien intracellular calcium chelators attenuate neurotransmitter release at the squid giant synapse. J Neurosci. 1991;11:1496–1507. doi: 10.1523/JNEUROSCI.11-06-01496.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellen HJ, Levis RW, Liao G, He Y, Carlson JW, Tsang G, Evans-Holm M, Hiesinger PR, Schulze KL, Rubin GM, et al. The BDGP gene disruption project: single transposon insertions associated with 40% of Drosophila genes. Genetics. 2004;167:761–781. doi: 10.1534/genetics.104.026427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall WA, Few AP. Calcium channel regulation and presynaptic plasticity. Neuron. 2008;59:882–901. doi: 10.1016/j.neuron.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Cousin MA. Synaptic vesicle endocytosis: calcium works overtime in the nerve terminal. Mol Neurobiol. 2000;22:115–128. doi: 10.1385/MN:22:1-3:115. [DOI] [PubMed] [Google Scholar]
- Cousin MA, Robinson PJ. Ba2+ does not support synaptic vesicle retrieval in rat cerebrocortical synaptosomes. Neurosci Lett. 1998;253:1–4. doi: 10.1016/s0304-3940(98)00610-7. [DOI] [PubMed] [Google Scholar]
- Cousin MA, Robinson PJ. Ca(2+) influx inhibits dynamin and arrests synaptic vesicle endocytosis at the active zone. J Neurosci. 2000;20:949–957. doi: 10.1523/JNEUROSCI.20-03-00949.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado R, Maureira C, Oliva C, Kidokoro Y, Labarca P. Size of vesicle pools, rates of mobilization, and recycling at neuromuscular synapses of a Drosophila mutant, shibire. Neuron. 2000;28:941–953. doi: 10.1016/s0896-6273(00)00165-3. [DOI] [PubMed] [Google Scholar]
- Dickman DK, Horne JA, Meinertzhagen IA, Schwarz TL. A slowed classical pathway rather than kiss-and-run mediates endocytosis at synapses lacking synaptojanin and endophilin. Cell. 2005;123:521–533. doi: 10.1016/j.cell.2005.09.026. [DOI] [PubMed] [Google Scholar]
- Dickman DK, Lu Z, Meinertzhagen I, Schwarz TL. Altered synaptic development and active zone spacing in endocytosis mutants. Curr Biol. 2006;16:591–598. doi: 10.1016/j.cub.2006.02.058. [DOI] [PubMed] [Google Scholar]
- Fabian-Fine R, Verstreken P, Hiesinger PR, Horne JA, Kostyleva R, Zhou Y, Bellen HJ, Meinertzhagen IA. Endophilin promotes a late step in endocytosis at glial invaginations in Drosophila photoreceptor terminals. J Neurosci. 2003;23:10732–10744. doi: 10.1523/JNEUROSCI.23-33-10732.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas R, Sutakova G. Ultrastructural changes of Drosophila larval and prepupal salivary glands cultured in vitro with ecdysone. In Vitro Cell Dev Biol Anim. 1998;34:813–823. doi: 10.1007/s11626-998-0036-7. [DOI] [PubMed] [Google Scholar]
- Fergestad T, Davis WS, Broadie K. The stoned proteins regulate synaptic vesicle recycling in the presynaptic terminal. J Neurosci. 1999;19:5847–5860. doi: 10.1523/JNEUROSCI.19-14-05847.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figler RA, Omote H, Nakamoto RK, Al-Shawi MK. Use of chemical chaperones in the yeast Saccharomyces cerevisiae to enhance heterologous membrane protein expression: high-yield expression and purification of human P-glycoprotein. Arch Biochem Biophys. 2000;376:34–46. doi: 10.1006/abbi.2000.1712. [DOI] [PubMed] [Google Scholar]
- Gad H, Low P, Zotova E, Brodin L, Shupliakov O. Dissociation between Ca2+-triggered synaptic vesicle exocytosis and clathrin-mediated endocytosis at a central synapse. Neuron. 1998;21:607–616. doi: 10.1016/s0896-6273(00)80570-x. [DOI] [PubMed] [Google Scholar]
- Guatimosim C, Romano-Silva MA, Gomez MV, Prado MA. Recycling of synaptic vesicles at the frog neuromuscular junction in the presence of strontium. J Neurochem. 1998;70:2477–2483. doi: 10.1046/j.1471-4159.1998.70062477.x. [DOI] [PubMed] [Google Scholar]
- Guo X, Macleod GT, Wellington A, Hu F, Panchumarthi S, Schoenfield M, Marin L, Charlton MP, Atwood HL, Zinsmaier KE. The GTPase dMiro is required for axonal transport of mitochondria to Drosophila synapses. Neuron. 2005;47:379–393. doi: 10.1016/j.neuron.2005.06.027. [DOI] [PubMed] [Google Scholar]
- Gwack Y, Srikanth S, Feske S, Cruz-Guilloty F, Oh-hora M, Neems DS, Hogan PG, Rao A. Biochemical and functional characterization of Orai proteins. J Biol Chem. 2007;282:16232–16243. doi: 10.1074/jbc.M609630200. [DOI] [PubMed] [Google Scholar]
- Heidelberger R, Heinemann C, Neher E, Matthews G. Calcium dependence of the rate of exocytosis in a synaptic terminal. Nature. 1994;371:513–515. doi: 10.1038/371513a0. [DOI] [PubMed] [Google Scholar]
- Henkel AW, Betz WJ. Monitoring of black widow spider venom (BWSV) induced exo- and endocytosis in living frog motor nerve terminals with FM1-43. Neuropharmacology. 1995;34:1397–1406. doi: 10.1016/0028-3908(95)00126-q. [DOI] [PubMed] [Google Scholar]
- Hoenderop JG, Voets T, Hoefs S, Weidema F, Prenen J, Nilius B, Bindels RJ. Homo- and heterotetrameric architecture of the epithelial Ca2+ channels TRPV5 and TRPV6. Embo J. 2003;22:776–785. doi: 10.1093/emboj/cdg080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingauf J, Kavalali ET, Tsien RW. Kinetics and regulation of fast endocytosis at hippocampal synapses. Nature. 1998;394:581–585. doi: 10.1038/29079. [DOI] [PubMed] [Google Scholar]
- Koenig JH, Ikeda K. Disappearance and reformation of synaptic vesicle membrane upon transmitter release observed under reversible blockage of membrane retrieval. J Neurosci. 1989;9:3844–3860. doi: 10.1523/JNEUROSCI.09-11-03844.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh TW, Korolchuk VI, Wairkar YP, Jiao W, Evergren E, Pan H, Zhou Y, Venken KJ, Shupliakov O, Robinson IM, et al. Eps15 and Dap160 control synaptic vesicle membrane retrieval and synapse development. J Cell Biol. 2007;178:309–322. doi: 10.1083/jcb.200701030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh TW, Verstreken P, Bellen HJ. Dap160/intersectin acts as a stabilizing scaffold required for synaptic development and vesicle endocytosis. Neuron. 2004;43:193–205. doi: 10.1016/j.neuron.2004.06.029. [DOI] [PubMed] [Google Scholar]
- Kuromi H, Honda A, Kidokoro Y. Ca2+ influx through distinct routes controls exocytosis and endocytosis at drosophila presynaptic terminals. Neuron. 2004;41:101–111. doi: 10.1016/s0896-6273(03)00815-8. [DOI] [PubMed] [Google Scholar]
- Kuromi H, Kidokoro Y. Selective replenishment of two vesicle pools depends on the source of Ca2+ at the Drosophila synapse. Neuron. 2002;35:333–343. doi: 10.1016/s0896-6273(02)00777-8. [DOI] [PubMed] [Google Scholar]
- Lahey T, Gorczyca M, Jia XX, Budnik V. The Drosophila tumor suppressor gene dlg is required for normal synaptic bouton structure. Neuron. 1994;13:823–835. doi: 10.1016/0896-6273(94)90249-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai MM, Hong JJ, Ruggiero AM, Burnett PE, Slepnev VI, De Camilli P, Snyder SH. The calcineurin-dynamin 1 complex as a calcium sensor for synaptic vesicle endocytosis. J Biol Chem. 1999;274:25963–25966. doi: 10.1074/jbc.274.37.25963. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Raghavachari S, Tsien RW. The sequence of events that underlie quantal transmission at central glutamatergic synapses. Nat Rev Neurosci. 2007;8:597–609. doi: 10.1038/nrn2191. [DOI] [PubMed] [Google Scholar]
- Littleton JT, Stern M, Schulze K, Perin M, Bellen HJ. Mutational analysis of Drosophila synaptotagmin demonstrates its essential role in Ca(2+)-activated neurotransmitter release. Cell. 1993;74:1125–1134. doi: 10.1016/0092-8674(93)90733-7. [DOI] [PubMed] [Google Scholar]
- Macleod GT, Hegstrom-Wojtowicz M, Charlton MP, Atwood HL. Fast calcium signals in Drosophila motor neuron terminals. J Neurophysiol. 2002;88:2659–2663. doi: 10.1152/jn.00515.2002. [DOI] [PubMed] [Google Scholar]
- Macleod GT, Marin L, Charlton MP, Atwood HL. Synaptic vesicles: test for a role in presynaptic calcium regulation. J Neurosci. 2004;24:2496–2505. doi: 10.1523/JNEUROSCI.5372-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marie B, Sweeney ST, Poskanzer KE, Roos J, Kelly RB, Davis GW. Dap160/intersectin scaffolds the periactive zone to achieve high-fidelity endocytosis and normal synaptic growth. Neuron. 2004;43:207–219. doi: 10.1016/j.neuron.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Marks B, McMahon HT. Calcium triggers calcineurin-dependent synaptic vesicle recycling in mammalian nerve terminals. Curr Biol. 1998;8:740–749. doi: 10.1016/s0960-9822(98)70297-0. [DOI] [PubMed] [Google Scholar]
- Meyer T, Wensel T, Stryer L. Kinetics of calcium channel opening by inositol 1,4,5-trisphosphate. Biochemistry. 1990;29:32–37. doi: 10.1021/bi00453a004. [DOI] [PubMed] [Google Scholar]
- Moiseenkova VY, Hellmich HL, Christensen BN. Overexpression and purification of the vanilloid receptor in yeast (Saccharomyces cerevisiae) Biochem Biophys Res Commun. 2003;310:196–201. doi: 10.1016/j.bbrc.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Moiseenkova-Bell VY, Stanciu LA, Serysheva II, Tobe BJ, Wensel TG. Structure of TRPV1 channel revealed by electron cryomicroscopy. Proc Natl Acad Sci U S A. 2008;105:7451–7455. doi: 10.1073/pnas.0711835105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale EA, Bowers LM, Jia M, Bateman KE, Williamson LC. Botulinum neurotoxin A blocks synaptic vesicle exocytosis but not endocytosis at the nerve terminal. J Cell Biol. 1999;147:1249–1260. doi: 10.1083/jcb.147.6.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkura M, Matsuzaki M, Kasai H, Imoto K, Nakai J. Genetically encoded bright Ca2+ probe applicable for dynamic Ca2+ imaging of dendritic spines. Anal Chem. 2005;77:5861–5869. doi: 10.1021/ac0506837. [DOI] [PubMed] [Google Scholar]
- Ohyama T, Verstreken P, Ly CV, Rosenmund T, Rajan A, Tien AC, Haueter C, Schulze KL, Bellen HJ. Huntingtin-interacting protein 14, a palmitoyl transferase required for exocytosis and targeting of CSP to synaptic vesicles. J Cell Biol. 2007;179:1481–1496. doi: 10.1083/jcb.200710061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owsianik G, Talavera K, Voets T, Nilius B. Permeation and selectivity of TRP channels. Annu Rev Physiol. 2006;68:685–717. doi: 10.1146/annurev.physiol.68.040204.101406. [DOI] [PubMed] [Google Scholar]
- Petrovich TZ, Merakovsky J, Kelly LE. A genetic analysis of the stoned locus and its interaction with dunce, shibire and Suppressor of stoned variants of Drosophila melanogaster. Genetics. 1993;133:955–965. doi: 10.1093/genetics/133.4.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakriya M, Feske S, Gwack Y, Srikanth S, Rao A, Hogan PG. Orai1 is an essential pore subunit of the CRAC channel. Nature. 2006;443:230–233. doi: 10.1038/nature05122. [DOI] [PubMed] [Google Scholar]
- Ramaswami M, Krishnan KS, Kelly RB. Intermediates in synaptic vesicle recycling revealed by optical imaging of Drosophila neuromuscular junctions. Neuron. 1994;13:363–375. doi: 10.1016/0896-6273(94)90353-0. [DOI] [PubMed] [Google Scholar]
- Ramjeesingh M, Huan LJ, Garami E, Bear CE. Novel method for evaluation of the oligomeric structure of membrane proteins. Biochem J. 1999;342(Pt 1):119–123. [PMC free article] [PubMed] [Google Scholar]
- Reiff DF, Ihring A, Guerrero G, Isacoff EY, Joesch M, Nakai J, Borst A. In vivo performance of genetically encoded indicators of neural activity in flies. J Neurosci. 2005;25:4766–4778. doi: 10.1523/JNEUROSCI.4900-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankaranarayanan S, Ryan TA. Calcium accelerates endocytosis of vSNAREs at hippocampal synapses. Nat Neurosci. 2001;4:129–136. doi: 10.1038/83949. [DOI] [PubMed] [Google Scholar]
- Sather WA, McCleskey EW. Permeation and selectivity in calcium channels. Annu Rev Physiol. 2003;65:133–159. doi: 10.1146/annurev.physiol.65.092101.142345. [DOI] [PubMed] [Google Scholar]
- Schneggenburger R, Neher E. Intracellular calcium dependence of transmitter release rates at a fast central synapse. Nature. 2000;406:889–893. doi: 10.1038/35022702. [DOI] [PubMed] [Google Scholar]
- Schulze KL, Broadie K, Perin MS, Bellen HJ. Genetic and electrophysiological studies of Drosophila syntaxin-1A demonstrate its role in nonneuronal secretion and neurotransmission. Cell. 1995;80:311–320. doi: 10.1016/0092-8674(95)90414-x. [DOI] [PubMed] [Google Scholar]
- Stowers RS, Schwarz TL. A genetic method for generating Drosophila eyes composed exclusively of mitotic clones of a single genotype. Genetics. 1999;152:1631–1639. doi: 10.1093/genetics/152.4.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tweedie S, Ashburner M, Falls K, Leyland P, McQuilton P, Marygold S, Millburn G, Osumi-Sutherland D, Schroeder A, Seal R, Zhang H. FlyBase: enhancing Drosophila Gene Ontology annotations. Nucleic Acids Res. 2009;37:D555–559. doi: 10.1093/nar/gkn788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstreken P, Kjaerulff O, Lloyd TE, Atkinson R, Zhou Y, Meinertzhagen IA, Bellen HJ. Endophilin mutations block clathrin-mediated endocytosis but not neurotransmitter release. Cell. 2002;109:101–112. doi: 10.1016/s0092-8674(02)00688-8. [DOI] [PubMed] [Google Scholar]
- Verstreken P, Koh TW, Schulze KL, Zhai RG, Hiesinger PR, Zhou Y, Mehta SQ, Cao Y, Roos J, Bellen HJ. Synaptojanin is recruited by endophilin to promote synaptic vesicle uncoating. Neuron. 2003;40:733–748. doi: 10.1016/s0896-6273(03)00644-5. [DOI] [PubMed] [Google Scholar]
- Verstreken P, Ohyama T, Bellen HJ. FM 1-43 labeling of synaptic vesicle pools at the Drosophila neuromuscular junction. Methods Mol Biol. 2008;440:349–369. doi: 10.1007/978-1-59745-178-9_26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vig M, Beck A, Billingsley JM, Lis A, Parvez S, Peinelt C, Koomoa DL, Soboloff J, Gill DL, Fleig A, et al. CRACM1 multimers form the ion-selective pore of the CRAC channel. Curr Biol. 2006;16:2073–2079. doi: 10.1016/j.cub.2006.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Gersdorff H, Matthews G. Inhibition of endocytosis by elevated internal calcium in a synaptic terminal. Nature. 1994;370:652–655. doi: 10.1038/370652a0. [DOI] [PubMed] [Google Scholar]
- Wagh DA, Rasse TM, Asan E, Hofbauer A, Schwenkert I, Durrbeck H, Buchner S, Dabauvalle MC, Schmidt M, Qin G, et al. Bruchpilot, a Protein with Homology to ELKS/CAST, Is Required for Structural Integrity and Function of Synaptic Active Zones in Drosophila. Neuron. 2006;49:833–844. doi: 10.1016/j.neuron.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Xiong J, Verkhratsky A, Toescu EC. Changes in mitochondrial status associated with altered Ca2+ homeostasis in aged cerebellar granule neurons in brain slices. J Neurosci. 2002;22:10761–10771. doi: 10.1523/JNEUROSCI.22-24-10761.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Oprian DD. Tertiary interactions between transmembrane segments 3 and 5 near the cytoplasmic side of rhodopsin. Biochemistry. 1999;38:12033–12040. doi: 10.1021/bi9909492. [DOI] [PubMed] [Google Scholar]
- Zefirov AL, Abdrakhmanov MM, Mukhamedyarov MA, Grigoryev PN. The role of extracellular calcium in exo- and endocytosis of synaptic vesicles at the frog motor nerve terminals. Neuroscience. 2006;143:905–910. doi: 10.1016/j.neuroscience.2006.08.025. [DOI] [PubMed] [Google Scholar]
- Zhai RG, Hiesinger PR, Koh TW, Verstreken P, Schulze KL, Cao Y, Jafar-Nejad H, Norga KK, Pan H, Bayat V, et al. Mapping Drosophila mutations with molecularly defined P element insertions. Proc Natl Acad Sci U S A. 2003;100:10860–10865. doi: 10.1073/pnas.1832753100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Koh YH, Beckstead RB, Budnik V, Ganetzky B, Bellen HJ. Synaptic vesicle size and number are regulated by a clathrin adaptor protein required for endocytosis. Neuron. 1998;21:1465–1475. doi: 10.1016/s0896-6273(00)80664-9. [DOI] [PubMed] [Google Scholar]
- Zinsmaier KE, Eberle KK, Buchner E, Walter N, Benzer S. Paralysis and early death in cysteine string protein mutants of Drosophila. Science. 1994;263:977–980. doi: 10.1126/science.8310297. [DOI] [PubMed] [Google Scholar]
- Zipursky SL, Venkatesh TR, Teplow DB, Benzer S. Neuronal development in the Drosophila retina: monoclonal antibodies as molecular probes. Cell. 1984;36:15–26. doi: 10.1016/0092-8674(84)90069-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.