Abstract
The voltage-gated Kv1.3 channel and the Ca2+ -activated IKCa1 K+ channel are expressed in T cells in a distinct pattern that depends on the state of lymphocyte activation and differentiation. The channel phenotype changes during the progression from the resting to the activated cell state and from naïve to effector memory cells, affording promise for specific immunomodulatory actions of K+ channel blockers. In this article, we review the functional roles of these channels in both naïve cells and memory cells, describe the development of selective inhibitors of Kv1.3 and IKCa1 channels, and provide a rationale for the potential therapeutic use of these inhibitors in immunological disorders.
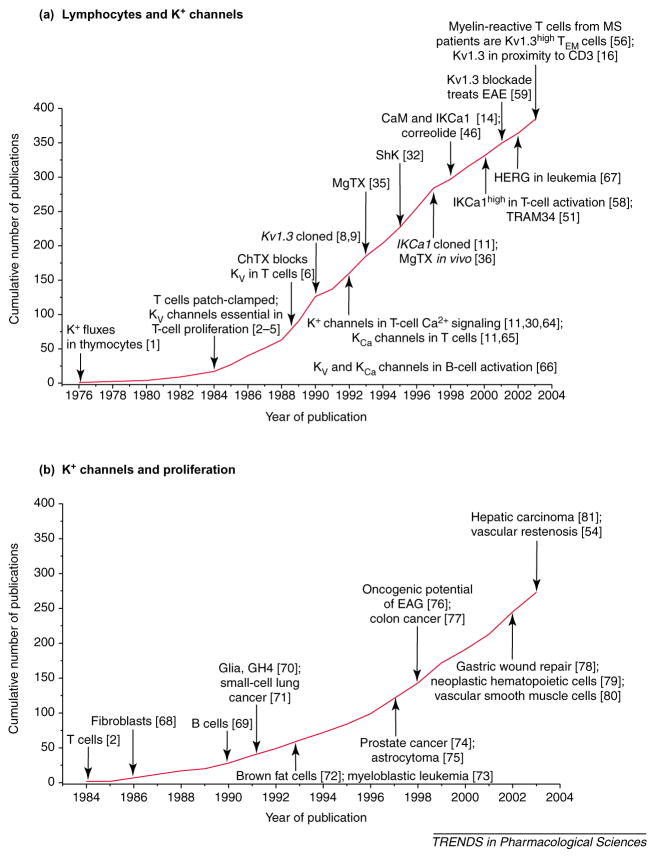
In the 1950s, James Gowans’ research group defined the central role of lymphocytes in immune responses, and in the 1960s the seminal work of Jacques Miller and Robert Good and their colleagues elucidated the crucial role of thymus-derived lymphocytes (T cells). Ionic movements associated with mitogen activation of lymphocytes were first reported in the 1970s [1], but ion channels and electrophysiological studies were far removed from the consciousness of immunologists during this period, being the domain of those investigating excitable cells (nerves and muscles). This barrier was breached in 1984 with the first patch-clamp studies on K+ channels in lymphocytes [2–4], and during the past two decades there has been a steady growth of publications in this research area (Figure 1a).
Figure 1.
The cumulative number of publications as of November 2003 on (a) ‘lymphocytes and K+ channels’ and (b) ‘K+ channels and proliferation’. Key discoveries in both fields are highlighted. Abbreviations: CaM, calmodulin; ChTX, charybdotoxin; EAE, experimental autoimmune encephalomyelitis; EAG, ether a-go-go; IKCa1, intermediate-conductance Ca2+ -activated K+ channel subtype 1; HERG, human ether-à-go-go-related gene; Kv1.3high, high levels of Kv1.3 channels; MgTX, margatoxin; MS, multiple sclerosis; ShK, Stichodactyla helianthus toxin; TEM cells, effector memory T cells. (Data are from [1–6,8,9,11,14,16,30,32,35,36,46,51,54,56,58,59,64–81].)
In addition to characterizing the biophysical properties of the voltage-gated KV channel in T cells, we showed that these channels are important for T-cell activation by demonstrating that several chemically unrelated K+ channel blockers suppressed proliferation and cytokine production with potencies paralleling channel blockade [2,5]. The serendipitous discovery in 1989 that charybdotoxin (ChTX), a peptide from the venom of the scorpion Leiurus quinquestriatus, blocks the KV channel at nanomolar concentrations [6] led to the isolation of increasingly potent and selective channel inhibitors from scorpion venom, sea anemone extracts and plants [7]. The pace of discovery accelerated with the demonstration that the KCNA3 (Kv1.3; http://www.iuphar-db.org/iuphar-ic/KV1x.html) gene encodes the lymphocyte KV channel [8,9]. An intermediate-conductance Ca2+ -activated K+ channel was identified in T cells in 1992 [10–12], and shown to be a product of the KCNN4 (IKCa1, KCa3.1; http://www.iuphar-db.org/iuphar-ic/KCa.html) gene in 1997 [13]. Subsequent studies by our group identified calmodulin as the Ca2+ sensor of the IKCa1 channel [14]. The salient features of both channels were summarized in a recent review [15].
Following the discovery that K+ channels are essential for T-cell function, several other K+ channels have been implicated in the proliferation of a wide variety of normal and malignant cells (Figure 1b). In this review, we define the physiological roles of Kv1.3 and IKCa1 channels in T cells, describe the development of increasingly selective channel inhibitors and discuss the rationale for their possible use as therapeutic immunomodulators.
Physiological roles of Kv1.3 and IKCa1 channels in T cells
Molecular interactions at the immunological synapse
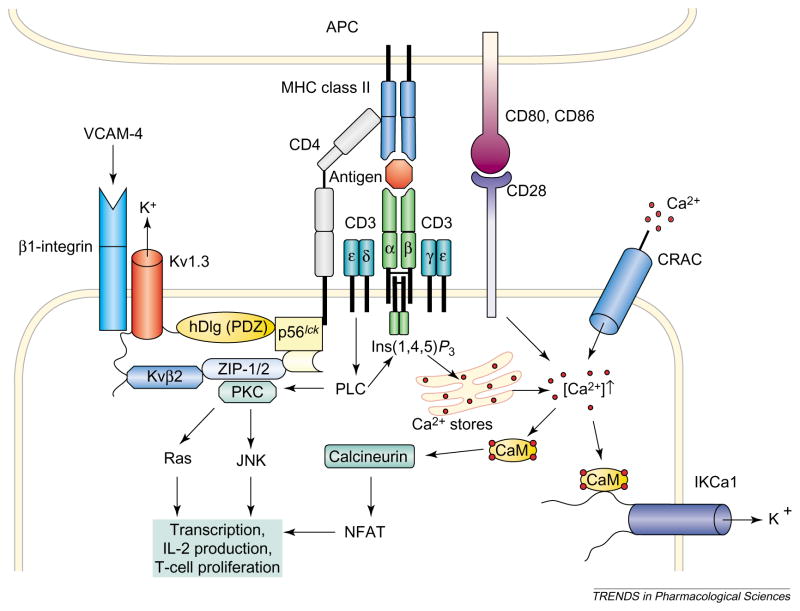
The T-cell-mediated immune response is initiated by recognition of processed antigenic peptide bound to major histocompatibility complex (MHC) proteins on antigen-presenting cells (APCs) by the antigen-specific multi-subunit T-cell receptor (TCR)–CD3 complex on T cells (Figure 2). Within minutes the TCR–CD3 complex and accessory proteins cluster at the zone of contact with the APC in a region termed the ‘immunological synapse’ (IS). Antigen-induced redistribution of key receptors and signaling molecules at the IS facilitates transduction of signals that activate T cells. A recent fluorescence resonance energy transfer (FRET) study in lymphocytes transfected with Kv1.3 channels showed that the channel was in close proximity to CD3 [16]. Biochemical experiments [17–22] also suggest that the Kv1.3 channel is part of a signaling complex that includes enzymes [tyrosine kinase p56lck and protein kinase C (PKC)], adaptor proteins [Kvβ2 and hDlg (human homolog of the Drosophila discs large tumor suppressor protein), PSD-95 (postsynaptic density 95), ZIP-1 (Zrt/Irt-like protein) and ZIP2] and the accessory protein CD4. Tyrosine phosphorylation of Kv1.3 channels and modulation of Kv1.3 channel currents by p56lck further suggests that the clustering of Kv1.3 channels and p56lck could provide a mechanism for regulating channel function [17–19,21,22]. Clustering interactions of Kv1.4 channels with PSD-95 inhibit the internalization of Kv1.4 channels [23] and the same could be true for Kv1.3 channels. Because CD4 and CD3 aggregate at the IS, it is conceivable that the envisaged CD4–Kv1.3 channel complex localizes at the IS and regulates early events in T-cell activation. A recent study of cytotoxic T cells transfected with Kv1.3 channels supports this notion by demonstrating clustering of Kv1.3 channels at the site of interaction of cytotoxic T cells with the target cell [24]. The Kv1.3 channel has also been reported to be physically and functionally coupled to β1-integrins [25] that stabilize the IS and are also important in lymphocyte adhesion and migration.
Figure 2.
The involvement of voltage-dependent Kv1.3 channels, intermediate-conductance Ca2+ -activated IKCa1 channels and voltage-independent Ca2+ release-activated Ca2+ (CRAC) channels in the activation of a CD4+ T cells by an antigen-presenting cell (APC) is shown. Engagement of the T-cell receptor–CD3 complex through an antigenic peptide presented in the context of major histocompatibility complex (MHC) class II activates phospholipase C (PLC), which leads to the activation of protein kinase C (PKC) and the production of inositol (1,4,5)-trisphosphate [Ins(1,4,5) P3], which liberates Ca2+ from intracellular stores. Simultaneous activation of CD28 by the co-stimulatory molecules CD80 or CD86 further amplifies the resulting Ca2+ signal. The rise in the intracellular concentration of Ca2+ activates the phosphatase calcineurin, which then dephosphorylates the transcription factor nuclear factor of activated T cells (NFAT), enabling it to accumulate in the nucleus and bind to the promoter of the gene encoding interleukin 2 (IL-2). Parallel activation of the c-JUN N-terminal kinase (JNK) and Ras by PKC results in the activation of other transcription factors and initiates transcription of various genes and finally T-cell proliferation. CRAC, Kv1.3 and IKCa1 channels regulate Ca2+ signaling. Depletion of internal Ca2+ stores causes CRAC channels in the membrane to open, and the ensuing Ca2+ influx sustains elevated levels of cytosolic Ca2+. Ca2+ influx through CRAC channels is reduced following membrane depolarization. The driving force for Ca2+ entry is restored by membrane hyperpolarization brought about by the opening of Kv1.3 channels in response to membrane depolarization and the opening of IKCa1 channels as a consequence of elevated concentrations of cytosolic Ca2+. Selective blockade of K+ channels leads to membrane depolarization, inhibits Ca2+ influx and shuts down cytokine production and cell proliferation. Abbreviations: CaM, calmodulin; hDlg, human homolog of the Drosophila discs large tumor suppressor protein; VCAM-4, vascular cell adhesion molecule 4; ZIP-1, Zrt/Irt-like protein.
K+ channels and Ca2+ signaling
The activation events following T-cell engagement with antigen and the role of Kv1.3 and IKCa1 channels in this signaling cascade are shown in Figure 2 [15,22]. Antigen recognition leads to the activation of tyrosine kinases and phospholipase C (PLC), resulting in the generation of inositol (1,4,5)-trisphosphate [Ins(1,4,5)P3] and diacylglycerol, which induce the release of Ca2+ from internal stores, and the activation of PKC. Depletion of internal Ca2+ stores causes voltage-independent Ca2+ release-activated Ca2+ (CRAC) channels to open in the membrane, and the ensuing Ca2+ influx sustains elevated levels of cytosolic Ca2+. The physiological significance of the Ca2+ signal mediated by CRAC channels is highlighted by their absence in severe-combined immunodeficiency patients [26,27] and by the requirement of sustained Ca2+ influx for 75% of new gene expression via the phosphatase calcineurin [27,28]. Coordinated activity of Ca2+ - and PKC-dependent signaling pathways culminates in cell proliferation.
Kv1.3 and IKCa1 channels regulate Ca2+ signaling events via the control of membrane potential [15,22]. Ca2+ influx through CRAC channels is reduced at depolarized potentials and consequently membrane depolarization attenuates the Ca2+ signal. The driving force for Ca2+ entry is restored by membrane hyperpolarization brought about by the opening of Kv1.3 channels in response to membrane depolarization and the opening of IKCa1 channels as a consequence of elevated concentrations of cytosolic Ca2+. Selective blockade of K+ channels leads to membrane depolarization, inhibition of Ca2+ influx and inhibition of cytokine production and cell proliferation.
Pharmacology of Kv1.3 and IKCa1 channels
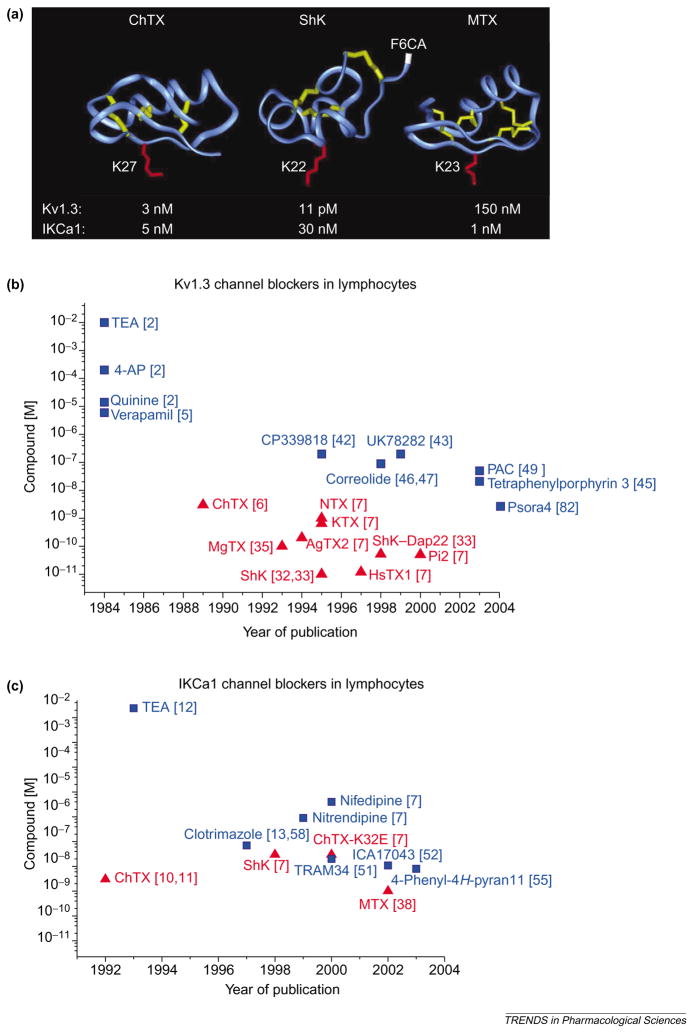
The roles of Kv1.3 and IKCa1 channels have been defined in T cells with the help of structurally diverse peptides, small molecules and metal ions that block these channels with potencies ranging from picomolar to millimolar values. Figures 3a and 4 display the chemical structures of selected peptide and small organic inhibitors, whereas Tables 1 and 2 provide lists of Kv1.3 and IKCa1 channel modulators in order of decreasing potency. For Kv1.3, but not IKCa1, channels the potency of peptide inhibitors exceeds by orders of magnitude the potency of the small organic inhibitors described thus far, as shown graphically in plots of blocker potency versus the year of publication for both channels (Figures 3b,c).
Figure 3.
(a) Backbone structures of charybdotoxin (ChTX), Stichodactyla helianthus toxin (ShK) and maurotoxin (MTX), which are peptide inhibitors of voltage-dependent Kv1.3 channels and intermediate-conductance Ca2+ -activated IKCa1 channels. The crucial lysine that occludes the channel pore is highlighted in red in each structure: Lys27 in ChTX; Lys22 in ShK; and Lys23 in MTX. The disulfide bonds are highlighted in yellow. Arg1 in ShK, to which the fluorophore fluorescein-6-carboxylic acid (F6CA) is attached, is highlighted in white. Such fluorophores attached to specific inhibitors can be used to visualize these K+ channels. (b,c) Plots of potency versus year of publication for peptide (red; triangles) and small-molecule (blue; squares) blockers of Kv1.3 (b) and IKCa1 (c) channels in lymphocytes are shown. Abbreviations: AgTX2, agio-toxin-2; 4-AP, 4-aminopyridine; ChTX-K32E, charybdotoxin derivative with glutamate at position 32 in place of the native lysine; HsTX1, Heterometrus spinnifer toxin 1; KTX, kaliotoxin; MgTX, margatoxin; NTX, noxiustoxin; PAC, 4-phenyl-4-[3-(2-methoxyphenyl)-3-oxo-2-azaprop-1-yl]cyclohexanone; Pi2, Pandinus imperator toxin 2; ShK–Dap22, Stichodactyla helianthus toxin with diaminopropionic acid introduced at position 22 in place of the native lysine; TEA, tetraethylammonium chloride. See Chemical names. (Data are from [2,5–7,10–13,32,33,35,38,42,43,45–47,49,51,52,55,58,82].)
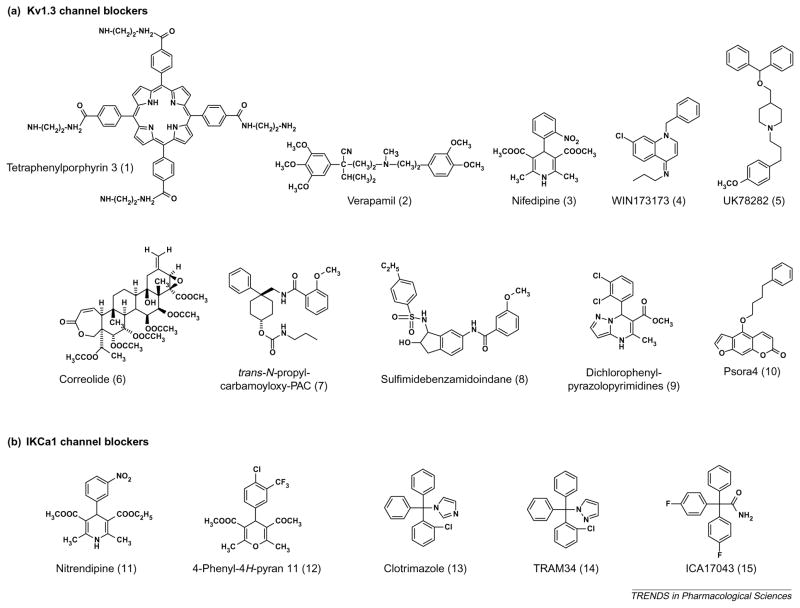
Figure 4.
Structures of small-molecule Kv1.3 channel and IKCa1 channel blockers. See Tables 1 and 2 for their Kd values on the respective channels.
Table 1.
| Inhibitor | Kd value | Inhibitor | Kd value |
|---|---|---|---|
| Stichodactyla helianthus toxin | 11 pM | Parabuthus toxin 3 | 492 nM |
| Heterometrus spinnifer toxin 1 | 12 pM | Parabuthus toxin 1 | 800 nM |
| ShK–F6CA | 48 pM | Resiniferatoxin | 3 μM |
| Pandinus imperator toxin 2 | 50 pM | Nifedipine (3) | 5 μM |
| ShK–Dap22 | 52 pM | Nitrendipine (11) | 5 μM |
| Hongotoxin | 86 pM | Ibu8 | 5 μM |
| Margatoxin | 110 pM | Phencyclidine | 5 μM |
| Agiotoxin-2 | 200 pM | Verapamil (2) | 6 μM |
| Pandinus imperator toxin 3 | 500 pM | H37 | 10 μM |
| Kaliotoxin | 650 pM | Hg2+ | 10 μM |
| Noxiustoxin | 1 nM | Quinine | 14 μM |
| Psora4 (10) | 3 nM | Cicutotoxin | 18 μM |
| Charybdotoxin | 3 nM | La3+ | 20 μM |
| Titystoxin-Kα | 4 nM | Trifluoperazine | 20 μM |
| Pandinus imperator toxin 1 | 11 nM | Capsaicin | 26 μM |
| Tetraphenylporphyrin 3* (1) | 20 nM | Diltiazem | 27 μM |
| Bunodosoma granulifera toxin | 39 nM | Progesterone | 30 μM |
| trans-N-propyl-carbamoyloxy-PAC (7) | 50 nM | κ-Hefutoxin | 40 μM |
| Correolide (6) | 90 nM | Luteolin | 50 μM |
| Sulfamidbenzamidoindane (8) | 100 nM | Flecainide | 60 μM |
| Maurotoxin | 150 nM | 4-AP | 190 μM |
| CP339818 | 150 nM | Zn2+, Co2+ | 200 μM |
| WIN173173 (4) | 200 nM | Ba2+, Cd2+ | 2 mM |
| UK78282 (5) | 200 nM | TEA | 10 mM |
| Dendrotoxin | 250 nM | Mn2+ | 20 mM |
| PAC | 270 nM |
Inhibitors are shown in order of decreasing potency. All Kd values with the exception of those marked with an asterisk (* = binding data) were determined through whole-cell patch-clamp or two-electrode voltage-clamp on the cloned channels expressed in mammalian cells or oocytes. The numbers in brackets correspond to structures in Figure 4.
Abbreviations: 4-AP, 4-aminopyridine; ShK–Dap22, Stichodactyla helianthus toxin with diaminopropionic acid introduced at position 22 in place of the native lysine; ShK–F6CA, Stichodactyla helianthus toxin–fluorescein-6-carboxylic acid; trans-N-propyl-carbamoyloxy-PAC, trans-N-propyl-carbamoyloxy-4-phenyl-4-[3-(2-methoxyphenyl)-3-oxo-2-azaprop-1-yl]cyclohexanone; TEA, tetraethylammonium chloride.
See Chemical names.
Table 2.
| Inhibitor | Kd value | Inhibitor | Kd value |
|---|---|---|---|
| Maurotoxin | 1 nM | Nimodipine | 1 μM |
| Charybdotoxin | 5 nM | ShK–Dap22 | 2.6 μM |
| 4-Phenyl-4H-pyran 11 (12) | 8 nM | Nifedipine | 4 μM |
| ICA17043 (15) | 11 nM | Econazole | 12 μM |
| TRAM34 (14) | 20 nM | Ketoconazole | 30 μM |
| Stichodactyla helianthus toxin | 30 nM | Verapamil | 28 μM |
| ChTx–Glu32 | 33 nM | Berberine | 50 μM |
| TRAM39 | 60 nM | Cetiedil | 79 μM |
| Clotrimazole (13) | 70 nM | 4-AP | >1 mM |
| Budonosoma granulifera toxin | 172 nM | La3+ | 2 mM |
| TRAM3 | 520 nM | Ba2+ | 10 mM |
| DiS-C2 | 700 nM | Cd2+ | 5 mM |
| Nitrendipine (11) | 900 nM | TEA | 24 mM |
| Activator | |||
| Dichloro-EBIO | 1 μM | ||
| Riluzole | 3 μM | ||
| EBIO | 80 μM | ||
| Zoxazolamine | 98 μM | ||
| Chlorzoxazone | 100 μM | ||
| Theophylline | 1 mM | ||
| Caffeine | 1 mM | ||
Inhibitors and activators are shown in order of decreasing potency. All Kd values were determined through whole-cell patch-clamp or two-electrode voltage-clamp on the cloned channels expressed in mammalian cells or oocytes. The numbers in brackets correspond to structures in Figure 4.
Abbreviations: 4-AP, 4-aminopyridine; ChTx–Glu32, charybdotoxin derivative with glutamate at position 32 in place of the native lysine; dichloro-EBIO, 5,6-dichloro-1-ethyl-1,3-dihydro-2H-benzimidazol-2-one; ShK–Dap22, Stichodactyla helianthus toxin with diaminopropionic acid introduced at position 22 in place of the native lysine; TEA, tetraethylammonium chloride.
See Chemical names.
Peptide blockers
Although the use of ChTX (Figure 3a) has demonstrated a crucial role for K+ channels in T-cell activation [11,29,30], it inhibits both Kv1.3 [6,29,31] and IKCa1 [11,12] channels at low nanomolar concentrations and does not distinguish between the functional contributions of the two channels. The most potent Kv1.3 inhibitor, ShK from the Caribbean sea anemone Stichodactyla helianthus [32], blocks the channel under physiological conditions with a Kd value of 11 pM [33] and exhibits >1000-fold selectivity over IKCa1 channels (Tables 1,2). ShK contains 35 amino acids held together by three disulfide bonds (Figure 3a) [33,34]. Margatoxin (MgTX) (Table 1) from the venom of Centruroides margaritatus is also used widely as a Kv1.3 channel inhibitor [35,36]. The most potent inhibitor of IKCa1 channels maurotoxin (MTX), from the venom of Scorpio maurus (Kd = 1 nM), exhibits >150-fold selectivity over Kv1.3 channels (Tables 1,2) [37,38]. MTX contains 34 amino acids held together by four disulfide bonds (Figure 3a) [39]. Despite differences in their structural fold, most toxins that block K+ channels use a crucial lysine to occlude the channel pore [7] (Figure 3a).
The lack of channel-specific antibodies that bind to extracellular epitopes on Kv1.3 or IKCa1 channels has precluded visualization of these channels in live cells. However, the affinity of peptide inhibitors of Kv1.3 channels is extremely high, and their ability to interact with all four subunits of the tetramer has made them attractive as fluorophore-tagged tools for channel visualization. Attachment of fluorescein-6-carboxylic acid (F6CA) through a nine-atom hydrophilic linker to Arg1 of ShK (Figure 3a) yielded a potent and specific inhibitor of Kv1.3 channels (Table 1) that detected, using flow cytometry, high levels of Kv1.3 channels (Kv1.3high) in chronically activated T cells [40]. Single Kv1.3 channels were also visualized in Jurkat T cells with hongotoxin conjugated to Cy5 via a spinster Cys19 on the ‘backside’ of the peptide [41].
Small-molecule Kv1.3 channel blockers
L-type Ca2+ channel blockers of the dihydropyridine (e.g. nifedipine; compound 3 in Figure 4) and phenylalkylamine (verapamil; compound 2 in Figure 4) classes were the first small molecules found to inhibit lymphocyte K+ channels with low micromolar affinity [5,7]. Verapamil, dilitazem and nifedipine inhibit IL-2 secretion and T-cell proliferation at concentrations between 10 and 50 μM [5], potencies that are consistent with K+ channel blockade. The first small-molecule Kv1.3 channel blockers with nanomolar affinity were the iminodihydroquinolines WIN173173 (see Chemical names; compound 4 in Figure 4) and CP339818 [42], and the benzhydryl piperidine UK78282 (compound 5 in Figure 4) [43]. However, the iminodihydroquinolines also block Na+ channels [44]and UK78282 blocks Kv1.4 channels [43], and thus further development of these compounds was abandoned. The tetraphenylporphyrins [45] are promising drug candidates, the most potent (compound1 in Figure 4) blocking Kv1.3 channels with a Kd of 20 nM in binding studies (Table 1), but their selectivity remains to be determined. The attachment of one or more fluorophores to these compounds might constitute interesting alternatives to fluorophore-tagged peptide inhibitors [45] because they mimic toxins by using a porphyrin ring as a scaffold to position charged groups at the optimal distance to form salt bridges with aspartate residues in the outer vestibule of each of the four Kv1.3 channel subunits. Other blockers with low micromolar to submicromolar potency for Kv1.3 channels include correolide (compound 6 in Figure 4), trans-N-propylcarbamoyloxy-PAC (compound 7 in Figure 4), sulfamidebenzamidoindanes (compound 8 in Figure 4), dichlorophenylpyrazolopyrimidines (compound 9 in Figure 4) and the 5-(4-phenylbutoxy)psoralen Psora4 (compound 10 in Figure 4) [15,82]. The binding sites for these drugs on Kv1.3 channels and the mechanism of channel block have been described in a recent review [15].
In 1998, scientists at Merck (http://www.merck.com/) discovered correolide, a pentacyclic nortriterpene, in extracts of the Costa Rican tree Spachea correa [46,47]. Correolide blocks Kv1.3 channels with a Kd of ~100 nM and inhibits mitogen-induced proliferation of T cells, and an analog with more favorable pharmacokinetic properties suppresses in vivo delayed-type hypersensitivity immune responses in Yucatan mini-pigs [48]. However, correolide might not be a suitable drug candidate because it blocks other KV1 channels with potencies equivalent to those that block Kv1.3 channels, and its molecular complexity makes synthesis of new analogs both challenging and expensive. The Merck group recently identified the chemically simpler cyclohexyl-substituted benzamides [PACs (4-phenyl-4-[3-(2-methoxyphenyl)-3-oxo-2-azaprop-1-yl] cyclohexanones)] in 86Rb+ flux experiments [49]. These compounds are distinguished from other Kv1.3 channel inhibitors by their Hill coefficient of 2 and interesting structural isomer activity profile [49]. The parent compound PAC is not selective for Kv1.3 channels, but substitutions introduced at position 1 of the cyclohexanone ring yield cis or trans isomer pairs with differing properties [49]. The cis-isomers are not selective for Kv1.3 channels, whereas the trans-isomers show two- to sixfold selectivity for Kv1.3 channels over Kv1.1 and Kv1.2 channels and demonstrate a well-defined structure–activity relationship. The most potent compound in this series (compound 7 in Figure 4) blocks Kv1.3 channels with a Kd of 50 nM. Although the trans-PACs appear promising, their specificity for Kv1.3 channels needs improvement and their in vivo immunomodulatory activity has yet to be reported.
The most potent small-molecule inhibitor of Kv1.3 channels is Psora4 (compound 10 in Figure 4). Psora4 blocks the channel in a use-dependent manner with a Hill coefficient of 2 and an EC50 value of 3 nM, by preferentially binding to the C-type inactivated state of the channel [82]. It exhibits 17–70-fold selectivity for Kv1.3 channels over closely related Kv1 family channels (Kv1.1, Kv1.2, Kv1.4 and Kv1.7), with the exception of Kv1.5 channels (EC50 = 7.7 nM), and shows no effect on HERG (human ether-à-go-go-related gene), Kv3.1 or Ca2+ -activated K+ channels (IKCa1, SK1–SK3 and BKCa) or the neuronal NaV1.2 channel. The limited selectivity of Psora4 for Kv1.3 channels over the cardiac channel Kv1.5 channel precludes its use as an immunomodulator, although future analogs might exhibit the requisite selectivity and oral bioavailability to make it a useful therapeutic.
Small-molecule IKCa1 channel blockers
The azole antimycotic clotrimazole (compound 13 in Figure 4) and the dihydropyridines nitrendipine (compound 11 in Figure 4) and nifedipine (compound 3 in Figure 4) were the first potent small-molecule inhibitors of IKCa1 channels [15]. Clotrimazole is particularly interesting because it ameliorates rheumatoid arthritis in patients [50], but it was abandoned because of adverse effects resulting from blockade of cytochrome P450 enzymes. Using clotrimazole as a template, our group [51] and chemists at Icagen (http://www.icagen.com/) [52] independently developed selective IKCa1 channel inhibitors that lack cytochrome P450 blocking activity. We designed a pyrazole-substituted triarylmethane called TRAM34 (compound 14 in Figure 4) that exhibits >100-fold selectivity for IKCa1 channels over other K+ channels [51,53]. TRAM34 did not produce obvious toxic side-effects when administered to rats in a trial that demonstrated its effectiveness in preventing vascular restenosis following balloon angioplasty [54]. The Icagen group developed another clotrimazole analog called ICA17043 (compound 15 in Figure 4), which is now in Phase II clinical trials for sickle cell anemia [52]. More recently, scientists at Bayer (http://www.bayer.com/) used nifedipine as a template to develop a novel 4-phenyl-4H-pyran (compound 12 in Figure 4) that blocks IKCa1 channels with a Kd of 8 nM [55] and reduces infarct volume in a rat subdural hematoma model, suggesting that it could have use in the treatment of traumatic brain injury. Lastly, several activators of IKCa1 channels have been identified (Table 2) but the therapeutic utility of these compounds in immunomodulation remains unclear.
K+ channels in T cells: targets for immunomodulation
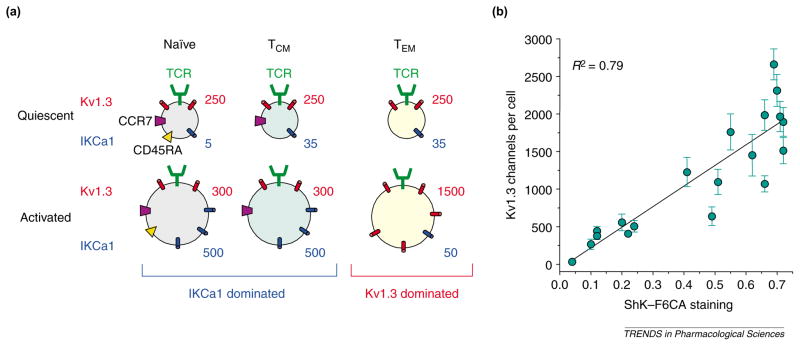
The immunomodulatory effects of channel blockers depend on the expression levels of Kv1.3 and IKCa1 channels, which change dramatically when T cells transition from resting to activated cells, and during differentiation from the naïve to the memory state [15,40,56]. Figure 5a shows the three human T-cell subsets that are distinguished based on the expression of the chemokine receptor CCR7 and the phosphatase CD45RA [57]: naïve cells (CCR7+ CD45RA+ ); central memory cells (TCM) (CCR7+ CD45RA−); and effector memory cells (TEM) (CCR7−CD45RA−).
Figure 5.
(a) Schematic showing the average numbers of Kv1.3 channels and IKCa1 channels per cell in naïve (CCR7+ CD45RA+ ), central memory (TCM) (CCR7+ CD45RA−) and effector memory (TEM) (CCR7−CD45RA−) cells. Naïve and TCM cells increase IKCa1 channel expression following activation, whereas TEM cells increase Kv1.3 channel expression. (b) Plot of Kv1.3 channel numbers per cell determined by whole-cell patch-clamp in different T-cell populations versus difference (D) values of Stichodactyla helianthus toxin (ShK)–fluorescein-6-carboxylic acid (F6CA) staining obtained by flow cytometry; these data were obtained from [40]. The D value is a measure of the difference in fluorescence intensity between ShK–F6CA-stained cells and background fluorescence from unstained cells of the same population. Abbreviation: TCR, T-cell receptor.
Kv1.3 and IKCa1 channels in naïve, TCM and TEM cells
In the quiescent state, cells belonging to all three T-cell subsets express ~250 Kv1.3 channels and 5–35 IKCa1 channels per cell [56] and, because Kv1.3 channels dominate functionally in quiescent cells, ShK and Psora4 suppress their activation [56,82], whereas TRAM34 has no effect [58]. Activation induces differential expression of K+ channels in these subsets, leading to an altered channel phenotype and consequently to altered responsiveness to Kv1.3 and IKCa1 channel blockers. IKCa1 channels are upregulated to ~500 per cell in naïve and TCM cells [56] via PKC-dependent transcription of the gene encoding the IKCa1 channel [58]. Transcriptional upregulation is detectable three hours after antigen stimulation and is independent of Ca2+ signaling because its blockade does not prevent IKCa1 channel augmentation [56,58]. IKCa1 channel levels are augmented even if the initial activation of naïve and TCM cells is suppressed by ShK or cyclosporin A (agents that attenuate Ca2+ signaling or activation of calcineurin) because IKCa1 channel upregulation is only minimally dependent on Ca2+ [15,56]. As a consequence of IKCa1 channel upregulation, naïve and TCM cells escape further Kv1.3 channel inhibition and become sensitive to IKCa1 channel blockade [15,56]. By contrast, when TEM cells are activated, Kv1.3 channel expression is increased in these cells to 1500 per cell with little change in IKCa1 channel levels [40,56], and consequently ShK (IC50 = 400 pM) and Psora4 (IC50 = 25 nM) persistently suppress TEM proliferation, whereas TRAM34 (IC50 > 6 μM) is ineffective [15,56,82]. Increased Kv1.3 channel expression is a result of new Kv1.3 channel tetramers being inserted into the cell membrane and is dependent on both Ca2+ - and PKC-dependent signaling cascades [40]. Flow cytometry with ShK–F6CA (Table 1) can detect this Kv1.3 channel upregulation, and the results from flow cytometry correlate well with measurements obtained in patch-clamp studies (Figure 5b). Because flow cytometry is more rapid than patch-analysis and samples bulk populations, it could have use in screening tissues for Kv1.3high TEM cells that have been implicated in autoimmune disorders. The functional dominance of IKCa1 channels in activated naïve and TCM cells versus Kv1.3 channels in TEM cells provides a powerful way to manipulate the activity of these subsets by administration of specific IKCa1 and Kv1.3 channel inhibitors.
IKCa1 and Kv1.3 channel blockers as possible therapeutics
Naïve and TCM cells are likely to be involved in immune-mediated acute rejection of transplanted organs and acute graft-versus-host disease. Because these cells are initially sensitive to Kv1.3 channel blockers and then become dependent on IKCa1 channels, initial combination therapy with Kv1.3 and IKCa1 channel blockers followed by IKCa1 channel blockade alone might be beneficial in the management of these clinical problems. Furthermore, cyclosporin A, a widely used immunosuppressant, synergizes with TRAM34 in T-cell proliferation assays [51], and combining these compounds could reduce the level of toxic side-effects that complicate cyclosporin A therapy.
In a recent study we demonstrated that the majority of pathogenic myelin-reactive T cells from patients with multiple sclerosis (MS) are Kv1.3highIKCa1low TEM cells [56], whereas T cells that are specific for control antigens in these patients express the naïve and/or TCM cell phenotype. Myelin-specific T cells from healthy individuals or from normal rats are naïve and/or TCM cells, but can be converted into Kv1.3high TEM cells by repeated activation in vitro by myelin antigens [56,59]. These findings suggest that pathogenic myelin-reactive T cells acquire the Kv1.3high TEM phenotype as a consequence of repeated in vivo exposure to myelin antigens during the course of disease. Activated autoreactive TEM cells probably contribute to MS by migrating to inflamed tissues where they secrete interferon γ and tumor necrosis factor α. In keeping with this idea, adoptive transfer of Kv1.3high rat memory T cells into naïve recipients causes severe experimental autoimmune encephalomyelitis (EAE), a model for MS [59]. Selective targeting of these disease-causing cells with Kv1.3 channel blockers prevents and reverses EAE in this rat model [59], and no side-effects are observed despite the expression of Kv1.3 channels in the brain, arteries [60], bladder [61] and epithelia [62]. Together, these findings lead to the prediction that Kv1.3 channel blockers will ameliorate symptoms of MS and other autoimmune disorders mediated by TEM cells in humans. Kv1.3 channel blockers have also been shown recently to be effective in preventing inflammatory bone resorption in experimental periodontal disease [63], and these blockers could also have use in managing chronic graft rejection and chronic graft-versus-host disease that are probably sustained by chronically activated TEM cells [83]. A Kv1.3 channel-based therapeutic approach would have an advantage over agents that cause generalized immunomodulation because naïve and TCM cells would escape inhibition through upregulation of IKCa1 channels, leaving the bulk of the immune response intact.
Concluding remarks
Much work remains to be done to bring IKCa1 and Kv1.3 channel blockers to the clinic. The situation with small-molecule IKCa1 channel blockers is encouraging because ICA17043 is already in Phase II clinical trials for sickle cell disease and TRAM34 has been used successfully in an in vivo animal trial [54]. Both these agents and the newly described 4-phenyl-4H-pyran need to be evaluated in animal models of organ graft rejection, acute active EAE and rheumatoid arthritis.
Existing small-molecule Kv1.3 channel inhibitors have not shown the requisite selectivity, potency or oral bioavailability to make them viable drug candidates. By contrast, peptide inhibitors such as ShK have exquisitely high potency for Kv1.3 channels and once bound to the channel on lymphocytes are only slowly released, but have short circulating half-lives [59]. A slow-release depot formulation or implantable pump might render these peptides therapeutically useful. Although ShK was effective in adoptive EAE trials [59], its efficacy needs to be assessed in models that exhibit the relapsing-remitting clinical course observed in MS and its toxicity profile has to be determined. Acute active EAE, the workhorse of drug-screening researchers, might not be useful for this purpose because it involves an acute immune response mediated by naïve and TCM cells, and IKCa1, but not Kv1.3, channel inhibitors might be effective in this model of MS.
In conclusion, Kv1.3 and IKCa1 channels are emerging as important targets for therapeutic manipulation of selective lymphocyte subsets and also as possible diagnostic targets for the detection of pathogenic Kv1.3high TEM cells in autoimmune disorders. The therapeutic promise of this avenue of research remains to be realized but the future does look encouraging.
Chemical names
CP339818: N-[1-(phenylmethyl)-4(1H)-quinolinylidene]-1-pentanamine monohydrochloride
diS-C2: 3-ethyl-2-[(1E,3E,5E)-5-(3-ethyl-2(3H)-benzothiazolylidene)-1,3-pentadienyl]-benzothiazolium iodide
H37: 4,9-diethoxy-7H-furo[3,2-g ] [1]benzopyran-7-one
Ibu8: 8-methoxy-5-methyl-2-(1-methylethyl)-furo[3,2-c] quinolin-4(5H)-one
ICA17043: 4-fluoro-α-(4-fluorophenyl)-α-phenyl-benzene-acetamide
Psora4: 4-(4-phenylbutoxy)-7H-furo[3.2-g ][1]benzopyrane-7-one
TRAM3: (2-chlorophenyl)diphenylmethanol
TRAM34: 1-[(2-chlorophenyl)diphenylmethyl]-1H-pyrazole
TRAM39: (2-chlorophenyl)diphenylacetonitrile
UK78282: 4-[(diphenylmethoxy)methyl]-1-[3-(4-methoxyphenyl)propyl]-piperidine
WIN173173: N-[7-chloro-1-(phenylmethyl)-4(1H)-quinolinylidene]-1-propanamine monohydrochloride
References
- 1.Iversen JG. Unidirectional K+ fluxes in rat thymocytes stimulated by concanavalin A. J Cell Physiol. 1976;89:267–276. doi: 10.1002/jcp.1040890210. [DOI] [PubMed] [Google Scholar]
- 2.DeCoursey TE, et al. Voltage-gated K+ channels in human T lymphocytes: a role in mitogenesis? Nature. 1984;307:465–468. doi: 10.1038/307465a0. [DOI] [PubMed] [Google Scholar]
- 3.Matteson DR, Deutsch C. K+ channels in T lymphocytes: a patch clamp study using monoclonal antibody adhesion. Nature. 1984;307:468–471. doi: 10.1038/307468a0. [DOI] [PubMed] [Google Scholar]
- 4.Fukushima Y, et al. Potassium current in clonal cytotoxic T lymphocytes from the mouse. J Physiol. 1984;351:645–656. doi: 10.1113/jphysiol.1984.sp015268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chandy KG, et al. Voltage-gated potassium channels are required for human T lymphocyte activation. J Exp Med. 1984;160:369–385. doi: 10.1084/jem.160.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sands SB, et al. Charybdotoxin blocks voltage-gated K+ channels in human and murine T lymphocytes. J Gen Physiol. 1989;93:1061–1074. doi: 10.1085/jgp.93.6.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chandy KG, et al. Potassium channels in T lymphocytes: toxins to therapeutic immunosuppressants. Toxicon. 2001;39:1269–1276. doi: 10.1016/s0041-0101(01)00120-9. [DOI] [PubMed] [Google Scholar]
- 8.Grissmer S, et al. Expression and chromosomal localization of a lymphocyte K + channel gene. Proc Natl Acad Sci U S A. 1990;87:9411–9415. doi: 10.1073/pnas.87.23.9411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Douglass J, et al. Characterization and functional expression of a rat genomic DNA clone encoding a lymphocyte potassium channel. J Immunol. 1990;144:4841–4850. [PubMed] [Google Scholar]
- 10.Grissmer S, et al. Ca2+ -activated K+ channels in human leukemic T cells. J Gen Physiol. 1992;99:63–84. doi: 10.1085/jgp.99.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leonard R, et al. Selective blockers of voltage-gated K+ channels depolarize human T lymphocytes: mechanism of the antiproliferative effect of charybdotoxin. Proc Natl Acad Sci U S A. 1992;89:10094–10098. doi: 10.1073/pnas.89.21.10094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grissmer S, et al. Calcium-activated potassium channels in resting and activated human T lymphocytes. Expression levels, calcium dependence, ion selectivity, and pharmacology. J Gen Physiol. 1993;102:601–630. doi: 10.1085/jgp.102.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Logsdon NJ, et al. A novel gene, hKCa4, encodes the calcium-activated potassium channel in human T lymphocytes. J Biol Chem. 1997;272:32723–32726. doi: 10.1074/jbc.272.52.32723. [DOI] [PubMed] [Google Scholar]
- 14.Fanger CM, et al. Calmodulin mediates calcium-dependent activation of the intermediate conductance KCa channel, IKCa1. J Biol Chem. 1999;274:5746–5754. doi: 10.1074/jbc.274.9.5746. [DOI] [PubMed] [Google Scholar]
- 15.Wulff H, et al. Potassium channels as therapeutic targets for autoimmune disorders. Curr Opin Drug Discov Dev. 2003;6:640–647. [PubMed] [Google Scholar]
- 16.Panyi G, et al. Colocalization and nonrandom distribution of the Kv1.3 potassium channel and CD3 molecules in the plasma membrane of human T lymphocytes. Proc Natl Acad Sci U S A. 2003;100:2592–2597. doi: 10.1073/pnas.0438057100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Szabo I, et al. Tyrosine phosphorylation-dependent suppression of a voltage-gated K+ channel in T lymphocytes upon Fas stimulation. J Biol Chem. 1996;271:20465–20469. doi: 10.1074/jbc.271.34.20465. [DOI] [PubMed] [Google Scholar]
- 18.Gulbins E, et al. Ceramide-induced inhibition of T lymphocyte voltage-gated potassium channel is mediated by tyrosine kinases. Proc Natl Acad Sci U S A. 1997;94:7661–7666. doi: 10.1073/pnas.94.14.7661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanada T, et al. Human homologue of the Drosophila discs large tumor suppressor binds to p56lck tyrosine kinase and Shaker type Kv1.3 potassium channel in T lymphocytes. J Biol Chem. 1997;272:26899–26904. doi: 10.1074/jbc.272.43.26899. [DOI] [PubMed] [Google Scholar]
- 20.Gong J, et al. Differential stimulation of PKC phosphorylation of potassium channels by ZIP1 and ZIP2. Science. 1999;285:1565–1569. doi: 10.1126/science.285.5433.1565. [DOI] [PubMed] [Google Scholar]
- 21.Cayabyab F, et al. Suppression of the rat microglia Kv1.3 current by src-family tyrosine kinases and oxygen/glucose deprivation. Eur J Neurosci. 2000;12:1949–1960. doi: 10.1046/j.1460-9568.2000.00083.x. [DOI] [PubMed] [Google Scholar]
- 22.Cahalan MD, et al. Molecular properties and physiological roles of ion channels in the immune system. J Clin Immunol. 2001;21:235–252. doi: 10.1023/a:1010958907271. [DOI] [PubMed] [Google Scholar]
- 23.Jugloff DG, et al. Internalization of the Kv1.4 potassium channel is suppressed by clustering interactions with PSD-95. J Biol Chem. 2000;275:1357–1364. doi: 10.1074/jbc.275.2.1357. [DOI] [PubMed] [Google Scholar]
- 24.Panyi G, et al. Kv1.3 potassium channels are localized in the immunological synapse formed between cytotoxic and target cells. Proc Natl Acad Sci U S A. 2004;101:1285–1290. doi: 10.1073/pnas.0307421100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levite M, et al. Extracellular K+ and opening of voltage-gated potassium channels activate T cell integrin function: physical and functional association between Kv1.3 channels and beta1 integrins. J Exp Med. 2000;191:1167–1176. doi: 10.1084/jem.191.7.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Partiseti M, et al. The calcium current activated by T cell receptor and store depletion in human lymphocytes is absent in a primary immunodeficiency. J Biol Chem. 1994;269:32327–32335. [PubMed] [Google Scholar]
- 27.Feske S, et al. Gene regulation mediated by calcium signals in T lymphocytes. Nat Immunol. 2001;2:316–324. doi: 10.1038/86318. [DOI] [PubMed] [Google Scholar]
- 28.Lewis RS. Calcium signaling mechanisms in T lymphocytes. Annu Rev Immunol. 2001;19:497–521. doi: 10.1146/annurev.immunol.19.1.497. [DOI] [PubMed] [Google Scholar]
- 29.Price M, et al. Charybdotoxin inhibits proliferation and interleukin 2 production in human peripheral blood lymphocytes. Proc Natl Acad Sci U S A. 1989;86:10171–10175. doi: 10.1073/pnas.86.24.10171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin CS, et al. Voltage-gated potassium channels regulate calcium-dependent pathways involved in human T lymphocyte activation. J Exp Med. 1993;177:637–645. doi: 10.1084/jem.177.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deutsch C, et al. Characterization of high affinity binding sites for charybdotoxin in human T lymphocytes. Evidence for association with the voltage-gated K+ channel. J Biol Chem. 1991;266:3668–3674. [PubMed] [Google Scholar]
- 32.Pennington M, et al. Chemical synthesis and characterization of ShK toxin: a potent potassium channel inhibitor from a sea anemone. Int J Pept Protein Res. 1995;46:354–358. doi: 10.1111/j.1399-3011.1995.tb01068.x. [DOI] [PubMed] [Google Scholar]
- 33.Kalman K, et al. ShK-Dap22, a potent Kv1.3-specific immunosuppressive polypeptide. J Biol Chem. 1998;273:32697–32707. doi: 10.1074/jbc.273.49.32697. [DOI] [PubMed] [Google Scholar]
- 34.Tudor JE, et al. Solution structure of ShK toxin, a novel potassium channel inhibitor from a sea anemone. Nat Struct Biol. 1996;3:317–320. doi: 10.1038/nsb0496-317. [DOI] [PubMed] [Google Scholar]
- 35.Garcia-Calvo M, et al. Purification, characterization, and biosynthesis of margatoxin, a component of Centruroides margaritatus venom that selectively inhibits voltage-dependent potassium channels. J Biol Chem. 1993;268:18866–18874. [PubMed] [Google Scholar]
- 36.Koo GC, et al. Blockade of the voltage-gated potassium channel Kv1.3 inhibits immune responses in vivo. J Immunol. 1997;158:5120–5128. [PubMed] [Google Scholar]
- 37.Kharrat R, et al. Chemical synthesis and characterization of maurotoxin, a short scorpion toxin with four disulfide bridges that acts on K+ channels. Eur J Biochem. 1996;242:491–498. doi: 10.1111/j.1432-1033.1996.0491r.x. [DOI] [PubMed] [Google Scholar]
- 38.Castle NA, et al. Maurotoxin – a potent inhibitor of the intermediate conductance Ca2+ -activated potassium channel. Mol Pharmacol. 2003;63:409–418. doi: 10.1124/mol.63.2.409. [DOI] [PubMed] [Google Scholar]
- 39.Blanc E, et al. Solution structure of maurotoxin, a scorpion toxin from scorpio maurus, with high affinity for voltage-gated potassium channels. Proteins. 1997;29:321–333. [PubMed] [Google Scholar]
- 40.Beeton C, et al. A novel fluorescent toxin to detect and investigate Kv1.3-channel up-regulation in chronically activated T lymphocytes. J Biol Chem. 2003;278:9928–9937. doi: 10.1074/jbc.M212868200. [DOI] [PubMed] [Google Scholar]
- 41.Freudenthaler G, et al. Ultrasensitive pharmacological characterisation of the voltage-gated potassium channel K(V)1.3 studied by single-molecule fluorescence microscopy. Histochem Cell Biol. 2002;117:197–202. doi: 10.1007/s00418-001-0374-y. [DOI] [PubMed] [Google Scholar]
- 42.Nguyen A, et al. Novel nonpeptide agents potently block the C-type inactivated conformation of Kv1.3 and suppress T cell activation. Mol Pharmacol. 1996;50:1672–1679. [PubMed] [Google Scholar]
- 43.Hanson DC, et al. UK-78,282, a novel piperidine compound that potently blocks the Kv1.3 voltage-gated potassium channel and inhibits human T cell activation. Br J Pharmacol. 1999;126:1707–1716. doi: 10.1038/sj.bjp.0702480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wanner S, et al. WIN 17317-3, a new high-affinity probe for voltage-gated sodium channels. Biochemistry. 1999;38:11137–11146. doi: 10.1021/bi990336p. [DOI] [PubMed] [Google Scholar]
- 45.Gradl SN, et al. Protein surface recognition by rational design: nanomolar ligands for potassium channels. J Am Chem Soc. 2003;125:12668–12669. doi: 10.1021/ja036155z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goetz MA, et al. Potent Nor-triterpenoid blockers of the voltage-gated potassium channel Kv1.3 from Spachea correae. Tetrahedron Lett. 1998;39:2895–2898. [Google Scholar]
- 47.Felix JP, et al. Identification and biochemical characterization of a novel nortriterpene inhibitor of the human lymphocyte voltage-gated potassium channel, Kv1.3. Biochemistry. 1999;38:4922–4930. doi: 10.1021/bi982954w. [DOI] [PubMed] [Google Scholar]
- 48.Koo GC, et al. Correolide and derivatives are novel immunosuppressants blocking the lymphocyte Kv1.3 potassium channels. Cell Immunol. 1999;197:99–107. doi: 10.1006/cimm.1999.1569. [DOI] [PubMed] [Google Scholar]
- 49.Schmalhofer WA, et al. Identification of a new class of inhibitors of the voltage-gated potassium channel, Kv1.3, with immunosuppressant properties. Biochemistry. 2002;41:7781–7794. doi: 10.1021/bi025722c. [DOI] [PubMed] [Google Scholar]
- 50.Wojtulewski JA, et al. Clotrimazole in rheumatoid arthritis. Ann Rheum Dis. 1980;39:469–472. doi: 10.1136/ard.39.5.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wulff H, et al. Design of a potent and selective inhibitor of the intermediate-conductance Ca2+ -activated K+ channel, IKCa1: A potential immunosuppressant. Proc Natl Acad Sci U S A. 2000;97:8151–8156. doi: 10.1073/pnas.97.14.8151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stocker JW, et al. ICA-17043, a novel Gardos channel blocker, prevents sickled red blood cell dehydration in vitro and in vivo in SAD mice. Blood. 2003;101:2412–2418. doi: 10.1182/blood-2002-05-1433. [DOI] [PubMed] [Google Scholar]
- 53.Wulff H, et al. Delineation of the clotrimazole/TRAM-34 binding site on the intermediate conductance calcium-activated potassium channel IKCa1. J Biol Chem. 2001;276:32040–32045. doi: 10.1074/jbc.M105231200. [DOI] [PubMed] [Google Scholar]
- 54.Kohler R, et al. Blockade of the intermediate-conductance calcium-activated potassium channel as a new therapeutic strategy for restenosis. Circulation. 2003;108:1119–1125. doi: 10.1161/01.CIR.0000086464.04719.DD. [DOI] [PubMed] [Google Scholar]
- 55.Urbahns K, et al. 4-Phenyl-4H-pyrans as IKCa channel blockers. Bioorg Med Chem Lett. 2003;13:2637–2639. doi: 10.1016/s0960-894x(03)00560-2. [DOI] [PubMed] [Google Scholar]
- 56.Wulff H, et al. The voltage-gated Kv1.3 K+ channel in effector memory T cells as new target for MS. J Clin Invest. 2003;111:1703–1713. doi: 10.1172/JCI16921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sallusto F, et al. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 58.Ghanshani S, et al. Up-regulation of the IKCa1 potassium channel during T-cell activation: molecular mechanism and functional consequences. J Biol Chem. 2000;275:37137–37149. doi: 10.1074/jbc.M003941200. [DOI] [PubMed] [Google Scholar]
- 59.Beeton C, et al. Selective blockade of T lymphocyte K+ channels ameliorates experimental autoimmune encephalomyelitis, a model for multiple sclerosis. Proc Natl Acad Sci U S A. 2001;98:13942–13947. doi: 10.1073/pnas.241497298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cox R, et al. Differential expression of voltage-gated K+ channel genes in arteries from spontaneously hypertensive and Wistar-Kyoto rats. Hypertension. 2001;37:1315–1322. doi: 10.1161/01.hyp.37.5.1315. [DOI] [PubMed] [Google Scholar]
- 61.Davies AM, et al. Potassium channel KV 1 subunit expression and function in human detrusor muscle. J Urol. 2002;167:1881–1886. [PubMed] [Google Scholar]
- 62.Grunnet M, et al. The voltage-gated potassium channel subunit, Kv1.3, is expressed in epithelia. Biochim Biophys Acta. 2003;1616:85–94. doi: 10.1016/s0005-2736(03)00198-6. [DOI] [PubMed] [Google Scholar]
- 63.Valverde P, et al. Selective blockade of voltage-gated potassium channels reduces inflammatory bone resorption in experimental periodontal disease. J Bone Miner Res. 2004;19:155–164. doi: 10.1359/JBMR.0301213. [DOI] [PubMed] [Google Scholar]
- 64.Hess SD, et al. Calcium oscillations in human T and natural killer cells depend upon membrane potential and calcium influx. J Immunol. 1993;150:2620–2633. [PubMed] [Google Scholar]
- 65.Grissmer S, et al. Ca2+ -activated K+ channels in human leukemic T cells. J Gen Physiol. 1992;99:63–84. doi: 10.1085/jgp.99.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Partiseti M, et al. Differential regulation of voltage- and calcium-activated potassium channels in human B lymphocytes. J Immunol. 1992;148:3361–3368. [PubMed] [Google Scholar]
- 67.Pillozzi S, et al. HERG potassium channels are constitutively expressed in primary human acute myeloid leukemias and regulate cell proliferation of normal and leukemic hemopoietic progenitors. Leukemia. 2002;16:1791–1798. doi: 10.1038/sj.leu.2402572. [DOI] [PubMed] [Google Scholar]
- 68.Gray PT, et al. Ion channels in rabbit cultured fibroblasts. Proc R Soc Lond B Biol Sci. 1986;227:1–16. doi: 10.1098/rspb.1986.0005. [DOI] [PubMed] [Google Scholar]
- 69.Amigorena S, et al. Ion channels and B cell mitogenesis. Mol Immunol. 1990;27:1259–1268. doi: 10.1016/0161-5890(90)90030-4. [DOI] [PubMed] [Google Scholar]
- 70.Ramsdell JS. Voltage-dependent calcium channels regulate GH4 pituitary cell proliferation at two stages of the cell cycle. J Cell Physiol. 1991;146:197–206. doi: 10.1002/jcp.1041460203. [DOI] [PubMed] [Google Scholar]
- 71.Pancrazio JJ, et al. Verapamil-induced blockade of voltage-activated K+ current in small-cell lung cancer cells. J Pharmacol Exp Ther. 1991;257:184–191. [PubMed] [Google Scholar]
- 72.Pappone PA, Ortiz-Miranda SI. Blockers of voltage-gated K channels inhibit proliferation of cultured brown fat cells. Am J Physiol. 1993;264:C1014–C1019. doi: 10.1152/ajpcell.1993.264.4.C1014. [DOI] [PubMed] [Google Scholar]
- 73.Lu L, et al. Alterations in a voltage-gated K+ current during the differentiation of ML-1 human myeloblastic leukemia cells. J Membr Biol. 1993;132:267–274. doi: 10.1007/BF00235743. [DOI] [PubMed] [Google Scholar]
- 74.Skryma RN, et al. Potassium conductance in the androgen-sensitive prostate cancer cell line, LNCaP: involvement in cell proliferation. Prostate. 1997;33:112–122. doi: 10.1002/(sici)1097-0045(19971001)33:2<112::aid-pros5>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 75.Chin LS, et al. 4-Aminopyridine causes apoptosis and blocks an outward rectifier K+ channel in malignant astrocytoma cell lines. J Neurosci Res. 1997;48:122–127. [PubMed] [Google Scholar]
- 76.Pardo LA, et al. Oncogenic potential of EAG K(+ ) channels. EMBO J. 1999;18:5540–5547. doi: 10.1093/emboj/18.20.5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yao X, Kwan HY. Activity of voltage-gated K+ channels is associated with cell proliferation and Ca2+ influx in carcinoma cells of colon cancer. Life Sci. 1999;65:55–62. doi: 10.1016/s0024-3205(99)00218-0. [DOI] [PubMed] [Google Scholar]
- 78.Shin VY, et al. Nicotine suppresses gastric wound repair via the inhibition of polyamine and K+ channel expression. Eur J Pharmacol. 2002;444:115–121. doi: 10.1016/s0014-2999(02)01610-2. [DOI] [PubMed] [Google Scholar]
- 79.Smith GA, et al. Functional up-regulation of HERG K+ channels in neoplastic hematopoietic cells. J Biol Chem. 2002;277:18528–18534. doi: 10.1074/jbc.M200592200. [DOI] [PubMed] [Google Scholar]
- 80.Neylon CB. Potassium channels and vascular proliferation. Vascul Pharmacol. 2002;38:35–41. doi: 10.1016/s1537-1891(02)00124-6. [DOI] [PubMed] [Google Scholar]
- 81.Zhou Q, et al. Blockage of voltage-gated K+ channels inhibits adhesion and proliferation of hepatocarcinoma cells. Int J Mol Med. 2003;11:261–266. [PubMed] [Google Scholar]
- 82.Vennekamp J, et al. Kv1.3 blocking 5-phenylalkoxypsoralens: a new class of immunomodulators. Mol Pharm. doi: 10.1124/mol.65.6.1364. (in press) [DOI] [PubMed] [Google Scholar]
- 83.Yamashita K, et al. Severe chronic graft-versus-host disease is characterized by a preponderance of CD4+ effector memory cells relative to central memory cells. Blood. doi: 10.1182/blood-2003-09-3286. (in press) [DOI] [PubMed] [Google Scholar]