Abstract
Platelet hyperactivity associated with hyperlipidemia may contribute to development of a pro-thrombotic state. We previously showed that oxidized LDL (oxLDL) formed in the setting of hyperlipidemia and atherosclerosis activated platelets in a CD36 dependent manner. We now show that MAP kinase JNK2 and its upstream activator MKK4 were phosphorylated in platelets exposed to oxLDL. Using apoe -/- mice as a model of hyperlipidemia we showed that JNK was constitutively phosphorylated in platelets in a CD36-dependent manner. Inhibition of src kinase activity reduced JNK phosphorylation by oxLDL. Immunoprecipitations revealed that active phosphorylated forms of src kinases Fyn and Lyn were recruited to CD36 in platelets exposed to oxLDL. Pharmacological inhibition of the MAP kinase JNK or src family kinases abolished platelet activation by oxLDL in vitro. Using a murine carotid artery thrombosis model we demonstrated CD36-dependent phosphorylation of platelet JNK within thrombi. Furthermore, pharmacological inhibition of JNK prolonged thrombosis times in wild type but not cd36 null mice in vivo. These findings suggest that a specific CD36-dependent signaling pathway is required for platelet activation by oxLDL and may provide insights related to development of novel anti-platelet therapies more relevant to atherothrombosis than to normal hemostasis.
Keywords: CD36, JNK, thrombosis, hyperlipidemia
Introduction
CD36 is an integral membrane protein expressed on monocytes/macrophages1, platelets2, microvascular endothelium1, fat and muscle3, 4. Although initially identified as a receptor for thrombospodin-1 (TSP-1) and malaria infected erythrocytes5, 6, it is now known to be a class B scavenger receptor that recognizes several unrelated ligands, including TSP-1 and -27, 8, oxidized phospholipids expressed on oxidized low density lipoprotein (oxLDL) and apoptotic cell surfaces9, 10, long chain fatty acids11, amyloidogenic peptides12, and specific components of microbial cell walls or cell surfaces13.
CD36 is involved in a variety of biological processes including lipid metabolism, inflammation, atherosclerosis, and angiogenesis, depending on the nature of the ligand to which it is exposed and the cell or tissue type on which it is expressed14. Although CD36 was first isolated and structurally characterized from platelets2, its functional role on platelets remains incompletely characterized. OKM5, a monoclonal antibody directed against CD36, was observed many years ago to induce platelet activation and aggregation15. Although the effect was dependent on expression of Fc receptors, it could be blocked by F(ab′)2 fragments of the antibody, suggesting that the CD36 epitope was required and that CD36 may transduce platelet activating signals. Many other CD36 monoclonal antibodies have also been shown to have stimulatory effects on platelets16. Our group in collaboration with others recently showed that platelets bind oxLDL via CD36 and this interaction leads to platelet activation, contributing to a pro-thrombotic state in the setting of hyperlipidemia17. The mechanisms by which interactions between CD36 and its ligands activate platelets remain unknown, however.
It is now well established that despite having very short intra-cytoplasmic domains, CD36 can serve as a signaling molecule. Antibodies to platelet CD36 were shown to co-precipitate the non-receptor protein tyrosine kinases Fyn, Lyn and Yes18. Studies in other cellular systems have linked the signaling function of CD36 to recruitment/activation of src-family kinases and activation of specific mitogen activated protein (MAP) kinases. For example, on microvascular endothelial cells TSP-1 induces a CD36-dependent anti-angiogenic, pro-apoptotic signal via activation of Fyn, caspase-3, and p38 MAP kinase19. On macrophages, exposure to oxLDL leads to recruitment of Lyn and activation of JNK-2 in a CD36 dependent manner. Inhibition of JNK resulted in significant reduction in uptake of oxLDL and foam cell formation20. These studies suggest a context-dependent mechanism for CD36 signaling involving specific src and MAP kinases. In platelet biology, MAP kinases have not been studied in detail, although it has been demonstrated that the P2Y1 ADP receptor activates p38 MAP kinase, and p38 deficient mice have prolonged thrombotic occlusion time in a ferric chloride (FeCl3)-induced thrombosis model21, 22. We thus hypothesized that CD36-mediated platelet activation might involve specific members of the src and MAP kinase families.
CD36 recognizes a variety of pathological ligands including oxLDL23, advanced glycation end products24, 25, apoptotic cells26, 27, and cell-derived microparticles28. We focused on oxLDL because of its essential role in the pathogenesis of atherosclerosis and the known association of oxidative stress, hyperlipidemia and a pro-thrombotic phenotype17, 29, 30. In studies outlined here, we identified a CD36-dependent signaling cascade responsible for oxLDL dependent activation of platelets that includes the src kinases Fyn and Lyn, the upstream MAP kinase kinase (MKK) 4, and the MAP kinase JNK2. These data indicate that a CD36-dependent signaling pathway is required for activation of platelets by oxLDL and shed new light on the mechanism of platelet hypersensitivity in the setting of atherosclerosis and/or hyperlipidemia.
Materials and Methods
Native LDL (nLDL) and oxidized LDL (oxLDL) was prepared as described previously20. Whole blood was collected from healthy human volunteers in 0.109 M sodium citrate (1:9 dilution) and platelets were separated by sedimentation, washed and resuspended in Modifed Tyrode's Buffer. CaCl2 and MgCl2 were added immediately before platelets were stimulated with various agonists and the activated platelets were analyzed by flow cytometry. Human or murine platelets were lysed, and 40-60μg of lysate protein was used for immunoblotting analysis of phosphorylated JNK, total JNK, phospho-MKK4 and MKK4. In some studies pre-cleared lysates containing 500 μg protein were incubated with protein-A-agarose beads conjugated to anti-Lyn or anti-CD36 IgG overnight at 4°C. Beads were washed, boiled in 2 × SDS-PAGE loading buffer, and bound material was analyzed by immunoblotting.
Blood from wild type, apoe null, or apoe/cd36 double null mice maintained on chow or high-fat diet for 3 months beginning at 6 weeks of age was obtained by cardiac puncture after animals were anesthetized with ketamine (90 mg/kg) and xylazine (15mg/kg). Platelets in platelet-rich plasma (PRP) were allowed to adhere to silanized slides (DAKO, Carpinteria, CA) and then double immunocytofluorescence staining was performed using antibodies to phospho-JNK and CD41. Images were obtained with a laser confocal microscope. In some studies platelets were lysed and analyzed by immunoblot for JNK activation. Li-Cor Odyssey Infrared Imaging System was used for signal detection and quantification.
Carotid artery thrombosis was induced in 12 week old male WT and cd36 null mice by topical application of 12.5% ferric chloride as previously described17. After the vessel was allowed to become completely occluded the carotid arteries were removed, sectioned (4-6 μm) across the thrombi, and analyzed by immunohistochemistry using an antibody to phospho-JNK. For quantification, images were scored on the basis of staining intensity by a blind observer (0 = negative; 1 = weak; 2 = moderate; 3 = intense)31. In some studies thrombi were dissected away from the vessel wall, pooled (9 per group), and total proteins extracted for analysis by immunoblot.
To study the effect of JNK in thrombosis in vivo, WT or CD36 null mice were exposed to11Gy of external beam irradiation from a Cesium 137 source to induce thrombocytopenia with platelet counts <5% of normal after 5 days17. Platelets obtained from syngeneic donor mice were labeled with Calcein AM (final concentration of 0.5 mg/ml) in the presence or absence of the JNK inhibitor SP6000125 (final concentration of 400 nM for 30 min). This dose is the minimum active dose and was chosen to avoid potential off-target effects. 2 × 109 donor platelets were injected through the jugular vein of thrombocytopenic mice 10 minutes prior to carotid injury with 12.5% FeCl3 to allow the transfused platelets to reach equilibrium in the circulation. For further details, please see the Data Supplement available at http://circres.ahajournal.org.
Results
OxLDL induces phosphorylation of JNK2 and MKK4 in platelets
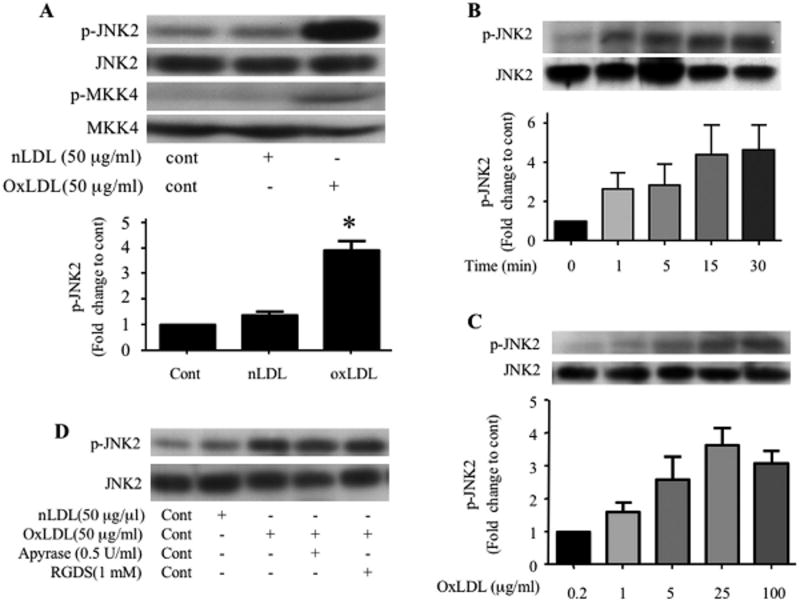
Previously we showed that oxidized LDL formed in the setting of hyperlipidemia and oxidant stress binds platelets and promotes platelet activation via CD36 (also see Supplement figure S1)17, however, the underlying mechanism by which platelet CD36 transduces signals and contributes to platelet activation is unknown. Since CD36 signaling in a variety of cell types involves MAP kinases and we recently showed that the specific MAP kinase JNK was a key component of a macrophage CD36-initiated signaling cascade induced by oxLDL20, we investigated whether JNK was involved in platelet CD36 signaling induced by oxLDL. We assessed the phosphorylation state of JNK by immunoblot and found that human platelets exposed to oxLDL (50 μg/ml) but not native LDL, had a 3-5 fold increase in phosphorylation of JNK2 and its upstream activator MKK4 (Figure 1A). Phosphorylation was time-dependent (Figure 1B) with detectable levels after one minute and reaching maximum by 15 minutes. At these time points neither native LDL or buffer alone effected JNK phosphorylation (data not shown). JNK phosphorylation was also concentration-dependent (Figure 1C). Concentrations as low as 5 μg/ml were effective at activating JNK with maximal effect seen at 25μg/ml. Treatment of platelets with an RGDS peptide (1 mM) to block fibrinogen binding, or the ADP scavenger apyrase (0.5 U/ml) did not block JNK activation by oxLDL, indicating that phosphorylation was not dependent on outside-in integrin signals or secreted ADP (Figure 1D).
Figure 1. OxLDL induces phosphorylation of JNK2 and MKK4 in platelets.
Washed human platelets (2 × 108/ml) containing 2 mM CaCl2 and 1 mM MgCl2 were incubated with native LDL or various concentrations of oxLDL over varying time points and then lysed. The lysates were analyzed by immunoblot with antibodies specific for phospho–JNK (p-JNK2, A, B, C), phospho-MKK4 (p-MKK4, A). The membranes were then stripped and re-probed with antibodies to the total relevant proteins to normalize the protein loaded. (D) Platelets were incubated with 50 μg/ml native LDL (Lane 2) or oxLDL with PBS (Lane 3), 0.5 U/ml apyrase (Lane 4) or 1 mM RGDS (Lane 5) for 5 minutes and then lysed. The lysates were analyzed by immunoblot as above for phospho-JNK (p-JNK) and total JNK. Results are representative of at least 3 independent experiments from different donors. The bar graph represents quantification of the phosphorylation of JNK2 (ratio of phosphorylated/total) expressed as relative values when compared with platelets without any treatment (A, B) or platelets treated with 0.2 μg/ml oxLDL (C). n=5 for A, n=3 for B and n=4 for C. c- control, nLDL- native LDL, oxLDL-oxidized LDL, * p < 0.05 when compared to control or nLDL treatment.
Elevated levels of platelet JNK phorphorylation in vivo are associated with hyperlipidemia and are CD36-dependent
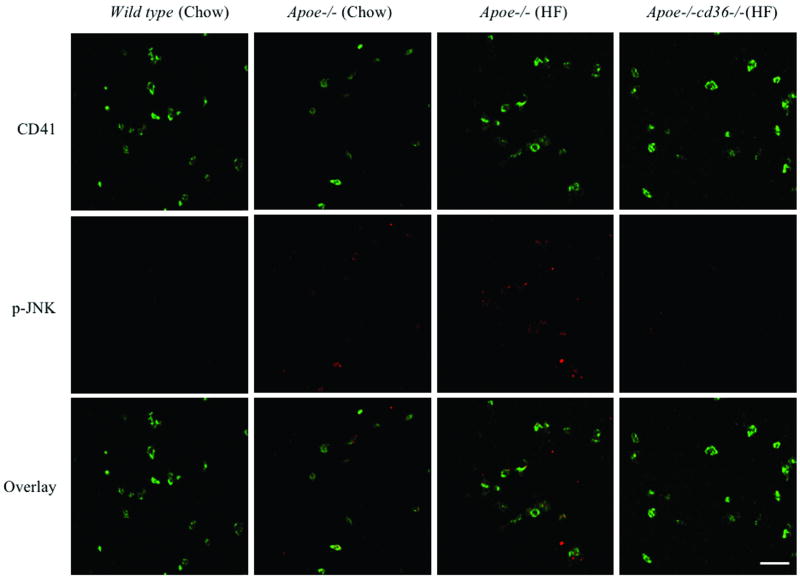
We recently identified specific oxidized phospholipids in oxLDL that serve as high affinity ligands for CD369. These oxidized lipids are present in the plasma of western diet fed mice rendered hyperlipidemic by genetic deletion of apoe and transduce pro-thrombotic signals in a CD36 dependent manner17. To characterize these signals we isolated PRP from WT, apoe-/-, and apoe-/-;cd36-/- mice fed normal chow or high fat “western” diets and examined the platelets by immunofluorescence microscopy for the presence of phospho-JNK. As shown in Figure 2A and 2B, there was minimal phospho-JNK2 expression in resting platelets from WT mice fed a chow diet. Platelets from apoe-/- mice on chow diet showed a modest increase in JNK phosphorylation whereas platelets from apoe-/- mice on the western diet showed a marked increase in phospho-JNK staining (p<0.001 compared to those from either of the chow fed strains). Diet-induced JNK phosphorylation was completely eliminated in apoe-/- mice that were also deficient in CD36. Flow cytometry analysis of platelets in suspension confirmed that those from apoe-/-;cd36-/- mice on western diet had significantly lower levels of phospho-JNK than those from apoe-/- mice on western diet did (n=3, P< 0.05, data not shown). As additional confirmation we also examined the level of phospho-JNK by immunoblot (Figure 2C) and found an increase in platelets from from apoe-/- mice on western diet compared to platelets from apoe-/- mice on chow diet. The increase was not seen in platelets from apoe-/-;cd36-/- mice on western diet. These data demonstrate that the interaction between endogenous oxidized lipid ligands and CD36 triggers a signaling cascade leading to JNK activation in platelets.
Figure 2. Basal phosphorylation of JNK is increased in resting platelets from hyperlipidemic mice in a CD36-dependent manner.
(A) Platelets from WT and apoe-/- mice maintained on normal chow diet and apoe-/- mice and apoe-/-;cd36-/- mice maintained on high fat (HF) diet for 3 months at the age of 6 weeks were stained for phosphorylated JNK (Red), CD41 (Green) was stained simultaneously for platelet identification. Original magnification was 63×4 for all panels. Scale bar=20 μm. (B) Fluorescence intensity of 30 randomly selected platelets from 3 different mice (10 for each from 3 random fields) were determined with ImageJ software, and adjusted to the area of CD41 staining of the same platelet, used for p-JNK quantification. The bar graph represents Mean ± SE fluorescence values. (C) The lysates of platelets from apoe-/- mice maintained on normal chow diet and apoe-/- mice and apoe-/-;cd36-/- mice maintained on high fat (HF) diet for 3 months were analyzed by immunoblotting for the level of phospho-JNK as well as total JNK. The bar graph represents quantification of the phosphorylation of JNK (ratio of phosphorylated/total).
OxLDL-induced JNK2 phosphorylation is mediated by src family kinases
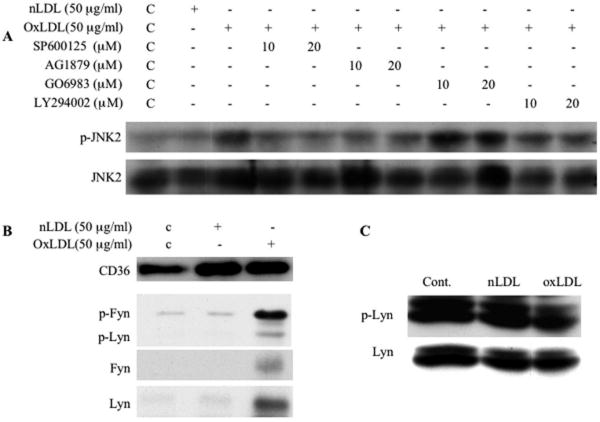
The mechanism by which oxLDL induces JNK phosphorylation was assessed using a panel of specific pharmacological inhibitors. Pre-treatment of platelets with the broad spectrum src kinase inhibitor AG1879 blocked oxLDL induced JNK2 activation, while the PI3 Kinase inhibitor LY294002 and the broadly active PKC inhibitor GO6983 had no effect (Figure 3A). These data suggest that JNK activation is downstream of src family kinases and independent of PI3K or PKC signaling pathways.
Figure 3. Recruitment of src family kinases to platelet CD36 is essential for oxLDL-mediated signaling.
(A) Human platelets were incubated with the JNK inhibitor SP600125, src inhibitor AG1879, PKC inhibitor GO6983 or PI3K inhibitor LY294002 for 30 minutes prior to incubation with 50 μg/ml oxLDL. The platelet lysates were then analyzed by immunoblot as in figure 1 for JNK phosphorylation. (B) Platelets were incubated with oxLDL or native LDL and then lysed. CD36 was precipitated by FA6 anti-CD36 IgG. Precipitates were analyzed by immunoblot with antibodies to CD36, phospho-src (Y416), Fyn and Lyn. (C) Platelets were incubated with 50 μg/ml oxLDL or native LDL and then lysed. Lyn was immunoprecipitated from lysates and the precipitates were analyzed by immunoblot with a phosphotyrosine antibody (4G10) and Lyn antibody.
Ligand induced recruitment of Fyn and Lyn kinases by platelet CD36 is essential for oxLDL-induced signaling events
Previous studies demonstrated that the specific src family kinases Fyn, Lyn, and Yes were co-precipitated from platelet membrane lysates with anti-CD36 antibodies18. In addition, Fyn and/or Lyn has/have been shown to be involved in CD36-initiated signaling leading to MAP kinase activation in macrophages, microglia and endothelial cells19, 20, 32. These studies suggest that the association between CD36 and Fyn and/or Lyn may have a functional role in CD36-mediated signaling in platelets. To test this hypothesis, we performed immunoprecipitation with anti-CD36 monoclonal antibody FA6 and examined the precipitates for the presence of src kinases and their activation state. The amounts of Fyn and Lyn in CD36 immunoprecipitates were markedly increased upon oxLDL treatment (Figure 3B). Src family kinases have two tyrosine phosphorylation sites; phosphorylation in the activation loop increases kinase activity while phosphorylation in the C terminus renders the kinases inactive. We used an antibody specific to the phosphotyrosine in the activation loop and found that the Fyn and to a lesser extent Lyn recruited to CD36 after oxLDL exposure were in the “active” state (Figure 3B). In contrast, oxLDL did not increase the total amount of “active” tyrosine phosphorylated Fyn or Lyn in the non-CD36-associated fraction (data not shown). We also performed immunoprecipitation with anti-Lyn antibody and probed the tyrosine phosphorylation state with a phosphotyrosine specific antibody 4G10 and found that there was no significant increase in total Lyn tyrosine phosphorylation after exposure to oxLDL (Figure 3C). In sum, these data suggest that recruitment of activated Fyn and Lyn to CD36 in response to oxLDL is a key step in CD36-mediated signaling leading to JNK2 phosphorylation.
OxLDL-induced activation of platelets in vitro is mediated by JNK and src family kinases
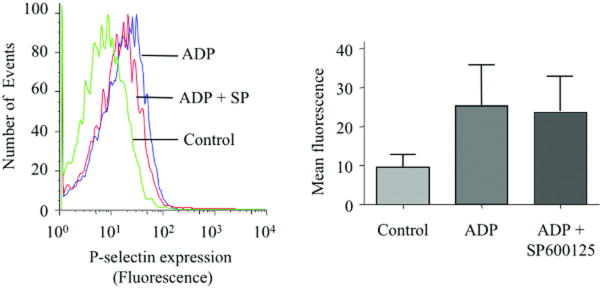
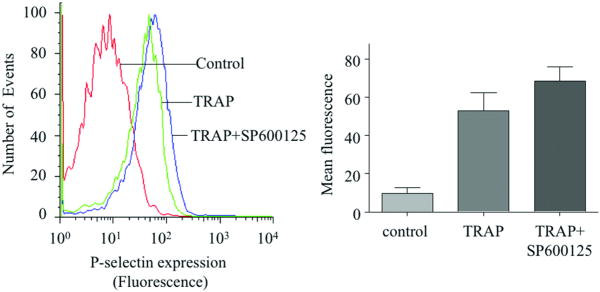
We next used pharmacological inhibitors to study the functional role of JNK and src kinases in oxLDL-induced platelet activation, using a flow-cytometry based assay for surface exposure of P-selectin as a marker for platelet activation. We found that specific pharmacological inhibition of JNK by SP600125 markedly reduced platelet activation in response to oxLDL (∼40% inhibition) (Figure 4A). The inhibitor had minimal effect on platelet activation by other agonists as exemplified by ADP or TRAP (SFLLRN) (Figure 4B, C). We also found that inhibition of src family kinases by AG1879 blocked oxLDL-induced platelet activation (∼55% inhibition) (Figure 4D). These results show that JNK and src family kinases are required for oxLDL-induced platelet activation and suggest that JNK is specific to oxLDL-initiated platelet signaling.
Figure 4. OxLDL-induced platelet activation of platelets is mediated by JNK and src kinases.
(A-C) Human platelets were incubated with the specific JNK inhibitor SP600125 (final concentration of 20 uM) for 30 minutes prior to incubation with 50 μg/ml oxLDL (A) or 10 μM ADP (B) or 2 μM TRAP (SFLLRN) (C) and analyzed by flow cytometry with PE-labeled anti-P-selectin antibody. (D) Platelets were incubated with src family kinase inhibitor AG1879 (10 uM) for 30 minutes prior to incubation with 50 μg/ml oxLDL. Control platelets were treated with the vehicle, DMSO. Results are representative of at least 3 independent experiments from different donors. The bar graph represents mean fluorescence intensities measured with flow cytometry. The error bars are expressed as Mean ± SE. n=4 for A or n=3 for B, C, D.
CD36-dependent activation of JNK promotes in vivo thrombus formation
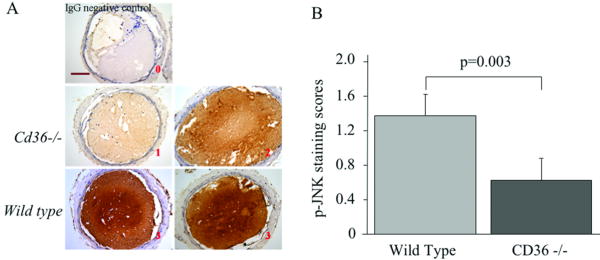
To determine if JNK signaling occurs during thrombosis in vivo we performed immunohistochemical analysis of carotid artery thrombi induced in mice by FeCl3 injury using a specific antibody for phospho-JNK. As shown in Figure 5A, phospho-JNK was detected in thrombi from both WT and cd36-/- mice. The staining intensity, however, was significantly lower (p=0.003) in thrombi from cd36-/- mice, suggesting that CD36-mediated JNK signaling occurred during thrombus formation in vivo (Figure 5B). As an alternative approach to quantify phospho-JNK levels we dissected carotid artery thrombi from WT and cd36-/- mice and examined pooled lysates from 9 thrombi in each group by immunoblot. Thrombi from cd36-/- mice had ∼16% less phospho-JNK than those from WT mice (supplement figure S2). These data suggest that CD36 contributed to JNK phosphorylation during thrombus formation in vivo.
Figure 5. JNK is phosphorylated during thrombus formation in a CD36-dependent manner.
(A) Representative images of Immunohistochemical detection of phosphorylated JNK (p-JNK) in carotid thrombi from WT and cd36-/- mice. No staining with non-immune IgG control showed specificity. Brown indicated positive staining. Red font number indicates the score of the appropriate image. Scale bar=100 μm. (B) The bar graph represents Mean ± SE of staining score of 5 sections from 3 thrombi in each group.
Platelet JNK inhibition prolonged time to thrombosis in a CD36-dependent manner
To define the functional effect of JNK signaling in thrombosis we transfused platelets pre-treated with the JNK inhibitor SP600125 into mice rendered severely thrombocytopenic by irradiation, and then monitored carotid artery thrombus formation in vivo in response to injury with 12.5% FeCl3. As shown in Figure 6, inhibition of platelet JNK significantly prolonged the time to thrombosis in mice transfused with WT platelets (2-sample t test, p=0.01) but had no effect in mice transfused with cd36-/- platelets (2–sample t test, p=0.37). Supplement figure S3 shows representative fluorescence images from these studies. These data strongly suggest that CD36 mediated JNK signaling promotes platelet activation and thrombus formation in vivo.
Figure 6. JNK inhibition prolongs occlusion times in a CD36 dependent manner in vivo after carotid artery injury.
Carotid thrombosis times were assessed by intravital video microscopy in irradiated thrombocytopenic WT and cd36-/- mice transfused with platelets from donor animals of identical genotype. Arteries were injured by topical application of FeCl3 (12.5%). Platelets were incubated with the JNK inhibitor SP600125 (400 nM) or vehicle control for 30 minutes prior to transfusion. Bar graph represents occlusion time. Data are expressed as Mean ± SE (n=5).
Discussion
Earlier studies demonstrated that engagement of platelet CD36 with oxLDL in vitro or with endogenous oxidized phospholipid ligands generated in vivo under hyperlipidemic conditions induced platelet activation17, 33, 34, but the mechanisms by which platelet CD36 acts as a modulator of platelet activity have not been defined. The studies reported here identify the MAP kinase JNK2 as a critical mediator of CD36-dependent platelet signaling. Our work also sheds light on the specific mechanisms linking CD36 and JNK. Earlier studies demonstrated that antibodies to platelet CD36 co-precipitated Fyn, Lyn and Yes, suggesting a physical association with these non-receptor protein tyrosine kinases18. Consistent with this work, we showed that pharmacological inhibition of src kinases abolished CD36-dependent JNK2 phosphorylation and subsequent platelet activation (Figure 3A, 4D). Furthermore, we showed that the “active” phosphorylated form of Fyn and Lyn were recruited to CD36 upon oxLDL treatment (Figure 3B). There was no change in the levels of “active” Fyn or Lyn in the fractions not associated with CD36 (data not shown). These studies thus suggest that CD36 functions to assemble a signaling complex in a ligand-dependent manner.
Using apoe null mice fed a high fat “western” diet as a model of hyperlipidemia and oxidant stress, we showed that the CD36 signaling pathway was activated in vivo, leading to increased basal levels of JNK phosphorylation in resting platelets (Figure 2). With mesenteric and carotid thrombosis models we previously demonstrated that the time to thrombotic occlusion after induction of injury was significantly shorter in hyperlipidemic apoe null mice than in WT mice17. This hyperlipidemia induced pro-thrombotic phenotype was rescued by genetic deletion of CD36 in the apoe null background. We thus hypothesized that the increased basal JNK activity contributed to CD36-dependent platelet hyper-reactivity associated with hyperlipidemia26. We also showed with a carotid injury model in chow-fed mice that phosphorylation of platelet JNK during thrombus formation in vivo was, in part, CD36-dependent and that pharmacological inhibition of platelet JNK produced a significant anti-thrombotic effect, supporting a role for the CD36-JNK signaling axis even under non-hyperlipidemia conditions (Figure 5 and 6). These latter studies suggest that CD36 ligands are generated during arterial injury and are consistent with recent data from our laboratory showing that cd36 null mice are less sensitive to carotid injury (i.e. have longer times to thrombosis)28. The nature of the CD36 ligands remains to be defined, although recent studies suggest the endothelial cell-derived microparticles could function in this capacity28.
Our findings are consistent with recent studies from other laboratories showing that MAP kinases, including JNK have a significant role in platelet biology35-39. Pharmacological inhibition of JNK in a model of arteriolar and venular thrombosis in mice suggested a role in arterial but not venular thrombosis39. It was recently demonstrated that JNK was activated after thrombin exposure and during collagen-induced platelet aggregation39, 40. In the latter process, ADP release was required for JNK activation, although ADP alone was not sufficient to induce JNK activation. In contrast, we demonstrated that oxLDL-induced JNK activation was not dependent on ADP release (Figure 1D). Integrin outside-in signaling was also not required for JNK activation induced by oxLDL, suggesting oxLDL-platelet interactions directly trigger the signaling cascade. We also showed that while pharmacological inhibition of JNK blocked platelet activation by oxLDL, it did not inhibit ADP or TRAP-induced activation (Figure 4A-C), suggesting that JNK may be specific to CD36 signaling. Our studies (not shown) and those of others have shown that another member of the MAP kinase family, p38, is phosphorylated in platelets after exposure to oxLDL33, 41, 42. Whether p38 and JNK work synergistically or independently in the process of platelet activation by oxLDL remains to be determined. The precise function of JNK in platelet biology also remains to be determined. It is known to be involved in a wide variety of diverse cellular processes through transcription-dependent and transcription independent mechanisms. Since platelets are anucleate cells it is unlikely that JNK action would be transcription-dependent. It will be important to define substrates of JNK in platelets to further define its functional role.
Several studies have shown that LDL subjected to various methods of in vitro oxidation, including by exposure to metal ions or HOCL (Hydrochlorous acid), can influence platelet function41-44. Our data clearly identify a central role for the interaction of CD36 with specific oxidized phospholipids within oxLDL in platelet signaling. CD36-specific ligands are generated when LDL is oxidized in vitro and in vivo and have been shown to accumulate in atherosclerotic plaque and to circulate in the blood of patients with hyperlipidemia and atherosclerosis29, 45, 46. OxLDL, however, is a complex particle and can contain a variety of biologically active lipids other than CD36 ligands, including lysophosphatidylcholine (LPC), platelet-activating factor (PAF), lysophosphatidic acid (LPA), and 9- and 13-HODE47. The role of these other lipids in JNK activation remains unknown. Others have shown that LPA in oxLDL can induce platelet shape change via a specific G-protein coupled LPA receptor44, 48. The signaling pathways triggered by LPA involve tyrosine phosphorylation of specific proteins including Syk and an increase of cytosolic Ca2+49, 50. It is also possible that LPA or PAF receptors may work in a synergic way with CD36 and contribute to JNK activation.
Acknowledgments
Sources of Funding. This work was supported by NIH HL81011; NHLBI Specialized Center for Clinically Oriented Research (SCCOR) in Thrombosis (RLS and MF) and American Heart Association Predoctoral Fellowship (0715088B) (KC).
Footnotes
Disclosures: None.
Subject codes: [92] Platelets; [138] Cell signal/Signal transduction; [186] Platelet function inhibitors.
References
- 1.Knowles DM, 2nd, Tolidjian B, Marboe C, D'Agati V, Grimes M, Chess L. Monoclonal anti-human monocyte antibodies OKM1 and OKM5 possess distinctive tissue distributions including differential reactivity with vascular endothelium. J Immunol. 1984;132:2170–2173. [PubMed] [Google Scholar]
- 2.Rhinehart-Jones T, Greenwalt DE. A detergent-sensitive 113-kDa conformer/complex of CD36 exists on the platelet surface. Archives of biochemistry and biophysics. 1996;326:115–118. doi: 10.1006/abbi.1996.0054. [DOI] [PubMed] [Google Scholar]
- 3.Abumrad NA, el-Maghrabi MR, Amri EZ, Lopez E, Grimaldi PA. Cloning of a rat adipocyte membrane protein implicated in binding or transport of long-chain fatty acids that is induced during preadipocyte differentiation. Homology with human CD36. The Journal of biological chemistry. 1993;268:17665–17668. [PubMed] [Google Scholar]
- 4.Van Nieuwenhoven FA, Verstijnen CP, Abumrad NA, Willemsen PH, Van Eys GJ, Van der Vusse GJ, Glatz JF. Putative membrane fatty acid translocase and cytoplasmic fatty acid-binding protein are co-expressed in rat heart and skeletal muscles. Biochemical and biophysical research communications. 1995;207:747–752. doi: 10.1006/bbrc.1995.1250. [DOI] [PubMed] [Google Scholar]
- 5.Silverstein RL, Asch AS, Nachman RL. Glycoprotein IV mediates thrombospondin-dependent platelet-monocyte and platelet-U937 cell adhesion. The Journal of clinical investigation. 1989;84:546–552. doi: 10.1172/JCI114197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oquendo P, Hundt E, Lawler J, Seed B. CD36 directly mediates cytoadherence of Plasmodium falciparum parasitized erythrocytes. Cell. 1989;58:95–101. doi: 10.1016/0092-8674(89)90406-6. [DOI] [PubMed] [Google Scholar]
- 7.Silverstein RL, Nachman RL. Thrombospondin binds to monocytes-macrophages and mediates platelet-monocyte adhesion. The Journal of clinical investigation. 1987;79:867–874. doi: 10.1172/JCI112896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simantov R, Febbraio M, Silverstein RL. The antiangiogenic effect of thrombospondin-2 is mediated by CD36 and modulated by histidine-rich glycoprotein. Matrix Biol. 2005;24:27–34. doi: 10.1016/j.matbio.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 9.Podrez EA, Poliakov E, Shen Z, Zhang R, Deng Y, Sun M, Finton PJ, Shan L, Gugiu B, Fox PL, Hoff HF, Salomon RG, Hazen SL. Identification of a novel family of oxidized phospholipids that serve as ligands for the macrophage scavenger receptor CD36. The Journal of biological chemistry. 2002;277:38503–38516. doi: 10.1074/jbc.M203318200. [DOI] [PubMed] [Google Scholar]
- 10.Greenberg ME, Sun M, Zhang R, Febbraio M, Silverstein R, Hazen SL. Oxidized phosphatidylserine-CD36 interactions play an essential role in macrophage-dependent phagocytosis of apoptotic cells. The Journal of experimental medicine. 2006;203:2613–2625. doi: 10.1084/jem.20060370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ibrahimi A, Bonen A, Blinn WD, Hajri T, Li X, Zhong K, Cameron R, Abumrad NA. Muscle-specific overexpression of FAT/CD36 enhances fatty acid oxidation by contracting muscle, reduces plasma triglycerides and fatty acids, and increases plasma glucose and insulin. The Journal of biological chemistry. 1999;274:26761–26766. doi: 10.1074/jbc.274.38.26761. [DOI] [PubMed] [Google Scholar]
- 12.Medeiros LA, Khan T, El Khoury JB, Pham CL, Hatters DM, Howlett GJ, Lopez R, O'Brien KD, Moore KJ. Fibrillar amyloid protein present in atheroma activates CD36 signal transduction. The Journal of biological chemistry. 2004;279:10643–10648. doi: 10.1074/jbc.M311735200. [DOI] [PubMed] [Google Scholar]
- 13.Hoebe K, Georgel P, Rutschmann S, Du X, Mudd S, Crozat K, Sovath S, Shamel L, Hartung T, Zahringer U, Beutler B. CD36 is a sensor of diacylglycerides. Nature. 2005;433:523–527. doi: 10.1038/nature03253. [DOI] [PubMed] [Google Scholar]
- 14.Febbraio M, Hajjar DP, Silverstein RL. CD36: a class B scavenger receptor involved in angiogenesis, atherosclerosis, inflammation, and lipid metabolism. The Journal of clinical investigation. 2001;108:785–791. doi: 10.1172/JCI14006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aiken ML, Ginsberg MH, Byers-Ward V, Plow EF. Effects of OKM5, a monoclonal antibody to glycoprotein IV, on platelet aggregation and thrombospondin surface expression. Blood. 1990;76:2501–2509. [PubMed] [Google Scholar]
- 16.Silverstein RL, LaSala J, Pearce SF. CD36 cluster report. In: Schlossman SF, Boumsell L, Gilks W, Harlan JM, Kishimoto T, Morimoto C, Ritz J, Silverstein RL, Springer TA, Tedder TF, Todd RF, editors. Leucocyte Typing V: White Cell Differentiation Antigens. Oxford, England: Oxford University Press; 1995. pp. 1271–1274. Vol. [Google Scholar]
- 17.Podrez EA, Byzova TV, Febbraio M, Salomon RG, Ma Y, Valiyaveettil M, Poliakov E, Sun M, Finton PJ, Curtis BR, Chen J, Zhang R, Silverstein RL, Hazen SL. Platelet CD36 links hyperlipidemia, oxidant stress and a prothrombotic phenotype. Nature medicine. 2007;13:1086–1095. doi: 10.1038/nm1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang MM, Bolen JB, Barnwell JW, Shattil SJ, Brugge JS. Membrane glycoprotein IV (CD36) is physically associated with the Fyn, Lyn, and Yes protein-tyrosine kinases in human platelets. Proc Natl Acad Sci U S A. 1991;88:7844–7848. doi: 10.1073/pnas.88.17.7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jimenez B, Volpert OV, Crawford SE, Febbraio M, Silverstein RL, Bouck N. Signals leading to apoptosis-dependent inhibition of neovascularization by thrombospondin-1. Nature medicine. 2000;6:41–48. doi: 10.1038/71517. [DOI] [PubMed] [Google Scholar]
- 20.Rahaman SO, Lennon DJ, Febbraio M, Podrez EA, Hazen SL, Silverstein RL. A CD36-dependent signaling cascade is necessary for macrophage foam cell formation. Cell Metab. 2006;4:211–221. doi: 10.1016/j.cmet.2006.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dangelmaier C, Jin J, Daniel JL, Smith JB, Kunapuli SP. The P2Y1 receptor mediates ADP-induced p38 kinase-activating factor generation in human platelets. Eur J Biochem. 2000;267:2283–2289. doi: 10.1046/j.1432-1327.2000.01235.x. [DOI] [PubMed] [Google Scholar]
- 22.Sakurai K, Matsuo Y, Sudo T, Takuwa Y, Kimura S, Kasuya Y. Role of p38 mitogen-activated protein kinase in thrombus formation. Journal of receptor and signal transduction research. 2004;24:283–296. doi: 10.1081/rrs-200040324. [DOI] [PubMed] [Google Scholar]
- 23.Endemann G, Stanton LW, Madden KS, Bryant CM, White RT, Protter AA. CD36 is a receptor for oxidized low density lipoprotein. The Journal of biological chemistry. 1993;268:11811–11816. [PubMed] [Google Scholar]
- 24.Kuniyasu A, Ohgami N, Hayashi S, Miyazaki A, Horiuchi S, Nakayama H. CD36-mediated endocytic uptake of advanced glycation end products (AGE) in mouse 3T3-L1 and human subcutaneous adipocytes. FEBS letters. 2003;537:85–90. doi: 10.1016/s0014-5793(03)00096-6. [DOI] [PubMed] [Google Scholar]
- 25.Ohgami N, Nagai R, Ikemoto M, Arai H, Miyazaki A, Hakamata H, Horiuchi S, Nakayama H. CD36, serves as a receptor for advanced glycation endproducts (AGE) Journal of diabetes and its complications. 2002;16:56–59. doi: 10.1016/s1056-8727(01)00208-2. [DOI] [PubMed] [Google Scholar]
- 26.Platt N, da Silva RP, Gordon S. Recognizing death: the phagocytosis of apoptotic cells. Trends in cell biology. 1998;8:365–372. doi: 10.1016/s0962-8924(98)01329-4. [DOI] [PubMed] [Google Scholar]
- 27.Savill J, Hogg N, Ren Y, Haslett C. Thrombospondin cooperates with CD36 and the vitronectin receptor in macrophage recognition of neutrophils undergoing apoptosis. The Journal of clinical investigation. 1992;90:1513–1522. doi: 10.1172/JCI116019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghosh A, Li W, Febbraio M, Espinola RG, McCrae KR, Cockrell E, Silverstein RL. Platelet CD36 mediates interactions with endothelial cell-derived microparticles and contributes to thrombosis in mice. The Journal of clinical investigation. 2008;118:1934–1943. doi: 10.1172/JCI34904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Witztum JL, Steinberg D. Role of oxidized low density lipoprotein in atherogenesis. The Journal of clinical investigation. 1991;88:1785–1792. doi: 10.1172/JCI115499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eitzman DT, Westrick RJ, Xu Z, Tyson J, Ginsburg D. Hyperlipidemia promotes thrombosis after injury to atherosclerotic vessels in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2000;20:1831–1834. doi: 10.1161/01.atv.20.7.1831. [DOI] [PubMed] [Google Scholar]
- 31.Erden O, Imir A, Guvenal T, Muslehiddinoglu A, Arici S, Cetin M, Cetin A. Investigation of the effects of heparin and low molecular weight heparin on E-cadherin and laminin expression in rat pregnancy by immunohistochemistry. Human reproduction (Oxford, England) 2006;21:3014–3018. doi: 10.1093/humrep/del262. [DOI] [PubMed] [Google Scholar]
- 32.Moore KJ, El Khoury J, Medeiros LA, Terada K, Geula C, Luster AD, Freeman MW. A CD36-initiated signaling cascade mediates inflammatory effects of beta-amyloid. The Journal of biological chemistry. 2002;277:47373–47379. doi: 10.1074/jbc.M208788200. [DOI] [PubMed] [Google Scholar]
- 33.Korporaal SJ, Van Eck M, Adelmeijer J, Ijsseldijk M, Out R, Lisman T, Lenting PJ, Van Berkel TJ, Akkerman JW. Platelet activation by oxidized low density lipoprotein is mediated by CD36 and scavenger receptor-A. Arteriosclerosis, thrombosis, and vascular biology. 2007;27:2476–2483. doi: 10.1161/ATVBAHA.107.150698. [DOI] [PubMed] [Google Scholar]
- 34.Volf I, Moeslinger T, Cooper J, Schmid W, Koller E. Human platelets exclusively bind oxidized low density lipoprotein showing no specificity for acetylated low density lipoprotein. FEBS letters. 1999;449:141–145. doi: 10.1016/s0014-5793(99)00437-8. [DOI] [PubMed] [Google Scholar]
- 35.Li Z, Zhang G, Feil R, Han J, Du X. Sequential activation of p38 and ERK pathways by cGMP-dependent protein kinase leading to activation of the platelet integrin alphaIIb beta3. Blood. 2006;107:965–972. doi: 10.1182/blood-2005-03-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mazharian A, Roger S, Berrou E, Adam F, Kauskot A, Nurden P, Jandrot-Perrus M, Bryckaert M. Protease-activating receptor-4 induces full platelet spreading on a fibrinogen matrix: involvement of ERK2 and p38 and Ca2+ mobilization. The Journal of biological chemistry. 2007;282:5478–5487. doi: 10.1074/jbc.M609881200. [DOI] [PubMed] [Google Scholar]
- 37.Song S, Freedman J, Mody M, Lazarus AH. Porcine von Willebrand factor and thrombin induce the activation of c-Jun amino-terminal kinase (JNK/SAPK) whereas only thrombin induces activation of extracellular signal-related kinase 2 (ERK2) in human platelets. British journal of haematology. 2000;109:851–856. doi: 10.1046/j.1365-2141.2000.02126.x. [DOI] [PubMed] [Google Scholar]
- 38.Shankar H, Garcia A, Prabhakar J, Kim S, Kunapuli SP. P2Y12 receptor-mediated potentiation of thrombin-induced thromboxane A2 generation in platelets occurs through regulation of Erk1/2 activation. J Thromb Haemost. 2006;4:638–647. doi: 10.1111/j.1538-7836.2006.01789.x. [DOI] [PubMed] [Google Scholar]
- 39.Kauskot A, Adam F, Mazharian A, Ajzenberg N, Berrou E, Bonnefoy A, Rosa JP, Hoylaerts MF, Bryckaert M. Involvement of the mitogen-activated protein kinase c-Jun NH2-terminal kinase 1 in thrombus formation. The Journal of biological chemistry. 2007;282:31990–31999. doi: 10.1074/jbc.M701596200. [DOI] [PubMed] [Google Scholar]
- 40.Bugaud F, Nadal-Wollbold F, Levy-Toledano S, Rosa JP, Bryckaert M. Regulation of c-jun-NH2 terminal kinase and extracellular-signal regulated kinase in human platelets. Blood. 1999;94:3800–3805. [PubMed] [Google Scholar]
- 41.Coleman LG, Jr, Polanowska-Grabowska RK, Marcinkiewicz M, Gear AR. LDL oxidized by hypochlorous acid causes irreversible platelet aggregation when combined with low levels of ADP, thrombin, epinephrine, or macrophage-derived chemokine (CCL22) Blood. 2004;104:380–389. doi: 10.1182/blood-2003-08-2961. [DOI] [PubMed] [Google Scholar]
- 42.Korporaal SJ, Gorter G, van Rijn HJ, Akkerman JW. Effect of oxidation on the platelet-activating properties of low-density lipoprotein. Arteriosclerosis, thrombosis, and vascular biology. 2005;25:867–872. doi: 10.1161/01.ATV.0000158381.02640.4b. [DOI] [PubMed] [Google Scholar]
- 43.Ardlie NG, Selley ML, Simons LA. Platelet activation by oxidatively modified low density lipoproteins. Atherosclerosis. 1989;76:117–124. doi: 10.1016/0021-9150(89)90094-4. [DOI] [PubMed] [Google Scholar]
- 44.Siess W, Zangl KJ, Essler M, Bauer M, Brandl R, Corrinth C, Bittman R, Tigyi G, Aepfelbacher M. Lysophosphatidic acid mediates the rapid activation of platelets and endothelial cells by mildly oxidized low density lipoprotein and accumulates in human atherosclerotic lesions. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:6931–6936. doi: 10.1073/pnas.96.12.6931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Esterbauer H, Gebicki J, Puhl H, Jurgens G. The role of lipid peroxidation and antioxidants in oxidative modification of LDL. Free Radic Biol Med. 1992;13:341–390. doi: 10.1016/0891-5849(92)90181-f. [DOI] [PubMed] [Google Scholar]
- 46.Sevanian A, Bittolo-Bon G, Cazzolato G, Hodis H, Hwang J, Zamburlini A, Maiorino M, Ursini F. LDL- is a lipid hydroperoxide-enriched circulating lipoprotein. J Lipid Res. 1997;38:419–428. [PubMed] [Google Scholar]
- 47.Siess W. Platelet interaction with bioactive lipids formed by mild oxidation of low-density lipoprotein. Pathophysiology of haemostasis and thrombosis. 2006;35:292–304. doi: 10.1159/000093222. [DOI] [PubMed] [Google Scholar]
- 48.Essler M, Retzer M, Bauer M, Zangl KJ, Tigyi G, Siess W. Stimulation of platelets and endothelial cells by mildly oxidized LDL proceeds through activation of lysophosphatidic acid receptors and the Rho/Rho-kinase pathway. Inhibition by lovastatin. Annals of the New York Academy of Sciences. 2000;905:282–286. doi: 10.1111/j.1749-6632.2000.tb06561.x. [DOI] [PubMed] [Google Scholar]
- 49.Maschberger P, Bauer M, Baumann-Siemons J, Zangl KJ, Negrescu EV, Reininger AJ, Siess W. Mildly oxidized low density lipoprotein rapidly stimulates via activation of the lysophosphatidic acid receptor Src family and Syk tyrosine kinases and Ca2+ influx in human platelets. The Journal of biological chemistry. 2000;275:19159–19166. doi: 10.1074/jbc.M910257199. [DOI] [PubMed] [Google Scholar]
- 50.Retzer M, Siess W, Essler M. Mildly oxidised low density lipoprotein induces platelet shape change via Rho-kinase-dependent phosphorylation of myosin light chain and moesin. FEBS letters. 2000;466:70–74. doi: 10.1016/s0014-5793(99)01762-7. [DOI] [PubMed] [Google Scholar]